Highlights
-
•
Xenobiotic metabolic process was significantly associated with resistance to PD-1/PD-L1 inhibitors in HNSCC.
-
•
ABCB11 was accumulated in immature TLSs and showed reduced PFS and OS after PD-1/PD-L1 inhibitors therapy in HNSCC.
-
•
ABCB11 participated in xenobiotic metabolic process and was associated with immunosuppressive cells infiltration.
-
•
Co-expression of ABCB11 and CYP1A2 in immature TLSs had positive correlation with immunosuppressive Treg cells infiltration.
Keywords: ABCB11, CYP1A2, Tertiary lymphoid structures, Xenobiotic metabolic process, PD-1/PD-l1 inhibitor, Treg, HNSCC
Abstract
Head and neck squamous cell carcinomas (HNSCC) are at a high risk of recurrence and multimodal therapy have not significantly improved survival in recent decades. Although immune checkpoint inhibitors (ICIs) are effective in a small proportion of HNSCC patients, the majority do not respond. In this study, we for the first time revealed that xenobiotic metabolic process was significantly associated with resistance to programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors in HNSCC and found that ATP binding cassette subfamily B member 11 (ABCB11) accumulated in immature tertiary lymphoid structures (TLSs) predicted worse progression-free survival (PFS) and overall survival (OS) after PD-1/PD-L1 inhibitors therapy. Moreover, the expression of cytochrome P450 1A2 (CYP1A2), a cytochrome P450 (CYP) enzyme that participates in xenobiotic metabolic process, was significantly upregulated in CD45+ABCB11+ tumor-infiltrating lymphocytes (TILs) compared with CD45+ABCB11−TILs in HNSCC tissues. Whole slide scans of 110 HNSCC tissues with hematoxylin-eosin (HE) and multispectral immuno-fluorescent (mIF) staining revealed that ABCB11 had a high co-expression with CYP1A2 in immature TLSs, and colocalization of ABCB11 and CYP1A2 in immature TLs significantly associated with high infiltration of immunosuppressive T-regulatory (Treg). Our study revealed that ABCB11 accumulated in immature TLSs might upregulate CYP1A2 to mediate xenobiotic metabolic process, thus increase the immunosuppressive Treg infiltration, and induce resistance to PD-1/PD-L1 inhibitors in HNSCC.
Introduction
Head and neck squamous cell carcinomas (HNSCC) is the sixth most common malignancy worldwide and carries a high risk of recurrence [1]. The programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) have approved by the Food and Drug Administration for the treatment of metastatic or unresectable HNSCC, but only about 20% of patients achieve a clinical benefit, highlighting the need for new therapeutic targets [2]. Xenobiotic metabolism plays a critical role in the progression of HNSCC. As two important xenobiotics, tobacco and alcohol are strong risk factors for developing HNSCC. Xenobiotic-metabolizing enzymes mainly activate the carcinogens contained in tobacco and alcohol through oxidation, convert them into active metabolites, and irreversibly react with macromolecules, resulting in mutations and potential carcinogenic effects. Interestingly, xenobiotics may also activate inflammatory cells to release inflammatory mediators. It has been reported that xenobiotic metabolism could affect the tumor microenvironment (TME) of colon cancer and immune regulation may be one of the mechanisms of xenobiotic metabolism affecting cancer process [3]. However, no studies have yet focused on the correlation between xenobiotic metabolism and immune microenvironment in HNSCC.
Tertiary lymphoid structures (TLSs), clusters of immune cells located around tumor tissue, have been shown to be involved in anti-tumor immunity. The formation of TLSs relies on several chemokines produced in response to various inflammatory stimuli. Pathologic studies have identified several TLS maturation stages [4], [5], [6], and one feature associated with mature TLS is the formation and presence of germinal centers [6]. Immature TLSs showed elevated expression of immune Inhibitory and immunosuppressive molecules, and favor immune evasion and cancer progression in hepatocellular carcinoma [7], but the role of immature TLSs in HNSCC is unknown.
In this study, we for the first time revealed that xenobiotic metabolic process was significantly associated with resistance to programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors in HNSCC and found that ATP binding cassette subfamily B member 11 (ABCB11) accumulated in immature tertiary lymphoid structures (TLSs) predicted worse progression-free survival (PFS) and overall survival (OS) after PD-1/PD-L1 inhibitors therapy. Moreover, the expression of cytochrome P450 1A2 (CYP1A2), a cytochrome P450 (CYP) enzyme that participates in xenobiotic metabolic process, was significantly upregulated in CD45+ABCB11+ tumor-infiltrating lymphocytes (TILs) compared with CD45+ABCB11−TILs in HNSCC tissues. Whole slide scans of 110 HNSCC tissues with hematoxylin-eosin (HE) and multispectral immuno-fluorescent (mIF) staining revealed that ABCB11 had a high co-expression with CYP1A2 in immature TLSs, and colocalization of ABCB11 and CYP1A2 in immature TLs significantly associated with high infiltration of immunosuppressive T-regulatory (Treg). Our study revealed that ABCB11 accumulated in immature TLSs might upregulate CYP1A2 to mediate xenobiotic metabolic process, thus increase the immunosuppressive Treg infiltration, and induce resistance to PD-1/PD-L1 inhibitors in HNSCC.
Methods
Bioinformatics analysis
The bulk RNA-seq data (FPKM value) of 102 and 28 HNSCC tissues with treatment of PD-1/PD-L1 inhibitors was downloaded from the Gene Expression Omnibus (GEO, GSE159067) and Mendeley Data (https://data.mendeley.com/datasets/yk8wj7xgdg/1) respectively. The bulk RNA-seq data and clinical-related information of HNSCC tissues were collected from The Cancer Genome Atlas (TCGA) dataset obtaining from the online data portal UCSC Xena (https://xenabrowser.net/datapages/). Gene Set Enrichment Analysis (GSEA) was performed with an online tool (https://www.omicstudio.cn/tool), and Gene Ontology (GO) enrichment analysis was performed using the DAVID online analysis tool (https://david.ncifcrf.gov/conversion.jsp?VFROM=NA). Immune Cell Abundance Identifier (ImmuCellAI, http://bioinfo.life.hust.edu.cn/ImmuCellAI#!/analysis) was used to estimate the immune cell infiltration from gene expression dataset [8].
