Abstract
Background
Most patients with resected bile tract cancers (BTCs) survive for less than 5 years; however, some achieve better prognosis. The tumor microbiome can improve survival by regulating the tumor immune microenvironment. However, whether the tumor microbiome promotes immune cell infiltration in BTCs is unknown. This study aimed to determine the association between CD8+ T lymphocyte infiltration and the tumor microbiome in patients with resected BTCs.
Methods
Archived formalin-fixed paraffin-embedded tumor specimens were collected from patients with resected BTCs and analyzed using 16S rRNA gene sequencing to identify that prognosis-related and significantly differentially enriched taxa. Gene ontology (GO) analysis of the differentially enriched taxa was used to assess how CD8+ T lymphocyte infiltration is affected by the tumor microbiome of BTCs.
Results
We enrolled 32 patients with resected BTCs. The high CD8+ lymphocyte-infiltration (CD8hi) group had four significantly enriched taxa, and in the low CD8+ lymphocyte-infiltration (CD8low) group comprised one significantly enriched taxon. Patients with higher Clostridia abundance (enriched in the CD8hi group) experienced longer overall survival than those with lower abundance. The enrichment of Clostridia in the CD8hi group corresponded with lower CCL2 expression and downregulation of phosphatidylinositol 3-kinase activity, which might decrease myeloid-derived suppressor cell recruitment to the tumor milieu, thus increasing CD8+ lymphocyte infiltration in BTCs.
Conclusions
The tumor microbiome is related to CD8+ T lymphocyte infiltration in patients with resected BTCs. The relationship between tumor Clostridia and high infiltration of CD8+ T lymphocytes might reflect decreased recruitment of myeloid-derived suppressor cells via the PI3K-CCL2-CCR2 axis.
Keywords: Bile tract cancers, Tumor microbiome, CD8+ T lymphocyte, Myeloid-derived suppressor cell, Phosphatidylinositol 3-kinase activity
Introduction
Biliary tract cancers (BTCs) include intrahepatic, perihilar, and distal cholangiocarcinoma, as well as gallbladder cancer [1]. Most patients present with unresectable disease and have a poor prognosis, with an overall 5-year survival rate of only 5 to 20% [2,3]. Only about 30–40% of BTCs patients have a chance to undergo radical resection. Even if the tumor can be resected, the recurrence rate can reach 67% at one year after surgery, and the 5-year survival varies from 5 to 15% [1,4]. However, some patients with BTC survive for more than 5 years after surgery [5,6]. The mechanism underlying this long-term survival is unclear. The cancer genomic landscape has been reported to predict overall survival and the response to therapy in cholangiocarcinoma [7], [8], [9]. Indeed, isocitrate dehydrogenase (IDH) 1 and fibroblast growth factor receptor (FGFR) inhibitors have recently been explored for BTCs, with exciting results [1,10]. However, less than 10% of all BTCs patients experienced survival benefits [11]. Encouraging survival improvements achieved by immune checkpoint inhibitors were only observed in cancers with a high-mutation burden cancers, e.g., bladder cancer, lung cancer, and melanoma. By contrast, they show poor effectiveness in cancers with a low-mutation burden, such as BTCs: the response rate was only 6–17% when using anti-PD-1 (programmed cell death protein 1) therapy according to phase II clinical trials for BTCs [11]. Therefore, improving the response of BTCs to immunotherapy has emerged the key to improve the long-term prognosis of BTCs.
A recent study of patients with pancreatic ductal adenocarcinoma with long term survival showed that they had increased infiltration and activation of CD8+ T lymphocytes and an increased abundance and quality of neoantigens [12]. Interestingly, the neoantigens shared molecular similarities with infectious disease-derived peptides [12]. Thus, similar to the composition of the tumor genome, host microbial factors might also influence tumor progression and patient prognosis. Additionally, Bulajic et al. found that changes in the biliary microbiome increase the risk of BTCs [13,14]. Thus, we wondered whether the cholangiocarcinoma commensal microbiome could activate the immune system in response to BTCs? To help solve this problem, the present study focused on whether the immune environment of BTCs could be remodeled by the tumor microbiome, ultimately affecting the survival of BTCs patients.
Materials and methods
Biliary tract cancer specimens
The study protocols were approved by the institutional review committee of West China Hospital, Sichuan University. Thirty-two BTCs patients who underwent radical resection were randomly chosen. Table 1 shows the clinical and pathological data for the patient cohort. The disease stage was reviewed according to the 8th Edition of the American Joint Committee on Cancer classification [15].
Table 1.
Clinicopathological characteristics of the CD8low and CD8hi groups.
| Variable, n (%) | CD8low (n = 16) | CD8hi (n = 16) | P value |
|---|---|---|---|
| Male/female | 8/8 | 10/6 | 0.48 |
| Age (year)a | 64 (32–83) | 62 (46–76) | 0.99 |
| Tumor type | |||
| ICC | 2 (12.5) | 0 (0.0) | 0.16 |
| GBC | 5 (31.3) | 1 (6.3) | |
| HCCA | 3 (18.8) | 7 (43.8) | |
| DCC/CAMP | 6 (37.5) | 8 (50.0) | |
| pTb | |||
| 1 | 3 (18.8) | 0 (0.0) | 0.13 |
| 2 | 6 (37.5) | 10 (62.5) | |
| 3 | 7 (43.8) | 6 (37.5) | |
| pNb | |||
| N0 | 9 (56.3) | 10 (62.5) | 0.81 |
| N1 | 6 (37.5) | 4 (25.0) | |
| N2 | 1 (6.3) | 2 (12.5) | |
| pStageb | |||
| I | 2 (12.5) | 0 (0.0) | 0.043* |
| II | 6 (37.5) | 13 (81.3) | |
| III | 5 (31.3) | 2 (12.5) | |
| IV | 3 (18.8) | 1 (6.3) | |
| Histological grade | |||
| G2 (moderately) | 3 (18.8) | 10 (62.5) | 0.012* |
| G3 (poorly) | 13 (81.3) | 6 (37.5) | |
| Post-operative adjuvant therapy | 7 (43.8) | 3 (18.8) | 0.13 |
| Antibiotics use (pre-surgery) | 0 (0.0) | 2 (12.5) | 0.48 |
| Biliary obstruction | 8 (50.0) | 13 (81.3) | 0.063 |
Group CD8low, low CD8+ lymphocyte-infiltration; group CD8hi, high CD8+ lymphocyte-infiltration; ICC, Intrahepatic cholangiocarcinoma; GBC, gallbladder carcinoma; HCCA, hilar cholangiocarcinoma; DCC, distal cholangiocarcinoma; CAMP, carcinoma of ampulla
*Significant difference
Median (range)
According to the AJCC TNM classification 8th edition
DNA extraction and library construction for bacterial 16S rDNA
A QIAGEN DNeasy PowerSoil Kit, a QIAamp DNA FFPE Tissue Kit, or an AllPrep DNA/RNA MicroKit (al QIAGEN GmbH, Hilden, Germany; 80284) were used to isolate DNA and RNA. Before use, pipettes, pipette tips, and nonenzymatic kit components were irradiated using UV light for at least 1 h.
