Abstract
Objective
The first biomarker associated with the rheumatoid arthritis is rheumatoid factor (RF) and since the earliest reports a role has been proposed in the diagnosis and in the prediction of clinical features and outcome. The study of RF isotypes has further attempted to improve diagnostic accuracy and identify specific subgroups of patients. The main objective of this study is to provide an analysis of the literature on the role of RF isotypes in the diagnosis and prognosis of rheumatoid arthritis (RA).
Methods
We performed a systematic literature review and meta-analysis on the role of RF isotypes in RA (only in English, from PubMed, search terms: “rheumatoid factor isotypes”, “diagnosis”, “prognosis” and “rheumatoid arthritis”, last search 31 July 2022, two independent assessment of quality and biases, results included in tables and in the meta-analysis).
Results
Thirty-six articles were examined (7517 patients). Testing all RF isotypes with latex test or nephelometry allows for the highest sensitivity (68.6%, 95% CI 66.2% to 71.0%); nonetheless, the determination of IgA isotype provides the highest specificity (91.4%, 95% CI 90.8% to 92.0%) and the highest positive likelihood ratio (7.7, 95% CI 5.7 to 10.4). When testing IgM isotype the highest diagnostic OR (21.7, 95% CI 16.1 to 29.3) is reached. When analysing anti-citrullinated protein antibodies, RF isotype determination increases diagnostic accuracy. On the other hand, these do not provide relevant prognostic information, as results are conflicting.
Conclusions
Testing RF allows the highest sensitivity, while IgA isotype the highest specificity and positive likelihood ratio for RA diagnosis. On the other hand, determination of RF isotypes dose not allow prognostic information, as data are limited and heterogeneous.
Keywords: rheumatoid factor; arthritis, rheumatoid; autoantibodies
WHAT IS ALREADY KNOWN ON THIS TOPIC
Rheumatoid factor is widely used in daily practice and is included in the classification criteria for rheumatoid arthritis but the role of its isotypes is controversial.
WHAT THIS STUDY ADDS
This is the first systematic literature review and metanalysis on the role of rheumatoid factor isotypes in the diagnosis and management of rheumatoid arthritis.
Testing rheumatoid factor by latex test or nephelometry allows for the highest sensitivity, while determination of IgA isotype provides the highest specificity and the highest positive likelihood ratio. Testing IgM isotype provides the highest diagnostic OR.
Rheumatoid factor isotype determination increases the diagnostic accuracy also when anti-citrullinated protein antibodies are tested while not providing prognostic information.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The determination of rheumatoid factors isotypes may be useful in patients with seronegative arthritis, potentially modifying the diagnosis and improve the management.
Further longitudinal studies are needed to determine the role of these biomarkers in the prognosis and in the management of rheumatoid arthritis.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease affecting 0.5%–1% of the population worldwide. The disease mainly involves the joints potentially leading to functional disability but can also present extra-articular manifestations in 40% of cases.1
Rheumatoid factor (RF) includes antibodies which recognises the fragment crystallisable region of IgG and represent the first biomarkers detected in RA.2 RF was first described in 1940 by Waaler, as a factor with haemagglutinating activity in the serum of a patient with RA. In fact, RF agglutinates sheep red blood cells sensitised with rabbit IgG (the classic Waaler-Rose test).3 It was then named by Pike in 1949 for its associations with RA.4
Agglutination techniques were initially used to detect RF, based on the ability of IgM to induce agglutination. Then, the technique was developed with other IgG carriers such as bentonite and latex particles. Automated methods as nephelometry and ELISA gradually replaced it, for their simplicity and greater reproducibility.5 Improved techniques for the detection of RF have been developed also for the important role of RF in the diagnosis of RA, as it was included in the 1987 American College of Rheumatology (ACR) classification criteria for RA.6 However, its relevance has been partly reconsidered as it is not uncommon in other diseases, including Sjögren’s syndrome, systemic lupus erythematosus, in chronic infectious diseases as syphilis, tuberculosis, liver diseases and in healthy population, with increased prevalence in the elderly.2
RF role in RA has been partly overtaken by anti-citrullinated protein antibodies—ACPA, also named anti cyclic citrullinated peptide antibodies—with high specificity for the disease.7 Nonetheless, several analyses have demonstrated the important role of RF, also complementary to ACPA, as described below.
Consistent with this, in the 2010 ACR/European Alliance of Associations for Rheumatology classification criteria for RA, RF and ACPA are considered equivalent disease markers, and RF positivity allows the same score as ACPA positivity. In fact, in patients with synovitis, ACPA status does not add information for classification as RA beyond those that are provided by a positive RF, despite ACPA being more specific.8
Currently, the sensitivity of RF for RA is estimated to be around 41%–66% for early RA and 62%–87% for established RA, while the specificity accounts for 43%–96%,9 with most studies reporting specificity higher than 70%, as described below. Moreover, the identification of IgM-RF, IgA-RF and IgG-RF isotypes has allowed a more subtle evaluation of the role of RF in different aspects of the disease. Several studies have evaluated whether one or more isotypes could be relevant for the diagnosis and management of RA, but a comprehensive evaluation of these studies has not been performed.
The aim of this systematic literature review and meta-analysis is to provide a comprehensive analysis of the literature reported to date on the role of RF and its isotypes in the diagnosis and prognosis of RA, in order to identify their role in the disease in terms of diagnostic accuracy and prognostic capability.
