Abstract
Background
An urgent need remains for antiviral therapies to treat patients hospitalized with COVID-19. PF-07304814—the prodrug (lufotrelvir) and its active moiety (PF-00835231)—is a potent inhibitor of the SARS-CoV-2 3CL protease.
Method
Eligible participants were 18 to 79 years old and hospitalized with confirmed COVID-19. This first-in-human phase 1b study was designed with 2 groups: single ascending dose (SAD) and multiple ascending dose (MAD). Participants could receive local standard-of-care therapy. In SAD, participants were randomized to receive a 24-hour infusion of lufotrelvir/placebo. In MAD, participants were randomized to receive a 120-hour infusion of lufotrelvir/placebo. The primary endpoint was to assess the safety and tolerability of lufotrelvir. The secondary endpoint was to evaluate the pharmacokinetics of lufotrelvir and PF-00835231.
Results
In SAD, participants were randomized to receive 250 mg lufotrelvir (n = 2), 500 mg lufotrelvir (n = 2), or placebo (n = 4) by continuous 24-hour infusion. In MAD, participants were randomized to receive 250 mg lufotrelvir (n = 7), 500 mg lufotrelvir (n = 6), or placebo (n = 4) by continuous 120-hour infusion. No adverse events or serious adverse events were considered related to lufotrelvir. At doses of 250 and 500 mg, concentrations for the prodrug lufotrelvir and active moiety PF-00835231 increased in a dose-related manner. Unbound concentrations of the lufotrelvir active metabolite reached steady state approximately 2- and 4-fold that of in vitro EC90 following 250- and 500-mg doses, respectively.
Conclusions
These safety and pharmacokinetic findings support the continued evaluation of lufotrelvir in clinical studies.
Clinical Trials Registration. ClinicalTrials.gov NCT04535167.
Keywords: COVID-19, PF-00835231, PF-07304814, SARS-CoV-2, antiviral
Single and multiple ascending PF-07304814 (lufotrelvir) infusions were generally safe and well tolerated in participants hospitalized with COVID-19. Unbound concentrations of the lufotrelvir active metabolite reached steady state approximately 2- and 4-fold that of in vitro EC90 following 250 and 500 mg/d, respectively.
COVID-19 continues to be a leading cause of hospitalization and death worldwide despite the availability of effective vaccines [1–3]. Several therapies became available under emergency use authorization, including neutralizing monoclonal antibodies (mAbs) and antivirals such as nirmatrelvir, for nonhospitalized patients at high risk of severe COVID-19 during the early stages of infection [4–7]. Sotrovimab and bebtelovimab were mAbs that were recommended during the Omicron surge, while other mAbs, such as bamlanivimab and etesevimab, were previously available under emergency use authorization but have since been removed due to diminished efficacy against emergent SARS-CoV-2 variants [4, 8–10]. Antivirals available for the treatment of COVID-19 in hospitalized patients remain limited. Although intravenous (IV) remdesivir has been shown to improve time to clinical recovery in patients hospitalized with COVID-19, clinical trials have not shown a mortality benefit [11–13]. Other therapeutics recommended in patients hospitalized with COVID-19, such as dexamethasone, baricitinib, and tocilizumab, are directed at modulating systemic inflammation [4]. Thus, an urgent need remains for additional effective antiviral therapies to treat patients hospitalized with COVID-19.
Coronaviruses such as SARS-CoV-2 produce 2 large viral polyproteins processed by 2 virally encoded cysteine proteases: the main protease, also called the 3C-like protease (Mpro), and the papain-like protease [14]. Mpro is crucial for processing viral polyproteins into functional units [15], is highly conserved across SARS-CoV-2 and other coronaviruses [16, 17], and has no identified human analogs [18], making it an attractive target for viral inhibitors. Nirmatrelvir [19], used in combination with ritonavir in outpatient settings, has been shown to be highly effective at reducing hospitalization and death [20], validating that Mpro inhibitors can be effective at preventing the severe outcomes of COVID-19 when administered within 5 days of symptom onset. Lufotrelvir is a phosphate prodrug of PF-00835231 (active moiety), which is also a potent and selective inhibitor of the SARS-CoV-2 Mpro. Lufotrelvir has been investigated as a continuous IV infusion for the treatment of patients hospitalized with COVID-19 [14]. This first-in-human study evaluated the safety, tolerability, and pharmacokinetics (PK) of escalating doses of lufotrelvir over 1 or 5 days in adult patients hospitalized with COVID-19 during the first year of the pandemic.
METHODS
Study Design and Participants
This phase 1b sponsor-open study, which was double-blind, randomized, and placebo controlled, was conducted with parallel groups to evaluate the safety, tolerability, and PK of lufotrelvir and active moiety PF-00835231 in participants hospitalized for COVID-19 (NCT04535167). Participants were recruited from the United States, Belgium, and Spain. Eligible participants were 18 to 79 years of age and hospitalized with confirmed COVID-19. Participants were excluded if they required mechanical ventilation or extracorporeal membrane oxygenation. Additional eligibility criteria are summarized in the Supplementary Appendix.
