Abstract
Folium Sennae are widely used around the world, mainly in purging and removal of endogenous active substances, such as anthraquinone and its derivatives. However, the potential toxicity of anthraquinones to the liver, kidney, and intestinal limits the application of Folium Sennae. In this study, we aimed at safe regulation of Folium Sennae to degrade anthraquinones, boosting medicinal properties and reducing toxicity and potency with Monascus fermentation. Monascus strains H1102 for Folium Sennae fermentation were selected as the initial strain which was capable of producing high yields of functional pigment and low yields of hazardous citrinin. The anthraquinone degradation rate reached 41.2%, with 212.2 U mL−1 of the pigment and approximately 0.038 mg L−1 of the citrinin under optimal fermentation conditions followed by response surface streamlining, which met the requirements of reducing toxicity, increasing efficiency of Monascus fermented Folium Sennae. Furthermore, the Monascus/Folium Sennae culture had no observable toxic effect on HK-2 and L-02 cells in vitro and further inhibited cell apoptosis and necrosis. Overall, our results showed that Monascus fermentation could provide an alternative strategy for toxicity reduction of herbal medicines as well as efficacy enhancement.
Keywords: Monascus, Folium sennae, Anthraquinone, Toxicity reduce and effect enhancement
1. Introduction
Anthraquinones are found in herbal plants such as Folium Sennae harboring a broad spectrum of biological activities that hold a number of roles including laxation [1], anti-inflammatory [2], immunomodulatory [3], anti-hyperlipidemic [4] and anti-cancer agents [5]. However, the long-term use of anthraquinone laxatives is reported to cause morphological changes in the colonic muscular system particularly the enteric nervous systems and smooth muscles [6,7]. In addition, high doses of anthraquinones may inhibit hepatocyte proliferation, leading to cell shrinkage, vacuolization, mitochondrial membrane reduction, and hepatotoxicity [8,9]. Excessive anthraquinones can also cause morphological changes in HK-2 cells inhibiting cell growth, block changes of the cell cycle and triggering apoptosis, which leads to nephrotoxicity in turn. Due to these safety and health concerns, the European Medicines Agency published a draft report on the evaluation of Folium Sennae in 2017 (https://www.ema.europa.eu/en/medicines/herbal/sennae-fructus), stating that long-term administration of free anthraquinone-containing drugs (such as emodin and aloe-emodin) could lead to hyperpigmentation of the colon and cecum intestinal wall within 4–13 months. In addition, due to their potential genotoxic and carcinogenic properties, the daily dose and cycle length of free anthraquinone-containing laxatives have been restricted and banned for children and lactating women. Therefore, it is crucial to reduce the toxicity of anthraquinones in Folium Sennae.
Fermentations of herbal medicines may increase the content of active ingredients, enhancing the efficacy of medicines. It can also trigger the generation of novel active ingredients, improving taste as well as reducing the toxic side effects of herbal medicines. Toxic aconitine in raw aconite which could be transformed into low-toxic or non-toxic components through fermentation [10]. Fermentation can also improve the medicinal properties of Rheum palmatum L., effectively degrading anthraquinone content and reducing toxicity to improve its utilization. However, studies on the attenuation of anthraquinones in Folium Sennae by microbial fermentation have not been well reported.
Monascus, a small filamentous saprophytic fungus, plays an important role in household food consumption and industrial food manufacture [[11], [12], [13]], including the production of rice fermented products [14], natural colorants [15,16], and medicines [17]. Zhao et al. found that fermented Panax ginseng by Monascus ruber regulated lipid metabolism and intestinal flora structure in high fat diet rats, which reduced obesity characteristics and lowered serum total cholesterol, low-density lipoprotein cholesterol and IgA levels [18]. Therefore, the versatility of Monascus offers excellent application potentials for the fermentation of herbal medicines.
In this study, we first tested the performance of Monascus strains stock in our laboratory to screen out Monascus strains with high pigment production and low citrinin production for endogenous anthraquinones degradation with Folium Sennae fermentation. Then, the fermentation condition was optimized based on anthraquinones degradation rate. Moreover, a single-factor optimization of the fermentation process was carried out. In the end, we conducted cytotoxicity test to verify the attenuation and efficiency of Folium Sennae/Monascus fermentation. Our study evidenced that fermentation processing with Monascus paved an effective way for reducing the toxicity and enhancing the efficacy of herbal medicines.
