Abstract
Objectives
The prognosis of invasive micropapillary carcinoma (IMPC) of the breast is determined by many clinicopathological factors. This study aims to identify prognostic factors and develop reliable nomogram to predict the overall survival (OS) in patients with IMPC.
Design
Log-rank test and Cox proportional hazards analysis were used to identify variables and construct a nomogram based on the training cohort. C-index and calibration curves were performed to evaluate the performance of the model in the training cohort and validation cohorts.
Setting
We collected the patient data from the Surveillance, Epidemiology and End Results (SEER) database. This database holds data related to the cancer incidence from 18 population-based cancer registries in the USA.
Participants
The SEER database was used to screen 754 eligible patients as the study cohort. The whole cohort was randomly divided into a training cohort (n=377) and a validation cohort (n=377).
Results
Age at diagnosis, hormone receptors, number of positive regional lymph nodes and clinical stage were independent prognostic factors for patients with IMPC. The calibration curves presented excellent consistency between the actual and nomogram-predict survival probabilities in the training and validation cohorts. The C-index values of the nomogram were 0.794 and 0.774 for OS in the training and validation cohorts, respectively.
Conclusions
The novel nomogram provides new insights of the risk of each prognostic factor and can assist doctors in predicting the 1-year, 3-year and 5-year OS in patients with IMPC.
Keywords: Breast tumours, Epidemiology, Adult oncology
Strengths and limitations of this study.
The data was downloaded from the Surveillance, Epidemiology and End Results database, which provides a representative population-based cohort.
Prognostic factors were determined by univariate and multivariate Cox proportional hazards regression analyses and used to develop nomograms to predict 1-year, 3-year and 5-year overall survival of patients with invasive micropapillary carcinoma.
We used the Harrell concordance index (C-index), the area under the receiver operating characteristic curve and the calibration curve to assess the discrimination of the nomograms.
This research was a retrospectively large-sample study, the casual basis of this research was difficult to conclude.
Introduction
Breast cancer is the most prevalent cancer in women and one of the most rapidly increasing human malignancies worldwide. In the USA, the number of newly estimated diagnosed cases and deaths were 290 560 and 43 780, respectively, in 2022.1 The invasive micropapillary carcinoma (IMPC) of the breast, which characterised by aggressive lymphovascular invasion and metastasis, accounting for less than 2% of all invasive breast cancers.2–5 Hormonal and HER-2 positivity in IMPC of the breast is also commoner when compared with other non-specific type (NST) carcinomas. IMPC occurs either as a pure form or more often as a component of mixed NST carcinoma.6–8 This cancer type has varying classifications and has no available standardised treatment guidelines.
Considering the rarity of this disease, the conduct of clinical trials to evaluate prognostic factors and optimal treatments is difficult. A few studies have discussed the potential pathological predictors of survival for IMPC.5 9–16 However, the discrepancies caused by the limited IMPC cases in the reported prevalence of overall survival (OS) and significant clinicopathological factors were difficult to exclude.
A nomogram, a simple visual prediction tool based on a prognostic model that includes related clinicopathological factors, allows doctors to access the probabilities of the clinical outcomes of particular individuals.17 18 Moreover, compared with the American Joint Committee on Cancer (AJCC) tumour, node, metastases (TNM) stage system, nomograms can provide a more precise estimation of prognosis for some malignancies19 20 and help clinicians to make decisions in complex situations in an alternative or novel standard.21–23
In this study, we investigated the Surveillance, Epidemiology and End Results (SEER) database to evaluate the prognostic clinicopathological indicators on OS in patients with IMPC. A novel nomogram was constructed to predict the prognosis for patients with IMPC.
Methods
Study cohorts
The data for this study were obtained from 18 registries of the SEER programme, and 1480 patients diagnosed with IMPC of the breast between 1973 and 2013 were included. The personal information from the SEER database is untracked and unavailable. The inclusion criteria for the data screening were as follows: (1) female patients who accepted surgery treatment; (2) age older than 18 years; (3) diagnosis confirmed by histopathological report; (4) IMPC as the first and primary cancer determined by international rules; (5) survival data with complete and available dates and more than 0 days of survival; and (6) clear clinicopathological information for all the variables of interest including age at diagnosis, race, marital status, primary site, hormone receptors (HRs) (oestrogen receptor and progesterone receptor), tumour size, grade, laterality, number of positive regional lymph nodes, surgery record and clinical stage (the sixth edition of AJCC system).
