Abstract
Introduction
Glioblastoma is the most common aggressive primary central nervous system cancer in adults characterised by uniformly poor survival. Despite maximal safe resection and postoperative radiotherapy with concurrent and adjuvant temozolomide-based chemotherapy, tumours inevitably recur. Imaging with O-(2-[18F]-fluoroethyl)-L-tyrosine (FET) positron emission tomography (PET) has the potential to impact adjuvant radiotherapy (RT) planning, distinguish between treatment-induced pseudoprogression versus tumour progression as well as prognostication.
Methods and analysis
The FET-PET in Glioblastoma (FIG) study is a prospective, multicentre, non-randomised, phase II study across 10 Australian sites and will enrol up to 210 adults aged ≥18 years with newly diagnosed glioblastoma. FET-PET will be performed at up to three time points: (1) following initial surgery and prior to commencement of chemoradiation (FET-PET1); (2) 4 weeks following concurrent chemoradiation (FET-PET2); and (3) within 14 days of suspected clinical and/or radiological progression on MRI (performed at the time of clinical suspicion of tumour recurrence) (FET-PET3). The co-primary outcomes are: (1) to investigate how FET-PET versus standard MRI impacts RT volume delineation and (2) to determine the accuracy and management impact of FET-PET in distinguishing pseudoprogression from true tumour progression. The secondary outcomes are: (1) to investigate the relationships between FET-PET parameters (including dynamic uptake, tumour to background ratio, metabolic tumour volume) and progression-free survival and overall survival; (2) to assess the change in blood and tissue biomarkers determined by serum assay when comparing FET-PET data acquired prior to chemoradiation with other prognostic markers, looking at the relationships of FET-PET versus MRI-determined site/s of progressive disease post chemotherapy treatment with MRI and FET-PET imaging; and (3) to estimate the health economic impact of incorporating FET-PET into glioblastoma management and in the assessment of post-treatment pseudoprogression or recurrence/true progression. Exploratory outcomes include the correlation of multimodal imaging, blood and tumour biomarker analyses with patterns of failure and survival.
Ethics and dissemination
The study protocol V.2.0 dated 20 November 2020 has been approved by a lead Human Research Ethics Committee (Austin Health, Victoria). Other clinical sites will provide oversight through local governance processes, including obtaining informed consent from suitable participants. The study will be conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. Results of the FIG study (TROG 18.06) will be disseminated via relevant scientific and consumer forums and peer-reviewed publications.
Trial registration number
ANZCTR ACTRN12619001735145
Keywords: Glioblastoma, FET, prognostic marker, pseudoprogression, chemoradiation
Strengths and limitations of this study.
Largest multicentre prospective study addressing the impact of O-(2-[18F]-fluoroethyl)-L-tyrosine (FET) positron emission tomography (PET) in the management of glioblastoma, including adjuvant radiation planning, differentiating pseudoprogression from recurrent and/or progressive disease, the role of FET-PET in prognostication, as well as a robust health economic analysis.
Development and implementation of robust multisite national credentialling and on-trial quality assurance programmes addressing both nuclear medicine and radiation oncology delivery.
Development of integrated FET-PET and MRI-specific criteria for assessment of treatment response in the management of study participants with newly diagnosed glioblastoma.
A limitation of the study includes varying levels of site experience with FET-PET interpretation and reporting, although this is addressed via a robust trial credentialling programme assessing both technical capability and upskilling of nuclear medicine specialist and radiation oncologist expertise. Ongoing quality assurance in the prospective phase of the trial will also serve to reduce interobserver variability.
Introduction
Background and rationale
Glioblastoma multiforme (GBM) is the most common primary brain cancer in adults1 with poor survival outcomes resulting in a median survival of 15 months and a 5-year survival of less than 5%.2 Since the introduction of concurrent temozolomide (TMZ) chemotherapy with postsurgical radiation in 2005, there has been little progress in improving outcomes.2 3 There remains a pressing need for the incorporation of accurate and timely imaging as a cornerstone in optimal management,4 prognostication and effective decision-making to help improve the current dismal outcomes in adult glioblastoma.
Amino acid (AA) positron emission tomography (PET) imaging tracers (such as O-(2-[18F]-fluoroethyl)-L-tyrosine (FET-PET)) has been shown to be accurate in detecting the site and extent of GBM in both initial diagnostic and recurrent disease settings. Figure 1 demonstrates imaging from serial FET-PET scans including baseline, retest (1 week) and post-therapy (see figure 1),5 although studies to date have been almost exclusively single-centre with relatively small sample sizes.6–8 The utility of AA FET-PET imaging tracers is based on the observation that AA transport, primarily mediated by the L-Type AA transporter, is increased in malignant transformation independent of a disrupted blood-brain barrier (BBB) and is also present in non-enhancing tumour sites, therefore yielding a high tumour to normal tissue contrast and potentially allowing more sensitive detection of tumour in non-gadolinium contrast enhancing areas.4 9 10
Figure 1.
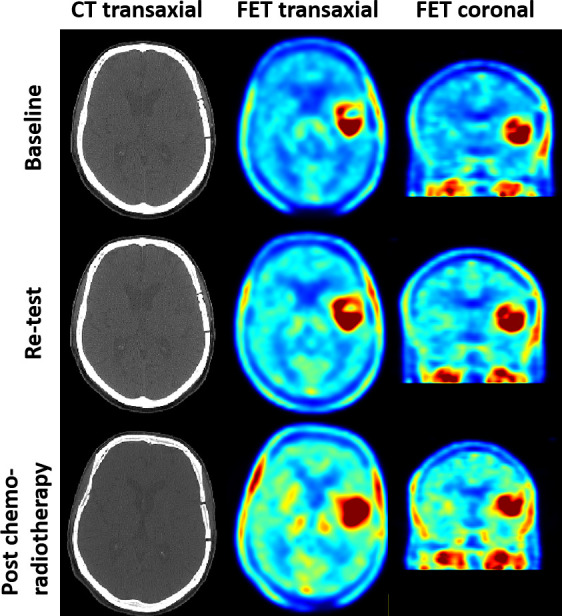
Serial O-(2-[18F]-fluoroethyl)-L-tyrosine positron emission tomography scans including baseline, retest (1 week) and post-therapy.