2. Materials and methods
2.1. Microorganisms and materials
Monascus strains (H1102, WM95101, WM95102, ZK-2, ZK-3, ZH4, ZH11, UK, M2, M2-2, 9AC05, PHH-1, IMI250763, ZMI, WUX3, JH2, 9901, 9085, 981, 9909, SJS-3, SJS-32, SJS-21, ZH2002) were all from laboratory storage that were screened during 2019–2021. Folium Sennae samples were provided by Guangzhou Infinity (China) Co. Citrinin standard was purchased from Sigma Aldrich (Shanghai) Co., LTD in June 2019. The four anthraquinone standards were purchased from Shanghai Maclean Biochemical Technology Co. Medium and chemical analysis reagents were purchased from Sinopharm Chemical Reagent Co., LTD. (Shanghai, China). Cell Counting Kit-8 (CCK-8 kit), Annexin V-FITC Apoptosis Detection Kit purchased from Shanghai Biyuntian Biotechnology Co Ltd; Human normal hepatocytes (L-02 cells), provided by Nanjing Agricultural University, Human renal cortical proximal tubular epithelial cells (HK-2 cells), purchased from Kunming Cell Bank, Chinese Academy of Sciences.
2.2. Seed media and cultivation
The 24 strains of Monascus were inoculated onto slant medium (potato dextrose agar) and separately cultured at 30 °C for 5–7 days in a constant temperature incubator. The spores were washed from the inclined surface medium with normal saline and diluted into 1 × 107 spore suspension. The spores were inoculated in liquid seed medium (glucose 60 g, peptone 25 g, NaNO3 1 g, MgSO4 1 g, K2HPO4 1 g, corn pulp 10 mL, distilled water 1000 mL, pH natural) and cultured at 30 °C in a constant temperature incubator. The regular temperature inside the incubator was set at 160 rpm for 2 days.
2.3. Liquid shaker fermentation
The liquid medium (80 g rice flour, 150 mL soybean hydrolysate, MgSO4 2 g, MnSO4 0.03 g, ZnSO4 0.03 g, 12 mL maize pulp, 850 mL distilled water, pH 7.0) was sterilized at 121 °C for 20 min, Then, the cultured seed solution was inoculated at 10% inoculum into 500 mL shake flasks containing 100 mL fermentation medium for 5 days in a rotating shaker at 30 °C, 180 rpm.
2.4. Fermentation of Folium Sennae by Monascus
The medium for Folium Sennae fermentation (10 g Folium Senna powder, 10 g sucrose, 3 g peptone, MgSO4 0.2 g, MnSO4 0.003 g, ZnSO4 0.003 g, 1.2 mL corn pulp, pH 4.5, 100 mL distilled water) was sterilized at 121 °C for 20 min. The cultured seed solution was inoculated at 10% inoculum into a 500 mL triangular flask containing 100 mL of fermentation medium for 5 days in a rotary shaker at 30 °C, 180 rpm.
2.5. Metabolite analyses and calculations
One mL of fermentation broth (mixture of bacteria and fermentation substrate) was taken into a test tube with a certain amount of 70% ethanol solution. Followed by ultrasonic extract for 30 min, the culture was placed in a constant temperature water bath at 60 °C for 1 h of accurate soaking. Then, the filtrate was diluted by 70% ethanol solution after cooling and filtering. An ultraviolet–visible spectrophotometer was employed to perform spectral scanning ranged from 300 to 550 nm, where the characteristic wavelength of Monascus pigment dropped at 505 nm. The content of Monascus pigment was calculated by Equation (1).
| (1) |
A Hitachi Chromater system (Hitachi Company of Japan) was used for HPLC analysis. Two mL of fermentation broth was mixed with 70% ethanol. Then, the broth was extracted in a water bath at 60 °C for 1 h. The supernatant was filtered by 0.22 μm organic membrane for HPLC analysis. Detection conditions: The column was an Agilent Eclipse Plus C18 column (4.6 μm × 150 mm, 5 μm) with a fluorescence detector, λex = 331 nm, λem = 500 nm, mobile phase of acetonitrile: water = 35:65 (v/v), with pH adjusted to 2.5 at a flow rate of 1.0 mL∙min−1. The sample chamber and column temperature were both set at 28 °C, and the injection volume was 10 μL.
2.6. Cell experiments
The L-02 cells and HK-2 cells were chosen to explore inhibitions of by Folium Sennae extract as well as Monascus/Folium Sennae culture. The cells during logarithmic growth were digested with trypsin, followed by centrifugation and resuspension at 5.0 × 104 mL−1. The cells were seeded in 96-well plates containing 200 μL per well, and were incubated for an additional 24 h at 37 °C in a CO2 incubator. Then the culture was treated with Folium Sennae extract and Monascus/Folium Sennae culture for further incubation of extra 48 h. The viability and survival rate of the cells were measured using the cck-8 kit.
Cells in log-phase were digested with trypsin, centrifuged, and resuspended in cell culture medium at 1.0 × 105 mL−1 in small 6 cm diameter dishes at 5 mL per well. After 24 h of culture in a CO2 incubator, the medium was continued for incubation of 48 h when added with Folium Sennae extract or Monascus/Folium Sennae culture. The cells were digested with trypsin and collected, and were stained with both Annexin V-FITC and propidium iodide (PI) stains for 10–20 min, and the staining was performed on a flow cytometer afterwards.
