Abstract
Interferons and chemokines play a critical role in regulating the host response to viral infection. Measles virus, a member of the Paramyxoviridae family, induces RANTES expression by astrocytes. We have examined the mechanism of this induction in U373 cells derived from a human astrocytoma. RANTES was induced in a dose- and time-dependent manner by measles virus infection. Inhibition of receptor binding by the anti-CD46 antibody TRA-2.10 and of virus-membrane fusion by the tripeptide X-Phe-Phe-Gly reduced RANTES expression. Formalin-inactivated virus, which can bind but not fuse, and extensively UV-irradiated virus, which can bind and fuse, were both ineffective. Therefore, virus binding to the cellular receptor CD46 and subsequent membrane fusion were necessary, but not sufficient, to induce RANTES. UV irradiation of virus for less than 10 min proportionally inhibited viral transcription and RANTES expression. RANTES induction was decreased in infected cells treated with ribavirin, which inhibits measles virus transcription. However, RANTES mRNA was superinduced by measles virus in the presence of cycloheximide. These data suggest that partial transcription of the viral genome is sufficient and necessary for RANTES induction, whereas viral protein synthesis and replication are not required. This hypothesis was supported by the fact that RANTES was induced through transient expression of the measles virus nucleocapsid gene but not by measles genes encoding P or L proteins or by leader RNA in A549 cells. Thus, transcription of specific portions of measles virus RNA, such as the nucleocapsid gene, appears able to generate the specific signaling required to induce RANTES gene expression.
Activation of pro- and anti-inflammatory cytokine genes is a critical host cell response to virus infection. Paramyxoviruses, a family of negative-stranded RNA viruses, are used extensively to study cytokine gene induction. This family includes important neurotropic pathogens, all with the potential to cause demyelinating disease, such as measles virus (MV), Newcastle disease virus (NDV), Sendai virus (SV), and canine distemper virus. Studies on the induction of beta interferon (IFN-β) gene transcription by SV revealed a complex promoter element requirement to which activating transcription factor 2 (ATF-2)–c-Jun, HMG-I(Y), NF-κB, and IRF-family proteins bind (9, 12, 48). Among these transcription factors, NF-κB and IRF proteins are activated by the double-stranded RNA (dsRNA)-activated protein kinase PKR (22, 23). Activation of PKR requires dimerization mediated by the binding of dsRNA (50). Single-stranded RNA viruses, including paramyxoviruses, presumably form the required dsRNA during the process of transcription and replication (20). Previous studies using primary glial cells and glial cell lines have demonstrated that MV, NDV, and SV induce multiple cytokines, including interleukin 1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), IFN-α and -β, and the chemokines IP-10 and RANTES (6, 13, 25, 32, 42, 51).
RANTES is a β-chemokine which attracts monocytes and T cells, including memory T cells, during inflammation and immune response (40, 41). RANTES is expressed in T cells, astrocytes, and microglia in experimental autoimmune encephalitis, and its expression correlates with the clinical onset and severity of demyelination (15, 30). RANTES expression has also been demonstrated in T cells surrounding multiple sclerosis lesions of the human brain (19). In addition, RANTES is a potent inhibitor of human immunodeficiency virus type 1 (HIV-1) replication in CD4+ cells through competition for binding to chemokine receptors, now known to be cofactors for HIV-1 fusion (10, 33). Murine RANTES is induced equally by live and UV-inactivated NDV in primary rat astrocytes and microglia through a tyrosine kinase-dependent pathway in the absence of new protein synthesis (13). Cross-linking of NDV RNA by UV irradiation does not interfere with murine RANTES induction. Therefore, RANTES induction may rely on the virus-receptor interaction and not on the formation of dsRNA.
We have investigated the mechanisms by which MV induces RANTES in a human astrocytoma cell line, U373. Experiments to inhibit virus-cell interaction showed that the CD46 receptor binding was required, but not sufficient, for RANTES induction. However, RANTES was induced, at a reduced level, by MV exposed to limited UV irradiation, which completely inhibited viral replication but allowed partial transcription of the viral genome. Ribavirin, an MV transcription inhibitor, also reduced MV-induced RANTES expression in a dose-dependent manner. Furthermore, transient expression of the MV nucleocapsid gene, but not of the P or L protein gene, induced RANTES. These data suggest that, in contrast with the induction of RANTES by NDV, limited transcription of the viral genome plays a key role in the induction of RANTES gene expression by MV.
MATERIALS AND METHODS
Infection of cultured cells with MV and NDV.
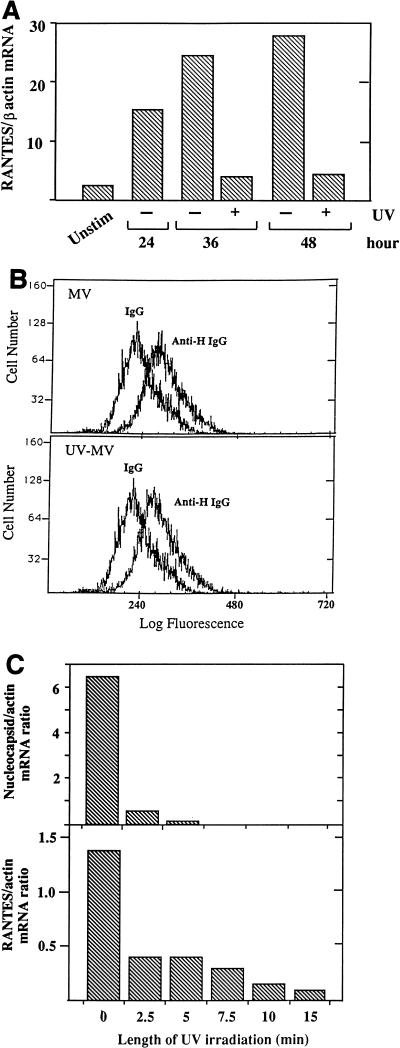
All reagents and chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.), unless stated otherwise. The human astrocytoma cell line U373-MG from the American Type Culture Collection (Manassas, Va.) was grown in Dulbecco’s modified Eagle medium (DMEM; Gibco-BRL, Gaithersburg, Md.) with 10% heat-inactivated fetal bovine serum (FBS), 25 mM HEPES buffer (pH 7.4), and penicillin and streptomycin (100 U/ml each) at 37°C in 5% CO2. MV Edmonston strain was obtained from S. Dhib-Jalbut (University of Maryland at Baltimore) and grown in Vero cells. Virus was titrated by plaque assay according to standard methods. The New Jersey La Sota strain of NDV (American Type Culture Collection) was grown in 9-day-old fertilized chick eggs and titrated by hemagglutination assay, and the multiplicity of infection (MOI) equivalent was calculated, as described previously (45). For UV cross-linking, 1 ml of virus in a 35-mm2 petri dish on ice was exposed to 400 μW of short-wave UV light/cm2 in a 4°C cold room for the time periods indicated, with constant shaking. The binding of MV and UV-irradiated virus to the cell was assessed by fluorescence-activated cell sorter (FACS) analysis using a monoclonal antibody to the MV hemagglutinin (HA) glycoprotein (2). Formalin-inactivated MV was prepared by mixing 1 ml of MV with 0.1 ml of 37% formalin for 24 h at 4°C as described elsewhere (3). Cells were infected with virus in a small volume of serum-free DMEM (1 ml for 24- or 6-well plates and 5 ml for 75-cm2 flasks), and plates were rocked every 20 min for 2 h; then additional DMEM with 10% FBS was added.
