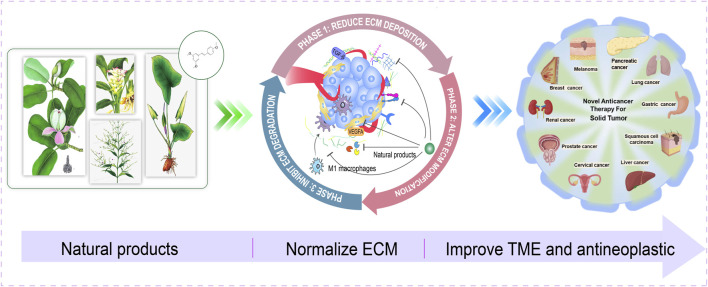
Abstract
Extracellular matrix (ECM) plays a pivotal and dynamic role in the construction of tumor microenvironment (TME), becoming the focus in cancer research and treatment. Multiple cell signaling in ECM remodeling contribute to uncontrolled proliferation, metastasis, immune evasion and drug resistance of cancer. Targeting trilogy of ECM remodeling could be a new strategy during the early-, middle-, advanced-stages of cancer and overcoming drug resistance. Currently nearly 60% of the alternative anticancer drugs are derived from natural products or active ingredients or structural analogs isolated from plants. According to the characteristics of ECM, this manuscript proposes three phases of whole-process management of cancer, including prevention of cancer development in the early stage of cancer (Phase I); prevent the metastasis of tumor in the middle stage of cancer (Phase II); provide a novel method in the use of immunotherapy for advanced cancer (Phase III), and present novel insights on the contribution of natural products use as innovative strategies to exert anticancer effects by targeting components in ECM. Herein, we focus on trilogy of ECM remodeling and the interaction among ECM, cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs), and sort out the intervention effects of natural products on the ECM and related targets in the tumor progression, provide a reference for the development of new drugs against tumor metastasis and recurrence.
Keywords: TME, tumor microenvironment; ECM, extracellular matrix; CAFs, cancer-associated fibroblasts; TAMs, tumor-associated macrophages; HA, hyaluronic acid; MMPs, matrix metalloproteinases; HSPGs, heparan sulfate proteoglycans; TGFβ, transforming growth factor beta
Graphical Abstract
Highlights
1 Based on extensive researches on extracellular matrix (ECM), this review provides a reference for the subsequent research on the mechanism of tumor occurrence and development and the development of new drug targets in the future.
2 Based on the coincidence between the whole-process of tumor progression and the ECM remodeling trilogy, we propose three phases of whole-process management of cancer and the necessity of targeting ECM remodeling trilogy in the treatment of tumors.
3 This paper is the first time comprehensively to summarize the current situation of natural products targeting ECM research, providing more new perspectives and research fields for following researchers.
1 Introduction
Statistics showed that there were 19.3 million new cancer diagnoses and 10 million new deaths worldwide in 2020. The cancer burden is predicted to reach 28.4 million by 2040, a 47% increase from 2020 (Sung et al., 2021). Malignant tumors spring up with high morbidity and mortality but lack of effective therapies (Huang et al., 2020; Tiwari et al., 2022). Current systemic antitumor therapy still has a high probability of tumor recurrence and distant metastasis (D'Alterio et al., 2020; Basu et al., 2022). Immune escape, early metastasis, and drug resistance under tumor microenvironment (TME) protection are the main reasons for the frustration of cancer therapy (Senthebane et al., 2017; Chen and Dey, 2022). There are dynamic interactions between cellular and non-cellular components in the TME. Their constant variations contribute to the generation of tumor cell heterogeneity, clonal evolution, enhanced multidrug resistance of tumor cells, and ineffectiveness of immunotherapy (Dzobo et al., 2023). Reprogramming of TME components is one of the possible exits. Coincidentally, extracellular matrix (ECM) is the major non-cellular component of TME, gradually becoming a hotspot in tumor therapy (Walker et al., 2018; Karamanos et al., 2021).
ECM is an insoluble structural component of stroma in mesenchyma and epithelial blood vessels, including collagen, elastin, proteoglycan and glycoprotein (Chaudhuri et al., 2020). It interacts with tumor and stromal cells to trigger uncontrolled cell proliferation, migration, invasion and immune evasion of during the lengthy process of precancerous lesions progressing to malignancy (Pickup et al., 2014). This whole process is accompanied by variations in the ECM’s own structure.
ECM remodeling includes the deposition of cells and components in the ECM, the abnormal modification caused by increasing ECM-modifying enzymes, and the degradation of ECM conducted by proteases (Winkler et al., 2020). The dysregulation of the above mechanisms, especially the amount and cross-linking state of ECM components, induce ECM stiffness variation (Meng et al., 2016; Hua et al., 2020; Hua et al., 2021; Tzanakakis et al., 2021; Yang et al., 2021). ECM stiffness and ECM degradation are the main contributors to cancer metastasis which also contribute to the deterioration of tumor (Najafi et al., 2019). The trilogy of ECM remodeling are exist simultaneously throughout the tumor progression cycle, with different emphases occurring in the early-, middle-, and advanced-stages.
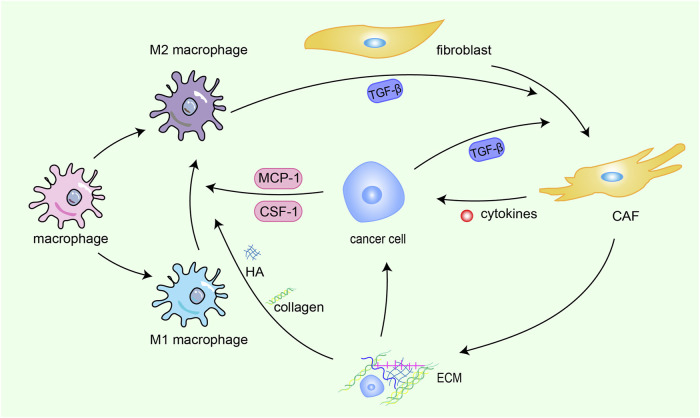
In addition, the signaling loop formed by TAMs, CAFs and tumor cells contribute to ECM remodeling. Fibroblasts, the fundamental cell types in the TME and the producer of ECM, play a pivotal role in the construction and remodeling of the ECM (Kalluri and Zeisberg, 2006). Fibroblasts are attracted to migrate toward the TME by transforming growth factor beta (TGF-β) derived from cancer cells, then switch into CAFs with greater abilities to proliferate and promote ECM accumulation (Voloshenyuk et al., 2011; Terra et al., 2018). CAFs promote the synthesis of collagen and fibronectin and chemokines related to tumor promotion (Ignotz and Massagué, 1986; Kuzet and Gaggioli, 2016), which heighten cancer cell invasion and ultimately contribute to the occurrence of organ-specific metastases. This interaction between cancer cells and CAFs constitute s a forward circulation that fuels rapid tumor development. TAMs are the most common immune cells in the TME, and TAMs are constantly affected by physical, chemical, and biological signals from cancer cells and the ECM (Tajaldini et al., 2022). ECM components can drive M2 polarization (Tariq et al., 2017), TAMs can weave an immunosuppressive network by secreting immunosuppressive factors are released into the TME, providing more fuel to this vicious cycle, thereby promoting the activation of CAFs and leading to malignant transformation (Figure 1).
FIGURE 1.
Crosstalk among CAFs, TAMs, ECM and cancer cells. Fibroblasts are attracted to migrate toward the TME by TGF-β derived from cancer cells and M2 macrophages, then switch into CAFs with greater abilities to proliferate and promote ECM accumulation. CAFs promote the synthesis of collagen and fibronectin and chemokines related to tumor promotion, which heighten cancer cell invasion and ultimately contribute to the occurrence of organ-specific metastases. This interaction between cancer cells and CAFs constitute s a forward circulation that fuels rapid tumor development. TAMs are constantly affected by physical, chemical, and biological signals from cancer cells and the ECM. ECM components drive M2 polarization. TAMs can weave an immunosuppressive network by secreting immunosuppressive factors, providing more fuel to this vicious cycle, thereby promoting the activation of CAFs and leading to malignant transformation.
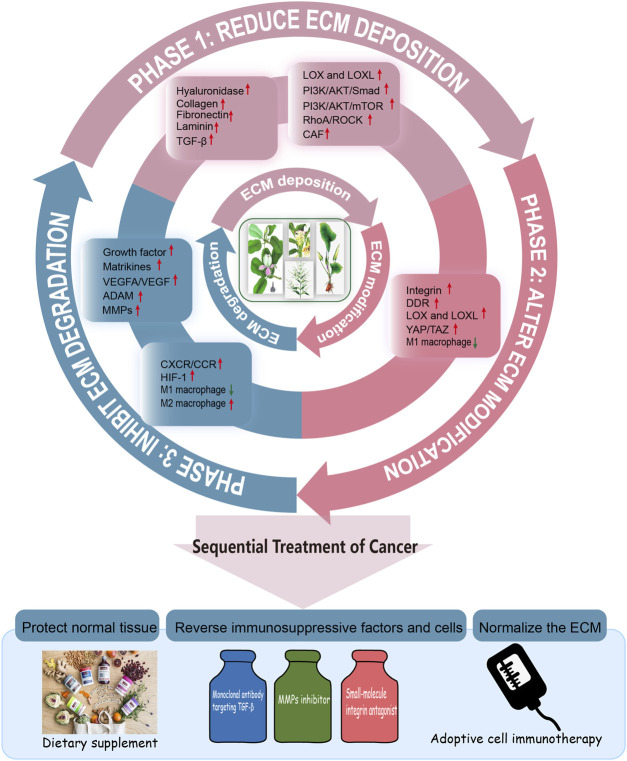
Since the ECM remodeling trilogy exhibits different emphases at different stages, the focus of pharmacotherapy in tumor sequential treatment should be coincident with different emphases at different stages. Thus, we put forward the concept of three phases of the whole-process management of cancer around ECM, controlling three phases of cancer progression is key to reducing cancer-related deaths. Phase I: Reducing ECM deposition in an early-stage contribute to improve ECM stiffness and delay the cancerization, such as fibrillation, which effectively reverse the carcinogenesis of precancerous lesions. Targeting MMPs can theoretically inhibit the likelihood of early metastasis. Phase II: During the middle stages of cancer progression, reversing the tumorigenic ECM modification and inhibiting ECM degradation can decrease the possibility of advanced cancer metastasis and recurrence. Inhibiting ECM deposition facilitates chemotherapy drugs reach the inside of the tumor through dense ECM, so that improving clinical efficacy. Phase III: In the advanced stages, regulating ECM degradation is beneficial to reduce tumor neovascularization, alleviate widespread metastasis and facilitate therapeutic drugs enter the dense microenvironment of solid tumors, such as pancreatic cancer, etc., thereby improving quality of life.
In the past decade, the invention and application of advanced technologies such as large-scale rapid screening, combinatorial chemistry, and genetic engineering have accelerated the process of drug development. The research and development of antineoplastic drugs has entered a new era. Nature is the richest source of structurally novel anticancer drug candidates. Natural products and drugs that are derivatives or mimic natural products and their pharmacophores account for a considerable proportion of anticancer drugs (Mokbel et al., 2019; Atanasov et al., 2021), served as a huge treasure trove of medicines can prepare for ECM preclinical research and new drug development. Clinical practice has shown that active ingredients extracted from natural products such as medicinal plants and Marine organisms, such as flavonoids, terpenoids, alkaloids, polysaccharides, essential oils and peptides, effectively inhibit the growth of tumor cells (Deng et al., 2020; Dong et al., 2022). Many natural products have small side effects and high safety, and could be used as dietary supplements to regulate ECM and curb the occurrence of cancer. There are also problems such as low solubility, low bioavailability and low tumor targeting, leading to the necessity of using large doses of most natural products, which increases the risk of side effects. Nanodrug delivery systems (liposomes, micelles and nanoparticles) provide a powerful backup for the design of advanced drug delivery systems for natural drugs due to their excellent tumor targeting, anti-tumor activity, immune sensitization, stability and safety. Study has shown the inhibitory effect of chitosan nanoparticles enriched with limonene essential oil on melanoma and breast cancer cells (Alipanah et al., 2021).
Currently, there have been reviews of natural products targeting CAF, whereas ECM remodeling is a core transit station in the TME. It’s necessary to review the natural products targeting ECM remodeling and help achieve the whole-process management of cancer. In cancer cell-centric TMEs, regulating angiogenesis, tumor metastasis, and drug resistance by manipulating the composition and structure of the ECM. Natural products can modulate the ECM, cut off cellular crosstalk between CAFs, cancer cells and TAMs, and regulate ECM remodeling to normalize it to inhibit angiogenesis, thereby meliorating the delivery of chemotherapeutic drugs to cancer cells.
2 Trilogy of tumorigenic ECM remodeling
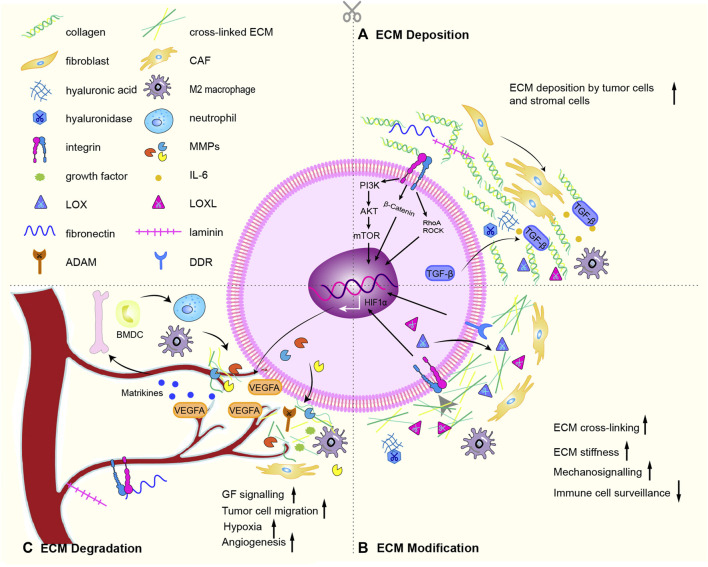
The core components of ECM are collagen, proteoglycans, and glycoproteins. About 300 unique matrix macromolecules, including fibronectin, elastin, tenascin, and hyaluronic acid, constitute the core matrix of the ECM (Hynes and Naba, 2012). These ECM components are post-translationally modified by a series of secreted modifying enzymes, such as oxidases and proteases. A dynamically regulated physical scaffold built by these embellished components also exist dynamically biochemical and biomechanical properties of the ECM. Cell surface receptors (such as integrins, discoid domain receptors, and multimers) interact with ECM components, binding factors, sense the biochemical and mechanical properties of the ECM, mediate cell adhesion and cell signaling, thereby regulating proliferation, differentiation, migration and apoptosis (Hastings et al., 2019). In the physiological state, the production and degradation of ECM proteins and receptors maintain a homeostasis to ensure normal cell function. During the tumorigenic ECM remodeling, proteins and receptors undergo tumorigenic changes in the processes of ECM deposition (Figure 2A), ECM modification (Figure 2B) and ECM degradation (Figure 2C), which promote the growth, proliferation and metastasis of tumor cells (Winkler et al., 2020).
FIGURE 2.
