Structured Abstract
Introduction
The PTGS2 gene codes for the cyclooxygenase-2 (COX-2) enzyme that catalyzes the committed step in prostaglandin (PG) synthesis. Various in-vivo and in-vitro data suggest that prostaglandin E2 mediates as a signaling molecule for activating the VEGF signaling pathway (VSP), forming an association between COX-2 and VSP. Several chemotherapy regimens increasingly rely on preventing the synthesis of PGs. The targeted and metronomic chemotherapy agents, which suppress the COX-2 enzymes, have a major role in suppressing the oral cancer cascade. Hence, this study was designed to understand the pattern of PTGS2 expression and genes regulating VSP in head and neck cancers.
Methods
PTGS2 expression was analyzed in the TCGA database computationally with the help of the UALCAN web-server. The expression of VEGF signaling pathway genes was mined, and their expression pattern was determined. Co-expression analysis was done to elucidate the association between VEGF signaling genes and PTGS2. The ShineyGo web server was used for gene set enrichment.
Results
Significantly high PTGS2 expression was observed in tumor samples. Further genes regulating VEGF signaling were significantly overexpressed in tumor samples. Co-expression analysis results showed a significant positive correlation between PTGS2 and angiogenesis-regulating genes. The majority of the genes were enriched for angiogenesis pathways.
Conclusion
PTGS2 was significantly expressed in head and neck cancer, and its expression was associated with genes regulating angiogenesis.
Keywords: PTGS2, VEGF, Head and neck, Angiogenesis, Gene expression, Cancer
Graphical abstract
1. Introduction
Squamous cell carcinoma of the head and neck is the sixth most frequent cancer worldwide and the most prevalent cancer in South Asia accounting for about 890,000 reported new cases and around 450,000 reported fatalities occurring globally. According to the Global Cancer Observatory (GLOBOCAN), 1.08 million new cases of malignancies of the Head and Neck region will be diagnosed each year by 2030, and about a 30% increase in the disease's occurrence.1,2 Since the proliferation and migration of cancer cells depend on an adequate supply of oxygen and nutrients, angiogenesis is an important occurrence in the natural history of cancer.3 Numerous malignancies, including squamous cell carcinoma of the head and neck, have been studied to determine the function of angiogenesis. Numerous pro- and anti-angiogenic molecules control the highly dynamic and complicated angiogenic process. The angiogenic switch to an angiogenic phenotype, in which proangiogenic pathways overcome or avoid negative regulators of angiogenesis, is regarded as a distinguishing feature of cancer progression.4 To date, many molecules have been isolated that regulate angiogenesis and have a significant role in promoting cancer.
One such molecule is the cyclooxygenase-2 (COX-2) enzyme, which is coded by the gene Prostaglandin-endoperoxide synthase 2 (PTGS2). COX-2 expression is negligible in normal tissue but can be induced with the help of a variety of stimuli for inflammatory responses. COX-2 enhances apoptotic resistance, proliferation, angiogenesis, inflammation, invasion, and metastasis of cancer cells. It also causes cancer stem cell (CSC)-like activities.5 Human tumors have an increase in the number of blood vessels, which is related to COX-2, which is produced in tumor endothelial cells and is necessary for cancer cell migration.6
Prostaglandins (PGs) are metabolites of arachidonic acid whose synthesis is regulated by COX-2. PGs take part in various biological pathways mainly as lipid mediators in various tissues. Many studies suggest the association of various PGs to regulate angiogenesis by inducing vascular endothelial growth factor (VEGF) expression in endothelial cells, especially prostaglandin E2 (PGE2), which is a product synthesized as a downstream pathway of arachidonic acid metabolism regulated by the COX-2 enzyme (Fig. 1).8 Due to the ability of PGs to bind with VEGF, COX-2 inhibitors can reduce angiogenesis in cancer cells.7 The most potent inducer of angiogenesis, endothelial cell proliferation, and capillary permeability is the VEGF which differs from other growth factors since it is a secreted protein, an endothelial cell-specific mitogen in-vitro, and the only reported growth factor capable of increasing vascular permeability. In osteoblasts, synovial fibroblasts, and other cell types cultured, PGE2 stimulates VEGF expression.9 Inhibiting COX-2 activity and hence prostaglandin generation has been demonstrated in studies to drastically lower VEGF expression, block angiogenesis, and restrict the growth of cancer cells.10
Fig. 1.