Detection of RANTES protein.
Supernatants were stored at −20°C until an assay for RANTES protein using the human RANTES Quantikine kit (R & D Systems, Minneapolis, Minn.) was performed according to the manufacturer’s instructions. Lactate dehydrogenase released into the medium was measured to assess cell death, as described elsewhere (4).
Northern blot analysis.
Cells (0.4 × 107 to 1 × 107) in a 75-cm2 flask were infected as described above. Total RNA was extracted by the guanidine isothiocyanate method, followed by ultracentrifugation through a cesium chloride cushion (7). Northern analysis was carried out as described elsewhere (39) by using 20 μg of RNA per lane. Specific mRNA expression was determined by using the following probes. For MV, nucleocapsid cDNA as a 1.597-kb SacI/SacII fragment and the fusion protein cDNA as a 0.721-kb SacI/EcoRV fragment were derived from plasmids (38). The β-Actin probe was a 1.8-kb HindIII fragment of plasmid provided by P. Pitha (Johns Hopkins Medical School, Baltimore, Md.), and the cyclophilin probe was a 1.1-kb HindIII/EcoRI fragment provided by D. Hilt (Amgen, Thousand Oaks, Calif.). The human RANTES probe was from R & D Systems, and glucose-3-phosphate dehydrogenase (G3PDH) was from Clontech Inc. (Palo Alto, Calif.). Probes were labeled with [α-32P]dCTP (New England Nuclear, Wilmington Del.) by using an oligolabeling kit (Pharmacia, Piscataway, N.J.), T4 polynucleotide kinase (New England Biolabs, Beverly, Mass.), and 35 to 150 μCi of [γ-32P]ATP (New England Nuclear). Labeled probe was purified on a Sephadex G-50 Pharmacia Biotech Nick Spin column prior to use. The blots were exposed to X-ray film. mRNA bands on autoradiograms were quantified on a computing densitometer (Molecular Dynamics, Sunnyvale, Calif.), and the integrated volume was calculated by using ImageQuant software (Molecular Dynamics). The result was expressed as the ratio of the mRNA density to that of actin, cyclophilin, or G3PDH.
Inhibition of MV binding and cell fusion.
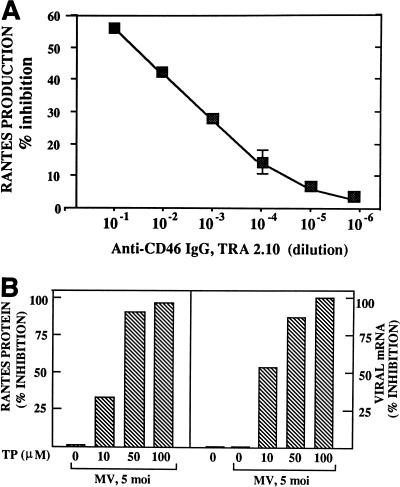
U373 cells were plated at 7.5 × 104/well in a 24-well plate. Twenty-four hours later, cells were incubated with serial dilutions of monoclonal anti-CD46 immunoglobulin G (IgG) directed to the SCR-1 domain of CD46 (a gift from J. Atkinson, Washington University, St. Louis, Mo.) for 1 h at 37°C. Cells were washed with phosphate-buffered saline, then infected with MV at an MOI of 2 for 24 h. Supernatants were then assayed for RANTES by enzyme-linked immunosorbent assay (ELISA). To inhibit virus-cell fusion, 5 × 106 cells were plated on a 10-cm2 dish for 24 h, followed by incubation with varying doses of the tripeptide X-Phe-Phe-Gly (American Peptide Co., Inc., Sunnyvale, Calif.), which inhibits the fusion protein activity (37), together with MV at an MOI of 5.
Transient transfection of expression vectors encoding single MV genes.
The A549 cell line, derived from a human lung carcinoma, was infected at an MOI of 2.5 with recombinant vaccinia virus expressing T7 polymerase for 1 h at 37°C. After a wash, the cells were transiently transfected with 2 μg of cDNA by using Lipofectin reagents (Gibco-BRL), according to the manufacturer’s instructions. The pBS vector, containing cDNA of single MV genes, and the pGEM vector, containing the SV NP or P gene, were used as described elsewhere (17). Expression of the genes in these vectors is under the control of the T7 promoter. After overnight incubation, RANTES protein in supernatants was determined by ELISA.
RESULTS
RANTES induction by MV in U373 cells.