2.7. Data analysis
SPSS 23.0 statistical software was applied to analyze the data. Response surfaces were designed using Design-Expert 10.0 software, and graphs were plotted using Origin 9.0. All samples were subjected to three replicate experiments.
3. Results
3.1. Comparison of degradation of anthraquinone in Folium Sennae fermented by different Monascus strains
In order to screen out the best functional initial strain for Monascus fermentation, Monascus pigment and citrinin were used as evaluation criteria. Laboratory preserved strains H1102, WM95101, WM95102, ZK-2, ZK-3, ZH4, ZH11, UK, M2, M2-2, 9AC05, PHH-1, JN, ZMI, WUX3, JH2, 9901, 9085, 981, 9909, SJS-3, SJS-32, SJS-21 and ZH2002 were tested by liquid shaker fermentation. The inoculum of the above 24 strains of Monascus was 10% for thermostatic incubation for 5 days at 30 °C, 160 rpm. At the end of fermentation, the fermentation broth of 24 strains of Monascus was isolated to determine the content of pigment and citrinin (Fig. 1). The results showed that Monascus H1102 had the highest pigment content of 302.1 U mL−1 and a relatively low citrinin content of 0.64 mg g−1.
Fig. 1.
Determination of performance of series Monascus strains. (a) Monascus pigment content of series Monascus strains. (b) Citrinin content of series Monascus strains.
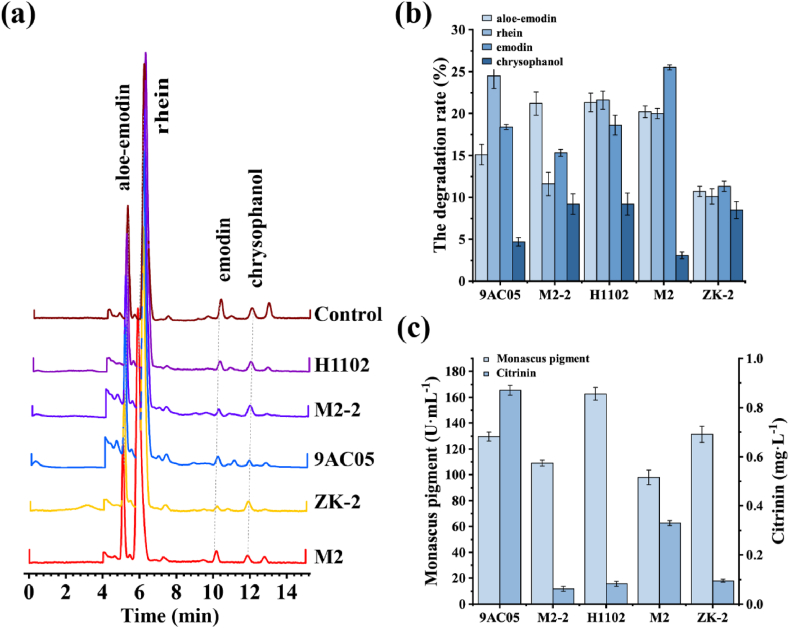
In order to screen the best Monascus strain that ferment Folium Sennae to degrade anthraquinone, five strains H1102, 9AC05, M2-2, M2 and ZK-2 with excellent fermentation performance (high yield of Monascus pigment and low yield of citrinin) were selected for the re-screening validation. At the end of fermentation, the content of anthraquinones, pigment and citrinin were measured in the fermentation broth to identify the best initial fermentation strain (Fig. 2). All the above five strains of Monascus were able to degrade the anthraquinones in Folium Sennae to a certain extent (Fig. 2a–b), among which Monascus H1102, Monascus M2 and Monascus 9AC05 had comparable degradation efficiency, and the degradation rates of aloe-emodin, emodin and rhein of Folium Sennae were all between 15% and 25%. Due to sterilization and constant temperature culture at 121 °C, the glycosidic bonds binding the anthraquinones together might have been broken or hydrolyzed resulting in the release of excess anthraquinones, which led to 2–3 fold of increase in anthraquinones content (Table S1). The fermentation broth was also tested for pigment and citrinin determination (Fig. 2c). Monascus H1102 showed superiority over Monascus M2 and Monascus 9AC05 with a pigment content of 162.6 U mL−1. It was noteworthy that the citrinin content of each strain was significantly reduced, which was only 0.064 mg L−1 detected in Monascus H1102. These observations were consistent with previous research by He et al. who investigated the effect of different flavonoids on citrinin production of Monascus based on 1H NMR, and found that adding flavonoids could reduce the yield of citrinin [19]. Therefore, based on the above measurements of fermentation performance, Monascus H1102 was selected as the initial strain for the fermentation of Folium Sennae.
Fig. 2.
Comparison of anthraquinone degradation performance of Folium Sennae fermented among different Monascus strains. (a) Liquid chromatograms of anthraquinones; (b) Degradation efficiency of four anthraquinones in Folium Sennae by five Monascus strains; (c) Contents of pigment and citrinin in 5 strains of Monascus.
