Abstract
Objective
We report an outbreak of 14 cases of Q fever among tourists caused by an infected calf and characterized by respiratory transmission and a high attack rate in humans.
Materials and methods
Following the identification of an index case of Q fever in September 2021, an epidemiological investigation was conducted in collaboration with local Health and Veterinary authorities and an unknown outbreak was discovered and terminated.
Results
The outbreak originated from the delivery by an infected cow, with spread of C. burnetii by air and infection via the respiratory route. The transmission period was calculated, and 25 potentially exposed tourists were identified: 14 were infected (56%) based on serological investigations, four were hospitalized, there were no deaths. All the 22 cows were tested by PCR for C. burnetii: 3 cows (14%) were positive on milk samples and one, the index animal, was also positive on blood.
Conclusions
Timely diagnosis in a human patient was pivotal to identify the outbreak since involved animals were asymptomatic. The close collaboration between veterinary and human Public Health services in six different geographical areas of two countries was crucial for the rapid termination of the outbreak.
Keywords: Q fever, Coxiella burnetii, Attack rate
Highlights
-
•
Q fever may have a very high attack rate in humans, much higher than that in cattle.
-
•
Many outbreaks in tourist facilities may remain undiagnosed.
-
•
Outbreaks discovery and control requires close collaboration between veterinary and human Public Health services.
1. Introduction
Q fever is a zoonosis caused by Coxiella burnetii, a small Gram-negative intracellular bacteria infecting a wide range of animals worldwide, either domestic or wild, including mammals, birds, and ticks [1,2]. The main reservoirs for human infection are goats, cattle, sheep, cats, rabbits, dogs, and ticks [1,3]. Viable organisms are shed in placenta, urine, milk, and faeces of infected animals [4]. Humans acquire the infection through inhalation of aerosols directly from birth fluids of infected animals, inhalation of dust contaminated with dried birth fluids or excreta, by ingestion of raw milk and dairy products, or tick bite [1,4,5].
In 2019, 958 confirmed cases of Q fever were reported in Europe mostly from Spain, France, and Germany [6]. From 2007 to 2010, more than 4000 cases were reported in the southern parts of the Netherlands [7,8]. Although Italy only reported 6 cases in 2019, 1 in 2018, and 7 in 2017, a recent serosurvey conducted in the area surrounding the city of Rome (Lazio, Italy) found a seroprevalence of 37% in sheep and 12% in cattle, suggesting a wide circulation of C. burnetii among livestock [6,9,10].
We describe an outbreak of Q fever among the guests of a holiday farm in Madonna di Senales (Bolzano, Alto Adige, Italy).
2. Methods
At the end of August 2021, a couple returning from a farm holiday on the Alps was admitted to Spedali Civili Hospital (Brescia, Lombardy, Italy) for SARS-CoV2 negative interstitial pneumonia. Diagnostic investigations showed a positive Coxiella burnetii serology for both of them (C. burnetii phase I and phase II IgG, by an IFA test). All other causes of infectious interstitial pneumonia were excluded (Legionella spp., viruses, Chlamydia spp., Mycoplasma spp.).
An epidemiological investigation was started. Hygiene, Public Health, and Veterinary Health services of Merano (Trentino Alto Adige, Italy) were involved as well as the Hygiene Service of Heidelberg (Germany) and Brescia (Lombardia, Italy). The index event was identified. The public health investigation involved all subject who visited the area in a period of 30 days after the index event. Data and consent to data treatment were collected by phone contacts. The veterinary investigations involved the heard of the index case, with a follow up of 15 weeks after the index event. It consisted of serologic tests and antigen research on milk of each cow and on heard milk. Moreover, a PCR on vaginal swab of all animals delivering in the follow up period was performed.
3. Results
The farm visited by the couple was remote and the nearest village was Madonna di Senales. It was composed of few buildings grouped in a single compound: few residential houses and a stable that hosted 22 cows, 2 pigs, some chickens and one dog. At the farm, visitors could join rural life, including caring for the animals. On the 8th of August 2021 a calf was borne that was visited by all the guests of the farm.
We identified and examined 10 people (including the index couple) resident in Brescia who had stayed at the farm in the period from the 4th to the 16th of August 2021. Tests for C. burnetii phase I and phase II IgG (IFA test) were performed at baseline and after four weeks for the whole group except for a small child who tested negative at the first control (results are shown in Table 1, Table 2, patient 1 to 10). Eight of 10 subjects had a serological diagnosis of Coxiella infection defined as a fourfold increase of the phase II IgG title. Three tested negatives at a first control and seroconverted at the second one. The eight infected tourists were all symptomatic complaining of fever, cough, headache. Five had X-ray defined interstitial pneumonia.