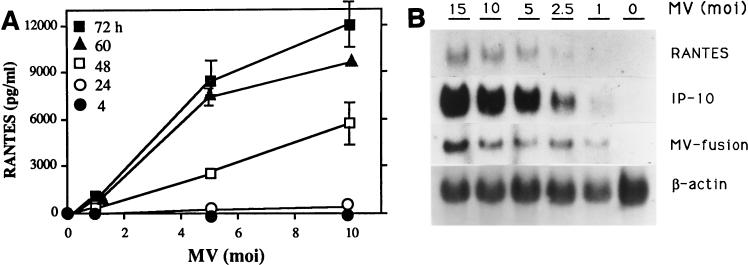
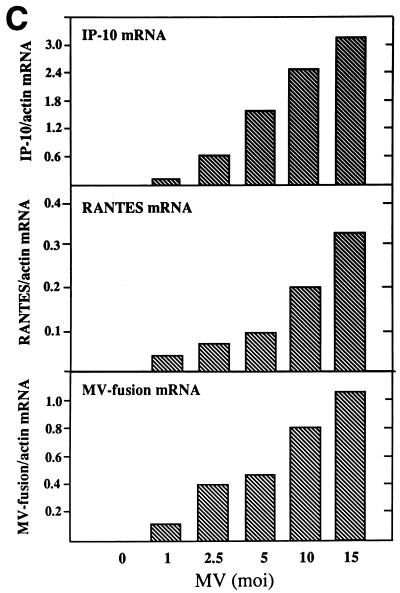
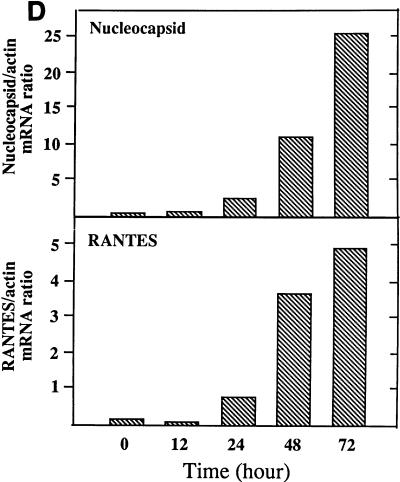
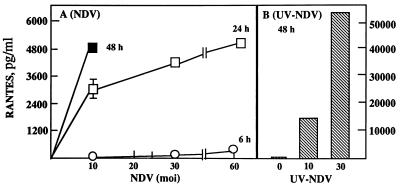
U373 cells infected with MV produced RANTES protein (Fig. 1A) and expressed RANTES mRNA (Fig. 1B and C). The induction was dependent on the viral dose and correlated with MV fusion (F) gene mRNA accumulation. Expression of the α chemokine IP-10 is shown for comparison. Expression of this chemokine correlates with data from a previous study in which IP-10 induction by MV was examined (32). RANTES mRNA expression increased from 24 to 72 h postinfection in parallel with increased viral load, determined by MV nucleocapsid (N) mRNA (Fig. 1D). At a low dose of MV (an MOI of 2.5), RANTES was produced continuously for up to 100 h (data not shown). Lipopolysaccharide (LPS), NDV, and UV-irradiated NDV, known to induce RANTES in primary murine astrocytes, microglia, and macrophages, also induced RANTES in human U373 cells. LPS at 1 μg/ml was 100-fold less effective than MV at an MOI of 2.5 (data not shown). RANTES was induced by NDV earlier than by MV, and live NDV at an MOI of 30 was about 10-fold less effective then NDV irradiated for 10 min (Fig. 2).
FIG. 1.
RANTES induction by MV in U373 cells. (A) RANTES protein. U373 cells (105/ml of 10% DMEM/well) in 24-well plates were infected with MV at an MOI of 1, 5, or 10 for 4, 24, 48, 60, or 72 h. Supernatants were then assayed for RANTES by ELISA. RANTES was not detected in mock-infected samples (MV at an MOI of 0). Data are means ± standard errors (SE) from three separate experiments performed in duplicate. (B) RANTES mRNA. U373 cells (107/75-cm2 flask) were infected with the indicated dose of MV for 48 h. Total cellular RNA was isolated and examined for RANTES mRNA by Northern blotting (20 μg of RNA/lane). MV fusion mRNA was used as an indicator of viral infection, and β-actin was used for normalization. The α-chemokine IP-10 was shown for comparison. Results represent one of two separate experiments. (C) The mRNA densities on an autoradiogram from panel B were quantitated, as described in Materials and Methods, and expressed as a ratio of the density of RANTES mRNA to that of β-actin mRNA. (D) Kinetics of RANTES mRNA expression. U373 cells were infected with MV at an MOI of 2.5, as in panel B, for the times noted. Total RNA was examined for RANTES and MV nucleocapsid mRNAs by Northern blotting (20 μg of RNA/lane). The mRNA bands on the autoradiogram were quantitated, and results are expressed as ratios of their densities to that of β-actin mRNA. A representative result from four separate experiments is shown.
FIG. 2.
RANTES induction by NDV and UV-irradiated NDV in U373 cells. U373 cells (3 × 105/ml of 10% DMEM/well) in 24-well plates were incubated with increasing doses of NDV for 6 (○), 24 (□), or 48 (■) h (A) or with NDV that had been irradiated with UV for 10 min, at an MOI of 10 or 30, for 48 h (B). Supernatants were assayed for RANTES protein by ELISA. Data are means ± SE from two experiments performed in duplicate. Cells infected with NDV at an MOI of 30 or 60 for 48 h showed significant cell death (data not shown).
Requirement of receptor binding and membrane fusion of MV for RANTES induction.
The role of the MV cellular receptor CD46 in RANTES induction was examined by preincubation of cells with serial dilutions of TRA-2.10, a monoclonal antibody directed to the SCR1 domain of CD46 which blocks MV binding (1, 29). RANTES production was inhibited in a dose-dependent manner by anti-CD46 antibody (Fig. 3A), indicating that binding of the viral HA protein to CD46 is necessary for the induction. To assess the role of virus-cell fusion, cells were infected with MV in the presence of the synthetic tripeptide X-Phe-Phe-Gly, which inhibits MV membrane penetration and virus-induced cell fusion (37). Expression of RANTES protein (Fig. 3B, left panel) and mRNA (data not shown) decreased proportionally with increasing doses of the peptide. Increases in the peptide dose also correlated with decreased levels of MV fusion and nucleocapsid mRNA expression (Fig. 3B, right panel). Since formalin inactivation blocks MV fusion but allows virus uptake through an endocytic pathway (3), formalin-inactivated MV was used to determine RANTES induction. The U373 cells exposed to formalin-inactivated virus showed no cytopathic effect by plaque assay and failed to produce RANTES (data not shown).