Variables and definitions
The following data were extracted for each patient from the database: age at diagnosis, race (white and others), marital status at diagnosis, laterality, clinical stage, number of positive regional lymph nodes, tumour size, tumour grade (well-differentiated, moderately differentiated, poorly differentiated, undifferentiated or anaplastic), HRs (HR+ and HR−), surgery record, radiotherapy record, survival months and vital status. Marital status was classified as married or unmarried. The latter included single, separated, divorced, widowed and unmarried/domestic partners. OS was defined as the time from diagnosis to death from any cause or to the time of the last follow-up.
Construction and validation of the nomogram
The univariate and multivariate Cox regression analyses were performed to determine the potential prognostic factors. The independent factors were used to build the nomogram for the Wins by using the rms package in R software V.4.1.3. And the annual survival rates were analysed by using the survival and rms packages in R software. All the significant independent factors in the training cohort were used to build a nomogram to predict the survival rates. The nomogram was validated in the training and the validation cohorts. We used the Harrell concordance index (C-index), the area under the receiver operating characteristic (ROC) curve (AUC) and the calibration curve to assess the discrimination of the nomogram.
Statistical analysis
Our study consolidated the descriptive characteristics of the training and validation cohorts, respectively. χ2 test or Fisher’s exact test was used to confirm whether significant differences exist in the demographic and clinicopathological features between the training and validation cohorts. The variables were analysed using Kaplan-Meier survival curves and log-rank tests to evaluate their effects on OS. The ROC-AUC calculation was performed by the function of ‘ROC curve’ in SPSS V.26.0. All p values are two-sided, and p values under 0.05 are considered as statistically significant. The SEER data were extracted using SEERStat V.8.4.0, and statistical analyses were performed using SPSS V.26.0.
Patient and public involvement
No patient involved.
Results
Demographics and clinicopathological characteristics
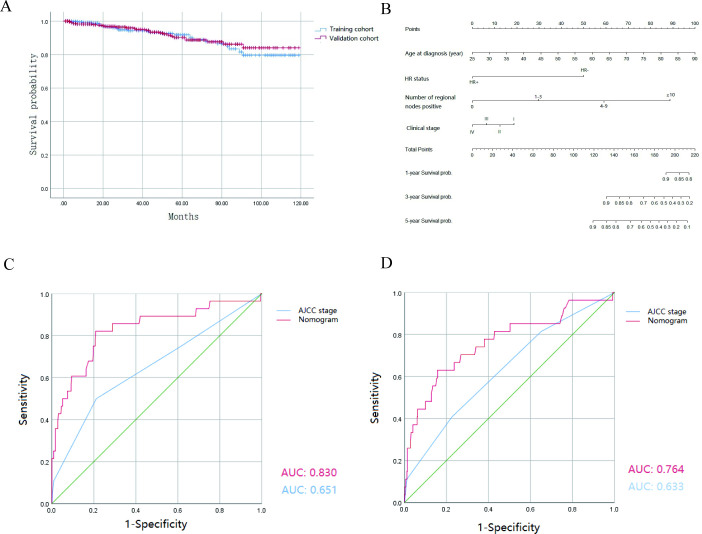
From the SEER database, a total of 754 cases of IMPC were eligible for inclusion criteria. The eligible patients were randomly divided into the training cohort (n=377) and the validation cohort (n=377) by applying ‘create Data Partition’ function in the package of ‘caret’ from R V.4.1.3. The demographic and clinicopathological characteristics of the training and validation cohorts are shown in table 1, and no statistically significant differences were found between the two cohorts. The estimated average OS values were 106.9 months (95% CI 102.7 to 111.1 months) in the 377 patients with IMPC in the training cohort, and 108.2 months (95% CI 104.4 to 112.1 months) in the validation cohort. The survival curve showed no significant differences between the two cohorts (figure 1A, p=0.786).
Table 1.
