Abstract
Studies of autism spectrum disorder (ASD) related to exposure to toxic levels of dietary phosphate are lacking. Phosphate toxicity from dysregulated phosphate metabolism can negatively impact almost every major organ system of the body, including the central nervous system. The present paper used a grounded theory-literature review method to synthesise associations of dysregulated phosphate metabolism with the aetiology of ASD. Cell signalling in autism has been linked to an altered balance between phosphoinositide kinases, which phosphorylate proteins, and the counteracting effect of phosphatases in neuronal membranes. Glial cell overgrowth in the developing ASD brain can lead to disturbances in neuro-circuitry, neuroinflammation and immune responses which are potentially related to excessive inorganic phosphate. The rise in ASD prevalence has been suggested to originate in changes to the gut microbiome from increasing consumption of additives in processed food, including phosphate additives. Ketogenic diets and dietary patterns that eliminate casein also reduce phosphate intake, which may account for many of the suggested benefits of these diets in children with ASD. Dysregulated phosphate metabolism is causatively linked to comorbid conditions associated with ASD such as cancer, tuberous sclerosis, mitochondrial dysfunction, diabetes, epilepsy, obesity, chronic kidney disease, tauopathy, cardiovascular disease and bone mineral disorders. Associations and proposals presented in this paper offer novel insights and directions for future research linking the aetiology of ASD with dysregulated phosphate metabolism and phosphate toxicity from excessive dietary phosphorus intake.
Keywords: Autism spectrum disorder, autism, cancer, dysregulated phosphate metabolism, epilepsy, gliosis, gluten-free casein-free diet, ketogenic diet, mitochondrial dysfunction, phosphate food additives, phosphate toxicity
Introduction
The aetiology of autism spectrum disorder (ASD), a group of neurodevelopmental disorders that cause persistent social impairment with difficulties in communication and repetitive behavioural patterns, involves both genetic and environmental factors (Ref. 1). Prevalence of ASD rapidly increased from 1 out of 150 U.S. children in 2000, to 1 out of 68 children in 2012 and 1 out of 59 children in 2018 (Ref. 2). A recent systematic review and meta-analysis found that the male-to-female ratio of autism in children is approximately 3 to 1, which could be lower due to diagnostic gender bias that is less likely to diagnose females meeting ASD criteria compared to males (Ref. 3). By 2016, black children in the United States were 1.5 times more likely to be identified with ASD than white or Hispanic children, suggesting that ASD is associated with social determinants of health involving socio-economic status, housing, physical environment and racial discrimination (Ref. 4).
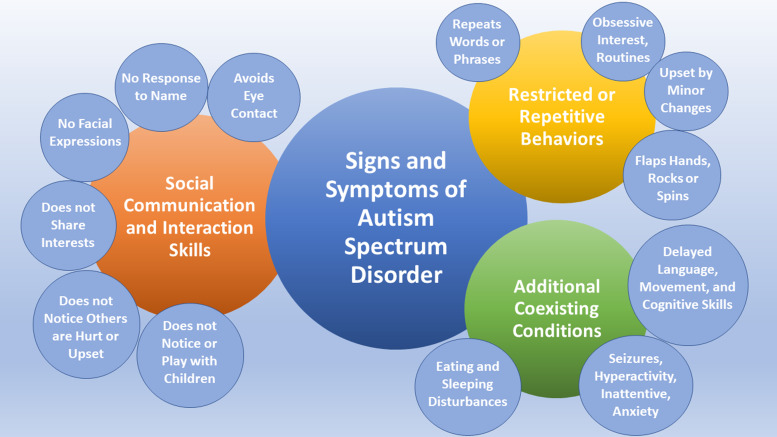
Figure 1 shows clinical signs and symptoms of ASD, based on information provided by the American Academy of Pediatrics Council on Children With Disabilities (Ref. 5). Age of onset of ASD symptoms ranges from the first year of infancy up to 2–3 years (Ref. 6). Adolescents with ASD have a higher risk of developing depression, anxiety and attention-deficit hyperactivity disorder (Ref. 7). With no known cause, treatments for ASD patients are mostly symptomatic to improve quality of life and daily functioning (Ref. 8).
Figure 1.
Signs and symptoms of autism spectrum disorder, based on American Academy of Pediatrics Council on Children With Disabilities (Ref. 5).
Among potential environmental factors in ASD aetiology, dietary associations with ASD merit further investigations. In particular, studies of neurodevelopmental disorders from exposure to toxic levels of dietary phosphate are lacking. Dietary phosphorus in the form of inorganic phosphate (Pi) is an essential mineral that is regulated in the body by a sensitive network of hormones released by the kidneys, intestines, parathyroid glands and bone (Ref. 9). Dysregulation of phosphate metabolism can lead to the accumulation of excess phosphate in the body causing a condition known as phosphate toxicity. Exposure to phosphate toxicity can negatively impact almost every major organ system in the body, including the central nervous system, with possible implications for the aetiology of neurodevelopmental disorders in ASD.
Method
The present narrative review used a grounded theory literature-review method (Ref. 10) to search, retrieve and analyse research findings using keywords related to dysregulated phosphate metabolism, phosphate toxicity and ASD. Grounded theory in this method replaces subjectivity and conjecture with a rigorous method to synthesise new information grounded in evidence. Through an iterative process of comparative analysis, findings from the research literature were developed into themes and formed into associated relationships, and additional keywords were searched to follow the trail of evidence and fill in knowledge gaps. The strength of this method's bottom-up inductive approach lies in discovering potential breakthrough knowledge grounded in findings from published literature (Ref. 11).
Nevertheless, ‘it is impossible to not be influenced by the background knowledge that one has’ (Ref. 10), and limitations of this method, as in most other research methods, include potential selection and information biases that influence data selection, analysis, interpretation and presentation of the research findings (Ref. 12). Findings may not be generalisable (external validity), and the researcher must successfully defend the work's internal validity under critical appraisal by peer experts. In the present paper, synthesised associations and proposals provide novel insights and directions for future research linking ASD aetiology and dysregulated phosphate metabolism.
ASD, cancer and phosphate
A retrospective cohort study of over 8000 children and adolescents with autistic disorder estimated that cancer incidence within the cohort was 94% higher than the expected cancer incidence (standardised incidence ratio 1.94) (Ref. 13). A more recent population-based study of 2.3 million individuals from Nordic countries found that overall risk from any cancer increased in ASD, but only in individuals with comorbid intellectual disability and/or birth defects (Ref. 14), implying involvement of other causative factors such as chromosomal abnormalities related to mutations in PTEN (Phosphatase and Tensin Homolog), a tumour suppressor phosphatase (Ref. 14). Phosphatases and protein kinases are enzymes that modulate cellular proteins by catalysing the transfer of phosphate in opposing directions (Ref. 15). A protein kinase catalyses the transfer of phosphate from ATP or GTP to phosphorylate a protein while a phosphatase catalyses the transfer of phosphate from a phosphoprotein to a water molecule. Similar to cell signalling in cancer (Ref. 16), autism has been linked to an altered balance between phosphoinositide kinases and the counteracting effect of phosphatases in neuronal membranes (Ref. 17).
Phosphorus is a growth-rate limiting factor in tumorigenesis (Ref. 18). High dietary phosphate activates phosphoinositide 3-kinase (PI3 K) which phosphorylates Akt (protein kinase B) leading to activation of mTOR kinase that upregulates protein synthesis in cancer cell proliferation while suppressing apoptosis (Ref. 19). High dietary phosphate also suppresses the counteracting effect of PTEN on PI3 K (Ref. 20). A similar cell-proliferation effect is seen in autism and epilepsy as PI3 K activates tuberous sclerosis complex 1/2 (TSC1/2) which activates mTOR kinase (Refs 21, 22). PTEN mutations in autism and epilepsy also suppress counteraction of PI3 K, leading to tumours and neurodevelopmental disorders in PTEN hamartoma tumour syndrome (Ref. 23). A study of brain analyses found that white matter in PTEN-ASD patients had a specific overgrowth pattern, with poor white matter development, reduced processing speed, increased deficits in working memory and extensive intellectual limitations (Ref. 24). Results of these brain tissue analyses imply that high concentrations of Pi may accumulate in the white matter of PTEN-ASD patients, who make up approximately 20% of children with ASD (Ref. 25), and future studies should investigate Pi brain concentrations in PTEN-ASD patients.
