Abstract
We previously reported that immunization with recombinant simian immunodeficiency virus SIVmne envelope (gp160) vaccines protected macaques against intravenous challenge by the cloned homologous virus E11S but that this protection was only partially effective against the uncloned virus, SIVmne. In the present study, we examine the protective efficacy of this immunization regimen against infection by a mucosal route. We found that the same gp160-based vaccines were highly effective against intrarectal infection not only with the E11S clone but also with the uncloned SIVmne. Protection against mucosal infection is therefore achievable by parenteral immunization with recombinant envelope vaccines. Protection appears to correlate with high levels of SIV-specific antibodies and, in animals protected against the uncloned virus, the presence of serum-neutralizing activities. To understand the basis for the differential efficacies against the uncloned virus by the intravenous versus the intrarectal routes, we examined viral sequences recovered from the peripheral blood mononuclear cells of animals early after infection by both routes. We previously showed that the majority (85%) of the uncloned SIVmne challenge stock contained V1 sequences homologous to the molecular clone from which the vaccines were made (E11S type), with the remainder (15%) containing multiple conserved changes (the variant types). In contrast to intravenously infected animals, from which either E11S-type or the variant type V1 sequences could be recovered in significant proportions, animals infected intrarectally had predominantly E11S-type sequences. Preferential transmission or amplification of the E11S-type viruses may therefore account in part for the enhanced efficacy of the recombinant gp160 vaccines against the uncloned virus challenge by the intrarectal route compared with the intravenous route.
Sexual transmission is the predominant route of human immunodeficiency virus type 1 (HIV-1) infection worldwide (45). For an AIDS vaccine to be effective, it must be able to prevent infection or disease resulting from mucosal as well as blood-borne transmissions. Although protection has been demonstrated for a number of vaccine approaches (1, 39), most of the evidence to date has come from intravenous challenge models. The requirements for an effective immunization regimen and the correlates of protection against mucosal transmission of HIV have yet to be adequately addressed.
Protection against mucosal transmission was first demonstrated experimentally in simian immunodeficiency virus (SIV) models. Macaques have been protected against intrarectal challenge with formalin-inactivated whole-virion vaccines (10). The use of microencapsulated whole inactivated virus vaccine in a regimen consisting of intramuscular priming and mucosal boosting has provided protection against vaginal challenge (25). However, because of the potential complications caused by cellular antigens associated with whole inactivated virus vaccines (2, 38), the mechanism of protection and the applicability of these findings to HIV vaccine development remain unclear.
Several investigators have also reported partial or complete protection against intravaginal or intrarectal challenge in macaques previously infected with live “attenuated” SIV (11, 24). In a few instances, protection against heterologous virus challenge was achieved. Cross-protection was observed in seronegative HIV-2-exposed animals against intrarectal SIVsm infection (33), in SIV-infected animals against intrarectal simian/human immunodeficiency virus (SHIV) infection (34), and in SHIV-infected animals against intravaginal SIV infection (26). Protection appears to be independent of virus-specific antibodies in some cases (33) or of immunity against viral envelope antigens in others (26, 34). Protection against intrarectal challenge by SIVmne E11S was also observed in macaques previously inoculated intravenously with low, subinfectious doses of the same virus (9). Protection in this case was associated only with SIV-specific T-cell proliferative responses.
Protection against intrarectal challenge was recently achieved with recombinant vaccines. Immunization with subunit envelope and core antigens targeted to the iliac lymph nodes protected macaques against intrarectal infection with the SIVmac32H clone J5 (22). Protection was associated with a significant increase in the iliac lymph node cells that secrete CD8-suppressor factor, β chemokines, and immunoglobulin A (IgA) antibodies to p27. A protective effect was observed in animals immunized with an attenuated recombinant vaccinia virus vector (NYVAC) expressing SIV gag, pol, and env genes (3). Transient infection was observed in a significant proportion of animals after intrarectal challenge with a highly virulent virus, SIVmac251. However, protection in this case was not attributable to any of the measured immunological parameters.
We previously reported that immunization with recombinant SIVmne envelope (gp160) vaccines in a “prime and boost” regimen protected macaques against an intravenous infection by the homologous pathogenic virus, clone E11S (16). However, only partial protection was achieved against the uncloned parental virus SIVmne (31). In the present study, we sought to determine the protective efficacy of this immunization regimen against infection by the same viruses through a mucosal route. The results indicate that parenteral immunization with gp160-based vaccines was highly effective against intrarectal infection not only by the E11S clone but also by the uncloned SIVmne. Analysis of viral sequences recovered from infected animals indicates that the enhanced efficacy of the vaccines against challenge with the uncloned virus by the intrarectal route, compared with the intravenous route, may be due in part to preferential transmission or amplification of the E11S-type viruses after mucosal exposure.
MATERIALS AND METHODS
Immunogens and immunization regimen.
Recombinant vaccinia virus vac-gp160 (v-SE5) contains the coding sequence of the full-length gp160 of SIVmne molecular clone 8 (GenBank accession number M32741 [7, 14]) in a New York City Board of Health strain (v-NY) of vaccinia virus (16, 17). v-SE5 was plaque purified and propagated on African green monkey kidney cells (BSC-40) (17). Cynomolgus macaques (Macaca fascicularis) were inoculated with 108 PFU of the recombinant virus by skin scarification at two or three sites along opposite sides of the midline of the back. Booster immunizations at 2.5, 20, and 22 months were done via intramuscular injections of gp160 produced in BSC-40 cells infected with recombinant vaccinia virus (19). Each booster dose contained 250 μg of total protein (corresponding to approximately 125 μg of gp160) formulated in Freund incomplete adjuvant.
Challenge virus and conditions.
SIVmne was isolated from a pig-tailed macaque (M. nemestrina) with lymphoma and was propagated on HuT 78 cells (4). E11S is a single-cell clone of SIVmne-infected HuT 78 cells that produces large amounts of envelope glycoproteins (7). Challenge was performed by an intrarectal inoculation 4 weeks after the last immunization with 2 to 20 animal infectious doses (AID) of SIVmne clone E11S. The in vivo infectivity of the virus was determined previously by a separate intrarectal titration experiment. Intrarectal inoculation was performed as described previously (21). The animals protected from the E11S challenge were held for 2 years to confirm their virus-negative status before they were boosted again with gp160 and rechallenged intrarectally 4 weeks later with 2 to 20 AID of uncloned SIVmne grown on HuT 78 cells. Blood samples were collected on the day of challenge; at 2, 4, 6, and 8 weeks after challenge; and monthly thereafter. Plasma and serum samples were collected and stored at −70 and −20°C, respectively, until used. Lymph node biopsy specimens were obtained at the indicated times after challenge and were frozen at −70°C for DNA analysis or fixed for in situ hybridization and histological analyses. Animals were housed in the Washington Regional Primate Research Center and were under the care of licensed veterinarians. All macaques were also tested negative for the presence of simian type D retrovirus by serology, PCR, and virus isolation. Euthanasia was performed on the basis of the following criteria: AIDS, termination of experiment, or deteriorating physical condition for reasons unrelated to infection. Euthanasia was considered to be AIDS-related if the animal exhibited peripheral blood CD4+ cell depletion and two or more of the following conditions: wasting, untreatable diarrhea, opportunistic infections, proliferative diseases (e.g., lymphoma), and abnormal hematology (e.g., anemia, thrombocytopenia, or leukopenia).
Virus isolation.
Peripheral blood mononuclear cells (PBMC) were isolated over Histopaque-1077 (Sigma Chemical Co., St. Louis, Mo.) as described previously (6, 8). Briefly, 4 × 106 PBMC were cocultivated with 5 × 106 AA-2CL5 cells, and cultures were maintained for 8 to 9 weeks. Virus was detected by reverse transcriptase (RT) assays performed as described previously (4). A positive value means positive results in RT assays, and a negative value means no RT activity was detected after 8 to 9 weeks of cocultivation.
ISH analysis.
