Abstract
In this study, three cooking methods (baking, boiling, sous vide (SV)) were applied to Turkish sweet potatoes with three flesh colors (white, orange, purple) to examine the effects of the product color and cooking methods on the total phenolics, antioxidant activity, sugars, phenolic acids, and anthocyanins. LC-MS-MS was employed in the characterization of these compounds. It was observed that the product color and cooking method significantly affected the concentrations of bioactive compounds like polyphenols. Both the highest total phenolic content (11.36 mg/g) and antioxidant activity (DPPH (50.3 μM TE/g) and ABTS (63.53 μM TE/g)) were determined in the purple sweet potato cooked with the SV method. 10 phenolic acids were quantified in all samples which were in the highest amounts in the orange colored samples followed by the purple samples. Baking resulted in the highest total phenolic acids in all samples. 13 anthocyanins were detected in the purple-colored samples, while the SV cooking best preserved the anthocyanins. In sum, purple sweet potatoes cooked by SV are recommended for higher phenolic contents, antioxidant capacity and anthocyanins.
Keywords: Sweet potato, Ipomoea batatas L., Phenolics, Antioxidant capacity, Anthocyanins
Graphical abstract
Highlights
-
•
Purple sweet potato samples had the highest total phenolic compounds (TPC) followed by orange and white potatoes.
-
•
The levels of phenolic compounds were lower in boiled samples due to the passage of compounds into boiling water.
-
•
A total of 10 phenolic acids were found in all samples with the highest quantity in orange and purple colored samples.
-
•
A total of 13 anthocyanins were detected in the purple sweet potato samples with mostly peonidin-type compounds (77%).
-
•
The purple-fleshed samples contained almost twice as much antioxidant content as orange or white-fleshed samples.
1. Introduction
Sweet potato (Ipomoea batatas L.), a vegetable belonging to the Convolvulaceae family, is grown in many countries in the tropical and subtropical regions of the world. About 89.5 million tons of sweet potatoes were grown on approximately 7.4 million hectares worldwide in 2020 [1]. The top three producer countries were China, Malawi and United Republic of Tanzania. InTürkiye, it is grown in some provinces, especially in Hatay, but statistical data on the production could not be found.
Sweet potato is used in both human and animal nutrition due to its high nutritional content as an inexpensive and easily-accessible natural source [2]. It is considered as one of the best agricultural products benefiting human health [3,4]. Even if its leaves are edible and offer health benefits [5,6], its tubers with different flesh colors such as white, yellow, orange, red, purple are mainly consumed. Color compounds have an important role in terms of bioactivity and usually darker colored agricultural products have a higher potential for bioactivity [6]. Sweet potato is rich in terms of starch, dietary fiber, minerals, vitamins, phenolic compounds (phenolic acids and anthocyanins), beta-carotene and tocopherol [7,8]. Among these, the major bioactive substances in purple sweet potato are phenolics and anthocyanins. Sweet potatoes contain high levels of phenolics along with low glycemic index that have potential for use as a functional food to improve human health [4]. These compounds are also directly related to their antioxidant potential, which is especially important for human health. Among the phenolic components, chlorogenic acid (ChlA), caffeic acid (CafA) and dicaffeoylquinic acid (diCQA) are the most dominant phenolics in sweet potatoes [9]. Violet-purple sweet potato varieties are rich in anthocyanins as strong antioxidants lowering blood sugar levels while the orange-colored types have high amounts of carotenoid which is a source of vitamin A and the red sweet potatoes are rich in terms of lycopene that mitigates the risk of heart disease and some cancers [4]. Purple sweet potato has an excellent source of anthocyanins whose chemical structure is mainly composed of cyanidin, peonidin, pelargonidin, and delphinidin derivatives in the form of monoacylation and diacetylation but the most abundant anthocyanins are peonidin derivatives [10].
Beyond varietal and color differences, phenolic content of agricultural products is also affected by other parameters including environmental factors, ripening degree, storage, processing, cooking, etc. [11]. Particularly, heat treatment, drying and type of cooking play an important role [11,12]. Sweet potato tubers are primarily consumed after cooking by different methods such as boiling, baking, frying, steaming, etc. Along with these classical methods, sous vide (means “under vacuum” in French) is a relatively newer cooking method in which the food is cooked inside a sealed vacuum bag in hot water under controlled temperature resulting in better preservation of bioactive compounds by cooking the food without contact with water and oxygen [13]. It is imperative that cooking methods should maintain the color and health-promoting compounds such as anthocyanins and phenolic compounds in sweet potato. Applications of drying and cooking have also diversified considerably focusing mainly on their effects on its aroma, nutritional and phenolic properties of sweet potato [14]. In a study by Jing et al. [15] the impacts of three drying methods were elucidated on the antioxidant activity of sweet potato tubers. In addition, Jiang et al. [16] reported that the degradation of anthocyanins was accompanied by an increase in polymeric color index due to the formation of melanoidin pigments and condensation reactions after heat treatment in purple sweet potato. Sun et al. [17] highlighted that the drying process broke the starch chain and damaged the structure of the crystal zone affecting the functional properties in sweet potato.
The product color and cooking methods influence the quality parameters and quantity of bioactive compounds of fruits and vegetables. Hence, we carried out this study to investigate and compare the effects of tuber color and the cooking methods on the changes in the phenolic substances, anthocyanin compounds, sugars, total phenolic contents, and antioxidant capacities (DPPH and ABTS) of sweet potato tubers. Turkish sweet potatoes with different colors (white, orange, purple) and three types of cooking (boiling, baking, and sous vide) were inspected for the first time with a view to understand the changes in these bioactive chemical and sugar constituents in detail and to determine the best cooking method and product color in terms of a higher degree of bioactive compounds and health benefits.
2. Materials and methods
2.1. Sweet potato samples and cooking methods
Sweet potatoes (Ipomoea batatas L.) with different flesh colors (purple (cv. Ilkmor), white (cv. Hatay Beyazı) and orange (cv. Regal)) in an amount of 3 kg of each type were obtained from farmers in the Uzunalic village (36°23′41.3″N, 36°11′48.8″E) of Hatay province, Türkiye in November of 2021.