Of relevance, phosphorus 31 magnetic resonance spectroscopy (MRS) was used in an earlier study to detect high concentrations of Pi in temporal lobe epilepsy (Ref. 26). More recently, increased Pi was detected in gliomas using MRS (Ref. 27), and dysregulated expression of the gene PHD-finger protein 3 (PHF3) in glioblastoma overlaps with similar dysregulated gene expression of PHF3 in autism (Ref. 28). Increased benign glioma risk of the central nervous system in children is also associated with neurofibromatosis type 1 (NF1) (Ref. 29), and autistic behaviour is elevated in children with NF1 (Ref. 30).
Feasibly, as in cancer, future research may show that high dietary phosphate associated with phosphate toxicity activates PI3 K and causes PTEN mutations in autism and epilepsy, leading to overgrowth and defective neuronal connectivity. Of relevance, phosphoric acid used as a common food additive was found to cause genotoxic damage to DNA in human lymphocytes (Ref. 31), which is an epigenetic factor that could increase PTEN mutations. Furthermore, lower urinary excretion of phosphoric acid was found in children with autism compared to controls (Ref. 32), possibly indicating higher retention of phosphoric acid in body tissue with greater genotoxic damage in children with autism. More research is needed in the role of dysregulated phosphate metabolism in PTEN mutations in ASD.
ASD, dietary phosphate and gliosis
Infants who have been breastfed for long periods have better cognitive development and lower risk of autistic traits (Ref. 33). Phosphorus is about six-times lower in human breast milk (15 mg per 100 ml) than in cow milk (Ref. 34), and phosphorus plasma levels were lower and calcium levels higher in breastfed newborn babies compared to newborn babies fed unmodified cow milk (Ref. 35). In infancy, high phosphorus, sodium, potassium, protein and chloride intake from inappropriate consumption of whole cow milk places a burden on developing kidneys' solute load (Ref. 36). These findings suggest that breastfeeding is associated with lower risk of renal burden and reduced incidence of dysregulated phosphate metabolism potentially related to autistic traits.
Casein in cow milk, a phosphoprotein with phosphate groups bonded to amino acid side groups, makes up about 80% of cow milk protein (Ref. 37). Accordingly, dietary patterns that reduce casein also reduce phosphorus, which may account for many of the suggested benefits of casein-free diets in children with ASD (Ref. 38). Although gluten protein in grain does not contain phosphorus, bread and baked goods are often high in phosphorus and phosphate additives (Ref. 39). While a negative effect from gluten ingestion remains unproven in ASD, gluten-free diets are suggested to have beneficial effects in ASD (Ref. 40), which might be attributed to reductions in overall intake of phosphorus in bread and baked goods.
The rise in ASD has been suggested to originate in changes to the gut microbiome from increasing consumption of additives in processed food. Abdelli et al. found that exposing human neuron stem cells to the common food preservative propionic acid increased inflammatory responses and gliosis (excessive proliferation of fibrous support cells in the central nervous system) as occurs in neuro-circuitry dysfunction in ASD (Ref. 41). Interestingly, the researchers also found that propionic acid decreased levels of PTEN by about 50% compared to controls, which allowed levels of activated p-Akt (phosphorylated Akt) to increase and stimulate glial cell proliferation. Abdelli et al. also noted decreased neurite outgrowth in neuronal cells, which differentiate into dendrites or axons, and decreased axonal expansion was also noted which the researchers attributed to a physical barrier caused by excessive glia. The researchers proposed that dysregulated outgrowth and expansion explained disrupted communication between neuronal cells reported in ASD.
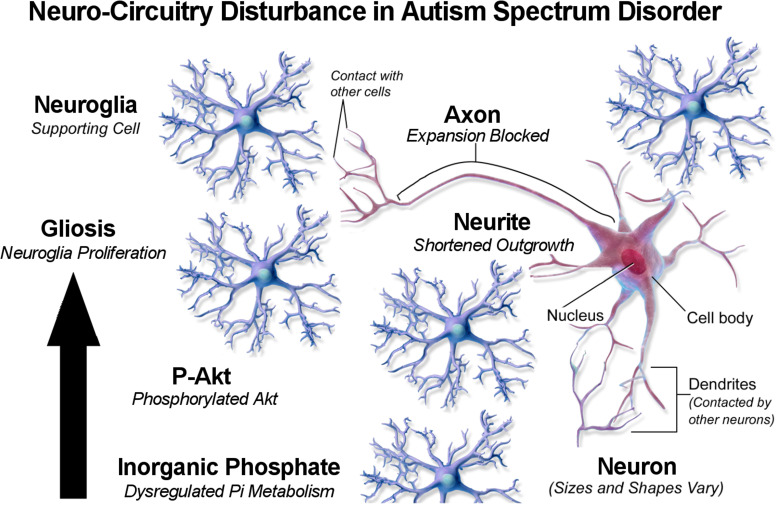
Figure 2 is a schematic diagram proposing that Pi from dysregulated phosphate metabolism phosphorylates Akt, leading to gliosis and neuro-circuitry disturbance in ASD. Future research should test this mechanism with in vitro experiments that compare glial cell proliferation in cultures exposed to concentrations of Pi, as well as in vivo experiments comparing levels of dietary phosphate and gliosis in lab animals. Although rodents are commonly used to model genetic mutations in autism, limited use of non-human primates comes closer to matching autistic behaviour in humans (Ref. 42).
Figure 2.
Neuro-circuitry disturbance in autism spectrum disorder. Based on https://upload.wikimedia.org/wikipedia/commons/7/73/Blausen_0672_NeuralTissue.png.
ASD, dysregulated phosphate and immune/neuroinflammatory responses
Abdelli et al. measured immune responses and neuroinflammation in human neural stem cells from exposure to propionic acid, including increased levels of inflammatory cytokines such as tumour necrosis factor alpha, with smaller increases in the anti-inflammatory cytokine interleukin 10 (Ref. 41). Rodriguez and Kern proposed that reducing neuroinflammation and microglial activation could lead to improvements in neurodevelopment outcomes in people with ASD (Ref. 43).
Feasibly, immune responses related to neuro-circuitry disturbances in ASD may be caused by dysregulated phosphate. The following text compares abnormalities of natural killer cells (NK-cells), T-cells, B-cells, macrophages and monocytes in patients with ASD (Ref. 44) with similar immune responses in conditions involving dysregulated phosphate. Although NK-cell proliferation was reported to increase in ASD, the NK-cells had reduced functioning ability (Ref. 45). Normally, NK-cells regulate neuron and glia functions, including proliferation and neurite outgrowth (Ref. 46), implying that NK-cell dysfunction and neuro-circuitry disturbances in ASD may share a common cause from exposure to phosphate toxicity. Of relevance, NK-cells are dysfunctional in end-stage kidney disease patients receiving dialysis therapy (Ref. 47), which is a procedure that helps control dysregulated phosphate (Ref. 48).
T-cell abnormalities in ASD include reduced proliferation of T-helper cells (Th) and reduced regulatory T cells (Treg) with upregulation of Th2 cells (Ref. 44). Interestingly, Th2 cells are involved in hypersensitivity to food antigens (Ref. 49) which may include hypersensitivity from exposure to excessive dietary phosphate. Of relevance to increased skin sensitivity in ASD (Ref. 50), high serum phosphate is associated with skin itching—pruritus (Ref. 51), and pruritus is associated with increased Th2 cytokines (Ref. 52). T cell exhaustion also occurs in kidney failure (Ref. 53), which is associated with harmful levels of serum phosphate (Ref. 54).