Methods
Study search strategy and selection
The Medline database was accessed from PubMed and systematically searched for articles published in English. The search strategy was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations10 to increase standardisation and quality in reporting. We followed the search strategy and article selection process illustrated in the flow charts in online supplemental figures 1 and 2, using the search terms: “rheumatoid factor isotypes”, “diagnosis”, “prognosis” and “rheumatoid arthritis”. The search strings used for each association are detailed in the respective flowchart. Only peer-reviewed articles in English accepted for publication were included in this search. The last search was run on 31 July 2022. From the initial search, we excluded papers not appropriate for the study design as ascertained by title and abstract. Reviews and case reports were also excluded. FM and NB have independently assessed the quality of the studies and the risk of biases, screened the full text reports and decided whether these met the inclusion criteria while resolving any disagreement through discussions. Neither of the authors were blind to the journal titles or to the study authors or institutions.
rmdopen-2022-002817supp001.pdf (658.3KB, pdf)
Finally, 36 studies providing data on RF isotypes were considered eligible for inclusion in the data analysis. All papers were assessed for methodological quality and statistical data on diagnostic accuracy of the tests.
Data extraction
Data retrieved from the articles included criteria for patient recruitment; the number of patients and healthy subjects enrolled; demographic and clinical data on the population investigated; the RF isotypes studied; specification of the analytical method used to detect RF isotypes (either in-house or commercial and, in this latter case, subdivided by the manufacturer); the cut-off used; sensitivity and specificity of the assay, and the number of true positive, false negative, true negative and false positive results. Missing data were calculated from the data available. Regarding the role of isotopes in defining prognosis, disease activity, radiographic progression and response to treatment were chosen. Data were reported in online supplemental tables 5–7 and were only described, to avoid risk of biases in a statistical analysis. Any missing or unclear information was not considered. Studies performed on the same population for comparison of two or more analytical methods were maintained. Therefore, the number of studies exceeds the number of selected articles.
Statistical analysis
Data were analysed with Meta-DiSc (V.1.4, Cochrane Colloquium, Barcelona, Spain) using the statistical model previously described.11 The accuracy of each study was measured as sensitivity, specificity, positive and negative likelihood ratio (LR+ and LR−) and diagnostic OR (DOR). Sensitivity was considered as the proportion of positives among people with disease, and specificity the proportion of negatives among people without disease. The LR express how much more frequent the respective result is among subjects with disease than among subjects without disease; LR+ was calculated by dividing the pooled sensitivity by 1-specificity; LR− was calculated by dividing 1-sensitivity by specificity. The DOR expresses how much greater the odds of having the disease are for subjects with a positive test than for subjects with a negative test result. The overall DOR was calculated by combining each study’s DOR, using a random-effects model. A correction factor of one-half was added to each cell to avoid calculation problems by having a value of 0 in the 2×2 table.
The threshold effect heterogeneity, that is, the differences in sensitivity and specificity occurring for the different cut-offs used in the studies, was explored by representation of accuracy estimates from each study in a summary receiver operating characteristic (sROC) space, and by calculating the Spearman correlation coefficient between the logit of sensitivity and logit of 1-specificity.
The sROC curve analysis was relied on a regression analysis of logit transformation of the data, which plots the difference (D) between the logit of the true-positive (TPR) and the logit of the false-positive (FPR) rates (D=logit TPR–logit FPR) on the y axis and the sum (S=logit TPR+logit FPR) on the x axis. The y axis (D) is equivalent to the log DOR, and the x axis (S) is a measure of how test features vary with test threshold. A regression equation (D = α+β∙S) derived from the sROC curve analysis can be used to determine the heterogeneity among study results. If the β coefficient is near zero and not statistically significant, then significant heterogeneity is not present. The symmetric sROC curve was created using the overall DOR of all the studies. The asymmetric sROC curve was generated using the (unweighted) method as described by Moses et al.12
The heterogeneity between the studies, other than threshold effect, was explored using the Cochran’s Q statistic and the Inconsistency index. DerSimonian and Laird methods13 were used to pool data in a random effects model. Forest plots and summary estimates with their 95% CI were created by constructing sROC regression curves, using each study’s paired sensitivity and specificity. The area under the curve (AUC) was also calculated.
Other potential causes for heterogeneity (such as variations in age of subjects or differences in analytical methods) were evaluated using subgroup and meta-regression analyses using DOR as a global measure of accuracy and also as a method to compare the overall diagnostic accuracy of different tests, according with Littenberg and Moses linear model. This model compares DOR in the subgroups, to evaluate significant differences. The outputs of meta-regression are ratios of DOR. If a specific study level covariate is significantly associated with diagnostic accuracy, then its coefficient will have a low p value, and the DOR ratio will give a measure of magnitude of the association. A relative DOR<1.0 indicates that studies with a particular feature have a lower DOR than studies without this feature.
Results
Diagnostic accuracy of RF isotypes in RA
Our review included 19 articles related to diagnostic accuracy of RF isotypes, which involved 4786 patients and 6994 controls.14–31 The IgM isotype was tested in 17/19 (89.4%) studies, IgA in 19/19 (100%) and IgG in 14/19 (73.6%). A solid phase immunoenzymatic test (ELISA) was used in 15/19 (78.9%) studies, an immunofluorimetric assay (fluorimetric enzyme-linked immunoassay, FEIA) was used in 5/19 (26.3%). In 8/19 (42.1%) articles RF was also tested by latex test (3/8–37.5%) or nephelometry (5/8–62.5%), which include all isotypes. Two articles18 31 described different analytical methods performed on the same population and were therefore considered more than once. Missing data were calculated from the data available. Of the ELISA methods used, 5 were manufactured in-house and 16 by seven different commercial companies. Data regarding RF isotypes retrieved from the articles included are detailed in online supplemental tables 1–4.