ECM remodeling mechanism. (A) Especially through active TGF-β signaling, CAFs deposit abundant collagen and express cytokines, promotes the recruitment and activation of M2 macrophages. The deposition of cells inevitably lead to deposition of ECM subassembly, including collagen, ECM modifying enzymes (LOX, LOX-like protein), fibronectin, hyaluronic acid, tenascin C and laminin. (B) Chemical modification alters the biochemical properties and structural characteristics of the ECM. The main proteins involved are LOX, LOXLs, and TG2. Force-mediated ECM remodeling affects ECM tissue by aligning ECM fibers and opening cell migration channels. The main mechanical sensors involved are integrins, ROCK, DDR, Piezo1, YAP/TAZ. Overexpression of LOX and LOXLs increases fibrosis and ECM stiffness, and promotes tumorigenesis and metastasis. During this modification, mechanical forces exerted by aggregated integrins would result in nonproteolytic destruction of the basement membrane, allowing invasion of cancer cells. (C) CAFs, cancer cells and recruited BMDC secrete proteases that degrade the ECM: such as MMPs, disintegrins and ADAMs. ECM degradation is an important driver of cancer cell motility. The binding of soluble signaling molecules such as growth factors to the ECM renders them insoluble and inactive, while proteases enables their release. Proteolytic ECM degradation produces bioactive matrikines and releases matrix-bound matrikines. These factors induce pro-tumor ECM signaling that promotes tumor proliferation, migration, invasion, and angiogenesis. Matrikines also induce the activation of BMDC to secrete neutrophils. Neutrophils secrete potent MMP-9, which degrades ECM and releases matrix-bound VEGF, creating a concentration gradient of new angiogenic sprouts. Finally, upon stimulation of dense ECM, tumor cells may acquire endothelial-like functions and mimic the vasculature connected to blood vessels. Some graphic elements refer to published literature (Winkler et al., 2020).
The abundance and composition of components are main variable during ECM deposition. Thus, the biochemical and mechanical properties are consist with these variation. ECM is produced mainly by CAFs, which are the main culprits in pathological changes such as fibrosis and canceration (Barbazan and Matic Vignjevic, 2019). CAFs are distinguished by α-smooth muscle actin (α-SMA) expression (Chen and Song, 2019). Various pro-fibrotic growth factors and inflammatory factors have an effect on the activation and differentiation of myofibroblast phenotype, such as TGF-α, TGF-β, fibroblast growth factor (FGF-2), platelet-derived growth factor (PDGF), epidermal growth factor (EGF) (Heneberg, 2016). Especially through active TGF-β signaling, CAFs deposit abundant collagen and express cytokines, promotes the recruitment and activation of immune cells, thereby establishing an oncogenic and pro-inflammatory cancer niche (Elyada et al., 2019). The deposition of cells inevitably lead to increase in ECM composition, including collagen, ECM modifying enzymes (such as lysyl oxidase (LOX) and LOX-like protein), fibronectin, hyaluronic acid, tenascin C and laminin. Deposition of fibrillar collagen is the most common tumorigenic change in the ECM and can directly promote tumor growth (Fang et al., 2014; Zhou et al., 2017). Deposition of fibronectin, hyaluronic acid and tenascin C lead to fibrotic phenotype (hyperplasia of connective tissue), a key feature of various cancers (Iacobuzio-Donahue et al., 2002). While laminin is involved in angiogenesis, especially in the process of vascular maturation. Deposition of ECM-modifying proteins hyaluronan and proteoglycan-linked protein 1 (HAPLN1) affects the integrity of the lymphatic vasculature, resulting in increased metastasis. Low-molecular-mass HA oligosaccharides (LMM-HA) have been linked with poor prognosis in several tumors (Lipponen et al., 2001; Schmaus et al., 2014). Dysregulated HA synthase and HA-degrading hyaluronidase lead to elevated LMM-HA, which interacts with the cell surface receptor CD44 to modulate pro-tumor signaling cascades (including glycolysis) (Sullivan et al., 2018).
Tumorigenic ECM modification including chemical modification at the post-translational level and force-mediated ECM remodeling (Wolf and Friedl, 2011; Nguyen-Ngoc et al., 2012). Chemical modification alters the biochemical properties and structural characteristics of the ECM(Karsdal et al., 2013). The main proteins involved are LOX, LOXLs, and tissue transglutaminase 2 (TG2) (Yuzhalin et al., 2018). Force-mediated ECM remodeling affects ECM tissue by aligning ECM fibers and opening cell migration channels. The main mechanical sensors involved are integrins, Rho kinase (ROCK), Disc domain receptors (DDR), Piezo1, YAP/TAZ (Jiang et al., 2022). In normal soft tissue, collagen fibers in the interstitial stroma that coil and run parallel to the epithelial layer may prevent cancer development by activating tumor suppressor phenotypes (Maller et al., 2013). However, collagen fibers near the tumor boundary are linear and vertically oriented, supporting the invasive growth of the tumor (Levental et al., 2009; Conklin et al., 2011). Overexpression of LOX and LOXLs increases fibrosis and ECM stiffness, and promotes tumorigenesis and metastasis (Barker et al., 2012). Overexpression of TG2 in cancer cells increases ECM cross-linking, affecting mechanical properties and cell-matrix signaling (Levental et al., 2009). Fibronectin glycosylation enhances the invasiveness of urothelial cancer cells (Richter et al., 2008). Increased glycosylation of transmembrane proteins results to bulky glycocalyx, applying tension on ECM-bound integrins which are important receptors for ECM components (Paszek et al., 2014). During this modification, mechanical forces exerted by aggregated integrins would result in nonproteolytic destruction of the basement membrane, allowing invasion of cancer cells (Kechagia et al., 2019).
CAFs, cancer cells and recruited bone marrow-derived cells secrete proteases that cleave and degrade the ECM: such as MMPs, disintegrins and metalloproteinases (ADAMs) (Lu et al., 2011). MMPs are involved in the physiological and pathological degradation of collagen (Mahalanobish et al., 2020). MMP-1, MMP-8, MMP-13 and MMP-14 cleave fibril-forming collagens I, II and III, while MMP-2 and MMP-9 cleave denatured collagen and collagen IV(Kessenbrock et al., 2010). ECM degradation is an important driver of cancer cell motility (Kai et al., 2019). The binding of soluble signaling molecules such as growth factors (GFs) to the ECM renders them insoluble and inactive, while proteases enables their release (Martino and Hubbell, 2010). The activity of proteases is counteracted by protease inhibitors (e.g., TIMP, a tissue inhibitor of metalloproteinases) (Saw et al., 2019). After ECM degradation, proteolytic fragment release matrix-bound matrikines (Mott and Werb, 2004; Wells et al., 2015). These factors induce pro-tumor ECM signaling that promotes tumor proliferation, migration, invasion, and angiogenesis (Ricard-Blum and Salza, 2014; Papadas et al., 2020). Matrikines also induce the expression and activation of MMPs, including membrane type-1 matrix metalloproteinase (MT1-MMP) and MMP-2 (Brassart et al., 1998). These combined changes in ECM make a hypoxic environment exist for cancer cells. Neutrophils secrete potent MMP-9, which degrades ECM and releases matrix-bound VEGF, creating a concentration gradient of new angiogenic sprouts (Ardi et al., 2007). Finally, upon stimulation of dense ECM, tumor cells may acquire endothelial-like functions and mimic the vasculature connected to blood vessels.
Overall, changes in the abundance of ECM components lead to different tissue densities and stiffnesses, ECM degradation leads to the release of GFs and cytokines secluded by ECM, which induce tumor cell growth, angiogenesis, and inflammation (Kai et al., 2019). Furthermore, the ECM interacts with neighboring cells and initiates multiple cellular signaling cascades. ECM deposition, modification, and degradation are important mechanisms that dynamically regulate ECM abundance and structure. The ECM remodeling trilogy is present simultaneously during tumorigenesis, but with different emphasis at different stages. Therefore, finding drugs to normalize ECM abundance and structure in the early, middle and late stages of cancer therapy is the research goal in the sequential treatment of cancer.
3 Natural products normalize ECM
3.1 Flavonoids
3.1.1 Reduce ECM deposition
Flavonoids reduce ECM deposition mainly by reducing ECM components such as hyaluronic acid, laminin, fibronectin, etc., They work by inhibiting the NF-kB, JAK2/STAT1/2, p38 MAPK, JNK and TGF-β signaling pathways, of which TGF-β signaling is the most critical target. Ampelopsin reduces ECM deposition by lessening the amount of collage I, α-SMA, TIMPs, p-Smad3, TGF-β1 and increasing the expression of MMP-9 (Ma et al., 2019). Pectolinarigenin, reported as a STAT3 inhibitor, blocks the phosphorylation of Smad3 and STAT3, and downregulates the major fibrotic gene and protein expression of TGF-β, α-SMA, COL-1, and fibronectin in TGF-β1-stimulated fibroblasts (Li et al., 2021b). Neohesperidin not only decreases the TGF-β1-induced myofibroblast differentiation and ECM production, but also inhibit fibroblast migration (Guo et al., 2019). Curcumin potently reduces levels of hyaluronic acid, laminin, fibronectin, α1 (1) collagen, procollagen III, α-SMA (Zhang et al., 2012). Further research revealed that the effect is associated with modulation of CBRs system (Zhang et al., 2013). Similarly, ginkgetin reduces the expression of collagen IV, fibronectin, and laminin via mediating AMPK/mTOR axis (Wei et al., 2021). Eupatilin increases the expressions of α-SMA, whereas expressions of ECM proteins type I collagen and fibronectin are reduced (Li et al., 2020b). Isoliquiritigenin suppresses inflammation cytokines, excessive deposition of ECM and oxidative stress-induced apoptosis via nuclear factor-erythroid 2 related factor 2 (Nrf2) and NF-kB pathways (Xiong et al., 2018). Morin inhibits ECM generation through restraining the activation of p38 MAPK and JNK signaling pathways (Ke et al., 2016).
Some flavonoids simultaneously reduce ECM deposition and inhibit ECM degradation. For example, apigenin is shown to reduce the ECM deposition, but also possess the potential of preserving collagen matrix from breakdown. Apigenin inhibit ECM production in TGF-β1-stimulated CFs, and the mechanisms partly ascribed to the reduction of miR-155-5p expression and subsequent increment of c-Ski expression, which lead to the inhibition of Smad2/3 and p-Smad2/3 (Wang et al., 2021). Furthermore, apigenin and wogonin primarily lessen ECM degradation by blocking c-Fos/activator protein-1 (AP-1) and JAK2/STAT1/2 pathways (Lim et al., 2011).
3.1.2 Alter ECM modification
In the force-mediated ECM remodeling, integrins play an important role by binding to the ECM molecules and applying mechanical force to ECM molecules and cancer cell. Silibinin interferes with the interaction between prostate cancer cells and fibronectin, thereby inhibiting their motility, invasiveness and survival. Studies have shown that silibinin modulates fibronectin induced expression of integrins (α5, αV, β1, and β3), actin remodeling (e.g., local adhesion kinase), apoptosis, EMT, and signaling molecules related to cell survival (Deep et al., 2014). Changes in surface integrins affect cell migration, proliferation, and ECM production. Quercetin alter the interaction between fibroblasts and ECM by up-regulating αV integrin and down-regulating β1 integrin on fibroblasts (Doersch and Newell-Rogers, 2017). Similarly, baicalein may be used to treat breast cancer by interfering with the interaction of ECM with cancer cells. Fibronectin-induced migration, invasion and F-actin remodeling are significantly inhibited by baicalein (Chen et al., 2019b).
3.1.3 Inhibit degradation of ECM
MMPs are important accessory molecules in the process of tumor cell metastasis, which can cause excessive ECM degradation and cancer cell invasion. MMPS are also considered as promising targets for tumor therapy. Most flavonoids are potential candidates for MMPs inhibitors. MMP-2 and MMP-9 degrade ECM to release essential components of neovascularization. Silibinin, naringin and naringenin can inhibit MMP2/9 to reduce angiogenesis. Silibinin inhibits the expression of urokinase-type plasminogen activator (u-PA) and MMP-2 in human osteosarcoma MG-63 cells by down-regulating the induction of adhesion spot kinase and ERK-dependent c-jun/activator protein-1 (AP-1) (Hsieh et al., 2007). Simultaneously, silibinin significantly lessens the expression and activity of MMP-9 promoted by EGFR signaling (Liang et al., 2012). Naringin attenuates MAPK signaling pathways including p38, JNK and ERK, leading to downregulation of MMP-2/9 (Aroui et al., 2016). The main effect of naringenin is to inhibit pERK1/2, TLR and TGF-β pathways, which significantly reduces ECM synthesis and deposition. Other effects include inhibition of VEGF, induction of apoptosis and regulation of MAPK pathway. (Jung et al., 2013; Hernandez-Aquino and Muriel, 2018).
Oroxylin A also inhibits MMP-2/9 in breast cancer. Further elucidation of the mechanism reveals that it increases the expression of TIMP-2 and represses the PMA-induced translocation of PKC δ, phosphorylation of ERK1/2 and binding activity of the transcription factor AP-1 which are upstream signaling molecules in MMP-2/9 expression (Sun et al., 2009; Lu et al., 2012). Similarly, kaempferol inhibits MMP-2 and TIMP-2 transcription by suppressing c-Jun activity and phosphorylation of ERK1/2. It also reduces human breast carcinoma cells invasion through blocking the PKC δ/MAPK/AP-1 cascade and subsequent MMP-9 (Lin et al., 2013; Li et al., 2015b). In addition, kaempferol can inhibit other matrix degrading enzymes (MMP-1, MMP-3, MMP-13, disintegrin, etc.), consequently abolishes the degradation of collagen II (Huang et al., 2018). Wogonin exerts its anticancer effects in breast cancer may through targeting 5-LO/BLT2/ERK/IL-8/MMP-9 signaling cascade. By inhibiting the BLT2/ERK-linked cascade, wogonin attenuates the expression of IL-8 and MMP-9 (Chen et al., 2011; Go et al., 2018). Furthermore, wogonin causes suppression of melanoma cell B16-F10 migration, adhesion, invasion and actin remodeling by inhibiting MMP-2 (Zhao et al., 2014). Orientin suppresses MMP-9 expression through inhibiting TPA-induced PKC α and ERK activation, as well as the nuclear translocation of AP-1 and STAT3 (Kim et al., 2018). In the glioblastoma multiforme (GBM), tricetin reduces MMP-2 production at transcriptional level. Specificity protein-1 (SP-1) is an promising object of tricetin for inhibiting MMP-2-mediated cell motility. Preventing the ERK pathway would inhibit the effect, and further enhances the anti-invasive ability of tricetin against GBM cells. The combination of tricetin and an ERK inhibitor may be a good strategy for preventing GBM invasion (Chao et al., 2015). In the A549 cell line, fisetin induces apoptosis by decreasing the expression of MMP-2/9, increases the expression of E-cadherin via targeting the ERK signaling pathway (Wang and Huang, 2018). In colorectal cancer, baicalein suppresses cell invasion via inhibiting the ERK signaling pathways and the expression of the MMP-2/-9 (Chai et al., 2017). Chrysin and daidzein perform their antitumor effect in part due to the downregulation of CXCL-1, ERK, AKT, and MMP-9 (Salama and Allam, 2021).