Patients and samples
A total number of 113 HNSCC patients who had no prior antitumor therapy and underwent curative resection at Tianjin Medical University Cancer Institute and Hospital were enrolled in this study. Among them, 110 HNSCC patients (39 oral cancer, 33 throat cancer, 38 tongue cancer) provided paraffin-embedded tissues for HE and mIF staining. 3 HNSCC patients (1 oral cancer, 1 throat cancer, 1 tongue cancer) provided fresh tumor tissues for flow cytometry and western blotting. All cases were diagnosed as HNSCC by 2 experienced pathologists separately, and more than 50% of the cancer cell content was determined for all paraffin-embedded tissues. We also collected clinical data including age, sex, tobacco and alcohol consumption, tumor size, differentiation grade and lymph node metastasis. Current smokers of ≥20 cigarettes/day for ≥30 years were defined as person with active tobacco consumption [9], others were defined as person with unactive tobacco consumption. Current drinkers of 100 g/day for ≥10 years were defined as person with active alcohol consumption, others were defined as person with unactive alcohol consumption. This study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital, and informed consent was obtained from all patients. TLS located inside tumors were assessed on HE stained sections, and they were defined as clusters of immune cells [7]. They were further classified as published previously: 1) immature TLSs: vague, ill-defined clusters of lymphocytes or round-shaped clusters of lymphocytes without significant germinal center; 2) mature TLSs: follicles with germinal center formation [4,10,11].
MIF staining and analysis
We performed mIF staining as previously described [12]. In brief, the paraffin-embedded slides were heated, deparaffinized using xylene, and rehydrated in graded alcohols. After antigen retrieval and blocking, the primary antibody (Table S1) was applied and incubated at 4℃ overnight. Opal polymer horseradish peroxidase (HRP) was used as the secondary antibody. The slides were washed, and tyramide signal amplification (TSA) dye (Opal 7 Color Kit, Akoya Biosciences) was applied. The slides were then microwaved to strip the primary and secondary antibodies, washed, and blocked again using blocking solution. Afterward, a second primary antibody and 4′,6′-diamidino-2-phenylindole (DAPI) were applied. Finally, slides were coverslipped using ProLong Gold Antifade Reagent (P36930, Invitrogen).
Whole slide scans were performed using a multispectral microscope (Pannoramic MIDI, 3DHISTECH), and were imaged to observe the landscape of ABCB11 and CYP1A1 expression, as well as infiltration of Treg and T helper 2 (Th2) cells. Briefly, ABCB11+ cells>10% in a TLS was defined as ABCB11+TLS. CYP1A2+ cells>10% in a TLS was defined as CYP1A2+TLS. Cells with colocalization of ABCB11 and CYP1A2 >10% in a TLS was defined as ABCB11+CYP1A2+TLS. Treg cells were counted for positively stained FOXP3 cells per 200 × magnification.
Flow cytometry
Tumor tissues preparation and flow cytometry were performed as previously described [13]. Tumor tissues were prepared into single-cell suspension by enzymatic digestion (collagenase IV 1 mg/ml and Dnase I 50 μg/ml). All single-cell suspension was passed through a 70-μm cell strainer (BD Falcon, New Jersey, USA) to remove impurities. Live/Death cells were selected/excluded based on Zombie Violet™ Fixable Viability Kit (423,113, Biolegend). PerCP/Cyanine5.5 anti-human CD45 antibody was used to mark TILs. Staining of intracellular ABCB11 protein was performed by use of the Fixation/Permeabilization Solution Kit (BD Biosciences) according to the manufacturer's instructions and then, were stained with ABCB11 antibody (1:500, ab255605, Abcam). A Goat anti rabbit IgG (Alexa Fluor® 488, ab150077) at 1/2000 dilution was used as the secondary antibody. Flow cytometry was performed to isolate CD45+ABCB11+TILs and CD45+ABCB11−TILs to compare CYP1A2 expression.
Western blotting
Western blotting was performed as previously described [12]. Briefly, we generated total protein from each group by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and transferred the proteins to polyvinylidene fluoride membranes. The membranes were incubated with the primary antibody CYP1A2 (1:1000, 19,936–1-AP, Proteintech) and GAPDH (1:2000, sc-47,724, Santa Cruz Biotechnology,) overnight at 4 °C and then incubated with secondary antibodies at room temperature for 1 h. Afterward, the membranes were exposed using an enhanced chemiluminescence reagent.
Statistical analysis
Statistical analysis was performed using GraphPad software (GraphPad Prism 9.0.0). We used Student's t-test to compare quantitative data between groups and the chi-square test to compare categorical variables. The Kaplan-Meier method was used to calculate OS and PFS, and the log-rank test was used to analyze any differences. p values < 0.05 were considered statistically significant.
Results
Xenobiotic metabolic process was significantly associated with resistance to PD-1/PD-L1 inhibitors in hnscc
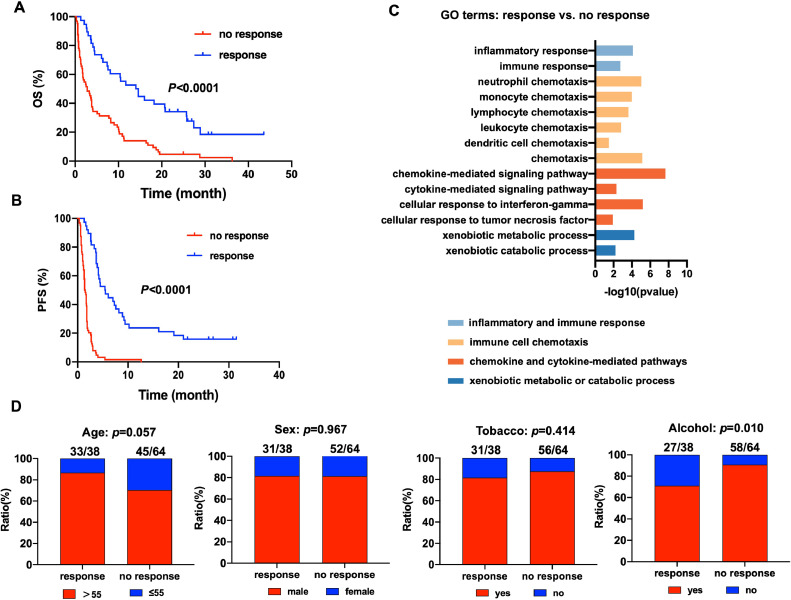
To explore the molecular mechanism underlying the resistance of HNSCC cells to anti-PD-1 therapy, we collected the gene expression profiles of 102 biopsied or surgically resected HNSCC tumor tissues which collected prior to treatment of PD-1/PD-L1 inhibitors from previously published datasets. We defined the patients with complete response, partial response and stable disease after treatment of PD-1/PD-L1 inhibitors as “response” group and defined the patients with progressive disease as “no response” group. Kaplan-Meier survival analysis demonstrated that the OS and PFS of “no response” group were significantly shorter than “response” group (Fig.1A-B). GO enrichment analysis revealed that besides pathways involved in regulating immune functions, such as inflammatory and immune response, immune cell chemotaxis, chemokine and cytokine-mediated pathways, pathways about xenobiotic metabolic or catabolic process were also enriched in “response” group compared with “no response” group (Fig.1C). The patients with drinking history tends to no response to PD-1/PD-L1 inhibitors compared with those without drinking history (p = 0.010, Fig.1D). These results suggested that xenobiotic metabolic process might play a key role in mediating resistance to PD-1/PD-L1 inhibitors in HNSCC.