FIG. 3.
Inhibition of MV binding to CD46 receptor and of virus-cell membrane fusion. (A) U373 cells (7.5 × 105) were first incubated with serial dilutions of monoclonal anti-CD46 IgG for 1 h, then infected with MV at an MOI of 2 for 24 h. Inhibition of RANTES production by anti-CD46 IgG was calculated by taking the amount of RANTES produced in the absence of antibody (∼370 pg/ml) as indicating 0% inhibition. (B) U373 cells (5 × 106) were incubated with varying concentrations of the tripeptide X-Phe-Phe-Gly together with MV at an MOI of 5 for 48 h. Supernatants were assayed for RANTES expression by ELISA (left panel), and cell pellets were examined for MV nucleocapsid and fusion protein mRNA expression by Northern blotting (right panel). The amount of RANTES protein produced in the absence of the tripeptide (TP) (12,000 pg/ml) was taken as indicating 0% inhibition (left panel). The band densities of MV nucleocapsid and fusion mRNAs expressed without tripeptide treatment were taken as indicating 0% inhibition (right panel). The nucleocapsid and fusion mRNAs were similarly inhibited by the tripeptide.
Requirement of viral transcription for RANTES induction studied by using UV irradiation and ribavirin.
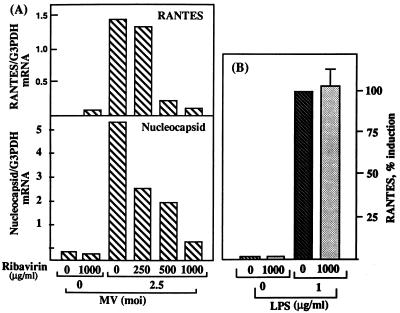
MV irradiated with UV for 60 min was unable to induce RANTES mRNA expression, although the binding of irradiated virus to U373 cells was not affected (Fig. 4A and B). UV irradiation for 2.5 min was sufficient to inhibit MV growth, as determined by plaque assay (data not shown). Therefore, MV irradiated for limited periods was used to determine whether UV dose-dependent inhibition of viral transcription would affect RANTES induction. Levels of MV nucleocapsid mRNA were decreased in cells infected with MV irradiated for 2.5 min, and it was no longer detected when MV was irradiated for 7.5 min or longer (Fig. 4C). The RANTES mRNA (Fig. 4C) and protein (data not shown) levels were affected similarly. The requirement of MV transcription for RANTES induction was further determined by using ribavirin, which inhibits paramyxovirus transcription (34). In cells infected in the presence of ribavirin, both RANTES expression and MV nucleocapsid mRNA accumulation were inhibited in a dose-dependent manner (Fig. 5A). This was not due to the inhibition of RANTES transcription by ribavirin directly, as shown by its failure to inhibit RANTES induction by LPS (Fig. 5B).
FIG. 4.
Failure to induce RANTES expression by infection with UV-irradiated MV. (A) MV was exposed to UV irradiation for 60 min. U373 monolayers were treated with UV-irradiated MV at the equivalent of an MOI of 2.5 for 24, 36, or 48 h. RANTES mRNA expression was then assessed by Northern blotting (20 μg of total RNA/lane). The mRNA bands on the autoradiogram were quantitated, and results are presented as density ratios of RANTES mRNA to β-actin mRNA. Unstim, unstimulated. (B) Binding of UV-irradiated and live MV to U373 cells. Cells were incubated on ice for 2 h with live MV or MV exposed to UV for 60 min, each at an MOI of 10. Cells were reacted with monoclonal anti-MV HA IgG or IgG isotype and then with fluorescein isothiocyanate-conjugated anti-IgG, followed by FACS analysis. The increases in specific mean fluorescence in cell-bound MV and UV-irradiated MV (UV-MV) were 60.10 and 56.77, respectively. (C) MV irradiated by UV for 2.5 to 15 min was used at an MOI of 2.5 to infect U373 cells for 48 h in 75-cm2 flasks. Expression of RANTES and MV-nucleocapsid mRNAs was determined by Northern blotting (20 μg of total RNA/lane). Representative results from one of three experiments are shown as RANTES/β-actin or MV nucleocapsid/β-actin mRNA density ratios.
FIG. 5.
Inhibition of MV transcription and RANTES induction by ribavirin. (A) U373 cells (5 × 106/75-cm2 flask) were infected with MV at an MOI of 2.5 for 2 h. Twenty milliliters of medium containing varying doses of ribavirin was added to yield the indicated final concentration. Cells were further incubated for 24 h, and then RANTES and MV nucleocapsid mRNA levels were examined by Northern blotting (20 μg of total RNA/lane). G3PDH was used for normalization. Results are representative of findings from two separate experiments. The background density of the nucleocapsid blot (an MOI of 0 for MV) was not subtracted. (B) Effect of ribavirin on RANTES induction by LPS. U373 cells as above (panel A) were stimulated with LPS at 1 μg/ml for 48 h, with or without ribavirin at 1,000 μg/ml. Supernatants were examined for RANTES expression by ELISA. Results are means ± SE from two separate experiments, performed in duplicate. The level of RANTES produced by LPS in the absence of ribavirin was taken as 100% induction.
Effect of CHX on RANTES induction by MV.
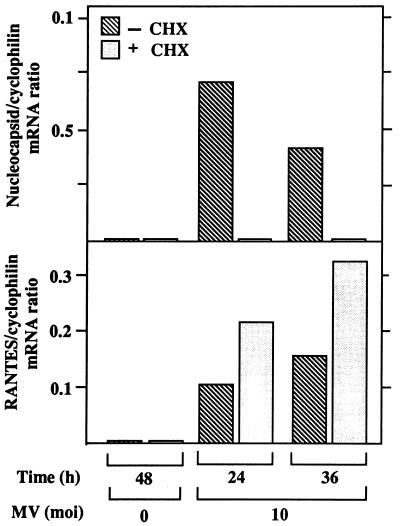
To determine whether RANTES induction requires viral and/or host protein synthesis, U373 cells were treated for 30 min prior to infection with cycloheximide (CHX) at 100 μg/ml, a concentration that inhibits more than 95% of protein synthesis in U373 cells without causing cell death (data not shown). In the presence of CHX, RANTES mRNA was superinduced by MV at 24 and 36 h (Fig. 6). RANTES was not induced by CHX alone. Accumulation of MV nucleocapsid mRNA was below the level detected by Northern blotting, although low levels of primary viral gene transcription occur in the presence of CHX.