The study was designed as a 2-part study based on a single ascending dose (SAD) and a multiple ascending dose (MAD; Supplementary Figure 1). All participants were allowed to receive local standard-of-care therapy, including anticoagulation prophylaxis. In SAD, participants were randomized to receive a 24-hour infusion of lufotrelvir or placebo. The study was planned such that at each dose level, 8 participants would be randomized to receive lufotrelvir or placebo in a 6:2 ratio, with 2 sentinel participants randomized in a 1:1 ratio in the dose-escalating cohorts. In MAD, participants were randomized to receive a 120-hour infusion of lufotrelvir or placebo. The study was planned such that at each dose level, 8 participants would be randomized to receive lufotrelvir or placebo in a 6:2 ratio.
Patient Consent Statement
Written informed consent was obtained from all participants before any study activity. The study was conducted in accordance with ethical principles derived from the Declaration of Helsinki and in compliance with the International Council for Harmonisation’s guidelines for good clinical practice. All local regulatory requirements were followed, including those affording greater protection to the safety of trial participants. The study protocol and amendments were approved by the institutional review board or ethics committee.
Randomization, Blinding, and Study Drug Preparation
Participants were randomized to treatment groups via an interactive response technology system. Lufotrelvir was provided as a solution for infusion in a single-use vial for the SAD group or as a powder in a single-use vial to be prepared into a dosing solution for infusion for the MAD group. Investigators were blinded to each participant's assigned study intervention throughout the course of the study. To maintain the blind, a third party prepared and dispensed the study intervention and ensured no differences in time to dispense.
Safety Evaluations
The primary endpoint was the assessment of safety and tolerability of lufotrelvir following SAD and MAD. Safety monitoring included the evaluation of adverse events (AEs) and serious AEs (SAEs) and the assessment of vital signs (including pulse oximetry/oxygen saturation), electrocardiograms, and laboratory tests at prespecified time points.
Measurement of biomarkers associated with safety was an exploratory endpoint. The change from baseline in coagulation studies (activated partial thromboplastin time [aPTT], prothrombin time [PT], and D-dimer level), fibrinogen, haptoglobin, high-sensitivity C-reactive protein (hs-CRP), ferritin, and procalcitonin was evaluated on days 1, 2, 3, and 6 for SAD and days 1, 2, 3, 5 to 7, 10, 14, and 34 to 41 for MAD.
PK Evaluations
Evaluation of the plasma PK of the prodrug lufotrelvir and the active moiety PF-00835231 was a secondary endpoint. Plasma samples obtained at prespecified time points were analyzed with a validated, sensitive, and specific method of liquid chromatography‒tandem mass spectrometry [21]. In the SAD group, plasma samples were collected before treatment was started and then at 6, 24, 28, and 48 hours of the start of infusion. In the MAD group, samples were collected before treatment was started and then at 24, 48, 96, 120, 122, and 126 hours after the start of infusion and on day 7 after treatment began. Definitions for PK parameters are provided in Supplementary Table 1.
Clinical Status and COVID-19 Signs and Symptoms
Clinical status on an 8-point ordinal scale (Supplementary Appendix) and COVID-19 signs and symptoms were evaluated as exploratory endpoints on days 1, 2, 3, and 6 and at the last follow-up visit (days 30‒37) in SAD and on days 1‒7, 10, and 14 and at the last follow-up visit (days 34‒41) in MAD. Clinical virology endpoints were also measured and will be reported separately (viral load and viral sequencing).
Statistical Methods
No statistical hypothesis testing was planned for this study. Safety data were summarized descriptively for the safety population (Supplementary Table 2). Plasma concentrations of lufotrelvir and PF-00835231 were assessed for the PK concentration population and descriptively summarized by nominal PK sampling time and dose. Concentrations below the limit of quantification were set to zero. Plasma PK parameters of lufotrelvir and PF-00835231 were derived from concentration-time data according to standard noncompartmental methods in oNCA version 2.4.33. PK parameters for the PK parameter population were descriptively summarized for lufotrelvir and PF-00835231 by dose. Absolute values and changes from baseline in biomarkers were evaluated for the biomarker analysis population and summarized by treatment and time after dose.
Nonclinical Studies
Methods for in vivo nonclinical studies are described in the Supplementary Appendix.