Fig. 1.
Xenobiotic metabolic process was significantly associated with resistance to PD-1/PD-L1 inhibitors in HNSCC. (A) Kaplan-Meier survival analysis of the OS of “no response” group and “response” group. (B) Kaplan-Meier survival analysis of the PFS of “no response” group and “response” group. (C) GO enrichment analysis of the pathways enriched in “response” group compared with “no response” group. (D) The correlations of age, sex, tobacoo and alcohol states with response to PD-1/PD-L1 inhibitors.
ABCB11 was accumulated in immature TLSs and showed reduced PFS and OS after PD-1/PD-L1 inhibitors therapy in HNSCC
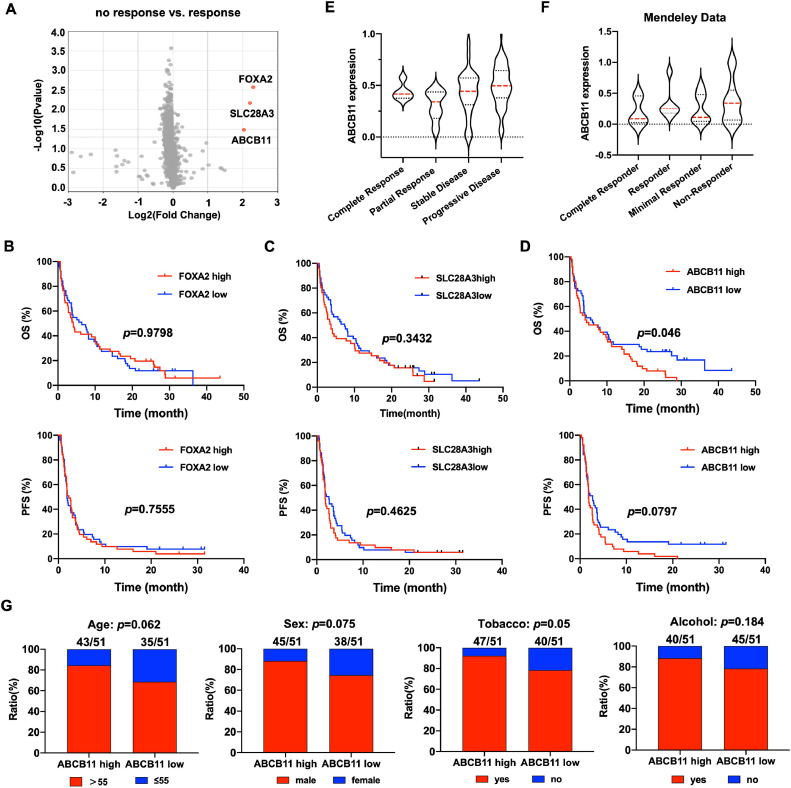
To further explore the key molecules regulating the resistance to PD-1/PD-L1 inhibitors therapy, we compared the differentially expressed genes (DEGs) in “response” group and “no response” group, and found that FOXA2, SLC28A3 and ABCB11 were significantly upregulated in “no response” group compared with “response” group (FOXA2: log2FC=2.296, p = 0.0027; SLC28A: log2FC=2.203, p = 0.0068; ABCB11: log2FC=2.029, p = 0.0329)(Fig.2A). To examine the effect the FOXA2, SLC28A3 and ABCB11 genes on the PD-1/PD-L1 inhibitors efficacy, the Kaplan-Meier survival analysis was used to analyze the OS and PFS of patients with different expression level of the 3 genes after PD-1/PD-L1 inhibitors therapy. The results showed that patients with ABCB11 high expression showed shorter PFS and OS compared with those with ABCB11 low expression after PD-1/PD-L1 inhibitors therapy, while no difference of OS and PFS were observed in patients with different expression of FOXA2 and SLC28A3 (Fig.2B-D). Moreover, ABCB11 was higher in patients with progressive disease than those with complete response, partial response and stable disease (Fig.2E). The consistent result was observed in another dataset of 28 HNSCC tissues with treatment of PD-1 inhibitors, ABCB11 was higher in non-responder than complete responder, responder and minimal responder (Fig.2F). Moreover, ABCB11 high expression was related to tobacco history (Fig.2G).
Fig. 2.
HNSCC patients with ABCB11 high expression showed reduced PFS and OS after PD-1/PD-L1 inhibitors therapy. (A) The volcano map of differentially expressed genes in “response” group and “no response” group. The Kaplan-Meier survival analysis was used to analyze the OS and PFS of patients with different expression level of the (B) FOXA2, (C) SLC28A and (D) ABCB11 after PD-1/PD-L1 inhibitors therapy. The expression of ABCB11 was compared in HNSCC patients with different response to PD-1/PD-L1 inhibitors therapy in (E) GEO cohort and (F) Mendeley Data cohort. (G) The correlations of age, sex, tobacoo and alcohol states with ABCB11 expression in GEO cohort.
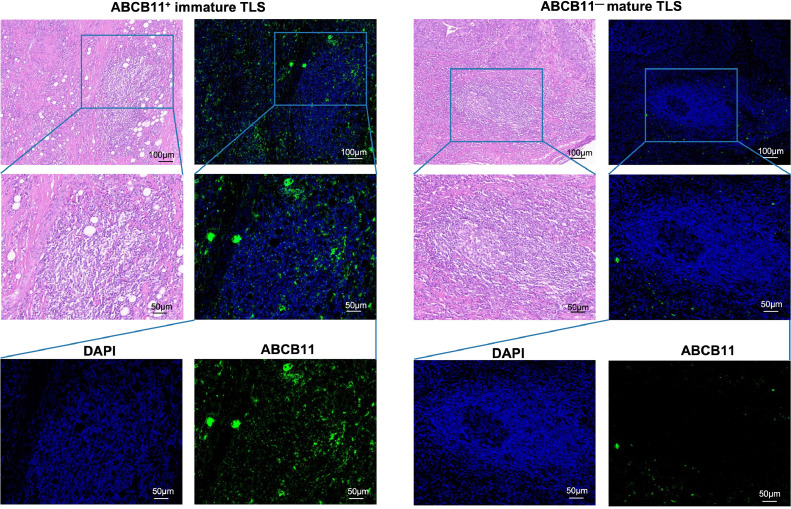
To detect the location of ABCB11, we performed HE and IF staining in 110 HNSCC tissues. The results showed that ABCB11 was mainly expressed in immune cells and accumulated in TLSs. Interestingly, we found that almost all ABCB11+ TLSs were immature TLSs which have no significant germinal center formation (Fig.3). Importantly, the active status of alcohol and tobacco consumption, larger tumor size, poor differentiation and lymphatic metastasis were significantly observed in the tissues with ABCB11+ TLSs than those without ABCB11+ TLSs (including tissues with ABCB11− TLSs and tissues without TLS) (p<0.05, Table 1). The above evidence showed that ABCB11 accumulated in immature TLSs might was conductive to tumor progression and induced resistance to PD-1/PD-L1 inhibitors in HNSCC.