FIG. 6.
Effects of CHX on RANTES mRNA induction by MV in U373 cells. U373 cells (107/25 ml of 10% DMEM/75-cm2 flask) were treated with CHX at 100 μg/ml for 30 min and were then infected with MV at an MOI of 10 in the presence of CHX for the times noted. The dose of CHX was predetermined by assessing the maximum level of protein synthesis inhibition by CHX in U373 cells in the absence of cell death, as described in Results. RANTES and MV nucleocapsid mRNAs were examined by Northern blotting (20 μg of RNA/lane). Cyclophilin was used as a control. Results are representative of findings from four separate experiments. The mRNA bands on the autoradiogram were quantitated, and the ratio of the density of RANTES or MV nucleocapsid mRNA to that of cyclophilin mRNA is shown as a histogram.
RANTES induction by transient expression of MV transcripts.
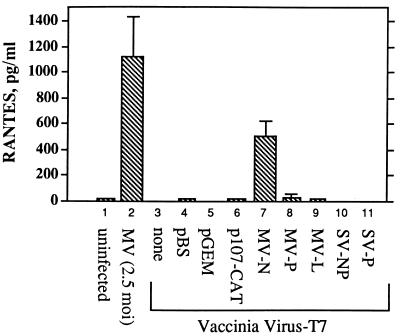
To examine the hypothesis that transcription of just a portion of the MV genome may be sufficient to induce RANTES, cells were transiently transfected with MV gene expression vectors. Transient expression of the MV nucleocapsid gene was sufficient for RANTES induction in A549 cells (Fig. 7). Transfection with vectors carrying the MV leader gene linked to chloramphenicol acetyltransferase (p107CAT) (46) or the P or L protein gene failed to induce RANTES. Vectors expressing the SV nucleocapsid or phosphoprotein gene were also ineffective. These vectors have been shown to express each gene transcript at similar levels in A549 cells (17). U373 and other astrocytoma cell lines were not infected by the recombinant vaccinia virus; therefore, the transfected genes were not expressed, and no RANTES was induced (data not shown).
FIG. 7.
RANTES induction in A549 cells transiently expressing MV genes. Approximately 80% confluent A549 cell monolayers in 35-mm2 dishes were infected for 60 min at an MOI of 2.5 with recombinant vaccinia virus expressing T7 polymerase. Cells were then transiently transfected with 2 μg of pBS vectors containing the individual MV genes: the nucleocapsid (N), phosphoprotein (P), or large protein (L) gene or p107CAT, expressing the MV leader gene linked to (−) sense chloramphenicol acetyltransferase. Cells were also transfected with pGEM vectors containing the SV nucleocapsid (NP) or phosphoprotein (P) gene. In both vectors the genes were under the control of the T7 promoter, which allows transcription by the vaccinia virus-encoded T7 polymerase. Cells infected with MV at an MOI of 2.5 were used as a positive control. Transfection with pBS and pGEM empty vectors (pBS and pGEM) was used as negative controls. After overnight incubation supernatants were examined for RANTES protein by ELISA. Results are shown as means ± SE from two separate experiments, with transfections performed in duplicate.
RANTES expression in neonatal rat brains infected with virus.
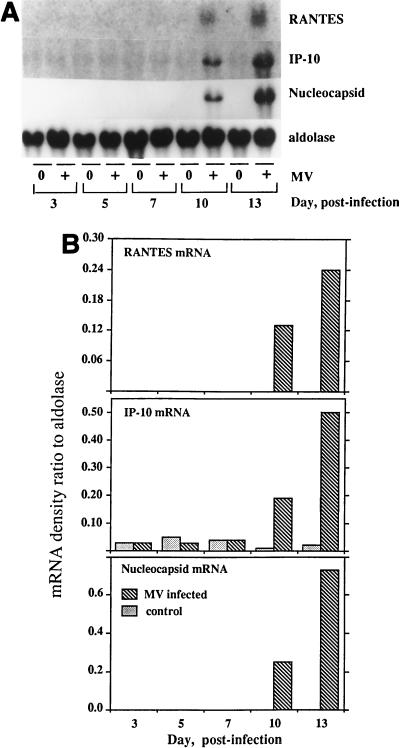
To determine whether RANTES is induced in brains infected with MV in vivo, Northern analysis was performed with total RNA derived from brains of 3-week-old Lewis rats inoculated intracerebrally with 4 × 103 50% tissue culture infective doses (TCID50) of the CAM/RB strain of MV, as described elsewhere (26, 49). Expression of RANTES and IP-10 mRNAs was observed on days 10 and 13 postinfection, which paralleled the accumulation of MV nucleocapsid mRNA (Fig. 8).
FIG. 8.
RANTES mRNA expressed in rat brains following MV infection. Lewis rats, 21 to 24 days old, were infected with 4 × 103 TCID50 of the MV CAM/RB strain by intracerebral inoculation as described elsewhere (26). (A) Brain tissues obtained 3 to 13 days postinfection were examined for the expression of murine RANTES and MV nucleocapsid mRNAs by Northern blotting using total RNA isolated from the tissue (20 μg of RNA/lane). IP-10 mRNA induced by MV is shown for comparison, and aldolase A was used for normalization. (B) Quantitation of the mRNA bands is shown as a histogram.
DISCUSSION
MV induces RANTES gene expression in U373 cells; induction requires binding to the MV cellular receptor CD46, membrane fusion, and viral transcription.