3.2. Optimization of fermentation medium for Folium Sennae of Monascus
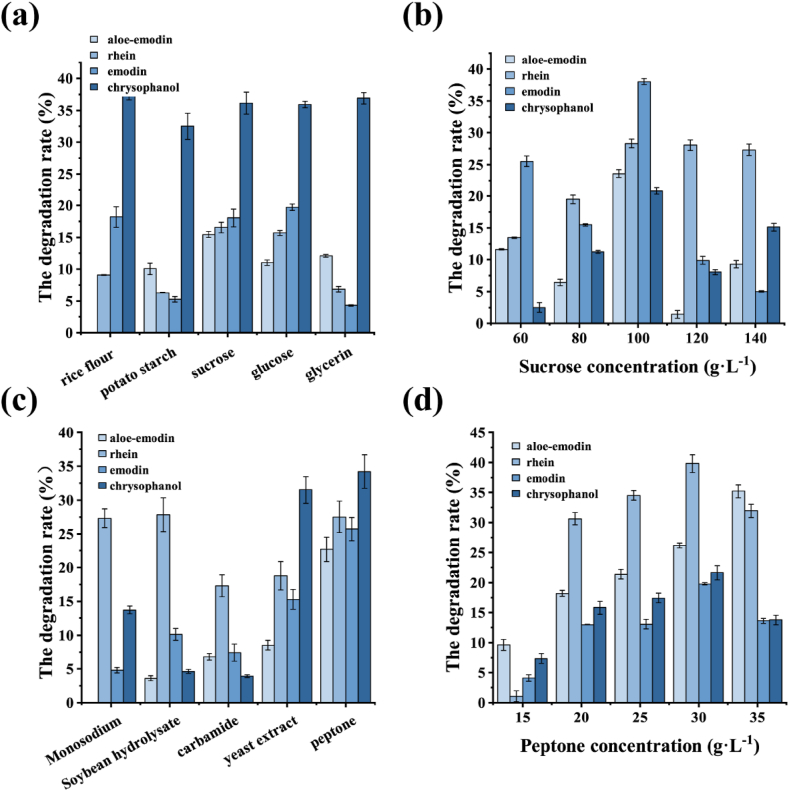
Five carbon sources, sucrose, glucose, rice flour, glycerol and potato starch were selected as estimate factors and added at a uniform level of 80 g L−1. The degradation of four anthraquinones by the five carbon sources was shown in Fig. 3a, chrysophanol pronounced obvious degradation of about 35%, while sucrose was the best carbon source for rhein and aloe-emodin, with 23.6% and 28.4% of degradation rate, respectively. Accordingly, sucrose was selected as the added carbon source for the degradation of anthraquinones given the degradation efficiency of the four anthraquinones.
Fig. 3.
Effects of carbon and nitrogen sources on anthraquinone degradation in Folium Sennae fermented by H1102. (a) Different sorts of carbon source; (b) Different sucrose concentrations; (c) Different sorts of nitrogen source; (d) Different concentrations of peptone.
The effects of sucrose addition at different concentrations on the Monascus H1102 fermentation degradation of anthraquinone were further investigated. Five different levels of sucrose concentrations were investigated (Fig. 3b). The degradation rate trend of four anthraquinones in Folium Sennae with Monascus H1102 showed gradual improvement in the early stages fermentation and decreased later on with increasing sucrose concentration. The four anthraquinones had the highest degradation rates when sucrose concentration was 100 g L−1, with 28.2% and 23.6% of the degradation rates for aloe-emodin and emodin, respectively. In contrast, the degradation efficiency decreased as sucrose concentration increased further, of which the degradation rate of aloe-emodin was only 9.3% when sucrose concentration was 140 g L−1. The possible reason was that at lower sucrose concentration levels, the overall growth of cells was poor, resulting in a low degradation rate of anthraquinone with subsequent increase in cell growth as the sucrose concentration increased. However, high concentrations of sucrose could produce large viscosity and osmotic pressure, affecting the initial growth of the Monascus. Besides, “glucose effect” from sucrose utilization could also lead to large accumulation of organic acid [20]. Therefore, a sucrose concentration of 100 g L−1 was chosen for optimum concentration.
