Abstract
Introduction
The purpose of TheShinISS-Vax|Flu study is to examine the association between influenza vaccines and adverse events requiring hospital admission or emergency care during the influenza vaccination campaigns 2021/2022 and 2022/2023 in Italy.
Methods and analysis
This is a Self-Controlled Case Series multiregional study using linked routinely collected data from regional healthcare databases of the participating regions. Study participants will be persons aged ≥6 months, unvaccinated or who have received influenza vaccine during the influenza vaccination campaigns in the seasons 2021/2022 and 2022/2023 in Italy and who have experienced the outcome of interest for the first time during the study period (1 September 2021–30 June 2022 and 1 September 2022–30 June 2023 for the first and second vaccination campaigns, respectively). Risk periods will be specifically defined for each outcome and further subdivided into periods of 7 days. The exposures will be the first or second dose of the influenza vaccines administered during the two vaccination campaigns. Statistical analysis will be conducted separately for the data of the two campaigns. Exposure risk period will be compared with baseline risk period defined as any time of observation out of the risk periods. The modified SCCS method will be applied to handle event-dependent exposure and mortality and fitted using unbiased estimating equations to estimate relative incidences and excess of cases per 100 000 vaccinated by dose, age, sex and type of vaccine. Calendar period will be included as time-varying confounder in the model, where appropriate.
Ethics and dissemination
The study received the approval from the National ethics committee for clinical trials of public research bodies and other national public institutions (PRE BIO CE n.0036723, 23/09/2022). Results will be published in peer-reviewed journals and reports in accordance with the publication policies of the Italian National Institute of Health and of the Italian Medicines Agency.
Keywords: epidemiology, public health, statistics & research methods
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Large sample size and long follow-up for detecting rare adverse events of influenza vaccines.
An R-based statistical tool, TheShinISS, enabled distributed analyses on real-world data to overcome privacy issues.
Use of modified Self-Controlled Case Series method to handle event-dependent exposure and mortality, and to control for time-independent confounders.
It was not possible to validate outcomes through clinical records review.
Only serious adverse events requiring emergency care or hospital admission were included.
Introduction
Seasonal influenza is a viral respiratory disease in human, caused by A or B virus. Influenza epidemics occur annually worldwide with substantial burden of disease.
Influenza vaccination campaigns remain an important public health intervention to reduce influenza viruses’ circulation during epidemic and pandemic. They are organised annually since the waning of immunity and the yearly changes in viral antigenic configuration requires annual updating of the vaccines.1
Vaccines are rigorously evaluated in preregistrative randomised clinical trials, but their wide scale introduction may provide the opportunity to identify rare adverse events that can be undetected in clinical trials. Therefore, it is essential to have continuous monitoring of adverse events potentially associated with influenza vaccines, using both passive and active surveillance systems, as a key element of any vaccination campaign.2
New safety concerns may arise since composition of influenza vaccines changes yearly according to WHO recommendations (https://www.who.int/teams/global-influenza-programme/vaccines/who-recommendations).
The Italian National Institute of Health (Istituto Superiore di Sanità-ISS) and the Italian Medicines Agency (Agenzia Italiana del Farmaco-AIFA) coordinated TheShinISS-Vax|Flu study, a post-marketing active surveillance of the adverse events following immunisation of influenza vaccines in place in Italy. This is a collaborative project which aims to cover a large population using linked healthcare databases of the participating Italian regions.
ISS has a long history of monitoring the safety of vaccines using ad hoc studies to collect and analyse data, also involving networks of local health authorities, general practitioners and paediatricians.3–6 These past experiences have offered the possibility to gain insights into areas where the existing surveillance system can be strengthened, and the development of large-linked database monitoring system has resulted a major challenge.
ISS has pioneered a new model to conduct active surveillances of influenza and COVID-19 vaccines in Italy. This model applies a distributed analysis framework using TheShinISS, an R-based open-source statistical tool that locally processes data collected and updated periodically from regional healthcare databases according to a study-tailored, Common Data Model (CDM).7 The advantages of this model consist of: the inclusion of a large population; the timely access and ease of regional data sharing with a reduction of workload of health professionals; and the enhancement in the quality control of the regional healthcare data. Recently, multiregional studies have been conducted by TheShinISS using regional routinely collected and linked health data from vaccination registries, hospital discharges and emergency care admissions and pharmacy claims databases.8 9
In Italy, the Ministry of Health annually releases recommendations for the prevention and control of influenza. The recommendations on 2021/2022 and 2022/2023 influenza vaccination campaign10 11 have expanded the vaccine eligible population comparing with the previous vaccination campaigns, also considering the challenges of the COVID-19 pandemic scenario.