In situ hybridization (ISH) was performed essentially as described previously for SIVagm (15). Digoxigenin-labeled RNA probes were generated by SP6 or T7 polymerase transcription reactions by using subclones of SIVmac239 as templates that spanned the entire genome in 1- to 2-kb fragments (Lofstrand Laboratories, Gaithersburg, Md.). Formalin-fixed, paraffin-embedded tissue sections were hybridized with 1.75 ng of SIVmac239 riboprobe (sense or anti-sense) per ml at 52°C overnight, washed sequentially in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–50% formamide solution and then 2× SSC, and treated for 30 min at 37°C in a solution containing RNase T1 and RNase A. The slides were blocked with a buffer containing 2% horse serum, 150 mM NaCl, and 100 mM Tris (pH 7.4) for 1 h. After the serum block, the slides were incubated for 1 h with sheep anti-digoxigenin alkaline phosphatase conjugate (Boehringer Mannheim) at 1:500 dilution, rinsed in Tris (pH 7.4), and incubated with nitroblue tetrazolium–5-bromo-4-chloro-3-imdolyl phosphate (Vector Laboratories, Burlingame, Calif.) substrate in the dark at room temperature overnight. The stained slides were rinsed in water, counterstained with nuclear fast red, dehydrated, and mounted with coverslips. All of the stained samples were viewed and photographed with a Zeiss Axiophot microscope. Controls included SIVmac239 sense probe hybridized on SIVmne-infected tissue, anti-sense SIVmac239 probe on uninfected tissues, and substitution of the sheep antibody conjugate with phosphate-buffered saline.
Serum neutralization assays.
Neutralizing antibodies against uncloned SIVmne and SIVmne clone E11S were measured in CEM-X174 cells by methods similar to those described previously (28). The uncloned SIVmne used for neutralization studies was grown on HuT 78 cells and was identical to the challenge stock but was prepared at different times. The E11S virus used for neutralization assays was derived from the same stock as the challenge virus but was grown on macaque (M. fascicularis) PBMC. Twofold serum dilutions (heat-inactivated at 56°C for 30 min) were tested in 96-well plates. The neutralization titer is expressed as the reciprocal serum dilution that inhibits 50% of SIVmne-induced cytopathic effect in CEM-X174 cells.
ELISA.
SIV-specific antibodies were measured by enzyme-linked immunosorbent assay (ELISA) as described earlier (16), except that the gradient-purified and disrupted whole SIVmne clone E11S virion was used as an antigen in the ELISA. Endpoint titers were determined as the reciprocal of the highest serum dilution that resulted in an optical density reading threefold greater than that obtained with negative control sera.
Immunoblot assay.
Proteins from sucrose gradient-purified SIVmne Cl E11S grown in HuT 78 cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon paper (Millipore Corp., Bedford, Mass.), and processed as described earlier (5). Macaque plasma was diluted 1:100 before the assay.
Nested PCR analysis.
PBMC were isolated from EDTA-treated blood by Hypaque-Ficoll gradient centrifugation, and nucleic acid was extracted by standard techniques. One microgram of total nucleic acid was used as a template for a two-step amplification by PCR by using a nested set of oligonucleotide primers specific for the env region. The conditions for the first and second rounds of amplification were as described previously (16). The final amplified fragment was approximately 642 bp in length. Amplified products were resolved by agarose gel electrophoresis and visualized by ethidium-bromide staining. Results for a subset of samples were also confirmed by PCR with primers from env and long terminal repeat (LTR)-gag regions.
Semiquantitative PCR analysis of proviral DNA load.
Proviral DNA in PBMC was measured by PCR with the use of radiolabeled primer incorporation for quantification (32) and was expressed as copies of proviral genome detected per million PBMC. Briefly, 1 μg of DNA from each sample was amplified in a PCR mixture that contained 0.2 μM concentrations of each primers, 200 μM concentrations of each of four deoxynucleoside triphosphates, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 1.0 U of Taq polymerase (Perkin-Elmer Cetus, Branchburg, N.J.) in a volume of 50 μl. The reaction was subjected to 30 cycles of denaturation for 1 min at 94°C, annealing for 2 min at 60°C, and elongation for 3 min at 70°C. The oligonucleotide primers used were derived from the nucleotide sequence of SIVmne (GenBank accession number M32741 [14]). They consist of a primer pair specific for the envelope region, env10 (nucleotides 7191 to 7211 [sense]) and env12 (nucleotides 7541 to 7561 [antisense]), and a pair of primers specific for the LTR-gag region, S1 (nucleotides 228 to 251 [sense]) and S8 (nucleotides 536 to 559 [antisense]). The amplified products for the env and the LTR-gag sequences are 370 and 330 bp, respectively. One oligonucleotide of each complementary pair was 5′ end labeled with [32P]ATP by using polynucleotide kinase (New England Biolabs, Inc., Beverly, Mass.). The 32P-labeled PCR products obtained by amplification were analyzed by electrophoresis on 8% nondenaturing polyacrylamide gels and quantified by autoradiography with PhosphorImager (PIA) analysis (32). Quantification of SIV-DNA was determined with a standard curve generated by known quantities of a plasmid clone of E11S.
RT-QC-PCR determination of plasma viral RNA.
Plasma viral RNA was prepared as described earlier (42). The viral RNA samples were serially diluted in a 96-well PCR microplate into a reaction buffer containing a fixed copy number of a competitor RNA with an internal deletion. The template and the competitor were subjected to reverse transcription followed by quantitative competitive-PCR (QC-PCR). The primers used are from the SIVmne gag sequence: 5′ primer (5G) from nucleotides 675 to 698 (AAAGCCTGTTGGAGAACAAAGAAG) and 3′ primer (3Diii) from nucleotides 993 to 1011 (AATTTTACCCAGGCATTTA). The internal RNA control contains a deletion of 82 bp which enables the discrimination between products amplified from the viral (336-bp) and the control (254-bp) templates. The conditions for the RT and QC-PCR reactions were as described by Watson et al. (42).
Analysis of the proviral DNA sequence in PBMC or lymph node cells from animals infected with uncloned SIVmne.
Proviral DNA sequences in infected macaques were analyzed by PCR amplification with radiolabeled primers as described earlier (31). Two oligonucleotide probes (nucleotides 6471 to 6499) were used, one specific for the E11S-like sequence (E11Sp, 5′-TTTATTGCCTCTGCTTTTGTTGGTATTGC-3′ [antisense]) and the other for the variant-type sequences (Variantp, 5′-TCTATTTTCTTTGTTGTTGGTTTTGGTGT-3′ [antisense]). The E11Sp probe hybridizes with the proviral cDNA of E11S and uncloned SIVmne, while the Variantp probe hybridizes only to the latter. By using primers specific for the E11S- or the variant-type sequences, we amplified V1 sequences in the PBMC or the lymph node cells of infected macaques. A radiolabeled primer specific for the V1 region (Env71, nucleotides 6097 to 6120, 5′-TTATCGCCATCTTGTTTCTAAGTC-3′ [sense]) was used in combination with the antisense primers specific for the E11S or the variant sequences. The amplified product, which was 397 bp in length, was resolved by electrophoresis on 8% nondenaturing polyacrylamide gels, and the relative abundance of the E11S-type and the variant-type sequences was determined by autoradiography by using PIA analysis as described above. For each PCR amplification, DNA from E11S-infected and uncloned SIVmne-infected cells were used as controls.
Lymphocyte subset analysis.
Cell surface immunofluorescence was quantified by use of a FACScan flow cytometer and Lysis II software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Lymphocyte subsets (CD4, CD8, CD2, and CD20) of whole heparinized blood samples were evaluated by conventional methods.
Statistical analysis.
Differences in proportions were tested by Fisher’s exact test. Differences in SIV-specific antibody titers in infected versus protected animals were tested by constructing a 95% confidence interval for the mean of the protected animals and determining whether the antibody level of the infected animal fell within that interval. The mean percentage of E11S-like sequences in intrarectally challenged animals was compared with the mean percentage of E11S-like sequences in intravenously challenged animals (31) by nonparametric permutation tests. Finally, declines in CD4+ cell counts were compared between groups using repeated measures analysis of variance with fixed group and time factors. The statistical significance of a decline within a group was tested using group by time interaction terms in the analysis of variance.
RESULTS
Protection against intrarectal challenge with homologous clone E11S.
To determine the protective efficacy of envelope-based vaccines against mucosal challenge, we used a combination immunization strategy that was shown previously to protect macaques against intravenous infections (16, 31). Briefly, we immunized four cynomolgus macaques first with a live recombinant vaccinia virus expressing the full-length envelope protein gp160 of SIVmne and then with subunit gp160 as a booster immunogen. As observed previously, all animals developed low levels of SIV-specific antibody responses (as determined by ELISA, immunoblots, and serum neutralization assays) after the recombinant vaccinia virus immunization. However, levels of SIV-specific antibodies increased 10- to 30-fold after the first subunit protein immunization (references 16 and 31 and data not shown). Four weeks after the last booster immunization, all four immunized animals, together with three naive controls, were challenged intrarectally with the homologous pathogenic virus clone E11S grown on HuT 78 cells. Infection was monitored by nested PCR, virus isolation by coculture, anamnestic response, and seroconversion to nonvaccine antigens.