Table 1.
Epidemiological data regarding 35 investigated individuals.
| ID | Age | Origin | Stay at the farm | Visit to the Stable | Drinking unpasteurized milk |
|---|---|---|---|---|---|
| 1 | 62 | Bz | Resident | Yes | Yes |
| 2 | 74 | Bz | Resident | Yes | Yes |
| 3 | 70 | Bz | Resident | Yes | No |
| 4 | 56 | Bz | Resident | Yes | Yes |
| 5 | 31 | Bz | Resident | Yes | Yes |
| 6 | 27 | Bz | Resident | Yes | No |
| 7 | 2 | Bz | Resident | Yes | Yes |
| 8 | 32 | DE | 01/07–10/08 | Yes | N/A |
| 9 | 59 | Bs | 07/08–15/08 | Yes | No |
| 10 | 57 | Bs | 07/08–15/08 | Yes | No |
| 11 | 64 | Bs | 07/08–16/08 | Yes | Yes |
| 12 | 61 | Bs | 07/08–16/08 | Yes | Yes |
| 13 | 57 | Fi | 07/08–21/08 | Yes | Yes |
| 14 | 45 | Fi | 07/08–21/08 | Yes | Yes |
| 15 | 11 | Fi | 07/08–21/08 | Yes | No |
| 16 | 54 | Bs | 09/08–10/08 | Yes | No |
| 17 | 38 | Bs | 09/08–17/08 | Yes | No |
| 18 | 36 | Bs | 09/08–17/08 | Yes | Yes |
| 19 | 5 | Bs | 09/08–17/08 | Yes | N/A |
| 20 | 31 | Bs | 10/08–18/08 | Yes | Yes |
| 21 | 31 | Bs | 10/08–18/08 | Yes | Yes |
| 22 | 15 | Ts | 11/08–21/08 | No | Yes |
| 23 | 48 | Ts | 11/08–21/08 | N/A | Yes |
| 24 | 13 | Ts | 11/08–21/08 | Yes | Yes |
| 25 | 46 | Ts | 11/08–21/08 | N/A | Yes |
| 26 | 46 | Ge | 21/08–28/08 | No | Yes |
| 27 | 14 | Ge | 21/08–28/08 | No | Yes |
| 28 | 11 | Ge | 21/08–28/08 | No | Yes |
| 29 | 46 | Ge | 21/08–28/08 | No | Yes |
| 30 | 54 | DE | 21/08–28/08 | No | Yes |
| 31 | 77 | DE | 21/08–28/08 | No | Yes |
| 32 | 17 | DE | 21/08–28/08 | No | Yes |
| 33 | 15 | DE | 21/08–28/08 | No | Yes |
| 34 | 66 | DE | 31/08–09/09 | No | Yes |
| 35 | 66 | DE | 31/08–09/09 | No | Yes |
N/A: data not available.
Origin: Bs = Brescia; Fi = Florence; Ts = Trieste; DE = Germany; Ge = Genoa; Bz = Bozen.
Table 2.
Serological results, clinical data and treatment in 35 investigated individuals.