Sweet potatoes with similar sizes and shapes (170 ± 20 gr, 15–17 cm length, 4–5 cm diameter) were selected and cooked as a whole without peeling or slicing by three different methods (baking, boiling and sous vide, SV). Potatoes were washed with tap water and freed from soil particles before cooking. Each cooking process was carried out in triplicate using six potatoes in each cooking. The cooking time and temperature were determined in the trials before the real experimental cooking to obtain sweet potatoes with suitable palatability and softness. The cooking procedures and conditions were as follows for each method:
Boiling: Sweet potatoes were boiled in distilled water at 90 °C for 100 min in thermostatic water bath (Buchi Heating Bath B-100, Flawil, Switzerland). All samples were boiled in 4 L distilled water at constant temperature and the boiling water was discarded after the process.
Baking: This cooking procedure was carried out using a fan-assisted oven (Tefal Twenty L, France). The oven was heated to 190 °C before baking. The whole potatoes were placed on a steel baking tray and baked at 190 °C for 70 min.
Sous vide (SV) cooking: Sweet potatoes are first packed as a whole in polyethylene packages in a vacuum of −90 kPa (Seles VS100, Istanbul, Türkiye). Packaged samples were cooked at 85 °C for 100 min in a thermostatic hot water bath (Buchi Heating Bath B-100, Flawil, Switzerland).
The internal temperature of the potatoes was monitored with a type K thermocouple (Verth RS232, Taiwan) during all three cooking operations. The thermocouple was positioned in the center of the potato sample. In the SV cooking, the needle was placed before packaging and then vacuum and heat sealing was established. The tightness of the packages has been verified in the pre-experiment trials and applied in the same manner in the cooking experiments. The cooked samples were cooled at room temperature before the analysis. The photos of the cooked samples are presented in Fig. 1.
Fig. 1.
Sweet potato samples with different flesh colors (purple, white, orange) cooked with three different techniques. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2. Chemicals
Chemicals as methanol (HPLC grade), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate, Folin–Ciocalteu reagent, sodium carbonate, and standard compounds like gallic acid, trolox, glucose, fructose, sucrose and maltose used in the study were procured from Sigma-Aldrich Chemical Co. (St. Louis, USA). Phenolic standards were also obtained from Sigma-Aldrich Chemical Co. (St. Louis, USA).
2.3. Extraction of phenolic compounds
Phenolic extraction of, white, orange and purple-fleshed sweet potato samples was carried out according to Franková et al. [18]. 5 g of potato samples were weighed and 10 ml of 80% methanol was added. This mixture was shaken in a shaker for 1 h and centrifuged at 4 °C, 5500 rpm for 15 min and then, the samples were filtered through a membrane filter to obtain the extract.
2.3.1. Total phenolic analysis
Total phenolics of the sweet potato samples were analyzed by using the Folin Ciocalteu (FC) reagent. 1 ml of each sample was taken and 5 ml of FC solution, 15 ml of 20% sodium bicarbonate and distilled water were added and waited for 2 h in the dark. Measurement was done at 765 nm in spectrophotometer. Data were calculated as milligram gallic acid equivalents (GAE) per kilogram (mg GAE/kg) [19,20]. All measurements were repeated three times and the results were expressed as mean ± standard deviation.
2.3.2. Determination of antioxidant activity
Antioxidant potentials of the samples were studied by two methods, DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis [3-ethylbenzthiazoline-6-sulfonic acid]).
DPPH assay can determine the sample's ability to inhibit free radicals and was carried out at 515 nm by using UV–Vis spectrophotometer (Agilent-Cary 60 UV–Vis, Agilent Tech., Palo Alto, CA, USA) in reference to Kelebek et al. [19].
ABTS assay was employed in reference to Sen & Sonmezdag [21]. ABTS of 7 mM was added to 2.45 mM potassium bisulfate and kept in the dark for 12–16 h. The solution was then diluted by means of sodium acetate (pH 4.5) buffer to 0.70 ± 0.01 absorbance at 734 nm wavelength in UV-VIS spectrophotometer. 20 μL of extract was added with a 2.98 ml of the prepared buffer and incubated at room temperature in the dark. After that, the absorbance value was read at 734 nm in a UV–visible spectrophotometer (Agilent-Cary 60 UV–Vis, Agilent Tech., Palo Alto, CA, USA). All measurements were repeated three times and the antioxidant capacity values were computed from calibration curves.
2.4. Analysis of phenolic compounds by LC-MS/MS
Phenolic compounds were determined according to the method of Sen & Sonmezdag [21] using LC-DAD-ESI-MS/MS with negative ionization mode. An HPLC instrument (Model 1260; Agilent Tech., Palo Alto, CA, USA) was employed with a diode array detector (G1351D 1260 DAD VL) that included a binary pump (G1312 B, 1260 Bin pump), an autosampler (G1367 E, 1260 HIP ALS) and a degasser (G1322 A, 1260 Degasser). A Phenomenex Luna reversed-phase C-18 column was utilized with 4.6 × 250 mm and 5 μm size (Torrance, California, USA). Two mobile phases were used as solvent A (water/formic acid; 99:1; v/v) and solvent B (acetonitrile/solvent A; 60:40; v/v). Curves were obtained by utilizing commercial standards with concentrations in the extracts (around 1–100 mg/L) and with R2 values higher than 0.99. In case of the absent reference compound, the calibration of similar elements was utilized along with the molecular weight correction factor. The limits of quantification (LOQ) and detection (LOD) were computed based on the signal-to-noise values of 10 and 3, respectively [19].
2.5. Analysis of sugar compounds
The samples were extracted and analyzed for sugar content according to the method described by Tanriseven et al. [22] An HPLC system (Agilent 1260 HPLC system, Palo Alto, CA, USA) equipped with a pump system and a refractive index detector was used for the sugar analysis. Sugars were analyzed using HRC NH2 (Biorad) 150 × 4.6 mm, 5μ (Bio-Rad, CA, USA). The analytical conditions included a flow rate of 0.6 ml/min and an eluent of 0.09 mol/L H2SO4 with 6% acetonitrile (v/v). A calibration curve was prepared using standards (maltose, sucrose, glucose and fructose) to determine the relationship between the peak area and concentration.