Libraries of 16S rDNA genes were prepared for Illumina sequencing as described previously, with slight modifications [16]. Briefly, the target region of 16S rDNA was amplified from 1 ng of template DNA using 10 cycles of PCR with barcoded primers. Excess primers were removed using AMPure XP Beads (Beckman Coulter, Danvers, MA, USA; A63880). After bead cleanup, the samples were pooled for 20 cycles of second round PCR using universal primers. The final PCR products were purified using AMPure XP Beads and then quantified using a Qubit dsDNA HS kit (ThermoFisher Scientific, Waltham, MA, USA; Q32854). The BioAnalyzer 2100 system (Agilent Technologies Inc., Santa Cara CA, USA) was used to evaluate the library quality. The libraries were then sequenced on an Illumina Hiseq instrument (Illumina, San Diego, CA, USA) to generate 150 bp paired end reads.
Microbiome pipeline analysis
Resphera Insight [17,18] and the SILVA Database v128 [19] were then used to assign the high-quality 16S rRNA sequences to a high-resolution taxonomic lineage. QIIME and R were then used for alpha- and beta-diversity analysis and principal coordinates analysis, respectively. A nonparametric difference test was used to analyze the differential abundance of alpha diversity features of interest. The negative binomial test (DESeq) [20] was used to analyze differential taxonomic abundances. Multiple hypothesis testing [21] was corrected using the false discovery rate (FDR). Linear discriminant analysis (LDA) was carried out using the linear discriminant analysis effect size (LEfSe) [22]. A heatmap was used to visualize the differentially abundant functional categories (GO Level 2, FDR adj.p < 0.05). Functional category was used to mean center the relative abundance values, which were colored according to enrichment or depletion between groups showing high and low abundance of Clostridia [23]. We added statistical annotations to indicate significant correlations with the metadata, allowing rapid assessment of many variables.
Chromogenic immunohistochemistry (IHC)
Immunohistochemistry was carried out as described in our previous article [24]. Avidin-biotin-peroxidase complexes were used to detect Clostridia, and the expression levels of human CD8 (CD8+ T cells, GeneTex), protein kinase B (Akt, Boster, Wuhan, China), phosphorylated (p)-Akt (Cell Signaling Technology, USA), forkhead box O1 (FOXO1, Abcam), p-FOXO1 (Abcam), AMP-activated protein kinase, catalytic, alpha-1 (AMPKα, Cell Signaling Technology), and c-Myc (Boster). Briefly, tissue sections were incubated at 4 °C overnight with primary antibodies recognizing the above proteins, and then incubated with biotinylated secondary antibodies at 37 °C for 1 h. The proportions of cells expressing CD8, Akt, p-Akt, FOXO1, p-FOXO1, AMPKα, and c-Myc were determined by counting positively stained cells in five randomly selected square areas (1 mm2 each) in the tumor section. For each marker, the average total number of positive cells selected areas was calculated as the positive cell number/total cell number × 100%.
Multiplex Immunofluorescence Staining (Multiplex IF)
We performed the staining manually using the same primary antibodies as were used for IHC analysis and those detecting certain other proteins: Clostridia, CD8, C-X3-C motif chemokine receptor 1 (CX3CR1), killer cell lectin like receptor G1 (KLRG1), PD-1, and CD101 (for TCF-1−CX3CR1highKLRG1+PD1−CD101− T effector cells (Teff) and TCF-1−CX3CR1lowKLRG1−PD1+CD101+ terminal T exhausted cells (Tex)), and CD11b, CD14, CD15, CD33, and human leucocyte antigen DR (HLA-DR) (for CD11b+CD14+CD15–CD33+HLA-DR−/lo, monocytic myeloid-derived suppressor cells, M-MDSCs). Binding of the antibodies was detected using the Opal Polymer HRP Ms + Rb immunohistochemistry detection reagent (Perkin Elmer, USA).
The same protocol of IHC was used for the continuous staining, and the next antibody was applied only after complete detection of the previous marker. Primary antibody detection used the Opal Polymer HRP Ms + Rb detection reagent and Opal 7-Color Manual IHC, including six reactive fluorophores: Opal 480, 520, 570, 620, 690, and 780. Nuclei were counterstained using 4′,6-diamidino-2-phenylindole (DAPI) according to the manufacturer's manual. The same protocols were used to stain the positive and negative control groups. The multiplex-stained sections were scanned using the Vectra Polaris system (Akoya Biosciences, USA).
Cell culture, bacterial culture, and bacteria/cell co-culture (BCCC)
Human cholangiocarcinoma cell lines, HCCC-9810 and HuCCT-1, were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (GIBCO, USA) supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin (100 units/ml) and streptomycin (100 μg/ ml) at 37 °C in a humidified 5% CO2 atmosphere. Human gallbladder carcinoma cell lines, GBC-SD and SGC-996 were grown in Dulbecco's modified Eagle's medium (DMEM)/F12 medium (GIBCO) with same 10% FBS and antibiotics as mentioned.
Extracted Clostridia, including Clostridium perfingens, Clostridium sordellii, Clostidrium novyi, Clostridium septicum, Clostridium chauvoei were isolated from BTC tissues of our cohort. A frozen stock of extracted Clostridia was plated on brain-heart infusion (BHI) agar (Anaerobe Systems, USA) and incubated at 37°C in an anaerobic chamber (GeneScience, China). Clostridia was also plated on yeast casitone fatty acids agar with carbohydrates (Anaerobe Systems) and incubated in an anaerobic chamber at 37°C. Inoculating a single colony of Clostridia into 5 ml of BHI broth (Anaerobe Systems) was used to prepare liquid cultures, which were incubated at 37°C anaerobically for 48 h before use, producing Clostridia at a bacterial density of approximately 4 × 106 colony forming units (CFUs/ml). To initiate BCCCs, the liquid cultures were pelleted, the supernatants were discarded, the pellets were resuspended in the same volume of BHI broth, and 4μL of the suspension was transferred to each plate of BTC cells (at a multiplicity of infection (MOI) of approximately 1.8:1 for each cell line). BCCCs were then incubated for 48 h at 37°C in 5% CO2 in a standard humidified incubator.
RNA-Seq technology and bioinformatics analysis
Co-cultured BTC cells were collected for RNA analysis. Total RNA was extracted with TRIzol reagent (Invitrogen, Canada) [25]. RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Bioanalyzer 2100 system (Agilent Technologies, USA). Quantified samples were sent for RNA-Seq analysis at Seqhealth Technology Co., Ltd., Wuhan, China.
Differential expression analysis
FPKM (Fragments Per Kilobase Million) were considered to estimate the gene expression level [26]. DESeq2 was applied to determine differential expression in the digital gene expression data. An adjusted P < 0.05 was defined as differentially expressed [27]. In addition to FPKM hierarchical clustering analysis of differentially expressed genes (DEGs), we further analyzed the subclusters based on log2 (ratios) of their gene expression level relative to that of the control group. The log2 (ratios) in the D and the Hgroups of ≥ 1 or ≤ − 1 was applied as a cutoff for subcluster analysis. The clustering algorithm divided the DEGs with similar gene expression trends into several subclusters.