After determining the optimal carbon source and its concentration for the degradation of anthraquinone by fermentation of Folium Sennae by Monascus strain H1102, the effect of nitrogen source on its fermentation for the degradation of anthraquinone was further investigated. A total of 5 organic and inorganic nitrogen sources were chosen as candidates, including sodium glutamate, soybean hydrolysate, urea, yeast extract and peptone. Except for the additional amount of soybean hydrolysate was 200 mL:1000 mL, the remaining amounts were all set at 20 g mL−1. The degradation rate of anthraquinone in Folium Sennae was the highest with peptone, in which the degradation rate of rhein and aloe-emodin were 27.5% and 22.7%, respectively (Fig. 3c). Therefore, peptone was selected as the initial nitrogen source. Furthermore, we explored the effects of different concentrations of peptone on fermentation results. As shown in Fig. 3d, the degradation efficiency of Monascus H1102 on anthraquinone also increased at the beginning with the increase of peptone content. When the concentration of peptone was 30 g L−1, the degradation rate of anthraquinone reached its peak with 39.8% degradation rate of rhein, while the degradation rate of anthraquinones showed a negative correlation with the increase of peptone concentration. It may be resulted from excessive nitrogen source, which caused the hypoxia of the medium to affect the growth of Monascus H1102 and reduce anthraquinones degradation [21]. Therefore, the optimum dosage of peptone was 30 g L−1.
3.3. Optimization of fermentation conditions of Folium Sennae by Monascus
As shown in Fig. 4a, when the inoculation amount was low, the Monascus strain H1102 did not show well growth but with decreased anthraquinones degradation rate. The highest anthraquinone degradation rate was achieved at an inoculum level of 8%. The anthraquinones degradation rate decreased instead as the inoculum level increased. This could be associated with insufficient dissolved oxygen and nutrients due to high cell density and turbidity, which did not meet with the growth and metabolism of the Monascus, thus leading to poor fermentation performance [22]. The yield of Monascus pigment was closely related to thallus growth, of which the pigment content reached to peak when the inoculation amount was 8% (Fig. 4b). Moreover, the content of citrinin was relatively low, with the overall content between 0.054 and 0.087 mg L−1. Monascus H1102 had the best effect on anthraquinones degradation when the ratio of Folium Senna to fermentation broth was 12.5% (Fig. 4c). The degradation rates of aloe-emodin and rhein were 37.4% and 32.7%, respectively, while that of chrysophanol and emodin were both more than 25%. When the solid-liquid ratio was low, the fermentation substrate had a low Folium Sennae content, which was not conducive to the fermentation of Folium Sennae by Monascus H1102. When the solid-liquid ratio was high, the water and dissolved oxygen levels were low due to the water-absorbent properties of Folium Sennae, which affected growth and metabolism. The pigment and citrinin content of Monascus H1102 fermented Folium Sennae were shown in Fig. 4d. In general, the pigment content increased with an increase in solid-liquid ratio, reaching a maximum of 214.5 U mL−1. On the contrary, citrinin decreased with an increased solid-liquid ratio, ranging from 0.026 to 0.057 mg L−1.
Fig. 4.
Effects of inoculation amount, solid-liquid ratio and fluid volume on anthraquinones degradation and pigment production of Folium Sennae fermented H1102. (a) Effects of different inoculum amounts on the degradation rate of anthraquinones; (b) Effects of different inoculum amount on pigment and citrinin contents; (c) Effects of different solid-liquid ratios on the degradation rate of anthraquinones; (d) Effects of solid-liquid ratios on pigment and citrinin contents; (e) Effects of different charge amount on degradation of anthraquinones; (f) Effects of different fluid amount on pigment and citrinin contents.
The effect of the charge volume on the overall fermentation was shown in Fig. 4e. With the increase in the charge volume, the efficiency of the fermentation of Folium Sennae by Monascus increased at the beginning and then decreased. The highest degradation rate of anthraquinones occurred when the fluid volume was 80 mL. With a further increase in the charge volume, the degradation effect of fermented anthraquinones gradually decreased. When the volume of charge was low, it could also lead to insufficient nutrients for growth of Monascus H1102, which slowed its growth and reduced the degradation efficiency of anthraquinone. However, when the fluid volume was high, the lack of dissolved oxygen due to the water absorption of Folium Sennae could not meet the growth requirements of Monascus [21]. It could be seen from Fig. 4f that the pigment content of H1102 showed the same trends after fermentation. Therefore, the optimal fluid volume was 80 mL per 500 mL triangular bottle.
3.4. Response surface optimization of Folium Sennae fermented by Monascus H1102
In order to obtain the optimal fermentation conditions for the degradation of anthraquinones in Folium Sennae by Monascus H1102 strain, we performed response surface optimization for the above single factor optimization of the related technical parameters of Folium Sennae by fluid volume, inoculation amount and solid-liquid ratio. Three levels were selected for each factor, and the degradation rate of anthraquinones was used as the response value. A response surface analysis scheme for three factors and three levels was designed by design-Expert 10.0 software. The experimental scheme was shown in Table S2. The regression model of the anthraquinone degradation rate (Y) of the fermentation of Folium Sennae by Monascus H1102 with the fermentation condition parameters (charge volume, solid-liquid ratio, inoculum amount) was established by fitting the experimental data in Table S3. The regression equation obtained was: Y = 42.67 + 1.78 A-1.31 B + 0.75 C+0.0 AB+ 1.92 AC+1.42 BC-6.11 A2-5.86 B2-2.14 C2. The results of each ANOVA of the method were shown in Table S4. The F-value of the model was 8.70 with a P-value of 0.0047, indicating that the model was significant. According to the ANOVA results, the simulated terms A2 and B2 had an important influence on the response values (p < 0.05), while the remaining values had no significant effect.