Clinicopathological characteristics of the training and validation cohorts
| Variables | Training cohort (n=377) (%) | Validation cohort (n=377) (%) | P value |
| Age (years) | 58.73±13.19 | 59.90±12.94 | 0.22 |
| Race | 0.11 | ||
| White | 276 (73.2) | 295 (78.2) | |
| Other | 101 (26.8) | 82 (21.8) | |
| Marital status | 0.55 | ||
| Unmarried | 159 (42.2) | 151 (40.1) | |
| Married | 218 (57.8) | 226 (59.9) | |
| Laterality | 0.17 | ||
| Left | 205 (54.4) | 186 (49.3) | |
| Right | 172 (45.6) | 191 (50.7) | |
| Grade | 0.26 | ||
| I/II | 236 (62.6) | 221 (58.6) | |
| III/IV | 141 (37.4) | 156 (41.4) | |
| Hormone receptor status | 0.55 | ||
| Positive | 335 (88.9) | 340 (90.2) | |
| Negative | 42 (11.1) | 37 (9.8) | |
| Tumour size (mm) | 24.44±22.78 | 24.71±21.93 | 0.87 |
| <20 | 223 (59.2) | 204 (54.1) | 0.29 |
| 20–50 | 114 (30.2) | 134 (35.5) | |
| >50 | 40 (10.6) | 39 (10.3) | |
| Number of positive regional nodes | 0.99 | ||
| 0 | 179 (47.5) | 175 (46.4) | |
| 1–3 | 118 (31.3) | 121 (32.1) | |
| 4–9 | 45 (11.9) | 47 (12.5) | |
| ≥10 | 35 (9.3) | 34 (9.0) | |
| Stage | 0.73 | ||
| I | 141 (37.4) | 127 (33.7) | |
| II | 148 (39.2) | 160 (42.4) | |
| III | 82 (21.8) | 83 (22.0) | |
| IV | 6 (1.6) | 7 (1.9) | |
| Surgery | 0.34 | ||
| Conserving surgery | 208 (55.2) | 195 (51.7) | |
| Mastectomy | 169 (44.8) | 182 (48.3) | |
| Radiotherapy | 0.06 | ||
| Yes | 238 (63.1) | 213 (56.5) | |
| No | 139 (36.9) | 164 (43.5) |
Figure 1.
(A) Kaplan-Meier survival curves of the patients with IMPC in the training and validation cohorts. The survival curves showed no significant differences between the two cohorts (p=0.786). (B) Nomogram for predicting 1-year, 3-year, 5-year OS for patients with the prognosis factors. The total points are calculated by summing up the points for each factor. The predicted probability of OS can be obtained by projecting the location of the total points to the bottom scales. (C, D) ROC curves for discrimination in the training and validation cohorts. (C) In the training cohort, the AUC of the ROC curve of the nomogram and the sixth edition AJCC tumour, node, metastases (TNM) staging classification was 0.830 and 0.651, respectively (p<0.001). (D) In the validation cohort, the AUC of the ROC curve of the nomogram and the sixth edition AJCC TNM staging classification was 0.764 and 0.633, respectively (p<0.001). AJCC, American Joint Committee on Cancer; AUC, area under the curve; HR, hormone receptor; OS, overall survival; ROC, receiver operating characteristic.
Univariate and multivariate Cox proportional hazards analyses
The hazard ratios for OS according to all variables in the univariate or multivariate Cox proportional hazards model are listed in tables 2 and 3. According to the results of univariate analysis, we found that the race, marital status, laterality and radiotherapy were not significant factors for OS. After excluding the aforementioned variables, age at diagnosis, grade, HR status, tumour size, number of positive regional lymph nodes, clinical stage and surgery were determined as prognostic factors in the multivariate Cox proportional hazards model for the OS analysis. As shown in table 3, age at diagnosis could be a negative prognostic factor for the OS of patients with IMPC. The HR negative special type exhibited higher risk of death. Compared with patients with IMPC and negative regional node, patients with positive regional lymph nodes suffered from higher risk of poor prognosis. Interestingly, the subgroups of stages II and III had a significantly lower risk than the stage I group.
Table 2.