GO enrichment analysis of DEGs
Gene Ontology (GO) enrichment analysis of DEGs were implemented using the clusterProfiler R package, in which gene length bias was corrected. P < 0.05 were considered significantly enriched by DEGs.
Protein extraction and western blotting
Cell lysis and western blotting were carried out as previously described [24,28]. Antibodies recognizing the following proteins were used: Akt (1:1000, Boster, Wuhan, China), p-Akt (Thr308) (1:1000, Cell Signaling Technology), FOXO1 (1:1000, Abcam), p-FOXO1 (S256) (1:1000, Abcam), AMPKα (1:1000, Cell Signaling Technology), c-Myc (1:1000, Boster), C-C motif chemokine ligand 2 (CCL2) (1:1000, Abcam), C-C motif chemokine ligand 10 (CXCL10) (1:1000, Abcam), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000, Abways Technology, China).
Quantification and statistical analyses
The chi-squared test and Fisher's exact test were used to compare the patients’ demographic and clinical information to investigate the relationship between the CD8low (low CD8+ lymphocyte-infiltration) and CD8hi (high CD8+ lymphocyte-infiltration) groups. Wilcoxon's rank sum test was used to compare the distributions of continuous variables between the two groups (CD8low versus CD8hi). Overall survival (OS) was determined as the period between the date of surgery to the date of any cause death or the most recent follow-up. The Kaplan–Meier method and the log-rank test were used to estimate survival probabilities. To identify factors that were independently associated with OS, those factors identified as significant at P < 0.20 in the univariate analysis were subjected to multivariate analysis using the Cox proportional hazards model. Hazard ratios (HR) and 95% confidence intervals (CIs) were determined. All tests were two-sided. Statistical significance was accepted at P < 0.05. SPSS version 26 (IBM Corp., USA) was used for all statistical analyses. QIIME [29] was used to process the raw 16S rRNA sequences. Silva v128 [19] was used to align the sequences. LEfSe was performed under the bioconda environment [22] to identify those genomic features that were most likely to explain the differences between CD8low and CD8hi groups. The FDR algorithm was used to adjust all the p values for multiple comparisons [21]. Candidate taxa were identified, validated, and prioritized as follows: all genera/species from our cohort that had an FDR-adjusted p value < 0.05 between the CD8low and CD8hi groups were included. Finally, a generalized linear model was used to check whether the candidate genera/species remained significantly associated with CD8low/CD8hi status (p < 0.01), allowing prioritization of the final list of candidates. GraphPad Prism 9 (GraphPad Software, Inc., San Diego, CA) was used to analyze the IHC data, which were expressed as the mean ± standard deviation (SD).
Results
High CD8+ lymphocyte infiltration correlates positively with improved survival in patients with resected BTCs
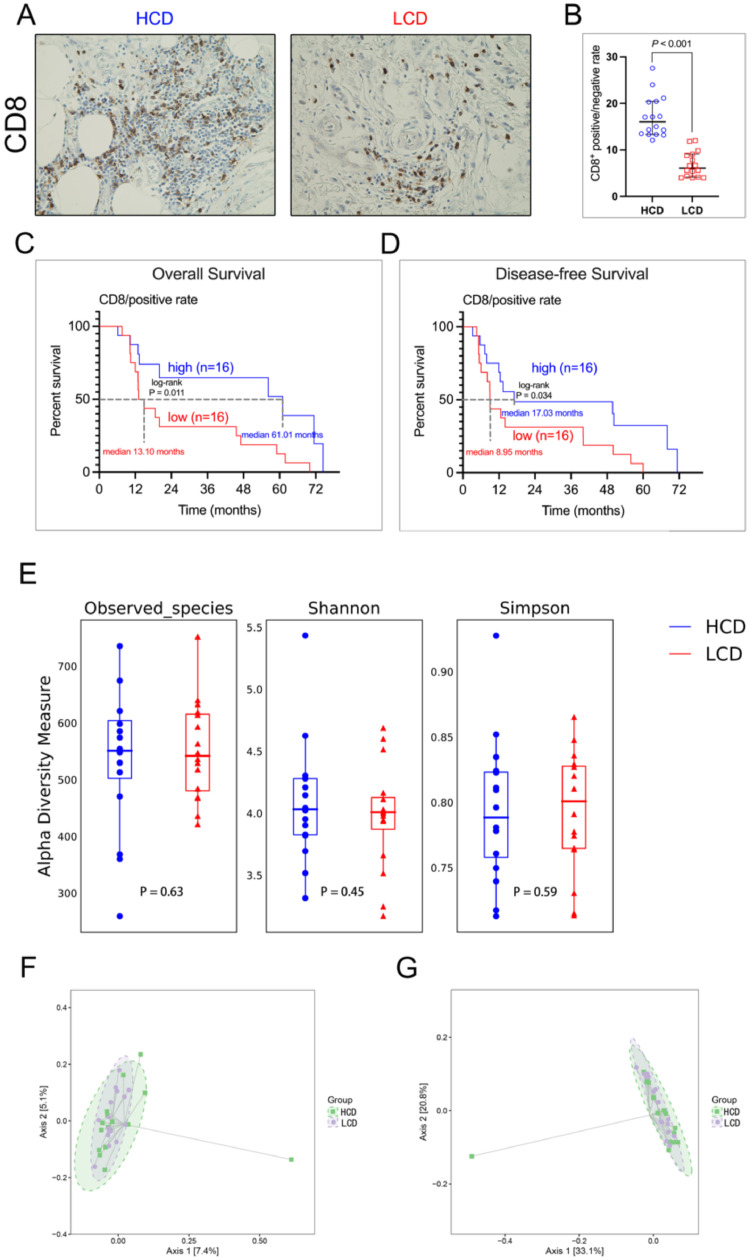
To investigate the impact of CD8+ lymphocyte infiltration on the prognosis of BTCs patients, we grouped the patients into CD8low and CD8hi groups according to their densities of CD8+ T cells (Figure 1A and 1B). We found that high densities of CD8+ cells correlated with lower TNM stage and better histological grade (Table 1), and better OS (CD8low vs. CD8hi: median, 13.10 vs. 61.01 months, P = 0.011) and disease-free survival (CD8low vs. CD8hi: median, 8.95 vs. 17.03 months, P = 0.034) (Figs. 1C and 1D).
Fig. 1.