RESULTS
Participants
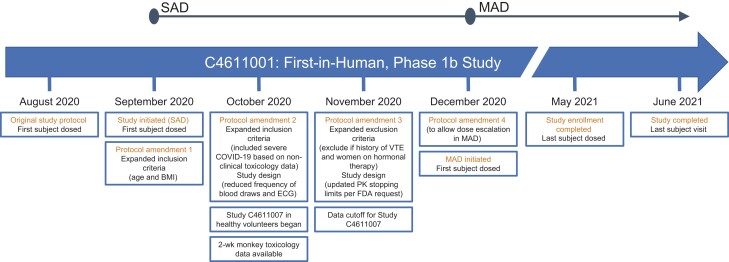
A timeline of key events is shown in Figure 1. Dosing for this study began September 2020 and ended May 2021. Participants were enrolled from the United States, Belgium, and Spain. In SAD, 8 participants were enrolled and randomized to receive 250 mg lufotrelvir (n = 2), 500 mg lufotrelvir (n = 2), or placebo (n = 4) as a continuous infusion for 24 hours. All 8 participants in the SAD group completed the study treatment phase and entered the follow-up phase. One participant who received placebo was lost to follow-up.
Figure 1.
Timeline of key events. BMI, body mass index; COVID-19, coronavirus disease 2019; ECG, electrocardiogram; FDA, US Food and Drug Administration; MAD, multiple ascending dose; PK, pharmacokinetics; SAD, single ascending dose; VTE, venous thrombotic event.
In MAD, 17 participants were enrolled and randomized to receive 250 mg lufotrelvir (n = 7), 500 mg lufotrelvir (n = 6), or placebo (n = 4) as a continuous infusion for 120 hours. All participants who received lufotrelvir (250 or 500 mg) completed the study treatment and follow-up phases. Of the 4 participants who received placebo, 1 discontinued from study treatment due to an increase in liver enzymes, and 1 who entered the follow-up phase discontinued due to death attributed to hypoxia.
Table 1 shows the demographic and baseline characteristics of the participants at the time of enrollment for each part of the study. Overall, the median duration since the first COVID-19 sign and symptom was 7 days from time of screening in both parts of the study. The most common concomitant medications were dexamethasone (88% of SAD and 82% of MAD groups) and remdesivir (100% of SAD and 47% of MAD groups).
Table 1.
Participant Demographics and Baseline Characteristics
| Characteristic | SAD (24 h) (n = 8) | MAD (120 h) (n = 17) |
|---|---|---|
| Age, y | ||
| 18‒44 | 2 (25.0) | 5 (29.4) |
| 45‒64 | 4 (50.0) | 7 (41.2) |
| ≥65 | 2 (25.0) | 5 (29.4) |
| Mean (SD) | 50.6 (12.3) | 54.6 (13.9) |
| Median (range) | 48.0 (34‒71) | 54.0 (33‒77) |
| Male | 5 (62.5) | 14 (82.4) |
| Race | ||
| White | 6 (75.0) | 13 (76.5) |
| Black or African American | 0 | 2 (11.8) |
| Asian | 0 | 1 (5.9) |
| Not reported | 2 (25.0) | 1 (5.9) |
| Ethnicity | ||
| Hispanic/Latinx | 2 (25.0) | 2 (11.8) |
| Not Hispanic/Latinx | 6 (75.0) | 12 (70.6) |
| Not reported | 0 | 3 (17.6) |
| Body mass index, kg/m2 | ||
| Mean (SD) | 30.2 (3.8) | 26.2 (3.6) |
| Median (range) | 30.6 (22.2‒35.0) | 25.3 (19.2‒33.9) |
| ≤29.9 | 2 (25.0) | 14 (82.4) |
| 30.0‒34.9 | 5 (62.5) | 3 (17.6) |
| 35.0‒39.9 | 1 (12.5) | 0 |
| Comorbidities | ||
| Autoimmune disorder | 0 | 1 (5.9) |
| Asthma | 1 (12.5) | 1 (5.9) |
| Diabetes mellitus type 2 | 0 | 2 (11.8) |
| Hypertension | 1 (12.5) | 5 (29.4) |
| Medication usea | ||
| Dexamethasone | 7 (87.5) | 14 (82.4) |
| Metoclopramide | 5 (62.5) | 5 (29.4) |
| Remdesivir | 8 (100.0) | 8 (47.1) |
| Anticoagulants | ||
| Enoxaparin | 3 (37.5) | 4 (23.5) |
| Low molecular weight heparin | 0 | 3 (17.6) |
| Bemiparin | 0 | 1 (5.9) |
| Clinical status by ordinal scaleb (day 1) | ||
| 3 | 1 (12.5) | 1 (5.9) |
| 4 | 6 (75.0) | 13 (76.5) |
| 5 | 1 (12.5) | 3 (17.6) |
| Duration since first COVID-19 sign and symptom, d (since screening) | ||
| Median (range) | 7 (2‒10) | 7 (1‒15) |
All data are No. (%) unless stated otherwise.
Abbreviations: MAD, multiple ascending dose; SAD, single ascending dose.
In ≥50% in either group or anticoagulant medication.
Assessed on an 8-point ordinal scale.
Safety
Table 2 A summarizes treatment-emergent AEs (TEAEs) in SAD. Only 1 TEAE, mild hematuria in a participant receiving placebo, was considered treatment related by the principal investigator. Most all-causality TEAEs (14/19) were mild in severity, except for 1 moderate AE of coagulopathy and 1 severe AE of respiratory failure in 1 participant, as well as 3 severe SAEs.