Children with ASD also have higher levels of pro-inflammatory B-cells [34]. B-cell infiltrates that occur in renal interstitial inflammation (Ref. 55) are potentially related to B-cell infiltrates in kidney burden from phosphate toxicity (Ref. 56). Macrophage and monocyte immune responses are also common in ASD (Ref. 44) and in conditions with dysregulated phosphate such as kidney disease. For example, accumulation of macrophages and monocytes in renal tissue causes inflammation and is associated with renal tissue damage, scarring and reduced kidney function (Ref. 57).
Dysregulated Pi in chronic kidney disease (CKD) also shares many other enzymes, immune responses and inflammatory markers with ASD. Compared to children with normal development, children with ASD were found to have elevated protein expression for IL-1β, IL-4, IL-9, interferon gamma (IFN-γ), Janus kinase 1 (JAK1), phosphorylated JAK1 (pJAK1), signal transducer and activator of transcription 5 (STAT5) and pSTAT5 (Ref. 58). Similarly, patients with diabetic nephropathy, a leading cause of end-stage renal disease with dysregulated Pi, have increased expression of IL-1β and IL-4 (Ref. 59), and overproduction of IL-9 and IFN-γ was found in patients with acute glomerular injury of the kidneys (Ref. 60). Additionally, JAK1 expression is enhanced in diabetic nephropathy (Ref. 61), phosphorylated JAK1 stimulates production of large amounts of FGF23, a regulator of phosphate metabolism (Ref. 62), abnormal amounts of STAT5 are expressed in autosomal dominant polycystic kidney disease (Ref. 63), and pSTAT5 is increased in focal segmental glomerulosclerosis of the kidneys (Ref. 64).
Compared to children with normal development, children with ASD showed elevated expression of chemokine receptors in CD4 + T cells, including chemokine receptor 2 + (CXCR2 + ), CXCR3 + , CXCR5 + and CXCR7 + and C-C motif chemokine receptor 3 + (CCR3 + ), CCR5 + , CCR7 + and CCR9+ (Ref. 65). Correspondingly, chemokine receptors CXCR1-7 are highly expressed in clear cell renal cell carcinoma (Ref. 66), CCR3 is upregulated in renal cell carcinoma (Ref. 67), CCR5 + contributes to the pathogenesis of CKD (Ref. 68), and children with CKD have increased expression of CCR7 and other chemokine receptors (Ref. 69). Furthermore, CCR9 + is associated with food allergens (Ref. 70), which may include reactions to exposure to excessive dietary phosphate.
Nuclear factor-erythroid 2 related factor 2 (Nrf2), a transcription factor that protects immune cells against inflammation and oxidation, was reduced in children with autism (Ref. 71). Similarly, impaired production of Nrf2 in CKD increases oxidation and inflammation leading to nephritis (Ref. 72). Severity of symptoms in children with autism is associated with upregulation of IL-6 receptors and IL-17A in CD4 + T cells (Ref. 73), and upregulated IL-17A expression in neutrophils increases inflammation and oxidation in children with autism (Ref. 74). Likewise, IL-17A plays a central role in kidney disease pathogenesis (Ref. 75) while IL-6 increases expression of FGF23 in acute and CKD (Ref. 76). Autism in children is also associated with elevated expression of IL-16 in CD4 + , CD8 + , CD14 + , CCR3 + and CXCR7 + cells (Ref. 77). Similarly, IL-16 contributes to inflammation of the kidneys which can lead to impaired kidney function (Ref. 78). Furthermore, immune dysfunction in children with autism is associated with upregulated T cell immunoglobulin and mucin domain 3 (TIM-3) (Ref. 79), and overexpression of TIM-3 is associated with severe kidney inflammation (Ref. 80).
Research is needed to explore common mechanisms that explain how dysregulated phosphate metabolism may contribute to immune and inflammatory responses in both kidney disease and ASD. Other environmental factors and toxic conditions can also contribute to ASD—nevertheless, evidence implicating dysregulated phosphate metabolism in ASD warrants further investigation.
ASD, phosphate additives and ultraprocessed food
U.S. consumption of ultra-processed foods, defined as industrially made, ready-to-eat or heat-and-serve products that contain food additives and generally lack whole foods, has continuously increased over the past two decades (Ref. 81), concurrent with increases in ASD prevalence. Children with ASD often prefer highly processed foods with phosphate additives, including chicken nuggets, pizza, macaroni with cheese, breakfast cereals, pancakes, waffles, hot dogs, crackers and bread, with less preference for fresh produce (Ref. 82). Compared to children with typical development, children with ASD were found to consume approximately 20–30% more ultra-processed food (Ref. 83). Of relevance, consumption of ultraprocessed food high in inorganic phosphates is associated with renal function decline in older adults (Ref. 84).
Additionally, a recent systematic review and meta-analysis found a 58% increased risk of obesity associated with ASD in children compared to controls (Ref. 85). Higher prevalence of childhood obesity in ASD may be related to higher energy intake and weight gain caused by ultra-processed food intake, as demonstrated in a controlled study of ultra-processed food consumed by adults with obesity (Ref. 86). Maternal intake of ultra-processed food is also associated with increased obesity in offspring (Ref. 87). Furthermore, a national cross-sectional study found that ultra-processed food consumption is greater among younger aged, lower income and less educated Americans (Ref. 88), and more research is needed to compare these demographic findings with ASD prevalence. Because neurodevelopment in newborns is critically influenced by maternal prenatal diet, which is suggested to play a role in ASD aetiology (Ref. 89), research should investigate whether younger mothers with less education, lower income, obesity and greater consumption of ultra-processed food have a higher risk of ASD in their offspring.
Many sugar-sweetened beverages (SSBs), such as colas, contain phosphoric acid, and increased consumption of SSBs is associated with stronger and more difficult emotional problems in ASD (Ref. 90). In addition, baking powder, chocolate, cocoa and beer are common sources of dietary phosphate that may contribute to excessive phosphate intake (Ref. 91). A 2019 national survey of food items in Finland (representative of Europe) found phosphate additives in 36% of sampled foods, and 17 different phosphate additives were observed overall (Ref. 92). Phosphorus-containing food additives are listed in Table 1.
Table 1.
Phosphorus-containing food additives
| Inorganic phosphates | Organic phosphates |
|---|---|
| Orthophosphate | Starch phosphates |
| Phosphoric acid | Monostarch phosphate |
| Sodium phosphate, mono-, di-, tri- | Distarch phosphate |
| Potassium phosphate, mono-, di-, tri- | Phosphated distarch phosphate |
| Calcium phosphate, mono-, di-, tri- | Acetylated distarch phosphate |
| Magnesium phosphates | Hydroxypropyl distarch phosphate |
| Magnesium phosphate, mono-, di- | Natural phosphorus-containing additives |
| Pyrophosphates | Ribonucleotides |
| Sodium diphosphate, di-, tri-, tetra- | Guanylic acid |
| Tetrapotassium diphosphate | Disodium guanylate |
| Dicalcium diphosphate | Dipotassium guanylate |
| Calcium dihydrogen phosphate | Calcium guanylate |
| Magnesium dihydrogen phosphate | Inosinic acid |
| Triphosphates | Disodium inosinate |
| Pentasodium phosphate | Dipotassium inosinate |
| Pentapotassium diphosphate | Calcium inosinate |
| Polyphosphates | Calcium-5′-ribonucleotides |
| Sodium polyphosphate | Disodium-5′-ribonucleotides |
| Potassium polyphosphate | Other |
| Sodium calcium polyphosphate | Lecithin |
| Calcium polyphosphate | Ammonium phosphatide |
| Other | Riboflavin-5′-phosphate |
| Sodium aluminium phosphate |
Based on Tuominen et al. (Ref. 92).