Study hypotheses, aims, objectives and related end points
Primary aim 1: to quantify the impact of FET on radiotherapy planning volumes relative to MRI alone
The first hypothesis is that incorporation of FET-PET imaging into radiation therapy treatment planning, compared with standard MRI planning alone, will lead to a clinically significant change, defined as >10% change in absolute gross tumour volume (GTV) and/or planning target volume (PTV) for radiotherapy in participants with GBM, particularly in areas lacking BBB disruption.5 Adjuvant radiation planning is currently performed using predominantly anatomical T1 post-contrast MRI sequences.11 The volume of residual tumour at the time of initiation of chemoradiation is highly predictive of subsequent patient outcome.5 7 The most promising nuclear medicine imaging agent is FET, shown to accurately detect the location and extent of GBM in both initial diagnosis and recurrent disease settings. Multiple single-centre studies have shown that the incorporation of FET-PET imaging can lead to significant change and discordance in radiation target volumes for participants with GBM when compared with standard MRI-based radiation planning alone.6 12–14
Niyazi et al12 retrospectively compared the MRI-based GTVs to biological tumour volumes (BTVs), based on pathological FET radiotracer uptake, subsequent clinical target volumes (CTVs), and PTVs for the radiotherapy planning of 17 participants with GBM. In 11 cases, there were major differences between GTV/BTV when FET was incorporated with standard MRI-based imaging, with significantly larger FET-based BTVs (median 43.9 cm3) compared with corresponding GTVs (median 34.1 cm3). Similarly, Rieken et al13 investigated the volumetric size and uniformity of MRI versus FET-derived GTVs and PTVs of 41 participants with GBM. They reported that the congruence of MRI and FET signals for the identification of glioma GTVs was poor, with mean uniformity indices of 0.39, and furthermore that MRI-based PTVs missed 17% of FET/CT-based GTVs.
Primary aim 2: to demonstrate the accuracy of pseudoprogression assessment using FET
The second hypothesis is that FET-PET imaging will be more accurate than routine MRI and clinical follow-up in differentiating tumour pseudoprogression from true tumour progression.13 15–18 Chemo-radiotherapy can induce pseudoprogression, defined as progressive enhancing lesions due to treatment-induced changes in the BBB, resulting in MRI findings mimicking progressive tumour.19 20 Pseudoprogression can occur in up to 20–30% of chemoradiation participants and may or may not be accompanied by clinical deterioration.
Despite the advent of the Response Assessment in Neuro-Oncology (RANO)21 criteria and modified RANO criteria for standard MRI interpretation22 in high-grade glioma,23 this remains predominantly criteria used in research and/or clinical trials. Clinically, the interpretation and assessment of disease status remains challenging. Therefore, it is important to have access to improved imaging biomarkers that can more accurately distinguish disease activity from post-therapy changes which enable optimal management decisions. Since FET uptake is independent of a disrupted BBB, this imaging modality may be more sensitive in distinguishing true progression from pseudoprogression. Indeed, FET-PET has been shown to be superior to MRI in detecting pseudoprogression across multiple single-site studies5 16–18 24 25 and a meta-analysis,26 but large, prospective multicentre studies are still needed.
Maurer et al24 retrospectively evaluated 127 participants with grade II–IV glioma who underwent FET-PET imaging to distinguish between tumour progression and treatment-related changes who then underwent either re-resection (n=40) or clinical/MRI follow-up. The slope of the time-activity curves (20–50 mins following injection), time to peak activity (objective parameter describing the slope of tracer uptake) and maximum tumour-to-brain ratios (TBRmax) of FET uptake were determined. Treatment-related change was observed in 26% of participants, with an optimal FET-PET TBRmax cut-off value of 1.95 for differentiating tumour progression from treatment-related change (sensitivity 70%, specificity 71%, accuracy 70%). The accuracy of FET PET was significantly higher in isocitrate dehydrogenase (IDH)-wild-type gliomas. The diagnosis based on FET-PET turned out to be incorrect in 33% of the IDH-mutant tumours, but in only 9% of the IDH-wild-type tumours. The FET-PET rating, the WHO grade, the IDH status and the Karnofsky performance status remained independent prognostic factors. O6-Methylguanine-DNA methyltransferase (MGMT) promoter methylation did not significantly affect the diagnostic performance of FET-PET.
The combination of perfusion MRI and FET-PET may improve the diagnostic accuracy in interpreting treatment-related changes. Steidl et al27 evaluated sequential perfusion MRI and FET-PET in 104 participants with WHO grade II–IV glioma and suspected tumour progression. Static (TBRmax) and dynamic FET-PET parameters (slope of the time-activity curves) were calculated, as well as leakage-corrected maximum relative cerebral blood volumes (rCBVmax) from dynamic susceptibility contrast Perfusion Weighted Imaging (PWI). The combined FET-PET parameters (TBRmax and slope) discriminated tumour progression from treatment-related change in 78% of participants, with an rCBVmax cut-off value>2.85 showing a positive predictive value for tumour progression of 100%.
Table 1 summarises the key retrospective and single centre prospective studies addressing the role of FET-PET in radiotherapy treatment planning and in distinguishing pseudoprogression from tumour progression in the management of glioblastoma.
Table 1.