Table 2.
Treatment-Emergent AEs in the Single and Multiple Ascending Dose Groups
| Lufotrelvir | Placebo | |||
|---|---|---|---|---|
| 250 mg | 500 mg | 250 mga | 500 mgb | |
| A: Single Ascending Dose | ||||
| All causality | ||||
| Evaluable participants | 2 | 2 | 2 | 2 |
| No. of AEs | 2 | 3 | 5 | 9 |
| AEs | 1 (50.0) | 1 (50.0) | 2 (100.0) | 2 (100.0) |
| SAEs | 0 | 1 (50.0) | 1 (50.0) | 1 (50.0) |
| Severe AEs | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) |
| Discontinued from study due to AEs | 0 | 0 | 0 | 0 |
| Treatment relatedc | ||||
| Evaluable participants | 2 | 2 | 2 | 2 |
| No. of AEs | 0 | 0 | 1 | 0 |
| AEs | 0 | 0 | 1 (50.0)d | 0 |
| SAEs | 0 | 0 | 0 | 0 |
| Severe AEs | 0 | 0 | 0 | 0 |
| B: Multiple Ascending Dose | … | |||
| All causality | ||||
| Evaluable participants | 7 | 6 | 4 | … |
| No. of AEs | 13 | 4 | 9 | … |
| AEs | 4 (57.1) | 2 (33.3) | 3 (75.0) | … |
| SAEs | 2 (28.6) | 1 (16.7) | 1 (25.0) | … |
| Severe AEs | 2 (28.6) | 0 | 1 (25.0) | … |
| Discontinued from study due to AEs | 0 | 0 | 1 (25.0)e | … |
| Discontinued drug due to AE and continued study | 0 | 0 | 0 | … |
| Dose reduction or temporary discontinuation due to AE | 0 | 1 (16.7) | 0 | … |
| Treatment relatedc | ||||
| Evaluable participants | 7 | 6 | 4 | … |
| No. of AEs | 0 | 0 | 1 | … |
| AEs | 0 | 0 | 1 (25.0)a | … |
| SAEs | 0 | 0 | 0 | … |
| Severe AEs | 0 | 0 | 0 | … |
All data are presented as No. (%) unless stated otherwise. Results are for the safety analysis set. Except for the number of AEs, participants were counted only once per treatment in each row.
Abbreviations: AE, adverse event; SAE, serious AE.
Placebo group for lufotrelvir, 250 mg.
Placebo group for lufotrelvir, 500 mg.
As determined by the investigator.
Mild hematuria.
Increased liver function test.
One participant who received 250 mg lufotrelvir began high-flow nasal cannula oxygen therapy 1 day before the single dose of the study drug, experienced respiratory failure requiring mechanical ventilation on day 16 relative to infusion of study drug, required extracorporeal membrane oxygenation on day 17, and had onset of moderate coagulopathy on day 18; both AEs occurred >2 weeks after investigational product administration. Both AEs were considered nonserious by the investigator. The 3 SAEs were COVID-19 pneumonia (placebo, n = 1) and 2 events of subclavian vein thrombosis (500 mg lufotrelvir, n = 1; placebo, n = 1). In the 2 subclavian vein thrombosis SAEs, both participants had received anticoagulation prophylaxis and had midlines placed for blood draws; they experienced the subclavian vein thrombosis in the same upper extremity of midline placement. None of the SAEs were considered treatment related. No discontinuations from the study or deaths were reported in the SAD group.
TEAEs in MAD are summarized in Table 2B. No dose-related increase in AE occurrence was observed. Among all TEAEs, 13 were mild in severity, 8 were moderate, and 5 were severe. One participant who received placebo was discontinued from the study on day 4 of infusion due to an AE of increased liver enzymes, which was considered treatment related by the principal investigator. Six SAEs were reported in 4 participants (23.5%): 2 (28.6%) in the 250-mg lufotrelvir group, 1 (16.7%) in the 500-mg lufotrelvir group, and 1 (25.0%) in the placebo group. The most frequent SAE was pulmonary embolism (PE), which occurred in 2 participants (500 mg lufotrelvir, n = 1; placebo, n = 1), both of whom were receiving prophylactic anticoagulation. A 77-year-old participant randomized to 500 mg lufotrelvir with a medical history of diabetes mellitus and hyperlipidemia, who had an elevated D-dimer level at the time of presentation, was diagnosed with a moderate SAE of PE on day 4 of investigational product administration. The other participant who experienced an SAE of PE was a 74-year-old participant randomized to placebo with a medical history of hypertension and decreased glomerular filtration rate. This participant had onset of an SAE of hypoxia on day 5 of placebo, which required mechanical ventilation, and an SAE of PE on day 8 and subsequently died on day 16 due to COVID-19‒related hypoxia. None of the SAEs were considered treatment related.