Sodium aluminium phosphate is commonly added to baking powder and processed cheese (Ref. 93). Excessive environmental exposure to aluminium within the body causes aluminium ions to bind with phosphate, which interferes with phosphorylation and dephosphorylation mechanisms in kinase and phosphatase enzymes, and contributes to mitochondrial dysfunction, microglia activation and immune and neuroinflammatory responses (Ref. 94). Compared to controls, greater levels of aluminium in brain tissue have been detected in autism and neurodegenerative diseases (Ref. 95), and intracellular aluminium in autism is concentrated within microglial cells and other non-neuron cells of brain tissue (Ref. 96).
In addition to higher dietary phosphate intake, average concentrations of urinary phosphorus excretion are 34% lower in children with ASD (Ref. 97), suggesting positive net phosphate absorption and tissue storage in ASD. Importantly, serum phosphate levels do not always accurately reflect tissue Pi storage levels (Ref. 98), and abnormal amounts of phosphate shifted from serum to tissue storage could explain hypophosphatemia found in some children with ASD compared to healthy children (Ref. 99). More studies are needed using phosphorus 31 MRS for detection of phosphate tissue storage in autism during brain development (Ref. 100).
Additionally, dietary fat is free of phosphorus, and lower phosphorus intake levels may explain benefits in ASD from high-fat ketogenic diets (Ref. 101). Ketogenic diets are effective in reducing or preventing seizures in epilepsy (Ref. 102), and ASD is associated with epilepsy (Ref. 103), implying that both ASD and epilepsy may share a common pathophysiological cause related to dysregulated phosphate metabolism and phosphate toxicity.
Evidence suggests that excessive dietary protein intake above optimal levels for neurodevelopment can be as harmful as deficient protein intake in perinatal care (Ref. 104). Of relevance, each gram of protein from dietary sources is directly related to approximately 12–14 mg phosphorus (Ref. 105). Consequently, dietary protein intake levels above optimal amounts for neurodevelopment are likely to involve excessive amounts of phosphorus with increased risk of dysregulated phosphate metabolism.
Higher ASD prevalence in males is consistent with higher mean dietary phosphorus intake in U.S. male children aged 2–11 years compared to females, with significantly higher phosphorus intake in male adolescents aged 12–19 years (Ref. 106). Interestingly, some children with ASD show improvements in behaviour during fever (Ref. 107). Coincidently, tumours also regress following fever (Ref. 108), and fever is associated with reduced appetite (Ref. 109), inferring lower food intake. Accordingly, reduction in dietary phosphate intake associated with fevers may act as a mediating factor that temporarily improves behaviours in ASD and may reduce tumours as lower phosphate levels limit the rate of cancer cell growth.
ASD, diabetes and dysregulated phosphate
Large studies have found that ASD is associated with increased prevalence of type 1 and type 2 diabetes mellitus (Ref. 110). Of relevance, neuronal degeneration and other complications in diabetes are associated with dysregulated phosphate metabolism (Ref. 111). These findings infer that dysregulated phosphate metabolism is a pathophysiological mechanism common to diabetes mellitus and neuronal degeneration in ASD. Maternal diabetes in rats induces autism-like behaviour in offspring (Ref. 112). In humans, maternal obesity comorbid with diabetes is associated with a greater risk of ASD in offspring compared to obesity or diabetes alone (Ref. 113). This finding implies that high dietary phosphate intake in obesity may interact with dysregulated phosphate metabolism in diabetes, leading to greater associated risk of ASD. Further research is needed to confirm an interactive relationship in ASD between obesity and diabetes mediated by excessive phosphate intake.
Prevalence of neurodevelopmental disorders, including ASD, is higher in children with type 1 diabetes, and diabetic nephropathy and retinopathy are also higher in type 1 diabetic children with neurodevelopment disorders (Ref. 114). Of relevance, children with ASD have a higher risk of ophthalmologic diagnosis (Ref. 115) and 25% of adults with ASD were found to have CKD (Ref. 116), providing further support for dysregulated phosphate metabolism as a common pathophysiological cause in diabetic complications and ASD. Furthermore, low muscle tone or hypotonia is an established marker of individuals with ASD (Ref. 117). Similarly, hypotonia affects children with dysregulated phosphate metabolism in CKD (Ref. 118), and hypotonia is associated with increased mortality in patients with renal failure receiving dialysis therapy for hyperphosphatemia (Ref. 119).
ASD, mitochondrial dysfunction and tauopathy
Mitochondrial dysfunction is associated with pathogenesis of ASD, and occurs in up to 80% of children with ASD (Ref. 120). Coincidentally, mitochondrial dysfunction is associated with dysregulated phosphate metabolism in the pathogenesis of diabetes (Ref. 111) and Parkinson's disease (Ref. 121). Precipitates of calcium phosphate collect within the mitochondria matrix of pancreatic beta cells and substantia nigra cells that produce insulin and dopamine in diabetes and Parkinson's disease, respectively, which interfere with the function of complex I in the electron transfer chain during oxidative phosphorylation. The result of mitochondrial dysfunction in cells includes reduced ATP biosynthesis, apoptosis and cell death. Importantly, the majority of children with autism in an exploratory controlled study had below-normal values of complex 1 activity (Ref. 122). Research is needed to examine the pathophysiologic effect of dysregulated phosphate metabolism, phosphate toxicity and calcium phosphate precipitation in mitochondrial dysfunction affecting neuronal cells of individuals with ASD.
In tauopathies associated with neurodegenerative diseases like Alzheimer disease and Parkinson's disease (Ref. 123), hyperphosphorylation of tau, a protein that assists in neuron cell maturation and cytoarchitecture, causes microtubule dysfunction which is linked with tau aggregation and neurofibrillary tangles (Ref. 124). Autism-like behaviours have been linked to increased levels of frontotemporal lobe tau and neurofibrillary pathology in late-life dementia (Ref. 125). Genetically lowering or removing tau in mouse models prevented or reduced key features of ASD, including abnormally enlarged head size, epilepsy and overactivation of the PI3 K/Akt/mTOR signalling pathway, which resulted in disinhibition of PTEN (Ref. 126). Drugs that interfere with tau phosphorylation are proposed to treat tauopathies (Ref. 124), but increased attention should focus on dietary interventions that reduce excessive phosphate intake, which may act as a rate-limiting factor to reduce tau hyperphosphorylation in the aetiology of neurodegenerative diseases and neurodevelopmental diseases like ASD. Importantly, hyperphosphorylation of tau is associated with glial-tau pathology in glial cells (Ref. 127), further implicating hyperphosphorylation as a cause of excessive glial cell proliferation and gliosis, which disrupts neuro-circuitry in ASD.
ASD, vitamin D, bone disorders and cardiovascular disease
The bioactive form of vitamin D, 1,25(OH)2D3, also known as calcitriol, is synthesised by the kidneys from the storage form of vitamin D, 25-hydroxy vitamin D, 25(OH)D3 (Ref. 9). Calcitriol increases intestinal absorption of Pi during digestion, raising serum phosphorus levels as needed. To reduce excessive levels of serum Pi, the kidneys produce less bioactive vitamin D, which decreases phosphorus absorption in the intestines. Low levels of 25(OH)D3, are common in ASD (Ref. 128), and lower serum levels of calcitriol and calcium were also found in ASD compared to normal controls (Ref. 129)—a potential biomarker indicating a kidney response in ASD that attempts to reduce serum Pi by lowering phosphate intestinal absorption when dietary phosphate intake is excessive.