Key studies addressing the role of FET in radiotherapy treatment planning and in distinguishing pseudoprogression from tumour progression in the management of glioblastoma
| First author | Publication year | Sample size (n) |
Study design | Study outcomes/findings |
| Niyazi12 | 2011 | 17 | Retrospective | FET-PET versus MRI in GTV/BTV for radiation planning. |
| Rieken13 | 2013 | 41 | Retrospective | FET-PET versus MRI in GTV/PTV for radiation planning. |
| Hayes6 | 2018 | 26 | Retrospective | FET-PET versus MRI in CTV/BTV for radiation planning. |
| Lau30 | 2010 | 21 (n=11 with glioma) |
Prospective | Diagnostic value of FET-PET versus FDG-PET in differentiating pseudoprogression from tumour progression: sensitivity 93%, specificity 100%, accuracy 96%, PPV 100%, NPV 91% for FET-PET. |
| Galldiks31 | 2012 | 31 | Retrospective | Diagnostic value of FET-PET for differentiating recurrence from radiation necrosis: TBRmax accuracy 78%. |
| Yu26 | 2018 | 48 studies n=23 in FET-PET |
Retrospective meta-analysis |
18F-FDOPA and FET-PET to differentiate tumour progression from pseudoprogression: sensitivity 85 versus 82%, specificity 77 versus 80%. |
| Maurer,24 | 2020 | 127 | Retrospective | FET-PET to differentiate tumour progression from pseudoprogression: TBRmax sensitivity 70% and accuracy 81%. |
| Lohmann18 | 2020 | 34 | Retrospective | FET-PET to differentiate tumour progression from pseudoprogression: TBRmax sensitivity 81% and NPV 80%. |
| Steidl27 | 2021 | 104 | Retrospective | Sequential PWI MRI and FET-PET to differentiate tumour progression from pseudoprogression: rCBVmax PPV 100%, TBRmax sensitivity 70% and NPV 32%. |
BTV, biological target volume; CTV, clinical target volume; FDG, (18)F-fluorodeoxyglucose; FDOPA, 18F-FDOPA (6-[18F]-L-fluoro-L-3, 4-dihydroxyphenylalanine; FET, O-(2-[18F]-fluoroethyl)-L-tyrosine; GTV, gross tumour volume; NPV, negative predictive value; PET, positron emission tomography; PPV, positive predictive value; PTV, planning target volume; PWI, Perfusion Weighted Imaging; rCBVmax, maximum relative cerebral blood volumes; TBRmax, maximum tumour-to-brain ratios.
Co-primary outcome
The comparison of the radiation target volume delineation determined by MRI compared with FET-PET imaging.
Co-primary outcome 2
To determine the accuracy and management impact of FET-PET in distinguishing pseudoprogression from true tumour progression and/or tumour recurrence.
Treating clinicians will complete a management intent questionnaire prior to knowledge of the FET-PET3 result, and then again at 4–8 weeks after FET-PET3 results are known, to establish the impact of FET-PET3 on patient management.
Follow-up (6 months later) will be performed to confirm whether final management aligns with that indicated in the post-FET-PET3 management impact questionnaire. This methodology has been previously established as the reference standard for patient management impact assessment of PET imaging studies.
Secondary aim 1: to assess the prognostic value of FET-PET parameters
The third hypothesis is that FET-PET imaging parameters of dynamic uptake, tumour-to-background ratio and metabolic tumour volume will be associated with progression-free survival (PFS) and overall survival (OS). Lundemann et al28 prospectively evaluated 16 participants with GBM undergoing multiparametric [18F]Fluorodeoxyglucose (FDG)-PET, FET-PET and diffusion and dynamic contrast-enhanced MRI at the time of radiation treatment planning. Within the radiotherapy target, median differences of imaging parameters in recurring and non-recurring voxels were calculated for the contrast-enhancing lesion, non-enhancing lesion and normal-appearing grey and white matter. Logistic regression models were created to predict the patient-specific probability of recurrence. The most pronounced correlations were observed for FDG and FET uptake in contrast-enhancing lesions and non-contrast-enhancing lesions. Voxel-wise modelling of recurrence probability resulted in an area under the receiver operating characteristic curve of 0.77 from scans prior to therapy.
Secondary outcomes
To investigate the relationships between FET-PET parameters (including dynamic uptake, tumour to background ratio, metabolic tumour volume, radiomics features) and PFS and OS outcomes in glioblastoma.
Assessing the change in the blood and tissue biomarkers as determined by serum assay when comparing FET-PET imaging data acquired prior to initial chemoradiation with other prognostic markers of PFS and OS.
To look at the relationships of FET-PET versus MRI-determined site/s of progressive disease post radiation and chemotherapy treatment.
To estimate the health economic impact of incorporating FET-PET imaging into the management strategy of patients with GBM undergoing chemo-radiotherapy and inthe assessment of post-treatment pseudoprogression or recurrence/progression.
Exploratory aims
There is an unmet need for improved prognostic and predictive biomarkers in GBM. The most validated biomarkers in GBM currently are MGMT promoter methylation and IDH gene mutation. A biobank of serum and /or tumour samples pre-chemoradiation, during-chemoradiation and post-chemoradiation will be subjected to multiomics analyses and the findings will be correlated with FET-PET and MRI radiomics features for the development of multiomics predictive models that may guide optimal therapy in participants with GBM.
Exploratory outcomes
Correlation of local and remote central nervous system relapses visualised on FET-PET imaging with radiotherapy treatment parameters (fields, target volumes).
Quantification of the differences in dose to normal tissues (including brainstem, chiasm, optic nerves, lenses) resulting from FET-PET planning compared with MRI planning alone.
Development of a biobank of serum and/or tumour samples pre, during and post-chemoradiation in participants with GBM and correlate these with FET-PET imaging parameters.
A comparison of FET-PET3 to FDG-PET in terms of tumour response assessment.
A comparison and correlation of FET-PET with MRI techniques and with histopathology at subsequent surgery when performed for suspected tumour recurrence.
Methods and analysis
Study design
The FET-PET in Glioblastoma (FIG) study (TROG 18.06) is a longitudinal prospective, non-randomised, phase II study undertaken in up to 10 metropolitan hospitals around Australia.