A pooled analysis of data from the studies was performed for each isotype.
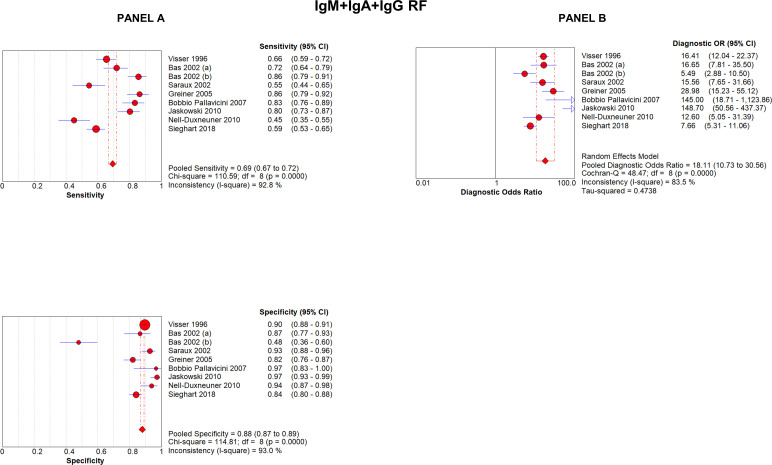
When considering the detection of all isotypes (by latex test or nephelometry), described in eight articles analysing nine methods, RF had an overall sensitivity of 68.6% (95% CI 66.2% to 71.0%) and a specificity of 88.3% (95% CI 87.1% to 89.4%); DOR was 18 (95% CI 10.7 to 30.5) (figure 1). The overall positive LR for having RA in RF-positive patients was 5.8 (95% CI 3.6 to 9.5) and negative LR was 0.34 (95% CI 0.25 to 0.43). The AUC for symmetric sROC curve was 0.859, while it was 0.853 for the asymmetric sROC curve, indicating that a very moderate threshold effect was present and that the modest heterogeneity was due to the low number of studies, whereas most studies gave homogeneous results (online supplemental figure 3).
Figure 1.
Forest plots of sensitivity and specificity (A) and diagnostic OR (B) of analytical methods testing all RF isotypes (IgM+IgA+IgG RF). RF, rheumatoid factor.
The diagnostic accuracy of testing IgM isotype alone (by ELISA or FEIA) was described in 17/19 articles. IgM-RF had an overall sensitivity of 63.4% (95% CI 62.1% to 64.6%) and a specificity of 90% (95% CI 89.5% to 90.7%); DOR was 21.7 (95% CI 16.1 to 29.3) (figure 2). Positive LR for having RA in IgM-RF-positive subjects was 7.09 (95% CI 5.5 to 9.1) and negative LR was 0.35 (95% CI 0.31 to 0.39). The AUC for symmetric sROC curve was 0.855, while it was 0.846 for the asymmetric sROC curve (online supplemental figure 4).
Figure 2.
Forest plots of sensitivity and specificity (A) and diagnostic OR (B) of analytical methods testing IgM-RF. RF, rheumatoid factor.
IgA isotype was studied in all the 19 articles. IgA-RF sensitivity was 49.1% (95% CI 48% to 50%) and specificity was 91.4% (95% CI 90.8% to 92.0%); DOR was 16.0 (95% CI 11.3 to 22.7) (figure 3). Positive LR was 7.7 (95% CI 5.7 to 10.4) and negative LR was 0.50 (95% CI 0.47 to 0.55). The AUC for symmetric sROC curve was 0.781, while it was 0.772 for the asymmetric sROC curve (online supplemental figure 5).
Figure 3.
Forest plots of sensitivity and specificity (A) and diagnostic OR (B) of analytical methods testing IgA-RF. RF, rheumatoid factor.
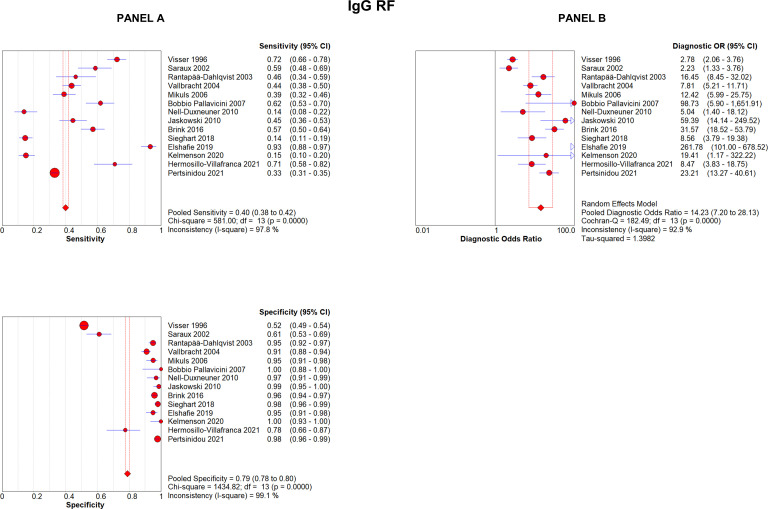
IgG-RF was analysed in 14 articles. Its sensitivity was 39.9% (95% CI 38.3% to 41.5%), with a specificity of 78.9% (95% CI 77.7% to 80.0%); DOR was 14.2 (95% CI 7.2 to 28.1) (figure 4). Positive LR was 7.4 (95% CI 3.4 to 16.2), negative LR was 0.56 (95% CI 0.48 to 0.66). The AUC for symmetric sROC curve was 0.831, while it was 0.830 for the asymmetric sROC curve (online supplemental figure 6).