The purpose of this study is to examine the association between rare, serious adverse events and adverse events of special interest and influenza vaccines during the 2021/2022 and 2022/2023 influenza vaccination campaigns in Italy.
Methods and analysis
Study population
Study population will include persons of ≥6 months of age of seven Italian regions (Piemonte, Friuli Venezia Giulia, Emilia-Romagna and Toscana of Northen Italy; Lazio of Central Italy; Puglia and Campania of Southern Italy), unvaccinated or who received influenza vaccine during the 2021/2022 and 2022/2023 influenza vaccination campaigns (from October to March), and who were admitted to emergency care or hospital for at least one of the outcomes of interest from the beginning of the vaccination campaigns to the end of the study periods. Participation in the study of the Italian regions is voluntary. AIFA invited all the Italian regions to participate in the study but participation depended on the availability of the healthcare databases, data update and personnel to be dedicated to the study.
Study period
For the vaccination campaign 2021/2022: 1 September 2021 –30 June 2022.
For the vaccination campaign 2022/2023: 1 September 2022– 30 June 2023.
Type of vaccine studied
All influenza vaccines were administered to the study population in the seven participating Italian Regions during the two campaigns, in accordance with the recommendations of the Ministry of Health10 11 and the provision of AIFA decision.12 13
Data sources
The following healthcare databases will be used:
Vaccination registry to identify influenza vaccination exposure and exposure to other vaccines which were administered from 1 September 2021 and 1 September 2022 for the first and second influenza vaccination campaigns, respectively, to the last data update.
Population registry to identify information on age, sex, date of registration and deregistration (where applicable) in the regional healthcare system, and vital status (causes of death are not recorded in this registry) to the last data update.
Pharmacy claims database to characterise the study population by obtaining information on the use of drugs (coded with Anatomical Therapeutic Chemical code) during the periods preceding the two influenza vaccination programmes (from 1 September 2020 and 1 September 2021 for the first and second influenza campaign, respectively, to the last data update).
Hospital discharges database to identify the outcomes of interest prevaccination and postvaccination (from 1 September 2021 and 1 September 2022 for the first and the second influenza campaigns, respectively, to the last data update) and also to obtain information on the comorbidities of the study population in the 5 years preceding influenza vaccination (from 1 October 2016 and 1 October 2017 for the first and the second influenza campaigns, respectively, to the last data update) coded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM).
Admissions to the emergency care database to identify the outcomes of interest (from 1 September 2021 and 1 September 2022 for the first and the second influenza campaign, respectively, to the last data update) coded with ICD-9-CM.
Exemptions from healthcare service copayment database to obtain information on comorbidities of the study population (to the last data update).
Study design
TheShinISS-Vax|Influenza study will use a Self-Controlled Case Series (SCCS) design.14–19
The SCCS design is best suited to evaluate the safety of vaccines and other medicinal products when the relationship between transient exposures and acute events is investigated. This method requires only data on individuals, vaccinated and unvaccinated, who have experienced an event (cases). Estimation is within individuals and consequently, any time-invariant unknown or unmeasured potential confounders are controlled for.
Therefore, it represents a valid epidemiological design alternative to the cohort and case–control study in the research on vaccine safety, particularly in situations where it is difficult to identify an appropriate comparison group, for example when vaccinated population has different characteristics from unvaccinated or most of the population has received the vaccine.