The role of COX-2 in inducing sustained angiogenesis and tumor progression by activating the VEGF signaling pathway with the aid of prostaglandin E2 (PGE2).
Various in-vitro and in-vivo data suggest the association of COX-2 with the VEGF pathway in various cancers, but the genetic basis is yet to be explored in detail, especially with head and neck squamous cell carcinoma (HNSCC) tumor samples. Hence, this study was designed to assess the relationship between PTGS2 gene expression and the expression of genes regulating the VEGF signaling pathway with their paired normal by averaging the expression of HNSCC tumor samples in-silico. The link between PTGS2 and the VEGF signaling pathway was further established by gathering data from gene enrichment analysis and pathway analysis.
2. Methodology
2.1. Data acquisition and gene expression pattern
Data sets with information on mRNA expression in HNSCC from The Cancer Genome Atlas (TCGA), PanCancer Atlas data were selected, and the genes of interest were queried in cBioportal data sets (https://www.cbioportal.org/) for mRNA expression z-scores relative to normal samples (log RNA Seq V2 RSEM) with a Z score threshold of 2.0 and a protein expression Z score. Oncoprint was generated to illustrate the mRNA expression of PTGS2 and VEGF signaling pathway genes (genes regulating angiogenesis).
2.2. Gene expression analysis in HNSCC
Gene expression of the PTGS2 gene and VEGF signaling pathway was analyzed in normal vs. tumor samples and across tumor grade in TCGA HNSCC data sets using the University of Alabama at Birmingham Cancer Data Analysis Portal (UALCAN)11,12 (http://ualcan.path.uab.edu). Gene expression of PTGS2 and the VEGF signaling pathway was assayed in TCGA-HNSCC samples based on critical molecular signatures, i.e., TP53 mutation and Human papillomavirus (HPV) infection status, using the UALCAN cancer data analysis portal. TCGA whole exome sequencing data are used to determine the status of the TP53 mutation. From the Genomic Data Commons site, we downloaded Mutation Annotation Format (MAF) data (derived from VarScan 2). Using RNA-seq data, the samples with and without the TP53 mutation were compared. The p16 and in-situ hybridization data were considered while considering the HPV status in the HNSCC data sets. The significance of the difference between normal and tumor tissue was estimated using the student's t-test considering unequal variance. One-way analysis of variance (ANOVA) was used to determine the significant difference between the expression of normal samples and the expression across tumor grades (based on the American Joint Committee on Cancer for pathologic tumor grade) and in critical molecular markers, such as the status of the TP53 mutation and HPV status (p16 and ISH). The mRNA expression is expressed as transcription per million (TPM) for all the selected genes.
2.3. Co-expression analysis
Co-expression of the PTGS2 gene with VEGF signaling pathway genes was done using cBioportal online database (https://www.cbioportal.org/.) in HNSCC (TCGA, PanCancer Atlas) data sets. Pearson's correlation analysis was used to compare the expression between two genes [RSEM (Batch normalized from Illumina HiSeq_RNASeqV2): VEGFA (log2)].
2.4. Pathway analysis
A graphical tool for gene enrichment analysis ShinyGO 0.76 (http://bioinformatics.sdstate.edu) was used, and the gene of interest was queried for gene ontology enrichment analysis done in the GO Biological Process database with a false detection rate (FDR) cutoff of 0.05.
3. Results
3.1. mRNA expression pattern of PTGS2 and genes regulating VEGF signaling pathway in HNSCC
In the data of HNSCC (TCGA, PanCancer Atlas), with a sample size of 523 samples, around 328 of the samples had been shown to have altered genes in the queried genes. Out of all the altered genes, VEGFA showed the highest percent of alteration in mRNA expression at about 28% in the selected data sets, followed by PTGS2 with 23% of the alteration. In the C-X-C motif chemokine receptor 1 (CXCR1) and C-X-C motif chemokine receptor 2 (CXCR2) genes, the alteration was observed to be 18% and 17%, respectively. Kinase insert domain receptor (KDR) showed alteration in 4% of the sample, followed by VEGFB with the lowest alteration.