2.6. Statistical data analysis
Data were analyzed by using SPSS package program (version 24, SPSS Inc., Chicago, Illinois, USA) at 95% confidence level (p = 0.05) after checking for normality and homogeneity of variances. Significant differences were examined according to Duncan's multiple comparison test. Also, principal component analysis (PCA) was applied in XLSTAT software (Trial version, 2022).
3. Results and discussion
3.1. Total phenolic and antioxidant properties
Total phenolic compounds (TPC) and antioxidant capacities of the sweet potato samples according to the three different flesh colors and three different cooking methods are enlisted in Table 1. Purple sweet potato samples were determined to have the highest TPC compared to the other two colored samples (Table 1). From this, it was concluded that the flesh color is an important factor affecting the total amount of phenols in sweet potatoes. A similar finding was reported by Frankova et al. [18]. When cooking methods were compared, it was observed that the TPC was the highest in the sous vide (SV) cooking in purple sweet potatoes (11.36 mg GAE/g fw). This result is thought to be due to the absence of direct contact of food and water in the SV technique and hence, water-soluble anthocyanins remain in the product. Also, since there is no contact of the food with oxygen in this method, the oxidation of anthocyanins is reduced and thus, they are better preserved. In another study on the effects of cooking techniques on the phenolics of cruciferous (Brassica) vegetables, it was reported that phenolics were preserved the most in the SV method whereas they were the lowest in the boiling similar to the current study [13]. It was also observed in the present study that the baking method resulted in the highest TPC in orange (6.19 mg GAE/g fw) and white (4.16 mg GAE/g fw) samples as contrary to the purple samples (Table 1).
Table 1.
Total phenolic contents (TPC), antioxidant activity values (DPPH, ABTS) and sugar profiles of the sweet potato samples according to their flesh color and cooking methods (mean ± standard deviation).
| White-fleshed sweet potato |
Orange-fleshed sweet potato |
Purple-fleshed sweet potato |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baking | Boiling | Sous vide | Baking | Boiling | Sous vide | Baking | Boiling | Sous vide | |
| TPC (mg GAE/g fw) | 4.16 ± 0.24d | 1.68 ± 0.01e | 1.70 ± 0.12e | 6.19 ± 0.14c | 2.50 ± 0.08e | 4.56 ± 0.09d | 9.82 ± 0.07b | 10.78 ± 0.08a | 11.36 ± 0.04a |
| DPPH (μM TE/g fw) | 17.42 ± 0.30f | 7.96 ± 0.70h | 8.62 ± 0.62h | 30.11 ± 0.64d | 14.96 ± 0.56g | 20.69 ± 0.20e | 42.31 ± 0.94c | 47.92 ± 1.14b | 50.26 ± 1.05a |
| ABTS (μM TE/g fw) | 22.69 ± 0.55f | 10.50 ± 0.71h | 11.21 ± 0.63h | 37.71 ± 0.77d | 18.48 ± 0.51g | 26.43 ± 0.30e | 53.86 ± 0.93c | 60.56 ± 1.10b | 63.53 ± 1.06a |
| Sugars | |||||||||
| Maltose | 5.20 ± 0.02a | 5.16 ± 0.04b | 4.90 ± 0.16c | 4.78 ± 0.04e | 3.88 ± 0.03h | 3.80 ± 0.07i | 4.82 ± 0.03d | 4.74 ± 0.08f | 4.64 ± 0.04g |
| Sucrose | 3.12 ± 0.01b | 3.10 ± 0.02c | 2.94 ± 0.09d | 3.35 ± 0.03a | 2.72 ± 0.02h | 2.66 ± 0.05i | 2.89 ± 0.02e | 2.84 ± 0.05f | 2.78 ± 0.03g |
| Glucose | 1.13 ± 0.03b | 1.03 ± 0.01d | 1.11 ± 0.03c | 1.26 ± 0.02a | 0.92 ± 0.01e | 0.66 ± 0.01f | 0.40 ± 0.01i | 0.43 ± 0.06h | 0.45 ± 0.00g |
| Fructose | 0.62 ± 0.00b | 0.51 ± 0.00d | 0.59 ± 0.02c | 0.69 ± 0.01a | 0.46 ± 0.01e | 0.37 ± 0.01f | 0.32 ± 0.01g | 0.31 ± 0.01g | 0.29 ± 0.01h |
| Total | 10.1 ± 0.07a | 9.8 ± 0.08b | 9.6 ± 0.21c | 10.1 ± 0.09a | 8.0 ± 0.07g | 7.5 ± 0.10h | 8.4 ± 0.06d | 8.3 ± 0.08e | 8.2 ± 0.08f |
TPC: Total phenolic content; DPPH: Antioxidant activity based on DPPH (2,2-diphenyl-1-picrylhydrazyl); ABTS: Antioxidant activity based on ABTS (2,2′-azino-bis [3-ethylbenzthiazoline-6-sulfonic acid]); fw: fresh weight. a-i Different letters in same row indicate significant differences (p < 0.05).
The antioxidant capacities were determined by using two methods (DPPH and ABTS) and the results are presented in Table 1. It was found that the DPPH activities of all samples were considerably lower than the ABTS activity values. A similar finding was reported by Teow et al. [7] in sweet potatoes with varying flesh colors. It was also found in the present study that the highest antioxidant capacity was found in the purple sweet potato cooked with the SV cooking in both methods (63.53 and 50.26 μM TE/g fw for ABTS and DPPH, respectively) as similar to the TPC (Table 1). Likewise, Makori et al. [23] determined that purple sweet potatoes had the strongest scavenging capacity in both DPPH and ABTS radicals and reported that orange and white sweet potatoes showed lower antioxidant properties than purple varieties. In the current study, the highest antioxidant value in the orange flesh-colored sweet potato was found in the baking method (37.71 and 30.11 μM TE/g fw for ABTS and DPPH, respectively). Similarly, the baking method yielded the highest antioxidant value in white sweet potatoes (22.69 and 17.42 μM TE/g fw for ABTS and DPPH methods) (Table 1). In sum, the purple sweet potatoes had almost twice more antioxidant capacity as compared to the white and orange-colored samples. Regarding the cooking methods, the highest antioxidant capacity was obtained by SV method in purple flesh colored samples and baking in white and orange potato samples.