The SCCS model was originally developed to investigate the association between vaccines and adverse events with the key assumption that the occurrence of an event does not influence postevent exposures, for example by delaying or even cancelling the subsequent exposures. This assumption may be violated for vaccine safety studies when the occurrence of the outcome of interest is a contra-indication to vaccination. To handle event-dependent exposures a modified SCCS method has been developed.17–19
In the modified SCCS model for event-dependent exposures, unlike the standard model, it is essential to include unvaccinated cases. This is because the absence of vaccination may indicate cancelled vaccination that occurs more often for events that occur earlier. As a result, the absence of vaccination can be informative on the timing of the event, and excluding unvaccinated cases may introduce bias.19
TheShinISS-Vax|Flu is a multiregional study using routinely collected data from regional healthcare databases/registries linked in each region at individual level. The study applies TheShinISS, the R-based open-source statistical tool which was developed by the researchers of ISS.7 The tool is currently maintained and customised by the ‘TheShinISS Network’ that includes researchers from ISS, the Department of Epidemiology of the Lazio Regional Health Service and the Universities of Verona and Messina. TheShinISS allows to carry out distributed analyses in multidatabase pharmacoepidemiological studies according to a CDM strategy which is study tailored.20 It has been already employed in large real-world studies with different epidemiological designs.8 9 21–25
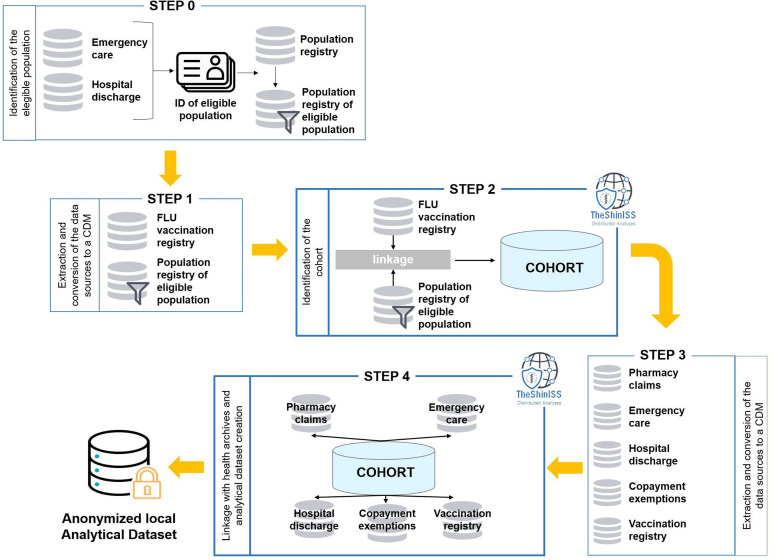
Going into further detail, figure 1 illustrates the relational scheme of the study, including all the steps, which use TheShinISS to locally process healthcare databases structured according to a CDM: Step 0—identification of the eligible population from the hospital discharges database and admissions to the emergency care database; Step 1—extraction and preparation of the CDM of the vaccination registry and the population registry related to the eligible population identified in Step 0; Step 2—identification of the study cohort by vaccination status, data quality control and descriptive analysis (by execution of TheShinISS); Step 3—extraction and conversion of healthcare databases, and preparation of the CDM related to the cohort; Step 4—execution of TheShinISS on CDM to perform: data quality control, linkage of the cohort with healthcare databases, anonymisation, aggregation and creation of a minimal set of exposure and outcome variables, and specific covariates of interest for the study, which will constitute the local anonymised analytical datasets.
Figure 1.
Diagram showing the data flow when using TheShinISS to locally process healthcare data structured according to a CDM. *Vaccination registry related to those registered in the regional population. CDM, Common Data Model.
Definition of the study outcomes
We will focus on 13 different outcomes considering the guidelines issued by AIFA26 and hypothetical concerns regarding analogous vaccines or complications associated with the disease itself.
The outcomes will be identified from the diagnosis of emergency care admission or hospital discharge using ICD-9-CM code.
The outcomes of interest will be ascertained during the study period. Each case will be followed up from the beginning of the vaccination campaign (1 September 2021 and 1 September 2022 for the first and second vaccination campaigns, respectively) to the date of last regional health data update, for each individual alive; conversely, when a case dies, the end of the observation period will be defined according to the SCCS methodology to deal with mortality.19
Cases will be defined as those patients who have experienced the outcome for the first time during the study period (incident cases). This means that patients who have an emergency care admission or a hospital discharge, for the same outcome, within the 5 years prior to the start of the study period (look back) will be excluded. A time window of 5 years provides a sufficiently look back period to selectively identify incident cases. Deaths for any causes will be also considered.