Univariate analysis of overall survival in the training cohort
| Variables | Hazard ratio | 95% CI | P value |
| Age (years) | 1.035 | 1.005 to 1.065 | 0.023 |
| Race | |||
| White | Reference | ||
| Other | 1.202 | 0.529 to 2.732 | 0.660 |
| Marital status | |||
| Unmarried | Reference | ||
| Married | 0.721 | 0.343 to 1.512 | 0.386 |
| Laterality | |||
| Left | Reference | ||
| Right | 0.915 | 0.435 to 1.923 | 0.816 |
| Grade | |||
| I/II | Reference | ||
| III/IV | 2.180 | 1.030 to 4.611 | 0.042 |
| Hormone receptor status | |||
| Positive | Reference | ||
| Negative | 4.150 | 1.914 to 8.998 | <0.001 |
| Tumour size (mm) | <0.001 | ||
| <20 | Reference | ||
| 20–50 | 1.931 | 0.728 to 5.119 | 0.186 |
| >50 | 7.960 | 3.339 to 18.973 | <0.001 |
| Number of positive regional nodes | <0.001 | ||
| 0 | Reference | ||
| 1–3 | 1.679 | 0.609 to 4.632 | 0.317 |
| 4–9 | 3.145 | 0.998 to 9.914 | 0.050 |
| ≥10 | 8.350 | 3.016 to 23.115 | <0.001 |
| Stage | <0.001 | ||
| I | Reference | ||
| II | 1.040 | 0.365 to 2.967 | 0.941 |
| III | 3.529 | 1.262 to 8.419 | 0.015 |
| IV | 19.576 | 4.982 to 76.921 | <0.001 |
| Surgery | |||
| Conserving surgery | Reference | ||
| Mastectomy | 2.530 | 1.144 to 5.596 | 0.022 |
| Radiotherapy | |||
| Yes | Reference | ||
| No | 0.780 | 0.368 to 1.649 | 0.515 |
Table 3.
Multivariate analysis of overall survival in the training cohort
| Variables | Hazard ratio | 95% CI | P value |
| Age (year) | 1.054 | 1.020 to 1.090 | 0.020 |
| Grade | |||
| I/II | Reference | ||
| III/IV | 1.159 | 0.504 to 2.666 | 0.728 |
| Hormone receptor status | |||
| Positive | Reference | ||
| Negative | 5.368 | 2.084 to 13.830 | 0.001 |
| Tumour size (mm) | |||
| <20 | Reference | ||
| 20–50 | 2.292 | 0.631 to 8.322 | 0.208 |
| >50 | 4.807 | 0.919 to 25.153 | 0.063 |
| Number of positive regional nodes | |||
| 0 | Reference | ||
| 1–3 | 18.314 | 1.387 to 241.811 | 0.027 |
| 4–9 | 10.340 | 1.044 to 102.388 | 0.046 |
| ≥10 | 26.776 | 3.300 to 23.115 | 0.002 |
| Stage | |||
| I | Reference | ||
| II | 0.057 | 0.004 to 0.802 | 0.034 |
| III | 0.096 | 0.100 to 0.964 | 0.046 |
| IV | 0.211 | 0.170 to 2.641 | 0.228 |
| Surgery | |||
| Conserving surgery | Reference | ||
| Mastectomy | 1.119 | 0.393 to 3.190 | 0.833 |
Construction and validation of the nomograms
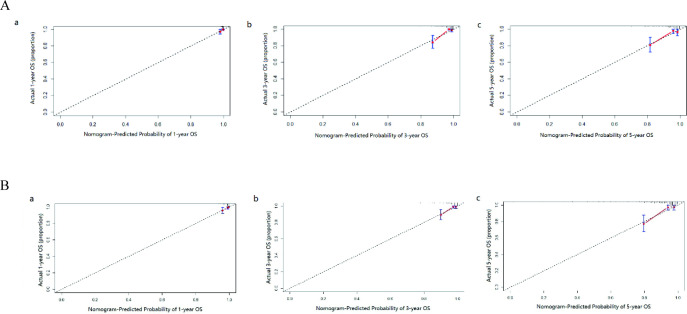
The nomogram for 1-year, 3-year and 5-year OS was developed by using the multivariate Cox proportional hazards models as the final prognostic models after factor selection (figure 1B). The nomogram was internally validated in the training cohort and externally validated in the validation cohort. The AUC values of the ROC curve, which exhibited the discrimination capacity, were 0.830 and 0.764 in the training and validation cohorts, respectively (figure 1C,D). Moreover, compared with the discriminative ability of the sixth edition AJCC TNM staging classification, the discriminative ability of the nomogram was significantly superior in the training and validation cohorts (p<0.001). The results indicated that the nomogram can efficiently predict OS in patients with IMPC. The calibration plots also showed great consistency between the actual and nomogram-predicted survival rates in the training and testing cohorts (figure 2A,B). The C-index values of the nomogram for OS were 0.794 in the training cohort and 0.774 in the validation cohort.
Figure 2.