Universal Trial Number: U1111-1222-4710. The trial sponsor is Trans Tasman Radiation Oncology Group (TROG) Cancer Research with date of registration: 9 December 2019.
The FIG study aims to recruit up to 210 participants, namely 140 participants in group 1 (pre-chemoradiation); and up to 70 participants in group 2 (post-chemoradiation). Up to 70 additional participants may be recruited into group 2. As the trial focus is imaging-based (rather than a therapeutic intervention), there are no interim analyses planned. The study will received oversight by the FIG Trial Management Committee, as well as the TROG Independent Data and Safety Monitoring Committee and the TROG Scientific Advisory Committee. The first patient was recruited to the study on 27 January 2021 with study recruitment expected to be completed in 2024.
Credentialling procedures
All participating centres must successfully complete pre-trial quality assurance procedures before enrolling participants, including ARTnet (Australasian Radiopharmaceutical Trials Network) validation of all PET scanners. Key credentialling items completed by FIG study sites will be overseen by TROG and cover both radiation oncology and nuclear medicine aspects, outlined in table 2. FET is provided by a commercial manufacturer or produced on-site according to agreed standard operating procedures (SOP). All aspects of FET provision (production, scan acquisition, imaging, etc) are being done in accordance with the joint European Association of Nuclear Medicine (EANM)/European Association of Neuro-Oncology (EANO)/RANO practice guidelines/ Society of Nuclear Medicine and Molecular Imaging (SNMMI) procedure standards for imaging of gliomas using PET with radiolabelled AAs and [(18)F]FDG: V.1.0.10
Table 2.
FIG study—summary of credentialling and quality assurance programme
| Radiation therapy quality assurance | ||
| Activity | Number of cases | Comments |
| Phantom dosimetry audit | N/A | Evidence of appropriate end-to-end audit using an anthropomorphic phantom to confirm delivered radiation therapy doses |
| Facility questionnaire | N/A | Documentation of site radiation therapy facilities and processes |
| Benchmarking exercise—radiation therapy contouring | 1 | (Part A) Contour a test case using standard imaging to demonstrate understanding of the protocol and ability to meet protocol contouring constraints |
| Benchmarking exercise—FET-PET imaging interpretation and incorporation into RT target volume delineation | 3 | (Part B) Delineation of a biological treatment volume using FET-PET imaging (incorporation of the FET-PET volumes into standard MRI-derived target volumes) |
| Benchmarking exercise—radiation therapy treatment planning | 1 | Develop a radiotherapy plan using a pre-contoured data set to demonstrate understanding of the protocol and ability to meet protocol planning and dosimetry constraints |
| Nuclear medicine quality assurance | ||
| Activity | Number of cases | Comments |
| ARTnet PET-CT certification | N/A | ARTnet validation of PET and MRI scanners |
| FIG—technical survey; nuclear medicine and radiology capacities | N/A | Technical survey to determine site imaging facilities and processes |
| Benchmarking exercise—FET-PET image interpretation target volume delineation | 3 | Nuclear medicine physician delineation of target volumes using FET-PET imaging |
| Benchmarking exercise—FET-PET imaging interpretation and response criteria/scoring | 3 | Nuclear medicine physician interpretation of response criteria, scoring and assessment of disease status using FET-PET imaging |
ARTnet, Australasian Radiopharmaceutical Trials Network; FET, O-(2-[18F]-fluoroethyl)-L-tyrosine; FIG, FET-PET in Glioblastoma; PET, positron emission tomography; RT, radiotherapy.
Study interventions
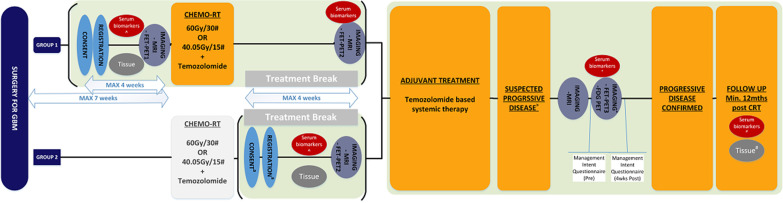
Following consent and screening, eligible participants will be offered enrolment at one of 10 credentialled study sites across Australia, as either a Group 1 participant pre-chemoradiation or a Group 2 participant, who enter and undergo FET-PET2 and study MRI2 at one month post-chemoradiation completion.
Adjuvant chemoradiation will be administered as per standard of care and should start after registration and within 7 weeks from the date of surgery. Radiotherapy will consist of conventionally fractionated radiotherapy delivered either as 60 Gy/30 daily fractions over 6 weeks28 or 40.05 Gy/15 daily fractions over 3 weeks for elderly participants and/or those with poor performance status3 (see online supplemental material S1). TMZ will be 75 mg/m2 oral daily for either: (1) 6 weeks concurrent with radiotherapy (60 Gy/30 daily fractions), or (2) 3 weeks concurrent with radiotherapy (40.05 Gy/15 daily fractions) for elderly and/or poorer performance status participants. Once concurrent chemoradiation has been completed, the participant will have a 4-week rest period before commencing adjuvant TMZ.
bmjopen-2022-071327supp001.pdf (192.4KB, pdf)
All participants
Adjuvant TMZ will be administered as per standard of care at 150–200 mg/m2 days 1–5 every 28 days until either disease progression, unacceptable toxicity or completion of 6 months of treatment. Dose interruptions and/or reductions, as well as ongoing treatment after discontinuation and/or cessation of study treatment is at the discretion of the participant’s treating clinician. Concurrent recruitment to other which are TMZ-based therapeutic trials is permitted.