Figure 4.
Forest plots of sensitivity and specificity (A) and diagnostic OR (B) of analytical methods testing IgG-RF. RF, rheumatoid factor.
To summarise, nine studies analysed the diagnostic accuracy of testing all the three isotypes by nephelometry or latex test, 17 articles studied IgM-RF, 19 IgA-RF and 14 IgG-RF isotypes tested by ELISA or FEIA. The highest sensitivity was achieved when all three isotypes were tested by nephelometry or latex test (68.6%, 95% CI 66.2% to 71.0%), while the highest specificity was reached by IgA-RF (91.4%, 95% CI 90.8% to 92.0%). DOR was greater when IgM-RF was tested (21.7, 95% CI 16.1 to 29.3). LR+ and LR− were more relevant for IgA-RF (7.7, 95% CI 5.7 to 10.4) and IgM-RF (0.35, 95% CI 0.31 to 0.39), respectively; the ROC AUC was greatest when all three isotypes were tested (0.859) (table 1).
Table 1.
Summary of sensitivity, specificity, positive and negative likelihood ratio, diagnostic OR, and AUC described in the included studies for each RF isotype
| All three isotypes | IgM-RF | IgA-RF | IgG-RF | |
| Test | Nephelometry/latex test | ELISA/FEIA | ELISA/FEIA | ELISA/FEIA |
| No of studies | 8 | 17 | 19 | 14 |
| Sensitivity % (95% CI) | 68.6 (66.2 to 71.0) | 63.4 (62.1 to 64.6) | 49.1 (47.8 to 50.4) | 39.9 (38.3 to 41.5) |
| Specificity % (95% CI) | 88.3 (87.1 to 89.4) | 90 (89.5 to 90.7) | 91.4 (90.8 to 92.0) | 78.9 (77.7 to 80.0) |
| Likelihood ratio+ (95% CI) | 5.8 (3.6 to 9.5) | 7.09 (5.5 to 9.1) | 7.7 (5.7 to 10.4) | 7.4 (3.4 to 16.2) |
| Likelihood ratio− (95% CI) | 0.3 (0.25 to 0.43) | 0.35 (0.31 to 0.39) | 0.50 (0.47 to 0.55) | 0.56 (0.48 to 0.66) |
| Diagnostic OR (95% CI) | 18 (10.7 to 30.5) | 21.7 (16.1 to 29.3) | 16.0 (11.3 to 22.7) | 14.2 (7.2 to 28.1) |
| AUC ROC (95% CI) | 0.859 (0.856 to 0.862) | 0.855 (0.85 to 0.860) | 0.781 (0.779 to 0.791) | 0.831 (0.826 to 0.836) |
AUC, area under the curve; FEIA, fluorimetric enzyme-linked immunoassay; RF, rheumatoid factor; ROC, receiver operating characteristic.
All eight studies that analysed nephelometry or latex test reported significant diagnostic value. Even when compared with ACPA testing alone, RF isotypes increased diagnostic accuracy, as IgM-RF, studied in 13 papers, proved to add diagnostic value in 8 (61.5%) of them and IgA-RF added diagnostic information in 7/14 studies (50%). IgG-RF added diagnostic information in only 1/11 (9.1%) of the articles included (table 2).
Table 2.