One participant treated with 250 mg lufotrelvir had mild AEs of hyperglycemia and thrombocytopenia; neither was considered treatment related. No other laboratory changes were reported as AEs in participants treated with lufotrelvir. In the SAD and MAD groups, there were no clinically significant changes in vital signs or electrocardiogram results. The incidence and severity of all TEAEs for the SAD and MAD groups are presented in Supplementary Table 3.
Biomarkers of Coagulation and Inflammation
During SAD, the mean values of aPTT and PT were generally within the reference ranges (Supplementary Figure 2A). There were no observed trends in D-dimer levels in participants receiving lufotrelvir as compared with placebo. Overall fibrinogen, haptoglobin, and hs-CRP values remained stable or decreased from baseline during treatment across groups.
In MAD, the mean values of aPTT and PT were generally within the reference ranges across treatment groups (Supplementary Figure 2B). D-dimer levels were above the upper limit of normal across treatment groups during treatment and at variable levels during follow-up. Across treatment groups, fibrinogen, haptoglobin, and hs-CRP values were above the reference ranges at baseline and remained stable or decreased during the study, with hs-CRP maintaining decreased levels in participants who received lufotrelvir compared with those taking the placebo. The limited number of participants in the SAD and MAD parts of the study precluded formal statistical analysis of the laboratory results among the treatment and placebo groups. However, no detectable differences in inflammatory markers or coagulation studies were observed across treatment groups.
Pharmacokinetics
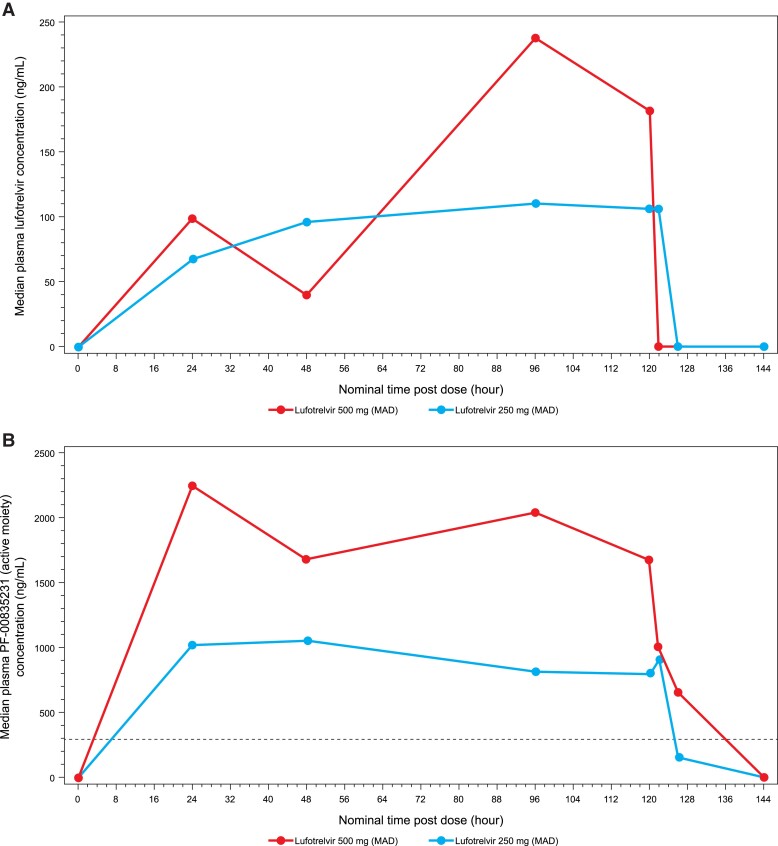
Table 3 summarizes the PK parameters for SAD and MAD for lufotrelvir and PF-00835231. In MAD, concentrations increased in a dose-related manner across both doses, with mean concentrations at 120 hours (C120) of 91.64 and 197.2 ng/mL for the 250- and 500-mg doses, respectively. For concentration at steady state (Css), mean values were 102.2 and 229.2 ng/mL for the 250- and 500-mg doses. In MAD, PF-00835231 concentrations increased in an approximately dose-proportional manner across both doses, with mean C120 values of 800.8 and 1338 ng/mL for the 250- and 500-mg doses. Mean Css values were 970.2 and 1720 ng/mL for the 250- and 500-mg doses. Mean t½ for the 250-mg dose was 2.317 hours, and only 1 participant in the 500-mg treatment group had a reportable t½ (1.79 hours). Median lufotrelvir and PF-00835231 concentration-time profiles are shown in Figure 2. The median concentration of PF-00835231 remained above the EC90 (adjusted for plasma protein binding) of 463 ng/mL for SARS-CoV-2 [14] from 8 through 120 hours postdose, and unbound concentrations of lufotrelvir reached steady state approximately 2- and 4-fold that of in vitro EC90 following 250 and 500 mg/d, respectively.
Table 3.