Fig. 3.
ABCB11 was accumulated in immature TLSs of NHSCC tissues. Representative images of ABCB11+ immature TLS and ABCB11— mature TLS in HNSCC tissues.
Table 1.
Correlations between ABCB11+TLS C and clinicopathologic parameters.
| Clinical parameters | Tissues with ABCB11+TLSs (34) | Tissues without ABCB11+TLSs (96) | Total (110) | P value |
|---|---|---|---|---|
| Age(year) | ||||
| <56 | 10 (27.8%) | 26 (72.2%) | 36(100%) | 0.620 |
| ≥56 | 24 (32.4%) | 50 (67.6%) | 74(100%) | |
| Gender | ||||
| male | 33(35.9%) | 59(64.1%) | 92(100%) | 0.011* |
| female | 1(5.6%) | 17(94.4%) | 18(100%) | |
| Tobacco | ||||
| active | 21(53.8%) | 18(46.2%) | 39(100%) | 0.000* |
| unactive | 8(15.1%) | 45(84.9%) | 53(100%) | |
| Alcohol | ||||
| active | 24(63.2%) | 14(36.8%) | 38(100%) | 0.000* |
| unactive | 2(5.7%) | 33(94.3%) | 35(100%) | |
| Tumor size(cm) | ||||
| ≤2.5 | 12(18.5%) | 53(81.5%) | 65(100%) | 0.000* |
| >2.5 | 21(51.2%) | 20(48.8%) | 41(100%) | |
| Differentiation | ||||
| good | 6(27.3%) | 16(72.7%) | 22(100%) | 0.033* |
| moderate | 14(24.6%) | 43(75.4%) | 57(100%) | |
| poor | 12(54.5%) | 10(45.5%) | 22(100%) | |
| Lymphatic metastasis | ||||
| yes | 18(48.6%) | 19(51.4%) | 37(100%) | 0.003* |
| no | 13(20.6%) | 50(79.4%) | 63(100%) |
ABCB11 participated in xenobiotic metabolic process and was associated with immunosuppressive cells infiltration
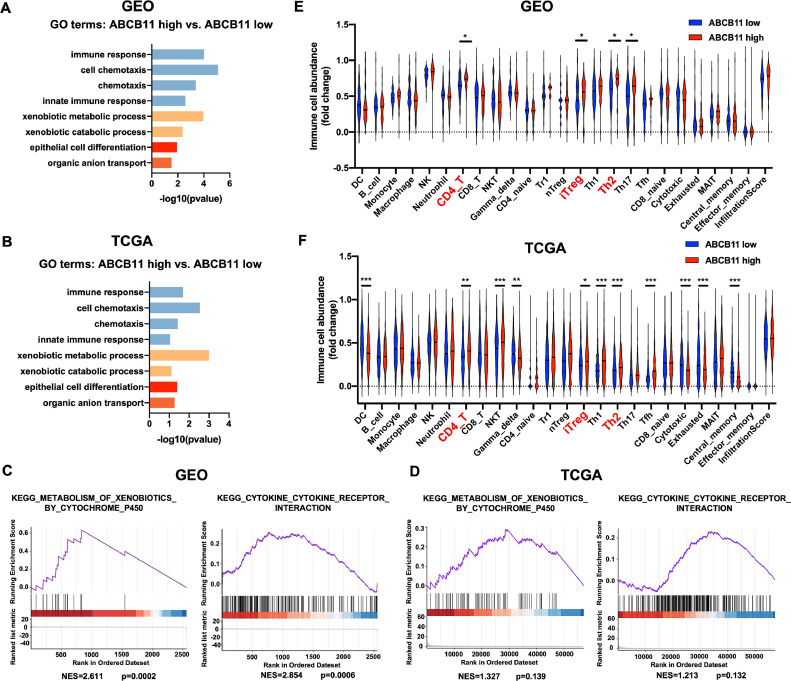
We found the DEGs between samples with high and low ABCB11 expression in GEO dataset and TCGA database and performed GO enrichment analysis of DEGs. The results showed that pathways involved in xenobiotic metabolic or catabolic process, and regulating immune cells, including immune response and cell chemotaxis, were significantly enriched in samples with high ABCB11 expression compared with those with low ABCB11 expression both in GEO dataset and TCGA database (Fig.4A-B). GSEA enrichment revealed that pathways of metabolism of xenobiotics by cytochrome P450 and cytokine-cytokine receptor interaction were significantly enriched in HNSCC tissues with ABCB11 high expression compared with those with ABCB11 low expression both in GEO dataset and TCGA database (Fig.4C-D). Next, we used the ImmuCellAI web tool to estimate the immune cell infiltration and found that CD4+ T cell, and immunosuppressive induced Treg (iTreg) and Th2 cells were significantly increased in tissues with ABCB11 high expression compared with tissues with ABCB11 low expression both in GEO dataset and TCGA database (Fig.4E-F). The results revealed that ABCB11 might participate in xenobiotic metabolic process and ABCB11 high expression was associated with immunosuppressive cells infiltration in HNSCC.
Fig. 4.
ABCB11 participated in xenobiotic metabolic process and was associated with immunosuppressive cells infiltration. GO enrichment analysis of the pathways enriched in tissues of ABCB11 high expression compared with ABCB11 low expression in (A) GEO dataset and (B) TCGA dataset respectively. GSEA enrichment analysis of the pathways about metabolism of xenobiotics by cytochrome P450 and cytokine-cytokine receptor interaction in HNSCC tissues with ABCB11 high expression compared with those with ABCB11 low expression in (C) GEO dataset and (D) TCGA database. ImmuCellAI web tool was used to estimate the immune cell infiltration in tissues with ABCB11 high expression compared with tissues with ABCB11 low expression in (E) GEO dataset and (F) TCGA database.