3.2. Expression of PTGS2 and VEGF pathway genes in HNSCC
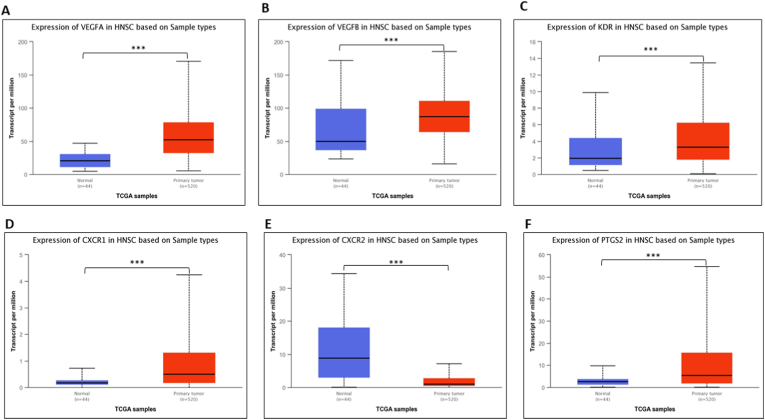
Based on TCGA-HNSCC gene expression data, the expression of PTGS2 and VEGF signaling pathway genes was explored by comparing normal and tumor tissue samples. All the genes, i.e., VEGFA, VEGFB, KDR, CXCR1, CXCR2, and PTGS2, showed a significant difference in expression between normal and tumor tissue samples (Fig. 2). All genes, including PTGS2, showed higher median expression in tumor tissue when compared to normal tissue, except CXCR2, where higher median expression was observed in normal tissue when compared to tumor tissue.
Fig. 2.
The gene expression analysis of genes, i.e., A. VEGFA, B. VEGFB, C. KDR, D. CXCR1, E. CXCR2, and F. PTGS2, in HNSCC in tumor samples as compared to normal samples using the UALCAN web server. (***p < 0.0001).
3.3. Gene expression based on tumor grade in HNSCC
The expression of PTGS2 and VEGF signaling pathway genes was carried out with the help of the UALCAN cancer analysis portal. A significant difference in expression was observed between different tumor-grade samples when compared to normal (Fig. 3). VEGFA gene expression was observed more in grade 2 and grade 3 tumors, with median expression being 52.401 TPM and 52.281 TPM, whereas normal tissue samples had a median expression of 20.59 TPM. In the VEGF-B gene, grade 3 tumor samples showed higher expression, with a median expression of 101.28 TPM in tumor samples compared to normal samples with 49.65 TPM. Grade 4 tumor samples expressed KDR genes with an expression of 5.217 TPM, whereas the normal tissue sample expression was 1.93 TPM. CXCR1 was more expressed in grade 1 tumors compared to other tumor-grade samples when compared to the normal expression of 0.167 TPM. The expression trend shown by CXCR2 was different when compared to other genes, where the CXCR2 gene was more expressed in normal tissue samples, i.e., a median expression of 8.807 TPM when compared to the tumor. Grade 1 tumor samples were found to have more expression, with the median being 2.249 TPM for the CXCR2 gene. PTGS2 gene expression was highest in grade 4 cancers, with a median expression of 18.73 TPM. Normal samples were observed to have a median gene expression of 2.58 TPM for the PTGS2 gene.
Fig. 3.
The gene expression analysis of genes, i.e., A. VEGFA, B. VEGFB, C. KDR, D. CXCR1, E. CXCR2, and F. PTGS2, in HNSCC in tumor grades as compared to normal samples using the UALCAN web server. (***p < 0.0001).
3.4. Gene expression in critical molecular signatures in HNSCC
In patients with HPV-negative status, PTGS2, KDR, CXCR1, and CXCR2 expression was significantly high compared to its paired normal. Similarly, VEGFA and VEGFB were found to be more expressed in patients with HPV-positive status. In tumor samples with TP53 mutation, high expression of VEGFA, VEGFB, CXCR1, and PTGS2 was observed whereas KDR and CXCR2 was more expressed in TP53 non-mutant samples. The results obtained are shown graphically in Fig. 4, Fig. 5.
Fig. 4.
The gene expression analysis of genes, i.e., A. VEGFA, B. VEGFB, C. KDR, D. CXCR1, E. CXCR2, and F. PTGS2, in HNSCC with HPV status using the UALCAN web server. (***p < 0.0001).
Fig. 5.