High infiltration of CD8+ lymphocytes correlates with better outcomes in patients with resected BTC. (A) IHC staining for CD8 in tumors from intrahepatic cholangiocarcinoma patients with CD8hi and CD8low BTCs (representative image). (B) IHC quantification of positive number of CD8 lymphocytes for patients with CD8hi and CD8low BTCs. (C) OS probability from Kaplan–Meier estimates based on the density of CD8 in patients with BTCs (p = 0.011). (D) Disease-free survival probability from Kaplan–Meier estimates based on the density of CD8 in patients with BTCs (p = 0.034). (E) Alpha diversity boxplot (Observed species, Shannon and Simpson reciprocal) of patients with BTCs. (F) Beta diversity as assessed using principal coordinate analysis (PCoA) with unweighted-UniFrac. (G) Beta diversity as assessed using PCoA with Bray-Curtis metric distances.
Bacterial DNA was extracted from 32 radically resected BTC tumors (16 CD8low vs. 16 CD8hi), and 16S rRNA gene sequencing was used to carry out taxonomic profiling. Observed taxonomic units [OTUs], and Shannon and Simpson indices were first used to measure the tumor microbial diversity. In our cohort, the CD8low and CD8hi groups did not differ significantly for the number of species in the tumor microbiome (alpha-diversity) [30] (OTUs: p = 0.63, Shannon: p = 0.45, and Simpson: p = 0.59) (Fig. 1E). High gut microbiome diversity could improve the effectiveness of anti-PD-1 immunotherapy in patients with pancreatic ductal adenocarcinoma and melanoma [31,32]. However, in our study, the tumoral microbial diversity did not correlate with the density of CD8+ cell in BTCs.
We next investigated the impact of microbiome diversity on CD8+ cell infiltration in BTCs. Beta-diversity analysis was performed using principal coordinate analysis (PCoA) through unweighted-UniFrac distances [33] and Bray-Curtis metric distances [34]. Unfortunately, there was no clear clustering between OTUs from CD8low and CD8hi using the two methods (Figs. 1F, G), suggesting that there is no obvious association between the tumor microbial communities within the CD8low and CD8hi groups (p > 0.05).
Tumor-associated Clostridia levels differ significantly between the CD8low and CD8hi groups
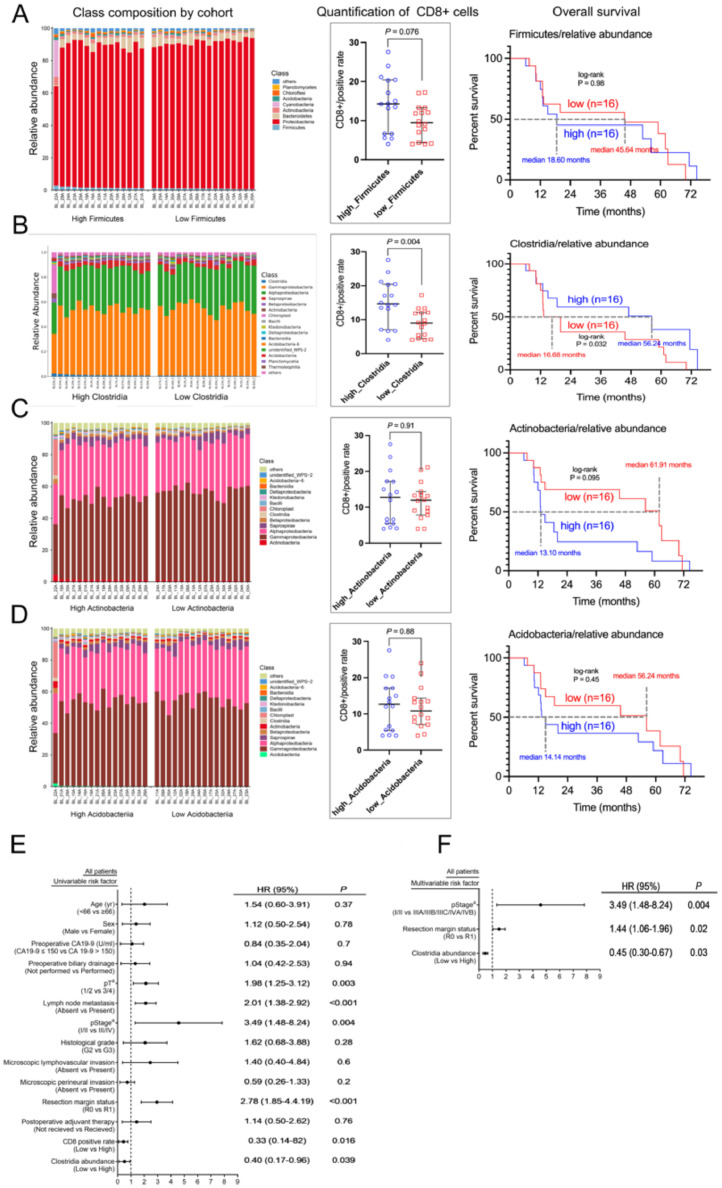
Specific members of the tumor microbiome contribute to the recruitment of CD8+ lymphocytes [32]. First, the overall tumor microbiome profile in all patients was assessed to investigate the differences in the tumor microbiome composition between the CD8low and CD8hi groups (Fig. 2A). Enrichment of special bacterial species in the two groups at the taxonomic level was investigated by comparing enrichment of OTUs (Figure S1A–E). To further validate these results, high dimensional class comparisons were conducted using LEfSe [22], which detected marked differences of bacterial communities between CD8low and CD8hi (Figs. 2B, C): The CD8hi tumors significantly high abundances of Firmicutes, Clostridia, Actinobacteria, and Acidobacteria at the class level. There was a significantly higher abundance of Gitt_GS in the CD8low group (Figs. 2B, C). A comparative heatmap based on the OTU abundance was used to explore whether the tumor microbiome can be isolated at the genus level grouped by densities of CD8+ cell infiltration. However, no significant difference was found (Fig. 2D).
Fig. 2.
Tumor Clostridia differs significantly between CD8low and CD8hi. (A) Bar plots showing the class taxonomic levels in our cohort of patients with BTCs. For each tumor, the relative abundance was plotted. (B) LEfSe-derived taxonomic cladogram showing the taxonomic associations between microbiome communities from the CD8hi and CD8low groups of patients with BTCs. Each specific taxonomic type is represented by a node. Yellow nodes represent those taxonomic features that are not significantly different between CD8hi and CD8low. Red nodes denote those taxonomic types with higher abundance in CD8hi than in CD8low, while green nodes represent those taxonomic types with higher abundance in CD8low. (C) The LDA score calculated using differentially abundant features between the CD8hi and CD8low groups, using the feature selection criterion of log LDA score > 4. (D) Heatmap showing the most differentially abundant features at the genus level. Blue represents lower abundance, lighter white represents intermediate abundance, and red represents high abundance.
Then, the patients were separated into high and low categories according to their median relative abundance of the four mentioned taxa. Significantly higher densities of CD8+ lymphocytes were only correlated positively with higher abundance of Clostridia (P = 0.004, Figure 3A-D). BTCs patients with higher abundance of Clostridia were predicted to have significantly better outcomes (HR = 2.50, 95% CI 1.04–5.88, Figs. 3B, 3E). The abundance of Clostridia was also an independent factor for patient prognosis (Fig. 3F). However, no significant difference was found for BTCs patients with a higher abundance of Acidobacteria, Actinobacteria, and Firmicutes (Fig. 3A-D).