Other endocrine hormones downregulate serum Pi by increasing renal phosphaturia, including fibroblast growth factor 23 (FGF23) released from bone and parathyroid hormone (PTH) released from the parathyroid glands (Ref. 9). A recent case study reported elevated serum PTH and serum phosphate in a 14-year old boy with ASD having hypocalemic seizures following a prolonged diet of processed snack foods (Ref. 130). Researchers also found structural changes in the frontal lobes associated with increased FGF23, likely in response to high levels of Pi which could also explain increased vascular calcification and microangiopathic lesions found in the brain leading to white matter loss (Ref. 131). In general the brain's frontal lobes control social and cognitive behaviour, and frontal cortex damage is known to lead to the onset of persistent and repetitive behaviour, insistence for sameness, and impulsive behaviours which are clinical features of ASD (Ref. 132). Interestingly, low serum levels of basic fibroblast growth factor, fibroblast growth factor 2 (FGF2), which regulates neurodevelopment, were found in children with ASD (Ref. 133). Future research is needed to investigate how dysregulated Pi impacts high FGF23 and low FGF2 levels in young children with ASD.
Odds of hip fractures in a case-control study were higher in children and adults with ASD compared to non-ASD individuals, and odds of fractures of the forearm and spine were greater in women with ASD (Ref. 134). Compared to typically developing children, a meta-analysis found that children with ASD had up to 13% lower bone mineral density of the total body, which is associated with increased risk of fractures (Ref. 135). Osteoporosis and fracture are also associated with dysregulated phosphate metabolism in CKD-mineral and bone disease (CKD-MBD) (Ref. 136), suggesting a common cause with fractures in ASD.
Bone mineral disorders related to dysregulated phosphate metabolism also impact poor oral health and periodontal disease, and dental plaque associated with gingivitis and periodontitis often forms from excessive calcium phosphate in saliva (Ref. 137). A meta-analysis in children and young adults with ASD found a pooled prevalence of approximately 70% for periodontal disease (Ref. 138), implicating phosphate toxicity in ASD as a potential pathophysiological factor in poor oral health.
Autism is also associated with endothelial dysfunction of the arterial system, which contributes to disruption of neurovascular coupling mechanisms in neurodevelopment (Ref. 139). In another potential link between ASD and dysregulated phosphate metabolism, hyperphosphatemia has a deleterious effect on endothelial dysfunction (Ref. 140). Of relevance, risk factors for cardiovascular disease (CVD) are increased in CKD, and the primary treatment goal in CKD is control of phosphate load (Ref. 141). Increased risk for heart failure and atrial fibrillation in CKD (Ref. 142) overlaps with similar risk for heart failure and atrial fibrillation in ASD (Ref. 143). Moreover, a higher risk of death in people with coronary disease was associated with higher serum phosphate levels (Ref. 144). Even people with normal renal function were found to have an increased risk of cardiovascular events associated with higher serum phosphate (Ref. 145). Although many environmental factors that contribute to ASD pathogenesis are shared with kidney disease, studies are lacking that investigate the association of paediatric kidney disease with ASD in children (Ref. 146), especially related to dysregulated phosphate metabolism.
Directed acyclic graph
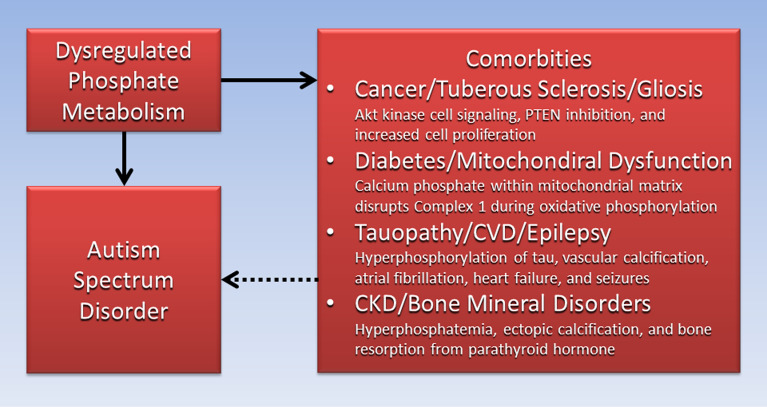
Figure 3 is a directed acyclic graph (DAG), often used in epidemiological and clinical research to illustrate causal pathways and mediating factors that help explain the association of outcomes with exposures to risk factors (Ref. 147). The DAG in Figure 3 shows that dysregulated phosphate metabolism is a potential mediating factor causatively linking (solid arrows) associations between ASD and comorbidities reviewed in this paper (dotted arrow). The DAG helps identify new pathways mediated by dysregulated phosphate metabolism for future research in the cause and prevention of ASD and associated comorbidities.
Figure 3.
Dysregulated phosphate metabolism is causatively linked to both autism spectrum disorder and comorbidities associated with autism spectrum disorder.
Conclusion
Evidence reviewed in this paper suggests that the effects of dysregulated phosphate metabolism and phosphate toxicity in ASD appear across the full lifespan, from the maternal stages of early development, throughout childhood and into adulthood. Dysregulated phosphate is proposed to stimulate gliosis of the brain in ASD, which disrupts neuro-circuitry by blocking axon extension and shortening neurite outgrowth. Children with ASD were found to have a wide variety of immune and neuroinflammatory responses. The rapid increase in ASD prevalence coincidences with the general population's increased consumption of ultraprocessed foods that are high in phosphate additives. Dysregulated phosphate metabolism is also causatively linked to comorbidities associated with ASD such as abnormal proliferation of cells, Akt kinase cell signalling and PTEN inhibition in cancer and tuberous sclerosis. Comorbidities also include calcium phosphate precipitation in mitochondrial dysfunction, impaired hormone secretion in diabetes, seizures in epilepsy, inflammatory and immune responses in CKD, obesity linked to excessive intake of ultraprocessed food, hyperphosphorylation in tauopathy, atrial fibrillation and heart failure in CVD, and bone resorption and ectopic calcification in bone mineral disorders.
Although other environmental factors and toxic conditions besides phosphate toxicity may contribute to ASD, findings implicating dysregulated phosphate metabolism in ASD warrant further investigation. The evidence presented in the present paper offers novel insights and directions for future research linking dysregulated phosphate metabolism with the aetiology of ASD. Additionally, future studies examining the effect of reduced dietary phosphorus in ASD are warranted.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The author declares no conflict of interest.