Calibration curves for predictions for the 1-year (a), 3-year (b), 5-year (c) OS in the training cohort (A) and in the testing cohort (B). The nomogram-predicted probability of OS is plotted on the X-axis, and the actual OS is plotted on the Y-axis. OS, overall survival.
Discussion
IMPC of breast is a rare variant of invasive breast carcinoma (IBC).4 Histologically, it is a special type characterised by small papillary structures that lack true central fibrovascular cores and lie within empty stromal spaces.24 25 Historically, patients with IMPC were usually treated with standard IBC treatment. However, notable differences in histological characters and prognosis exist between IMPC and IBC26; as such, treating IMPC as IBC would be inappropriate. Accurate predictions of prognosis of patients with IMPC patients could effectively help clinicians to take proper treatment modalities. This study aims to build a nomogram capable of predicting the prognosis of IMPC based on a larger population database of the SEER programme.
In this study, we equally divided 754 patients with IMPC from the SEER database into two cohorts. We developed an effective nomogram that contains four independent prognostic factors including age at diagnosis, HR, number of positive regional lymph nodes and clinical stage. The nomogram, derived from the Cox regression model to predict the 1-year, 3-year and 5-year OS of patients with IMPC, was verified to have good discrimination capacity. Moreover, the nomogram showed better prediction ability for OS than that of the sixth edition AJCC TNM staging classification (AUCs in the ROC curve: 0.830 and 0.651 in the training cohort and 0.764 and 0.633 in the validation cohort, respectively).
HRs play important role in prognosis of breast cancer.27 28 A previous study showed that the 5-year OS was 59% in 100 patients with IMPC with a mean age of 50 years and 46% hour positivity.12 In another study, 72 patients with IMPC with a mean age of 46 years and 75% hour positivity had 86% 5 year OS.10 In comparison, our study population was older (mean age of 59.3 years) and had a higher percentage of HR positivity (89.5%). The higher HR positivity in the present study may contribute to the better 5 year OS (91.1%).29 The Cox-regression analysis result also proved that HR negativity could lead to significantly poor OS in patients with IMPC (hazard ratio 5.368; 95% CI 2.084 to 13.830; p=0.001).
Lymph node metastasis is widely considered as an unfavourable prognostic factor in clinical practice.30 31 Axillary lymph node metastasis is commonly seen in patients with IMPC at first diagnosis. The rate of lymphatic and lymph nodal spread ranged from 33% to 95%.4 24 32 33 The value and necessity of sentinel lymph node biopsy (SLNB) or axillary dissection in patients with IMPC remains controversial. Walsh and Bleiweiss found that regional lymph nodes can be involved even at early stage of IMPC lesions. The team highly recommended a thorough regional lymph node examination to patients with IMPC.25 However, Paterakos et al were sceptical to the utility of SLNB for patients with IMPC due to the high frequency of multiple positive regional lymph nodes.34 In the present study, we found that patients with IMPC with even one positive regional lymph node would suffer higher risk than patients with negative lymph node. Patients with IMPC and 10 or more positive lymph nodes are at the highest risk (OR 26.776; 95% CI 3.300 to 23.115; p=0.002). Thus, axillary dissection, or SLNB at minimum, should be performed to correctly access the risk and adopt suitable treatment regimens for patients with IMPC.
This study has some limitations. First, retrospective SEER data lack a pathological review to identify the diagnosis for each case. Second, we cannot consider the types of systemic therapy administered to patients. Hormonal blockade therapy and chemotherapy could significantly affect the outcome of patients. Third, the relationship between the degree of micropapillary involvement and clinical outcomes among patients with IMPC remains unclear. Although some previous small case series studies have revealed that an increasing percentage of micropapillary component was not associated with more lymph node metastasis and worse survival,32 35 it needs to be further validated in large-scale studies.
Conclusions
In conclusion, age at diagnosis, HR status, number of positive regional lymph nodes and clinical stage were independent prognostic factors for patients with IMPC. We constructed a nomogram to predict OS in patients with IMPC based on a large-scale population from the SEER database. This accessible nomogram will help doctors to adopt proper treatment regimens in clinical practice.
Supplementary Material
Footnotes
JL and WX contributed equally.
Contributors: JL and WX contributed equally. JL and WX designed and conducted the study. JZ provide suggestions in revision. WG analysed and interpreted the data. WX drafted, revised, finalised and submitted the manuscript. JL is responsible for the critical revision. QW approved the submission of the manuscript. QW has full responsibility for the overall content as the guarantor, had access to the data and controlled the decision to publish.