Table 1 lists the selected adverse events which are potentially associated with influenza vaccination and the corresponding ICD-9-CM codes and the risk period, which are derived from the Brighton Collaboration26 and the AIFA report.27 The list will be updated in case of emerging signals on new adverse events potentially associated with influenza immunisation and the participant Regions will be requested to provide further specific data.
Table 1.
Definition of the adverse events potentially associated with influenza vaccines
| Adverse events potentially associated with influenza vaccines | ICD-9-CM | Risk period (days after the vaccination) |
| Bell’s palsy | 351.0 | 60 |
| Acute hepatitis | 570; 572.2; 573.3; 573.9 | 60 |
| Guillain-Barré syndrome | 357.0; 357.8; 357.9 | 42 |
| Encephalitis and encephalomyelitis | 323; 348.3 | 42 |
| Thrombocytopaenia | 283.0; 286.5; 287 (excl. 287.39); V83.01; V83.02 | 42 |
| Vasculitis | 136.1; 273.2; 287.0; 446.0; 446.2; 446.4; 446.5; 446.6; 446.7; 709.1 | 42 |
| Demyelinating diseases | 323.81; 340; 341.0; 341.1; 341.2; 341.9; 377.3; 377.49; 377.9; 725 | 42 |
| Convulsions | 780.39 | 14 |
| Anaphylaxis | 995.0; 999.4 | 2 |
| Neuritis (brachial neuritis, neuralgic amyotrophy) | 353.5; 723.4 | 28 |
| Narcolepsy | 347 | 42 |
| Swelling of limb | 729.81 | – |
| Syncope and collapse | 780.2 | – |
ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
Definition of comorbidities and drugs
Table 2 reports codes of hospital discharges, drugs use and exemptions derived from the local health care databases/registries which are necessary for definition of comorbidities. Table 3 shows codes of definition of drug use.
Table 2.
Definition of comorbidities
| Hospital discharge code: ICD-9-CM (in the last 5 years) | Exemptions code | Pharmacy claim code: ATC (in the last 12 months) | |
| Chronic pulmonary disease | 480–488; 491; 495; 518.81–518.84 | 024 | J05AH |
| Chronic obstructive pulmonary disease* | 490; 492; 494; 496 | 057 | R03 |
| Asthma* | 493 | 007 | – |
| Cardiovascular and cerebrovascular diseases | 390–398; 406–459 | 002; 021; 0A02; 0B02; 0C02; 036 | B01AC; C01B; C01DA; C08DA; C08DB |
| Hypertension | 401–405 | 031; 0A31 | C02; C03; C07; C08; C09 |
| Chronic kidney diseases | 580; 582–585; 593; 753.12–753.14 | 023; 022; 061; 062 | – |
| Dementia/Alzheimer’s disease | 290; 294.1; 331.2 | 011; 029 | N06DA; N06DX |
| Diabetes | 250 | 013 | A10 |
| Rheumatic diseases | 446.5; 696; 710; 714; 720; 725 | 006; 028; 030; 045; 054; 067 | L04 |
| Haematological disease | 280–289 (excl. 285.1) | 003 | B01AA; B01AB; B01AE; B01AF; B01AX; B02BD; B03 |
| Neurological diseases | 238.7; 296.3; 311; 332; 345; 340; 348.39 | 017; 038; 044; 046 | N03A; N04B; N05A; N06A |
| Neoplasms | 140–209; V10 | 048 | L01 |
| Metabolic disorders | 272; 278 | 025 | C10 |
| Moderate/severe hepatopathy | 456.0–456.2; 571–572; 573.0 | 008; 016 | – |
| Cystic fibrosis | 277.0 | 018 | R07AX |
| Ulcer disease | 531; 532; 533 | A02B | |
| Colitis | 555; 556 | 009 | – |
| HIV | 042 | 020 | J05AE; J05AF; J05AG; J05AR |
| Infections* | 053; 599.0; 010–018; 031; 078.5; 052–054; 136.3; 117.5 | 055 | J01; J02; J04; J05 (excl. J05AE; J05AF; J05AG; J05AH; J05AR) |
*ICD-9-CM in 365 days.
ATC, Anatomical Therapeutic Chemical; HIV, Human Immunodeficiency Virus; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
Table 3.