The gene expression analysis of genes, i.e., A. VEGFA, B. VEGFB, C. KDR, D. CXCR1, E. CXCR2, and F. PTGS2, in HNSCC with TP53 mutation status using the UALCAN web server. (***p < 0.0001).
3.5. Co-expression analysis using cBioPortal
The co-expression analysis was done using cBioPortal to use Pearson correlation to measure the strength of the linear relationship between the expression of two genes. Co-expression analysis was done between PTGS2 expression and VEGF signaling pathway genes. A positive correlation was observed between PTGS2 and VEGF pathway genes except for VEGFB, where a negative correlation was observed with the PTGS2 gene (Fig. 6).
Fig. 6.
Co-expression analysis using cBioPortal between A. VEGFA vs. PTGS2, B. VEGFB vs. PTGS2, C. KDR vs. PTGS2, D. CXCR1 vs. PTGS2, and E. CXCR2 vs. PTGS2.
3.6. Gene set enrichment analysis
To assess the pathways associated with PTGS2 genes and to decipher the role of PTGS2 in oncogenesis, gene set enrichment analysis was done using ShinyGo gene ontology software. The queried gene sets for GO biological processes were found to be associated with various biological pathways, i.e., sprouting angiogenesis, regulation of angiogenesis, regulation of chemotaxis, and various other pathways linked to angiogenesis (Fig. 7). Details of the result obtained by the GO biological process are given in Table 1.
Fig. 7.
The PTGS2-correlated transcripts and enrichment analysis using ShinyGO, the lollipop plot illustrating the enriched pathways (FDR 0.05).
Table 1.
List of top significant pathways in GO Biological process with FDR cutoff 0.05.
| Enrichment FDR | nGenes | Pathway Genes | Fold Enrichment | Pathway |
|---|---|---|---|---|
| 4.86E-06 | 4 | 131 | 116.0102 | Sprouting angiogenesis |
| 4.86E-06 | 4 | 164 | 92.66667 | Positive regulation of angiogenesis |
| 4.86E-06 | 4 | 154 | 98.68398 | Positive regulation of chemotaxis |
| 4.86E-06 | 3 | 28 | 407.0714 | Regulation of positive chemotaxis |
| 4.86E-06 | 3 | 27 | 422.1481 | Positive regulation of positive chemotaxis |
| 4.86E-06 | 5 | 343 | 55.38387 | Cell chemotaxis |
| 4.86E-06 | 3 | 27 | 422.1481 | Vascular wound healing |
| 4.86E-06 | 3 | 22 | 518.0909 | Positive regulation of cell migration involved in sprouting angiogenesis |
| 4.86E-06 | 4 | 164 | 92.66667 | Positive regulation of vasculature development |
| 5.10E-06 | 5 | 544 | 34.92034 | Angiogenesis |
| 5.14E-06 | 5 | 556 | 34.16667 | Positive regulation of cell migration |
| 5.14E-06 | 2 | 2 | 3799.333 | Interleukin-8-mediated signaling pathway |
| 5.14E-06 | 5 | 597 | 31.82021 | Positive regulation of locomotion |
| 5.14E-06 | 5 | 595 | 31.92717 | Positive regulation of cellular component movement |
| 5.14E-06 | 3 | 36 | 316.6111 | Angiogenesis involved in wound healing |
| 5.14E-06 | 5 | 581 | 32.6965 | Positive regulation of cell motility |
| 5.81E-06 | 5 | 622 | 30.54126 | Positive regulation of response to external stimulus |
| 5.81E-06 | 5 | 635 | 29.91601 | Blood vessel morphogenesis |
| 5.81E-06 | 3 | 39 | 292.2564 | Regulation of cell migration involved in sprouting angiogenesis |
| 6.51E-06 | 6 | 1590 | 14.33711 | Cell migration |
| 6.77E-06 | 3 | 43 | 265.0698 | Vascular endothelial growth factor signaling pathway |
| 7.50E-06 | 5 | 717 | 26.49465 | Blood vessel development |
| 7.50E-06 | 5 | 699 | 27.17692 | Chemotaxis |
| 7.50E-06 | 5 | 702 | 27.06078 | Taxis |
| 7.50E-06 | 4 | 239 | 63.58717 | Regulation of chemotaxis |
| 7.50E-06 | 2 | 3 | 2532.889 | Response to interleukin-8 |
| 7.50E-06 | 2 | 3 | 2532.889 | Cellular response to interleukin-8 |
| 8.17E-06 | 5 | 748 | 25.39661 | Vasculature development |
| 8.17E-06 | 6 | 1776 | 12.83559 | Cell motility |
| 8.17E-06 | 6 | 1776 | 12.83559 | Localization of cell |
4. Discussion