3.2. Sugar compounds
A total of four sugar compounds were quantified as maltose, sucrose, glucose, and fructose in the samples (Table 1). Maltose was the most abundant followed by sucrose, glucose, and fructose, respectively. Similar results were also reported by Chan et al. [24] in sweet potatoes cooked by seven different heat treatments in China. These researchers emphasized that the amount of maltose in fresh sweet potatoes was lower but it increased as a result of heat treatment and became the most dominant sugar because of the formation of new maltose molecules. On the contrary, the authors reported a partial decrease in sucrose with cooking. In the present study, the total sugar content was found to be the highest in the samples obtained by baking (Table 1). This may be due to the fact that the baking process converts starch molecules more into disaccharides and monosaccharides than the other two methods.
3.3. Phenolic profile
Phenolic compounds can inhibit the negative reactivity of undesired reactive oxygen/nitrogen species produced by metabolic activities in the body. They also have anti-inflammatory, antioxidant, antimutagenic, antimicrobial, hypoglycemic, and anti-platelet properties with a potential to prevent cardiovascular diseases, oxidative diseases, and various cancers [25].
In the present study, 10 phenolic acids in all samples (Table 2) and 13 anthocyanin compounds in purple sweet potatoes (Table 3) were detected by LC-DAD-ESI-MS/MS analysis. As seen in Table 2, the phenolic acid contents differed in the analyzed sweet potato samples as a function of flesh color and cooking method. In regards to the total concentrations of phenolic acids, the highest amount was detected in the orange colored samples (up to 263.5 mg/100 g), followed by the purple and white samples (up to 195.2 and 133.6 mg/100 g, respectively). Regarding the cooking methods, the highest total phenolic acid was found in orange-colored sweet potato cooked by baking method (263.5 mg/100 g) while the lowest amount was quantified in the white sweet potato (26.7 mg/100 g) cooked by boiling method (Table 2). It was stated in previous studies that some phenolics pass into water in significant amounts during boiling and the nutritional value of the food decreases [11,26].
Table 2.
Phenolic profile of the sweet potato samples according to their flesh color and cooking methods (mean ± standard deviation).
| White-fleshed sweet potato |
Orange-fleshed sweet potato |
Purple-fleshed sweet potato |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic Compounds (mg/100 g) | RT* | m/z (−)* | Baking | Boiling | Sous vide | Baking | Boiling | Sous vide | Baking | Boiling | Sous vide | |
| 1 | p-Coumaroylquinic acid | 8.28 | 337 | 1.39 ± 0.03ab | 2.11 ± 0.05a | 1.28 ± 0.03ab | 0.82 ± 0.02b | 0.73 ± 0.02b | 0.94 ± 0.02 b | 1.53 ± 0.03ab | 0.95 ± 0.02b | 1.41 ± 0.03 ab |
| 2 | Caffeic acid derivative I | 11.6 | 487 | 2.80 ± 0.06d | 2.44 ± 0.06d | 2.60 ± 0.06d | 3.09 ± 0.06cd | 2.71 ± 0.06d | 4.30 ± 0.09 b | 10.47 ± 0.22a | 3.80 ± 0.08bc | 4.07 ± 0.09 b |
| 3 | Caffeic acid derivative II | 12.66 | 377 | 10.37 ± 0.21d | 5.47 ± 0.11g | 7.64 ± 0.16f | 14.17 ± 0.29b | 12.29 ± 0.25c | 17.35 ± 0.36a | 17.75 ± 0.36a | 9.30 ± 0.19e | 10.22 ± 0.21d |
| 4 | 3-O-caffeoylquinic acid | 21.68 | 353 | 7.61 ± 0.16d | 1.98 ± 0.04g | 2.40 ± 0.06g | 13.82 ± 0.28b | 3.30 ± 0.08f | 4.54 ± 0.09e | 12.92 ± 0.27c | 12.11 ± 0.25c | 15.84 ± 0.33a |
| 5 |
5-O-caffeoylquinic acid (chlorogenic acid) | 31.23 | 353 | 36.46 ± 0.75f | 4.66 ± 0.10h | 7.04 ± 0.15g | 80.88 ± 1.67a | 35.66 ± 1.34f | 65.14 ± 0.73 b | 60.62 ± 1.25c | 45.01 ± 1.10e | 50.80 ± 1.06d |
| 6 | 4-O-caffeoylquinic acid | 36.85 | 353 | 2.94 ± 0.06de | 2.54 ± 0.05e | 2.68 ± 0.06e | 8.09 ± 0.17c | 3.72 ± 0.08d | 1.00 ± 0.02f | 9.73 ± 0.20b | 11.95 ± 0.25a | 11.42 ± 0.24a |
| 7 | 4,5 di-CQA (dicaffeoylquinic acids) | 51.03 | 515 | 25.39 ± 0.52c | 1.95 ± 0.04g | 5.18 ± 0.11f | 43.80 ± 0.90a | 11.75 ± 0.24e | 4.52 ± 0.09f | 28.10 ± 0.68b | 22.60 ± 0.71d | 22.27 ± 0.46d |
| 8 | 3,5 di-CQA (dicaffeoylquinic acids) | 53.14 | 515 | 18.80 ± 0.39c | 1.09 ± 0.03g | 4.15 ± 0.09f | 37.84 ± 0.78a | 16.74 ± 0.34d | 5.20 ± 0.11e | 21.32 ± 0.44b | 19.24 ± 0.90c | 17.18 ± 1.57d |
| 9 | 3,4 di-CQA (dicaffeoylquinic acids) | 55.25 | 515 | 24.07 ± 0.50c | 1.68 ± 0.04g | 5.47 ± 0.11f | 48.41 ± 1.00a | 15.45 ± 0.32e | 5.43 ± 0.11f | 25.25 ± 0.54b | 22.66 ± 0.91d | 23.10 ± 0.48d |
| 10 | Caffeic acid | 37.23 | 179 | 3.74 ± 0.08e | 2.78 ± 0.06f | 15.05 ± 0.31a | 12.53 ± 0.26b | 2.96 ± 0.06ef | 1.18 ± 0.03 g | 7.47 ± 0.15d | 11.20 ± 0.23c | 11.06 ± 0.23c |
| Total phenolic acids | 133.6 ± 2.75e | 26.7 ± 0.55i | 53.5 ± 1.11h | 263.5 ± 5.41a | 105.3 ± 2.77g | 109.6 ± 1.65f | 195.2 ± 4.02b | 158.8 ± 2.29d | 167.4 ± 3.67c | |||
*RT: retention time (min); m/z: mass/charge value. a-h Different letters in the same row indicate significant differences (p < 0.05).