Descriptive Summary of Pharmacokinetic Parameters for Plasma Lufotrelvir and PF-00835231
| Lufotrelvir | ||
|---|---|---|
| 250 mg | 500 mg | |
| Plasma lufotrelvir | ||
| Single ascending dose | ||
| N, N2a | 2, 0 | 2, 0 |
| AUClast, ng·h/mL | … | 2550, 5120 |
| Cmax, ng/mL | … | 562, 878 |
| tmax, h | … | 0.500, 2.12 |
| C24, ng/mL | 53.7, 130 | 0b |
| Css, ng/mL | … | 155, 319 |
| Multiple ascending dose | ||
| N, N2a | 7, 7 | 6, 5 |
| Cmax, ng/mL | 272.6 (281) | 345.3 (70) |
| tmax, h | 118 (45.5‒140) | 95.0 (45.1‒117) |
| Css, ng/mL | 102.2 (35) | 229.2 (61) |
| Css (dn), ng/mL/mg | 0.4086 (35) | 0.4591 (61) |
| C120, ng/mLc | 91.64 (51) | 197.2 (72) |
| C120 (dn), ng/mL/mgc | 0.3666 (51) | 0.3944 (72) |
| Plasma PF-00835231 | ||
| Single ascending dose | ||
| N, N2a | 2, 0 | 2, 0 |
| AUCinf, ng·h/mL | … | 40000, 73300 |
| AUClast, ng·h/mL | … | 39900, 71700 |
| Cmax, ng/mL | … | 3230, 3530 |
| t1/2, h | … | 2.01, 2.80 |
| tmax, h | … | 0.500, 6.12 |
| C24, ng/mL | 632, 1140 | 1350b |
| Css, ng/mL | … | 1680, 3000 |
| Multiple ascending dose | ||
| N, N2, N3a | 7, 7, 3 | 6, 6, 1 |
| Cmax, ng/mL | 1265 (20) | 2382 (36) |
| t1/2, h | 2.317 ± 0.96547 | 1.79b |
| tmax, h | 45.0 (21.5‒128) | 23.0 (20.9‒95.0) |
| Css, ng/mL | 970.2 (16) | 1720 (44) |
| Css (dn), ng/mL/mg | 3.884 (16) | 3.439 (44) |
| C120, ng/mL | 800.8 (19) | 1338 (84) |
| C120 (dn), ng/mL/mg | 3.203 (19) | 2.675 (84) |
Values are presented as geometric means (percentage coefficient of variation) except tmax, median (range); t1/2, arithmetic mean ± SD; and otherwise, as indicated. Individual values are presented where N, N2, or N3 is <3. Results are for the pharmacokinetic parameter set. Parameters are further defined in Supplementary Table 1.
Abbreviations: AUCinf, area under the plasma concentration-time curve from time 0 extrapolated to infinity; AUClast, area under the plasma concentration-time curve from time 0 to the time of the last quantifiable concentration; C24, concentration at 24 hours; C120, concentration at 120 hours; Cmax, maximum plasma concentration; Css, concentration at steady state; dn, dose normalized to 1 mg lufotrelvir; t1/2, terminal half-life; tmax, time to Cmax.
N is the total number of participants in the treatment group in the indicated population; N2 is the number of participants contributing to the summary statistics; and N3 is the number of participants contributing to the summary statistics for t1/2.
Only 1 participant had a value.
Arithmetic mean (percentage coefficient of variation).
Figure 2.
Median concentration-time curves of (A) lufotrelvir and (B) PF-00835231 (active moiety) following multiple ascending doses of lufotrelvir. The dotted line in panel B is the EC90 of 463 ng/mL for SARS-CoV-2. MAD, multiple ascending dose.
Nonclinical Safety Studies Supporting Clinical Trials
Nonclinical studies conducted to assess the safety of lufotrelvir and PF-00835231 are listed in Supplementary Table 4. In vitro results were previously reported [14]. The major findings from in vivo safety studies in rats and monkeys administered the drug via continuous IV infusions, as well as systemic drug exposures, are outlined in Supplementary Tables 5‒11. No effects on cardiovascular or pulmonary function were observed. No target organs of toxicity were identified, and observed effects were those commonly associated with the infusion procedure (rats and monkeys) or exacerbation of infusion procedure‒related effects at higher doses (monkeys) [22–25].