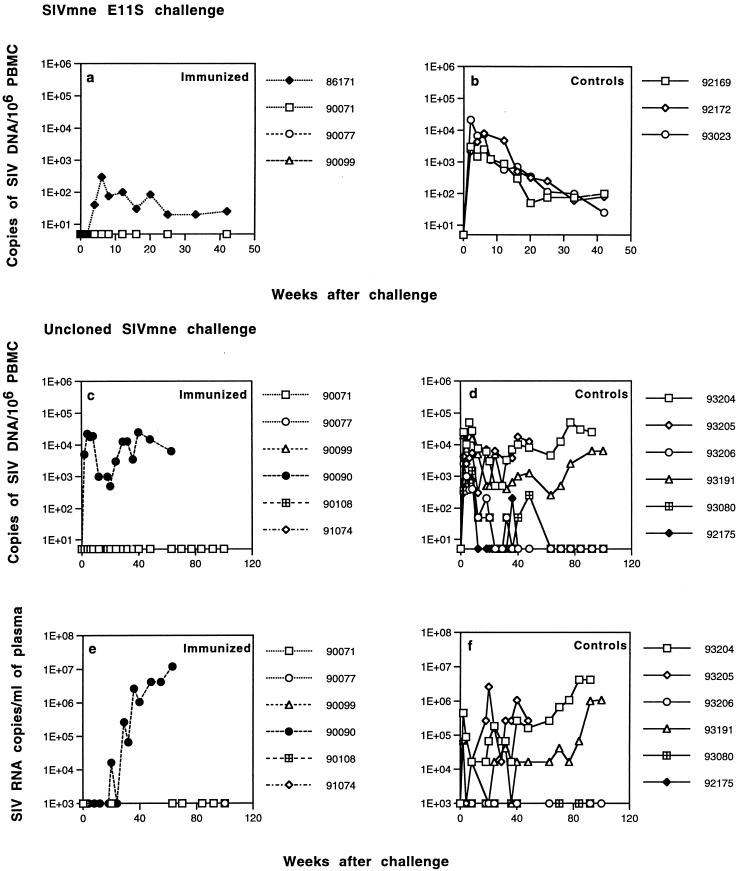
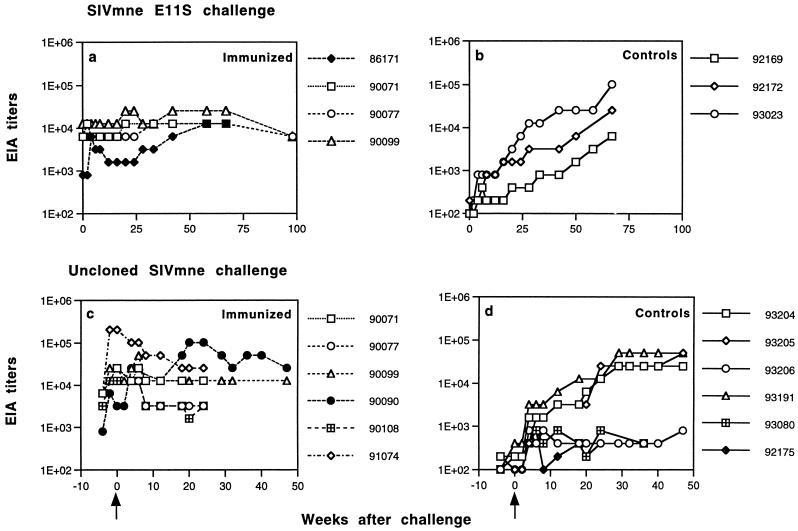
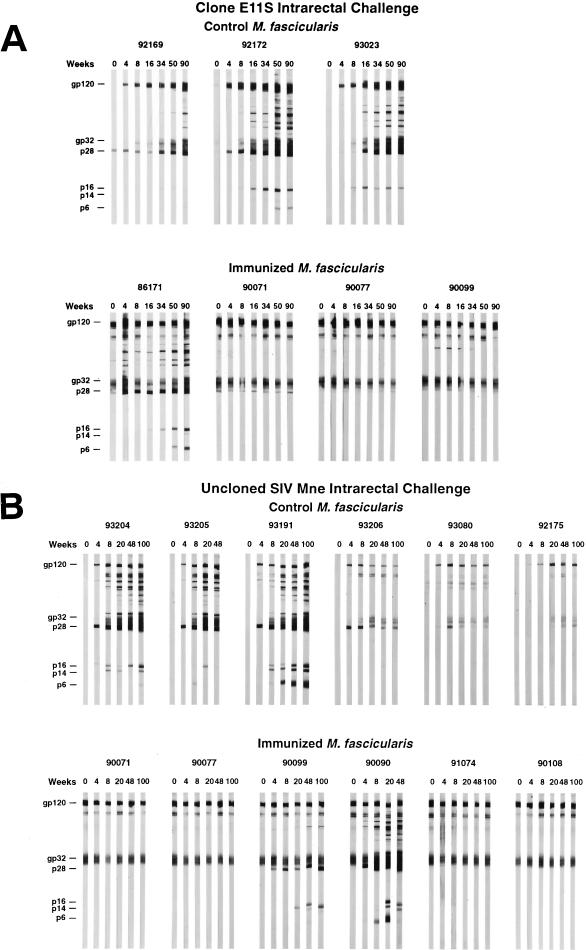
After intrarectal challenge with E11S, all three control animals became persistently infected, whereas three of four immunized macaques were completely protected (Table 1). Virus was isolated from the PBMC of one immunized animal (macaque 86171) only once (at week 4) after challenge. Detection of virus in this animal by nested PCR was intermittent (Table 1), and its viral load was significantly reduced (10- to 100-fold) compared with the controls (Fig. 1). Serological analyses confirmed these results. Although macaque 86171 clearly showed an anamnestic response, its antibody titers were not maintained but instead declined steadily beginning 2 months after challenge. In contrast, all three immunized and protected animals had relatively high levels of SIV-specific antibodies already at the time of challenge. They showed no significant change in antibody titers after challenge (Fig. 2). Macaque 86171 was also the only immunized animal that developed antibodies to nonenvelope antigens as a result of the challenge, albeit the onset of these antibodies was delayed compared to the control animals (Fig. 3). These results are consistent with the notion that vaccine-induced responses in this animal were partially protective. Therefore, although the proportion of protected animals did not reach statistical significance due to the small sample sizes (three of four immunized animals versus zero of three control animals, P = 0.143 [Table 2]), these results together indicate that the “prime and boost” immunization regimen with SIVmne gp160 vaccines was highly effective against intrarectal infection by the homologous virus clone E11S.
TABLE 1.
Virus isolation and nested PCR analysis of PBMC and lymph node cells from macaques after intrarectal challenge with E11S clone or with uncloned SIVmne
| Challenge virus and macaque no. | Results of analysis at (wks postchallenge)a:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 12 | 16 | 20 | 34 | 42 | 75 | 82 | 90 | 98 | 112 | 171 | 210 | |
| E11S clone | |||||||||||||||
| Control | |||||||||||||||
| 92169 | +/+ | +/+ | +/+ | +/+ | −/+ | −/+ | −/+ | −/+ | +/+ | −/+ | +/+ | +/+ | +/+ | +/NT | E |
| 92172 | +/+ | +/+ | +/+ | +/+ | +/+ | −/+ | NT/NT | +/+ | +/+ | +/+ | +/+ | +/+ | U | ||
| 93023 | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | −/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | E | |
| Immunized | |||||||||||||||
| 86171 | −/− | +/+ | −/+ | −/+ | −/+ | −/+ | −/+ | −/+ | −/− | −/+ | −/− | −/− | −/− | −/NT | E |
| 90071 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | R | ||
| 90077 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | R | ||
| 90099 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | R | ||
| 2 | 4 | 6 | 12 | 20 | 32 | 40 | 55 | 70 | 92 | 109 | 124 | 133 | 141 | 180 | |
| Uncloned SIVmne | |||||||||||||||
| Control | |||||||||||||||
| 93204 | +/+/+ | +/+/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | A |
| 93205 | +/+/+ | +/+/+ | +/+ | +/+ | +/+ | +/+ | +/+ | A | |||||||
| 93191 | +/+/+ | +/+/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | A |
| 93206 | +/+/+ | +/+/+ | −/+ | −/+ | −/+ | −/+ | −/− | −/− | −/− | −/− | −/− | −/− | NT/− | NT/− | NT |
| 93080 | +/+/+ | +/+/+ | +/+ | −/+ | −/+ | −/+ | −/+ | −/+ | −/+ | −/+ | −/− | −/− | NT/− | NT/− | NT |
| 92175 | +/+/+ | +/+/+ | +/+ | −/− | −/− | −/+ | −/− | −/+ | −/− | −/− | −/− | −/NT | NT/− | NT/− | NT |
| Immunizedb | |||||||||||||||
| 90071 | −/−/NT | −/−/NT | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | NT |
| 90077 | −/−/− | −/−/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | NT |
| 90099 | −/−/− | −/+/− | −/+ | −/− | −/− | −/− | −/− | −/− | −/? | −/? | −/− | −/− | −/− | −/− | NT |
| 90090 | +/+/+ | +/+/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | A | ||||||
| 91074 | −/−/− | −/−/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | NT |
| 90108 | −/−/− | −/−/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | NT |
Symbols within each column denote positive (+) or negative (−) results in the following assays: virus isolation from PBMC, PCR analysis of PBMC DNA, and PCR analysis of lymph node DNA. When only two data are indicated, they refer to results from the first two assays. R, reassigned to be rechallenged with uncloned SIVmne. Animals 93204, 93205, and 93191 were euthanatized due to AIDS at, respectively, 160, 55, and 178 weeks after challenge. Letters within the column denote the cause of death as follows: A, AIDS-related euthanasia; E, elective euthanasia; U, unrelated death. NT, not tested; ?, results inconclusive.