Patients’ characteristics and additional value of testing RF isotypes
| Study | Nephelometry/latex test | IgM | IgA | IgG | Median age of patients (range or SD) | Time from diagnosis | Median age of controls (range or SD) | Notes |
| Visser 199617 | Yes | No | No | No | 60 (16–89) | 6 months (median) | 47 (12–92) | |
| Saraux 200219 | Yes | Yes | No | No | 49 (33–65) | n.a. | n.a | Combination more predictive of RA: nephelometry+IgM RF (sensitivity+15%, specificity −7%). |
| Bas 200218 | Yes | Yes | Yes | – | 64 (22–87) | n.a. | 58 (4–191) | IgM-RF+IgA-RF: increased LR for RA. |
| Bas 200330 | – | No | Yes | – | 54 (22–77) | n.a | 42 (4–101) DC 60 (50–75) HC |
CCP+IgA-RF: increased specificity for RA diagnosis (CCP specificity 90%, IgA-RF 90%, IgM-RF 82%; CCP+IgA RF 98%; CCP+IgA-RF+IgM RF 98%). |
| Rantapää-Dahlqvist 200320 | – | No | Yes | No | 54 (31–77) | 3 years (median) | 55 (30–69) | IgA-RF predicts RA diagnosis. |
| Vallbracht 200447 | – | No | No | No | 62 (±15) | n.a | 56 (±18) 52 (±19) 50 (±20) |
|
| Greiner 200526 | Yes | – | n.a. | – | 58.6 (19–84) | n.a. | n.a | |
| Mikuls 200627 | – | Yes | Yes | No | 51 (±13) | <2 years | 45 (±14) HC 39 (±12) SLE |
CCP sensitivity 62%, specificity 94%. IgM-RF sensitivity 70%, specificity 80%. IgA-RF sensitivity 65%, specificity 87%. IgG-RF sensitivity 38%, specificity 85%. CCP+IgM RF sensitivity 55%, specificity 98%. CCP+IgA RF sensitivity 59%, specificity 98%. |
| Bobbio Pallavicini 200716 | Yes | n.a. | n.a. | n.a. | 57 (±12) | 8.3 (±6.9) | n.a. | |
| Jaskowski 201022 | Yes | Yes | No | No | n.a. | 8.6 years (mean) | 33 (16–70) | In 48 CCP-negative subjects, 57% were FR positive (IgM-RF+IgA RF or triple positive). |
| Nell-Duxneuner 201014 | Yes | – | No | No | 50 (18–83) | < 3 months | 43 (18–87) | |
| Brink 201621 | – | Yes | No | No | 50.3 (IQR 20.1) |
6.2 years (median) | 51.1 (IQR 20) |
In pre-symptomatic individuals, OR IgM-RF 11.1, IgM-RF+IgA RF 21.9, IgM-RF+IgA-RF+IgG RF 34.5. OR CCP 20.2. OR CCP+IgA RF 50.7, CCP+IgG RF 30.2, CCP+IgM RF 67.6. |
| Sieghart 201823 | Yes | Yes | Yes | No | 57 (47–66) EA 53 (44–63) RA |
<3 months >2 years |
55 (43–64) DC 50 (42.55) HC |
14/290 (4.8%) patients negative at nephelometry resulted positive for IgM-RF. 14/109 (12.8%) ACPA-IgM-RF negative were IgA-RF+. |
| Elshafie 201915 | – | Yes | Yes | Yes | 49 (18–80) | 3 years (1–420 months) |
35 (n.a.) | CCP sensitivity 70.7%. CCP+IgM RF sensitivity 80.1%. CCP+IgM-RF+IgA RF sensitivity 89.5%. CCP+IgM-RF+IgA-RF+IgG RF sensitivity 100%. |
| Kelmenson 202024 | – | n.a. | n.a. | n.a. | 37 (±8) | 5.1 years | 37 (±8) | |
| Janssen 202028 | – | n.a. | n.a. | – | 55 (±11) | 5.5 years (3–10) | 42 (±15) DC 34 (±15) HC |
|
| Hermosillo-Villafranca 202125 | – | No | No | No | n.a. | n.a | n.a. | |
| Pertsinidou 202129 | – | n.a. | n.a. | n.a. | 57 M/54 F (18–70) | <2 months | n.a. | |
| Van Hoovels 202231 | – | Yes | Yes | No | 57 (18–86) | 16.8%<6 weeks 83.2%≥6 weeks |
53 (11–92) | IgA RF picked up 0.5% or 2.3% (Thermo Fisher or Orgentec assay, respectively) RA patients that were ACPA and IgM RF negative. Single IgA RF positivity was found in 1.5% or 5.6% (Thermo Fisher or Orgentec assay, respectively) of controls. CCP+high titers RF (IgA or IgM) LR 62-infinity. |
CCP, cyclic citrullinated peptide antibodies; DC, disease controls; EA, early arthritis; HC, healthy controls; Ig, Immunoglobulin; LR, likelihood ratio; n.a., not applicable; RA, rheumatoid arthritis; RF, rheumatoid factor; SLE, systemic lupus erythematosus.
Role of RF isotypes in RA prognosis
Seventeen articles related to RF isotypes in predicting prognosis were analysed, involving 2731 patients.14–17 30 32–43 IgM isotype was tested in 12/17 (70.5%) studies, IgA in 17/17 (100%) and IgG in 11/17 (64.7%). ELISA was used to determine isotypes in all but one study (94.1%), where FEIA was used. Of the ELISA methods used, 7 were manufactured in-house and 10 by eight different companies.
Correlation of IgM, IgA and IgG-RF isotypes with disease activity, radiographic progression and response to treatment were evaluated for each article. As studies used different criteria to define outcomes, pooled analysis has not been performed and results are detailed in online supplemental tables 5–7. Overall, IgM-RF was associated with disease activity in 3/5 studies (60%), IgA-RF in 4/8 (50%), IgG-RF in 1/3 (33.3%). Radiographic progression was associated to IgM-RF in 3/9 (33.3%) studies, to IgA-RF in 6/12 (50%) and to IgG-RF in 2/8 (25%). IgM-RF isotype predicted response to treatment in none of the 5 articles which analysed the issue, IgA-RF in 3/6 (50%) and IgG-RF in 1/4 (25%).
To recapitulate, results on the role of RF isotypes for RA prognosis are conflicting and not comparable, as not all isotypes were always considered and outcomes were defined differently. Therefore, data are only described, to avoid risk of biases in a statistical analysis.