Funding: This work was supported by a grant from the hospital incubation funding project of the First Affiliated Hospital of Wenzhou Medical University (No. FHY2019064).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The corresponding author has full access to all the data used in this study and had final responsibility for the decision to submit the study for publication.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was reviewed by the Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University. The data are anonymous, and the requirement for informed consent was therefore waived.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics. CA Cancer J Clin 2022;72:7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Luna-Moré S, Gonzalez B, Acedo C, et al. Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract 1994;190:668–74. 10.1016/S0344-0338(11)80745-4 [DOI] [PubMed] [Google Scholar]
- 3.Fu L, Ikuo M, Fu X, et al. Relationship between biologic behavior and morphologic features of invasive micropapillary carcinoma of the breast. Zhonghua Bing Li Xue Za Zhi 2004;33:21–5. [PubMed] [Google Scholar]
- 4.Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol 1993;6:660–2. [PubMed] [Google Scholar]
- 5.Chen AC, Paulino AC, Schwartz MR, et al. Prognostic markers for invasive micropapillary carcinoma of the breast: a population-based analysis. Clin Breast Cancer 2013;13:133–9. 10.1016/j.clbc.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 6.Verras G-I, Tchabashvili L, Mulita F, et al. Micropapillary breast carcinoma: from molecular pathogenesis to prognosis. Breast Cancer (Dove Med Press) 2022;14:41–61. 10.2147/BCTT.S346301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akrida I, Mulita F. The clinical significance of Her2 expression in DCIS. Med Oncol 2022;40:16. 10.1007/s12032-022-01876-9 [DOI] [PubMed] [Google Scholar]
- 8.Verras G-I, Mulita F, Tchabashvili L, et al. A rare case of invasive micropapillary carcinoma of the breast. Prz Menopauzalny 2022;21:73–80. 10.5114/pm.2022.113834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen E, Du Y, Chen Y, et al. A web-based novel model for predicting prognostic value in patients with invasive micropapillary carcinoma in breast cancer: a real-world data retrospective cohort study. Updates Surg 24, 2023. 10.1007/s13304-023-01530-7 [DOI] [PubMed] [Google Scholar]
- 10.Yu JI, Choi DH, Park W, et al. Differences in prognostic factors and patterns of failure between invasive micropapillary carcinoma and invasive Ductal carcinoma of the breast: matched case-control study. Breast 2010;19:231–7. 10.1016/j.breast.2010.01.020 [DOI] [PubMed] [Google Scholar]
- 11.Yu JI, Choi DH, Huh SJ, et al. Differences in prognostic factors and failure patterns between invasive micropapillary carcinoma and carcinoma with micropapillary component versus invasive Ductal carcinoma of the breast: retrospective multicenter case-control study [KROG 13-06]. Clinical Breast Cancer 2015;15:353–361. 10.1016/j.clbc.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Fan Y, Lang R, et al. Breast carcinoma with micropapillary features: clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol 2008;16:155–63. 10.1177/1066896907307047 [DOI] [PubMed] [Google Scholar]
- 13.Li D, Zhong C, Cheng Y, et al. A competing nomogram to predict survival outcomes in invasive micropapillary breast cancer. J Cancer 2019;10:6801–12. 10.7150/jca.27955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye F-G, Xia C, Ma D, et al. Nomogram for predicting preoperative lymph node involvement in patients with invasive micropapillary carcinoma of breast: a SEER population-based study. BMC Cancer 2018;18:1085. 10.1186/s12885-018-4982-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Yu C, Chen D, et al. A prognostic nomogram based on risk assessment for invasive micropapillary carcinoma of the breast after surgery. Cancer Med 2023;12:8050–62. 10.1002/cam4.5595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Li S, Yan L, et al. Nomogram for predicting overall survival in patients with invasive micropapillary carcinoma after breast-conserving surgery: a population-based analysis. Front Surg 2022;9. 10.3389/fsurg.2022.1009149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364–70. 10.1200/JCO.2007.12.9791 [DOI] [PubMed] [Google Scholar]