Macaques 90071, 90077, and 90099 were previously challenged intrarectally with SIVmne E11S (produced from a HuT 78 single-cell clone). Macaques 90090, 91074, and 90108 were challenged intravenously at the same time with the same virus grown on macaque PBMC (31). All six animals were protected from the first challenge and were consistently found to be virus negative by PBMC coculture and nested PCR for >2 years before rechallenge with uncloned SIVmne.
FIG. 1.
Viral load in macaques challenged intrarectally with E11S clone (a and b) or uncloned SIVmne (c to f). The proviral load in PBMC was determined by PCR analysis by using radiolabeled primer incorporation (a to d). Values are expressed as copies of proviral genome per 106 PBMC. Quantification of proviral DNA was determined by using an external standard containing a known copy number of SIVmne E11S proviral DNA. Plasma viral load was determined by RT-QC-PCR (e and f) with an internally controlled template as described in Materials and Methods.
FIG. 2.
SIV-specific antibody responses in immunized and control macaques after challenge. Dilutions of macaque sera collected at the indicated times were incubated with disrupted, gradient-purified SIVmne virion proteins immobilized on microtiter plates. Endpoint titers were defined as the reciprocal of the highest dilution that gave an optical absorbance value at least threefold higher than the average values obtained with SIV-negative macaque sera. Panels (a) and (b), immunized and control animals challenged with SIVmne E11S; panels (c) and (d), immunized and control animals challenged with uncloned SIVmne. Arrows indicate the time of challenge.
FIG. 3.
Immunoblot analysis of SIV-specific antibody responses in macaques challenged with SIVmne clone E11S (A) or uncloned SIVmne (B). Macaque plasma (diluted 100-fold) was reacted with SDS-PAGE-separated proteins from disrupted, sucrose gradient-purified SIVmne clone E11S as described earlier (5). At various times after SIV challenge, antibodies were detected in infected animals to envelope surface (gp120) and transmembrane (gp32) proteins; to the Gag proteins p28, p16, and p6; and to the Vpx protein p14. Antibodies to gp120 and gp32 were evident in all immunized macaques on the day of challenge (week 0). A weak antibody that cross-reacts with p28 was also present in one control animal at week 0 (animal 92169 [panel A]). This has occasionally been observed in naive M. fascicularis. The source of this cross-reactive antibody is unknown.
TABLE 2.
Summary of results from challenge studies
| Challenge route | No. of uninfected macaques/total no. of macaques challenged (% protection) with:
|
|||||
|---|---|---|---|---|---|---|
| E11S clone
|
Uncloned SIVmne
|
|||||
| Immunized | Control | P | Immunized | Control | P | |
| Intravenousa | 14/16 (88) | 1/15 (7) | <0.001 | 3/10 (30) | 0/10 (0) | 0.2 |
| Intrarectal | 3/4 (75) | 0/3 (0) | 0.143 | 5/6 (83) | 0/6 (0) | 0.015 |
Results of previous intravenous challenge studies (31) are included here for comparison.
Protection against intrarectal challenge with uncloned SIVmne.
To examine the breadth of the protective immunity, we rechallenged all three protected animals with uncloned SIVmne by the intrarectal route. In this experiment, we included three additional animals (macaques 90090, 90108, and 91074) that were immunized in parallel with the same gp160 vaccines but were protected against E11S challenge by the intravenous route (31). All of these animals met the following criteria for inclusion in the study: they had been virus negative for >2 years after challenge, as determined by nested PCR analysis and by virus isolation from PBMC coculture, and they had shown no anamnestic response and seroconversion to nonvaccine antigens (reference 31 and data not shown). Lymph nodes from these animals were also examined by nested PCR and by in situ hybridization and were shown to be virus negative (Table 1 and data not shown). All six animals were boosted again with recombinant gp160 approximately 2 years after the initial E11S challenge. Although none of these animals received any SIV antigen for >2 years, all of them showed significant recall responses upon receiving the booster immunization (Fig. 2c).
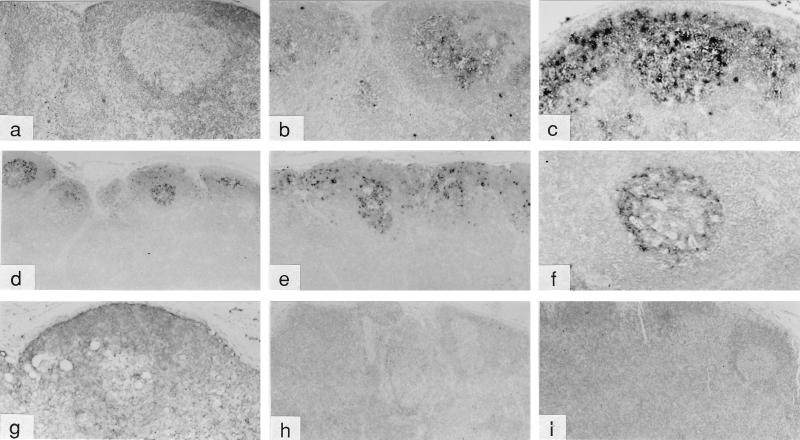
Four weeks after the last immunization, all six animals were rechallenged with uncloned SIVmne by the intrarectal route. Six control animals were challenged in parallel: five naive macaques and one macaque (92175) that had been exposed to E11S by intravenous inoculation but never showed any sign of infection. As shown in Table 1, all six control animals, including macaque 92175, became infected after intrarectal inoculation, albeit the virus was consistently isolated from only three animals (macaques 93204, 93205, and 93191). Virus was detected in the PBMC of the other three controls (macaques 93206, 93080, and 92175) by nested PCR and coculture but became intermittently positive or negative after the first 3 months (Table 1). Plasma viremia was only transiently detected in these three animals (Fig. 1f). ISH analysis of peripheral lymph nodes collected from control macaques 14 days after infection revealed SIV mRNA-positive cells within the paracortex and germinal centers of the follicles (Fig. 4b and c and data not shown). There was also diffuse staining in the follicular dendritic cells (FDC), a finding consistent with viral trapping and presentation. By 27 days after infection, prominent staining in the FDC of germinal centers was observed and numerous virus-positive cells were found predominantly in the cortex and germinal centers of the peripheral lymph nodes (Fig. 4d to f). In general, the animals with high plasma viral loads (macaques 93204, 93205, and 93191) also showed high numbers of virus-positive cells in their lymph nodes.
FIG. 4.
In situ hybridization analysis of lymph nodes from macaques challenged intrarectally with uncloned SIVmne. Peripheral lymph node samples from control (a to f) and immunized (g to i) animals were collected on day 14 (a to c) or day 26 or 27 (d to i) after challenge. Samples were examined with SIV-specific digoxigenin-labeled riboprobes as described in Materials and Methods. Panels: a and b, macaque 93191; c, macaque 93080; d, macaque 93204; e, macaque 93205; f, macaque 93206; g, macaque 90099; h, macaque 90108; and i, macaque 91074. Magnifications in panels a to i are, respectively, ×25, ×25, ×31.2, ×10, ×15, ×31.2, ×31.2, ×12.5, and ×12.5. The sample in panel a was hybridized with a “sense” probe as a control. All other samples shown were hybridized with “anti-sense” probes.