HNSCC is a very deadly malignancy that costs the lives of many people around the globe. Despite various advances in anti-cancer therapy, recurrence and metastasis of the disease are common among patients, leading to a poor prognosis for treatment.13 Angiogenesis is a well-studied hallmark of cancer and draws the attention of scientists working on anti-cancer therapy. Many studies have established the importance of angiogenesis in tumor progression and its pivotal role in locally advanced and higher grades of HNSCC. Various chemotherapeutic regimes aim to target angiogenesis by inhibiting various molecules regulating angiogenesis, which is a promising strategy leading to better survival in patients with HNSCC.10 The PTGS2 gene encodes the protein COX-2 enzyme, also known as prostaglandin epoxide synthase, which is needed for the conversion of arachidonic acid to PGs. Cyclooxygenase-1 (COX-1) and COX-2 are the two COX isoforms that have been identified. COX-1 is produced naturally in the gastric mucosa, whereas COX-2 is particularly inducible in areas of inflammation and malignancy.14 Data from in-vitro and in-vivo investigations show that PGs produced by the COX-2 enzyme are critical for cancer initiation, promotion, and progression.15 COX-2 converts arachidonic acid to prostaglandin H2 (PGH2), which is subsequently converted to PGE2, an eicosanoid molecule that stimulates the manufacture and release of VEGF, a powerful angiogenic growth factor.16 It was demonstrated in Apc/COX-2 double knockout mice that stromal COX-2 expression is required for the production of VEGF and, as a result, tumor angiogenesis.17 As a result, we hypothesized that PTGS2 expression and the expression of genes regulating VEGF signaling are associated, which serves as the foundation for this study because all of the evidence is based on in-vitro and in-vivo studies, making it necessary to validate the expression pattern of the selected genes in human tissue samples using in-silico analysis of the TCGA data in HNSCC tumor tissue samples.
In a study done by Cherie-Ann et al.,18 COX-2 was expressed in HNSCC when compared to normal; similarly, we observed a higher gene expression of PTGS2 (the gene coding COX-2) in HNSCC tumor samples when compared to normal samples, and even significantly higher expression was observed in grade 4 tumor samples when compared to normal. In a study carried out by Salven P. et al. and A. Giatromanolaki et al.,19 expression of VEGF and KDR was found to be overexpressed in HNSCC,19 and a similar trend was observed with other genes where the genes regulating angiogenesis were more expressed in tumor tissue when compared to normal. A different anomaly was observed with CXCR2 gene expression, where its expression was higher in normal samples when compared to tumor samples, contrary to the results obtained in various other studies.20 A similar trend was seen with CXCR2 expression in an in-silico study by Shen Y et al.21 aimed to decipher the expression levels of the CXC chemokine receptor gene family and to determine its prognostic value in HNSCC. It was found that CXCR2 expression in HNSCC was higher in normal tissue compared to tumor tissue in ONCOMINE datasets. When gene expression in different tumor grades is assessed, it is found that grade 4 tumors exhibit significantly greater levels of KDR expression. VEGFB had more expression in grade 3 tumors; similar results were observed in a similar study done by Vickie et al.22 where VEGF-B was significantly expressed in grade 3 tumor specimens. All genes were significantly expressed in grade 1 and 2 tumors.
In a study done by O. Gallo et al.,23 COX-2 mRNA and protein expression were higher as compared to normal, which was correlated to increased VEGF mRNA expression. Similarly, we observed the co-expression between the PTGS2 gene and the VEGF protein gene (both VEGF-A and VEGF-B). A positive correlation was observed with VEGF-A, whereas a negative correlation was observed between PTGS2 and VEGF-B expression. A positive correlation between the expression of PTGS2 and KDR, CXCR1, and CXCR2 was also apparent. Following the results obtained by the gene set enrichment analysis, PTGS2, and VEGF signaling genes showed positive enrichment in various pathways that are linked to the process of neovascularization, supporting the notion that PTGS2 genes and VEGF signaling pathways work synergistically to induce, support, and sustain tumor angiogenesis, leading to disease progression.