Fig. 3.
CD8 lymphocyte densities and overall survival were only significantly different according to the different abundance of tumor Clostridia, not Firmicutes, Actinobacteria, and Acidobacteria. (A-D) Bar plots of the class taxonomic levels, densities of CD8 lymphocytes, and overall survival in our cohort of patients with BTC for high and low abundance of Firmicutes (A), Clostridia (B), Actinobacteria (C), and Acidobacteria (D), respectively. For each tumor, the relative abundance was plotted (0–100%). (E) Univariate analysis of our cohort of patients with BTC. 95% confidence intervals are shown as bars. (F) Multivariate analysis of our cohort of patients with BTC. 95% confidence intervals are shown as bars.
Tumor Clostridia is associated with T cell activation in BTCs
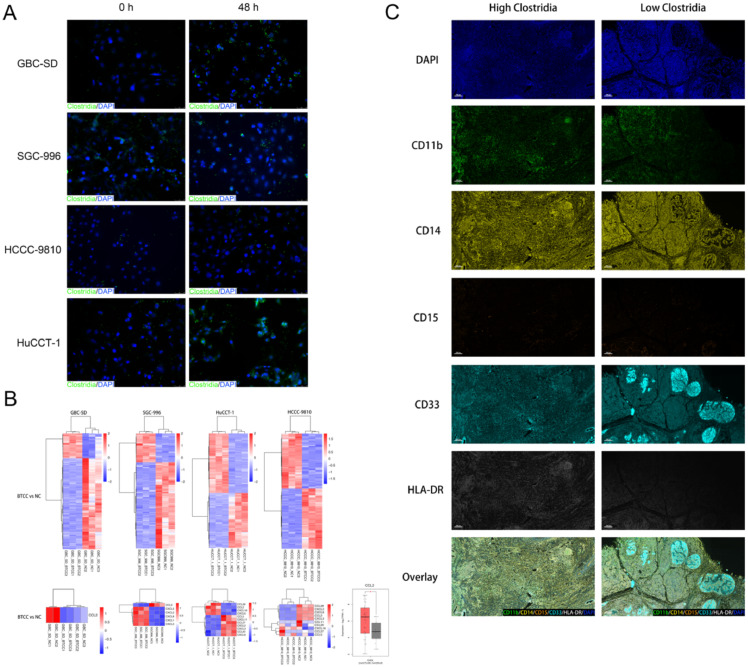
The gut composition of the microbiome plays an important role in shaping the immune system [35], in which it improves effectiveness of immunotherapy by shaping the immune system [35]. We identified a positive correlation between the extent of CD8+ T lymphocyte infiltration in BTCs tissues and the abundance of Clostridia. However, only effector CD8+ T lymphocytes (TCF-1−CX3CR1highKLRG1+PD1−CD101− T effector cells, Teff) play role in killing tumor cells, while exhausted CD8+ T lymphocytes (TCF-1−CX3CR1lowKLRG1−PD1+CD101+ terminal T exhausted cells, Tex) cannot kill tumor cells [36]. We found higher densities of Teff cells in the HCL (high Clostridia group) compared with that in the LCL (low Clostridia group) patients (Figure 4) through multiplex immunofluorescence staining. It suggested that Clostridia in tumors might contribute to the anti-tumor immune response by favoring CD8+ T cell recruitment and activation.
Fig. 4.
Tumor-associated Clostridia affect the immune responses by promoting T Cell activation in BTCs. Multiplex Immunofluorescence Staining of Clostridia, CD8, CX3CR1, KLRG1, PD1, and CD101 (TCF-1−CX3CR1highKLRG1+PD1− CD101− T effector cells, Teff and TCF-1−CX3CR1lowKLRG1−PD1+CD101+ terminal T exhausted cells, Tex).
Clostridia could spontaneously accumulate around BTC cells
Although we found a potential positive correlation between the abundance of tumor Clostridia and the extent of CD8+ T lymphocyte infiltration in BTCs tissues, it is unknown whether the tumor Clostridia could spontaneously accumulate around BTCs cells. After 48 h of co-culture of extracted clostridia and BTC cells, we found that the anaerobic extracted Clostridia spontaneously aggregated around the BTC cells (Fig. 5A).
Fig. 5.
Clostridium difficile correlates negatively with the expression of CCL2 in BTC cells and the recruitment of myeloid-derived suppressor cells in BTC tissues. (A) Immunofluorescence Staining of Clostridium difficile and biliary tract cancer cells. (B) Heatmap clustering analysis of differential expressed mRNAs after RNA-seq of co-culture of Clostridium difficile (Clostridia) and BTC cells. (C) Multiplex Immunofluorescence Staining of CD11b, CD14, CD15, CD33, and HLA-DR (CD11b+CD14+CD15–CD33+HLA-DR−/lo, monocytic myeloid-derived suppressor cells, M-MDSCs).
Clostridia reduces the expression of CCL2 in BTCs cells
To further observe the effect of Clostridia on the differences in mRNA expression of various BTCs cell lines, we performed heat map clustering analysis of differentially expressed mRNAs after co-culture (Fig. 5B). The directed migration of T lymphocytes relies mainly on chemokine-mediated chemokinesis, we focused on screening for changes in the gene expression profile of chemokines. Among them, no significant differences in expression were found among chemokines directly related to CD8+ T lymphocyte infiltration, such as CXCL9, CXCL10, and CXCL11. However, the expression of mononuclear chemistin-1 (CCL2) of myeloid-derived suppressor cells (MDSCs), the main cells that maintain the tumor immunosuppressive microenvironment, was significantly downregulated (Fig. 5B). High expression of CCL2 is known to correlate positively with the degree of malignancy of tumors in hepatocellular carcinoma and pancreatic cancer [37,38]. After further analysis of CCL2 in the TCGA public database, the expression of CCL2 in tumor tissues was found to be significantly higher than that in para-cancerous tissues in BTCs patients (Fig. 5B). That is, hepato-pancreatic-biliary malignancies tend to express CCL2 at a higher level to attract MDSCs to inhibit the tumor immune microenvironment, thereby alleviating the anti-tumor response of immune cells to tumor cells. However, the tumor-associated clostridia might reduce the chemotaxis of MDSCs by reducing CCL2 expression by BTC cells, thereby reducing the inhibition of the immune environment by MDSCs, which would improve the immune system's anti-tumor response to BTC cells.
Clostridia abundance correlated negatively with the infiltration of MDSCs in BTC tissues
Although we found that clostridia could downregulate CCL2 expression, thereby decreasing MDSCs to BTC cells, it was not confirmed that differences in the infiltration of M-MDSCs (CD11b+CD14+CD15– CD33+HLA-DR−/lo), which play a major immunosuppressive role in MDSCs [39], correlated negatively with the Clostridia abundance in clinical samples. After multiple immunohistochemistry/fluorescence,. we found that the infiltration of M-MDSCs in BTC tissues with high Clostridia abundance was indeed lower than that in BTC tissues with low Clostridia abundance tissues, i.e., there was a negative correlation between Clostridia abundance and M-MDSC infiltration in BTC tissues (Fig. 5C).