Response surface analysis for the interaction of the three factors was shown in Fig. 5a–c. The response surface of each group was downward. The degradation rate of anthraquinones in Folium Sennae showed an undulating trend with the increase of the selected three factors. The response surface of inoculum to solid-liquid ratio and charge volume was steep and the contour plot was elliptical, indicating that the interaction of inoculum to solid-liquid ratio and charge volume had remarkable effects on the degradation of anthraquinone in Folium Sennae.
Fig. 5.
Optimization of anthraquinones degradation. (a)–(c) Response surface diagram of the conditions of anthraquinones degradation; (d) Fermentation processes of anthraquinone, total pigment content and citrinin concentration in Folium Sennae with H1102 under response surface optimization conditions; (e) concentrations of four anthraquinone monomers in Folium Sennae fermented with H1102 under response surface optimization conditions.
Further analysis of the model regression equation showed that the maximum value of anthraquinone degradation in Folium Sennae by liquid fermentation of Monascus H1102 was obtained. The optimal fermentation parameters were as follows: fluid volume 83.64 mL, inoculation amount 8.45%, solid-liquid ratio 12.28%. According to the above regression analysis results, the degradation efficiency of anthraquinones was 42.98% after Monascus H1102 fermented Folium Sennae for 5 days. In the specific experiments predicted under the above conditions, the parameters were adjusted to 85 mL charge volume, 8.4% inoculum amount and 12.3% feed to liquor ratio for the fermentation of Monascus. The degradation rate of anthraquinone was also closely related to the growth of the Monascus H1102 as shown in Fig. 5d–e. The rate of anthraquinone degradation decreased rapidly from 1 to 3 days. After that, the total anthraquinone content decreased steadily as the growth of the Monascus strain slowed down. In the first half day of fermentation, the pigment content of the fermentation broth was only 11.2 U mL−1, and the overall anthraquinones content showed little degradation. But in the next 0.5–5 days, the degradation rate of anthraquinones increased significantly as the fermentation time increased, and the overall pigment content also increased and reached the maximum value of 212.2 U mL−1 in 5 days of fermentation. The level of citrinin also increased with the fermentation time, reaching a maximum at 5.5 days, but only at 0.041 mg L−1.
3.5. Verification of synergy and attenuation in fermented Folium Sennae culture with Monascus H1102
The effects on the apoptosis and necrosis of L-02 and HK-2 cells by Folium Sennae extract and Monascus/Folium Sennae culture by flow cytometry are shown in Table S5 and Fig. 6. Folium Sennae extract promoted apoptosis and necrosis of L-02 cells and HK-2 cells that were cultured in vitro to different degrees. When treated with Folium Sennae extract, the proportions of normal and apoptotic cells in L-02 cells were 87.1% and 5.72%, while that of the proportions in HK-2 cells were 72.40% and 3.77%, respectively (Fig. 6a). Monascus/Folium Sennae culture has no obvious toxic effect on L-02 and HK-2 cells in vitro, and even inhibited apoptosis and necrosis of cells to a certain extent. After the treatment of Monascus/Folium Sennae culture, the number of normal cells in both L-02 and HK-2 reached more than 97%, which was higher than that in the control group. Moreover, we used the cck-8 kit to detect the cell viability of senna leaf water extract and Monascus fermentation, and obtained similar results (Fig. 6b and S1), indicating that Monascus/Folium Sennae culture has synergistic and detoxification effects.
Fig. 6.
Cytotoxicity verification of Folium Sennae culture with Monascus H1102. (a) Effects of Monascus/Folium Sennae culture or Folium Sennae extracts on apoptosis and necrosis of L-02 and HK-2 cells by flow cytometry, control: cells treated nothing, extract: Folium Sennae extract, culture: Monascus/Folium Sennae culture; (b) Effects of Monascus/Folium Sennae culture and Folium Sennae extract on survival rate of L-02 and HK-2 cells.
4. Discussion
Fermentation has become an important method for processing traditional herbal medicine [23]. Microorganisms exhibit strong material transformation capabilities, producing abundant secondary metabolites through chemical composition esterification, oxidation, glycosylation, isomerization, methylation, and acetylation [[24], [25], [26]]. Microorganisms can produce a variety of enzymes that convert macromolecular substances into smaller molecules that are facilitated for absorption [27]. Some herbal medicines show both positive effects and certain toxic side effects, which is one of the major constraints for their applications. Encouragingly, the toxic side effects of herbal medicines can be alleviated by certain microbial fermentation. Fungal fermentation could degrade the toxic ingredient in Aristolochia debilis, reducing the amount of aristolochic acids [28]. The highly toxic substance could be converted into low-toxic or non-toxic components after microbial fermentation.