Table 3.
Anthocyanin compounds of the purple-flesh-colored sweet potato samples according to cooking methods (mean ± standard deviation).
| Anthocyanins (mg/100 g) | RT* | m/z (−)* | Baking | Boiling | Sous vide | |
|---|---|---|---|---|---|---|
| 1 | Cy 3-soph-5-glc | 16.53 | 773 | 12.8 ± 0.19c | 17.1 ± 0.26b | 21.3 ± 0.31a |
| 2 | Peo 3-soph-5-glc | 20.26 | 787 | 21.4 ± 0.31c | 46.1 ± 0.64b | 54.3 ± 0.76a |
| 3 | Cy 3-p-hydroxybenzoylsoph-5-glc | 29.82 | 893 | 24.5 ± 0.34b | 25.7 ± 0.36b | 38.5 ± 0.53a |
| 4 | Cy 3-(6‴-caffeoyl soph)-5-glc | 42.51 | 935 | 22.0 ± 0.31c | 31.1 ± 0.43b | 40.2 ± 0.56a |
| 5 | Peo 3-p-hydroxybenzoylsoph-5-glc | 35.43 | 907 | 93.2 ± 1.11c | 102.4 ± 1.41b | 118.2 ± 1.63a |
| 6 | Peo 3-(6‴-caffeoyl soph)-5-glc | 44.04 | 949 | 59.5 ± 0.82c | 109.3 ± 1.50b | 112.7 ± 1.55a |
| 7 | Cy 3-feruloyl soph-5-glc | 45.29 | 949 | 39.1 ± 0.54a | 34.9 ± 0.48b | 40.4 ± 1.65a |
| 8 | Peo 3-feruloyl soph-5-glc | 42.01 | 963 | 11.5 ± 0.18a | 10.5 ± 0.15a | 12.1 ± 0.17a |
| 9 | Peo 3-caf-fer soph-5-glc | 46.82 | 963 | 53.1 ± 0.73c | 77.3 ± 1.06b | 98.0 ± 1.35a |
| 10 | Cy 3-caf-p-hb soph-5-glc | 43.61 | 1055 | 21.3 ± 0.29b | 17.5 ± 0.24c | 27.5 ± 0.39a |
| 11 | Cy 3-caffeoyl-feruloylsoph-5-glc | 44.23 | 1111 | 19.2 ± 0.27b | 15.5 ± 0.22c | 24.2 ± 0.36a |
| 12 | Peo 3-caf-p-hb soph-5-glc | 45.55 | 1069 | 191.2 ± 2.33a | 176.7 ± 1.29c | 222.0 ± 3.05a |
| 13 | Peo 3-fer-p-hb soph-5-glc | 47.85 | 1125 | 40.4 ± 0.57b | 32.0 ± 0.44c | 46.6 ± 0.64a |
| Total anthocyanins (mg/100g) | 609.0 ± 7.8c | 696.1 ± 8.5b | 855.9 ± 11.8a | |||
| Groups of anthocyanins (mg/100g; %): | ||||||
| Non-acylated anthocyanin | 34.2 ± 0.50 5.6% ± 0.02% |
63.2 ± 0.89 9.1% ± 0.02% |
75.6 ± 1.07 8.8 ± 0.04% |
|||
| Monoacylated anthocyanin | 249.4 ± 3.43 41.0% ± 0.02% |
313.9 ± 4.32 45.1% ± 0.01% |
361.9 ± 5.14 42.3% ± 0.10% |
|||
| Diacylated anthocyanin | 324.5 ± 4.48 53.4% ± 0.00% |
319.7 ± 4.40 45.9% ± 0.01% |
418.3 ± 5.75 48.9 ± 0.07% |
|||
| Cyanidin-based anthocyanin | 138.9 ± 1.94 22.8% ± 0.03% |
141.8 ± 1.98 20.3% ± 0.03% |
192.1 ± 2.98 22.5% ± 0.14% |
|||
| Peonidin-based anthocyanin | 469.1 ± 6.46 77.1% ± 0.03% |
554.9 ± 7.64 79.6 ± 0.03% |
663.8 ± 9.13 77.5 ± 0.14% |
*RT: retention time (min); m/z: mass/charge value. a-c Different letters in same row indicate significant differences (p < 0.05).