Discussion
This is the first systematic review and meta-analysis addressing the role of RF isotypes in RA diagnosis and prognosis. This is of relevance since RF is included in the classification criteria for RA8 with the same importance of ACPA and its levels are relevant for the classification criteria, as values more than three times the upper limit of normal for the laboratory and the assay account for half the score required to classify an arthritis as RA. No specific assay is suggested to determine RF, in agreement with the finding that none proved globally superior to the others.18 Nonetheless, the isotype determination is not required in the classification criteria but according to the results of this meta-analysis this could be of some relevance for RA diagnosis. Whether testing all RF isotypes with latex test or nephelometry allows for the highest sensitivity (68.6%, 95% CI 66.2 to 71.0), the determination of IgA isotype provides the highest specificity (91.4%, 95% CI 90.8 to 92.0) and the highest LR− (7.7, 95% CI 5.7 to 10.4), while the determination of IgM isotype provides the higher DOR (21.7, 95% CI 16.1 to 29.3). Further, even when testing ACPA, RF isotype determination appears to increase diagnostic accuracy.15 22 23 27 30
Results on specificity deserve a separate comment, as a few studies show low RF specificity.18 25 Poor quality of the assay, low cut-off levels or inappropriate control cohorts could account for these results. Similarly, a paper showed a very high specificity with 95% CI 88% to 100%, likely due to the low number of healthy controls.16 We included all these studies in our analysis to provide a comprehensive review, despite some data should be critically evaluated. Further, relevant data can be gathered, as prognostic information according to RF isotype presence (online supplemental tables 5–7).
From the results of our study, therefore, a relevant role of RF isotypes could be in the evaluation of seronegative arthritis, adding further information on the serologic state of patients and potentially modifying the diagnosis to RA. In patients with synovitis, ACPA positivity alone allows higher specificity than RF positivity alone. However, when ACPA are negative, positivity for multiple RF isotypes allows higher specificity, despite being rarely seen in ACPA negative patients. This could be particularly relevant for early arthritis, where a portion of patients might be seronegative when testing RF and ACPA, but might reveal positive if tested for RF isotypes, thereby modifying the diagnosis. Reaching a prompt diagnosis, introducing an adequate treatment, and planning a tight control can impact prognosis and late outcome.
Limitations to the meta-analysis on the role of isotypes in diagnosis can be that it included retrospective studies, single-centre studies and small case numbers. Further, it considers a period of time during which the classificatory criteria for diagnosis have changed, therefore, disease diagnosis may not be uniform among studies.
As for the significance of RF isotypes for RA prognosis, results are conflicting and this is a limitation of this study. Overall, RF isotypes seem to be associated with disease activity, radiographic progression or response to treatment in 50% or less of the studies analysing the issue, with the exception of IgM-RF, which appeared to be associated with higher disease activity in 60% of the studies. These contrasting findings may in part be due to the scarce homogeneity of the papers analysed, as not all isotypes were always considered and outcomes were defined differently, therefore results are not easily comparable.
There are no previous systematic reviews or meta-analyses to compare the results of our study with; nonetheless, in the literature there are a few reviews addressing the topic. A meta-analysis comparing ACPA and RF stated that ACPA specificity is higher compared with RF, while IgM-RF sensitivity was slightly higher; LR among the three RF isotypes were similar; further, ACPA better predicted erosive disease.44 A subsequent study reported that ACPA are more specific than RF isotypes but that RF isotypes can be useful in predating the diagnosis; regarding prognosis, limited data did not allow a definite conclusion.5 In another study, ACPA sensitivity was higher than IgM-RF and IgA-RF, while specificity was similar; LR+ and LR− were better when testing ACPA.45 Another one concluded that in RA with high disease activity ACPA have higher sensitivity than all RF isotypes.46 47
In conclusion, from the result of our study, RF isotypes may add diagnostic information and prove useful in specific patient population, such as those with seronegative arthritis. From the studies analysed, RF isotypes do not appear to provide relevant prognostic information, as results are conflicting.
Footnotes
Contributors: FM, NB and CS conceived the study, performed the literature search and wrote the paper. DG performed the statistical analysis. MI, BP, FF and GDS revised the manuscript. All authors approved the final version for submission. NB is the author responsible for the overall content as guarantor.
Funding: This systematic review and meta-analysis was supported by ThermoFisher Diagnostics.