Colocalization of ABCB11 and CYP1A2 in immature TLSs had positive correlation with immunosuppressive Treg cells infiltration
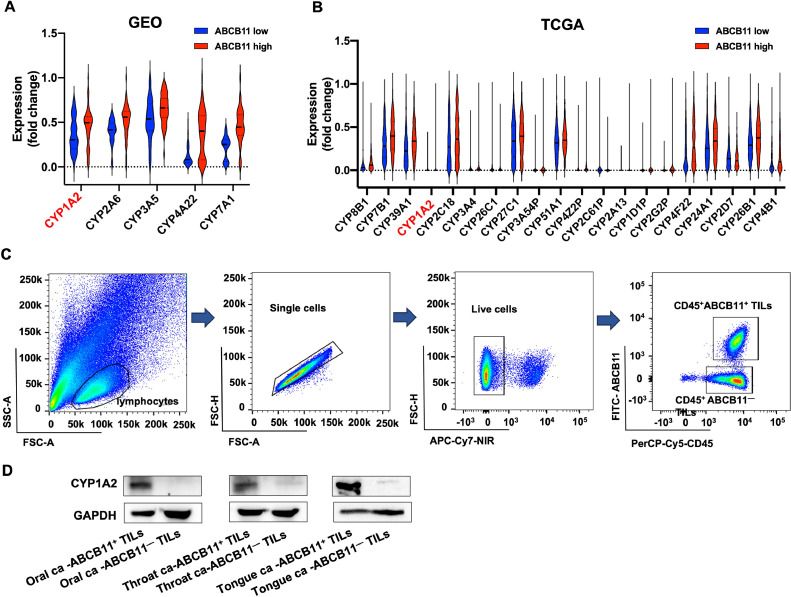
Of all the xenobiotic-metabolizing enzymes, the cytochrome P450 (CYP) enzymes are the most important due to their abundance and versatility [14]. We supposed that ABCB11 induce xenobiotic metabolic process by regulating CYP enzymes, therefore, we compared the gene expression of CYP enzymes between tissues with ABCB11 high expression and those with ABCB11 low expression. The results showed that multiple CYP family genes significantly increased in tissues with ABCB11 high expression in GEO dataset and TCGA database, and CYP1A2 was upregulated in both GEO dataset and TCGA database (Fig.5A-B).
Fig. 5.
CYP1A2 expression was significantly upregulated in CD45+ABCB11+ TILs compared with CD45+ABCB11−TILs. (A) The gene expression of CYP enzymes between tissues with ABCB11 high expression and those with ABCB11 low expression in (A) GEO dataset and (B) TCGA database. (C) Representative flow cytometry plots showing the process for sorting CD45+ABCB11+ TILs and CD45+ABCB11−TILs in NHSCC tissues. (D) Western bolt analysis of CYP1A2 expression in CD45+ABCB11+ TILs and CD45+ABCB11−TILs in HNSCC tissues.
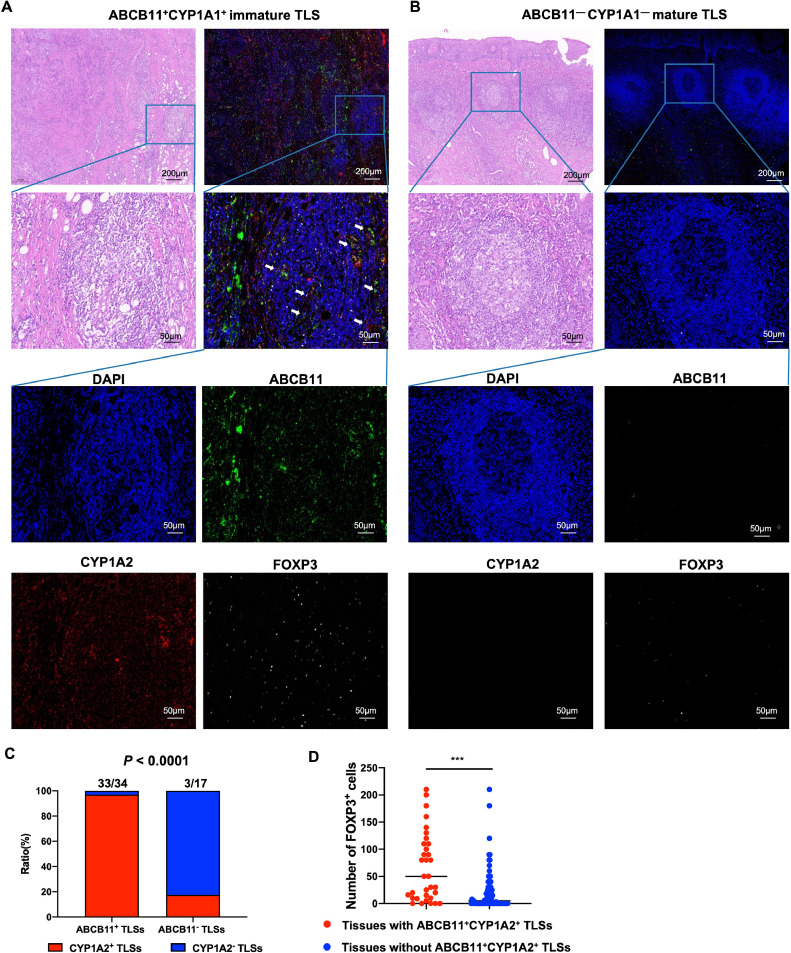
Next, we collected 3 HNSCC fresh tissues, including 1 oral cancer, 1 throat cancer and 1 tongue cancer, to isolate CD45+ABCB11+TILs and CD45+ABCB11−TILs by flow cytometry and compare CYP1A2 expression by western blot analysis. The result demonstrated that CYP1A2 expression was significantly upregulated in CD45+ABCB11+ TILs compared with CD45+ABCB11−TILs (Fig.5C-D). We further performed mIF staining of ABCB11, CYP1A2, FOXP3 and GATA3 in 110 HNSCC tissues, and whole slide scans were used to observe the landscape of ABCB11 and CYP1A1 expression, as well as infiltration of Treg and Th2 cells (Fig.6A-B). The results showed that 97.1% (33/34) tissues with ABCB11+ TLSs have colocalization of ABCB11 and CYP1A2 in TLSs, while only 17.6% (3/17) in tissues with ABCB11− TLSs expressed CYP1A2+ TLSs (p<0.0001, chi-squared test). The result revealed that ABCB11 was positively correlated with the expression of CYP1A2 in TLSs (Fig.6C). Moreover, FOXP3+ Treg cells were significantly increased in tissues with ABCB11+CYP1A2+ immature TLSs compared with tissues without ABCB11+CYP1A2+ immature TLSs (65.76±11.05 vs. 22.16±4.40, p<0.0001) (Fig.6D). However, we observed a low number of GATA3+ Th2 cells (data not shown). The results suggested that ABCB11 accumulated in immature TLSs might upregulate CYP1A2 to mediate xenobiotic metabolic process, thus increase the immunosuppressive Treg infiltration, and induce resistance to PD-1/PD-L1 inhibitors in HNSCC.