Among the phenolic acids, the most dominant was 5-O-caffeoylquinic acid (chlorogenic acid) followed by 4,5 di-CQA (dicaffeoylquinic acids), 3,4 di-CQA (dicaffeoylquinic acids) and 3,5 di-CQA (dicaffeoylquinic acids) in all samples (Table 2). The chlorogenic acid, a family of esters is usually formed from certain cinnamic acids and quinic acids in the plants. It is an important phenolic in coffee and available as a type of hydroxycinnamic acid derivative in fruits, vegetables, black tea and some Chinese medicines [27]. In the present study, the highest level of chlorogenic acid was found in baked (80.88 mg/100 g) and SV-cooked orange-colored (65.14 mg/100 g) samples (Table 2). Moreover, the baked purple (60.62 mg/100 g) and baked white (36.46 mg/100 g) samples had higher amounts, while the lowest amount was detected in the boiling method in all three samples. Similarly, Frankova et al. [18] reported that chlorogenic acid was prominent in sweet potatoes and heat treatment increased its content along with total polyphenols and antioxidant activity. Within a similar pattern, Xu et al. [26] reported that microwave, steaming, and their combination increased the phytochemicals, including phenolics, flavonoids, anthocyanins, and phenolic acids (except caffeic acid) in purple sweet potatoes after cooking.
4,5 di-CQA (dicaffeoylquinic acids), 3,5 di-CQA (dicaffeoylquinic acids) and 3,4 di-CQA (dicaffeoylquinic acids) are the other important phenolic acids (Table 2). The highest value was determined in the baked samples. It was reported in previous studies that chlorogenic and dicaffeoylquinic acids were the two most abundant phenolics and di- and tri-caffeoylquinic acids are biosynthesized through the conversion of chlorogenic acid and isolation of an enzyme which catalyzes the conversion of chlorogenic acid to dicaffeoylquinic acids in sweet potato root [9].
The phenolic acid detected at the lowest amount was p-coumaroylquinic acid, between 0.73 and 2.11 mg/100 g (Table 2). Cooking methods did not significantly affect the amount of this compound. Villegas & Kojima [28] reported that the trans-esterification enzymes in sweet potato root which catalyze the formation of l-O-t-cinnamoyl-d-glucose and the trans-esterification between 1-0-p-coumaroyl-d-glucose and d-quinic acid, produces p-coumaroylquinic acid.
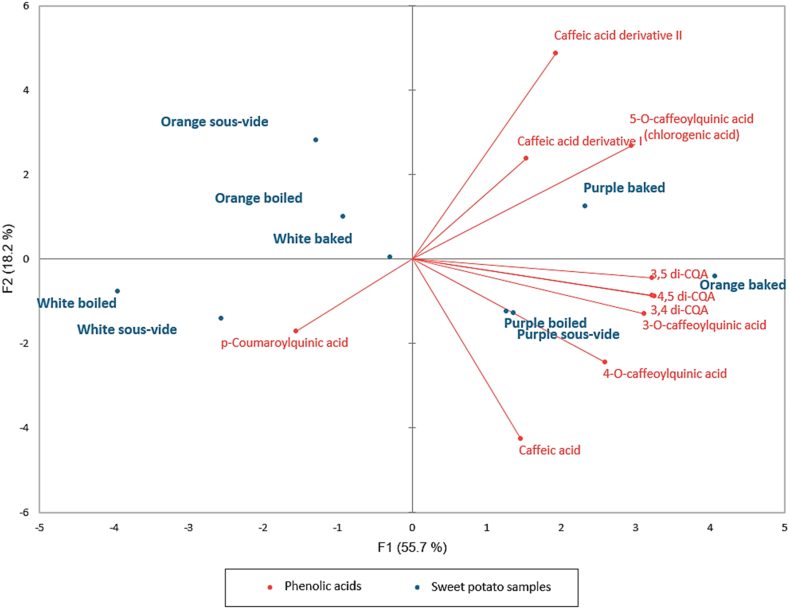
In addition, principal component analysis (PCA) was applied to study the relationship between cooking methods (baking, boiling and SV), flesh color and the phenolic acids (Fig. 2). The first two components explained about 74% of the total variance (F1: 56%, F2: 18%). It was observed that all purple potatoes and baked orange potatoes were located on the positive F1 axis while all the white potatoes in addition to boiled and SV cooked orange samples were on the negative side of the F1 axis. Compounds such as 3-O-caffeoylquinic acid, 3,5 di-CQA, 4,5 di-CQA, 3,4 di-CQA, 4-O-caffeoylquinic acid were found as correlated with the baked orange, boiled and SV cooked purple samples (Fig. 2). Also, baked purple potatoes are characterized with chlorogenic acid and caffeic acid derivatives.
Fig. 2.
PCA biplot of phenolic acids determined in the sweet potato samples according to their flesh color and cooking methods. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Anthocyanin compounds
Anthocyanins are highly sensitive pigments in foods and are easily degraded and discolored under changes in temperature, pH, light and oxygen among other factors [29,30]. In the present study, 13 anthocyanin compounds were determined in purple sweet potatoes and grouped as non-acylated, monoacylated, diacylated and cyanidin-peonidin-based anthocyanins (Table 3). Over 77% of the anthocyanins were peonidin derivatives in all cooking methods, which indicates that the peonidin–type was dominant in the studied sweet potato samples. A similar result was reported by Li et al. [10] in purple sweet potatoes indicating an abundance of peonidin-type anthocyanins.
The total amount of anthocyanins was the highest (855.9 mg/100 g) in the samples cooked with the SV method, followed by boiled (696.1 mg/100 g) and baked (609.1 mg/100 g) samples (Table 3). As can be seen, SV cooking preserved anthocyanins quite well compared to the other two methods. Iborra-Bernad et al. [31] reported that the color of the purple potato obtained by the SV cooking was quite similar to the raw potato while the color of the boiled sample differed greatly due to the dissolution of hydrophilic anthocyanins in water.
The most dominant anthocyanin was found as peonidine 3-caffeic-p-hydroxybenzoic sophoroside-5-glucoside with an amount of 191.2 mg/100 mg in the baked sample, 176.7 mg/100 mg in the boiled sample and 222.0 mg/100 mg in the SV sample. This was followed by 3-p-hydroxybenzoylsophoroside-5-glucoside. Similar anthocyanins were also reported in purple sweet potatoes grown in different countries [19,32].
4. Conclusions
In the current study, the phenolic compounds including anthocyanins, sugars, and antioxidant capacities of white, orange, and purple flesh-colored Turkish sweet potato tubers were analyzed for the first time by LC-MS-MS and the effects of three different cooking methods (baking, boiling and sous vide cooking) on these compounds were examined.