TGF-β/Smad pathway occupies an important position in ECM remodeling. Regarded as a TGF-β inhibitor, Hesperetin works on the TGF-β1/Smad pathway-mediated ECM progression and restores the upregulation of IL-1β, PTGS2, and MMP-13 induced by TNF-α, reverses the degradation of the ECM, therefore it has promising therapeutic effects on various cancers (Kong et al., 2018; Wu et al., 2021). Curcumin significantly suppresses the expression of TGF-β1 and phospho-smad2/3, upregulates smad7, increases the amount of α-SMA expressing myofibroblasts, inhibits activity of MMPs, preserves ECM from degradation and also attenuates collagen deposition (Wang et al., 2012). It inhibits NO, PGE2, IL-6, IL-8, and MMP-3 production in a concentration-dependent manner (Mathy-Hartert et al., 2009). In addition, some genes relating to ECM-receptor interaction pathway, such as GP1BB, COL9A3, and AGRN, could be inhibited by curcumin (Mao et al., 2021).
Quercetin inhibits the degradation of ECM via up-regulating SOD and TIMP-1, down-regulating MMP-13 and increasing the expression of collagen II (Wei et al., 2019; Guo et al., 2021). Noteworthy, quercetin also induces activation of MMP-2/9 (Hernandez-Ortega et al., 2012). Isoliquiritigenin decreases expression level of collagen X (Col X), MMP-13 and exerts anti-angiogenesis effects through direct suppression of MMP-2 (Ji et al., 2018). Glabridin upregulates the expression levels of ECM related genes, such as collagen II, proteoglycan 4, SRY-box 9 and aggrecan (Dai et al., 2021). The addition of genistein results in downregulation of the transcription of most of MMPs genes in MDA-MB-231 and MCF-7 cells (Kousidou et al., 2005). Isorhamnetin obviously blocks MDA-MB-231 cell invasion via downregulating MMP-2/9, which is potentially associated with the inhibition of p38 MAPK and STAT3 (Li et al., 2015a). At the same time, Isorhamnetin could reduce mRNA expression of TNF-α, IL-1β, IL-6, IL-12, and MMP-1 (Jnawali et al., 2016). Ononin inhibits the expression of MMP-13 and alleviates the decomposition of collagen II protein by downregulating the MAPK and NF-κB signaling pathways (Xu et al., 2022). Nobiletin suppresses MMP-9 expression through modulating p38 MAPK without cytotoxicity (Kim. et al., 2014).
These findings constitute the molecular basis for the antiinvasive and anti-metastatic effects of flavonoids, and shed light on the investigation of flavonoids on cancer metastasis. Therefore, we proposes that flavonoids might be considered as a therapeutic potential candidate for the treatment of cancer metastasis.
3.1.4 Targeting CAFs
A study found that curcumin effectively inhibits TGF-β1-induced fibroblasts differentiation through Smad-2 and p38 signaling pathways (Liu et al., 2016). Further research revealed that curcumin induces the apoptosis and cell cycle arrest of prostate-CAFs. The upregulation of ROS caused by curcumin triggers endoplasmic reticulum stress of CAFs via the PERK-eIF2 α-ATF4 axis (Zeng et al., 2020). Moreover, curcumin abrogates CAF-induced invasion and EMT, and lessens ROS production and CXCR4 and IL-6 receptor via blocking MAOA/mTOR/HIF-1α axis, thereby supporting the therapeutic effect of curcumin in prostate cancer (Du et al., 2015). Meanwhile, curcumin eliminate CAF’s ability of promoting invasion by increasing E-cadherin level and lowering vimentin level. In prostatic cancer, the observed increase in α-SMA and CAF-like phenotype is TGF-β2 dependent, which is strongly suppressed by silibinin. The finding emphasizes the potential application of silibinin in prostatic cancer prevention by targeting the CAF phenotype (Ting et al., 2015). Moreover, silibinin reduces MCP-1 and CAFs’ biomarkers (fibroblast activation protein, a-SMA, TGF-β2, vimentin, etc.), and significantly modulates the recruitment of immune cells in TME, inhibiting invasiveness in prostatic cancer (Ting et al., 2016).
3.2 Phenols
3.2.1 Reduce ECM deposition
Most Phenols reduces ECM-related protein expression via NF-kB signaling or TGF-β-induced cellular responses, demonstrating their potential to reduce ECM deposition. Noteworthy, some phenols exert dual-directional regulation on ECM remolding. Resveratrol decreases the mRNA levels of fibronectin and the protein expression of collagen I and α-SMA, as well as β-catenin (Chen et al., 2019a). It also inhibits the generation of VEGF, MMP-3, MMP-9 and COX-2 to counteract IL-1β-mediated degradation. These gene are regulated by NF-kB. Nuclear translocation of the p65 subunit of NF-kB and its phosphorylation are consist with IL-1β-induced proteasome function and the dissociation of IkBa which suppressed by Resveratrol. (Shakibaei et al., 2008; Frischholz et al., 2020). The production of TNF-a, IL-6, fibronectin and collagen IV is inhibited by daphnetin. In addition, daphnetin enhances the expression of Nrf2 and inhibits the levels of p-Akt and p-p65. Thus, daphnetin inhibits ECM accumulation, oxidative stress, and inflammatory response. The effect is partially intermediated by Nrf2/keap1 and Akt/NF-kB pathways (Xu et al., 2019).
Garcimultiflorone K inhibited Smad protein phosphorylation, cell migration, and ECM expression induced by TGF-β. Mechanistic studies demonstrated that garcimultiflorone K suppresses TGF-β signaling by downregulating TGF-β receptor II (Huang et al., 2021b). Histological studies revealed that sesamin reduces CAFs activation and collagen accumulation in the ECM. Sesamin inhibits TGF-β1-stimulated expression of α-SMA, the accumulation of α-SMA-positive cells and expression of collagen I, as well as TGF-β1-induced Smad3 phosphorylation (Lin et al., 2014). Osthole significantly mitigates fibronectin, collagen IV, and laminin increased by TGF-β2 stimulation, suggesting it’s capable of reducing ECM expression (Fan et al., 2020).
3.2.2 Alter ECM modification
Integrin-mediated ECM modification is crucial during the cell adhesion processes involved in carcinogenesis. Epigalloccatechin-3-gallate (EGCG) specifically antagonizes the migration of DAOY cells on collagen by up-regulating the expression of specific genes and proteins of the β-1 integrin subunit and improving the adhesion of cells (Pilorget et al., 2003). [10]-gingerol downregulates the expression of EGFR and β1-integrin, reverting the malignant phenotype of the breast cancer cells (Fuzer et al., 2017).
3.2.3 Inhibit degradation of ECM
In HTB94 chondrosarcoma cells and breast cancer cells, resveratrol significantly suppresses the expression of Cyclin D1, c-Myc, SOX-2, MMP-2/9, and inhibits MMP-induced differentiation via the p38 kinase and JNK pathways as well as activation of Akt and STAT3. At the same time, resveratrol induces the expression of collagen II, SOX-9 and the production of sulfated proteoglycans (Gweon and Kim, 2014; Suh et al., 2018). Some structural analogues of natural products may have better pharmacological effects. For instance, 4,4′-dihydroxy-trans-stilbene (DHS), a synthetic analog of resveratrol, exerts a strong reduction in MMP-2/9 activities, concomitantly with a pronounced inhibition of cell adhesion, migration and invasion to ECM components. Meanwhile, modulation of E-cadherin is also observed in DHS-treated cells. These outcomes suggested that the two 4,4′-hydroxyl groups bound to the stilbenic backbone make DHS a more active molecule than resveratrol (Maccario et al., 2012).
Phenolics extracted from tea include Epicatechin-3-gallate (ECG), EGCG and catechins. ECG suppresses the invasion of highly metastatic A549 cells by reducing the activities of MMP-2 and uPA, simultaneously inhibits fibronectin and p-FAK (Huang et al., 2016). Catechin significantly decreases circulatory MMP-9 levels at a dose of 50 mg/kg (Addepalli and Suryavanshi, 2018). Previous studies have shown that EGCG inhibit prostate CAFs differentiation. ECM contraction is an enhancer of tumor cell invasion. Further researches showed that EGCG and luteolin inhibit TGF-β-induced ECM contraction and the downstream signaling of ERK and AKT. Furthermore, both of them reduce Rho A activation which is necessary for fibronectin expression. Thus, combined clinical use of these compounds could prevent or reverse cancer progression by targeting the TME. (Gray et al., 2014).
MMPs and VEGF exert critical effects during the ECM remodeling and angiogenesis. EGCG protect ARPE-19 cells against apoptosis and attenuate mRNA and protein expressions of MMP-9, VEGF, VEGFR-2 by inhibiting production of ROS(Lee et al., 2014). Procyanidins crosslink vascular ECM proteins, protect them against proteolysis by MMPs (Zhai et al., 2011). Erianthridin has shown obviously antimetastatic activity against non-small-cell lung cancer through Akt/mTOR/p70S6K-induced actin reorganization and MMP-2/9 expression (Pothongsrisit et al., 2021). Treatment of cultured B-cell lymphocytic leukemia patients’ cells with hyperforin results in an obvious inhibition of their capacity to secrete MMP-9. The hyperforin acts by reducing the production of the latent 92 kDa pro-enzyme and decreasing VEGF released by the leukemic cells (Quiney et al., 2006). Ellagic acid and rosmarinic acid both downregulate the MMP-13 and upregulate the collagen of type II, aggrecan and sulfated-proteoglycan synthesis. Ellagic acid acts via the NF-κB signaling, while the rosmarinic acid via ERK-1/2 and p38 kinase signaling pathways (Eo and Kim, 2017; Lin et al., 2020). Esculetin and fraxetin are both dihydroxycoumarins. Esculetin’s antiangiogenic action in LM8 cells have been due to the inhibition of MMP-2, TGF-β1 and VEGF productions at tumor sites (Kimura and Sumiyoshi, 2015). The coumarin derivative 4-methylesculetin (4-ME) is known to inhibit the elevation of hyaluronidase, MMP-13, MMP-3 and MMP-9 levels that cause ECM degeneration (Hemshekhar et al., 2013). Magnolol has been recognized as an antitumor reagent. In HCC bearing animal model, expression of phospho-ERK, NF-κBp65, MMP-9, VEGF, and CyclinD1 are significantly decreased by it. In addition, caspase-8 and caspase-9, the major extrinsic and intrinsic apoptosis signaling factors, are both enhanced by magnolol (Tsai et al., 2020).
Zingerone is a phenolic alkanone with anti-inflammatory and antioxidant effects. Acetyl zingerone is a structural analogue of Zingerone. Both of them upregulate Notch pathway gene expression and downregulate expression of genes linked to ECM disassembly, thereby improving ECM integrity. After structural improvement, Acetyl zingerone possesses a greater activity of suppressing MMP-3 and MMP-12 (Swindell et al., 2020).
3.2.4 Targeting CAFs
Resveratrol can revoke the generation of IL-6 by CAFs. CAFs strongly induce IL-6-mediated cell motility in conditioned medium, but pretreatment with resveratrol completely prevented cancer cell motility, simultaneously reversing the N-to-E cadherin switch (Thongchot et al., 2018). Eugenol and decitabine can inhibit the expression of DNA methyltransferase genes DNMT1 and DNMT3A in breast CAF cells, and suppress the invasion/migration and proliferation potential of CAF cells by down-regulating E2F1, as well as their paracrine carcinogenic effects. The difference is that eugenol has a persistent inhibitory effect, whereas the effect of decitabine is transient. (Al-Kharashi et al., 2021).
3.3 Polysaccharides
Ascophyllan inhibits the migration and adhesion of B16 melanoma cells through lessening the expression of N-cadherin and enhancing the E-cadherin. Furthermore, ascophyllan inhibited the expression of MMP-9 mRNA and the secretion of MMP-9 protein, a process that may involve the ERK signaling pathway (Abu et al., 2015). Prunella vulgaris Polysaccharide could exert an antineoplastic effect on CAFs by inhibiting basic fibroblast growth factor (b-FGF) expression, thus inhibiting the growth of breast cancer cells indirectly (Hao et al., 2020).
3.4 Saponins
Polyphyllin II may be a potential antineoplastic drugs used in the prospective application by improving ECM. It arouses programmed cell death and suppresses metastases in murine lung adenocarcinoma, efficaciously attenuates hepatotoxic and inhibits pulmonary adenoma through down-regulating expression of MMP-9 and upregulating level of TIMP-2 (Man et al., 2015). Astragaloside Ⅳ can strongly inhibit the proliferation/migration/invasion-promoting capacities of gastric CAFs. It significantly upregulates microRNA-214 expression and downregulates microRNA-301a expression in gastric CAFs, reestablishes the microRNA expression balance subsequently elevates TIMP2 production and secretion, and suppresses macrophage colony-stimulating factors production and secretion (Wang et al., 2017b).
3.5 Terpenoids
Terpenoids regulate the ECM remodeling by decreasing the production of MMPs and reducing collagen deposition. Heparan sulfates are long, unbranched, negatively charged polysaccharides that are bound to core proteins. Heparanase cleaves the ECM by degrading heparan sulfate, which eventually leads to cell invasion and metastasis. In the melanoma, hinokitiol could suppress the expression of heparanase through lowering the phosphorylation of protein kinase B (Akt) and ERK, inhibiting the expression and activity of MMP-2/9 simultaneously (Huang et al., 2015; Wu et al., 2020). Artemisinin significantly inhibits the induction of extracellular matrix metalloproteinase inducer (EMMPRIN) and MMP-9 at both the transcriptional and translational levels via suppressing the PKCd/ERK/p38 cascade (Wang et al., 2011). Andrographolide inhibits MMP-2-mediated cell motility through blocking the ERK 1/2 pathway in GBM cells, as well as targeting the CREB. A combination of andrographolide and an ERK inhibitor might be a good strategy for preventing GBM metastasis (Yang et al., 2017). Asiatic acid could inhibit TGF-β1-induced expression of collagen I, suppress the phosphorylation of Smad2/3 and the expression of plasminogen activator inhibitor-1(PAI-1), while elevate Smad7 protein level (Bian et al., 2013).
3.6 Alkaloids
Most alkaloids inhibit MMP-2/9 through P38, PI3K/Akt and protein kinase C-a (PKCa)/ERK/nuclear factor kappa B (NF-jB) pathways respectively. The anticancer activity of α-Chaconine mainly includes inhibition of tumor cell proliferation, migration, invasion and induction of cell apoptosis. Researches reveals a new therapeutic potential for α-chaconine and α-solanine on anti-angiogenic therapy. They suppresses migration, invasion and tube formation of cancer cell through inhibiting MMP-2 activities, as well as JNK, PI3K/Akt and NF-kB signaling pathways (Lu et al., 2010a), (Lu et al., 2010b). Some reports demonstrated that α-tomatine prevent the invasion/migration of MCF-7 cells through blocking PKCa/ERK/NF-jB signaling pathways. Specifically, α-tomatine could inhibit the activation of ERK1/2 and PKCa involved in the downregulation of the enzyme activities and messenger RNA levels of MMP-2/9 induced by TPA. Next, α-tomatine also strongly inhibits TPA induced the activation of NF-jB and phospho-inhibitor of kappa Ba (Shi et al., 2013; Yelken et al., 2017). Aristolochic acid inhibits MMP-9 through the NF-jB signal pathway, implying it may be involved in alteration of matrix homeostasis (Wu et al., 2011).