Fig. 6.
Colocalization of ABCB11 and CYP1A2 in immature TLSs had positive correlation with immunosuppressive Treg cells infiltration. (A-B) Representative images of ABCB11+CYP1A2+ immature TLS and ABCB11−CYP1A2− mature TLS in HNSCC tissues. (C) The CYP1A2 expression pattern in tissues with ABCB11+ TLSs and tissues with ABCB11−TLSs. (D) FOXP3+ Treg infiltration in tissues with ABCB11+CYP1A2+ TLSs and tissues without ABCB11+CYP1A2+ TLSs.
Discussion
In addition to the metabolism of sugar, lipid, and protein, xenobiotic metabolism is an important part of the biological processes related to human metabolism. Except for drugs, most exogenous substances are harmful to human body. Toxic xenobiotics require metabolism to induce cancer. Therefore, the metabolic process of xenobiotics is particularly important for the prognosis of cancer. Studies have shown that excessive exposure of the human body to xenobiotic substances can lead to abnormal changes in the TME [3]. Most HNSCC are squamous cell carcinomas that develop in the upper aerodigestive epithelium after exposure to xenobiotics such as tobacco and alcohol [15]. However, little is known about the biological functional molecules or immune cells involved in the xenobiotic metabolic pathways in NHSCC. In this study, we for first time found that the association of xenobiotic metabolic process with resistance to PD-1/PD-L1 inhibitors in HNSCC. The expression of ABCB11+ immature TLSs was significantly correlated with larger tumor size, poor differentiation and lymphatic metastasis, which suggested the ABCB11+ immature TLSs indicates a more aggressive HNSCC subtype with a poor prognosis. At the mechanism level, ABCB11 accumulated in immature TLSs might upregulate CYP1A2 to mediate xenobiotic metabolic process, thus increase the immunosuppressive Treg infiltration, and induce resistance to PD-1/PD-L1 inhibitors in HNSCC.
ABCB11 enables ABC-type xenobiotic transporter activity and is the major exporter for the specific secretion of bile salts [16]. ABCB11 involved in several processes, including canalicular bile acid transport, xenobiotic export from cell and xenobiotic transmembrane transport [17]. A range of human diseases is associated with the malfunction of ABCB11, including fatal hereditary liver disorders and mild cholestatic conditions [18]. However, its role in HNSCC has not been reported. Our study demonstrated that ABCB11 was significantly accumulated in immature TLSs and mediated xenobiotic metabolic process to induce resistance to immunotherapy in HNSCC. Moreover, ABCB11+ immature TLSs was significantly correlated with the active status of alcohol and tobacco consumption. The results suggested that ABCB11 in TLSs might be activated by drinking and smoking, which would further mediate the xenobiotic metabolic process in HNSCC.
The CYP enzymes are among the most important xenobiotic-metabolizing enzymes [19]. CYP enzymes are expressed in many different tissues of the human body, mostly in intestinal and hepatic tissues [20]. Many reports show that CYP enzymes also play an important role in the development of tumors. The main enzymes involved in activating carcinogens are CYP1A1, CYP1A2, CYP1B1, CYP2A6, and CYP2E1, and genetic polymorphisms in these enzymes have been associated with an increased risk of certain types of cancer [21]. Elevated expression of CYP1A1, CYP1B1 and CYP2W1 has been detected in HNSCC patients as well [22]. However, the distribution and characteristics of CYP enzymes in HNSCC are still unclear. Our study detected the accumulation of CYP1A2 in immature TLSs of HNSCC tissues and found that the colocalization of ABCB11 and CYP1A2 in immature TLSs had positive correlation with immunosuppressive Treg cells infiltration, which reveals a new mechanism of CYP enzyme-mediated tumor promotion.
Patients with NHSCC are often immunocompromised, leading to cancer immune evasion and cancer cell proliferation. Recent data suggest that subsets of immune cells found in the TME may contribute to immune evasion, thereby attenuating the effects of PD-1/PD-L1 inhibitors. Treg cells are particularly important in driving immune escape during HNSCC progression [23]. In cancers, Treg cells are suppressors of the antitumor response, leading to tumor immune escape, thereby conferring resistance to anti-PD1 therapy [24]. It was reported that infiltration of FOXP3+ Treg cells is a strong and independent prognostic factor in HNSCC [25]. These studies provided strong evidence for our hypothesis that ABCB11+CYP1A2+ immature TLSs promoted immunosuppression to induce resistance to immunotherapy by upregulation the infiltration of Treg cells in HNSCC. It is worth noting that the gene expression profiles of biopsied or surgically resected HNSCC tumor tissues was collected prior to treatment of PD-1/PD-L1 inhibitors, and ABCB11+ TLSs were detected in HNSCC tissues with no prior antitumor therapy, which reflected the expression landscape of tumor tissues without the selection for therapy. It suggested that ABCB11+ TLSs might act as a marker to predict the treatment response before immunotherapy.
Previous studies revealed the double-edged sword role of TLSs in cancer [26]. Although most studies have shown a strong association between tumor-associated TLS and favorable clinical outcomes in most cancers, some studies have shown an association between TLS and poor prognosis. Presence of B cells and TLS in melanoma, renal cell carcinoma, sarcoma and HPV-associated HNSCC has been reported to be associated with better response to immunotherapy [27], [28], [29]. While increase of Th cells and macrophages in TLS is associated with disease relapse in advanced colorectal cancer, and these TLS are considered to be immature or just breaking up [30]. The studies suggested that the different cellular composition of TLSs and their functional differences affects the patient's prognosis [31]. Our study revealed that ABCB11+ immature TLSs indicates a more aggressive HNSCC subtype with a poor prognosis, which reflected dysfunction of the anti-tumor immune reaction of ABCB11+immature TLS. However, the factors that regulate the composition of ABCB11+immature TLS are not clear, and further investigation is needed to understand their heterogeneity and functions.