In a prospective phase II clinical trial to elucidate the association of VEGF expression with HPV status in patients with oropharyngeal squamous cell carcinomas, it was found that the expression of VEGF mRNA was significantly increased in HPV p16 positive samples.24 A similar trend was observed in-silico, where the expression of VEGFA and VEGFB genes in the HPV-positive samples was higher compared to HPV p16 negative samples in TCGA HNSCC data. In-vitro data suggest that p16 oncoproteins, i.e., E5, E6, and E7, can induce VEGF expression independent of the p53 pathway, providing an insight into high VEGF expression in HPV-negative samples.25 In a study done by Tarikh et al.,26 it was found that COX-2 expression is not correlated with HPV status in patients diagnosed with laryngeal squamous cell carcinoma. A similar trend was observed in our study; it was observed that PTGS2 expression was low in HPV-positive patients as compared to normal samples. According to in-vitro data, E6 and E7 oncoproteins were found to induce COX-2 enzyme expression, but a similar trend was not observed in-silico in patient tumor samples.27 Though PTGS2 expression was found to be higher in TP53 mutant cells, clinically, no correlation has been found between the two factors in disease progression and survival.28 Several studies support the hypothesis that p53 regulates the angiogenesis process via the expression of VEGF29; parallelly, in this study, a similar expression pattern was observed where the expression of VEGFA and VEGFB was higher in tumor samples with TP53 mutations.
The above findings aid us in deciphering that the PTGS2 gene might play a role in inducing and regulating angiogenesis in HNSCC. COX-2, the protein product of the PTGS2 gene, as such does not participate in tumor development, but the prostaglandins produced by the enzyme, especially PGE2, act as a signaling molecule to further initiate the expression of VEGF, which aids in sustained angiogenesis.30 It has been demonstrated experimentally that PGE2 can be considered a connecting link between COX-2 expression and activation of the VEGF signaling pathway, which further leads to angiogenesis in the in-vitro model.31 Although many unique compounds that block VEGF have been identified so far, their usage in routine clinical practice is limited, and newly discovered medications must first pass through a highly rigorous screening process before being adopted as standard practice. Therefore, it is crucial to use medications that are already in use in standard clinical practice. In that regard, the PTGS2 gene has emerged as a potent druggable target, and COX-2 inhibition has proven to be a promising strategy to treat malignancies of the head and neck region, having a further downstream effect on the VEGF signaling pathway.7,15 Clinically, COX-2 inhibition has proven to improve survival opportunities for patients. Many drugs have been developed that target PTGS2, especially non-steroidal anti-inflammatory drugs (NSAIDs), which act directly on the cyclooxygenase (both COX-1 and COX-2) enzymes. Drugs like celecoxib that specifically act on the COX-2 enzyme, inhibiting its function, have been proven clinically to be very efficient in curbing cancer progression, especially in head and neck cancers.32 COX-2 inhibitors have now been employed in various multi-drug chemotherapeutic regimens to treat head and neck cancers, like metronomic chemotherapy, which acts on various druggable targets, including COX-2, and has been shown to increase patient survival.32,33
The objective of the study to establish a relation between PTGS2 and angiogenesis using in-silico analysis (using various bioinformatics and gene ontology databases) was reached, but this study has various limitations where different databases with heterogeneous datasets have been used to assess the expression pattern of the queried genes. Except for the ShinyGO gene ontology database, all other analysis was done using TCGA datasets for HNSCC. Another major limitation of the in-silico gene expression approach is the cumbersome nature of the subsequent data analysis. The trend of expression identified in the above-mentioned datasets is particular to a specific population and may not apply to all ethnic groups; hence, the results thus obtained need to be further validated.
Nevertheless, this study was done with the perspective of predicting the role of PTGS2 as a potent druggable target, its expression pattern, and the associated molecular pathways linked to it. Although bioinformatics predictions can foretell expression patterns, the association of various genes with various pathways may cause us to augment or underestimate the role of PTGS2. To validate the aforesaid findings, future experiments aimed at validating the current predictions are required, like gene expression studies on patient samples. To further confirm the role of PTGS2 in angiogenesis, gene knockout studies using primary cell cultures from patient samples will be required. A transcriptomic examination of patient tissues can help us construct a more complete picture of PTGS2 and its role in disease progression. Hence, future research done to validate the role of PTGS2 in inducing angiogenesis will aid in increasing the utility of PTGS2 application clinically in HNSCC.