Purple sweet potato samples had the highest total phenolic concentration (TPC) (up to 11.36 mg GAE/g fw) due to the abundance of anthocyanins followed by orange and white colored potatoes (up to 6.19 and 4.16 mg GAE/g fw, respectively). When the effects of cooking methods were examined, the amounts of these compounds were found to be lower in boiled samples due to the transfer of these compounds to water during boiling. Sous vide (SV) cooking resulted in the highest TPC only in purple sweet potato sample.
The purple sweet potatoes had almost twice more antioxidant capacity than the white and orange colored samples. As for cooking methods, the highest antioxidant capacity (65.53 and 50.26 μM TE/g fw for DPPH and ABTS, respectively) was obtained by the SV method in purple colored samples. The antioxidant capacity values were found to be related to TPC.
A total of 10 phenolic acids were quantified in all samples. The highest amount was detected in the orange colored samples (up to 263.5 mg/100 g) followed by the purple (up to 195.2 mg/100 g) samples. Baking resulted in the highest total phenolic acid amounts in all three colored potato samples. The most dominant phenolic acid was 5-O-caffeoylquinic acid (chlorogenic acid).
Regarding the anthocyanins, peonidin-type anthocyanins were the most dominant with a ratio of about 77%. The SV cooking preserved anthocyanins (855.9 mg/100 g) best compared to the other two methods. As in other anthocyanin-rich foods, the SV method was found as the most suitable cooking method in terms of the preservation of these compounds.
In sum, purple flesh colored sweet potatoes cooked by SV method are recommended due to higher phenolic contents, antioxidant capacity and anthocyanins while baked purple samples had the highest amount of phenolic acids and sugar compounds. These findings highlight the importance of considering both flesh color and cooking methods in optimizing the bioactive compounds and health benefits of sweet potatoes.
Author contribution statement
Gamze Guclu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mumine Melike Dagli: Performed the experiments; Wrote the paper.
Ozge Aksay: Performed the experiments; Analyzed and interpreted the data.
Muharrem Keskin: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hasim Kelebek: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Serkan Selli: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors thank the farmers, Erkan Gezer and Erhan Duman, who provided the sweet potato samples used in the study.
Contributor Information
Hasim Kelebek, Email: hkelebek@atu.edu.tr.
Serkan Selli, Email: sselli@cu.edu.tr.
References
- 1.FAO . 2020. Crop Production Statistics.http://faostat.fao.org/ [Google Scholar]
- 2.Wang S., Nie S., Zhu F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016;89:90–116. doi: 10.1016/j.foodres.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 3.Amoanimaa-Dede H., Hongbo Z., Kyereko W.T., Yeboah A., Agyenim-Boateng K.A. Structure, Functions and biosynthetic pathway of naturally occurring anthocyanin in sweet potato -A Review. J. Plant. Biochem. Physiol. 2019;7:234. doi: 10.35248/2329-9029.19.7.234. [DOI] [Google Scholar]
- 4.Przybyl K., Adamski F., Wawrzyniak J., Gawrysiak-Witulska M., Stangierski J., Kmiecik D. Machine and deep learning in the evaluation of selected qualitative characteristics of sweet potatoes obtained under different convective drying conditions. Appl. Sci. 2022;12:7840. doi: 10.3390/app12157840. [DOI] [Google Scholar]
- 5.Dinu M., Soare R., Hoza G., Becherescu A.D., Băbeanu C. Bioactive compounds content and antioxidant activity in the leaves of some sweet potato cultivars (Ipomoea batatas L.) Scientific Papers. Series B, Horticulture. 2021;65:415–422. [Google Scholar]
- 6.Rivera-Espejel E.A., Oscar C.A., Mejia-Munoz J.M., Garcia-Mateos M.R., Colinas-Leon M.T., Martinez-Damian M.T. Physicochemical quality, antioxidant capacity and nutritional value in tuberous roots of some wild dahlia species. Not. Bot. Horti Agrobot. Cluj-Napoca. 2019;47(3):813–820. doi: 10.15835/nbha47311552. [DOI] [Google Scholar]
- 7.Teow C.C., Truong V.D., McFeeters R.F., Thompson R.L., Pecota K.V., Yencho G.C. Antioxidant activities, phenolic and β-carotene contents of sweet potato genotypes with varying flesh colours. Food Chem. 2007;103:829–838. doi: 10.1016/j.foodchem.2006.09.033. [DOI] [Google Scholar]
- 8.Bach D., Bedin A.C., Lacerda L.G., Nogueira A., Demiate I.M. Sweet potato (ipomoea batatas l.): a versatile raw material for the food industry. Braz. Arch. Biol. Technol. 2021;64 doi: 10.1590/1678-4324-2021200568. [DOI] [Google Scholar]
- 9.Padda M.S., Picha D.H. Methodology optimization for quantification of total phenolics and individual phenolic acids in sweet potato (Ipomoea batatas L.) roots. J. Food Sci. 2007;72:412–416. doi: 10.1111/j.1750-3841.2007.00448.x. [DOI] [PubMed] [Google Scholar]
- 10.Li A., Xiao R., He S. Research advances of purple sweet potato anthocyanins: extraction, identification, stability, bioactivity, application, and biotransformation. Mol. 2019;24:3816. doi: 10.3390/molecules24213816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sruthi N.U., Premjit Y., Pandiselvam R., Kothakota A., Ramesh S.V. An overview of conventional and emerging techniques of roasting: effect on food bioactive signatures. Food Chem. 2021;348 doi: 10.1016/j.foodchem.2021.129088. [DOI] [PubMed] [Google Scholar]