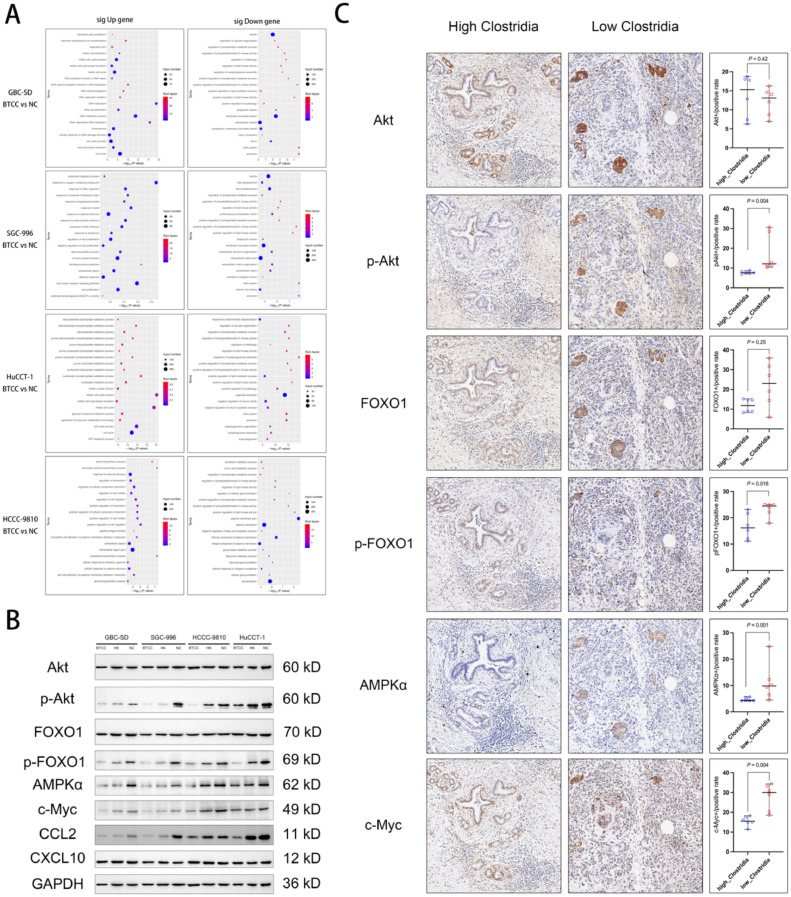
Tumor Clostridia correlates negatively with positive regulation of phosphatidylinositol 3-kinase activity (PI3K) of BTC cells
To explore the pathway by which Clostridia mediate the downregulation of CCL2, GO function classification of the differentially expressed genes was performed. In the annotation of the significant downregulated genes, we found that positive regulation of phosphatidylinositol 3-kinase activity (PI3K) was commonly downregulated in all four BTC cell lines. That is, Clostridia correlates negatively with the positive regulation of PI3K in BTC cells (Fig. 6A). Wong et al. reported that increased phosphorylation of Akt, FOXO1 and other molecules in the PI3K-Akt-FOXO1 pathway would increase the expression of CCL2 [40]. At the same time, CCL2 has been shown to directly activate cancer cells, and enhance the activity and proliferation of cancer cells via the PI3K-AKT phosphorylation pathway [41]. Therefore, we hypothesized that tumor-associated Clostridia facilitation of CD8+ T lymphocyte accumulation in BTCs sites is related to the downregulation of the CCL2-CCR2 axis and the reduction of the recruitment of MDSCs via downregulation of PI3K activity.
Fig. 6.
Tumor Clostridium difficile (Clostridia) correlates negatively with positive regulation of phosphatidylinositol 3-kinase (PI3K) activity of BTC cells. (A) Gene ontology function classification of differentially expressed genes after co-culture of Clostridium difficile and biliary tract cancer cells. (B) Western blotting for Akt, p-Akt, FOXO1, p-FOXO1, AMPKα, c-Myc, CCL2 (MCP-1), CXCL10, and GAPDH after co-culture of Clostridium difficile and biliary tract cancer cells. (C) Immunohistochemistry of Akt, p-Akt, FOXO1, p-FOXO1, AMPKα, and c-Myc in resected intr-hepatic cholangiocarcinoma tissues.
To verify the results of mRNA sequencing after co-culture of Clostridia and BTC cells, i.e., downregulation of PI3K activity, no significant difference in the expression of CXCL10, and downregulation of CCL2, western blotting was used to verify the levels of the above molecules. We found no significant differences in Akt and FOXO1 levels among Clostridia co-cultures with BTC cells (Bacteria-tumor co-cultures, BTCC group), inactivated Clostridia co-culture group (heat-killed, HK group), and the non-specific control group (NC group). However, p-Akt and p-FOXO1 (the active forms of Akt and FOXO1), were downregulated in both the BTCC group and the HK group, especially in the BTCC group. Furthermore, the levels of downstream AMPKα and c-Myc proteins, which are regulated by p-Akt and p-FOXO1, were also downregulated in the BTCC group and HK group, especially in in BTCC group. However, the level of CXCL10 did not differ significantly among the BTCC, HK, and NC groups. The level of CCL2 was significantly downgraded in the BTCC and HK groups, more significantly in the BTCC group (Fig. 6B).
In addition to western blotting, tumor-associated Clostridia downregulation of PI3K activity was further validated by immunohistochemistry of clinical tissue specimens. No significant difference was found in the levels of Akt and FOXO1 between the high- and low abundance tumor Clostridia group (Fig. 6C). However, in immunohistochemistry of phosphorylated proteins, the levels of p-Akt and p-FOXO1 in the high-abundance tumor Clostridia group were significantly lower compared with those in the low-abundance tumor Clostridia group. Moreover, the levels of downstream AMPKα and c-Myc, which are regulated by p-Akt and p-FOXO1, were lower in the high-abundance tumor Clostridia group than in the low-abundance tumor Clostridia group. (Fig. 6C). These results showed that the tumor-associated Clostridia downregulation of the positive regulation of PI3K activity correlated negatively with the abundance of Clostridia in BTCs tissues.
Discussion
The microbiome is reported to exert a regulatory role in areas other than in the gut [32,42,43]. Herein, we investigated the function exerted by the tumor microbiome in the immune microenvironment of BTCs. Multiple analyses, including bioinformatics analysis of the intra-tumoral microbiome of patients with CD8hi and CD8low BTCs, co-culture of Clostridia and BTC cells, and clinical specimen validation were performed. Generally, BTCs patients with higher infiltration of CD8+ T lymphocytes had significantly better prognosis. Furthermore, the CD8hi and CD8low groups each had a unique tumor microbiome, in which the abundance of Clostridia could predict prognosis via multi-factor analysis. Interestingly, this is the first report of higher tumor Clostridia abundance being positively associated with high infiltration of CD8+ T lymphocytes. Additionally, this positive correlation might be attributed to downregulation of the PI3K-CCL2-CCR2-MDSCs axis, which has an important function in suppressing immunity in the tumor microenvironment.