References
- 1.Hodges H, Fealko C and Soares N (2020) Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Translational Pediatrics 9(suppl. 1), S55–S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baio J et al. (2018) Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR. Surveillance Summaries 67, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loomes R, Hull L and Mandy WPL (2017) What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry 56, 466–474. [DOI] [PubMed] [Google Scholar]
- 4.Shaw KA et al. (2022) Progress and disparities in early identification of autism spectrum disorder: autism and developmental disabilities monitoring network, 2002–2016. Journal of the American Academy of Child & Adolescent Psychiatry 61, 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson CP and Myers SM and American Academy of Pediatrics Council on Children With Disabilities (2007) Identification and evaluation of children with autism spectrum disorders. Pediatrics 120, 1183–1215. [DOI] [PubMed] [Google Scholar]
- 6.Hyman SL, Levy SE and Myers SM (2020) Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics 145, e20193447. [DOI] [PubMed] [Google Scholar]
- 7.Accardo AL, Pontes NMH and Pontes MCF (2022) Heightened anxiety and depression among autistic adolescents with ADHD: findings from the national survey of children's health 2016–2019. Journal of Autism and Developmental Disorders 3, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Politte LC et al. (2015) Evidence-based treatments for autism spectrum disorder. Current Treatment Options in Psychiatry 2, 38–56. [Google Scholar]
- 9.Brown RB and Razzaque MS (2015) Dysregulation of phosphate metabolism and conditions associated with phosphate toxicity. BoneKEy reports 4, 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfswinkel JF, Furtmueller E and Wilderom CPM (2013) Using grounded theory as a method for rigorously reviewing literature. European Journal of Information Systems 22, 45–55. [Google Scholar]
- 11.Brown RB (2020) Breakthrough knowledge synthesis in the age of Google. Philosophies 5, 4. [Google Scholar]
- 12.Tripepi G et al. (2010) Selection bias and information bias in clinical research. Nephron Clinical Practice 115, c94–c99. [DOI] [PubMed] [Google Scholar]
- 13.Chiang H-L et al. (2015) Risk of cancer in children, adolescents, and young adults with autistic disorder. The Journal of Pediatrics 166, 418–423.e1. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q et al. (2022) Cancer risk in individuals with autism spectrum disorder. Annals of Oncology 33, 713–719. [DOI] [PubMed] [Google Scholar]
- 15.Cheng HC et al. (2011) Regulation and function of protein kinases and phosphatases. Enzyme Research 2011, 794089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown RB and Razzaque MS (2018) Phosphate toxicity and tumorigenesis. Biochimica et Biophysica Acta (BBA) – Reviews on Cancer 1869, 303–309. [DOI] [PubMed] [Google Scholar]
- 17.Gross C (2017) Defective phosphoinositide metabolism in autism. Journal of Neuroscience Research 95, 1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuang Y, Nagy JD and Elser JJ (2004) Biological stoichiometry of tumor dynamics: mathematical models and analysis. Discrete and Continuous Dynamical Systems Series B 4, 221–240. [Google Scholar]
- 19.Guertin DA and Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer cell 12, 9–22. [DOI] [PubMed] [Google Scholar]
- 20.Jin H et al. (2009) High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. American Journal of Respiratory and Critical Care Medicine 179, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis PE et al. (2015) Tuberous sclerosis: a new frontier in targeted treatment of autism. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics 12, 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxena A and Sampson JR (2015) Epilepsy in tuberous sclerosis: phenotypes, mechanisms, and treatments. Seminars in Neurology 35, 269–276. [DOI] [PubMed] [Google Scholar]
- 23.Yehia L, Keel E and Eng C (2020) The clinical spectrum of PTEN mutations. Annual Review of Medicine 71, 103–116. [DOI] [PubMed] [Google Scholar]
- 24.Frazier TW et al. (2015) Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Molecular Psychiatry 20, 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busch RM et al. (2019) Neurobehavioral phenotype of autism spectrum disorder associated with germline heterozygous mutations in PTEN. Translational Psychiatry 9, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laxer KD et al. (1992) Increased pH and seizure foci inorganic phosphate in temporal demonstrated by [31P]MRS. Epilepsia 33, 618–623. [DOI] [PubMed] [Google Scholar]
- 27.Peter SB and Nandhan VR (2021) 31-Phosphorus magnetic resonance spectroscopy in evaluation of glioma and metastases in 3 T MRI. The Indian Journal of Radiology & Imaging 31, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appel LM et al. (2021) PHF3 regulates neuronal gene expression through the Pol II CTD reader domain SPOC. Nature Communications 12, 6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa AA and Gutmann DH (2019) Brain tumors in neurofibromatosis type 1. Neuro-Oncology Advances 1, vdz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chisholm AK et al. (2022) Delineating the autistic phenotype in children with neurofibromatosis type 1. Molecular Autism 13, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yilmaz S et al. (2014) DNA damage in human lymphocytes exposed to four food additives in vitro. Toxicology and Industrial Health 30, 926–937. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q et al. (2019) Urine organic acids as potential biomarkers for autism-Spectrum disorder in Chinese children. Frontiers in Cellular Neuroscience 13, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boucher O et al. (2017) Association between breastfeeding duration and cognitive development, autistic traits and ADHD symptoms: a multicenter study in Spain. Pediatric Research 81, 434–442. [DOI] [PubMed] [Google Scholar]
- 34.Platt BS and Moncrieff A (1947) Nutritional comparison of human and cow's milk for infant-feeding. British Medical Bulletin 5, 177–180. [Google Scholar]
- 35.Oppé T and Redstone D (1968) Calcium and phosphorus levels in healthy newborn infants given various types of milk. The Lancet 291, 1045–1048. [DOI] [PubMed] [Google Scholar]
- 36.Leung AK and Sauve RS (2003) Whole cow's milk in infancy. Paediatrics & Child Health 8, 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarode AR et al. (2016) Casein and caseinate: methods of manufacture. In Caballero B, Finglas PM and Toldrá F (eds), Encyclopedia of Food and Health. Oxford: Academic Press, pp. 676–682. [Google Scholar]
- 38.Quan L et al. (2021) A systematic review and meta-analysis of the benefits of a gluten-free diet and/or casein-free diet for children with autism spectrum disorder. Nutrition reviews 80, 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.León JB, Sullivan CM and Sehgal AR (2013) The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. Journal of Renal Nutrition 23, 265–270.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Croall ID, Hoggard N and Hadjivassiliou M (2021) Gluten and autism spectrum disorder. Nutrients 13, 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdelli LS, Samsam A and Naser SA (2019) Propionic acid induces gliosis and neuro-inflammation through modulation of PTEN/AKT pathway in autism spectrum disorder. Scientific reports 9, 8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katsnelson A (2018) Modeling autism. Lab Animal 47, 41–44. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez JI and Kern JK (2011) Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron Glia Biology 7, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gevezova M et al. (2020) Inflammation and mitochondrial dysfunction in autism spectrum disorder. CNS & Neurological Disorders Drug Targets 19, 320–333. [DOI] [PubMed] [Google Scholar]
- 45.Ashwood P et al. (2011) In search of cellular immunophenotypes in the blood of children with autism. PloS one 6, e19299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebrahimi Meimand S, Rostam-Abadi Y and Rezaei N (2021) Autism spectrum disorders and natural killer cells: a review on pathogenesis and treatment. Expert Review of Clinical Immunology 17, 27–35. [DOI] [PubMed] [Google Scholar]
- 47.Nagai K (2021) Dysfunction of natural killer cells in end-stage kidney disease on hemodialysis. Renal Replacement Therapy 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cupisti A et al. (2013) Phosphate control in dialysis. International Journal of Nephrology and Renovascular Disease 6, 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellenbogen Y et al. (2018) The initiation of Th2 immunity towards food allergens. International Journal of Molecular Sciences 19, 1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jameson C et al. (2022) Eczema and related atopic diseases are associated with increased symptom severity in children with autism spectrum disorder. Translational Psychiatry 12, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swarna SS et al. (2019) Pruritus associated with chronic kidney disease: a comprehensive literature review. Cureus 11, e5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcovich S et al. (2021) Pruritus as a distinctive feature of type 2 inflammation. Vaccines (Basel) 9, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartzell S et al. (2020) Kidney failure associates with T cell exhaustion and imbalanced follicular helper T cells. Frontiers in Immunology 11, 583702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rastogi A et al. (2021) Management of hyperphosphatemia in end-stage renal disease: a new paradigm. Journal of Renal Nutrition 31, 21–34. [DOI] [PubMed] [Google Scholar]
- 55.Heller F et al. (2007) The contribution of B cells to renal interstitial inflammation. American Journal of Pathology 170, 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mironov N, Atfi A and Razzaque MS (2022) Phosphate burden and organ dysfunction. Frontiers in Aging 3, 890985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X et al. (2021) The role of macrophages in kidney fibrosis. Frontiers in Physiology 12, 705838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmad SF et al. (2017) Upregulation of IL-9 and JAK-STAT signaling pathway in children with autism. Progress in Neuro-Psychopharmacology & Biological Psychiatry 79, 472–480. [DOI] [PubMed] [Google Scholar]