FET-PET1 (along with study MRI1) will be performed following initial surgery and before starting chemoradiation in Group 1 participants. FET-PET2 (along with study MRI2) will be performed no earlier than 4 weeks (+ up to 7 days) following concurrent chemoradiation in both Group 1 and 2 participants. Study MRI3 will be performed at the time of clinical suspicion of tumour recurrence and/or progression, with FET-PET3 performed within 14 days of suspected radiological progression on MRI in both Group 1 and 2 participants. The timing of FET-PET1 is aligned with literature establishing the potential role of FET-PET in delineating the extent of residual tumour12,13). FET-PET2 timing was to establish a baseline after chemoradiation and to compare to FET-PET3 which is timed for when clinical suspicion of progression versus pseudoprogression arises. At the time of suspected recurrent disease, in addition to FET-PET3 and MRI3, study participants are requested to undergo an FDG-PET scan which has been made optional, as although a direct comparison of FDG-PET with FET-PET is planned, the study protocol requirements for participants are already quite substantial, with the FET-PET and MRI taking precedence.
Importantly, treating oncologists and imaging specialists are blinded to FET-PET1 and FET-PET2 results. Furthermore, FET-PET1 results will not be incorporated into actual radiotherapy target volumes used for treatment, given that FET-PET1 is being evaluated for this indication in the study.
Tissue will be obtained at baseline (archival or from debulking surgery) for MGMT methylation status, and at the time of recurrence if repeat surgery and/or biopsy is clinically indicated Formalin-Fixed Paraffin-Embedded(FFPE) samples are sent to the central laboratory). Blood for serum markers is obtained between registration and initial FET-PET, then on the day of each subsequent FET-PET. If further surgical resection or biopsy is required, a sample of the tissue will be requested to assist with confirmation of tumour recurrence versus pseudoprogression. EORTC QLQ C30 will be assessed at baseline (study entry) and at each assessment time point (see table 3, schedule of assessments). All participants will be followed for 12 months after the end of accrual to allow evaluation of PFS and OS, with analysis at 12 months after the final patient has completed chemoradiation treatment.
Table 3.
Schedule of assessments in the FIG study
| Time point | Registration | Pretreatment | Chemo-RT | Post chemo-RT | Suspected progression on MRI | |||
| 4 weeks | 4 months (or 18 weeks) |
7 months (or 30 weeks) |
12 months (or 52 weeks) |
|||||
| Assessment | Imaging time point 1 |
Imaging time point 2 |
(3 months adjuvant TMZ) | 6 months adjuvant TMZ) | Imaging time point 3 |
|||
| Visit window | After surgery, prior to chemo-RT | ≤7 weeks from surgery | +7 days | ±7 days | ±7 days | ±7 days | +2 weeks from progressive disease on MRI | |
| Informed consent | X | |||||||
| Eligibility assessment | X | |||||||
Clinical assessment
|
X | X | X | X | X | X | ||
| Signs and symptoms | X | X | X | |||||
| EORTC QLQ C30 | X | X | X | X | X | X | X | |
| MGMT and biomarker testing | X | |||||||
| Tissue collection | X | |||||||
| Serum biomarkers | X | X | X | |||||
| MRI* | X (MRI1) | X (MRI2) | X * | X * | X * | X (MRI3) | ||
| FET-PET† | X (FET-PET1) | X (FET-PET2) | X (FET-PET3) | |||||
| FDG-PET† | X | |||||||
| Management intent questionnaires | X | |||||||
| Survival status | ||||||||
*Where feasible, FIG participant MRI are performed as per online supplemental material S3, otherwise MRI protocol as per site standard protocol.
†FET-PET and FDG-PET performed as per online supplemental material S4.
‡
FDG, [18F]Fluorodeoxyglucose; FET, O-(2-[18F]-fluoroethyl)-L-tyrosine; FIG, FET-PET in Glioblastoma; MGMT, O6-Methylguanine-DNA methyltransferase (MGMT) promoter methylation; PET, positron emission tomography; RT, radiotherapy; TMZ, temozolomide.
The FIG study schema is shown in figure 2.
Figure 2.
The FIG study schema for screening and registration of both Group 1 (postoperative pre-concurrent chemoradiation) and Group 2 (prior to adjuvant temozolomide) participants. CRT, chemo-RT; GBM, glioblastoma multiforme; FET, O-(2-[18F]-fluoroethyl)-L-tyrosine; PET, positron emission tomography; [18F]Fluorodeoxyglucose (FDG) PET, RT, radiotherapy.
Eligibility
Participants must fulfil all inclusion and none of the exclusion criteria prior to registration and enrolment. Eligibility criteria are listed below.
Inclusion criteria
All participants
Age ≥18 years.
Histologically confirmed, newly diagnosed GBM (IDH1-R132H wild type or IDH mutant using immunohistochemistry (IHC) (2016 WHO grade IV glioma) following surgery, with methylated or non-methylated MGMT promoter gene.
Note: Participants with a previous grade I–III glioma which has progressed to GBM are eligible if they have not received prior cranial radiotherapy or TMZ for the treatment of glioma.
Eastern Cooperative Oncology Group (ECOG) performance status 0–2.
Life expectancy >12 weeks.
Adequate bone marrow reserve or organ function to allow TMZ-based chemotherapy.
Available tissue for MGMT and biomarker analysis.
Participants capable of childbearing are using adequate contraception.
Willing and able to comply with all study requirements, including treatment, timing and/or nature of required imaging and study assessments.
Has provided written informed consent (see online supplemental material S2).
bmjopen-2022-071327supp002.pdf (346.8KB, pdf)
Group 1 participants
Considered suitable for radiotherapy (with one of the two dose schedules of 60 Gy in 30 daily fractions or 40.05 Gy in 15 daily fractions) plus concurrent TMZ followed by adjuvant TMZ.
Group 2 participants (entering the study post chemoradiation at imaging time point 2)
Currently undergoing or have recently completed concurrent radiotherapy with TMZ, with one of the two radiation dose schedules of 60 Gy/30 daily fractions or 40.05 Gy/15 daily fractions and logistically able to be recruited.
Have commenced adjuvant chemoradiation ≤7 weeks from surgery.
Considered suitable for adjuvant TMZ-based chemotherapy.