Overproduction of MMPs and EMMPRIN by monocytes/macrophages leads to the degradation of ECM. IL-1β prompts the expression of MMPs and IL-6 through toll-like receptor 4 (TLR4)/NF-jB axis, while oxymatrine reduce the expression of MMPs and TNF-a induced by IL-1β(Wei et al., 2020). Berberine significantly inhibits IGF-1R expression and decreases MMP-2/9, a-SMA, but also reduces collagen I expression (Li et al., 2018a). Further research showed that berberine lessens MMP-9 and EMMPRIN generation through inhibiting the activation of p38 pathway in PMA-induced macrophages (Huang et al., 2011). Solasodine was found to inhibit proliferation in various tumor cells. It reduces the mRNA level of MMP-2/9 and EMMPRIN, increases the production of TIMP-1/2, and simultaneously suppresses PI3K and Akt phosphorylation (Shen et al., 2017). Evodiamine inhibits cell migration and invasion abilities by down-regulating expression of MMP-9, uPA and uPAR. Evodiamine-induced G0/G1 arrest and apoptosis are linked with a lessening in Bcl-2, cyclin D1 and cyclin-dependent kinase 6 (CDK6) expression and an increment in Bax and p27Kip1 expression. Furthermore, it also can regulates p-ERK and p-p38 MAPK expression (Du et al., 2013).
3.7 Quinones
Thymoquinone, a major active compound derived from the medicinal Nigella sativa, may reduce ECM accumulation by enhancing the phosphorylation AMPK. Thymoquinone significantly reduces protein and mRNA expression of α-SMA, collagen and TIMP-1, accompanied by downregulating the expression of TLR4 and proinflammatory cytokine levels, also inhibits PI3K phosphorylation (Bai et al., 2014). Analogously, shikonin significantly inhibits ECM formation by downregulating the TGF-β1 expression and maintaining the normal equilibrium between MMP-2 and TIMP-1 (Liu et al., 2019). Cross-linking formation play a critical role in ECM formation and homeostasis. Emodin was found to be a strong enhancer of LOXL1 expression that promotes ECM cross-linking formation by using the ZsGreen reporter system for drug screening, suggesting that emodin may be an effective drug or complement to ECM homeostasis (Jian et al., 2014).
4 Natural products affect the TAM-ECM-cancer cell crosstalk
4.1 Flavonoids
TAMs occupy an important position in modulating the TME and favoring cancer metastases. TAMs-targeted therapy is a promising approach that could be used to reverse the immunosuppressive tumor microenvironment. In osteosarcoma LM8-bearing mice, wogonin inhibits tumor growth and metastasis to multiple organs, angiogenesis, lymphangiogenesis and reduces TAM numbers. The effects are associated with the reduction in VEGF-C-induced VEGFR-3 phosphorylation by the inhibition of COX-2 expression and IL-1β production in TAMs (Kimura and Sumiyoshi, 2013). The therapeutic effect of silibinin in inhibiting lung tumor growth and regression through an antiangiogenic mechanism appears to be mediated by reduction of TAM and cytokines, inhibition of HIF-1α, NF-κB and STAT-3 activation, and upregulation of angiogenic inhibitors Ang-2 and Tie-2 (Tyagi et al., 2009). In glioblastomas, Isoliquiritigenin decreasing secretion of 20-HETE, VEGF and TGF-βreduces, accompanied with the reduction of tumor burden and normalization of vasculature. Furthermore, isoliquiritigenin intervenes crosstalk between TAMs and endothelial progenitor cells during angiogenesis (Wang et al., 2017a). TAMs achieves immunosuppression by secreting immunosuppressive molecules such as IL-10, TGF-β. Luteolin has been demonstrated that it exerted significant tumor suppressive activity on several cancers, by suppressing the secretion of IL-6, IL-12 and TNF-α in macrophages (Chen et al., 2018). Kumatakenin shows antineoplastic activities by eliciting apoptosis of ovarian cancer cells and suppressing the alternative activation of TAM. It inhibits the generation of M2 markers, IL-10, MMP-2/9, VEGF, and MCP-1, which is major determinants of macrophage recruitment at tumor sites (Woo et al., 2017).
Curcumin exhibits a potent anti-inflammatory activity which partly mediated by inhibiting the JNK signaling pathway. It is worth emphasizing the anti-inflammatory effects of curcumin in macrophages and the role of altering the macrophage M1/M2 equilibrium in TME. Mechanism of curcumin to immune modulation might be associated with its ability to enhance IL-10-mediated effects (Shiri et al., 2015; Somchit. et al., 2018). Baohuoside I is the key bioactive compound of XIAOPI formula,a newly approved drug by the State Food and Drug Administration of China. Mechanistic investigations demonstrated TAMs/CXCL1 is the crucial target of BHS in suppressing breast cancer metastasis. Baohuoside I can inhibit the M2 phenotype polarization of TAMs, thereby attenuating the expression and secretion of CXCL1 (Wang et al., 2020). Icariin impedes the development of pancreatic cancer in mice by regulating the tumor immune microenvironment. Icariin suppresses the polarization of RAW 264.7 cells into M2 macrophages by down-regulating ARG1 and MRC1 expression and blocking the IL4-STAT6 signaling pathway (Zheng et al., 2019).
4.2 Phenols
CD4+Foxp3+ regulatory T cells (Tregs) in the TME restrict antitumor immunity, contributing to tumor aggression and poor survival. CD8+CD122+ Tregs are more potent in immunosuppression than CD4+Foxp3+ Tregs. Studies have validated that resveratrol exerts its antineoplastic effects by suppressing CD4+Foxp3+ and M2-like macrophages. In the recent study, resveratrol inhibits the tumor growth in hepatocellular carcinoma model by decreasing the frequency of CD8+CD122+ Tregs. Furthermore, immunosuppressive cytokines such as TGF-β1 and IL-10 in tumor tissues were decreased by resveratrol, whereas antitumor cytokines TNF-α and IFN-γ were increased. Resveratrol also inhibits the activation of STAT3 signaling and reduces the percentage of M2-like macrophages (Zhang et al., 2020). Locally aggregated IFN-γ at the tumor site regulate TAM status. Under IFN-γ exposure, TAMs change their phenotypes to the M1-like morphology and intracellular granular pattern, accompanied with a reduction level of immunosuppressive and tumor progressive mediators. Synthetic resveratrol analog HS-1793 significantly increases IFN-γ secreting cells in splenocytes and decreases CD206+ macrophage infiltration (Jeong et al., 2014). Another study found that resveratrol significantly inhibited the tumor growth associated with decreased expression of p-STAT3 (Kimura and Sumiyoshi, 2016; Zhao et al., 2018).
The interaction between TAM and cancer cells provides a perspective of future therapeutic approaches. EGCG exerts anticancer activity by regulating of TAMs in TME. The expression levels of monocyte chemokines (csf-1 and ccl-2) in tumor cells of mice treated with EGCG were lower, and the cytokine was skewed from M2-to M1, which was manifested as the decrease of IL-6 and TGF-β, and the increase of TNF-α. EGCG upregulates miR-16 in tumor cells, enabling miR-16 translocate to TAM via exosomes and inhibit TAM invasion and M2 polarization (Jang. et al., 2013). Gallic acid and Caffeic acid block the processes of angiogenesis and tumor growth via protection of the tumoricidal efficacy of M1 macrophages and inhibition of proangiogenic factors, particularly VEGF, MMP-2/9, and cyclooxygenase-2 (COX-2) activity (Orsolic et al., 2020). Esculetin and fraxetin reduces the p-STAT3 and inhibits the production of IL-10, MCP-1 and TGF-β1 during the differentiation of M2 macrophages, thereby regulating the activation of TAM(Kimura and Sumiyoshi, 2015).
4.3 Polysaccharides
CMPB90-1, a novel natural polysaccharide from Cordyceps sinensis, activates p38, Akt and NF-κB by binding to TLR2, which resets TAMS from M2 phenotype to M1 phenotype. This process also involves the functional inhibition of the immune checkpoint programmed death ligand-1 (PD-L1)/programmed death 1 (PD-1) axis and T lymphocytes. These findings suggest that CMPB90-1 may be developed as a potential tumor immunotherapy reagent (Bi et al., 2020). Similarly, Astragalus polysaccharin inhibited the HCC-like phenotype in a mouse hepatocellular carcinoma model by inhibiting the M2 polarization of TAM. It enhanced the expression of M1 macrophage markers and the proportion of M1 macrophages, but the opposite effect was observed for M2 macrophages (Li et al., 2021a). It was shown that oral administration of granulosa yeast-derived β-Glucan significantly delayed cancer progress, which was linked with TAM phenotypic (Liu et al., 2015). These findings establish a new paradigm for macrophage polarization and immunosuppressive TAM conversion and shed light on the action mode of natural products treatment in cancer.
4.4 Saponins
Ginsenoside Rh2 is a promising anti-tumor monomer compound for lung cancer. In vivo, G-Rh2 can reduce the expression levels of M2 macrophage markers CD206 and VEGF, and contribute to the conversion of TAM from M2 subsets to M1, thereby preventing the migration of lung cancer cells. In addition, G-Rh2 can also reduce the expression levels of MMP-2 and MMP-9 (Li et al., 2018b). Dioscin is an herbal steroid saponin that promotes the secretion of proinflammatory cytokines (IL-6, TNF-α, and IL-1β) by promoting the M2 to M1 phenotypic transition (Kou et al., 2017).
4.5 Terpenoids
The inhibitory effect of terpenoids on TAM viability, differentiation, and cytokine production might elucidate the major mechanisms underlying its antitumor activity. The CCL2/CCR2 axis is an important mechanism of mediated macrophage infiltration. A natural product in fir, named 747, is structurally related to kaempferol and has sensitivity and selectivity similar to CCR2 antagonists. 747 blocked tumor-infiltrating macrophage-mediated immune suppression, increased the number of CD8+T cells in tumors, and inhibited in situ and subcutaneous tumor growth. In addition, 747 enhance the therapeutic effect of low-doses of sorafenib without significant toxicity. This study suggests that the combination of immunomodulators and chemotherapeutic agents may be a new way to treat tumors (Yao et al., 2017). Paeoniflorin (Wu et al., 2015), triptolide (Li et al., 2020a), corosolic acid (CA) and oleanolic acid (OA) can inhibit the differentiation of macrophages into M2 phenotype, suppress the expression of M2 markers and anti-inflammatory factors such as CD206, CD163, CD204, and IL-10. In addition, CA and OA also directly inhibit the proliferation of tumor cells and sensitize tumor cells to anti-cancer drugs such as doxorubicin and cisplatin (Fujiwara et al., 2011; Fujiwara et al., 2014).
4.6 Quinones
It has been shown that emodin delay tumor growth by inhibiting macrophage invasion and M2-like polarization, while increasing T-cell activation and reducing tumor angiogenesis. It is worth noting that emodin can interfere with the cancer-promoting feed-forward interaction between tumor cells and macrophages, thereby improving the immunosuppressive status of TME. Recent studies have found that emodin can block the TGF-β1-mediated crosstalk between TAMS and breast cancer cells, inhibit the epithelial cell transformation of breast cancer cells and the formation of cancer stem cells (Liu et al., 2020). Another study showed that emodin inhibited the secretion of MCP-1 and CSF-1 by tumor cells, thereby reducing macrophage migration to and adhesion to tumor cells. In conclusion, emodin inhibits tumor growth and metastasis by effectively blocking the feed-forward loop between breast cancer cells and macrophages (Iwanowycz et al., 2016). The specific dosage, other information and structural formula of all the natural products are shown in Tables 1, 2.
TABLE 1.
The Table of Natural products targeting ECM.