- 12.Keskin M., Guclu G., Sekerli Y.E., Soysal Y., Selli S., Kelebek H. Comparative assessment of volatile and phenolic profiles of fresh black carrot (Daucus carota L.) and powders prepared by three drying methods. Sci. Hortic. 2021;287 doi: 10.1016/j.scienta.2021.110256. [DOI] [Google Scholar]
- 13.Florkiewicz A., Socha R., Filipiak‐Florkiewicz A., Topolska K. Sous‐vide technique as an alternative to traditional cooking methods in the context of antioxidant properties of Brassica vegetables. J. Sci. Food Agric. 2018;99:173–182. doi: 10.1002/jsfa.9158. [DOI] [PubMed] [Google Scholar]
- 14.Musilova J., Lidikova J., Vollmannova A. Influence of heat treatments on the content of bioactive substances and antioxidant properties of sweet potato (Ipomoea batatas L.) tubers. J. Food Qual. 2020 doi: 10.1155/2020/8856260. [DOI] [Google Scholar]
- 15.Jing Y., Chen J.F., Zhao Y.Y., Mao L.C. Effects of drying processes on the antioxidant properties in sweet potatoes. Agric. Sci. China. 2010;9:1522–1529. doi: 10.1016/S1671-2927(09)60246-7. [DOI] [Google Scholar]
- 16.Jiang T., Mao Y., Sui L., Yang N., Li S., Zhu Z., He Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019;274:460–470. doi: 10.1016/j.foodchem.2018.07.141. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q., Song X., Arun S.M. Effects of blanching drying methods on the structure and physicochemical properties of starch in sweet potato slices. Food Hydrocolloids. 2022;127 doi: 10.1016/j.foodhyd.2022.107543. [DOI] [Google Scholar]
- 18.Franková H., Musilová J., Árvay J., Šnirc M., Jančo I., Lidiková J., Vollmannová A. Changes in antioxidant properties and phenolics in sweet potatoes (ipomoea batatas L.) due to heat treatments. Mol. 2022;27:1884. doi: 10.3390/molecules27061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelebek H., Sevindik O., Uzlasir T., Selli S. LC-DAD/ESI MS/MS characterization of fresh and cooked capia and aleppo red peppers (Capsicum annuum L.) phenolic profiles. Eur. Food Res. Technol. 2020;246:1971–1980. doi: 10.1007/s00217-020-03548-2. [DOI] [Google Scholar]
- 20.Tetik M.A., Sevindik O., Kelebek H., Selli S. Screening of key odorants and anthocyanin compounds of cv. okuzgozu (Vitis vinifera L.) red wines with a free run and pressed pomace using GC‐MS‐Olfactometry and LC‐MS‐MS. J. Mass Spectrom. 2018;53:444–454. doi: 10.1002/jms.4074. [DOI] [PubMed] [Google Scholar]
- 21.Sen K., Sonmezdag A.S. Elucidation of phenolic profiling of cv. Antep karasi grapes using LC-DAD-ESI-MS/MS. J. Raw Mater. Process Foods. 2021;1:1–6. [Google Scholar]
- 22.Tanriseven D., Kadiroglu P., Selli S., Kelebek H. LC-DAD-ESI-MS/MS-assisted elucidation of the phenolic compounds in shalgams: comparison of traditional and direct methods. Food Chem. 2020;305 doi: 10.1016/j.foodchem.2019.125505. [DOI] [PubMed] [Google Scholar]
- 23.Makori S.I., Mu T.H., Sun H.N. Total polyphenol content, antioxidant activity, and individual phenolic composition of different edible parts of 4 sweet potato cultivars. Nat. Prod. Commun. 2020;15:1–12. doi: 10.1177/1934578X20936931. [DOI] [Google Scholar]
- 24.Chan C.F., Chiang C.M., Lai Y.C., Huang C.L., Kao S.C., Liao W.C. Changes in sugar composition during baking and their effects on sensory attributes of baked sweet potatoes. J. Food Sci. Technol. 2014;51:4072–4077. doi: 10.1007/s13197-012-0900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelebek H., Kesen S., Selli S. Comparative study of bioactive constituents in Turkish olive oils by LC-ESI/MS/MS. Int. J. Food Prop. 2015;18(10):2231–2245. doi: 10.1080/10942912.2014.968788. 2015. [DOI] [Google Scholar]
- 26.Xu Y., Chen Y., Cao Y., Xia W., Jiang Q. Application of simultaneous combination of microwave and steam cooking to improve nutritional quality of cooked purple sweet potatoes and saving time. Innovat. Food Sci. Emerg. Technol. 2016;36:303–310. doi: 10.1016/j.ifset.2016.07.014. [DOI] [Google Scholar]
- 27.Olthof M.R., Hollman P.C., Katan M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001;131:66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]
- 28.Villegas R.J.A., Kojima M. Sweet potato root enzyme which catalyzes the formation of chlorogenic acid from 1-o-caffeoyl-d-glucoseand d-quinic acid. Agric. Biol. Chem. 1985;49:263–265. doi: 10.1080/00021369.1985.10866714. [DOI] [Google Scholar]
- 29.Kelebek H., Selli S. Characterization of phenolic compounds in strawberry fruits by RP-HPLC-DAD and investigation of their antioxidant capacity. J. Liq. Chromatogr. Relat. Technol. 2011;34:2495–2504. doi: 10.1080/10826076.2011.591029. [DOI] [Google Scholar]
- 30.Cody R.B., Tamura J., Downard K.M. Quantitation of anthocyanins in elderberry fruit extracts and nutraceutical formulations with paper spray ionization mass spectrometry. J. Mass Spectrom. 2018;53:58–64. doi: 10.1002/jms.4033. [DOI] [PubMed] [Google Scholar]
- 31.Iborra‐Bernad C., García‐Segovia P., Martínez‐Monzó J. Effect of vacuum cooking treatment on physicochemical and structural characteristics of purple‐flesh potato. Int. J. Food Sci. Technol. 2014;49:943–951. doi: 10.1111/ijfs.12385. [DOI] [Google Scholar]
- 32.Kano M., Takayanagi T., Harada K., Makino K., Ishikawa F. Antioxidative activity of anthocyanins from purple sweet potato, Ipomoera batatas cultivar Ayamurasaki. Biosci. Biotech. Biochem. 2005;69:979–988. doi: 10.1271/bbb.69.979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.