Exclusion criteria
Participants with implanted devices deemed by the radiologist to be a contraindication to performing a brain MRI.
Any concurrent comorbidities, conditions or illness, including severe infection or medical or psychiatric conditions that may jeopardise the ability of the participant to undergo the procedures outlined in this protocol with reasonable safety or that may compromise assessment of key outcomes.
-
History of another malignancy within 2 years prior to registration.
Note:
Participants with a history of adequately treated carcinoma in situ, basal cell carcinoma of the skin, squamous cell carcinoma of the skin or superficial transitional cell carcinoma of the bladder are eligible.
Participants with a history of other malignancies are eligible if continuously disease free for at least 2 years after definitive primary treatment.
Group 1 participants
Prior chemotherapy or cranial radiation within the last 2 years.
Outcome measures and assessments
Schedule of assessments
Assessments will be performed according to the schedule shown in table 3 for FIG study Group 1 and Group 2 participants.
Post progression follow-up consists of survival status verification at 1 year post chemoradiation completion and 6 monthly thereafter. For those participants proceeding to second surgery, tissue and serum blood biomarkers will be collected.
Patient and public involvement
Patients were involved in the design and conduct of this research. In particular, there was a consumer investigator named on competitive grant funding applications secured to support the FIG study. In addition, integral input was sought from a consumer representative during the design of the Patient and Information and Consent forms to facilitate a patient-centred approach to informed consent (see online supplemental material S2). A consumer representative is a member of the Trial Management Committee.
FET-PET1 analysis
Following treatment delivery, FET-PET data and FET-PET BTV will be delineated by the site nuclear medicine physician (using the dedicated FIG study V.7.0, MIM software, Workflow). This is sent to the TROG Radiation Therapy Quality Assurance Department for central approval before being made available to the radiation oncologist (RO) for fusion to radiotherapy planning CT and MRI, and delineation of a new PET-MRI defined GTV, CTV and PTV (without reference to actual treatment volumes). Each site will be provided with the FIG trial MIM Workflow (see online supplemental material S5). Central review of RO-derived hybrid volumes are also undertaken.
FET-PET2 and FET-PET3 analyses for tumour recurrence
An integrated MRI and FET-based treatment response criteria will be used in the FIG study (see table 4). When timepoint 3 is triggered, there is both site and central review of FET-PET3 within seven calendar days of image acquisition (see online supplemental material S6). Treating site clinicians will complete a management intent questionnaire prior to knowledge of the FET-PET3 result and then complete a follow-up questionnaire 4–8 weeks after the FET-PET3 results are known, to establish the impact of FET-PET3 on participant management. FET-PET2 will only be used for comparison to FET-PET3 at the time of evaluation of tumour recurrence/progression for further analysis of lesion uptake, following initial review of FET-PET3 alone.
Table 4.
Integrated MRI and FET-PET based treatment response criteria applied in the FIG study
| Treatment predominant | Tumour progression | |
| Static (20–40 min) | ||
| FET-PET activity in lesion | No focal activity | Focal and intense activity in suspected lesion |
| Compared with FET-PET2 | FET-PET3 has similar or less intense activity and distribution | FET-PET3 has more intense or extensive activity |
| Compared with Gd enhancement on MRI | FET-PET activity concordant with distribution of Gd enhancement | FET-PET activity discordant with Gd enhancement |
| TBR | TBR<2.3 | TBR>2.3 |
| Dynamic (0–40 min) | ||
| Time activity curve (TAC) | Pattern I: slow rising TAC with no identifiable peak | Pattern III: early peak in TAC (<20 min) with subsequent descent pattern |
FET, O-(2-[18F]-fluoroethyl)-L-tyrosine; FIG, FET-PET in Glioblastoma; Gd, Gadolinium; PET, positron emission tomography; TBR, tumour to brain ratio.
Time to event, toxicity and QOL measures
Time to event measures are defined as the interval between the date of initial surgery and the date of the event, with censoring at last follow-up if the event has not occurred. The time to first treatment for recurrent disease is defined as the interval between the date of initial surgery and the date of first salvage therapy (eg, re-resection, re-irradiation, second-line chemotherapy or a clinical trial treatment) or death from any cause, with censoring at last follow-up if alive with no treatment for recurrent disease.
As this is an imaging-based study with no therapeutic interventions delivered over and above standard of care, no treatment-related toxicity data will be collected. Only suspected reactions to FET radiopharmaceuticals will be reported as Adverse Events (AE) (collected 48 hours post-FET injection). The incidence of significant toxicities is anticipated to be very low, but could include a local reaction at the tracer injection site or minor systemic symptoms. Study discontinuation would occur in the circumstance that the participant decides to completely withdraw from all aspects of the trial.
Health-related Quality of Life (HRQoL) will be reported by participants using the EORTC QLQ C-30 at baseline (study entry) and at each assessment time point (as per table 2). These will also be used to estimate quality adjusted life years for a comparison with the costs of care including FET-PET delivery, MRI imaging, radiotherapy and outpatient services obtained by consenting participants for access to their administrative claims data (Medicare) for medical and pharmaceutical services use. This data will be used for a health economic analysis compared with published literature.29
The cost consequences of incorporating FET-PET imaging in the management of patients with GBM will be evaluated by quantifying the resource use associated with these tests. Data will include resource use associated with the delivery and interpretation of FET-PET scans; chemoradiation and subsequent treatment usage. Resource use associated with all multimodal imaging (FET-PET, MRI) as well as radiation therapy treatment plans will be available from trial-based case report forms. Data on outpatient and community-based services (pharmaceuticals and medical) will also be collected as well as prescribing data. Additionally, based on 10 of the 15 dimensions of the QLQ-C30, the QLU-C10D is a newly developed, cancer-specific multi-attribute utility instrument included in the EORTC assessment system and will be used for the health economic evaluations in cost-utility analyses relating to FIG trial participants.