The composition of tumor microbiome has been reported to improve the effectiveness of cancer immunotherapy by modulating the regional immune microenvironment [32,44]. Our results indicated that the BTC tumor microbiome can shape the immune microenvironment, ultimately affecting the prognosis of BTCs patients. Notably, we found a significantly high signature of Clostridia in CD8hi patients. High abundance of Clostridia was a key predictor of long-term survival in our BTCs patients. In the future, we will use tumor microbiome sequencing to stratify patients with BTC to identify those who could benefit from adjuvant immunotherapy via microbiome interventions.
Although the tumor microbiome has been found regulating the regional immune system, whether it affects the antitumor response of BTCs has not been confirmed. The Saccharopolyspora family, particularly Saccharopolyspora rectivirgula, has been described to promote CD8+ T cell infiltration into pancreatic adenocarcinoma tumors [32]. Additionally, analysis of The Cancer Genome Atlas (TCGA) RNA sequencing data showed that chemokine levels could be regulated by the intra-tumoral gut microbiome, thereby modifying CD8+ T cell infiltration in lung cancer, melanoma, and pancreatic ductal adenocarcinoma (PDAC), which then impacted patient survival [44]. Besides, many bacteria suppress the immune microenvironments in the TME. In PDAC tumors, distinct bacteria promote the differentiation of suppressive monocytes via Toll-like receptor ligation, which leads to T cell unresponsiveness [45]. In patients with hepatobiliary cancers, the effectiveness of anti-PD-1 immunotherapy was reported to be related to the gut microbiome [46]. However, no study has reported whether the tumor microbiome composition can affect the immune microenvironment in BTCs. Taking the fact that activation of PI3K resulted in increased CCL2 expression [47] together with our findings, we hypothesized that the presence of Clostridia might contribute toward CD8+ T cell infiltration by downregulation of CCL2 secretion by tumor cells through reducing PI3K activity, thereby suppressing the recruitment of MDSCs into BTCs tissues. However, further investigations should be performed to confirm whether this mechanism can regulate the regional immune microenvironment by tumor Clostridia in BTCs.
Why is the abundance of Clostridia higher in patients with CD8hi BCTs? Mao et al. observed a significant and positive correlation between Clostridium genus abundance and raised bile acid [46]. Serum bile acid levels correlated with Clostridium XIVa enrichment in high tumor burden HCC [48]. Clostridium species regulate the conversion of primary bile acid to secondary bile acid, particularly the 7α-dehydroxylation reaction process. This process could restrict the accumulation of hepatic natural kill T cell and the inhibition of liver cancer [49]. For BTCs patients, obstructive jaundice is one of the most important clinical manifestations. In our study, we found more biliary obstruction patients in CD8hi group. Taken together, these observations suggest that the abundance of tumor Clostridia might correlate positively with higher bile acid caused by biliary obstruction. The higher abundance of tumor Clostridia in turn stimulates the infiltration of CD8+ T lymphocytes. This might be a pathway by which certain bacteria activate the immune system. It seems that BTCs might cause biliary obstruction, which in turn might kill the BTCs. Further studies are needed to verify our hypothesis that Clostridia can lead to tumor immune responses through the PI3K-CCL2-CCR2-MDSCs axis in BTCs patients.
There were some limitations in our study. First, we did not control for dietary habits and geographical differences and other confounding factors, which have been reported to affect the gut microbiome composition. Second, our findings and hypothesis were based on a limited sample size and should be validated by a large-sample study. Third, some findings in BTCs patients were not entirely consistent. It remains unclear whether the difference was derived the small sample size in the subgroup or from different cancer types. Therefore, a further large-sample study is needed to investigate the differences among different types of BTC (including intrahepatic, perihilar, distal cholangiocarcinoma, and gallbladder cancer). Lastly, transplantation of the fecal microbiota from a BTC mouse model or BTCs patients with that from CD8hi group-enriched taxa or feces would be expected to verify whether the effectiveness of immunotherapy toward bile tract cancers is regulated by the gut microbiome.
In conclusion, we found that different tumor microbiomes have a powerful effect in determining CD8+ T lymphocyte infiltration in patients with BTC. Tumor-associated Clostridia is related to high infiltration of CD8+ T lymphocytes, which might correlate negatively with PI3K-CCL2-CCR2 axis, characterized by the lower myeloid-derived suppressor cells recruitment and activation in the tumor microenvironment. This might also be useful to predict the long-term outcomes of patients. In addition, our results indicate that the development of treatment options that manipulate the tumor microbiome could improve the life expectancy of BTCs patients.
Funding
W.J.M was supported by Young Scientists Fund of National Natural Science Foundation of China (82203650), Sichuan Science and Technology Program (2021YJ0132), Science and Technology Bureau of Sichuan Province (2020YFS0099), Natural Science Foundation of Sichuan Province (2022NSFSC0806), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18014, ZYJC21046), 1.3.5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2021HXFH001), and Sichuan University-Zigong School-local Cooperation project (2021CDZG-23).
CRediT authorship contribution statement
Wen-Jie Ma: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Zheng-Hua Li: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Zhen-Ru Wu: Investigation, Writing – review & editing. Fei Liu: Investigation, Writing – review & editing. Jun-Ke Wang: Investigation, Methodology, Writing – review & editing. Yu-Jun Shi: Conceptualization, Data curation, Formal analysis, Supervision, Funding acquisition, Writing – original draft, Writing – review & editing. Yan-Wen Jin: Conceptualization, Supervision, Funding acquisition, Methodology, Writing – review & editing. Fu-Yu Li: Conceptualization, Data curation, Formal analysis, Supervision, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2023.100920.
Contributor Information
Yan-Wen Jin, Email: yanwjin@126.com.
Fu-Yu Li, Email: lifuyu@scu.edu.cn.