DISCUSSION
Results from this first-in-human study showed that 24- and 120-hour continuous infusions of lufotrelvir (250 and 500 mg) appeared safe and well tolerated in this limited investigation in participants hospitalized with COVID-19. No AEs, SAEs, or severe AEs that occurred with SAD or MAD were considered related to lufotrelvir. The SAEs were attributed to the known clinical manifestations and complications associated with severe COVID-19 infection and disease progression. This included the 4 SAEs of venous thrombotic events (VTEs; subclavian vein thrombosis, n = 2; PE, n = 2), which occurred equally across the placebo and lufotrelvir groups. These events are in line with reports of thrombotic complications in patients with COVID-19 [26, 27]. Patients hospitalized with COVID-19 are at increased risk of VTEs, with widely ranging incidence rates, which can depend on various factors, such as host factors and the hospital setting. Two meta-analyses evaluating the incidence of VTEs reported that these events occurred in 7.9% to 13.0% of patients with COVID-19 in the non‒intensive care unit and 22.7% to 30.4% of patients with COVID-19 in the intensive care unit [26, 27]. Importantly, the VTEs in this study were distributed equally across the treatment and placebo groups. Thus, no unexpected safety findings were observed. Following 24-hour intravenous infusions of lufotrelvir and 120-hour continuous IV infusions at doses of 250 and 500 mg, concentrations for the prodrug lufotrelvir and its active moiety PF-00835231 increased in a dose-related manner, with dose-proportional increases for C120 and Css following multiple dosing.
This first-in-human study was unusual in that the first clinical administration was in patients with COVID-19 rather than healthy volunteers. This was done due to the urgent medical need for treatment. At the time of study initiation, good laboratory practice rat toxicology data were available to support SAD dosing in patients [14]. Subsequently, good laboratory practice data from 2-week continuous infusion toxicology studies in rats and monkeys became available to support the enrollment of participants in MAD. A study in healthy volunteers was also initiated (C4611007) that provided data to inform a starting MAD dose for C4611001 [21]. The notable finding in the monkey study was an exacerbation at higher doses of commonly observed infusion procedure‒related effects of inflammation and thromboemboli. As a result, the PK stopping limits were conservatively adjusted after consultation with the US Food and Drug Administration in the middle of conducting SAD. The initial PK stopping limit for PF-00835231 was a 24-hour area under the concentration-time curve of 272 μg·h/mL, which was modified to 28.2 μg·h/mL. Combined with the observed PK data from the first 4 participants (cohort 1; 500 mg or placebo), a decision was made to set the dose at 250 mg for the next cohort (cohort 2).
After a single 500-mg dose of lufotrelvir, the maximum plasma concentration, Css, and area under the curve for PF-00835231 were higher among participants in this study compared with healthy volunteers, whereas t½ was similar between healthy participants and patients [21]. However, the small number of participants in this study preclude definitive comparisons between the studies.
Limitations of this study include the small number of participants. Recruitment challenges arose due to various factors, such as the strict eligibility criteria, intensive monitoring required for the phase 1 study, and the continuous infusion requirement. At the outset of the study, only patients with mild or moderate COVID-19 could be enrolled; patients with severe COVID-19 could enroll later. Because this was a phase 1 study, multiple safety and PK assessments were required, which could be challenging for patients and health care workers, especially in settings with high numbers of COVID-19 cases. Similarly, the need to administer the study drug via continuous infusions over 1 or 5 days required significant hospital resources and training. Thus, it was challenging to recruit sites, and significant time was required to recruit patients; subsequently, a small number of participants were enrolled. Another limitation of the study was the ability to evaluate the primary endpoint of safety and tolerability of lufotrelvir in a population hospitalized with COVID-19, with a small number of participants across the SAD and MAD treatment arms, thus making it challenging to ascertain attribution of the AEs observed to study drug vs the underlying infection.
CONCLUSION
Continuous 24- and 120-hour IV infusions of lufotrelvir (250 and 500 mg) appeared to be safe and well tolerated in this limited investigation in hospitalized patients with COVID-19. At doses of 250 and 500 mg, concentrations of the prodrug lufotrelvir and its active moiety PF-00835231 increased in a dose-related manner. Mean Css values for the active moiety PF-00835231 were above the EC90 of 463 ng/mL for SARS-CoV-2. The safety and pharmacokinetic findings of this study support the continued evaluation of lufotrelvir in clinical studies.
Supplementary Material
Contributor Information
Philip Robinson, Infectious Disease, Hoag Memorial Hospital Presbyterian, Newport Beach, California, USA.
Sima S Toussi, Pfizer Worldwide Research, Development and Medical, Pfizer Inc, Pearl River, New York, USA.
Sudeepta Aggarwal, Early Clinical Development, Pfizer Inc, Cambridge, Massachusetts, USA.
Arthur Bergman, Pfizer Worldwide Research, Development and Medical, Pfizer Inc, Cambridge, Massachusetts, USA.
Tong Zhu, Pfizer Worldwide Research, Development and Medical, Pfizer Inc, Cambridge, Massachusetts, USA.
Frances Hackman, Pfizer Worldwide Research, Development and Medical, Pfizer Ltd, Cambridge, UK.
Jean G Sathish, Drug Safety Unit, Pfizer Inc, Pearl River, New York, USA.
Lawrence Updyke, Drug Safety Unit, Pfizer Inc, Cambridge, Massachusetts, USA.
Peter Loudon, Tenpoint Therapeutics, Cambridge, UK.
Ganesh Krishna, El Camino Health, Mountain View, California, USA.
Philippe Clevenbergh, University Hospital Brugmann, Brussels, Belgium.