In contrast, five of the six immunized animals showed little or no sign of infection after challenge. Virus isolation from the PBMC of these animals was consistently negative (Table 1). ISH (Fig. 4g to i) and nested PCR (Table 1) analyses revealed no virus infection in their peripheral lymph nodes. No anamnestic response was observed in any of these five animals after challenge (Fig. 2). One animal (macaque 90099) was positive in its PBMC by nested PCR analysis only at weeks 4 and 6 after challenge (Table 1). Although this animal developed antibodies to p28 by week 4 and to other Gag proteins by week 20 after challenge (Fig. 3), no viral nucleic acid was detected in the lymph node by ISH (Fig. 4g), nor was there any virus isolated by PBMC coculture at any time, indicating that this animal was at least partially, if not completely, protected. Only one of the six immunized animals (macaque 90090) showed robust and persistent infection by all measurements (Table 1 and Fig. 1 to 3). Taken together, these results indicate that significant protection was achieved in five of the six vaccinated animals against an intrarectal infection by the uncloned SIVmne (versus zero of the six control animals; P = 0.015 [Table 2]).
SIV-specific antibody responses in immunized animals.
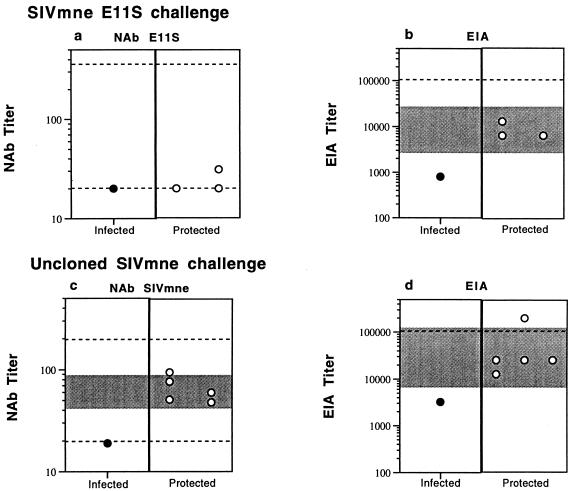
To determine whether SIV-specific antibodies correlated with protection, we analyzed sera from immunized animals by ELISA and by virus neutralization assays. On the day of challenge with E11S, all four immunized animals showed moderate levels of SIV-specific antibodies, with titers ranging from 10- to 100-fold lower than a pooled serum sample from SIV-infected macaques (Fig. 3 and 5 and unpublished data). The three animals protected against E11S challenge had significantly higher serum antibody titers than the one that was not protected (Fig. 5b). Although none of the four animals developed an appreciable level of serum neutralizing antibodies against the homologous challenge virus E11S (Fig. 5a), their sera showed significant neutralizing activities against a heterologous virus, SIVmac251, passaged in HuT 78 cells (21a). However, there was no apparent correlation between the challenge outcome and the level of serum neutralizing antibodies in either assay.
FIG. 5.
SIV-specific antibody responses in immunized macaques. Sera collected on the day of challenge were analyzed for neutralizing activities against the homologous challenge virus (E11S or uncloned SIVmne). Neutralizing titers are expressed as the reciprocal serum dilutions that resulted in >50% cytopathicity of E11S or uncloned SIVmne infection in CEMx174 cells (a and c). Serum reactivity with disrupted SIVmne E11S virion proteins was analyzed by ELISA, and the results are expressed as endpoint titers (b and d). Top panels (a and b), animals challenged with SIVmne E11S; bottom panels (c and d), animals challenged with uncloned SIVmne. Solid symbols denote persistently infected animals; open symbols show the protected animals. The shaded areas denote the 95% confidence interval for the mean titer of the protected animals. The upper and lower dotted lines represent, respectively, the titers of positive and negative control serum samples in each assay.
On the day of challenge with uncloned SIVmne, five of the six immunized animals had SIV-specific serum antibodies with titers within 5- to 10-fold of those in SIV-infected macaque serum pools (Fig. 5d). Moderate levels of neutralizing activity against the challenge virus uncloned SIVmne were also detected in these animals (Fig. 5c). In contrast to the findings after challenge with clone E11S, protection in this case appears to be correlated with the levels of serum SIV-specific antibodies, as well as the levels of neutralizing antibodies against the challenge virus. The only animal (macaque 90090) that became persistently virus positive after challenge had significantly lower titers of SIV-specific antibodies than the protected animals, as measured by ELISA and by SIVmne neutralization assay.
To avoid traumatizing the rectal mucosa of the animals and thus compromising the intended route of exposure, we did not collect rectal secretions on the day of challenge. We did, however, collect vaginal secretions from three female animals (macaques 93080 and 93191 from the control group and macaque 91074 from the experimental group). It is of interest to note that the immunized animal had SIV-specific IgG (0.55 μg/mg of total protein, or 0.72% total IgG) and IgA (4 pg/mg of total protein, or 0.08% total IgA) in its vaginal wash on the day of challenge (20a). These levels are comparable to those found in macaques infected intrarectally by SIVmne (0.43 μg and 1 pg of SIV-specific IgG and IgA, respectively, per mg of total protein) (21). No SIV-specific antibody was detected in the vaginal wash of either control macaque (data not shown).
Analysis of viral sequences recovered from intrarectally infected animals.
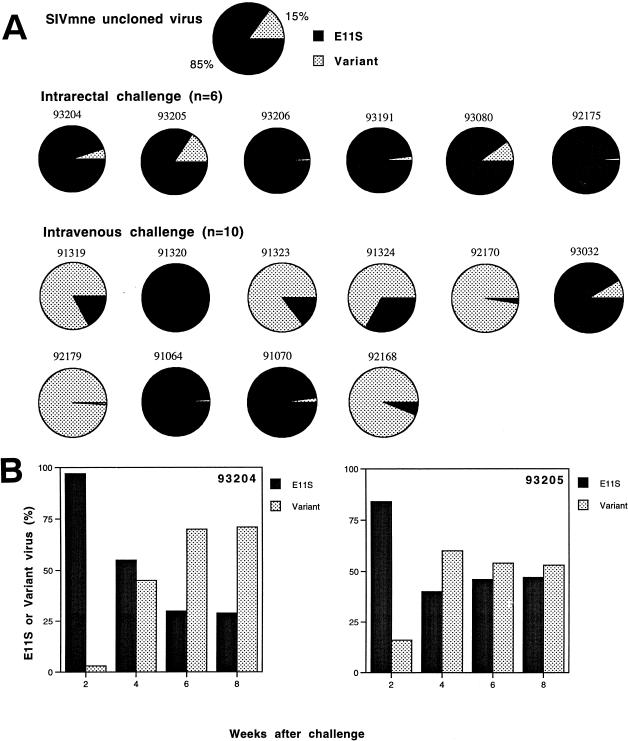
To gain a better understanding of the basis for vaccine success or failure, we examined proviral sequences recovered from naive control animals infected intrarectally by the uncloned SIVmne. In an earlier study (31), we showed that the majority (85%) of the uncloned SIVmne challenge stock contained V1 sequences homologous to the molecular clone from which the vaccines were made (E11S type), with the remainder (15%) containing multiple conserved changes (the variant types). We used labeled-primer amplification analyses to identify and quantify the proportion of E11S-like and “variant”-like sequences present in infected macaques. Both PBMC and lymph node samples were analyzed. First, we focused on the earliest samples from which we were able to detect >50 copies of viral sequences per microgram of total DNA (approximately 2 × 105 cells). These also represent preseroconversion samples, thus minimizing potential complications due to immune selection. In contrast to intravenously infected animals, from which either E11S-type or the variant-type V1 sequences could be recovered in significant proportions (31), all five intrarectally infected control animals had predominantly E11S-like sequences at 2 weeks after infection (Fig. 6A). The percentage of E11S-like sequences in the latter animals ranged from 84 to 99.5%, with a median of 96.5%. The mean percentage of E11S-like sequences in intrarectally infected animals was significantly higher than that in the PBMC of animals infected intravenously with the same virus (31) (P = 0.027). Both PBMC and lymph node samples collected concurrently from the same animal showed similar percentages of E11S-like and variant-like sequences (results not shown). These findings indicate preferential transmission and/or early amplification of viruses with E11S-like V1 sequences after intrarectal exposure.
FIG. 6.