Despite the promising findings obtained, our study has certain limitations. It is necessary for us to collect prognostic data for HNSCC patients receiving immunotherapy, to further verify the relationship between ABCB11+ TLSs expression and predicted prognosis. In addition, further in vitro and in vivo functional experiments are needed to demonstrate the regulatory relationships between ABCB11 and CYP1A2 in TLSs, and their effects on immunotherapy efficacy.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82,203,668,81,872,169,82,172,821,82,103,386), Tianjin Municipal Science and Technology Project (19JCYBJC27400, 21JCZDJC00360), and Beijing-Tianjin-Hebei Basic Research Cooperation Project (20JCZXJC00120), the Science &Technology Development Fund of Tianjin Education Commission for Higher Education (2021ZD033), Tianjin Medical Key Discipline (Specialty) Construction Project (TJYXZDXK-058B), Tianjin Health Research Project (TJWJ2022XK024).
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (No. EK2021149). Written informed consent was obtained from all subjects.
Patient consent for publication
Not applicable.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
CRediT authorship contribution statement
Junya Ning: Writing – original draft, Writing – review & editing. Jie Hao: Writing – original draft, Writing – review & editing. Fengli Guo: Investigation, Project administration, Writing – review & editing. Xiukun Hou: Methodology, Writing – review & editing. Lijuan Li: Investigation, Project administration, Writing – review & editing. Jinmiao Wang: Investigation, Project administration, Writing – review & editing. Shoujun Wang: Data curation, Writing – review & editing. Ying Gao: Data curation, Writing – review & editing. Xiangqian Zheng: Conceptualization, Writing – review & editing. Ming Gao: Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101747.
Appendix. Supplementary materials
References
- 1.Hunter K.D., Parkinson E.K., Harrison P.R. Profiling early head and neck cancer. Nat. Rev. Cancer. 2005;5(2):127–135. doi: 10.1038/nrc1549. [DOI] [PubMed] [Google Scholar]
- 2.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C., Worden F., Saba N.F., Iglesias Docampo L.C., Haddad R., Rordorf T., Kiyota N., Tahara M., Monga M., Lynch M., Geese W.J., Kopit J., Shaw J.W., Gillison M.L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen L., Han Z. Identification and validation of xenobiotic metabolism-associated prognostic signature based on five genes to evaluate immune microenvironment in colon cancer. J. Gastrointest. Oncol. 2021;12(6):2788–2802. doi: 10.21037/jgo-21-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderaro J., Petitprez F., Becht E., Laurent A., Hirsch T.Z., Rousseau B., Luciani A., Amaddeo G., Derman J., Charpy C., Zucman-Rossi J., Fridman W.H. Sautès-Fridman C. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J. Hepatol. 2019;70(1):58–65. doi: 10.1016/j.jhep.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Posch F., Silina K., Leibl S., Mündlein A., Moch H., Siebenhüner A., Samaras P., Riedl J., Stotz M., Szkandera J., Stöger H., Pichler M., Stupp R., van den Broek M., Schraml P., Gerger A., Petrausch U., Winder T. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7(2) doi: 10.1080/2162402X.2017.1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siliņa K., Soltermann A., Attar F.M., Casanova R., Uckeley Z.M., Thut H., Wandres M., Isajevs S., Cheng P., Curioni-Fontecedro A., Foukas P., Levesque M.P., Moch H., Linē A., van den Broek M. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer. Res. 2018;78(5):1308–1320. doi: 10.1158/0008-5472.CAN-17-1987. [DOI] [PubMed] [Google Scholar]
- 7.Meylan M., Petitprez F., Lacroix L., Di Tommaso L., Roncalli M., Bougoüin A., Laurent A., Amaddeo G., Sommacale D., Regnault H., Derman J., Charpy C., Lafdil F., Pawlotsky J.M., Sautès-Fridman C., Fridman W.H., Calderaro J. Early hepatic lesions display immature tertiary lymphoid structures and show elevated expression of immune inhibitory and immunosuppressive molecules. Clin. Cancer. Res. 2020;26(16):4381–4389. doi: 10.1158/1078-0432.CCR-19-2929. [DOI] [PubMed] [Google Scholar]
- 8.Miao Y.R., Zhang Q., Lei Q., Luo M., Xie G.Y., Wang H., Guo A.Y. ImmuCellAI: a unique method for comprehensive t-cell subsets abundance prediction and its application in cancer immunotherapy. Adv. Sci. (Weinh) 2020;7(7) doi: 10.1002/advs.201902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.G Di Credico, Edefonti V., Polesel J., Pauli F., Torelli N., Serraino D., Negri E., Luce D., Stucker I., Matsuo K., Brennan P., Vilensky M., Fernandez L., Curado M.P., Menezes A., Daudt A.W., Koifman R., Wunsch-Filho V., Holcatova I., Ahrens W., Lagiou P., Simonato L., Richiardi L., Healy C., Kjaerheim K., Conway D.I., Macfarlane T.V., Thomson P., Agudo A., Znaor A., LF Boaventura Rios, Toporcov T.N., Franceschi S., Herrero R., Muscat J., Olshan A.F., Zevallos J.P., C La Vecchia, Winn D.M., Sturgis E.M., Li G., Fabianova E., Lissowska J., Mates D., Rudnai P., Shangina O., Swiatkowska B., Moysich K., Zhang Z.F., Morgenstern H., Levi F., Smith E., Lazarus P., Bosetti C., Garavello W., Kelsey K., McClean M., Ramroth H., Chen C., Schwartz S.M., Vaughan T.L., Zheng T., Menvielle G., Boccia S., Cadoni G., Hayes R.B., Purdue M., Gillison M., Schantz S., Yu G.P., Brenner H., D'Souza G., Gross N.D., Chuang S.C., Boffetta P., Hashibe M., Lee Y.A., Dal Maso L. Joint effects of intensity and duration of cigarette smoking on the risk of head and neck cancer: A bivariate spline model approach. Oral. Oncol. 2019;94:47–57. doi: 10.1016/j.oraloncology.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkin S., Yuan D., Stein I., Taniguchi K., Weber A., Unger K., Browning J.L., Goossens N., Nakagawa S., Gunasekaran G., Schwartz M.E., Kobayashi M., Kumada H., Berger M., Pappo O., Rajewsky K., Hoshida Y., Karin M., Heikenwalder M., Ben-Neriah Y., Pikarsky E. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol. 2015;16(12):1235–1244. doi: 10.1038/ni.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakami J., Shimizu Y., Kashii Y., Kato T., Minemura M., Okada K., Nambu S., Takahara T., Higuchi K., Maeda Y., Kumada T., Watanabe A. Functional B-cell response in intrahepatic lymphoid follicles in chronic hepatitis C. Hepatology. 1999;30(1):143–150. doi: 10.1002/hep.510300107. [DOI] [PubMed] [Google Scholar]