- 59.Araújo LS et al. (2020) Renal expression of cytokines and chemokines in diabetic nephropathy. BMC Nephrology 21, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim MG et al. (2022) Kidney VISTA prevents IFN-γ/IL-9 axis-mediated tubulointerstitial fibrosis after acute glomerular injury. Journal of Clinical Investigation 132, e151189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brosius FC III and He JC (2015) JAK Inhibition and progressive kidney disease. Current Opinion in Nephrology and Hypertension 24, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daryadel A et al. (2021) Systemic Jak1 activation provokes hepatic inflammation and imbalanced FGF23 production and cleavage. FASEB Journal 35, e21302. [DOI] [PubMed] [Google Scholar]
- 63.Fragiadaki M et al. (2017) STAT5 drives abnormal proliferation in autosomal dominant polycystic kidney disease. Kidney International 91, 575–586. [DOI] [PubMed] [Google Scholar]
- 64.Tao J et al. (2018) JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney International 94, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmad SF et al. (2018) Upregulation of peripheral CXC and CC chemokine receptor expression on CD4(+) T cells is associated with immune dysregulation in children with autism. Progress in Neuro-Psychopharmacology & Biological Psychiatry 81, 211–220. [DOI] [PubMed] [Google Scholar]
- 66.Wu Z et al. (2020) Characterization of the prognostic values of the CXCR1-7 in clear cell renal cell carcinoma (ccRCC) microenvironment. Frontiers in Molecular Biosciences, 7, 601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jöhrer K et al. (2005) Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clinical Cancer Research 11, 2459–2465. [DOI] [PubMed] [Google Scholar]
- 68.Fuhro MI et al. (2022) The impact of intradialytic exercise on immune cells expressing CCR5 + in patients with chronic kidney disease: a cross-over trial. The International Journal of Artificial Organs 45, 221–226. [DOI] [PubMed] [Google Scholar]
- 69.Szczepańska M et al. (2015) Expression of chemokine receptors on peripheral blood T cells in children with chronic kidney disease. Mediators of Inflammation 2015, 536894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castan L et al. (2018) Food allergen-sensitized CCR9(+) lymphocytes enhance airways allergic inflammation in mice. Allergy 73, 1505–1514. [DOI] [PubMed] [Google Scholar]
- 71.Nadeem A et al. (2020) Differential regulation of Nrf2 is linked to elevated inflammation and nitrative stress in monocytes of children with autism. Psychoneuroendocrinology 113, 104554. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz S et al. (2013) Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney International 83, 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nadeem A et al. (2020) Dysregulation in IL-6 receptors is associated with upregulated IL-17A related signaling in CD4 + T cells of children with autism. Progress in Neuro-Psychopharmacology & Biological Psychiatry 97, 109783. [DOI] [PubMed] [Google Scholar]
- 74.Nadeem A et al. (2019) Oxidative and inflammatory mediators are upregulated in neutrophils of autistic children: role of IL-17A receptor signaling. Progress in Neuro-Psychopharmacology & Biological Psychiatry 90, 204–211. [DOI] [PubMed] [Google Scholar]
- 75.Cortvrindt C et al. (2017) The role of interleukin-17A in the pathogenesis of kidney diseases. Pathology 49, 247–258. [DOI] [PubMed] [Google Scholar]
- 76.Durlacher-Betzer K et al. (2018) Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney International 94, 315–325. [DOI] [PubMed] [Google Scholar]
- 77.Ahmad SF et al. (2019) Elevated IL-16 expression is associated with development of immune dysfunction in children with autism. Psychopharmacology 236, 831–838. [DOI] [PubMed] [Google Scholar]
- 78.Tavener SK, Jewell DE and Panickar KS (2022) The increase in circulating levels of pro-inflammatory chemokines, cytokines, and complement C5 in canines with impaired kidney function. Current Issues in Molecular Biology 44, 1664–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahmad SF et al. (2019) Dysregulation of T cell immunoglobulin and mucin domain 3 (TIM-3) signaling in peripheral immune cells is associated with immune dysfunction in autistic children. Molecular Immunology 106, 77–86. [DOI] [PubMed] [Google Scholar]
- 80.Lu C et al. (2021) An emerging role of TIM3 expression on T cells in chronic kidney inflammation. Frontiers in Immunology 12, 798683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Juul F et al. (2021) Ultra-processed food consumption among US adults from 2001 to 2018. The American Journal of Clinical Nutrition 115, 211–221. [DOI] [PubMed] [Google Scholar]
- 82.Mayes SD and Zickgraf H (2019) Atypical eating behaviors in children and adolescents with autism, ADHD, other disorders, and typical development. Research in Autism Spectrum Disorders 64, 76–83. [Google Scholar]
- 83.Buro A, Kakkad A and Gray H (2020) P120 children with autism Spectrum disorder who are picky eaters may consume more ultra-processed foods than non-picky eaters. Journal of Nutrition Education and Behavior 52(suppl. 7), S73. [Google Scholar]
- 84.Rey-García J et al. (2021) Ultra-processed food consumption is associated with renal function decline in older adults: a prospective cohort study. Nutrients 13, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sammels O et al. (2022) Autism spectrum disorder and obesity in children: a systematic review and meta-analysis. Obesity Facts 15, 305–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hall KD et al. (2019) Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metabolism 30, 67–77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Y et al. (2022) Maternal consumption of ultra-processed foods and subsequent risk of offspring overweight or obesity: results from three prospective cohort studies. BMJ 379, e071767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baraldi LG et al. (2018) Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open 8, e020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong C et al. (2020) Maternal dietary factors and the risk of autism spectrum disorders: a systematic review of existing evidence. Autism Research: Official Journal of the International Society for Autism Research 13, 1634–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tan S et al. (2022) The association between sugar-sweetened beverages and milk intake with emotional and behavioral problems in children with autism spectrum disorder. Frontiers in Nutrition 9, 927212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Picard K et al. (2023) Currently available handouts for low phosphorus diets in chronic kidney disease continue to restrict plant proteins and minimally processed dairy products. Journal of Renal Nutrition 33, 45–52. [DOI] [PubMed] [Google Scholar]
- 92.Tuominen M, Karp HJ and Itkonen ST (2022) Phosphorus-Containing food additives in the food supply-an audit of products on supermarket shelves. Journal of Renal Nutrition 32, 30–38. [DOI] [PubMed] [Google Scholar]
- 93.Saiyed SM and Yokel RA (2005) Aluminium content of some foods and food products in the USA, with aluminium food additives. Food Additives & Contaminants 22, 234–244. [DOI] [PubMed] [Google Scholar]
- 94.Morris G, Puri BK and Frye RE (2017) The putative role of environmental aluminium in the development of chronic neuropathology in adults and children. How strong is the evidence and what could be the mechanisms involved? Metabolic Brain Disease 32, 1335–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Exley C and Clarkson E (2020) Aluminium in human brain tissue from donors without neurodegenerative disease: a comparison with Alzheimer's disease, multiple sclerosis and autism. Scientific Reports 10, 7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mold M et al. (2018) Aluminium in brain tissue in autism. Journal of Trace Elements in Medicine and Biology 46, 76–82. [DOI] [PubMed] [Google Scholar]
- 97.Qureshi F et al. (2020) Urinary essential elements of young children with autism spectrum disorder and their mothers. Research in Autism Spectrum Disorders 72, 101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Osuka S and Razzaque MS (2012) Can features of phosphate toxicity appear in normophosphatemia? Journal of Bone and Mineral Metabolism 30, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Afroz S et al. (2020) Serum calcium and phosphate in children with autism spectrum disorder. Journal of Bangladesh Society of Physiologist 15, 72–77. [Google Scholar]
- 100.Minshew NJ and Pettegrew JW (1998) 31P Magnetic resonance spectroscopy and its application to autism and brain development. In Garreau B (ed.), Neuroimaging in Child Neuropsychiatric Disorders. Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 181–197. [Google Scholar]
- 101.Li Q et al. (2021) A ketogenic diet and the treatment of autism spectrum disorder. Frontiers in Pediatrics 9, 650624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D'Andrea Meira I et al. (2019) Ketogenic diet and epilepsy: what we know so far. Frontiers in Neuroscience 13, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Besag FM (2018) Epilepsy in patients with autism: links, risks and treatment challenges. Neuropsychiatric Disease and Treatment 14, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barreault S et al. (2019) Impact of early protein and energy intakes on neurodevelopment at 2 years of corrected age in very low birth weight infants: a single-center observational study. PLoS ONE 14, e0218887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.D'Alessandro C, Piccoli GB and Cupisti A (2015) The “phosphorus pyramid”: a visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrology 16, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moshfegh A, Kovalchik A and Clemens J (2016) Phosphorus intake of Americans: what we eat in America, NHANES 2011–2012. Food Surveys Research Group Dietary Data Brief No 15.