Competing interests: FM has received grant/research support from ThermoFisher Diagnostics. NB has received speaker honoraria for lectures from Werfen and ThermoFisher Diagnostics. DG has received speaker honoraria for lectures from DASIT and support for attending meetings and/or travel from Abbott, Stago, DiaSorin, Medical Systems/Snibe, Siemens, Werfen and Roche. FF has acted as a consultant for ThermoFisher Diagnostics. GDS has acted as a consultant for ThermoFisher Diagnostics. CS has received grant/research support from AbbVie, Amgen and Pfizer Inc. CS has acted as a consultant for and has received lecture fees from AbbVie, Amgen, Alfa-Wassermann, Biogen, Eli-Lilly, Galapagos, Janssen, Novartis, Pfizer and SOBI.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. 10.1056/NEJMra1004965 [DOI] [PubMed] [Google Scholar]
- 2.van Delft MAM, Huizinga TWJ. An overview of autoantibodies in rheumatoid arthritis. J Autoimmun 2020;110:102392. 10.1016/j.jaut.2019.102392 [DOI] [PubMed] [Google Scholar]
- 3.Waaler E. On the occurrence of a factor in human serum activating the specific agglutintion of sheep blood corpuscles. APMIS 2007;115:422–38; 10.1111/j.1600-0463.2007.apm_682a.x [DOI] [PubMed] [Google Scholar]
- 4.Pike RM, Sulkin SE, Coggeshall HC. Serological reactions in rheumatoid arthritis. J Immunol 1949;63:441–6. 10.4049/jimmunol.63.4.441 [DOI] [PubMed] [Google Scholar]
- 5.Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers 2013;35:727–34. 10.1155/2013/726598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 7.Lee DM, Schur PH. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann Rheum Dis 2003;62:870–4. 10.1136/ard.62.9.870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 9.Wu C-Y, Yang H-Y, Luo S-F, et al. From rheumatoid factor to anti-citrullinated protein antibodies and anti-carbamylated protein antibodies for diagnosis and prognosis prediction in patients with rheumatoid arthritis. IJMS 2021;22:686. 10.3390/ijms22020686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 11.Bizzaro N, Villalta D, Giavarina D, et al. Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of metanalysis. Autoimmun Rev 2012;12:97–106. 10.1016/j.autrev.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 12.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293–316. 10.1002/sim.4780121403 [DOI] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 14.Nell-Duxneuner V, Machold K, Stamm T, et al. Autoantibody profiling in patients with very early rheumatoid arthritis: a follow-up study. Ann Rheum Dis 2010;69:169–74. 10.1136/ard.2008.100677 [DOI] [PubMed] [Google Scholar]
- 15.Elshafie AI, Elbagir S, Aledrissy MIE, et al. Occurrence of anti-CCP2 and RF isotypes and their relation to age and disease severity among Sudanese patients with rheumatoid arthritis. Clin Rheumatol 2019;38:1545–53. 10.1007/s10067-019-04431-6 [DOI] [PubMed] [Google Scholar]
- 16.Bobbio-Pallavicini F, Caporali R, Alpini C, et al. High IgA rheumatoid factor levels are associated with poor clinical response to tumour necrosis factor alpha inhibitors in rheumatoid arthritis. Ann Rheum Dis 2007;66:302–7. 10.1136/ard.2006.060608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser H, Gelinck LB, Kampfraath AH, et al. Diagnostic and prognostic characteristics of the enzyme linked immunosorbent rheumatoid factor assays in rheumatoid arthritis. Ann Rheum Dis 1996;55:157–61. 10.1136/ard.55.3.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bas S, Perneger TV, Kunzle E, et al. Comparative study of different enzyme immunoassays for measurement of Igm and IgA rheumatoid factors. Ann Rheum Dis 2002;61:505–10. 10.1136/ard.61.6.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saraux A, Berthelot JM, Chalès G, et al. Value of laboratory tests in early prediction of rheumatoid arthritis. Arthritis Rheum 2002;47:155–65. 10.1002/art.10241 [DOI] [PubMed] [Google Scholar]
- 20.Rantapää-Dahlqvist S, de Jong BAW, Berglin E, et al. Antibodies against cyclic Citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. 10.1002/art.11223 [DOI] [PubMed] [Google Scholar]
- 21.Brink M, Hansson M, Mathsson-Alm L, et al. Rheumatoid factor isotypes in relation to antibodies against citrullinated peptides and carbamylated proteins before the onset of rheumatoid arthritis. Arthritis Res Ther 2016;18:43. 10.1186/s13075-016-0940-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaskowski TD, Hill HR, Russo KL, et al. Relationship between rheumatoid factor isotypes and IgG anti-cyclic citrullinated peptide antibodies. J Rheumatol 2010;37:1582–8. 10.3899/jrheum.091236 [DOI] [PubMed] [Google Scholar]
- 23.Sieghart D, Platzer A, Studenic P, et al. Determination of autoantibody isotypes increases the sensitivity of serodiagnostics in rheumatoid arthritis. Front Immunol 2018;9:876. 10.3389/fimmu.2018.00876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelmenson LB, Wagner BD, McNair BK, et al. Timing of elevations of autoantibody isotypes prior to diagnosis of rheumatoid arthritis. Arthritis Rheumatol 2020;72:251–61. 10.1002/art.41091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermosillo-Villafranca JA, Guillén-Lozoya AH, Vega-Morales D, et al. Role of rheumatoid factor isotypes and anti-citrullinated peptide antibodies in the differential diagnosis of non-selected patients with inflammatory arthralgia. Reumatol Clin (Engl Ed) 2021;17:12–5. 10.1016/j.reuma.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 26.Greiner A, Plischke H, Kellner H, et al. Association of anti-cyclic citrullinated peptide antibodies, anti-citrullin antibodies, and Igm and IgA rheumatoid factors with serological parameters of disease activity in rheumatoid arthritis. Ann N Y Acad Sci 2005;1050:295–303. 10.1196/annals.1313.031 [DOI] [PubMed] [Google Scholar]