| Patient ID | Serology T0 (type of test) |
Serology Week 4 (type of test) |
Symptoms | Chest X-Ray | Hospital admission | Therapy |
|---|---|---|---|---|---|---|
| 1 | (CLIA) IgM II neg IgG II borderline |
N/A | No | Not performed | No | No |
| 2 | (CLIA) IgM II neg IgG II pos |
N/A | No | Not performed | No | No |
| 3 | Not performed | N/A | No | Not performed | No | No |
| 4 | (CLIA) IgM II neg IgG II pos |
N/A | No | Not performed | No | No |
| 5 | (CLIA) IgG II neg |
N/A | No | Not performed | No | No |
| 6 | (ELISA) IgG neg |
N/A | No | Not performed | No | No |
| 7 | Not performed | N/A | No | Not performed | No | No |
| 8 | (not known) IgM positive IgG positive |
N/A | Bilateral pneumonia | Positive | Yes | Meropenem Vancomicyn Voriconazole |
| 9 | (IFA) IgM I N/A IgM II N/A IgG I neg IgG II neg |
(IFA) IgM I neg IgM II1/64 IgG I neg IgG II1/1024 |
Pneumonia | Positive | No | Levofloxacin 21 days |
| 10 | (IFA) IgM I neg IgM II neg IgG I neg IgG II neg |
(IFA) IgM I neg IgM II neg IgG I1/16 IgG II1/16 |
Fever | Negative | No | Doxycycline 14 days |
| 11 | (IFA) IgM I neg IgM II neg IgG I neg IgG II neg |
(IFA) IgM I neg IgM II neg IgG I neg IgG II neg |
No | Not performed | No | No |
| 12 | (IFA) IgM I1/4096 IgM II1/2048 IgG I neg IgG II1/256 |
(IFA) IgM I1/1024 IgM II1/512 IgG I1/64 IgG II1/512 |
Fever Headache |
Not performed | No | Doxycycline 14 days |
| 13 | N/A | N/A | Fever Headache Muscular pain |
N/A | No | N/A |
| 14 | N/A | N/A | Fever Headache Muscular pain Cough |
N/A | No | N/A |
| 15 | N/A | N/A | No | Not performed | No | No |
| 16 | (IFA) IgM I1/4096 IgM II 1/4096 IgG I neg IgG II 1/16 |
(IFA) IgM I 1/4096 IgM II 1/4096 IgG I neg IgG II 1/512 |
Pneumonia | Positive | Yes | Doxycycline 21 days |
| 17 | (IFA) IgM I N/A IgM II N/A IgG I neg IgG II 1/64 |
(IFA) IgM I 1/8182 IgM II 1/8182 IgG I neg IgG II 1/256 |
Bilateral pneumonia | Positive | Yes | Levofloxacin 21 days |
| 18 | (IFA) IgM I N/A IgM II N/A IgG I neg IgG II 1/1024 |
(IFA) IgM I 1/512 IgM II 1/1024 IgG I neg IgG II 1/256 |
Bilateral pneumonia | Positive | Yes | Levofloxacin 14 days |
| 19 | (IFA) IgM I neg IgM II neg IgG I neg IgG II neg |
N/A | No | Not performed | No | No |
| 20 | (IFA) IgM I 1/512 IgM II 1/128 IgG I neg IgG II 1/64 |
(IFA) IgM I 1/512 IgM II 1/128 IgG I neg IgG II 1/512 |
Pneumonia | Positive | No | Doxycycline 21 days |
| 21 | (IFA) IgM I 1/64 IgM II 1/32 IgG I neg IgG II neg |
(IFA) IgM I 1/256 IgM II 1/128 IgG I neg IgG II 1/256 |
Headache | Negative | No | Doxycycline 14 days |
| 22 | (ELISA) IgG II neg |
N/A | Fever | Negative | No | Amoxicillin/ clavulanate |
| 23 | (ELISA) IgG II 2,3 |
N/A | No | Not performed | No | No |
| 24 | (ELISA) IgG II 2,3 |
N/A | No | Not performed | No | No |
| 25 | (ELISA) IgG II 2,0 |
N/A | No | Not performed | No | No |
| 26 | Not performed | N/A | No | Not performed | No | No |
| 27 | Not performed | N/A | No | Not performed | No | No |
| 28 | Not performed | N/A | Headache Nausea Diarrhea |
Not performed | No | N/A |
| 29 | Not performed | N/A | No | Not performed | No | No |
| 30 | (ELISA) IgG neg |
N/A | Headache Cough Pharyngodynia |
Not performed | No | N/A |
| 31 | (ELISA) IgG neg |
N/A | Headache Cough Pharyngodynia |
Not performed | No | N/A |
| 32 | (ELISA) IgG neg |
N/A | Headache Cough Pharyngodynia |
Not performed | No | N/A |
| 33 | (ELISA) IgG neg |
N/A | Headache Cough Pharyngodynia |
Not performed | No | N/A |
| 34 | N/A | (01.12.2021) IgM I neg IgM II neg IgG I neg IgG II neg |
No | N/A | N/A | No |
| 35 | Not performed | N/A | No | N/A | N/A | No |
N/A: data not available.
An epidemiological investigation was performed. We censed all people who spent a night at the farm from 8th of August to 7th of September 2021. Of the 35 people included, 7 (the owners) were residents in the farm and 28 were tourists. Of the latter, 7 were from Germany and 21 from Italy: 4 from Trieste-Friuli Venezia Giulia, 4 from Genoa-Liguria, 3 from Florence-Tuscany, and 10 from Brescia. The mean age was 40.8 years; 9 were children and 5 were older than 65 years of age. A total of 17 (48.5%) persons reported symptoms (mostly fever, pneumonia, headache, and cough) after the stay at the farm; 4 of them were admitted to hospital. Twenty-two persons had visited the stable and 26 had drank the unpasteurised milk (epidemiological data are shown in Table 1).