- 12.Ning J., Ye Y., Bu D., Zhao G., Song T., Liu P., Yu W., Wang H., Li H., Ren X., Ying G., Zhao Y., Yu J. Imbalance of TGF-β1/BMP-7 pathways induced by M2-polarized macrophages promotes hepatocellular carcinoma aggressiveness. Mol. Ther. 2021;29(6):2067–2087. doi: 10.1016/j.ymthe.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S., Meng Y., Liu L., Lv Y., Yu W., Liu T., Wang L., Mu D., Zhou Q., Liu M., Ren Y., Zhang D., Li B., Sun Q., Ren X. CD4(+) T cells are required to improve the efficacy of CIK therapy in non-small cell lung cancer. Cell. Death. Dis. 2022;13(5):441. doi: 10.1038/s41419-022-04882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raunio H., Kuusisto M., Juvonen R.O., Pentikäinen O.T. Modeling of interactions between xenobiotics and cytochrome P450 (CYP) enzymes. Front. Pharmacol. 2015;6:123. doi: 10.3389/fphar.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argiris A., Karamouzis M.V., Raben D., Ferris R.L. Head and neck cancer. Lancet. 2008;371(9625):1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Hou W.T., Wang J., Xu D., Guo C., Sun L., Ruan K., Zhou C.Z., Chen Y. Structures of human bile acid exporter ABCB11 reveal a transport mechanism facilitated by two tandem substrate-binding pockets. Cell. Res. 2022;32(5):501–504. doi: 10.1038/s41422-021-00611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L., Hou W.T., Chen L., Jiang Y.L., Xu D., Sun L., Zhou C.Z., Chen Y. Cryo-EM structure of human bile salts exporter ABCB11. Cell. Res. 2020;30(7):623–625. doi: 10.1038/s41422-020-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telbisz Á., Homolya L. Recent advances in the exploration of the bile salt export pump (BSEP/ABCB11) function. Expert. Opin. Ther. Targets. 2016;20(4):501–514. doi: 10.1517/14728222.2016.1102889. [DOI] [PubMed] [Google Scholar]
- 19.Esteves F., Rueff J., Kranendonk M. The central role of cytochrome P450 in xenobiotic metabolism-A brief review on a fascinating enzyme family. J. Xenobiot. 2021;11(3):94–114. doi: 10.3390/jox11030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stavropoulou E., Pircalabioru G.G., Bezirtzoglou E. The Role of Cytochromes P450 in Infection. Front. Immunol. 2018;9:89. doi: 10.3389/fimmu.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stipp M.C., Acco A. Involvement of cytochrome P450 enzymes in inflammation and cancer: a review. Cancer. Chemother.. Pharmacol. 2021;87(3):295–309. doi: 10.1007/s00280-020-04181-2. [DOI] [PubMed] [Google Scholar]
- 22.Presa D., Khurram S.A., Zubir A.Z.A., Smarakan S., Cooper P.A., Morais G.R., Sadiq M., Sutherland M., Loadman P.M., McCaul J., Shnyder S.D., Patterson L.H., Pors K. Cytochrome P450 isoforms 1A1, 1B1 AND 2W1 as targets for therapeutic intervention in head and neck cancer. Sci. Rep. 2021;11(1):18930. doi: 10.1038/s41598-021-98217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maggioni D., Pignataro L., Garavello W. T-helper and T-regulatory cells modulation in head and neck squamous cell carcinoma. Oncoimmunology. 2017;6(7) doi: 10.1080/2162402X.2017.1325066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleh R., Elkord E. Treg-mediated acquired resistance to immune checkpoint inhibitors. Cancer. Lett. 2019;457:168–179. doi: 10.1016/j.canlet.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Seminerio I., Descamps G., Dupont S., de Marrez L., Laigle J.A., Lechien J.R., Kindt N., Journe F., Saussez S. Infiltration of FoxP3+ Regulatory T Cells is a Strong and Independent Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Cancers. (Basel) 2019;11(2) doi: 10.3390/cancers11020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang W., Feng Z., Luo J., He Z., Liu J., Wu J., Rong P. Tertiary lymphoid structures in cancer: the double-edged sword role in antitumor immunity and potential therapeutic induction strategies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.689270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helmink B.A., Reddy S.M., Gao J., Zhang S., Basar R., Thakur R., Yizhak K., Sade-Feldman M., Blando J., Han G., Gopalakrishnan V., Xi Y., Zhao H., Amaria R.N., Tawbi H.A., Cogdill A.P., Liu W., LeBleu V.S., Kugeratski F.G., Patel S., Davies M.A., Hwu P., Lee J.E., Gershenwald J.E., Lucci A., Arora R., Woodman S., Keung E.Z., Gaudreau P.O., Reuben A., Spencer C.N., Burton E.M., Haydu L.E., Lazar A.J., Zapassodi R., Hudgens C.W., Ledesma D.A., Ong S., Bailey M., Warren S., Rao D., Krijgsman O., Rozeman E.A., Peeper D., Blank C.U., Schumacher T.N., Butterfield L.H., Zelazowska M.A., McBride K.M., Kalluri R., Allison J., Petitprez F., Fridman W.H., Sautès-Fridman C., Hacohen N., Rezvani K., Sharma P., Tetzlaff M.T., Wang L., Wargo J.A. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petitprez F., de Reyniès A., Keung E.Z., Chen T.W., Sun C.M., Calderaro J., Jeng Y.M., Hsiao L.P., Lacroix L., Bougoüin A., Moreira M., Lacroix G., Natario I., Adam J., Lucchesi C., Laizet Y.H., Toulmonde M., Burgess M.A., Bolejack V., Reinke D., Wani K.M., Wang W.L., Lazar A.J., Roland C.L., Wargo J.A., Italiano A., Sautès-Fridman C., Tawbi H.A., Fridman W.H. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.S., Shen S., Miyauchi S., Sanders P.D., Franiak-Pietryga I., Mell L., Gutkind J.S., Cohen E.E.W., Califano J.A., Sharabi A.B. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin. Cancer. Res. 2020;26(13):3345–3359. doi: 10.1158/1078-0432.CCR-19-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi K., Ito M., Ohmura H., Hanamura F., Nakano M., Tsuchihashi K., Nagai S., Ariyama H., Kusaba H., Yamamoto H., Oda Y., Nakamura M., Akashi K., Baba E. Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal cancer. Oncoimmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1724763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieu-Nosjean M.C., Goc J., Giraldo N.A., Sautès-Fridman C., Fridman W.H. Tertiary lymphoid structures in cancer and beyond. Trends. Immunol. 2014;35(11):571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files.