Definition of drug use
| Pharmacy claims code: ATC (in the last 12 months) | |
| Other vaccines | J07 (excluding COVID-19 and J07BX03 influenza vaccines J07BB) |
| Anti-COVID-19 vaccines | J07BX03 |
| Glucocorticoids | H02AB |
| Non-steroidal anti-inflammatory drugs | M01A |
| Estroprogestinics | G03 |
ATC, Anatomical Therapeutic Chemical.
Definition of the exposure
The exposure variables will include the first or second dose of the influenza vaccines available in Italy during the vaccination campaigns 2021/2022 and 2022/2023.
The influenza vaccines will be categorised according to the available type of vaccines during the two vaccination campaigns: quadrivalent vaccine (egg-based and cell culture-based influenza vaccine), quadrivalent and trivalent with MF59 adjuvant vaccine, live attenuated influenza vaccine (the nasal spray influenza vaccine).
For each outcome of interest, we will define specific risk periods (table 1) which will be further subdivided into three subrisks periods.
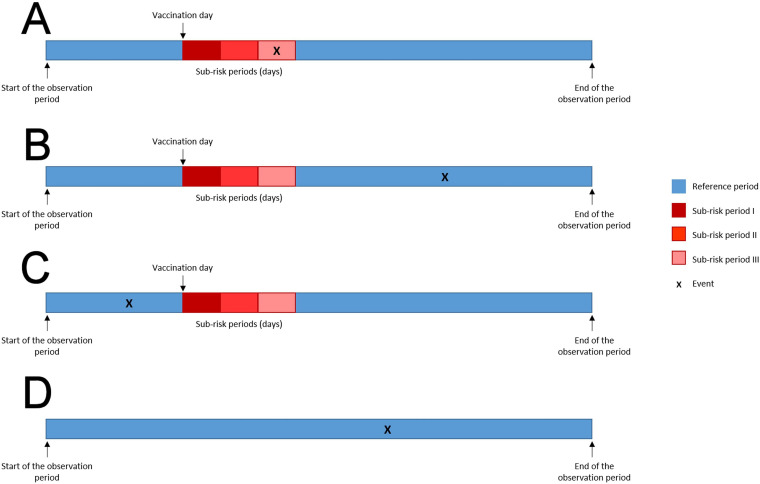
All remaining time within the individual observation period will define the no-exposure period for each outcome of interest, and will represent the baseline period to which the exposure risk period will be compared (figure 2).
Figure 2.
Schematic presentation of Self-Controlled Case Series method for hypothetical subjects included in the study. (A) Events occurring in vaccinated subjects during the risk period. (B) Events occurring in the reference period after vaccination. (C) Events occurring in the reference period before vaccination. (D) Events occurring in unvaccinated subjects.
Methods of analysis
Statistical analysis will be conducted separately for the data of the two vaccination programmes 2021/2022 and 2022/2023. Where appropriate, a pooled analysis will be conducted.
We will describe the characteristics of the cases as frequencies, percentages, medians and IQRs, in terms of age, sex, geographical areas, Charlson Index (based on hospitalisation in the 5 years prior vaccination), length of hospitalisation, number of hospital admissions for any causes in the 5 years prior to vaccination, number of drug prescriptions in the year prior to vaccination, and comorbidities.
We will describe the data extraction process in a flowchart reporting number of individuals at each stage of the process, for example those individuals potentially eligible, included, analysed and those excluded with reasons, indicating also numbers of individuals with missing or incoherent observations.
We will use the SCCS methodology, modified to event-dependent exposures,14–19 to examine the association between influenza vaccine and each outcome of interest in individuals aged ≥6 months during the observation period. The modified SCCS model addresses situations where the occurrence of an event affects the timing or the occurrence of subsequent exposures. It introduces a counterfactual scenario in which no exposure can occur after occurrence of an event.17–19
If patients died, the end of the observation period will be defined according to what is proposed by the modified SCCS methodology to handle mortality.19
The SCCS model will be fitted using unbiased estimating equations to estimate relative incidences (RIs) and their 95% CIs in the predefined risk periods compared with the baseline periods. Unbiased estimating equations theory generalises likelihood theory to estimate the parameters of interest and it is used when the likelihood function is difficult to obtain. Precision of the estimates can be calculated similarly to the methods of the maximum-likelihood estimate.28
To account for possible seasonal variation in the baseline incidence of each outcome, temporal effects will be included in the model as time-varying covariate.