- 107.Grzadzinski R et al. (2018) Children with autism spectrum disorder who improve with fever: insights from the simons simplex collection. Autism Research: Official Journal of the International Society for Autism Research 11, 175–184. [DOI] [PubMed] [Google Scholar]
- 108.Køstner AH et al. (2013) Regression in cancer following fever and acute infection. Acta Oncologica 52, 455–457. [DOI] [PubMed] [Google Scholar]
- 109.IOM (1993) Nutritional needs in hot environments: Applications for military personnel in field operations. Institute of Medicine (US) Committee on Military Nutrition Research. 1993. In. [PubMed]
- 110.Tromans S et al. (2020) The prevalence of diabetes in autistic persons: a systematic review. Clinical Practice and Epidemiology in Mental Health 16, 212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brown RB (2020) Diabetes, diabetic complications, and phosphate toxicity: a scoping review. Current Diabetes Reviews 16, 674–689. [DOI] [PubMed] [Google Scholar]
- 112.Wang X et al. (2019) Maternal diabetes induces autism-like behavior by hyperglycemia-mediated persistent oxidative stress and suppression of superoxide dismutase 2. Proceedings of the National Academy of Sciences of the USA 116, 23743–23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li M et al. (2016) The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics 137, e20152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu S et al. (2021) Neurodevelopmental disorders, glycemic control, and diabetic complications in type 1 diabetes: a nationwide cohort study. The Journal of Clinical Endocrinology & Metabolism 106, e4459–e4e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chang MY et al. (2021) Prevalence of ophthalmologic diagnoses in children with autism spectrum disorder using the optum dataset: aPopulation-based study. American Journal of Ophthalmology 221, 147–153. [DOI] [PubMed] [Google Scholar]
- 116.Miot S et al. (2019) Comorbidity burden in adults with autism spectrum disorders and intellectual disabilities – A report from the EFAAR (frailty assessment in ageing adults with autism spectrum and intellectual disabilities) study. Frontiers in Psychiatry 10, 00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gabis LV et al. (2021) The weak link: hypotonia in infancy and autism early identification. Frontiers in Neurology 12, 612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Abd El Naby SA et al. (2020) Neurophysiological and neuroradiological changes in children with chronic kidney disease. Frontiers in Pediatrics 8, 570708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wanic-Kossowska M and Czekalski S (2007) [Hypotonia in renal failure patients undergoing dialysis therapy]. Polskie Archiwum Medycyny Wewnetrznej 117, 58–63. [PubMed] [Google Scholar]
- 120.Castora FJ (2019) Mitochondrial function and abnormalities implicated in the pathogenesis of ASD. Progress in Neuro-Psychopharmacology and Biological Psychiatry 92, 83–108. [DOI] [PubMed] [Google Scholar]
- 121.Brown RB (2022) Parkinson's disease etiology: insights and associations with phosphate toxicity. International Journal of Molecular Sciences 23, 8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giulivi C et al. (2010) Mitochondrial dysfunction in autism. JAMA 304, 2389–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang X et al. (2018) Tau pathology in Parkinson's disease. Frontiers in Neurology 9, 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xia Y, Prokop S and Giasson BI (2021) “Don't Phos over Tau”: recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer's disease and other tauopathies. Molecular Neurodegeneration 16, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rhodus EK et al. (2022) Frontotemporal neurofibrillary tangles and cerebrovascular lesions are associated with autism spectrum behaviors in late-life dementia. Journal of Neurology 269, 5105–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tai C et al. (2020) Tau reduction prevents key features of autism in mouse models. Neuron 106, 421–437.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kahlson MA and Colodner KJ (2015) Glial Tau pathology in tauopathies: functional consequences. Journal of Experimental Neuroscience 9, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Petruzzelli MG et al. (2020) Vitamin D deficiency in autism spectrum disorder: a cross-sectional study. Disease Markers 2020, 9292560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meguid NA et al. (2010) Reduced serum levels of 25-hydroxy and 1,25-dihydroxy vitamin D in Egyptian children with autism. Journal of Alternative and Complementary Medicine 16, 641–645. [DOI] [PubMed] [Google Scholar]
- 130.Kota A, Kumar K and Bargman R (2019) Hypocalcemic seizures in an adolescent with autism spectrum disorder. Consultant360.com. 2019. In.
- 131.Marebwa BK et al. (2018) Fibroblast growth factor23 is associated with axonal integrity and neural network architecture in the human frontal lobes. PLoS ONE 13, e0203460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khadem-Reza ZK and Zare H (2022) Evaluation of brain structure abnormalities in children with autism spectrum disorder (ASD) using structural magnetic resonance imaging. The Egyptian Journal of Neurology, Psychiatry and Neurosurgery 58, 135. [Google Scholar]
- 133.Esnafoglu E and Ayyıldız SN (2017) Decreased levels of serum fibroblast growth factor-2 in children with autism spectrum disorder. Psychiatry Research 257, 79–83. [DOI] [PubMed] [Google Scholar]
- 134.Neumeyer AM et al. (2015) Brief report: bone fractures in children and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders 45, 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rostami Haji Abadi M et al. (2021) Bone health in children and youth with ASD: a systematic review and meta-analysis. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 32, 1679–1691. [DOI] [PubMed] [Google Scholar]
- 136.Pazianas M and Miller PD (2021) Osteoporosis and chronic kidney disease-mineral and bone disorder (CKD-MBD): back to basics. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation 78, 582–589. [DOI] [PubMed] [Google Scholar]
- 137.Brown RB (2019) Dysregulated phosphate metabolism, periodontal disease, and cancer: possible global health implications. Dentistry Journal 7, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.da Silva SN et al. (2017) Oral health status of children and young adults with autism spectrum disorders: systematic review and meta-analysis. International Journal of Paediatric Dentistry 27, 388–398. [DOI] [PubMed] [Google Scholar]
- 139.Ouellette J et al. (2020) Vascular contributions to 16p11.2 deletion autism syndrome modeled in mice. Nature Neuroscience 23, 1090–1101. [DOI] [PubMed] [Google Scholar]
- 140.Stevens KK et al. (2017) Deleterious effects of phosphate on vascular and endothelial function via disruption to the nitric oxide pathway. Nephrology Dialysis Transplantation 32, 1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ritter CS and Slatopolsky E (2016) Phosphate toxicity in CKD: the killer among us. Clinical Journal of the American Society of Nephrology 11, 1088–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rehm M et al. (2022) Chronic kidney disease and risk of atrial fibrillation and heart failure in general population-based cohorts: the BiomarCaRE project. ESC Heart Failure 9, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun X et al. (2021) Association of autism spectrum disorder, neuroticism, and subjective well-being with cardiovascular diseases: a two-sample Mendelian randomization study. Frontiers in Cardiovascular Medicine 8, 676030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tonelli M et al. (2005) Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112, 2627–2633. [DOI] [PubMed] [Google Scholar]
- 145.McGovern AP et al. (2013) Serum phosphate as a risk factor for cardiovascular events in people with and without chronic kidney disease: a large community based cohort study. PLoS ONE 8, e74996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Clothier J and Absoud M (2021) Autism spectrum disorder and kidney disease. Pediatric Nephrology 36, 2987–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Digitale JC, Martin JN and Glymour MM (2022) Tutorial on directed acyclic graphs. Journal of Clinical Epidemiology 142, 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]