5. Conclusion
We may infer from the data above that PTGS2 gene expression significantly affects the expression of the VEGF signaling pathway and further promotes angiogenesis in head and neck malignancies. Drugs that inhibit the activity of the PTGS2 enzyme have shown promising outcomes in delaying disease progression and maintaining tumor dormancy, although the mechanism of action of these drugs is still unclear. The in-silico investigation indicated conclusively that PTGS2 plays an indispensable role in modulating sustained angiogenesis in cancer tissue and is one of many pathways altered by PTGS2 expression. The aforementioned hypothesis needs to be tested and validated experimentally because of certain limitations of in-silico analysis.
Data availability statement
The data used in this article are freely available in The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov), cBioportal (https://www.cbioportal.org/), UALCAN (http://ualcan.path.uab.edu) and ShineyGO (http://bioinformatics.sdstate.edu/go/) database.
Ethics approval
This was a study based on public data from TCGA, cBioportal, and UALCAN databases. Thus, the study was exempt from approval of the Institutional Review Board since all data used had been authenticated and publicly available.
Funding
No funding was obtained for this study.
Declaration of competing interest
The authors declare no conflict of interest.
References
- 1.Johnson D.E., Burtness B., Leemans C.R., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6(1) doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Döme B., Hendrix M.J.C., Paku S., Tóvári J., Tímár J. Alternative vascularization mechanisms in cancer: pathology and therapeutic implications. Am J Pathol. 2007;170(1):1–15. doi: 10.2353/ajpath.2007.060302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lugano R., Ramachandran M., Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77(9):1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rumzhum N.N., Ammit A.J. Cyclooxygenase 2: its regulation, role and impact in airway inflammation. Clin Exp Allergy. 2016;46(3):397–410. doi: 10.1111/cea.12697. [DOI] [PubMed] [Google Scholar]
- 6.Hida K., Ohga N., Akiyama K., Maishi N., Hida Y. Heterogeneity of tumor endothelial cells. Cancer Sci. 2013;104(11):1391–1395. doi: 10.1111/cas.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L., Stevens J., Hilton M.B., et al. COX-2 inhibition potentiates antiangiogenic cancer therapy and prevents metastasis in preclinical models. Sci Transl Med. 2014;6(242) doi: 10.1126/scitranslmed.3008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyde C.A.C., Missailidis S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int Immunopharmacol. 2009;9(6):701–715. doi: 10.1016/j.intimp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Nör J.E., Christensen J., Mooney D.J., Polverini P.J. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154(2):375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tosetti F., Ferrari N., De Flora S., Albini A. ‘Angioprevention’: angiogenesis is a common and key target for cancer chemopreventive agents. Faseb J. 2002;16(1):2–14. doi: 10.1096/fj.01-0300rev. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekar D.S., Karthikeyan S.K., Korla P.K., et al. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia (United States) 2022;25(C):18–27. doi: 10.1016/j.neo.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrashekar D.S., Bashel B., Balasubramanya S.A.H., et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia (United States) 2017;19(8):649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meir H., Kenter G., Burggraaf J., et al. The need for improvement of the treatment of advanced and metastatic cervical cancer, the rationale for combined chemo-immunotherapy. Anti Cancer Agents Med Chem. 2014;14(2):190–203. doi: 10.2174/18715206113136660372. [DOI] [PubMed] [Google Scholar]
- 14.Hla T., Bishop-Bailey D., Liu C.H., Schaefers H.J., Trifan O.C. Cyclooxygenase-1 and -2 isoenzymes. Int J Biochem Cell Biol. 1999;31(5):551–557. doi: 10.1016/s1357-2725(98)00152-6. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh N., Chaki R., Mandal V., Mandal S.C. Cox-2 as a target for cancer chemotherapy. Pharmacol Rep. 2010;62(2):233–244. doi: 10.1016/s1734-1140(10)70262-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L., Wu Y., Xu Z., et al. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J Cell Mol Med. 2012;16(8):1840–1855. doi: 10.1111/j.1582-4934.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seno H., Oshima M., Ishikawa T.O., et al. Cyclooxygenase 2- and prostaglandin E2 receptor EP2-dependent angiogenesis in ApcΔ716 mouse intestinal polyps. Cancer Res. 2002;62(2):506–511. [PubMed] [Google Scholar]