Twenty-five persons were tested for C. burnetii antibodies: 11 were negative while 14 persons had a positive serology (8 with confirmed IgG phase II increase in two controls, 6 with positive serology at a single control). All visitors arriving after 21st of August had negative serological tests. The exposure period was therefore identified between 8th to 21st August. Over that period, there were 25 exposed people, 14 were infected, resulting in an attack rate of 56% (results are shown in Table 2 and Fig. 1). A PCR test for C. burnetii in blood was performed in 9 cases, all with negative results. Cases with positive serology were treated with doxycycline or levofloxacin for 14 to 21 days.
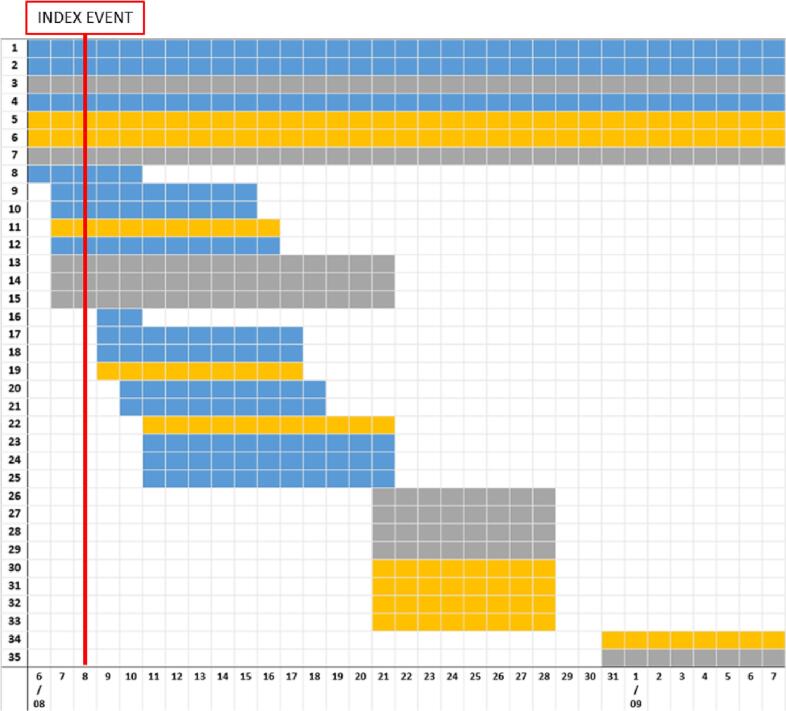
Fig. 1.
Results of serological tests and period of stay at the farm, 35 residents and visitors.
The red line represents the delivery of the infected calf (INDEX EVENT).
Each horizontal bars represents a resident (1–7) or a visitor (8–35) to the farm.
In the X axes is reported the period of stay, in days (range: 6th August to 7th September 2021).
The colour of the bar identifies: blue = subjects with a positive serology for C. burnetiid; yellow = negative serology; grey = serology results unknown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
A veterinary investigation was also performed. All the 22 resident cows and the dog underwent a serological test for C. burnetii (Ab-ELISA and Complement Fixation [CFR] phase I and II) and the milk of the 11 lactating cows was tested by qualitative real time PCR (qPCR). The tests were performed at four, seven and fifteen weeks from the index event.
The cow that delivered on 8th of August was the only animal that was positive by PCR on milk and that remained positive at all checkpoints. It was also positive by Ab-ELISA/phase I-II at CFR. This was identified as the index animal: it had been brought back to the stable on the 30th of July because of the imminent delivery.
Two more cows tested positive at one single checkpoint by PCR on milk: because for both this was an isolated result, we speculate that these might have been false positive results, possibly due to contamination.
The index animal always remained asymptomatic and was finally butchered for meat consumption as doxycycline treatment is not allowed in lactating or adult cows. The dog tested positive and was prophylactically treated with doxycycline.