MV induced RANTES mRNA and protein expression within 48 h after infection of U373 cells derived from a human astrocytoma. LPS at 1 μg/ml was far less effective than MV or NDV. This is similar to the inefficient induction of TNF-α by LPS in primary rat astrocytes, which is in part astrocyte specific (8, 25, 45). RANTES induction was inhibited by anti-CD46 antibody and by the tripeptide X-Phe-Phe-Gly, which inhibits viral fusion (37). Furthermore, formalin-treated MV, which binds to CD46 but cannot fuse with the host cell and internalizes by endocytosis (3), failed to induce RANTES expression. MV exposed to UV irradiation for 60 min, which can still bind to CD46 and fuse with the host cell membrane (14), was also ineffective in inducing RANTES expression. These data strongly suggest that while receptor binding and membrane fusion of MV are essential, they are not sufficient, and cytoplasmic delivery of the MV genome may be required. This is not due to an inability of MV binding to CD46 to activate signaling, as this binding has been shown to regulate IL-12 induction (21).
To determine whether viral transcription is required, RANTES induction was examined by using UV-irradiated MV and ribavirin. Since UV does not inhibit MV binding to CD46 or fusion with the host cell membrane (14), the effect of the length of UV exposure is expected to be correlated with the level of cross-linking in the viral genome. UV irradiation for 10 min was sufficient to block MV nucleocapsid gene transcription, while infection with MV irradiated for less than 7.5 min allowed the accumulation of significantly reduced levels of nucleocapsid and RANTES mRNAs. This is distinct from the efficient induction of RANTES by UV-irradiated NDV (13), another member of the Paramyxoviridae family. In fact, UV-irradiated NDV was more potent at higher doses than the cytopathic live NDV. Ribavirin, which selectively inhibits the RNA polymerase of paramyxoviruses (34), blocked nucleocapsid gene expression and RANTES mRNA accumulation induced by MV, but not by LPS. These findings suggest that RANTES induction requires viral transcription, and partial transcripts present at minimal levels appear sufficient. This is in agreement with the way vesicular stomatitis virus induces IFN-β, which requires transcription of minimally two-thirds of the viral nucleocapsid gene (29, 43). It was proposed that this amount of transcription may allow the mRNA transcript to pair with the encapsidated RNA genome to generate dsRNA.
RANTES induction by MV does not require new protein synthesis or viral replication.
In U373 cells infected with MV in the presence of CHX, RANTES mRNA was detected by Northern blotting in cells expressing only the very low levels of viral mRNA produced by primary transcription. Synthesis of viral proteins is required to shift the viral RNA polymerase from transcription to replication of the genome (24). Viral replication also requires the presence of host factors such as tubulin (31). Therefore, treatment of cells with CHX for 24 h is expected to prevent viral replication, secondary transcription, and accumulation of significant viral mRNA, none of which were required for RANTES induction. RANTES is also induced in the presence of CHX in a variety of primary and transformed cells stimulated with LPS or cytokines, indicating that RANTES is induced in response to activation of preexisting transcription factors (13, 35, 44). Interestingly, RANTES mRNA was superinduced by MV in the presence of CHX. In rat glia, the half-life of RANTES mRNA was estimated to be ∼3 h (13), which is consistent with the absence of AU-rich motifs associated with unstable mRNA (5) in its 3′ and 5′ untranslated regions (41). Superinduction of RANTES mRNA by CHX may also reflect transcriptional activity, possibly mediated by CHX-induced NF-κB activation (48), which enhances RANTES promoter activity (36).
Partial expression of the MV genome is sufficient for RANTES induction.
Induction of RANTES in A549 cells transiently transfected with vectors carrying the MV nucleocapsid gene is consistent with the hypothesis that limited transcription of the genome is sufficient for MV to induce RANTES. Since these vectors express each gene transcript similarly in A549 cells (17), it is significant that expression vectors other than that carrying the MV nucleocapsid gene were ineffective. Since UV-irradiated NDV and SV are as effective as live NDV and SV in inducing the RANTES and IP-10 chemokine genes (6, 13), the failure of SV nucleocapsid gene expression suggested a specific requirement for the MV nucleocapsid transcript. The SV nucleocapsid transcripts may not deliver the required signaling. The effect of differences in nucleocapsids of different MV strains was examined by using two wild isolates (the Chicago-1 and New Jersey strains) and two additional vaccine strains (Edmonston-Zagreb and Moraten) to infect U373 cells at comparable viral doses, determined by Vero cell plaque assay. RANTES was induced by all strains tested, but at widely varying levels, which correlated with nucleocapsid mRNA expression, except in cells infected with the Chicago-1 strain (data not shown). Although the use of receptors other than CD46 by wild isolates (18) was not examined, the potency of RANTES induction was not restricted to vaccine strains, and all strains tested were able to infect Vero cells and U373 cells. Therefore, studies to correlate each of the strain-specific nucleocapsid genes may provide further information on the role of MV nucleocapsid gene transcripts in inducing RANTES gene expression.
RANTES induction by MV infection in vivo.
RANTES mRNA was expressed in Lewis rat brain tissue infected with neuroadapted CAM/RB MV (26). This experiment was performed in order to demonstrate that MV can induce RANTES within the brain compartment, as does experimental autoimmune encephalomyelitis (15, 30). The appearance of RANTES mRNA on day 10 correlates with the development of acute MV encephalitis 10 to 12 days postinoculation in this model (26).
Expression of RANTES in glial cells by MV infection is induced by limited transcription of the MV genome. Since RANTES induction by MV does not require viral and host protein synthesis, or replication of MV, RANTES can function as an early host response factor immediately following MV infection of the brain. RANTES may play a critical role in the response to viral infection by recruiting virus-specific memory T cells, monocytes, and macrophages to the infection sites within the central nervous system (41, 47). IFN-β, concomitantly produced by infected glial cells and infiltrating inflammatory cells, induces the expression of major histocompatibility complex class I molecules on infected cells (11, 16). Subsequent activation of cytotoxic T cells at the site of infection may lead to recognition and removal of infected brain cells. Limitation of infective virus would result in effective viral clearance, where a higher viral dose and/or defective host response may produce tissue damage mediated by virus replication and proinflammatory cytokines and chemokines.
ACKNOWLEDGMENTS
The work was supported by U.S. Public Health Grants to M.L.S. (RO1-NS36231 and RO1-NS15662).