Analysis of env V1 sequences recovered from the PBMC of macaques infected intrarectally with uncloned SIVmne. (A) Percentages of E11S-like (solid area) and variant-like (stippled area) V1 sequences in each of the six intrarectally infected control macaques are represented in individual pie charts. For comparison, the composition of the uncloned challenge virus and the results of a similar analysis (31) of intravenously infected animals are included. Analyses of PBMC were done on samples collected 2 weeks after infection. (B) Change of V1 genotype in the PBMC of animals 93204 and 93205 during the acute phase of infection. Percentages of E11S-like (solid bar) and variant-like (stippled bar) V1 sequences are as indicated.
Composition of the V1 sequence in the PBMC and lymph node cells in intrarectally infected control animals was also examined biweekly for the first 2 months after infection. In two of the five intrarectally infected animals, we observed a rapid reversal of the relative percentage of E11S-like and variant-like sequences within this 8-week period (Fig. 6B). This reversal was not observed in the other three intrarectally infected animals. These results lend further support to the idea that preferential transmission, rather than amplification per se, was the basis for the predominance of E11S-like viruses observed at 2 weeks after intrarectal infection.
We also performed the same analysis on the only immunized animal (macaque 90090) that became persistently infected after challenge with uncloned SIVmne. V1 sequences from its PBMC (collected from weeks 2 through 8) and lymph nodes cells (collected at week 4) were predominantly (>99%) E11S-like (data not shown). This result is in agreement with the findings in naive control animals and is consistent with the vaccine failure seen in this animal.
Clinical outcome of infection.
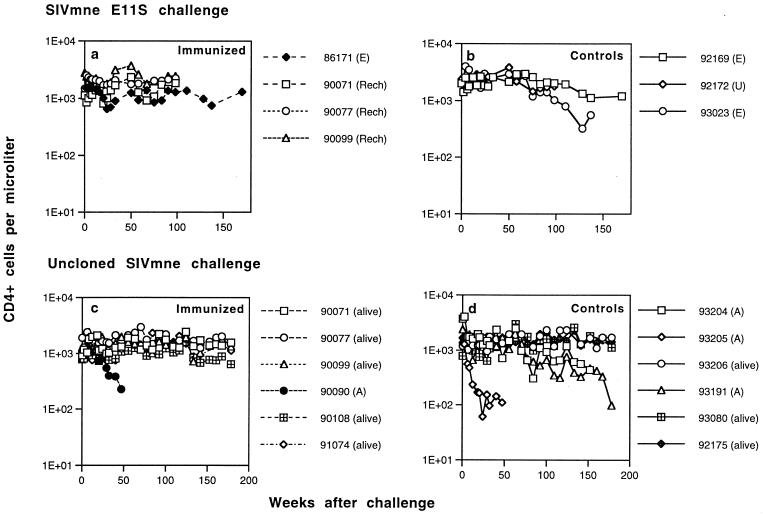
Infected animals were monitored for 3 or more years after challenge to determine the clinical outcome of infection and the effects of immunization. They were checked periodically for lymphocyte subsets, hematology, blood chemistry, body weight, opportunistic infections, and proliferative diseases. Figure 7 summarizes their peripheral blood CD4+ cell levels and survival time after challenge.
FIG. 7.
Peripheral blood CD4+ T-lymphocyte numbers in immunized and control macaques after intrarectal challenge with SIVmne E11S (a and b, respectively) or uncloned SIVmne (c and d, respectively). Animals euthanatized because of AIDS are labeled A; animals sacrificed at the end of the experimentation period are labeled E; and animals that died of causes unrelated to AIDS are labeled U. The last datum point for each animal represents the time of death or the termination of the experiment (euthanatized, alive, or rechallenged [Rech]).
Among the three animals infected with the E11S clone, there was little change in the general health or the peripheral blood CD4+ cell count during the study period (Fig. 7b). One animal (macaque 92172) died of reasons unrelated to SIV infection about 2 years after challenge. One animal (macaque 93023) showed moderate reduction in peripheral blood CD4+ cell numbers approximately 2 years after infection. These findings are in agreement with the previously reported rate of clinical progression of macaques infected intravenously with the pathogenic clone E11S (31). No significant change was observed in the only immunized but infected animal (macaque 86171 [Fig. 7a]).
Among the six control animals infected with uncloned SIVmne, one (macaque 93205) showed progressive and severe depletion of peripheral blood CD4+ cells beginning 2 months after challenge (Fig. 7d). It was euthanatized due to AIDS approximately 1 year after infection (Table 1). Another animal (macaque 93204) showed progressive CD4+ cell decline starting about 2 years after infection and died of AIDS about a year later. Its symptoms include wasting, weight loss (>25% in 4 months), fever, lymphadenopathy, and anemia. It is of interest to note that these were the same two animals that showed rapid changes in their viral V1 genotypes (i.e., a switch from E11S-type to variant-type) early after infection (Fig. 6B) and maintained relatively high viral loads in their PBMC and plasma (Fig. 1d and f). A third animal (macaque 93191) also showed CD4+ cell decline starting approximately 2 years after infection. This animal remained healthy and maintained moderate levels of viral load in its PBMC and plasma (Fig. 1d and f) for >1.5 years. However, it eventually developed AIDS and was euthanatized at week 178. At euthanasia, its peripheral blood CD4+ cell count was 98/mm3. Clinical findings include wasting, weight loss, anemia, thrombocytopenia, leukopenia, chronic enteritis, and lymphadenopathy. The other three infected control animals are clinically healthy, with normal CD4+ cell numbers. The only immunized but infected animal (macaque 90090) developed CD4+ cell depletion and died of AIDS about 1 year after challenge (Fig. 7c). The other five immunized animals remain healthy, with normal CD4+ cell counts. Overall, we did not observe significant differences in the time course of clinical development and in the proportion of animals that died of AIDS as a result of intrarectal or intravenous infection (31).
DISCUSSION
We have shown that a “prime and boost” immunization regimen with recombinant SIVmne gp160 vaccines is highly effective against intrarectal challenge by the homologous virus. Our findings thus support and extend earlier observations that protection against mucosal infection by a primate lentivirus is possible through parenteral immunizations. Such protection has been obtained with live attenuated virus (11, 24, 26, 33, 34), whole killed virus (10, 25), or complex immunogens (3). Our results show that protection is also achievable with parenterally administered envelope-based vaccines. It is possible that mucosally administered vaccines may be more desirable or effective than parenteral immunizations against mucosal infection under certain conditions (see reference 25), including natural transmission. However, this remains to be demonstrated in comparative studies.
Results from the present study also support the notion that protection against mucosal infection may be more readily achievable than that against blood-borne infection. The gp160 vaccines protected macaques against intrarectal infection by the homologous E11S clone as well as the uncloned SIVmne. This is in contrast to our previous finding that the same immunization regimen protected macaques against the E11S clone significantly better than the uncloned SIVmne given intravenously. Our findings are in agreement with the result of Benson et al. (3), who observed a better clinical outcome in a significant proportion of immunized macaques after intrarectal, but not after intravenous, infection with SIVmac251. It is therefore possible that vaccine strategies that fail to protect against intravenous challenge may still have some efficacy against mucosal infection. Since mucosal infection is the primary mode of natural transmission of HIV, such findings are potentially of significance.
Several mechanisms may contribute to the enhanced efficacy of the vaccines against the uncloned virus infection by the intrarectal route compared with the intravenous route. The relative inefficiency of mucosal transmission is not likely to account for such a difference because we compensated for the low efficiency by using 500- to 1,000-fold larger virus inocula for intrarectal challenges so that the same AID was used as for intravenous challenges. It is possible that, for a finite but significant time after the initial exposure, the infection is restricted locally in animals challenged through mucosal routes (37) and the process of dissemination is delayed compared with those challenged intravenously. The recall response in immunized animals would be better able to control and perhaps eradicate localized infection after mucosal exposure and before disseminated infection could be established.