- 18.Nathan C.A.O., Leskov I.L., Lin M., et al. COX-2 expression in dysplasia of the head and neck: correlation with eIF4E. Cancer. 2001;92(7):1888–1895. doi: 10.1002/1097-0142(20011001)92:7<1888::aid-cncr1706>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Giatromanolaki A., Sivridis E., Athanassou N., et al. The angiogenic pathway “vascular endothelial growth factor/flk-I (KDR)-receptor” in rheumatoid arthritis and osteoarthritis. J Pathol. 2001;194(1):101–108. doi: 10.1002/path.842. [DOI] [PubMed] [Google Scholar]
- 20.Chan L.P., Wang L.F., Chiang F.Y., Lee K.W., Kuo P.L., Liang C.H. IL-8 promotes HNSCC progression on CXCR1/2-meidated NOD1/RIP2 signaling pathway. Oncotarget. 2016;7(38):61820–61831. doi: 10.18632/oncotarget.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Y., Zhou C., Cao Y., et al. Expression profile and prognostic value of CXCR family members in head and neck squamous cell carcinoma. World J Surg Oncol. 2022;20(1):1–15. doi: 10.1186/s12957-022-02713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanrahan V., Currie M.J., Gunningham S.P., et al. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200(2):183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- 23.Gallo O., Franchi A., Magnelli L., et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3(1):53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo S., Juhasz A., Zhang K., et al. Human papillomavirus infection as a prognostic factor in oropharyngeal squamous cell carcinomas treated in a prospective phase II clinical trial. Anticancer Res. 2009;29(5):1467–1474. [PMC free article] [PubMed] [Google Scholar]
- 25.Pal A., Kundu R. Human papillomavirus E6 and E7: the cervical cancer hallmarks and targets for therapy. Front Microbiol. 2020;10(January) doi: 10.3389/fmicb.2019.03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartholomeusz A., Locarnini S. Associated with antiviral therapy. Antivir Ther. 2006;55(November 2005):52–55. [Google Scholar]
- 27.Subbaramaiah K., Dannenberg A.J. Cyclooxygenase-2 transcription is regulated by human papillomavirus 16 E6 and E7 oncoproteins: evidence of a corepressor/coactivator exchange. Cancer Res. 2007;67(8):3976–3985. doi: 10.1158/0008-5472.CAN-06-4273. [DOI] [PubMed] [Google Scholar]
- 28.Atula T., Hedström J., Ristimäki A., et al. Cyclooxygenase-2 expression in squamous cell carcinoma of the oral cavity and pharynx: association to p53 and clinical outcome. Oncol Rep. 2006;16(3):485–490. [PubMed] [Google Scholar]
- 29.Riedel F., Götte K., Schwalb J., Schäfer C., Hörmann K. Vascular endothelial growth factor expression correlates with p53 mutation and angiogenesis in squamous cell carcinoma of the head and neck. Acta Otolaryngol. 2000;120(1):105–111. doi: 10.1080/00016480060203334. [DOI] [PubMed] [Google Scholar]
- 30.Ma X., Holt D., Kundu N., et al. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE2-mediated immunosuppression and inhibits breast cancer metastasis. OncoImmunology. 2013;2(1) doi: 10.4161/onci.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai R., Soreghan B., Szabo I.L., Pavelka M., Baatar D., Tarnawski A.S. Prostaglandin E2, transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8(3):289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 32.Kamal M.V., Rao M., Damerla R.R., et al. A mechanistic Review of methotrexate and celecoxib as a potential metronomic chemotherapy for oral squamous cell carcinoma. Cancer Invest. 2022;0(0):1–11. doi: 10.1080/07357907.2022.2139840. [DOI] [PubMed] [Google Scholar]
- 33.Parikh P.M., Hingmire S.S., Deshmukh C.D. Selected current data on metronomic therapy (and its promise) from India. South Asian J Cancer. 2016;5(2):37–47. doi: 10.4103/2278-330X.181623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this article are freely available in The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov), cBioportal (https://www.cbioportal.org/), UALCAN (http://ualcan.path.uab.edu) and ShineyGO (http://bioinformatics.sdstate.edu/go/) database.