| 1.Flavonoid | ||||
|---|---|---|---|---|
| Name | Models | Dosage range | Targets/pathway/process | Reference |
| Curcumin | Ketamine and zylaxine (rats) | 150 mg/kg | MMPs, TGF-β1, phospho-Smad2/3, Smad7 | 100 |
| Second passage CFs | 20 µmol/l | Smad-2, p38, TGF-β1 | 113 | |
| Colorectal CSCs | 1, 5, 25 μM | TFAP2A | 102 | |
| Human chondrocytes and cartilage explants | 5-20 μM | NO, PGE2, IL-6, IL-8, MMP-3, 35S-GAG | 101 | |
| CCl4 (rats) | 100, 200, 400 mg/kg | CBR1 | 71 | |
| CCl4 (rats) | 200 mg/kg | HA, laminin, procollagen III, fibronectin, α-SMA, α1(1) collagen | 70 | |
| PC3 cells | 25 µM | MAOA/mTOR/HIF-1α, CXCR4, IL 6, ROS | 115 | |
| Prostate-CAFs, PC-3 | 0, 10, 20, 30 μM | ROS, PERK/eIF2α/ATF4, CAF | 114 | |
| BALB/c (rats) | 40, 80 mg/kg | IL-10, IL-12, STAT3, STAT4, M2 macrophage | 173 | |
| Silibinin | PC3 cells | 50–200 µM | Integrins (α5, αV, β1, β3) | 78 |
| Human RCC cell lines (ACHN, OS-RC-2 and SW839) | 0, 25, 50, 75 µM | ERK1/2, EGFR/MMP-9 | 82 | |
| MG-63 cells | 0, 5, 10, 15, 20, 25, 30 µM | u-PA, MMP-2, ERK1/2, AP-1, c-Jun | 81 | |
| Urethane injected mice | 742 mg/kg | Ang-2, Tie-2, TIMP-1, TIMP-2, HIF-1α, NF-κβ, STAT-3 | 168 | |
| PC3 cells | 30, 60, 90 µM | α-SMA, CAF | 116 | |
| RWPE-1, WPE-1 NA-22, WPE-1 NB-14 and PC3 | 90 µM | MCP-1, fibroblast activation protein, α-SMA, TGF-β2, vimentin | 117 | |
| Kaempferol | Rat chondrocytes | 0, 5, 10, 20 µM | IL-1β, MMP-1, MMP-3/13, disintegrin, p38 | 90 |
| MDA-MB-231,B16F10 | 10, 20, 40 µmol/L | PKCδ/MAPK/AP-1, MMP-9 | 88 | |
| C57BL/6 mice | 50,100, 200 mg/kg | |||
| SCC-4 | 0–100 μM | MMP-2, TIMP-2, ERK1/2, Jun | 89 | |
| Quercetin | C57Bl/6J mice | 10 μM | surface αV, β1 integrin | 79 |
| OA rabbits | 25 mg/kg | SOD, TIMP-1, MMP-13 | 104 | |
| CCl4/mineral oil injected rats | 100 mg/kg | MMP-2/9 | 106 | |
| Rat chondrocytes | 0, 12.5, 25, 50, 100, 200 μM | MMP-13, p110α/AKT/mTOR, collagen II gene | 105 | |
| Isoliquiritigenin | HK-2 human renal proximal tubule cells | 2.5, 5, 10 μM | Nrf2, NF-kB | 74 |
| ACLT mice | 10, 20, 40 mg/kg | Col X, MMP-13, MMP-2 | 107 | |
| C6 glioma cells, U87 glioma cells | 10, 20 mg/kg | 20-HETE, VEGF, TGF-β, TAM | 170 | |
| Isorhamnetin | INF-γ (MRC-5 Cells) | 20 μM | TNF-α, I L-1β, IL-6, IL-12, MMP1 | 111 |
| MDA-MB-231 | 10, 20, 40 μM | MMP-2/9, p38 MAPK, STAT3 | 110 | |
| Wogonin | MDA-MB-231 | 15, 30, 60 μM | MMP-9, PKCδ, ERK1/2 | 91 |
| B16-F10 | 15, 30, 60 μM | MMP-2, Rac1 | 93 | |
| B16-F10 (mice) | 15-60 mg/kg | |||
| MDA-MB-231 | 20 µM | IL-8, MMP-9, BLT2 /ERK, 5-LO/BLT2/ERK/IL-8/MMP-9 | 92 | |
| MDA-MB-231 (mice) | 20 mg/kg | |||
| LM8 cells (mice) | 25, 50 mg/kg | COX-2, IL-1β, VEGFR-3 | 168 | |
| LM8 cells | 0.1–100 µM | |||
| SW1353 cells | 10, 25 μM | c-Fos/AP-1, JAK2/STAT1/2, MMP-13 | 77 | |
| Apigenin | CFs | 6–24 μM | miR-155-5p, c-Ski, Smad2/3, p-Smad2/3 | 76 |
| SW1353 cells | 5-25 μM | c-Fos/AP-1, JAK2/STAT1/2, MMP-13 | 77 | |
| Oroxylin A | MDA-MB-435 | 1, 10, 100 µM | MMP-2/9, ERK1/2 | 86 |
| MDA-MB-231 | 1, 4, 16 µM | TIMP-2, PMA, PKCδ, ERK1/2, AP-1 | 87 | |
| B16-F10 (C57BL/6 mice) | 20, 40, 80 mg/kg | |||
| Hesperetin | Rat Chondrocytes | 1, 5, 10, 20, 50, 100 μM | IL-1β, PTGS2, MMP-13 | 99 |
| OA rats | 10 μM | |||
| BDL (mice) | 200 mg/kg | TGF-β1/Smad | 100 | |
| HSC-T6 cell | 0, 5, 10, 20, 50, 100 µM | |||
| Baicalein | CRC HT29 and DLD1 cell | 0–120 µM | ERK, MMP-2/9 mice | 97 |
| DLD1 implanted Balb/c athymic nude | 20 mg/kg | |||
| Mice | 30 mg/kg | calpain-2 | 80 | |
| MCF-10A | — | |||
| Tricetin | GBM 8401, U87 | 0, 20, 40, 80 µM | MMP-2, SP-1 | 95 |
| Chrysin | Colorectal cancer models (mice) | 125, 250 mg/kg | MMP-9, CXCL1, AREG, ERK, AKT | 98 |
| SW620 | — | |||
| Daidzein | Colorectal cancer models (mice) | 5, 10 mg/kg | MMP-9, CXCL1, AREG, ERK, AKT | 98 |
| SW620 | — | |||
| Glabridin | human OA chondrocytes | — | Collagen II, ACAN, SOX9, PRG4 | 108 |
| OA rats | 100 μL | |||
| Genistein | MCF-7, BT-20, MDA-MB-231, MCF-12A | 35, 100 μM | MMPs | 109 |
| Morin | rat glomerular MCs | 25, 50 μM | p38 MAPK, JNK | 75 |
| Icariin | pancreatic cancer models (mice) | 120 mg/kg | ARG1mRNA, MRC1mRNA, IL4-STAT6 | 176 |
| Kumatakenin | A2780, SKOV3 | — | IL-10, MMP-2/9, VEGF | 172 |
| Luteolin | rats | 20 mg/kg | IL-6, IL-12, TNF-α, NF-κB, M1 macrophage | 171 |
| Ononin | rat Chondrocytes | 0–100 μM | MAPK, NF-κB, TNF-α, IL-6, MMP-13 | 112 |
| Ampelopsin | CCl4 (mice) | 125, 250 mg/kg | collage I, α-SMA, TIMP-1, TGF-β1, p-Smad3, MMP-9, SIRT1SIRT1/TGF-β1/Smad3 | 67 |
| mouse hepatic stellate cells | 25, 50, 100 μM | |||
| Eupatilin | Human ASMCs | 0, 10, 20, 40, 80 μM | NF-κB, STAT3, AKT | 73 |
| Fisetin | A549 | 10, 40 µM | c-myc, cyclin-D1, COX-2, CXCR4, MMP-2/9, E-cadherin | 96 |
| Nobiletin | hDFs | — | p38 MAPK, MMP-9 | 113 |
| Orientin | TPA (MCF-7) | 50 nM | MMP-9, IL-8, PKCα, ERK, AP-1, STAT3 | 94 |
| Pectolinarigenin | UUO mice | 25 mg/kg | TGFβ, α-SMA, COL-1, FN, SMAD3, STAT3 | 68 |
| NRK-49F | 50 μМ | |||
| Neohesperidin | pulmonary fibrosis mice | 20 mg/kg | TGF-β1/Smad3 | 69 |
| CAGA-NIH-3T3 | — | |||
| MLg | 20 µM | |||
| Baohuoside i | MCF-10A, HBL100, BT549, Raw264.7 | — | TAMs/CXCL1 | 175 |
| Ginkgetin | HBZY- 1 | 2-80 μM | AMPK/mTOR | 72 |
| Naringin | U87 | 0, 5, 10, 15 μM | ERK, JNK, p38, MAPK, MMP-2, MMP-9 | 83 |
| Naringenin | fibroblasts | 0–160 μM | α-SMA, pERK1/2 | 85 |
| 2. Phenols | ||||
|---|---|---|---|---|
| Epicatechin-3-gallate (ECG) | A549, WI-38, MRC-5 | 10, 20 mg/kg | MMP-2, u-PA, E-cadherin, fibronectin, p-FAK | 131 |
| BALB/c mice | ||||
| Epigalloccatechin-3-gallate (EGCG) | ARPE-19, HRMECs | 1–100 μM | ROS, MMP-9, VEGF, VEGFR-2, PECAM/CD31 | 134 |
| BALB/c mice, SD rats | 200 mg/kg | |||
| WPMY-1 prostate fibroblast cell | 1.3–40 μM | ERK, AKT, RhoA, fibronectin | 133 | |
| DAOY medulloblastoma cell | 10, 20 μM | β1 integrins | 126 | |
| 4T1 | 100 μM | MicroRNA-16 | 181 | |
| BALB/c mice | 10 mg/kg | TAM infiltration, M2 polarization | ||
| Resveratrol | primary culture chondrocytes | 50μM | ECM, GAG, IL-1β | 120 |
| HTB94 | 50 μM | p38, JNK, MMP-2/9, type II collagen, SOX-9, sulfated proteoglycan | 128 | |
| Chondrocyte | 100 μM | MMP-3/9, COX-2, IL-1β, VEGF | 121 | |
| ELT-3-LUC, the primary cultures of human leiomyoma cells | 10, 50, 100 µM | PCNA, α-SMA, fibronectin, Bax, Bcl-2, COL1A1, β-catenin | 119 | |
| Nude (Foxn1nu) mice | 10 mg/kg | |||
| Hepa1-6, H22 | 10, 20, 40 µM | CD8+CD122+ Treg, TAMs/M2 macrophage | 177 | |
| C57BL/6 and BALB/c mice | 50 mg/kg | STAT3,TGF-β1, IL-10, TNF-α, IFN-γ | ||
| THP-1, LM8 | 5, 10, 25, 50 µM | IL-10, MCP-1, TGF-β1 | 118 | |
| C3H/He mice | 10, 25 mg/kg | M2 macrophage | ||
| MCF-7, MDA-MB-231 | 50 µM | Cyclin D1, c-Myc, MMP-2/9, Sox2, Akt, STAT3, self-renewal signaling molecules | 129 | |
| KKU-213, KKU-100, CAFs | 50, 100 µM | CAFs, IL-6 | 144 | |
| HS-1793 | FM3A, THP-1 | 1.25, 2.5, 5 μM | M-2 phenotype TAM | 178 |
| C3H/He mice | 1.5 mg/kg | IFN-γ, VEGF, MMP-9, IL-6, IL-12, IL-10, TFG-β, IL-1β, TNF-α, CD206, CD204 | ||
| 4,4′-dihydroxy-trans-stilbene | BALB/c 3T3 clone A31-1-1 cells, MCF-7 | 1, 2.5, 5, 7.5, 15, 30, 60, 90 µM | P53, p21, pRb, MMP-2/9, E-cadherin | 130 |
| Erianthridin | A549, H460 | 0–50 µM | Akt/mTOR/p70S6K, MMP-2/9 | 136 |
| CB17-Prkdcscid mice | ||||
| Hyperforin | Leukemic B-cells, HBMEC | 0–5 µg/uL | MMP-9, VEGF | 137 |
| 10-gingerol | HMT3522 S1, T4-2 | 0.25, 1, 2.5, 5, 10 µM | EGFR, β1-integrin | 127 |
| nude mice | ||||
| Catechin | SD rats | 25, 50 mg/kg | MMP-9, SOD, MDA, GSH | 132 |
| Ellagic acid | primary human chondrocytes | 12.5, 25, 50 μM | iNOS, COX-2, NO, TNF-α, PGE2, IL-6, MMP-13, ADAMTS-5, collagen II, NF-κB, aggrecan | 139 |
| C57BL/6 male wild-type (WT) mice | 40 mg/kg | |||
| Magnolol | HCC SK-Hep1 cells | 50, 100 mg/kg | p-ERK, NF-κB p65, MMP-9, XIAP, CyclinD1, caspase-8, caspase-9 | 142 |
| BALB/cAnN.Cg-Foxn1nu/CrlNarl (nude) mice | ||||
| Procyanidins | HUVECs, A549 cells | 0.75, 1.5, 3.1, 6.3, 12.5, 25, 50, 100 μg/ml | MMP-2, CD31 | 135 |
| Nude mice | 10, 30 mg/kg | |||
| Sesamin | A549, fibroblast cells | 10 μM | α-SMA, E-cadherin, fibronectin, vimentin, p-Smad3, collagen I | 124 |
| BALB/c mice | 1, 10, 20 mg/kg | |||
| Eugenol | CAF-64, CAF-180, MDA-MB-231, MCF-7 | 0.5, 1 μM | DNMT1/DNMT3A, E2F1 | 145 |
| nude mice | ||||
| Esculetin | LM8 cells | 10, 50, 100, 200 μM | Cyclin-D1, CDK4, MMP-2, TGF-β1, VEGF, p-Stat 3, IL-10, MCP-1 | 140 |
| LM8-bearing mice | 3, 10 mg/kg | |||
| Fraxetin | LM8 cells | 10, 50, 100, 200 μM | TGF-β1, p-Stat 3, IL-10, MCP-1 | 140 |
| LM8-bearing mice | 3, 10 mg/kg | |||
| Osthole | HTM cells | 1, 3, 10, 30, 100 μM | FN, COL-IV, LN | 125 |
| BALB/cJ mice | 30 mg/kg | |||
| 4-Methylesculetin | rats | 25, 50 mg/kg | MMP-3/9/13,ROS, GSH, H2O2, LPO, PCC, Thiol, e TNF-a, IL-1b, IL-6, COX-2 and PGE2 | 141 |
| Daphnetin | MCs | 0, 10, 20, 40 μM | Nrf2/keap1, Akt/NF-kB | 122 |
| Rosmarinic acid | Primary cultured chondrocytes | 0, 25, 50, 75, 100 μg/mL | MMP-13, COX-2, ERK-1/2 and p38, PEG2 | 138 |
| Gallic acid/Caffeic acid | EAT | 40, 80 mg/kg | M1 macrophages | 182 |
| mice | VEGF, MMP-2/9, COX-2 | |||
| zingerone | RHE tissues | 50 µg/ml | MMP-1/3/12, NOTCH1, MAML3, CTSV, PMAIP1, ARG2 | 143 |
| Acetyl zingerone | RHE tissues | 50 µg/ml | MMP-1/3/12, COL11A2, VCAN, SPARC, NOTCH1, MAML3, CTSV, PMAIP1, ARG2 | 143 |
| 3. Polysaccharides, Saponins and Terpenoid | ||||
|---|---|---|---|---|
| Astragalus polysacharin | MHCC97H, Huh7, THP-1 | 8, 16 mg/ml | CD68, CD86, CD206 | 184 |
| HCC mice | 50, 100, 200 mg/kg | |||
| Polyphyllin II | DEN-induced pulmonary adenoma rats | 50 mg/10ml/kg | MMP-9, TIMP-2 | 148 |
| Ginsenoside Rh2 | RAW267.4, THP-1, A549, H1299 | 60, 100, 140 µM | VEGF, MMP2/9, CD206, CD16/32,TNFα, iNOS, Arg-1 | 186 |
| Dioscin | B16, RAW264.7 | 1, 2 µM | E-Cad, N-Cad, Cx43, CD206, IL-6, TNFα, IL-1β | 187 |
| Melanoma metastasis model | 30, 60 mg/kg | |||
| Astragaloside IV | GCAFs, BGC-823 | 10, 20, 40 µM | Pan-CK, E-cadherin, Vimentin, PDGFR-β, α-SMA | 149 |
| miR-214/301a | ||||
| Andrographolide | GBM8401, U251 | 5, 10, 20, 40 µM | MMP-2, ERK1/2, Raf, MEK, JNK,CREB, c-Jun, c-fos, SP-1 | 153 |
| Hinokitiol | B16-F10, 4T1 | 1.25, 12.5, 125, 1250 nM | Akt, ERK | 150 |
| Melanoma/mammary carcinoma metastasis model | ||||
| B16-F10 | 1, 2, 5 µM | MMP-2, MMP-9 | 151 | |
| Artemisinin | THP-1 | 20, 40, 80 μg/mL | EMMPRIN, MMP-9, JNK, p38, ERK | 152 |
| Triptolide | 4T1, RAW264.7, THP-1 | 5, 10, 20 nM | CD206, Arg-1, IL-10, CD68, MCP-1, CD163 | 190 |
| Colon tumor model | 0.05, 0.2 mg/kg | |||
| Tumor syngeneic graft model | ||||
| Oleanolic acid | U373, THP-1 | 20, 30 µM | STAT3, JAK | 191, 192 |
| Corosolic acid | U373, macrophage | 10, 20, 30 μM | STAT3, CD163, IL-10 | 192 |
| Paeoniflorin | LLC | 1, 3, 10, 30, 100 μM | CD11c, F4/80 | 189 |
| LLC injected mice | ||||
| Asiatic Acid | keloid fibroblasts | 3, 10, 30 μM | PPAR-γ, PAI-1, Smad | 154 |
| 4. Alkaloids and Quinones | ||||
|---|---|---|---|---|
| α-Chaconine | BAECs | 1, 2, 3 μg/mL | JNK, PI3K, NF-κB, Akt, MMP-2 | 155 |
| α-Tomatine | MCF-7 | 0.5, 0.75, 1 µM | MMP-2/9, PKCα, ERK1/2, NF-κB | 157 |
| α-Solanine | A2058, A375 | 4.6, 9.2, 13.8, 18.4 µM | MMP-2/9, ERK, JNK, NF-κB, PI3K, Akt | 156 |
| Berberine | Type 2 diabetic animal models (rat) | 200mg·kg−1·day−1 | IGF-1R, MMP-2/9/14, ERK, a-SMA, Collagen I | 161 |
| THP-1 | 5, 10, 25, 50 μM | MMP-9, EMMPRIN, p38 | 162 | |
| Aristolochic acid | TIB-202, THP-1 | 1, 5, 10, 20 μM | NF-κB, MMP-9 | 59 |
| Solasodine | A549 | 4, 8, 12 μM | MMP/9, EMMPRIN, RECK, TIMP-1/2, PI3K, Akt, miR-21 | 163 |
| Evodiamine | MDA-MB-23 | 15, 30, 60, 90, 120 μM; 10 mg/kg | Bcl-2, Bax, P27, CDK6, Cyclin D1, MMP-9, ERK, p38, JNK | 164 |
| MDA-MB-23 injected mice | ||||
| Oxymatrine | nucleus pulposus cells | 0.25, 0.5, 1, 2 mg/mL | HMGB1, TLR4, IkBa, NF-κB | 160 |
| IDD rat | ||||
| Emodin | EO771, 4T1, MCF7, MDA-MB-231, TAMs | 40 mg/kg | TGF-β1, AKT, STAT3, ZEB1, Snail, Twist, Smad2/3/4 | 193 |
| Breast cancer model (mice) | ||||
| EO771, 4T1 | 25, 50 μM | JMJD3, IRF4, STAT6, H3K27m1/2/3 | 194 | |
| Breast cancer model (mice) | 40 mg/kg | |||
| hSf, hDF | 1, 2, 3, 4 μM | LOXL1 | 167 | |
| ICR female mice | 1 mg/L | |||
| Shikonin | liver fibrosis C57 mice | 2.5, 5 mg/kg | α-SMA, Beclin-1, LC3 I/II, TGF-β1, Smads | 166 |
| Thymoquinone | liver fibrosis Kunming mice | 20, 40 mg/kg | Collagen I, α-SMA, TIMP-1, TLR4, PI3K, MAPK, LKB1 | 165 |
TABLE 2.