Monascus has high medicinal value and wide application. Guo et al. used solid-state fermentation of Monascus with mulberry leaves, increasing the yield of quercetin and kaempferol. Fermented mulberry leaves showed higher antioxidant and antibacterial activities than non-fermented mulberry leaves [29]. Ling et al. screened 15 herbal medicines to investigate their effects on the production of lovastatin by Monascus M2-1 solid-state fermentation. The dosage and form of the added herbs significantly affected the fermentation results, and hawthorn juice, mulberry leaf powder, clove juice, and tangerine peel powder showed significant synergistic effects on the production of lovastatin by Monascus M2-1 fermentation [30]. Thus, Monascus can be widely used in herbal medicine for fermentation of pigment, lovastatin and or to improve the taste of herbal medicine, which improves the activity and medicinal value of traditional herbal medicine.
In this study, we explored reducing toxicity and increasing efficiency of Folium Sennae fermented by Monascus. The strain of Monascus H1102 stood out and was selected as the initial fermentation strain of Folium Sennae. The fermentation medium and fermentation conditions were optimized by single factors, and the optimal fermentation conditions were determined by the response surface optimization. The optimal fermentation parameters were as follows: fluid volume 83.64 mL, inoculation amount 8.45%, solid-liquid ratio 12.28%. Under best conditions, the degradation rate of anthraquinones reached 41.2%, the pigment content of Monascus reached 212.2 U mL−1 and citrinin was only 0.038 mg L−1, which met the requirements of reducing toxicity and increasing efficiency of Folium Sennae fermented by Monascus. Cell experiments revealed that culture of Monascus/Folium Sennae could inhibit apoptosis and necrosis on HK-2 and L-02 cells in vitro. However, there were still some limitations need to be settled. Medicinal fungal is an important part of herbal medicine, which is capable of decomposing and transforming active components with certain physiological activities. In terms of the types of microorganisms used, only a small proportion has been currently mined to be discovered. Moreover, herbal medicine microbial transformation from a single active compound could be expanded to the whole composition of medicine gradients. Most importantly, the degradation metabolism of risky compounds shall be determined and specific enzymes or genes shall be localized to expand the development and application of microbial fermentation in natural herbal medicine. Overall, our study proved the feasibility of fermentation for detoxification and improving efficiency of risky herbal medicine, and this simple approach is anticipated to make great progress with great vitality for further applications.
Author contribution statement
Mengfei Long: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xiaomei Pei: Performed the experiments; Wrote the paper.
Zhi Lu, Duo Xu, Nan Zheng, Yaxian Li, Hanxiao Ge, Osire Tolbert: Analyzed and interpreted the data; Wrote the paper.
Wentao Cao: Performed the experiments; Analyzed and interpreted the data.
Xiaole Xia: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data, Wrote the paper.
Data availability statement
Data will be made available on request.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2104001); the China Postdoctoral Science Foundation (2022M711368); the Fundamental Research Funds for the Central Universities (JUSRP122037); and the Natural Science Foundation of Jiangsu Province, Science, and Technology Department of Jiangsu Province, China (BK20221081).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e18735.
Abbreviations
- HK-2
human kidney-02
- L-02
human normal liver cell
- CCK-8 kit
Cell Counting Kit-8
- OD
Optical Density
- PI
propidium iodide
Appendix A. Supplementary data
Supplementary information related to this article can be found at online.
Appendix C. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Gong X.H., et al. The synergism mechanism of Rhubarb Anthraquinones on constipation elucidated by comparative pharmacokinetics of Rhubarb extract between normal and diseased rats. Eur. J. Drug Metab. Pharmacokinet. 2015;40(4):379–388. doi: 10.1007/s13318-014-0216-7. [DOI] [PubMed] [Google Scholar]
- 2.Li D., et al. Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-κB and MAPKs signal pathways. Eur. J. Pharmacol. 2013;705(1–3):79–85. doi: 10.1016/j.ejphar.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Abu N., et al. Subchronic toxicity, immunoregulation and anti-breast tumor effect of Nordamnacantal, an anthraquinone extracted from the stems of Morinda citrifolia L. BMC Compl. Alternative Med. 2018;18(1):31. doi: 10.1186/s12906-018-2102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin L., et al. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: a review. J. Ethnopharmacol. 2015;159:158–183. doi: 10.1016/j.jep.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., et al. Pharmacokinetics of anthraquinones from medicinal plants. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.638993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller-Lissner S.A. Adverse effects of laxatives: fact and fiction. Pharmacology. 1993;47(Suppl 1):138–145. doi: 10.1159/000139853. [DOI] [PubMed] [Google Scholar]