We appreciate the help of Suhayl Dhib-Jalbut (University of Maryland) in our studies of MV, John Atkinson (Washington University, St. Louis, Mo.) and Peter Andrews (University of Sheffield, Western Bank, Sheffield, Great Britain) for anti-CD46 IgG used to block MV binding to CD46, and Chantal Rabourdin-Combe (Laboratoire de Biologie Moleculaire et Cellulaire, CNRS-ENS, Lyon, France) for the detailed protocol for formalin inactivation of MV.
REFERENCES
- 1.Andrews P W, Banting G, Damyanov I, Amaud D, Avner P. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 1984;3:347–361. doi: 10.1089/hyb.1984.3.347. [DOI] [PubMed] [Google Scholar]
- 2.Bellini W, Englund G, Rammohan K, McFarlin D E. Evaluation of the structural proteins of the hamster neurotropic strain of measles virus with monoclonal antibodies. J Neuroimmunol. 1986;11:149–163. doi: 10.1016/0165-5728(86)90116-5. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso A I, Beauverger P, Gerlier D, Wild T F, Rabourdin-Combe C. Formaldehyde inactivation of measles virus abolishes CD46-dependent presentation of nucleoprotein to murine class I-restricted CTLs but not to class II-restricted helper T cells. Virology. 1995;212:255–258. doi: 10.1006/viro.1995.1479. [DOI] [PubMed] [Google Scholar]
- 4.Carney D F, Hammer C H, Shin M L. Elimination of terminal complement complexes in the plasma membrane of nucleated cells: influence of extracellular Ca2+ and association with cellular Ca2+ J Immunol. 1986;37:263–270. [PubMed] [Google Scholar]
- 5.Chen C-Y, Shyu A-B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 6.Cheng C, Nazar A S M I, Shin H S, Vanguri P, Shin M L. IP-10 gene transcription by virus in astrocytes requires cooperation of ISRE with adjacent κB site, but not IRF-1 or viral transcription. J Interferon Cytokine Res. 1998;18:987–997. doi: 10.1089/jir.1998.18.987. [DOI] [PubMed] [Google Scholar]
- 7.Chirgwin J M, Pryzbyla A E, McDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 8.Chung I Y, Benveniste E N. Tumor necrosis factor-α production by astrocytes: induction by lipopolysaccharide, IFN-γ, and IL-1β. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- 9.Daly C, Reich N C. Characterization of specific DNA-binding factors activated by double-stranded RNA as positive regulators of interferon-α/β-stimulated genes. J Biol Chem. 1995;270:23739–23746. doi: 10.1074/jbc.270.40.23739. [DOI] [PubMed] [Google Scholar]
- 10.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;38:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Dhib-Jalbut S, Cowan E P. Direct evidence that interferon-β mediates enhanced HLA-class I expression in measles virus-infected cells. J Immunol. 1993;151:6248–6258. [PubMed] [Google Scholar]
- 12.Du W, Thanos D, Maniatis T. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell. 1993;74:887–898. doi: 10.1016/0092-8674(93)90468-6. [DOI] [PubMed] [Google Scholar]
- 13.Fisher S N, Vanguri P, Shin H S, Shin M L. Regulatory mechanisms of MuRantes and CRG-2 chemokine gene induction in central nervous system glial cells by virus. Brain Behav Immun. 1995;9:331–344. doi: 10.1006/brbi.1995.1031. [DOI] [PubMed] [Google Scholar]
- 14.Gerlier D, Trescol-Biemont M C, Varior-Krishnan G, Naniche D, Fugier-Vivier I, Rabourdin-Combe C. Efficient major histocompatibility complex II-restricted presentation of measles virus relies on hemagglutinin-mediated targeting to its cellular receptor human CD46 expressed by murine B cells. J Exp Med. 1994;179:353–358. doi: 10.1084/jem.179.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godiska R, Chantry D, Dietsch G N, Gray P W. Chemokine expression in murine experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:167–176. doi: 10.1016/0165-5728(95)00008-p. [DOI] [PubMed] [Google Scholar]
- 16.Gogate N, Swoveland P, Yamabe T, Verma L, Woyciechowska J, Tarnowska-Dziduszko E, Dymecki J, Dhib-Jalbut S. Major histocompatibility complex class I expression on neurons in subacute sclerosing panencephalitis and experimental subacute measles encephalitis. J Neuropathol Exp Neurol. 1996;55:435–443. doi: 10.1097/00005072-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Horikami S M, Curran J, Kolakofsky D, Moyer S A. Complexes of Sendai virus NP-P and P-L proteins are required for defective interfering particle genome replication in vitro. J Virol. 1992;66:4901–4908. doi: 10.1128/jvi.66.8.4901-4908.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu E C, Sarangi F, Iorio C, Sidhu M S, Udem S A, Dillehay D L, Xu W, Rota P A, Bellini W J, Richardson C D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J Virol. 1998;72:2905–2916. doi: 10.1128/jvi.72.4.2905-2916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hvas J, McLean C, Justesen J, Kannourakis G, Steinman L, Oksenberg J R, Bernard C C. Perivascular T cells express the pro-inflammatory chemokine RANTES mRNA in multiple sclerosis lesions. Scand J Immunol. 1997;46:195–203. doi: 10.1046/j.1365-3083.1997.d01-100.x. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs B L, Langland J O. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology. 1996;219:339–349. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- 21.Karp C L, Wysocka M, Wahl L M, Ahearn J M, Cuomo P J, Sherry B, Trinchieri G, Griffin D E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A, Haque J, Lacoste J, Hiscott J, Williams B R. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc Natl Acad Sci USA. 1994;91:6288–6292. doi: 10.1073/pnas.91.14.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Yang Y L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissman C, Williams B R. Deficient cytokine signalling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fundamental virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. p. 577. [Google Scholar]