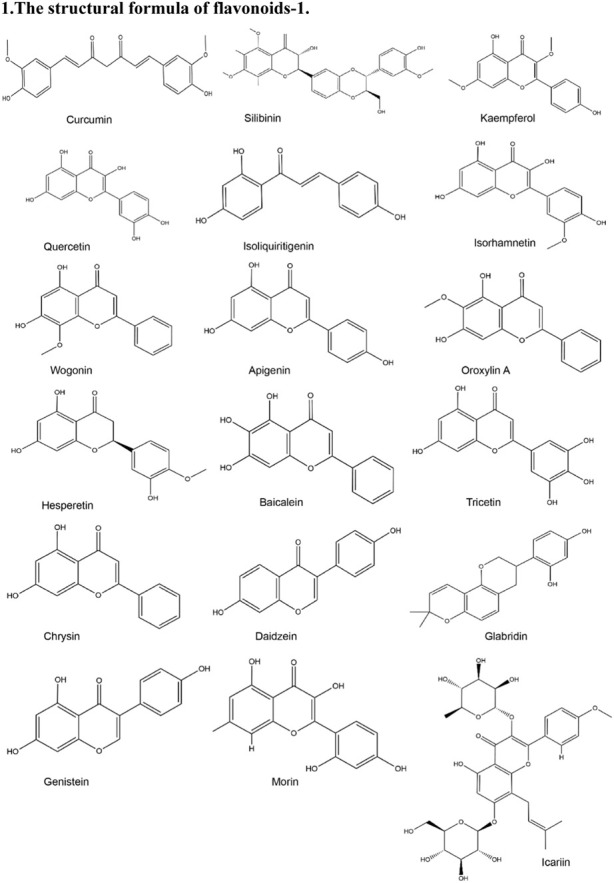
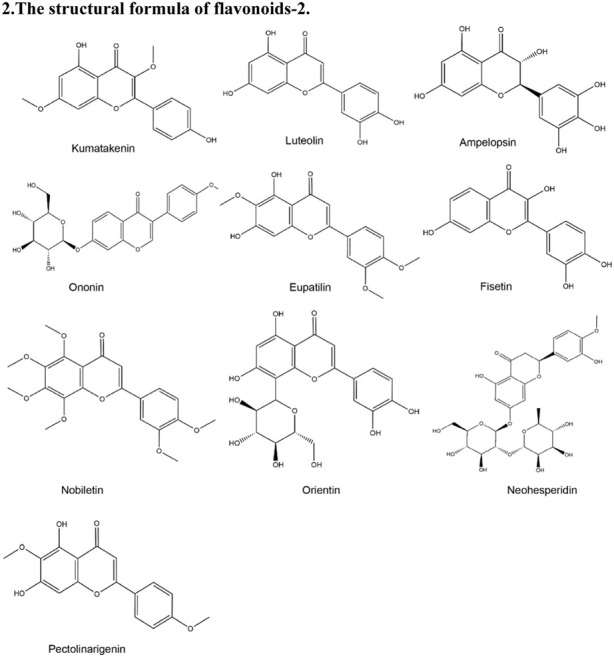
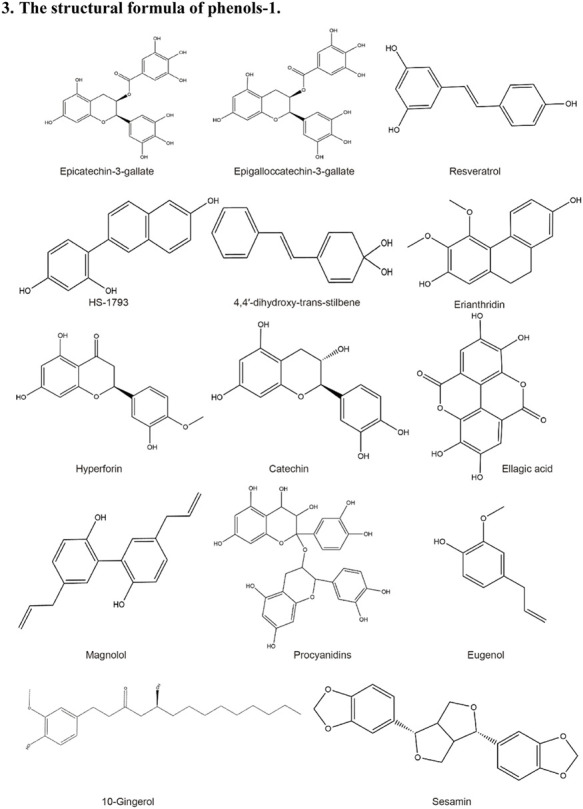
Structural formula of the Natural products.
| 1. The structural formula of flavonoids-1 |
|
| 2. The structural formula of flavonoids-2 |
|
| 3. The structural formula of phenols-1 |
|
| 4. The structural formula of phenols-2 |
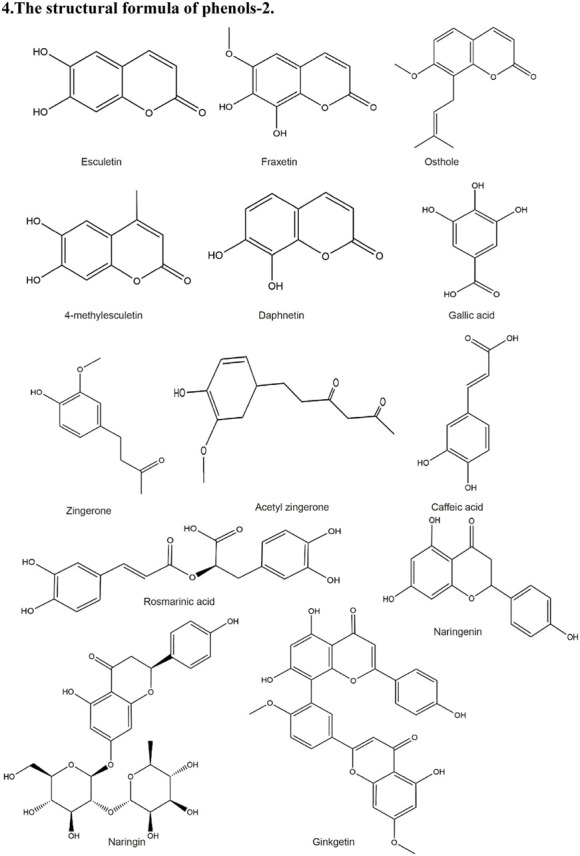
|
| 5. The structural formula of polysaccharides, saponins and terpenoid |
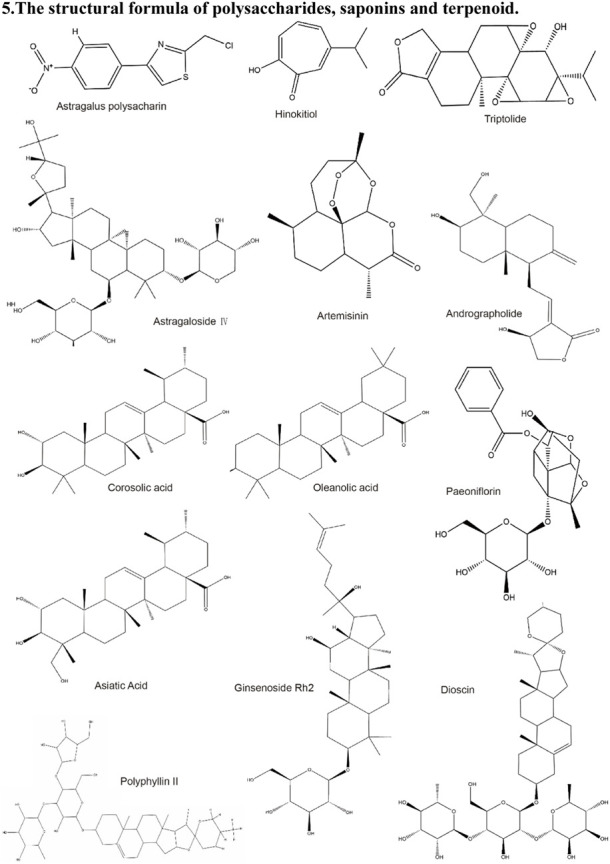
|
| 6. The structural formula of alkaloids and quinones |
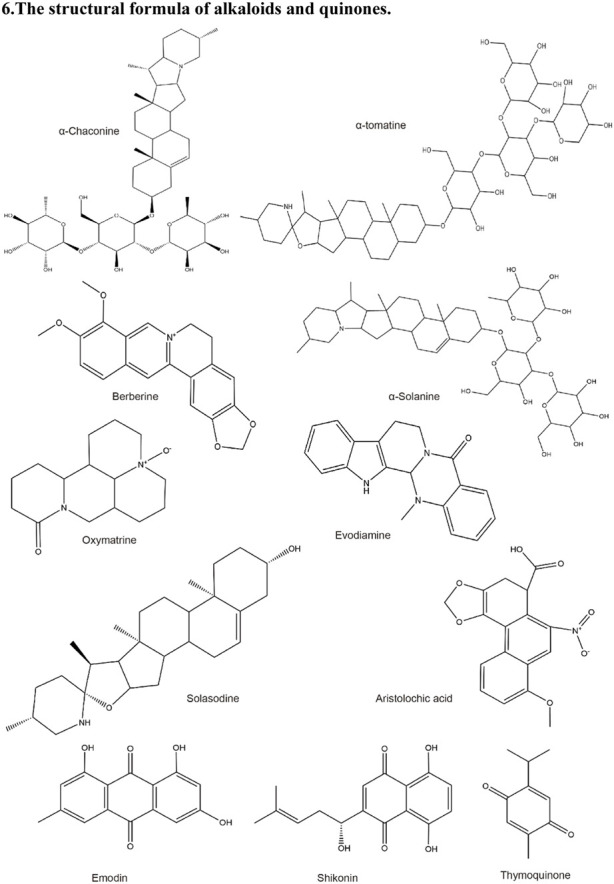
|
5 Discussion and outlook
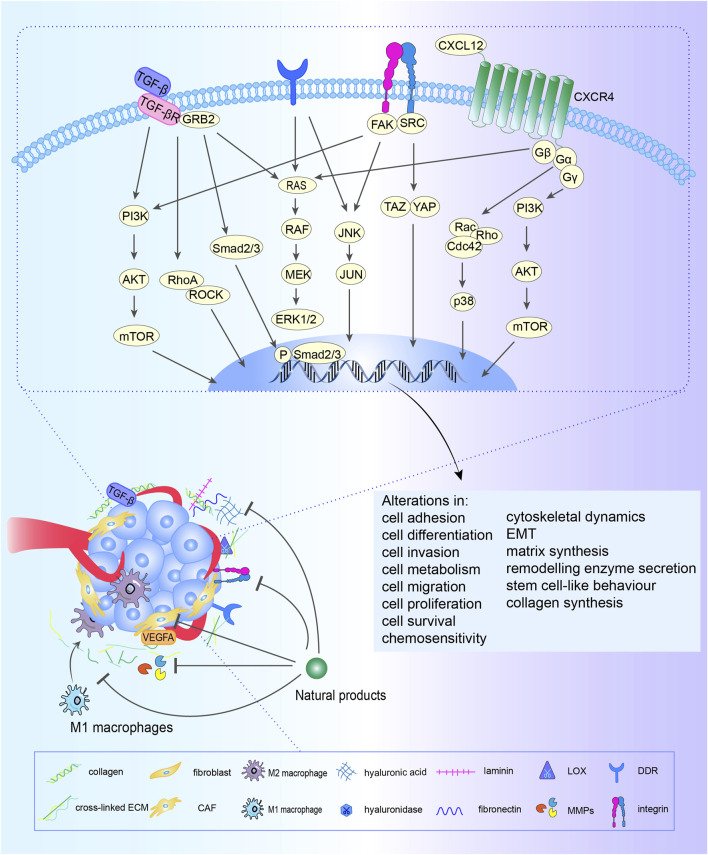
Compared to traditional approaches that target tumor cells, strategies of targeting ECM components and tumor stromal cells may have several potential advantages (Bonnans et al., 2014). First, the components of ECM and stromal cells is more stable in the genetic and therefore it is less likely to acquire resistance to cytotoxic agents. Second, many solid tumor malignancies possess common alterations in TME. Thus, approaches targeting the TME can be broadly applied to different tumor types. With the rapid development of natural product researches, the active ingredients therein have shown therapeutic effects in the clinical with fewer side effects. By means of collating natural products, we found that they showed great potential intervention on the matrix stiffness formed by the change of components and receptors during ECM remodeling, and the crosstalk between CAFs, TAMs and cancer cells. Flavonoids, polyphenols, alkaloids, terpenes and quinones mainly target the deposition and degradation of ECM, with collagen, fibronectin, integrins and MMPs as the main targets, while polysaccharides mainly target TAMs (Figure 3).
FIGURE 3.
Natural products target ECM. Natural products normalize ECM via reducing deposition, altering modification and inhibiting degradation of ECM. Existing research suggests that TGF-β, MMPs, VEGFA, integrins, DDR and so on, would be a drug target. These signaling pathways are intertwined. Simultaneously, flavonoids, phenols, polysaccharides, and saponins, etc. Could regulating ECM by triggering variations about cell biological behavior and phenotype. In addition, natural products also affect the macrophage polarization.