- 18.Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173–80. 10.1016/S1470-2045(14)71116-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diao J-D, Ma L-X, Sun M-Y, et al. Construction and validation of a nomogram to predict overall survival in patients with inflammatory breast cancer. Cancer Med 2019;8:5600–8. 10.1002/cam4.2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, Pan Z, Zhao F, et al. A nomogram for predicting survival in patients with nodular melanoma: a population-based study. Medicine 2019;98:e16059. 10.1097/MD.0000000000016059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Zheng S-Q, Li X-Y, et al. Derivation and validation of a nomogram to predict in-hospital complications in children with tetralogy of Fallot repaired at an older age. J Am Heart Assoc 2019;8:e013388. 10.1161/JAHA.119.013388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song J, Sun Y, Cao H, et al. A novel pyroptosis-related lncRNA signature for prognostic prediction in patients with lung adenocarcinoma. Bioengineered 2021;12:5932–49. 10.1080/21655979.2021.1972078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Chen L, Nie Y, et al. Nomogram for predicting the overall survival of patients with breast cancer with pathologic nodal status N3. Clin Breast Cancer 2020;20:e778–85. 10.1016/j.clbc.2020.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Pettinato G, Manivel CJ, Panico L, et al. Invasive micropapillary carcinoma of the breast: clinicopathologic study of 62 cases of a poorly recognized variant with highly aggressive behavior. Am J Clin Pathol 2004;121:857–66. 10.1309/XTJ7-VHB4-9UD7-8X60 [DOI] [PubMed] [Google Scholar]
- 25.Walsh MM, Bleiweiss IJ. Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Human Pathology 2001;32:583–9. 10.1053/hupa.2001.24988 [DOI] [PubMed] [Google Scholar]
- 26.Nassar H, Wallis T, Andea A, et al. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol 2001;14:836–41. 10.1038/modpathol.3880399 [DOI] [PubMed] [Google Scholar]
- 27.Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. JCO 1984;2:1102–9. 10.1200/JCO.1984.2.10.1102 [DOI] [PubMed] [Google Scholar]
- 28.Osborne CK, Fisher E, Redmond C, et al. Estrogen receptor, a marker for human breast cancer differentiation and patient prognosis. Adv Exp Med Biol 1981;138:377–85. 10.1007/978-1-4615-7192-6_23 [DOI] [PubMed] [Google Scholar]
- 29.Luna-Moré S, Casquero S, Pérez-Mellado A, et al. Importance of estrogen receptors for the behavior of invasive micropapillary carcinoma of the breast. Review of 68 cases with follow-up of 54. Pathol Res Pract 2000;196:35–9. 10.1016/S0344-0338(00)80019-9 [DOI] [PubMed] [Google Scholar]
- 30.Kuru B, Camlibel M, Dinc S, et al. Prognostic significance of axillary node and Infraclavicular lymph node status after mastectomy. Eur J Surg Oncol 2003;29:839–44. 10.1016/j.ejso.2003.08.003 [DOI] [PubMed] [Google Scholar]
- 31.Hur MH, Ko S. Metastatic axillary node ratio predicts recurrence and poor long-term prognosis in patients with advanced stage IIIC (pN3) breast cancer. Ann Surg Treat Res 2017;92:340–7. 10.4174/astr.2017.92.5.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zekioglu O, Erhan Y, Ciris M, et al. Invasive micropapillary carcinoma of the breast: high incidence of lymph node metastasis with extranodal extension and its immunohistochemical profile compared with invasive Ductal carcinoma. Histopathology 2004;44:18–23. 10.1111/j.1365-2559.2004.01757.x [DOI] [PubMed] [Google Scholar]
- 33.Adrada B, Arribas E, Gilcrease M, et al. Invasive micropapillary carcinoma of the breast: mammographic, sonographic, and MRI features. AJR Am J Roentgenol 2009;193:W58–63. 10.2214/AJR.08.1537 [DOI] [PubMed] [Google Scholar]
- 34.Paterakos M, Watkin WG, Edgerton SM, et al. Invasive micropapillary carcinoma of the breast: a prognostic study. Hum Pathol 1999;30:1459–63. 10.1016/s0046-8177(99)90168-5 [DOI] [PubMed] [Google Scholar]
- 35.Kim M-J, Gong G, Joo HJ, et al. Immunohistochemical and clinicopathologic characteristics of invasive Ductal carcinoma of breast with micropapillary carcinoma component. Arch Pathol Lab Med 2005;129:1277–82. 10.5858/2005-129-1277-IACCOI [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The corresponding author has full access to all the data used in this study and had final responsibility for the decision to submit the study for publication.