Appendix. Supplementary materials
References
- 1.Valle JW, Kelley RK, Nervi B, Oh D-Y, Zhu AX. Biliary tract cancer. The Lancet. 2021;397:428–444. doi: 10.1016/s0140-6736(21)00153-7. [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019;71:104–114. doi: 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Manzia TM, Parente A, Lenci I, Sensi B, Milana M, Gazia C, Signorello A, Angelico R, Grassi G, Tisone G, et al. Moving forward in the treatment of cholangiocarcinoma. World. J. Gastrointest. Oncol. 2021;13:1939–1955. doi: 10.4251/wjgo.v13.i12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma WJ, Wu ZR, Shrestha A, Yang Q, Hu HJ, Wang JK, Liu F, Zhou RX, Li QS, Li FY. Effectiveness of additional resection of the invasive cancer-positive proximal bile duct margin in cases of hilar cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2018;7:251–269. doi: 10.21037/hbsn.2018.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly J-P, Boudjema K, Fartoux L, Bouhier-Leporrier K, Jouve J-L, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III. Study J. Clin. Oncol. 2019;37:658–667. doi: 10.1200/jco.18.00050. [DOI] [PubMed] [Google Scholar]
- 6.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. The Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 7.Dong L, Lu D, Chen R, Lin Y, Zhu H, Zhang Z, Cai S, Cui P, Song G, Rao D, et al. Proteogenomic characterization identifies clinically relevant subgroups of intrahepatic cholangiocarcinoma. Cancer Cell. 2022;40(70–87):e15. doi: 10.1016/j.ccell.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabró R, Pinyol R, Torres-Martin M, Bassaganyas L, Moeini A, Peix J, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J. Hepatol. 2020;73:315–327. doi: 10.1016/j.jhep.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DVT, Borad MJ, Bridgewater JA, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021;7:1669–1677. doi: 10.1001/jamaoncol.2021.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carapeto F, Bozorgui B, Shroff RT, Chagani S, Solis Soto L, Foo WC, Wistuba I, Meric-Bernstam F, Shalaby A, Javle M, et al. The immunogenomic landscape of resected intrahepatic cholangiocarcinoma. Hepatology. 2022;75:297–308. doi: 10.1002/hep.32150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balachandran VP, Łuksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulajic M, Maisonneuve P, Schneider-Brachert W, Müller P, Reischl U, Stimec B, Lehn N, Lowenfels AB, Löhr M. Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer. 2002;95:1946–1953. doi: 10.1002/cncr.10893. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda K, Kuroki T, Tajima Y, Tsuneoka N, Kitajima T, Matsuzaki S, Furui J, Kanematsu T. Comparative analysis of Helicobacter DNAs and biliary pathology in patients with and without hepatobiliary cancer. Carcinogenesis. 2002;23:1927–1931. doi: 10.1093/carcin/23.11.1927. [DOI] [PubMed] [Google Scholar]
- 15.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, et al. Springer; New York: 2017. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. [Google Scholar]
- 16.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daquigan N, Seekatz AM, Greathouse KL, Young VB, White JR. High-resolution profiling of the gut microbiome reveals the extent of burden. NPJ Biofilms Microbiomes. 2017;3:35. doi: 10.1038/s41522-017-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drewes JL, White JR, Dejea CM, Fathi P, Iyadorai T, Vadivelu J, Roslani AC, Wick EC, Mongodin EF, Loke MF, et al. High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes. 2017;3:34. doi: 10.1038/s41522-017-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic. Acids. Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 22.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma W-J, Wu Z-R, Yang Q, Hu H-J, Wang J-K, Shi Y-J, Li F-Y, Cheng N-S. Biliary antibiotics irrigation for e. coli-induced chronic proliferative cholangitis and hepatolithiasis: a pathophysiological study in rabbits. Clin. Res. Hepatol. Gastroenterol. 2020;44:356–367. doi: 10.1016/j.clinre.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Zhao L, Chen C, Wang Y, Shen J, Ding Z. Conserved microRNA act boldly during sprout development and quality formation in pingyang tezaocha. Front. Genet. 2019;10:237. doi: 10.3389/fgene.2019.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Gao X, Yi J, Sang X, Dai Z, Tao Z, Wang M, Shen L, Jia Y, Xie D, et al. BTF3 confers oncogenic activity in prostate cancer through transcriptional upregulation of replication factor C. Cell Death. Dis. 2021;12:12. doi: 10.1038/s41419-020-03348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y-X, Wang S-N, Chen J, Hu J-G, Lü H-Z. A transcriptomic study of probenecid on injured spinal cords in mice. PeerJ. 2020;8:e8367. doi: 10.7717/peerj.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma W-J, Zhou Y, Shrestha A, Mao H, Li FY, Cheng NS, Zhang W, Xu R-H, Zhang YQ, Jiang T, et al. Applying chemical bile duct embolization to achieve chemical hepatectomy in hepatolithiasis: a further experimental study. J. Surg. Res. 2014;187:113–121. doi: 10.1016/j.jss.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 29.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurilshikov A, Wijmenga C, Fu J, Zhernakova A. Host genetics and gut microbiome: challenges and perspectives. Trends Immunol. 2017;38:633–647. doi: 10.1016/j.it.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (New York, NY) 2018:359. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178:795–806. doi: 10.1016/j.cell.2019.07.008. e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu Q, Lin Y, Ma Y, Li X, Liang J, Chen Z, Liu K, Huang Y, Luo H, Huang R, et al. Exploring the emerging role of the gut microbiota and tumor microenvironment in cancer immunotherapy. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.612202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, Huang AC, Johnson J, Staupe RP, Bengsch B, Xu C, et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity. 2019:51. doi: 10.1016/j.immuni.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, Medina-Echeverz J, Longerich T, Forgues M, Reisinger F, et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell. 2016;30:533–547. doi: 10.1016/j.ccell.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegde S, Leader AM, Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity. 2021;54:875–884. doi: 10.1016/j.immuni.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong P-P, Muñoz-Félix JM, Hijazi M, Kim H, Robinson SD, De Luxán-Delgado B, Rodríguez-Hernández I, Maiques O, Meng Y-M, Meng Q, et al. Cancer burden is controlled by mural cell-β3-integrin regulated crosstalk with tumor cells. Cell. 2020:181. doi: 10.1016/j.cell.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Pausch TM, Aue E, Wirsik NM, Freire Valls A, Shen Y, Radhakrishnan P, Hackert T, Schneider M, Schmidt T. Metastasis-associated fibroblasts promote angiogenesis in metastasized pancreatic cancer via the CXCL8 and the CCL2 axes. Sci. Rep. 2020;10:5420. doi: 10.1038/s41598-020-62416-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu A, Yao B, Dong T, Chen Y, Yao J, Liu Y, Li H, Bai H, Liu X, Zhang Y, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022 doi: 10.1016/j.cell.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, Ma L, Roy S, Fu Q, Brown ZJ, et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 2021;11:1248–1267. doi: 10.1158/2159-8290.CD-20-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu G, Su H, Johnson CH, Khan SA, Kluger H, Lu L. Intratumour microbiome associated with the infiltration of cytotoxic CD8+ T cells and patient survival in cutaneous melanoma. Eur. J. Cancer. 2021;151:25–34. doi: 10.1016/j.ejca.2021.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8:403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao J, Wang D, Long J, Yang X, Lin J, Song Y, Xie F, Xun Z, Wang Y, Wang Y, et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2021-003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao H, Yang Y, Kim KJ, Bethel-Brown C, Gong N, Funa K, Gendelman HE, Su T-P, Wang JQ, Buch S. Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood. 2010;115:4951–4962. doi: 10.1182/blood-2010-01-266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang H, Ren Z, Gao X, Hu X, Zhou Y, Jiang J, Lu H, Yin S, Ji J, Zhou L, et al. Integrated analysis of microbiome and host transcriptome reveals correlations between gut microbiota and clinical outcomes in HBV-related hepatocellular carcinoma. Genome Med. 2020;12:102. doi: 10.1186/s13073-020-00796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360 doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.