Collagen and HA are the main factors determining matrix stiffness during the ECM deposition mechanism (Huang et al., 2021a). Fibronectin also plays a vital role during malignant transformation. Reducing collagen synthesis can lessen ECM stiffness, and alleviate malignant fibrosis hyperplasia. Prompting collagen rupture can facilitate the penetration of many conventional chemotherapeutic agents and nanoparticles through the barrier of the hardened matrix in the TME. Among the polyphenolic compounds from nature, curcumin, resveratrol, eupatilin, isoliquiritigenin and morin can significantly reduce collagen, laminin and fibronectin deposition in Phase I of the management of cancer. Considering TGF-β′s crucial role during collagen synthesis, TGF-β signaling is the most promising target to inhibit collagen synthesis (Liu et al., 2012; Chauhan et al., 2013). There are multiple drugs targeting TGF-β being evaluated in clinical trials. Flavonoids such as ampelopsin, pectolinarigenin, neohesperidin, apigenin and other polyphenolic compounds such as sesamin, osthole and daphnetin can reduce collagen deposition by inhibiting TGF-β signaling. There are also small molecules targeting the TGF-β receptor (TGF-βR) that are widely used in cancer treatment trials, but they lead to severe systemic toxicity due to instability and low specificity (Tanaka et al., 2010; DaCosta Byfield et al., 2004). Garcimultiflorone K inhibits TGF-β signaling by down-regulating TGF-βR, showing no apparent toxicity. In consideration of the complex roles of TGF-β and TGF-βR in inflammation and tumorigenesis, a comprehensive understanding of tumor traits, stages of disease and TME is necessary for researchers to be cautious with therapeutic targets involving TGF-β and TGF-βR. Two therapeutic strategies for HA are also under investigation, including inhibition of HA synthesis and enhancement of HA degradation (Whatcott et al., 2011). Researches on fibronectin mainly focused on its application as a target for precise drug delivery, several therapies as targeting fibronectin EDA and extra domain B (EDB) have been developed in an attempt to inhibit tumor neovasculature (Castellani et al., 1994). In addition, ECM is the primary target of dicarbonyl (Zhou et al., 2015). For example, methylglyoxal (MG) is a highly reactive dicarbonyl group that interacts with proteins to form advanced glycation end products (AGEs), favoring ECM stiffness (Nowotny et al., 2018). Collagen is the primary target of MG modification in the ECM in vivo. Some natural products such as EGCG, phloridzin, as well as for quercetin, have been reported possess the trapping capacity of dicarbonyls (Li et al., 2014; Hung et al., 2018; Zhao et al., 2022).
During the Phase II, ECM modification stage, drugs can target matrix stiffness sensors such as integrins, DDR1, CD44-HA and RHAMM-HA interactions to reduce the generation of abnormal ECM structures and soften hardened ECM. Integrins can bind to various proteins, such as collagen, fibronectin, and laminin, triggering mechano-transduction and carcinogenesis (Xiong et al., 2021). Integrin inhibitors such as Vitaxi and volociximab can inhibit disease progression based on preclinical studies, showing therapeutic potential in various cancers (Posey et al., 2001; Ricart et al., 2008; Bell-McGuinn et al., 2011). Since many integrins are also expressed in immune cells, the impact of integrin inhibitors on immune surveillance needs to be considered (Su et al., 2016). Among natural products, only a few phenolic compounds can modulate integrins, such as silibinin, EGCE and [10]-gingerol, suggesting that more research should focus on integrins. Emodin can enhance the effect of LOX1, but no evidence has been observed for natural products to reverse abnormal ECM modification, and more researches should be conducted on the effects of natural products on ECM modifying enzymes and substrate stiffness sensors. Combinations of DDR1 inhibitors and classical chemotherapeutics reduce tumor burden in orthotopic xenograft and orthotopic pancreatic cancer models (Aguilera et al., 2017). If natural DDR inhibitors can be found and combined it with chemotherapeutic drugs, the clinical effect can be enhanced while the occurrence of adverse reactions can be reduced. CD44 is mainly functions receptor for HA, collagen, fibronectin, and growth factors. However, due to the lack of a comprehensive understanding of all CD44 isoforms and the consequences of knocking out a mixture of CD44 isoforms, some challenges remain for the clinical application of targeting CD44. Several therapeutic approaches targeting the RHAMM-HA interaction are under evaluation in preclinical studies in various cancers. Targeted collagen cross-linking can significantly reduce ECM stiffness in cancer (Huang et al., 2021a). LOX is a core target that catalyzes the covalent cross-linking and matrix hardening of collagen and elastin. Pan-LOX family inhibitors and specific inhibitors of a LOX family member have been developed. These LOX inhibitors have shown anticancer effects in preclinical studies, but long-term use can cause aortic damage and osteoporosis, limiting their clinical application (Chen et al., 2012). Currently there are not enough studies to find out the effect of natural products on LOX, thus further research can be done in this field.
In terms of regulating ECM degradation, studies on the intervention of MMPs by natural products are the most abundant. Most flavonoids have inhibitory effects on MMPs, especially MMP-2/9, to reduce ECM degradation and anti-angiogenesis in Phase III. For example, silibinin, oroxylin A, kaempferol, wogonin, baicalein, chrysin and daidzein target the ERK pathway and inhibit MMP-2/9-mediated ECM degradation and cell motility. Combining natural products with ERK inhibitors may be a good strategy to reduce cancer cell invasion. Hesperetin and curcumin can target the TGF-β/Smad pathway to reverse the degradation of ECM. Further research can focus on natural products targeting TGF-β or combining with TGF-β inhibitors. Since collagen degradation releases growth factors and cytokines in the ECM, triggering a cascade of inflammatory signals and tumor progression, inhibition of ECM degradation could theoretically reduce cell invasion. However, MMPs degrade collagen and reduce the stiffness of the matrix, and also help to deliver drugs more efficiently into solid tumors, so the optimal timing for the application of such therapy should be carefully considered. Furthermore, MMPs have pleiotropic activity, for example, MMP-8 has both pro-tumor and anti-tumor functions. MMPs may have opposite functions at different stages of the disease (Zeisberg et al., 2006). The poor efficacy or even negative effects of MMPs inhibitors in clinical trials may be due to the inappropriate application of broad-spectrum inhibitors, selection of patients with advanced metastatic disease, inadequate/insufficient evaluation of the therapeutic window, and reliance on monotherapy (Juurikka et al., 2019). Some natural products that simultaneously regulate the levels of MMPs and TIMPs to maintain the balance deserve further study to discover modulators of MMPs adapted to complex TAM. Some compounds showed no cytotoxicity when reducing ECM degradation by targeting the p38 MAPK pathway, providing ideas for the study of new drugs that target the ECM while protecting normal tissues. Regulating multiple targets is an advantage of natural products. For example, some compounds can increase normal collagen production while inhibiting MMPs (such as quercetin, glabridin, resveratrol, acetyl zingerone, ellagic acid, and rosmarinic acid) or decrease angiogenesis (such as EGCG, hyperforin, Esculetin, and fraxetin). The ability of EGCG and luteolin to synergistically target TGF-β and myofibroblasts suggests that the combined use of these compounds may enhance ECM targeting. Making adjustment based on the original structure of natural products can significantly increase the activity and improve the stability of the natural product, such as the synthetic resveratrol analog 4,4′-dihydroxy-trans-stilbene and the designed acetyl zingerone on the zingerone structure.
The combination of three phases sequential cancer therapy and the ECM remodeling trilogy makes it possible to manage cancer throughout the entire process (Figure 4). In the vicious circle of ECM remodeling that drives cancer progression, some natural products with bidirectional regulatory effects may have great therapeutic potential. How to grasp the optimal treatment window during ECM dynamic changes and tumor progression is the focus of further exploration. Overall, ECM is a promising direct therapeutic target in cancer treatment (Jiang et al., 2022), but Gordian Knots still exists in the field of scientific research and clinical practice. There is a lack of material that can accurately mimic ECM in vitro, and the detailed ECM components in various disease contexts remain unclear, as are the specific targets of different proteases. Given its importance in disease, further studies/researches should focus on a detailed biological understanding of ECM morphology and its components such as key enzymes, its structure and signaling molecules, therefore to inspire the identification of next-generation cancer diagnostic and therapeutic targets.
FIGURE 4.
The whole process management of cancer. Efforts to establish a whole-process management of cancer treatment is critical for global cancer control. Thus, we put forward the concept of three phases of the whole-process management of cancer with ECM remodeling. Phase I: Natura products reduce ECM deposition. Phase II: Natura products alter ECM modification. Phase III: Natura products regulate degradation of ECM. Therefore, the sequential treatment of cancer should be constructed to normalize the ECM, protect the normal tissue, reverse immunosuppressive factors and cells.
Funding Statement
This work was supported by National Natural Science Foundation of China (No. 82074397, China). The central government guides local science and technology development projects of Sichuan Provincial Science and Technology Department (No. 2021ZYD0107, China). Xinglin Scholar Research Promotion Project of Chengdu University of TCM (grant no. QJRC2022008).
Author contributions
DL and LL: contributed equally to this work. DL and LL: conceptualization, collecting references and writing; YZ and ST: collecting references and writing; ZL and YL: graph and table; YH: critical evaluation; JT: project administration, funding acquisition; NC: conceptualization, methodology, writing—review and editing, supervision, project administration. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| TME | tumor microenvironment |
| ECM | extracellular matrix |
| CAFs | cancer-associated fibroblasts |
| TAMs | tumor-associated macrophages |
| HA | hyaluronic acid |
| MMPs | matrix Metalloproteinases |
| HSPGs | heparan sulfate proteoglycans |
| TGF-β | transforming growth factor beta |
| CXCL12/10 | C-X-C motif chemokine ligand 12/10 |
| CXCR4/3 | C-X-C motif chemokine receptor 4/3 |
| CCR9 | C-C motif chemokine receptors 9 |
| CCL21/25 | C-C motif chemokine ligand 21/25 |
| MCP-1 | monocyte chemoattractant protein-1 |
| CSF-1 | colony stimulating factor-1 |
| IL-6 | interleukin-6 |
| PGE2 | prostaglandin E2 |
| α-SMA | α-smooth muscle actin |
| FGF-2 | fibroblast growth factor-2 |
| PDGF | platelet-derived growth factor |
| EGF | epidermal growth factor |
| LOX | lysyl oxidase |
| LOXL | LOX-like protein |
| IGF | insulin-like growth factor |
| HAPLN1 | hyaluronan and proteoglycan-linked protein 1; |
| HMM-HA | High molecular weight HA |
| LMM-HA | low-molecular-mass HA |
| PLODs | procollagen-lysine 1, 2-oxoglutarate 5-dioxygenases |
| TG2 | tissue transglutaminase 2 |
| ADAMs | disintegrin and metalloproteinases |
| IGFBP-3 | insulin-like growth factor-binding protein 3 |
| VEGF-A | vascular endothelial growth factor-A |
| TIMP | tissue inhibitor of metalloproteinases |
| GF | growth factors |
| MT1-MMP | membrane type-1 matrix metalloproteinase |
| AP-1 | activator protein-1 |
| JAK2 | Janus kinase 2 |
| PCA | prostate cancer |
| FAK | focal adhesion kinase |
| u-PA | urokinase-type plasminogen activator |
| GBM | glioblastoma multiforme |
| SP-1 | Specificity protein-1 |
| Col X | collagen X |
| Nrf2 | nuclear factor-erythroid 2related factor 2 |
| ECG | Epicatechin-3-gallate |
| EGCG | Epigalloccatechin-3-gallate |
| GCAFs | gastric cancer-associated fibroblasts |
| PAI-1 | plasminogen activator inhibitor-1 |
| EMMPRIN | extracellular matrix metalloproteinase inducer |
| TLR4 | toll-like receptor 4 |
| CDK6 | cyclin-dependent kinase 6 |
| SFDA | State Food and Drug Administration of China |
| PD-L1 | programmed death ligand-1 |
| PD-1 | programmed death-1 |
| TGF-βR | TGF-β receptor |
| EDB | extra domain B |
| DDR1 | discoid domain receptor 1 |
References
- Abu R., Jiang Z., Ueno M., Isaka S., Nakazono S., Okimura T., et al. (2015). Anti-metastatic effects of the sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on B16 melanoma. Biochem. Biophys. Res. Commun. 458, 727–732. 10.1016/j.bbrc.2015.01.061 [DOI] [PubMed] [Google Scholar]
- Addepalli V., Suryavanshi S. V. (2018). Catechin attenuates diabetic autonomic neuropathy in streptozotocin induced diabetic rats. Biomed. Pharmacother. 108, 1517–1523. 10.1016/j.biopha.2018.09.179 [DOI] [PubMed] [Google Scholar]
- Aguilera K. Y., Huang H., Du W., Hagopian M. M., Wang Z., Hinz S., et al. (2017). Inhibition of discoidin domain receptor 1 reduces collagen-mediated tumorigenicity in pancreatic ductal adenocarcinoma. Mol. Cancer Ther. 16, 2473–2485. 10.1158/1535-7163.MCT-16-0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kharashi L. A., Bakheet T., Alharbi W. A., Al-Moghrabi N., Aboussekhra A. (2021). Eugenol modulates genomic methylation and inactivates breast cancer-associated fibroblasts through E2F1-dependent downregulation of DNMT1/DNMT3A. Mol. Carcinog. 60, 784–795. 10.1002/mc.23344 [DOI] [PubMed] [Google Scholar]
- Alipanah H., Farjam M., Zarenezhad E., Roozitalab G., Osanloo M. (2021). Chitosan nanoparticles containing limonene and limonene-rich essential oils: Potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complement. Med. Ther. 21, 186. 10.1186/s12906-021-03362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi V. C., Kupriyanova T. A., Deryugina E. I., Quigley J. P. (2007). Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 104, 20262–20267. 10.1073/pnas.0706438104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroui S., Aouey B., Chtourou Y., Meunier A. C., Fetoui H., Kenani A. (2016). Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MMP-9) via the inhibition of ERK-P38-JNK signaling pathway in human glioblastoma. Chem. Biol. Interact. 244, 195–203. 10.1016/j.cbi.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Atanasov A. G., Zotchev S. B., Dirsch V. M., International Natural Product Sciences T., Supuran C. T. (2021). Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 20, 200–216. 10.1038/s41573-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T., Yang Y., Wu Y. L., Jiang S., Lee J. J., Lian L. H., et al. (2014). Thymoquinone alleviates thioacetamide-induced hepatic fibrosis and inflammation by activating LKB1-AMPK signaling pathway in mice. Int. Immunopharmacol. 19, 351–357. 10.1016/j.intimp.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Barbazan J., Matic Vignjevic D. (2019). Cancer associated fibroblasts: Is the force the path to the dark side? Curr. Opin. Cell. Biol. 56, 71–79. 10.1016/j.ceb.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Barker H. E., Cox T. R., Erler J. T. (2012). The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 12, 540–552. 10.1038/nrc3319 [DOI] [PubMed] [Google Scholar]