Tumour and blood will be analysed for multiomics (genomic, epigenomic and transcriptomic) markers including DNA methylation, circulating tumour DNA (ctDNA) and exosomal analysis.
Statistical design
Participant demographic, clinical and treatment characteristics and study outcomes will be presented using standard descriptive statistics (mean, SD and range for continuous variables, frequencies and percentages for categorical variables and Kaplan-Meier method for time-to-event endpoints (PFS/OS). Although there is no formal stratification performed as part of this non-randomised study, analysis of survival outcomes may be adjusted for known prognostic factors including ECOG performance status, age, extent of resection, standard versus hypofractionated radiation course, as well as biological factors including IDH1-R132H (via IHC) and MGMT methylation status.
Primary aim 1
FET-PET1 (Group 1 only) will be used to assess the impact of FET-PET on radiotherapy planning, described using the percentage volume of FET-PET-avid disease that would be excluded from the GTV and PTV participants if MRI data alone were used for GBM radiation treatment planning. If GTV and/or PTV volume changed by >10% in absolute terms (cc3), it would be concluded that the addition of FET-PET1 has a clinically meaningful impact on radiation planning. The proportion of participants in whom this occurs will be described with a 95% CI. It is anticipated that 140 participants will be available, enabling estimation of the proportion with a 95% CI of maximum width ±8%.
Primary aim 2
FET-PET3 will be used to categorise participants as undergoing pseudoprogression or true tumour progression. This will be compared with the final determination of progression, by clinical follow-up and sequential MRI, and calculating the total proportion of true positives and true negatives. If this accuracy FET-PET is ≤80%, then FET-PET would not be considered sufficiently accurate. If FET-PET3 is obtained in 120 participants, the study has 80% power at 2.5% one-sided alpha to rule out accuracy of 80% if the true accuracy is 90%, and will also enable accuracy to be estimated with a 95% CI of maximum width ±9%.
Secondary aim 1
In this study, use of FET-PET as a prognostic factor for PFS and OS will have power to detect only large differences in PFS between groups of participants categorised as having poor (non-responders) or good (responders) prognosis according to the information in FET-PET1. Assuming approximately equal numbers of non-responders and responders in the study, and if the true HR is 1.75 for PFS for FET-PET non-responders relative to responders, then 100 participants followed until progressive disease or death from any cause will enable a difference to be detected with 80% power at 5% (two-sided alpha).
Ethics and dissemination
Ethical and safety considerations
The FIG study was approved by the lead site, Austin Health Human Research Ethics Committee - HREC/56071/Austin-2019. HREA (V.3, 30 December 2019), Protocol (V.1.0, 01 October 2019). Protocol No. TROG 18.06. Other clinical sites will provide oversight through local governance processes, including obtaining informed consent from suitable participants (see online supplemental material 2). Any substantial amendments to the study protocol will be reported to the lead site ethics committee for approval prior to implementation, and updated on the trial registry, with study investigators being advised in writing.
Dissemination plan
The FIG Trial Co-Chairs and Trial Management Committee are responsible for presentations and publications arising from this trial with the TROG Publications Committee providing oversight and independent scientific review of all relevant material prior to submission. Study promotion and updates will be undertaken via relevant professional and consumer networks across Australia. Results will be disseminated in relevant scientific forums, peer-reviewed publications and using a range of media channels including newsletters and social media.
The FIG study publication policy is an overarching policy between participating researchers that governs the multisite collaborative effort. The FIG study will run under the auspices of the FIG Trial Management Committee and be open to input from all participating sites and researchers.
Supplementary Material
Footnotes
Contributors: AMS, AKN, RJF, HG, RJH, FF, E-SK, MR, MK and ME developed the study concept. E-SK, AMS, CS, EL, RJF and ME wrote the manuscript draft. STL, DLB, BM, GF, RJH, RC, RV, KMW, EHB, RDAL, LA, AL, AM, PT, PR, MB and RL reviewed the manuscript and provided critical input.
Funding: This work is supported by the Medical Research Future Fund (MRFF) Lifting Clinical Trials and Registries Capacity scheme ((MRF1152501), from the Australian Brain Cancer Mission Innovative Clinical Trials Scheme (MRF9500003) and a Cure Brain Cancer Foundation Innovation Grant (award/grant number N/A). RC acknowledges the support of funding from the National Cancer Institute (R35CA197579 and UH3CA241685). Support is also received from commercial partners Cyclotek for the provision of FET and also Telix Pharmaceuticals.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Ostrom QT, Cioffi G, Waite K, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol 2021;23(12 Suppl 2):iii1–105. 10.1093/neuonc/noab200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant Temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 3.Perry JR, Laperriere N, Mason WP. Radiation plus Temozolomide in patients with glioblastoma. N Engl J Med 2017;376. 10.1056/NEJMc1704726 [DOI] [PubMed] [Google Scholar]
- 4.Albert NL, Weller M, Suchorska B, et al. Response assessment in neuro-oncology working group and European Association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 2016;18:1199–208. 10.1093/neuonc/now058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galldiks N, Niyazi M, Grosu AL, et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients - a report of the PET/RANO group. Neuro Oncol 2021;23:881–93. 10.1093/neuonc/noab013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes AR, Jayamanne D, Hsiao E, et al. Utilizing 18F-Fluoroethyltyrosine (FET) positron emission tomography (PET) to define suspected Nonenhancing tumor for radiation therapy planning of glioblastoma. Pract Radiat Oncol 2018;8:230–8. 10.1016/j.prro.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 7.Pinkawa M, Piroth MD, Holy R, et al. Quality of life after whole pelvic versus prostate-only external beam radiotherapy for prostate cancer: a matched-pair comparison. Int J Radiat Oncol Biol Phys 2011;81:23–8. 10.1016/j.ijrobp.2010.05.054 [DOI] [PubMed] [Google Scholar]