Alternatively, the enhanced efficacy of the vaccines against intrarectal challenge by the uncloned SIVmne may be due to selective transmission and amplification of E11S-like virus after intrarectal exposure. Selective transmission and/or amplification has been proposed to account for the genotype restriction observed after natural infection with HIV-1 (36, 43, 44, 46, 47). However, due to the difficulties in obtaining both the inocula and early tissue samples (prior to seroconversion), it remains unclear to what extent such restriction is caused by sequestration of the donor’s virus (12, 48) and/or selection by the recipient’s immune responses (20). Although results from different investigators vary, studies in animal models have provided the most definitive evidence for selective transmission and/or amplification after intravaginal (13, 23, 27, 29) or intrarectal infection (40, 41) with SIV or SHIV chimeras. Our present findings are in agreement with these earlier reports and provide a strong indication for the preferential transmission and amplification of E11S-like viruses after intrarectal exposure. At present, we cannot distinguish between these two possible mechanisms for the enrichment of E11S-like viruses. However, since two of the six control macaques showed a rapid reversal from E11S-like viruses to variant types during the first month of infection, preferential amplification alone is not likely to account for the predominance of E11S-like viruses at 2 weeks after intrarectal exposure. It is possible that differential selective processes exist in the mucosa versus the peripheral lymph nodes, such that different viral genotypes are selected immediately after mucosal exposure or after disseminated infection has occurred. In HIV-infected individuals, viruses recovered early after infection often exhibit macrophage-tropic, “non-syncytium-inducing” phenotypes. Although there is as yet no evidence supporting preferential transmission of macrophage-tropic viruses in animals, it is of interest that a molecular clone derived from E11S (SIVmne CL8) was recently shown to be macrophage-tropic, whereas variants that evolved from CL8 and shared the same canonical variant V1 sequences as reported here were not (35). In any case, preferential transmission and/or amplification of E11S-like viruses, if confirmed, would at least partially account for the greater efficacy of the vaccine against the uncloned virus after intrarectal versus intravenous challenge. Our results therefore also point to the importance of selecting the relevant and appropriate isolates of HIV-1 for the development of candidate vaccines.
To conserve animals in the present study, we used macaques previously protected against E11S infection for the rechallenge with uncloned SIVmne. It is possible that prior exposure to virus inoculum, without resulting in an ongoing infection, may nevertheless contribute to protection against the rechallenge (9, 18, 30). We cannot exclude this possibility without a direct comparative study. However, it should be noted that we have not been able to demonstrate any sign of E11S infection in these animals by multiple and stringent assays, and we have confirmed their virus-negative status for over 2 years. Any such effect, if present, would have to be elicited by very transient and limited infection below the limit of our detection, which in itself would lend further support to the efficacy of the vaccination regimen described here. In any case, it is unlikely that any effect of prior exposure alone could account for the differential protective efficacy of vaccination against intrarectal versus intravenous challenge, since reduced efficacy was observed in intravenously challenged animals regardless of whether they were challenged for the first time or were protected against E11S and rechallenged (31).
Although we are not able to address the mechanism of protection in this relatively small study, it is of interest to note that in both challenge studies the only immunized animal that became infected had the lowest titer of SIV-specific serum antibodies as determined by ELISA. The only animal infected after the uncloned SIVmne challenge also had significantly lower serum neutralizing antibodies than the protected animals. However, there was no significant difference in the serum neutralizing titers between the protected animal and those infected with E11S, perhaps due to the relative insensitivity of this assay. The apparent correlation between SIV-specific antibody titers (and perhaps serum neutralizing activities) and protection contradicts findings from our previous studies in which no such correlation was observed with the intravenous route of challenge (31). The basis for such a discrepancy is not clear. However, it is possible that the kinetics and the initial events after intravenous or intrarectal infection are sufficiently different that the quantitative or qualitative requirements for immune protection may also differ. In this context, it is of interest to note that the only immunized animal from which the vaginal washes were analyzed had levels of SIV-specific IgG and IgA comparable to those present in chronically infected animals (21). It is possible that SIV-specific antibodies, including neutralizing antibodies, could be present in mucosal sites such as vaginal and rectal surfaces as a result of transudation and, if so, may contribute to protection against challenge at these sites. It is also possible that other effector mechanisms (such as T-helper cells, cytotoxic T lymphocytes, and antibody-dependent cellular cytotoxicity) may contribute to protection, especially in those animals with no apparent neutralizing antibodies.
Over the past decade, a number of clinical trials have been undertaken to examine the safety and immunogenicity of envelope-based vaccines, including those in combination regimens similar to those described here. The potential efficacy of these vaccines is unknown, but it has been the subject of much controversy. Results from the present study are therefore of potential importance in this regard. They indicate that parenterally administered envelope-based vaccines, when given in a combination immunization regimen, may elicit protection against mucosal infection by a pathogenic uncloned virus. Furthermore, contrary to some previous indications, protection may be achieved more easily against mucosal infections than against blood-borne infections. Although it remains to be determined whether and to what extent these findings will be applicable to the development of HIV-1 vaccines, our results provide a strong basis for further improvements and testing of recombinant vaccines in combination immunization strategies.
ACKNOWLEDGMENTS
We thank Randy Nolte, LaRene Kuller, and Tom Beck for assistance with veterinary studies; Susan Gallinger, Kia Kornas, Lynda Misher, Walter Knott, and Richard Hill for expert technical assistance; Bryan Kennedy for flow cytometry analysis; Sridhar Pennathur, Gail Sylva, and Jim Klaniecki for the preparation of immunogens; Bruce Travis and Andy Watson for advice on QC-PCR analyses; Li Wang for statistical analysis; Julie Overbaugh for critical reading of the manuscript; and Kate Elias and Marjorie Domenowske for manuscript preparation.
This work was supported in part by NIH grants AI26503 and RR00166 and by NIH contracts AI65302 and NCI-6S-1649.
REFERENCES
- 1.Almond N M, Heeney J L. AIDS vaccine development in primate models. AIDS. 1998;12:S133–S140. [PubMed] [Google Scholar]
- 2.Arthur L O, Bess J W, Jr, Urban R G, Strominger J L, Morton W R, Mann D L, Henderson L E, Benveniste R E. Macaques immunized with HLA-DR are protected from challenge with simian immunodeficiency virus. J Virol. 1995;69:3117–3124. doi: 10.1128/jvi.69.5.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo R C, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against highly pathogenic simian immunodeficiency virus SIVmac251 dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benveniste R E, Arthur L O, Tsai C C, Sowder R, Copeland T D, Henderson L E, Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986;60:483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benveniste R E, Morton W R, Clark E A, Tsai C C, Ochs H D, Ward J M, Kuller L, Knott W B, Hill R W, Gale M J, Thouless M E. Inoculations of baboons and macaques with simian immunodeficiency virus/Mne, a primate lentivirus closely related to human immunodeficiency virus type 2. J Virol. 1988;62:2091–2101. doi: 10.1128/jvi.62.6.2091-2101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benveniste R E, Raben D, Hill R, Knott W, Drummond J E, Arthur L O, Jahrling P B, Morton W R, Henderson L E, Heidecker G. Molecular characterization and comparison of simian immunodeficiency virus isolates from macaques, mangabeys, and African green monkeys. J Med Primatol. 1989;18:287–303. [PubMed] [Google Scholar]
- 7.Benveniste R E, Hill R W, Eron L J, Csaikl U M, Knott W B, Henderson L E, Sowder R C, Nagashima K, Gonda M A. Characterization of clones of HIV-1 infected HuT78 cells defective in gag gene processing and of SIV clones producing large amounts of envelope glycoprotein. J Med Primatol. 1990;19:351–366. [PubMed] [Google Scholar]
- 8.Benveniste R E, Kuller L, Rodman S T, Hu S-L, Morton W R. Long-term protection of macaques against high-dose type D retrovirus challenge after immunization with recombinant vaccinia virus expressing envelope glycoproteins. J Med Primatol. 1993;22:74–79. [PubMed] [Google Scholar]
- 9.Clerici M, Clark E A, Polacino P, Axberg I, Casey N I, Morton W R, Shearer G M, Benveniste R E. T-cell proliferation to subinfectious SIV correlates with lack of infection after challenge of macaques. AIDS. 1994;8:1391–1395. doi: 10.1097/00002030-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Cranage M P, Baskerville A, Asworth L A, Dennis M, Cook N, Sharpe S, Farrar G, Rose J, Kitchin P A, Greenaway P J. Intrarectal challenge of macaques vaccinated with formalin-inactivated simian immunodeficiency virus. Lancet. 1992;339:273–274. doi: 10.1016/0140-6736(92)91335-6. [DOI] [PubMed] [Google Scholar]
- 11.Cranage M P, Whatmore A M, Sharpe S A, Cook N, Polyanskaya N, Leech S, Smith J D, Rud E W, Dennis M J, Hall G A. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology. 1997;229:143–154. doi: 10.1006/viro.1996.8419. [DOI] [PubMed] [Google Scholar]
- 12.Delwart E L, Mullins J I, Gupta P, Learn G H, Jr, Holodniy M, Katzenstein D, Walker B D, Singh M K. Human immunodeficiency virus type 1 populations in blood and semen. J Virol. 1998;72:617–623. doi: 10.1128/jvi.72.1.617-623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enose Y, Okada M, Sata T, Ma W, Igarashi T, Ibuki K, Ido E, Hayami M. Restriction of viral population by intravaginal infection of simian immunodeficiency viruses in macaque monkeys. Arch Virol. 1997;142:37–51. doi: 10.1007/s007050050057. [DOI] [PubMed] [Google Scholar]
- 14.Heidecker G, Muñoz H, Lloyd P, Hodge D, Ruscetti F W, Morton W R, Hu S-L, Benveniste R E. Macaques infected with cloned simian immunodeficiency virus show recurring nef gene alterations. Virology. 1998;249:260–274. doi: 10.1006/viro.1998.9325. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch V M, Dapolito G, Johnson P R, Elkins W R, London W T, Goldstein S, Montali R, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with extent of in vivo replication. J Virol. 1994;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S-L, Abrams K, Barber G N, Moran P, Zarling J M, Langlois A J, Kuller L, Morton W R, Benveniste R E. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 17.Hu S-L, Stallard V, Abrams K, Barber G N, Kuller L, Langlois A J, Morton W R, Benveniste R E. Protection of vaccinia-primed macaques against SIVmne infection by combination immunization with recombinant vaccinia virus and SIVmne gp160. J Med Primatol. 1993;22:92–99. [PubMed] [Google Scholar]
- 18.Kent S J, Hu S-L, Corey L, Morton W R, Greenberg P D. Detection of simian immunodeficiency virus (SIV)-specific CD8+ T cells in macaques protected from SIV challenge by prior SIV subunit vaccination. J Virol. 1996;70:4941–4947. doi: 10.1128/jvi.70.8.4941-4947.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaniecki J, Dykers T, Travis B M, Schmitt R, Wain M, Watson A J, Sridhar P, McClure J, Morein B, Ulrich J T, Hu S-L, Lewis J B. Cross-neutralizing antibodies in rabbits immunized with HIV-1 gp160 purified from simian cells infected with recombinant vaccinia virus. AIDS Res Hum Retroviruses. 1991;7:791–797. doi: 10.1089/aid.1991.7.791. [DOI] [PubMed] [Google Scholar]
- 20.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Kuller, L. R. Unpublished data.