Miguel Gorgolas Hernandez-Mora, Hospital Universitario Fundación Jiménez Díaz, Universidad Autónoma de Madrid, Madrid, Spain.
Jose Miguel Cisneros Herreros, Hospital Universitario Virgen Del Rocio, Sevilla, Spain.
Timothy E Albertson, UC Davis Medical Center, Sacramento, California, USA.
Michael Dougan, Massachusetts General Hospital, Boston, Massachusetts, USA.
Amber Thacker, Regional One Health, Memphis, Tennessee, USA.
Mary Lynn Baniecki, Early Clinical Development, Pfizer Inc, Cambridge, Massachusetts, USA.
Holly Soares, Early Clinical Development, Pfizer Inc, Cambridge, Massachusetts, USA.
Mark Whitlock, Early Clinical Development, Pfizer Inc, Cambridge, UK.
Gianluca Nucci, Pfizer Worldwide Research, Development and Medical, Pfizer Ltd, Cambridge, UK.
Sandeep Menon, Pfizer Worldwide Research, Development and Medical, Pfizer Ltd, Cambridge, UK.
Annaliesa S Anderson, Pfizer Worldwide Research, Development and Medical, Pfizer Ltd, Cambridge, UK.
Michael Binks, Pfizer Worldwide Research, Development and Medical, Pfizer Ltd, Cambridge, UK.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Medical writing support was provided by Sheena Hunt, PhD, and Allison R. Gillies, PhD, of ICON and was funded by Pfizer. We thank William Reagan, Norimitsu Shirai, Daniel Lettiere, Frank Geoly, and Jeremy Dugas for expert input into the nonclinical studies. We also thank all the participants who volunteered for this study and all the study investigators and site personnel for their contributions to this study.
Data sharing. Upon request and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual deidentified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Financial support. This work was supported by Pfizer Inc.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention . Estimated COVID-19 burden. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html. Accessed 22 November 2021.
- 2. Harder T, Külper-Schiek W, Reda S, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Euro Surveill 2021; 26:2100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis 2022; 114:252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. COVID-19 Treatment Guidelines Panel, National Institutes of Health . Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed 10 March 2022. [PubMed]
- 5. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med 2021; 384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization for PAXLOVID. Available at: https://www.fda.gov/media/155050/download. Accessed 7 February 2022.
- 7. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration . Emergency use authorization 091 (casirivimab and imdevimab). Available at: https://www.fda.gov/media/145610/download. Accessed 21 October 2021.
- 9. US Food and Drug Administration . Emergency use authorization 094 (bamlanivimab and etesevimab). Available at: https://www.fda.gov/media/145801/download. Accessed 21 October 2021.
- 10. US Food and Drug Administration . Emergency use authorization 100 (sotrovimab). Available at: https://www.fda.gov/media/149532/download. Accessed 21 October 2021.
- 11. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for COVID-19—interim WHO solidarity trial results. N Engl J Med 2021; 384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis 2022; 22:209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boras B, Jones RM, Anson BJ, et al. Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19. Nat Commun 2021; 12:6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J 2014; 281:4085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang H, Xie W, Xue X, et al. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol 2005; 3:e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ning L, Liu L, Li W, et al. Novel coronavirus (SARS-CoV-2) infection in a renal transplant recipient: case report. Am J Transplant 2020; 20:1864–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 2003; 300:1763–7. [DOI] [PubMed] [Google Scholar]
- 19. Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021; 374:1586–93. [DOI] [PubMed] [Google Scholar]
- 20. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu T, Pawlak S, Toussi SS, et al. Safety, tolerability, and pharmacokinetics of intravenous doses of PF-07304814, a phosphate prodrug protease inhibitor for the treatment of SARS-CoV-2, in healthy adult participants. Clin Pharmacol Drug Dev 2022; 11:1382–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lilbert J, Burnett R. Main vascular changes seen in the saline controls of continuous infusion studies in the cynomolgus monkey over an eight-year period. Toxicol Pathol 2003; 31:273–80. [DOI] [PubMed] [Google Scholar]
- 23. Lilbert J, Mowat V. Common vascular changes in the jugular vein of saline controls in continuous infusion in the beagle dog. Toxicol Pathol 2004; 32:694–700. [DOI] [PubMed] [Google Scholar]
- 24. Resendez J, Rehagen D. Infusion toxicology and techniques. In: Faqi AS, editor. A comprehensive guide to toxicology in nonclinical drug development. Amsterdam: Elsevier, 2016:555–83. [Google Scholar]
- 25. Weber K, Mowat V, Hartmann E, et al. Pathology in continuous infusion studies in rodents and non-rodents and ITO (infusion technology organisation)—recommended protocol for tissue sampling and terminology for procedure-related lesions. J Toxicol Pathol 2011; 24:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chi G, Lee JJ, Jamil A, et al. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. J Clin Med 2020; 9:2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID-19: a systematic review and meta-analysis. Res Pract Thromb Haemost 2020; 4:1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.