- 27.Mikuls TR, Holers VM, Parrish L, et al. Anti-cyclic citrullinated peptide antibody and rheumatoid factor isotypes in African Americans with early rheumatoid arthritis. Arthritis Rheum 2006;54:3057–9. 10.1002/art.22200 [DOI] [PubMed] [Google Scholar]
- 28.Janssen KMJ, Hop H, Vissink A, et al. Levels of anti-citrullinated protein antibodies and rheumatoid factor, including IgA isotypes, and articular manifestations in ulcerative colitis and Crohn’s disease. Int J Environ Res Public Health 2020;17:8054. 10.3390/ijerph17218054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pertsinidou E, Manivel VA, Westerlind H, et al. Rheumatoid arthritis autoantibodies and their association with age and sex. Clin Exp Rheumatol 2021;39:879–82. 10.55563/clinexprheumatol/4bcmdb [DOI] [PubMed] [Google Scholar]
- 30.Bas S, Genevay S, Meyer O, et al. Anti‐cyclic citrullinated peptide antibodies, Igm and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology (Oxford) 2003;42:677–80. 10.1093/rheumatology/keg184 [DOI] [PubMed] [Google Scholar]
- 31.Van Hoovels L, Vander Cruyssen B, Sieghart D, et al. Iga rheumatoid factor in rheumatoid arthritis. Clin Chem Lab Med 2022;60:1617–26. 10.1515/cclm-2022-0244 [DOI] [PubMed] [Google Scholar]
- 32.Vittecoq O, Pouplin S, Krzanowska K, et al. Rheumatoid factor is the strongest Predictor of radiological progression of rheumatoid arthritis in a three-year prospective study in community-recruited patients. Rheumatology (Oxford) 2003;42:939–46. 10.1093/rheumatology/keg257 [DOI] [PubMed] [Google Scholar]
- 33.Manfredsdottir VF, Vikingsdottir T, Jonsson T, et al. The effects of tobacco smoking and rheumatoid factor seropositivity on disease activity and joint damage in early rheumatoid arthritis. Rheumatology (Oxford) 2006;45:734–40. 10.1093/rheumatology/kei240 [DOI] [PubMed] [Google Scholar]
- 34.Berglin E, Johansson T, Sundin U, et al. Radiological outcome in rheumatoid arthritis is predicted by presence of antibodies against cyclic citrullinated peptide before and at disease onset, and by IgA-RF at disease onset. Ann Rheum Dis 2006;65:453–8. 10.1136/ard.2005.041376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrawal S, Misra R, Aggarwal A. Autoantibodies in rheumatoid arthritis: association with severity of disease in established RA. Clin Rheumatol 2007;26:201–4. 10.1007/s10067-006-0275-5 [DOI] [PubMed] [Google Scholar]
- 36.Lequerré T, Jouen F, Brazier M, et al. Autoantibodies, Metalloproteinases and bone markers in rheumatoid arthritis patients are unable to predict their responses to Infliximab. Rheumatology (Oxford) 2007;46:446–53. 10.1093/rheumatology/kel262 [DOI] [PubMed] [Google Scholar]
- 37.Ateş A, Kinikli G, Turgay M, et al. Effects of rheumatoid factor isotypes on disease activity and severity in patients with rheumatoid arthritis: a comparative study. Clin Rheumatol 2007;26:538–45. 10.1007/s10067-006-0343-x [DOI] [PubMed] [Google Scholar]
- 38.Loët XL, Brazier M, Mejjad O, et al. Serum IgA rheumatoid factor and pyridinoline in very early arthritis as predictors of Erosion(S) at two years: a simple model of prediction from a conservatively treated community-based inception cohort. Arthritis Care Res 2010;62:1739–47. 10.1002/acr.20321 [DOI] [PubMed] [Google Scholar]
- 39.Fabris M, De Vita S, Blasone N, et al. Serum levels of anti-CCP antibodies, anti-MCV antibodies and RF IgA in the follow-up of patients with rheumatoid arthritis treated with rituximab. Auto Immun Highlights 2010;1:87–94. 10.1007/s13317-010-0013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markatseli TE, Voulgari PV, Alamanos Y, et al. Prognostic factors of radiological damage in rheumatoid arthritis: a 10-year retrospective study. J Rheumatol 2011;38:44–52. 10.3899/jrheum.100514 [DOI] [PubMed] [Google Scholar]
- 41.Can M, Najip A, Yılmaz N, et al. Immunoglobulin subtypes predict therapy response to the biologics in patients with rheumatoid arthritis. Rheumatol Int 2013;33:1455–60. 10.1007/s00296-012-2560-8 [DOI] [PubMed] [Google Scholar]
- 42.Sakthiswary R, Shaharir SS, Mohd Said MS, et al. Iga rheumatoid factor as a serological predictor of poor response to tumour necrosis factor Α inhibitors in rheumatoid arthritis. Int J Rheum Dis 2014;17:872–7. 10.1111/1756-185X.12443 [DOI] [PubMed] [Google Scholar]
- 43.Oka S, Higuchi T, Furukawa H, et al. Serum rheumatoid factor IgA, anti-citrullinated peptide antibodies with secretory components, and anti-carbamylated protein antibodies associate with interstitial lung disease in rheumatoid arthritis. BMC Musculoskelet Disord 2022;23:46. 10.1186/s12891-021-04985-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura K, Sugiyama D, Kogata Y, et al. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 2007;146:797–808. 10.7326/0003-4819-146-11-200706050-00008 [DOI] [PubMed] [Google Scholar]
- 45.Conrad K, Roggenbuck D, Reinhold D, et al. Profiling of rheumatoid arthritis associated autoantibodies. Autoimmun Rev 2010;9:431–5. 10.1016/j.autrev.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 46.Vallbracht I, Helmke K. Additional diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Autoimmun Rev 2005;4:389–94. 10.1016/j.autrev.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 47.Vallbracht I, Rieber J, Oppermann M, et al. Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann Rheum Dis 2004;63:1079–84. 10.1136/ard.2003.019877 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002817supp001.pdf (658.3KB, pdf)
Data Availability Statement
Data are available on reasonable request.