- 21.Kuller L R, Thompson J, Watanabe R, Iskandriati D, Alpers C E, Morton W R, Agy M. Mucosal antibody expression following rapid SIVmne dissemination in intrarectally infected Macaca nemestrina. AIDS Res Hum Retroviruses. 1998;14:1345–1356. doi: 10.1089/aid.1998.14.1345. [DOI] [PubMed] [Google Scholar]
- 21a.Langlois, A. J. Unpublished data.
- 22.Lehner T, Wang Y, Cranage M, Bergmeier L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Brosio P, Lafaile M, Li J, Collman R G, Sodroski J, Miller C J. Vaginal transmission of chimeric simian/human immunodeficiency viruses in rhesus macaques. J Virol. 1996;70:3045–3050. doi: 10.1128/jvi.70.5.3045-3050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marthas M L, Miller C J, Sutjipto S, Higgins J, Torten J, Lohman B L, Unger R E, Ramos R A, Kiyono H, McGhee J R, Marx P A, Pedersen N C. Efficacy of live-attenuated and whole-inactivated simian immunodeficiency virus vaccines against vaginal challenge with virulent SIV. J Med Primatol. 1992;21:99–107. [PubMed] [Google Scholar]
- 25.Marx P A, Compans R W, Gettie A, Stass J K, Gilley R M, Mulligan M J, Yamschivock G V, Chen D, Eldridge J H. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–1327. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 26.Miller C J, McChesney M B, Lu X, Dailey P J, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller C J, Marthas M, Greenier J, Lu D, Dailey P J, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72:3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montefiori D C, Robinson W E, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neildez O, Le Grand R, Caufour P, Vaslin B, Cheret A, Matheux F, Theodoro F, Roques P, Dormont D. Selective quasispecies transmission after systemic or mucosal exposure of macaques to simian immunodeficiency virus. Virology. 1998;243:12–20. doi: 10.1006/viro.1997.9026. [DOI] [PubMed] [Google Scholar]
- 30.Pauza C D, Emau P, Salvato M S, Trivedi P, MacKenzie D, Malkovsky M, Uno H, Schultz K T. Pathogenesis of SIVmac251 after atraumatic inoculation of the rectal mucosa in rhesus monkeys. J Med Primatol. 1993;22:154–161. [PubMed] [Google Scholar]
- 31.Polacino P, Stallard V, Klaniecki J E, Montefiori D C, Langlois A J, Richardson B A, Overbaugh J, Morton W R, Benveniste R E, Hu S-L. Limited breadth of the protective immunity elicited by SIVmne gp160 vaccines in a combination immunization regimen. J Virol. 1999;73:618–630. doi: 10.1128/jvi.73.1.618-630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polacino P S, Liang H A, Firpo E J, Clark E. T-cell activation influences initial DNA synthesis of simian immunodeficiency virus in resting T lymphocytes from macaques. J Virol. 1993;67:7008–7016. doi: 10.1128/jvi.67.12.7008-7016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putkonen P, Makitalo B, Bottinger D, Biberfeld G, Thorstensson R. Protection of human immunodeficiency virus type 2-exposed seronegative macaques from mucosal simian immunodeficiency virus transmission. J Virol. 1997;71:4981–4984. doi: 10.1128/jvi.71.7.4981-4984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quesada-Rolander M, Makitalo B, Thorstensson R, Zhang Y J, Castanos-Velez E, Biberfeld G, Putkonen P. Protection against mucosal SIVsm challenge in macaques infected with a chimeric SIV that expresses HIV type 1 envelope. AIDS Res Hum Retroviruses. 1996;12:993–999. doi: 10.1089/aid.1996.12.993. [DOI] [PubMed] [Google Scholar]
- 35.Rudensey L, Kimata J T, Long E M, Chackerian B, Overbaugh J. Changes in the extracellular envelope glycoprotein of variants that evolve during the course of simian immunodeficiency virus SIVmne infection affect neutralizing antibody recognition, syncytium formation, and macrophage tropism but not replication, cytopathicity, or CCR-5 coreceptor recognition. J Virol. 1998;72:209–217. doi: 10.1128/jvi.72.1.209-217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soto-Ramirez L E, Renijifo B, McLane M F, Marlick R, O’Hara C, Sutthent R, Wasi C, Vithayasai P, Vithayasai V, Apichartpiyakul C, Auewarakul P, Cruz V P, Chui D S, Osathanondh R, Mayer K, Lee T H, Essex M. HIV-1 Langerhans’ cell tropism associated with heterosexual transmission of HIV. Science. 1996;271:1291–1293. doi: 10.1126/science.271.5253.1291. [DOI] [PubMed] [Google Scholar]
- 37.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stott E J, Kitchin P A, Page M, Flanagan B, Taffs L F, Chan W L, Mills K H G, Silvera P, Rodgers A. Anti-cell antibody in macaques. Nature. 1991;353:393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- 39.Stott E J, Hu S-L, Almond N. Candidate vaccines protect macaques against primate immunodeficiency viruses. AIDS Res Hum Retroviruses. 1998;14:S265–S270. [PubMed] [Google Scholar]
- 40.Trivedi P, Meyer K K, Streblow D N, Preuninger B L, Schultz K T, Pauza C D. Selective amplification of simian immunodeficiency virus genotypes after intrarectal inoculation of rhesus macaques. J Virol. 1994;68:7649–7653. doi: 10.1128/jvi.68.11.7649-7653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trivedi P, Horejsh D, Hinds S B, Hinds II P W, Wu M S, Salvato M S, Pauza C D. Intrarectal transmission of simian immunodeficiency virus in rhesus macaques: selective amplification and host responses to transient or persistent viremia. J Virol. 1996;70:6876–6883. doi: 10.1128/jvi.70.10.6876-6883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P R, Hu S-L, Haigwood N L. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfs T F, Zwart G, Bakker M, Goudsmit J. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology. 1992;189:103–110. doi: 10.1016/0042-6822(92)90685-i. [DOI] [PubMed] [Google Scholar]
- 44.Wolinsky S M, Wike C M, Korber B T, Hutto C, Parks W P, Rosemblum L L, Kunstman K J, Furtado M R, Muñoz J L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. WHO report on the global HIV/AIDS epidemic. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 46.Zhang L Q, MacKenzie P, Cleland A, Holmes E C, Brown A J L, Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993;67:3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 48.Zhu T, Wang N, Carr A, Nam D S, Moor-Jankowski R, Cooper D A, Ho D D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]