- 25.Lieberman A P, Pitha P M, Shin H S, Shin M L. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebert U G, ter Meulen V. Virological aspects of measles virus-induced encephalomyelitis in Lewis and BN rats. J Gen Virol. 1987;68:1715–1722. doi: 10.1099/0022-1317-68-6-1715. [DOI] [PubMed] [Google Scholar]
- 27.Lokuta M A, Maher J, Noe K H, Pitha P M, Shin M L, Shin H S. Mechanisms of murine RANTES chemokine gene induction by Newcastle disease virus. J Biol Chem. 1996;271:13731–13738. doi: 10.1074/jbc.271.23.13731. [DOI] [PubMed] [Google Scholar]
- 28.Manchester M, Valsamakis A, Kaufman R, Liszewski M K, Alvarez J, Atkinson J P, Lublin D M, Oldstone M B. Measles virus and C3 binding sites are distinct on membrane cofactor protein (CD46) Proc Natl Acad Sci USA. 1995;92:2303–2307. doi: 10.1073/pnas.92.6.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus P I, Sekellick M J. Interferon induction by viruses. III. Vesicular stomatitis virus: interferon-inducing particle activity requires partial transcription of gene N. J Gen Virol. 1980;47:89–96. doi: 10.1099/0022-1317-47-1-89. [DOI] [PubMed] [Google Scholar]
- 30.Miyagishi R, Kikuchi S, Takayama C, Inoue Y, Tashiro K. Identification of cell types producing RANTES, MIP-1α and MIP-1β in rat experimental autoimmune encephalomyelitis by in situ hybridization. J Neuroimmunol. 1997;77:17–26. doi: 10.1016/s0165-5728(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 31.Moyer S A, Baker S C, Horikami S M. Host cell proteins required for measles virus reproduction. J Gen Virol. 1990;71:775–783. doi: 10.1099/0022-1317-71-4-775. [DOI] [PubMed] [Google Scholar]
- 32.Nazar A S, Cheng G, Shin H S, Brothers P N, Dhib-Jalbut S, Shin M L, Vanguri P. Induction of IP-10 chemokine promoter by measles virus: comparison with interferon-γ shows the use of the same response element but with differential DNA-protein binding profiles. J Neuroimmunol. 1997;77:116–127. doi: 10.1016/s0165-5728(97)00070-2. [DOI] [PubMed] [Google Scholar]
- 33.Oravecz T, Pall M, Norcross M A. Beta-chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 34.Patterson J L, Fernandez-Larsson R. Molecular mechanisms of action of ribavirin. Rev Infect Dis. 1990;12:1139–1146. doi: 10.1093/clinids/12.6.1139. [DOI] [PubMed] [Google Scholar]
- 35.Rathanaswami P, Hachicha M, Sadick M, Schall T J, McColl S R. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. J Biol Chem. 1993;268:5834–5839. [PubMed] [Google Scholar]
- 36.Ray P, Yang L, Zhang D H, Ghosh S K, Ray A. Selective up-regulation of cytokine-induced RANTES gene expression in lung epithelial cells by overexpression of IκB. J Biol Chem. 1997;272:20191–20197. doi: 10.1074/jbc.272.32.20191. [DOI] [PubMed] [Google Scholar]
- 37.Richardson C D, Scheid A, Choppin P W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequence similar to those at the N-terminal of the F1 or HA2 viral polypeptides. Virology. 1980;105:205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- 38.Rota J S, Wang Z D, Rota P A, Bellini W J. Comparison of sequences of the H, F, and N coding genes of measles virus vaccine strains. Virus Res. 1994;31:317–330. doi: 10.1016/0168-1702(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 7.3–7.84. [Google Scholar]
- 40.Schall T J, Bacon K, Toy K J, Goeddel D V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 41.Schall T J, Jongstra J, Bradley J D, Jorgensen J, Clayberger C, Davis M M, Krensky A M. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 42.Schneider-Schaulies J, Schneider-Schaulies S, ter Meulen V. Differential induction of cytokines by primary or persistent measles virus infections in human glial cells. Virology. 1993;195:219–228. doi: 10.1006/viro.1993.1363. [DOI] [PubMed] [Google Scholar]
- 43.Sekellick M J, Marcus P I. Interferon induction by viruses. VIII. Vesicular stomatitis virus: [±]DI-011 particles induce interferon in the absence of standard virions. Virology. 1982;117:280–285. doi: 10.1016/0042-6822(82)90530-x. [DOI] [PubMed] [Google Scholar]
- 44.Shin H S, Drysdale B-E, Shin M L, Noble P W, Fisher S N, Paznekas W A. Definition of a lipopolysaccharide-responsive element in the 5′-flanking regions of MuRantes and crg-2. Mol Cell Biol. 1994;14:2914–2925. doi: 10.1128/mcb.14.5.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin M L, Lieberman A P, Fisher S N. Methodological evaluation of tumor necrosis factor production in central nervous system glial cells. In: DeSouza E B, editor. Neurobiology of cytokines, part B. San Diego, Calif: Academic Press Inc.; 1993. pp. 16–34. [Google Scholar]
- 46.Sidhu M S, Chan J, Kaelin K, Spielhofer P, Radecke E, Schneider H, Masurekar M, Dowling P C, Billeter M A, Udem S A. Rescue of synthetic measles virus minireplicons: measles genomic termini direct efficient expression and propagation of a reporter gene. Virology. 1995;208:800–807. doi: 10.1006/viro.1995.1215. [DOI] [PubMed] [Google Scholar]
- 47.Vaddi K, Newton R C. Comparison of biological responses of human monocytes and THP-1 cells to chemokines of the intercrine-β family. J Leukoc Biol. 1994;55:756–762. doi: 10.1002/jlb.55.6.756. [DOI] [PubMed] [Google Scholar]
- 48.Wathelet M G, Lin C H, Parker B S, Ronco L V, Howley P M, Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 49.Wojcik W J, Swoveland P, Zhang X, Vanguri P. Chronic intrathecal infusion of phosphorothioate or phosphodiester antisense oligonucleotides against cytokine responsive gene/2IP-10 in experimental allergic encephalomyelitis of Lewis rat. J Pharmacol Exp Ther. 1996;278:404–410. [PubMed] [Google Scholar]
- 50.Wu S, Kaufman R J. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem. 1997;272:1291–1296. doi: 10.1074/jbc.272.2.1291. [DOI] [PubMed] [Google Scholar]
- 51.Yamabe T, Dhir G, Cowan E P, Wolf A L, Bergey G K, Krumholz A, Barry E, Hoffman P M, Dhib-Jalbut S. Cytokine gene expression in measles-infected adult human glial cells. J Neuroimmunol. 1994;49:171–179. doi: 10.1016/0165-5728(94)90193-7. [DOI] [PubMed] [Google Scholar]