- 7.Wald A. Is chronic use of stimulant laxatives harmful to the colon? J. Clin. Gastroenterol. 2003;36(5):386–389. doi: 10.1097/00004836-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Beuers U., Spengler U., Pape G.R. Hepatitis after chronic abuse of senna. Lancet. 1991;337(8737):372–373. doi: 10.1016/0140-6736(91)91012-j. [DOI] [PubMed] [Google Scholar]
- 9.Yang H.N., et al. Aloe-induced toxic hepatitis. J. Kor. Med. Sci. 2010;25(3):492–495. doi: 10.3346/jkms.2010.25.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo M.L., et al. Analytical aspects of diterpene alkaloid poisoning with monkshood. Nat. Prod. Commun. 2009;4(11):1551–1552. [PubMed] [Google Scholar]
- 11.Yang C.L., et al. Comparative analysis of genetic polymorphisms among Monascus strains by ISSR and RAPD markers. J. Sci. Food Agric. 2017;97(2):636–640. doi: 10.1002/jsfa.7780. [DOI] [PubMed] [Google Scholar]
- 12.Patakova P. Monascus secondary metabolites: production and biological activity. J. Ind. Microbiol. Biotechnol. 2013;40(2):169–181. doi: 10.1007/s10295-012-1216-8. [DOI] [PubMed] [Google Scholar]
- 13.Shao Y., Lu X., Chen F. Genetic diversity analysis of Monascus strains using SRAP and ISSR markers. Mycoscience. 2011;52(4):224–233. [Google Scholar]
- 14.Wang T.H., Lin T.F. Monascus rice products. Adv. Food Nutr. Res. 2007;53:123–159. doi: 10.1016/S1043-4526(07)53004-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen G., Wu Z. Production and biological activities of yellow pigments from Monascus fungi. World J. Microbiol. Biotechnol. 2016;32(8):136. doi: 10.1007/s11274-016-2082-8. [DOI] [PubMed] [Google Scholar]
- 16.Vendruscolo F., et al. Monascus: a reality on the production and application of microbial pigments. Appl. Biochem. Biotechnol. 2016;178(2):211–223. doi: 10.1007/s12010-015-1880-z. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C., et al. Ultrasonic and enzymatic pretreatments of Monascus fermentation byproduct for a sustainable production of Bacillus subtilis. J. Sci. Food Agric. 2021;101(9):3836–3842. doi: 10.1002/jsfa.11018. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C., et al. Monascus ruber fermented Panax ginseng ameliorates lipid metabolism disorders and modulate gut microbiota in rats fed a high-fat diet. J. Ethnopharmacol. 2021;278 doi: 10.1016/j.jep.2021.114300. [DOI] [PubMed] [Google Scholar]
- 19.He S., et al. (1)H NMR-based metabolomic study of the effects of flavonoids on citrinin production by Monascus. Food Res. Int. 2020;137 doi: 10.1016/j.foodres.2020.109532. [DOI] [PubMed] [Google Scholar]
- 20.Xiong Z., et al. An overview of the bioactivity of monacolin K/lovastatin. Food Chem. Toxicol. 2019;131 doi: 10.1016/j.fct.2019.110585. [DOI] [PubMed] [Google Scholar]
- 21.Chakraborty D. Production and evaluation of physicochemical properties of red pigment from Monascus purpureus MTCC 410. Internet J. Microbiol. 2008;7 [Google Scholar]
- 22.Kraboun K., et al. Factors and advances on fermentation of Monascus sp. for pigments and monacolin K production: a review. Int. Food Res. J. 2019:751–761. [Google Scholar]
- 23.Zhang H., et al. Fermentation characteristics and the dynamic trend of chemical components during fermentation of Massa Medicata Fermentata. Arab. J. Chem. 2022;15(1) [Google Scholar]
- 24.Liu Z., et al. Comparison study of the volatile profiles and microbial communities of Wuyi Qu and Gutian Qu, two major types of traditional fermentation starters of Hong Qu glutinous rice wine. Food Microbiol. 2018;69:105–115. doi: 10.1016/j.fm.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Hussain A., et al. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016;81:1–16. [Google Scholar]
- 26.Ouyang Y., et al. Effects of plants-associated microbiota on cultivation and quality of Chinese herbal medicines. Chinese Herbal Medicines. 2023 doi: 10.1016/j.chmed.2022.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y., et al. Monitoring of the bacterial and fungal biodiversity and dynamics during Massa Medicata Fermentata fermentation. Appl. Microbiol. Biotechnol. 2013;97(22):9647–9655. doi: 10.1007/s00253-013-5187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombo M.L., et al. Analytical aspects of diterpene alkaloid poisoning with monkshood. Nat. Prod. Commun. 2009;4(11) 1934578X0900401118. [PubMed] [Google Scholar]
- 29.Guo N., et al. Improvement of flavonoid aglycone and biological activity of mulberry leaves by solid-state fermentation. Ind. *. Prod. 2020;148 [Google Scholar]
- 30.Peng L., et al. Effects of Chinese medicines on monacolin K production and related genes transcription of Monascus ruber in red mold rice fermentation. Food Sci. Nutr. 2020;8(4):2134–2142. doi: 10.1002/fsn3.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.