- 8.Poulsen SH, Urup T, Grunnet K, et al. The Prognostic value of FET PET at radiotherapy planning in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging 2017;44:373–81. 10.1007/s00259-016-3494-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunet V, Rossier C, Buck A, et al. Performance of 18F-Fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and Metaanalysis. J Nucl Med 2012;53:207–14. 10.2967/jnumed.111.096859 [DOI] [PubMed] [Google Scholar]
- 10.Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with Radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging 2019;46:540–57. 10.1007/s00259-018-4207-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niyazi M, Brada M, Chalmers AJ, et al. “ESTRO-ACROP guideline "target delineation of Glioblastomas"” Radiother Oncol 2016;118:35–42. 10.1016/j.radonc.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 12.Niyazi M, Geisler J, Siefert A, et al. FET-PET for malignant glioma treatment planning. Radiother Oncol 2011;99:44–8. 10.1016/j.radonc.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 13.Rieken S, Habermehl D, Giesel FL, et al. Analysis of FET-PET imaging for target volume definition in patients with gliomas treated with Conformal radiotherapy. Radiother Oncol 2013;109:487–92. 10.1016/j.radonc.2013.06.043 [DOI] [PubMed] [Google Scholar]
- 14.Weber DC, Casanova N, Zilli T, et al. Recurrence pattern after [(18)F]Fluoroethyltyrosine-positron emission tomography-guided radiotherapy for high-grade glioma: a prospective study. Radiother Oncol 2009;93:586–92. 10.1016/j.radonc.2009.08.043 [DOI] [PubMed] [Google Scholar]
- 15.Delgado-López PD, Riñones-Mena E, Corrales-García EM. Treatment-related changes in glioblastoma: a review on the controversies in response assessment criteria and the concepts of true progression, Pseudoprogression, Pseudoresponse and Radionecrosis. Clin Transl Oncol 2018;20:939–53. 10.1007/s12094-017-1816-x [DOI] [PubMed] [Google Scholar]
- 16.Galldiks N, Dunkl V, Stoffels G, et al. Diagnosis of Pseudoprogression in patients with glioblastoma using O-(2-[18F]Fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging 2015;42:685–95. 10.1007/s00259-014-2959-4 [DOI] [PubMed] [Google Scholar]
- 17.Kebir S, Fimmers R, Galldiks N, et al. Late Pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]Fluoroethyl)-L-tyrosine PET. Clin Cancer Res 2016;22:2190–6. 10.1158/1078-0432.CCR-15-1334 [DOI] [PubMed] [Google Scholar]
- 18.Lohmann P, Elahmadawy MA, Gutsche R, et al. FET PET Radiomics for differentiating Pseudoprogression from early tumor progression in glioma patients post-Chemoradiation. Cancers 2020;12:3835. 10.3390/cancers12123835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandes AA, Tosoni A, Franceschi E, et al. Recurrence pattern after Temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol 2009;27:1275–9. 10.1200/JCO.2008.19.4969 [DOI] [PubMed] [Google Scholar]
- 20.Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of Pseudoprogression in malignant gliomas. Lancet Oncol 2008;9:453–61. 10.1016/S1470-2045(08)70125-6 [DOI] [PubMed] [Google Scholar]
- 21.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963–72. 10.1200/JCO.2009.26.3541 [DOI] [PubMed] [Google Scholar]
- 22.Boxerman JL, Quarles CC, Hu LS, et al. Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro Oncol 2020;22:1262–75. 10.1093/neuonc/noaa141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 2017;14:307–20. 10.1007/s13311-016-0507-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer GD, Brucker DP, Stoffels G, et al. 18)F-FET PET imaging in differentiating glioma progression from treatment-related changes: A single-center experience. J Nucl Med 2020;61:505–11. 10.2967/jnumed.119.234757 [DOI] [PubMed] [Google Scholar]
- 25.Pyka T, Hiob D, Preibisch C, et al. Diagnosis of glioma recurrence using Multiparametric dynamic 18F-Fluoroethyl-tyrosine PET-MRI. Eur J Radiol 2018;103:32–7. 10.1016/j.ejrad.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Zheng J, Xu W, et al. Accuracy of 18F-FDOPA positron emission tomography and 18F-FET positron emission tomography for differentiating radiation necrosis from brain tumor recurrence. World Neurosurg 2018;114:e1211–24. 10.1016/j.wneu.2018.03.179 [DOI] [PubMed] [Google Scholar]
- 27.Steidl E, Langen K-J, Hmeidan SA, et al. Sequential implementation of DSC-MR perfusion and dynamic [18F]FET PET allows efficient differentiation of glioma progression from treatment-related changes. Eur J Nucl Med Mol Imaging 2021;48:1956–65. 10.1007/s00259-020-05114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundemann M, Munck Af Rosenschöld P, Muhic A, et al. Feasibility of multi-parametric PET and MRI for prediction of tumour recurrence in patients with glioblastoma. Eur J Nucl Med Mol Imaging 2019;46:603–13. 10.1007/s00259-018-4180-3 [DOI] [PubMed] [Google Scholar]
- 29.Rosen J, Ceccon G, Bauer EK, et al. Cost effectiveness of (18)F-FET PET for early treatment response assessment in glioma patients after adjuvant Temozolomide chemotherapy. J Nucl Med 2022;63:1677–82. 10.2967/jnumed.122.263790 [DOI] [PubMed] [Google Scholar]
- 30.Lau EWF, Drummond KJ, Ware RE, et al. Comparative PET study using F-18 FET and F-18 FDG for the evaluation of patients with suspected brain tumour. J Clin Neurosci 2010;17:43–9. 10.1016/j.jocn.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 31.Galldiks N, Stoffels G, Filss CP, et al. Role of O-(2-(18)F-Fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med 2012;53:1367–74. 10.2967/jnumed.112.103325 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-071327supp001.pdf (192.4KB, pdf)
bmjopen-2022-071327supp002.pdf (346.8KB, pdf)