We will estimate, for each outcome of interest, the excess of cases per 100 000 vaccinated (EC) as the ratio of the number of excess cases due to the vaccine {[(RI − 1)/RI] × no. events in the risks period} divided by the number of vaccinated × 1 00 00029; while the 95% CI 95% calculated by non-parametric bootstrapping methodology (10 000 replications).
Subgroup analyses will be carried out by age group (<60 and ≥60 years), sex, and type of vaccine for each outcome of interest.
Several sensitivity analyses will be performed to assess the assumptions of the SCCS model regarding the event-dependent exposure and observation period, the seasonality and the pre-specification of risk periods. Moreover, we will carry out analyses on cases receiving only influenza vaccines, excluding those with both influenza and COVID-19 vaccines. This restriction will also be applied in cases where other vaccines are received concurrently.
Statistical analyses will be performed using R (R Core team 2021) with SCCS package30 and STATA software.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Time schedule
Time schedule of the study is presented in online supplemental table 1.
bmjopen-2022-069858supp001.pdf (222.5KB, pdf)
Ethics and dissemination
The study received the approval from the National ethics committee for clinical trials of public research bodies and other national public institutions (PRE BIO CE n.0036723, 23/09/2022). Results will be published in peer-reviewed journals and reports in accordance with the publication policies of the Italian National Institute of Health and of the Italian Medicines Agency.
Adverse reaction management
The adverse reaction reporting is not required according to the Guideline on Good Pharmacovigilance Practices (GVP) VI rev. 2 (VI.C.1.2.1.2. Non-interventional postauthorisation studies with a design based on secondary use of data).31
Supplementary Material
Acknowledgments
The authors thank Giuseppe Marano and Maria Cutillo of the Pharmacoepidemiology Unit of the Italian National Institute of Health who participated in the discussion and review of the study protocol. Research team of TheShinISS-Vax|Flu study: Italian National Institute of Health: Stefania Spila Alegiani, Maria Cutillo, Roberto Da Cas, Giuseppe Marano, Marco Massari, Flavia Mayer, Francesca Menniti Ippolito, Cristina Morciano (National Center for Drug Research and Evaluation). Italian Medicines Agency: Anna Rosa Marra, Patrizia Felicetti, Pasquale Marchione (Signals Management Office); Fiorella Petronzelli (Pharmacovigilance Office). TheShinISS network: Marco Massari, Stefania Spila Alegiani, Maria Cutillo, Roberto Da Cas, Flavia Mayer, Cristina Morciano (Italian National Institute of Health, Rome); Gianluca Trifirò, Luca L’Abbate, Salvatore Crisafulli, Ylenia Ingrasciotta, Nicoletta Luxi (University of Verona); Valentina Ientile (University of Messina); Valeria Belleudi, Alessandro De Rosa, Marco Finocchietti, Sara Lopes (Department of Epidemiology of the Lazio Regional Health Service, Rome); Andrea Fontana (IRCCS Casa del Sollievo e della Sofferenza, San Giovanni Rotondo).
Footnotes
SSA and CM contributed equally.
Contributors: SSA, CM, FM-I, RDC, PF, PM, FP, ARM and MM were involved in conception and study design. SSA, CM and MM were involved in drafting of the article. FMI, RDC, PF, PM, FP and ARM were involved in critical revision of the article for important intellectual content. All the authors were involved in final approval of the article. SSA, MM and CM provided statistical expertise.