The farm was promptly isolated and sanitized. A biosecurity procedure was implemented including performing qPCR on vaginal swab for all deliveries, correct disposal of placentas, disinfection of the delivery box, prohibition of raw milk consumption and of touristic visits to the stable, monitoring on the herd with ELISA/CFR serology and qPCR on milk at four, seven and fifteen weeks from the index event. qPCR for C. burnetii was negative in vaginal secretions of four cows that gave birth after the index event.
4. Conclusions
We report a Q fever human outbreak originated from the delivery of an infected cow, characterized by a high attack rate (56%) in humans but a low transmission rate in cattle. Timely etiological diagnosis in a human patient was pivotal to identify the outbreak since the involved animals were persistently asymptomatic. The close collaboration between veterinary and human Public Health services was crucial for the timely tracing of exposed individuals and rapid termination of the outbreak. Contact tracing and patient management involved six different geographical areas of two countries: a good European coordination allowed an effective management of this infectious disease outbreak.
Funding source
This is an independent work: no financial support was provided.
Ethical approval statement
Subject's data are managed in a completely anonymous form. In conducting this work we followed the principle contained in the Helsinki declaration. In this manuscript we describe the management of an outbreak, ethical approval was not necessary.
CRediT authorship contribution statement
Annacarla Chiesa: Conceptualization, Methodology, Validation, Investigation, Resources, Data curation, Writing – original draft. Ciro Onza: Conceptualization, Methodology, Validation, Investigation, Data curation, Visualization. Najada Sulcaj: Validation, Investigation, Data curation, Visualization. Agate Torggler: Validation, Investigation, Data curation, Visualization. Giulia Morosetti: Conceptualization, Methodology, Writing – review & editing, Visualization. Filippo Conforti: Validation, Investigation, Data curation, Visualization. Elisabeth Kofler: Validation, Investigation, Resources, Data curation, Visualization. Giacomo Moretto: Validation, Investigation, Data curation, Visualization. Paola Sinigaglia: Resources, Visualization. Liana Signorini: Methodology, Visualization. Christian Piffer: Methodology, Supervision, Visualization. Maria Grazia Zuccaro: Conceptualization, Methodology, Supervision, Visualization. Alexander Tavella: Conceptualization, Methodology, Validation, Resources, Writing – review & editing, Supervision, Visualization. Alberto Matteelli: Conceptualization, Methodology, Writing – original draft, Supervision.
Declaration of Competing Interest
The authors have no conflict of interests to declare for this paper.
Data availability
Data will be made available on request.
References
- 1.Raoult D., Marrie T. Q fever. Clin. Infect. Dis. 1995;20(3):489–496. doi: 10.1093/clinids/20.3.489. [DOI] [PubMed] [Google Scholar]
- 2.González-Barrio D., Ruiz-Fons F. Coxiella burnetii in wild mammals: a systematic review. Transbound. Emerg. Dis. 2019;66(2):662–671. doi: 10.1111/tbed.13085. [DOI] [PubMed] [Google Scholar]
- 3.Körner S., Makert G.R., Ulbert S., Pfeffer M., Mertens-Scholz K. The prevalence of Coxiella burnetii in hard ticks in Europe and their role in Q fever transmission revisited—a systematic review. Front. Vet. Sci. 2021;8(April):1–16. doi: 10.3389/fvets.2021.655715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson A., et al. Diagnosis and management of Q fever--United States, 2013. MMWR Recomm. Rep. 2013;62(3):1–30. [PubMed] [Google Scholar]
- 5.Armengaud A., et al. Urban outbreak of Q fever, Briancon, France, March to June 1996. Eurosurveillance. 1997;2(2):137. doi: 10.2807/esm.02.02.00137-en. [DOI] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control . Stockholm; 2021. Q fever. [Google Scholar]
- 7.Wegdam-Blans M.C.A., et al. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J. Inf. Secur. 2012;64(3):247–259. doi: 10.1016/j.jinf.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Van Der Hoek W., et al. Follow-up of 686 patients with acute Q fever and detection of chronic infection. Clin. Infect. Dis. 2011;52(12):1431–1436. doi: 10.1093/cid/cir234. [DOI] [PubMed] [Google Scholar]
- 9.Barlozzari G., et al. Cross-sectional serosurvey of Coxiella burnetii in healthy cattle and sheep from extensive grazing system in Central Italy. Epidemiol. Infect. 2020 doi: 10.1017/S0950268819002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzo F., et al. Q fever seroprevalence and risk factors in sheep and goats in Northwest Italy. Prev. Vet. Med. 2016;130:10–17. doi: 10.1016/j.prevetmed.2016.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.