Funding: This work was supported by the Italian Medicines Agency in the framework of the collaboration agreement 2022-2025 between Italian Medicines Agency and Italian National Institute of Health (FARV-AVPM n. 8). The funder had no role in study design, nor in writing and submission of the protocol for publication.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Uyeki TM, Hui DS, Zambon M, et al. Influenza. Lancet 2022;400:693–706. 10.1016/S0140-6736(22)00982-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Interim guidance on enhanced safety surveillance for seasonal influenza vaccines in the EU, EMA/PRAC/222346/2014. n.d. Available: https://www.ema.europa.eu/en/documents/scientific-guideline/interim-guidance-enhanced-safety-surveillance-seasonal-influenza-vaccines-eu_en.pdf
- 3.Spila-Alegiani S, Salmaso S, Rota MC, et al. Reactogenicity in the elderly of nine commercial influenza vaccines: results from the Italian SVEVA study. study for the evaluation of adverse events of influenza vaccination. Vaccine 1999;17:1898–904. 10.1016/s0264-410x(98)00467-8 [DOI] [PubMed] [Google Scholar]
- 4.Spila Alegiani S, Alfonsi V, Bella A, et al. Vaccino Antinfluenzale Stagionale in Italia: Misurare L’Efficacia Sul Campo E La Sicurezza. Stagione 2015-2016. (Rapporti ISTISAN 17/19). Roma: Istituto Superiore di Sanità, 2017. [Google Scholar]
- 5.Spila Alegiani S, Alfonsi V, Appelgren EC, et al. Active surveillance for safety monitoring of seasonal influenza vaccines in Italy, 2015/2016 season. BMC Public Health 2018;18:1401. 10.1186/s12889-018-6260-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galeotti F, Massari M, D’Alessandro R, et al. Risk of Guillain-Barré syndrome after 2010-2011 influenza vaccination. Eur J Epidemiol 2013;28:433–44. 10.1007/s10654-013-9797-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massari M, Spila Alegiani S, Da R, et al. “Theshiniss: UN Applicativo open-source per La Conduzione Di Analisi Distribuite in Studi Di Farmacoepidemiologia Di Tipo multi-database ("Theshiniss": an open-source tool for conducting distributed analyses within Pharmacoepidemiological multi-database studies)”. Boll Epidemiol Naz 2020;1:39–45. 10.53225/BEN_006 [DOI] [Google Scholar]
- 8.Spila Alegiani S, Morciano C, Belleudi V, et al. Valutazione Postmarketing Della Sicurezza del Vaccino Antinfluenzale Durante La campagna Di Vaccinazione Antinfluenzale 2020-2021 in Italia: Uno studio self-controlled case series Sulla Sindrome Di Guillain-Barré (post-marketing safety evaluation of flu vaccine during the 2020-2021 flu vaccination campaign in Italy: a self-controlled case series study of Guillain-Barré syndrome). Boll Epidemiol Naz 2022;3:1–9. 10.53225/BEN_042 [DOI] [Google Scholar]
- 9.Massari M, Spila Alegiani S, Morciano C, et al. Post-marketing active surveillance of myocarditis and Pericarditis following vaccination with COVID-19 mRNA vaccines in persons aged 12-39 years in Italy: a multi-database, self-controlled case series study. PLoS Med 2022;19:e1004056. 10.1371/journal.pmed.1004056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Circolare del Ministero Della salute Prevenzione E Controllo Dell’Influenza: Raccomandazioni per La Stagione 2021-2022. n.d. Available: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=79647&parte=1%20&serie=null
- 11.Circolare del Ministero Della salute Prevenzione E Controllo Dell’Influenza: Raccomandazioni per La Stagione 2022-2023. n.d. Available: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2022&codLeg=87997&parte=1%20&serie=null
- 12.Determina Dell’AIFA che Autorizza L’Aggiornamento, per La Stagione 2021-2021, Della Composizione Dei Vaccini Influenzali Autorizzati Secondo Procedura Di Mutuo Riconoscimento E Decentrata (Determina AAM/PPA N. 654/2021). n.d. Available: https://www.aifa.gov.it/documents/20142/1563396/Determinazione_AIFA_654-2021_GU.pdf
- 13.Determina Dell’AIFA che Autorizza L’Aggiornamento, per La Stagione 2022-2023, Della Composizione Dei Vaccini Influenzali Autorizzati Secondo Procedura Di Mutuo Riconoscimento E Decentrata (Determina AAM/PPA N. 652/2022). n.d. Available: https://www.aifa.gov.it/documents/20142/1754982/Det_AIFA_652-2022.pdf
- 14.Whitaker HJ, Farrington CP, Spiessens B, et al. Tutorial in bio-Statistics: the self-controlled case series method. Stat Med 2006;25:1768–97. 10.1002/sim.2302 [DOI] [PubMed] [Google Scholar]
- 15.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard Epidemiological study designs. BMJ 2016;354:i4515. 10.1136/bmj.i4515 [DOI] [PubMed] [Google Scholar]
- 16.Weldeselassie YG, Whitaker HJ, Farrington CP. Use of the self-controlled case-series method in vaccine safety studies: review and recommendations for best practice. Epidemiol Infect 2011;139:1805–17. 10.1017/S0950268811001531 [DOI] [PubMed] [Google Scholar]
- 17.Farrington CP, Whitaker HJ, Hocine MN. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics 2009;10:3–16. 10.1093/biostatistics/kxn013 [DOI] [PubMed] [Google Scholar]
- 18.Farrington CP, Whitaker H, Weldeselassie YG. Self-controlled case series studies. In: A modelling guide with R. Boca Raton, Florida : CRC Press, 2018: Chapman and Hall/CRC Press, 2018. 10.1201/9780429491313 [DOI] [Google Scholar]
- 19.Ghebremichael-Weldeselassie Y, Jabagi MJ, Botton J, et al. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med 2022;41:1735–50. 10.1002/sim.9325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gini R, Sturkenboom MCJ, Sultana J, et al. Different strategies to execute multi-database studies for medicines surveillance in real-world setting: a reflection on the European model. Clin Pharmacol Ther 2020;108:228–35. 10.1002/cpt.1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trifirò G, Massari M, Da Cas R, et al. Renin–angiotensin–aldosterone system inhibitors and risk of death in patients hospitalised with COVID-19: A retrospective Italian cohort study of 43,000 patients. Drug Saf 2020;43:1297–308. 10.1007/s40264-020-00994-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spila Alegiani S, Crisafulli S, Giorgi Rossi P, et al. Risk of COVID-19 hospitalization and mortality in rheumatic patients treated with hydroxychloroquine or other conventional Dmards in Italy. Rheumatology (Oxford) 2021;60:SI25–36. 10.1093/rheumatology/keab348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massari M, Spila-Alegiani S, Fabiani M, et al. Association of influenza vaccination and prognosis in patients testing positive to SARS-COV-2 SWAB test: a large-scale Italian multi-database cohort study. Vaccines (Basel) 2021;9:716. 10.3390/vaccines9070716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trifirò G, Isgrò V, Ingrasciotta Y, et al. Large-scale post-marketing surveillance of biological drugs for immune-mediated inflammatory diseases through an Italian distributed multi-database Healthcare network: the VALORE project. BioDrugs 2021;35:749–64. 10.1007/s40259-021-00498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belleudi V, Rosa AC, Finocchietti M, et al. An Italian Multicentre distributed data research network to study the use, effectiveness, and safety of immunosuppressive drugs in transplant patients: framework and perspectives of the CESIT project. Front Pharmacol 2022;13:959267. 10.3389/fphar.2022.959267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruppo Di Lavoro Sull’Analisi Dei Segnali DA Vaccino, Agenzia Italiana del Farmaco. Guida Alla Valutazione delle Reazioni Avverse Osservabili Dopo Vaccinazione. n.d. Available: https://www.aifa.gov.it/sites/default/files/Guida_valutazione_reazioni_avverse_osservabili_dopo_vaccinazione_2.pdf
- 27.Brighton collaboration case definitions. n.d. Available: https://brightoncollaboration.us/category/pubs-tools/case-definitions/
- 28.Cox DR. Unbiased estimating equations derived from statistics that are functions of a parameter. Biometrika 1993;80:905–9. 10.1093/biomet/80.4.905 [DOI] [Google Scholar]
- 29.Wilson K, Hawken S. Drug safety studies and measures of effect using the self-controlled case series design. Pharmacoepidemiol Drug Saf 2013;22:108–10. 10.1002/pds.3337 [DOI] [PubMed] [Google Scholar]
- 30.Weldeselassie YJ, Whitaker H, Farrington P. SCCS: the self-controlled case series method [R. package version 1.5]. 2021. Available: https://CRAN.R-project.org/package=SCCS
- 31.European Medicine Agency (EMA) . Guideline on good Pharmacovigilance practices (GVP) – 28 July 2017, EMA/873138/2011. n.d. Available: https://www.ema.europa.eu/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-069858supp001.pdf (222.5KB, pdf)