- Basu S., Dong Y., Kumar R., Jeter C., Tang D. G. (2022). Slow-cycling (dormant) cancer cells in therapy resistance, cancer relapse and metastasis. Semin. Cancer Biol. 78, 90–103. 10.1016/j.semcancer.2021.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Mcguinn K. M., Matthews C. M., Ho S. N., Barve M., Gilbert L., Penson R. T., et al. (2011). A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol. Oncol. 121, 273–279. 10.1016/j.ygyno.2010.12.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S., Huang W., Chen S., Huang C., Li C., Guo Z., et al. (2020). Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. Int. J. Biol. Macromol. 150, 261–280. 10.1016/j.ijbiomac.2020.02.050 [DOI] [PubMed] [Google Scholar]
- Bian D., Zhang J., Wu X., Dou Y., Yang Y., Tan Q., et al. (2013). Asiatic acid isolated from Centella asiatica inhibits TGF-β1-induced collagen expression in human keloid fibroblasts via PPAR-γ activation. Int. J. Biol. Sci. 9, 1032–1042. 10.7150/ijbs.7273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C., Chou J., Werb Z. (2014). Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell. Biol. 15, 786–801. 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassart B., Randoux A., Hornebeck W., Emonard H. (1998). Regulation of matrix metalloproteinase-2 (gelatinase A, MMP-2), membrane-type matrix metalloproteinase-1 (MT1-MMP) and tissue inhibitor of metalloproteinases-2 (TIMP-2) expression by elastin-derived peptides in human HT-1080 fibrosarcoma cell line. Clin. Exp. Metastasis 16, 489–500. 10.1023/a:1006550503612 [DOI] [PubMed] [Google Scholar]
- Castellani P., Viale G., Dorcaratto A., Nicolo G., Kaczmarek J., Querze G., et al. (1994). The fibronectin isoform containing the ED-B oncofetal domain: A marker of angiogenesis. Int. J. Cancer 59, 612–618. 10.1002/ijc.2910590507 [DOI] [PubMed] [Google Scholar]
- Chai Y., Xu J., Yan B. (2017). The anti-metastatic effect of baicalein on colorectal cancer. Oncol. Rep. 37, 2317–2323. 10.3892/or.2017.5437 [DOI] [PubMed] [Google Scholar]
- Chao R., Chow J. M., Hsieh Y. H., Chen C. K., Lee W. J., Hsieh F. K., et al. (2015). Tricetin suppresses the migration/invasion of human glioblastoma multiforme cells by inhibiting matrix metalloproteinase-2 through modulation of the expression and transcriptional activity of specificity protein 1. Expert Opin. Ther. Targets 19, 1293–1306. 10.1517/14728222.2015.1075509 [DOI] [PubMed] [Google Scholar]
- Chaudhuri O., Cooper-White J., Janmey P. A., Mooney D. J., Shenoy V. B. (2020). Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546. 10.1038/s41586-020-2612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan V. P., Martin J. D., Liu H., Lacorre D. A., Jain S. R., Kozin S. V., et al. (2013). Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4, 2516. 10.1038/ncomms3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Y., Lin P. H., Shih Y. H., Wang K. L., Hong Y. H., Shieh T. M., et al. (2019a). Natural antioxidant resveratrol suppresses uterine fibroid cell growth and extracellular matrix formation in vitro and in vivo . Antioxidants (Basel) 8, 99. 10.3390/antiox8040099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. C., Tu S. H., Huang C. S., Chen C. S., Ho C. T., Lin H. W., et al. (2012). Human breast cancer cell metastasis is attenuated by lysyl oxidase inhibitors through down-regulation of focal adhesion kinase and the paxillin-signaling pathway. Breast Cancer Res. Treat. 134, 989–1004. 10.1007/s10549-012-1986-8 [DOI] [PubMed] [Google Scholar]
- Chen P., Dey P. (2022). Co-dependencies in the tumor immune microenvironment. Oncogene 41, 3821–3829. 10.1038/s41388-022-02406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Lu N., Ling Y., Chen Y., Hui H., Lu Z., et al. (2011). Inhibitory effects of wogonin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Toxicology 282, 122–128. 10.1016/j.tox.2011.01.018 [DOI] [PubMed] [Google Scholar]
- Chen X., Lai Y., Song X., Wu J., Wang L., Zhang H., et al. (2018). Polysaccharides from Citrus grandis associate with luteolin relieves chronic pharyngitis by anti-inflammatory via suppressing NF-κB pathway and the polarization of M1 macrophages. Int. J. Immunopathol. Pharmacol. 32, 2058738418780593. 10.1177/2058738418780593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Song E. (2019). Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 18, 99–115. 10.1038/s41573-018-0004-1 [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen L., Hong D., Chen Z., Zhang J., Fu L., et al. (2019b). Baicalein inhibits fibronectin-induced epithelial-mesenchymal transition by decreasing activation and upregulation of calpain-2. Cell. Death Dis. 10, 341. 10.1038/s41419-019-1572-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin M. W., Eickhoff J. C., Riching K. M., Pehlke C. A., Eliceiri K. W., Provenzano P. P., et al. (2011). Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232. 10.1016/j.ajpath.2010.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'alterio C., Scala S., Sozzi G., Roz L., Bertolini G. (2020). Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin. Cancer Biol. 60, 351–361. 10.1016/j.semcancer.2019.08.019 [DOI] [PubMed] [Google Scholar]
- Dacosta Byfield S., Major C., Laping N. J., Roberts A. B. (2004). SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 65, 744–752. 10.1124/mol.65.3.744 [DOI] [PubMed] [Google Scholar]
- Dai J., Zhang Y., Chen D., Chen D., Li X., Wang J., et al. (2021). Glabridin inhibits osteoarthritis development by protecting chondrocytes against oxidative stress, apoptosis and promoting mTOR mediated autophagy. Life Sci. 268, 118992. 10.1016/j.lfs.2020.118992 [DOI] [PubMed] [Google Scholar]
- Deep G., Kumar R., Jain A. K., Agarwal C., Agarwal R. (2014). Silibinin inhibits fibronectin induced motility, invasiveness and survival in human prostate carcinoma PC3 cells via targeting integrin signaling. Mutat. Res. 768, 35–46. 10.1016/j.mrfmmm.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L. J., Qi M., Li N., Lei Y. H., Zhang D. M., Chen J. X. (2020). Natural products and their derivatives: Promising modulators of tumor immunotherapy. J. Leukoc. Biol. 108, 493–508. 10.1002/JLB.3MR0320-444R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doersch K. M., Newell-Rogers M. K. (2017). The impact of quercetin on wound healing relates to changes in αV and β1 integrin expression. Exp. Biol. Med. (Maywood) 242, 1424–1431. 10.1177/1535370217712961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Guo X., Han F., He Z., Wang Y. (2022). Emerging role of natural products in cancer immunotherapy. Acta Pharm. Sin. B 12, 1163–1185. 10.1016/j.apsb.2021.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Wang X. F., Zhou Q. M., Zhang T. L., Lu Y. Y., Zhang H., et al. (2013). Evodiamine induces apoptosis and inhibits metastasis in MDAMB-231 human breast cancer cells in vitro and in vivo . Oncol. Rep. 30, 685–694. 10.3892/or.2013.2498 [DOI] [PubMed] [Google Scholar]
- Du Y., Long Q., Zhang L., Shi Y., Liu X., Li X., et al. (2015). Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling. Int. J. Oncol. 47, 2064–2072. 10.3892/ijo.2015.3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzobo K., Senthebane D. A., Dandara C. (2023). The tumor microenvironment in tumorigenesis and therapy resistance revisited. Cancers (Basel) 15, 376. 10.3390/cancers15020376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyada E., Bolisetty M., Laise P., Flynn W. F., Courtois E. T., Burkhart R. A., et al. (2019). Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123. 10.1158/2159-8290.CD-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eo S. H., Kim S. J. (2017). Rosmarinic acid induces rabbit articular chondrocyte differentiation by decreases matrix metalloproteinase-13 and inflammation by upregulating cyclooxygenase-2 expression. J. Biomed. Sci. 24, 75. 10.1186/s12929-017-0381-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Wei J., Guo L., Zhao S., Xu C., Sun H., et al. (2020). Osthole reduces mouse IOP associated with ameliorating extracellular matrix expression of trabecular meshwork cell. Invest. Ophthalmol. Vis. Sci. 61, 38. 10.1167/iovs.61.10.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Yuan J., Peng C., Li Y. (2014). Collagen as a double-edged sword in tumor progression. Tumour Biol. 35, 2871–2882. 10.1007/s13277-013-1511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischholz S., Berberich O., Bock T., Meffert R. H., Blunk T. (2020). Resveratrol counteracts IL-1β-mediated impairment of extracellular matrix deposition in 3D articular chondrocyte constructs. J. Tissue Eng. Regen. Med. 14, 897–908. 10.1002/term.3031 [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Komohara Y., Kudo R., Tsurushima K., Ohnishi K., Ikeda T., et al. (2011). Oleanolic acid inhibits macrophage differentiation into the M2 phenotype and glioblastoma cell proliferation by suppressing the activation of STAT3. Oncol. Rep. 26, 1533–1537. 10.3892/or.2011.1454 [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Takeya M., Komohara Y. (2014). A novel strategy for inducing the antitumor effects of triterpenoid compounds: Blocking the protumoral functions of tumor-associated macrophages via STAT3 inhibition. Biomed. Res. Int. 2014, 348539. 10.1155/2014/348539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzer A. M., Lee S. Y., Mott J. D., Cominetti M. R. (2017). [10]-Gingerol reverts malignant phenotype of breast cancer cells in 3D culture. J. Cell. Biochem. 118, 2693–2699. 10.1002/jcb.25906 [DOI] [PubMed] [Google Scholar]
- Go J. H., Wei J. D., Park J. I., Ahn K. S., Kim J. H. (2018). Wogonin suppresses the LPSenhanced invasiveness of MDAMB231 breast cancer cells by inhibiting the 5LO/BLT2 cascade. Int. J. Mol. Med. 42, 1899–1908. 10.3892/ijmm.2018.3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A. L., Stephens C. A., Bigelow R. L., Coleman D. T., Cardelli J. A. (2014). The polyphenols (-)-epigallocatechin-3-gallate and luteolin synergistically inhibit TGF-β-induced myofibroblast phenotypes through RhoA and ERK inhibition. PLoS One 9, e109208. 10.1371/journal.pone.0109208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Yin W., Zou Z., Zhang C., Sun M., Min L., et al. (2021). Quercitrin alleviates cartilage extracellular matrix degradation and delays ACLT rat osteoarthritis development: An in vivo and in vitro study. J. Adv. Res. 28, 255–267. 10.1016/j.jare.2020.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Fang Y., Jiang F., Li L., Zhou H., Xu X., et al. (2019). Neohesperidin inhibits TGF-β1/Smad3 signaling and alleviates bleomycin-induced pulmonary fibrosis in mice. Eur. J. Pharmacol. 864, 172712. 10.1016/j.ejphar.2019.172712 [DOI] [PubMed] [Google Scholar]
- Gweon E. J., Kim S. J. (2014). Resveratrol attenuates matrix metalloproteinase-9 and -2-regulated differentiation of HTB94 chondrosarcoma cells through the p38 kinase and JNK pathways. Oncol. Rep. 32, 71–78. 10.3892/or.2014.3192 [DOI] [PubMed] [Google Scholar]
- Hao J., Ding X. L., Yang X., Wu X. Z. (2020). Prunella vulgaris polysaccharide inhibits growth and migration of breast carcinoma-associated fibroblasts by suppressing expression of basic fibroblast growth factor. Chin. J. Integr. Med. 26, 270–276. 10.1007/s11655-016-2587-x [DOI] [PubMed] [Google Scholar]
- Hastings J. F., Skhinas J. N., Fey D., Croucher D. R., Cox T. R. (2019). The extracellular matrix as a key regulator of intracellular signalling networks. Br. J. Pharmacol. 176, 82–92. 10.1111/bph.14195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemshekhar M., Sunitha K., Thushara R. M., Sebastin Santhosh M., Shanmuga Sundaram M., Kemparaju K., et al. (2013). Antiarthritic and antiinflammatory propensity of 4-methylesculetin, a coumarin derivative. Biochimie 95, 1326–1335. 10.1016/j.biochi.2013.02.014 [DOI] [PubMed] [Google Scholar]
- Heneberg P. (2016). Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Crit. Rev. Oncol. Hematol. 97, 303–311. 10.1016/j.critrevonc.2015.09.008 [DOI] [PubMed] [Google Scholar]
- Hernandez-Aquino E., Muriel P. (2018). Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 24, 1679–1707. 10.3748/wjg.v24.i16.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ortega L. D., Alcantar-Diaz B. E., Ruiz-Corro L. A., Sandoval-Rodriguez A., Bueno-Topete M., Armendariz-Borunda J., et al. (2012). Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J. Gastroenterol. Hepatol. 27, 1865–1872. 10.1111/j.1440-1746.2012.07262.x [DOI] [PubMed] [Google Scholar]
- Hsieh Y. S., Chu S. C., Yang S. F., Chen P. N., Liu Y. C., Lu K. H. (2007). Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis 28, 977–987. 10.1093/carcin/bgl221 [DOI] [PubMed] [Google Scholar]
- Hua H., Kong Q., Yin J., Zhang J., Jiang Y. (2020). Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: A challenge for cancer therapy. J. Hematol. Oncol. 13, 64. 10.1186/s13045-020-00904-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua H., Zhang H., Chen J., Wang J., Liu J., Jiang Y. (2021). Targeting Akt in cancer for precision therapy. J. Hematol. Oncol. 14, 128. 10.1186/s13045-021-01137-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A., Garraway L. A., Ashworth A., Weber B. (2020). Synthetic lethality as an engine for cancer drug target discovery. Nat. Rev. Drug Discov. 19, 23–38. 10.1038/s41573-019-0046-z [DOI] [PubMed] [Google Scholar]
- Huang C. H., Jayakumar T., Chang C. C., Fong T. H., Lu S. H., Thomas P. A., et al. (2015). Hinokitiol exerts anticancer activity through downregulation of MMPs 9/2 and enhancement of catalase and SOD enzymes: In vivo augmentation of lung histoarchitecture. Molecules 20, 17720–17734. 10.3390/molecules201017720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhang L., Wan D., Zhou L., Zheng S., Lin S., et al. (2021a). Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target Ther. 6, 153. 10.1038/s41392-021-00544-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. F., Horng C. T., Hsieh Y. S., Hsieh Y. H., Chu S. C., Chen P. N. (2016). Epicatechin-3-gallate reverses TGF-β1-induced epithelial-to-mesenchymal transition and inhibits cell invasion and protease activities in human lung cancer cells. Food Chem. Toxicol. 94, 1–10. 10.1016/j.fct.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Huang S. F., Wang Y. L., Chen J. J., Huang Y. B., Tai S. B., Chung C. L., et al. (2021b). Garcimultiflorone K from Garcinia multiflora attenuates hepatocellular carcinoma metastasis by suppressing transforming growth factor-beta signaling. Phytomedicine 84, 153502. 10.1016/j.phymed.2021.153502 [DOI] [PubMed] [Google Scholar]
- Huang X., Pan Q., Mao Z., Wang P., Zhang R., Ma X., et al. (2018). Kaempferol inhibits interleukin‑1β stimulated matrix metalloproteinases by suppressing the MAPK‑associated ERK and P38 signaling pathways. Mol. Med. Rep. 18, 2697–2704. 10.3892/mmr.2018.9280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Wang L., Meng S., Wang Y., Chen T., Wang C. (2011). Berberine reduces both MMP-9 and EMMPRIN expression through prevention of p38 pathway activation in PMA-induced macrophages. Int. J. Cardiol. 146, 153–158. 10.1016/j.ijcard.2009.06.023 [DOI] [PubMed] [Google Scholar]
- Hung W. L., Wang S., Sang S., Wan X., Wang Y., Ho C. T. (2018). Quantification of ascorbyl adducts of epigallocatechin gallate and gallocatechin gallate in bottled tea beverages. Food Chem. 261, 246–252. 10.1016/j.foodchem.2018.04.050 [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Naba A. (2012). Overview of the matrisome-an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 4, a004903. 10.1101/cshperspect.a004903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobuzio-Donahue C. A., Argani P., Hempen P. M., Jones J., Kern S. E. (2002). The desmoplastic response to infiltrating breast carcinoma: Gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res. 62, 5351–5357. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. (1986). Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 261, 4337–4345. 10.1016/s0021-9258(17)35666-1 [DOI] [PubMed] [Google Scholar]
- Iwanowycz S., Wang J., Hodge J., Wang Y., Yu F., Fan D. (2016). Emodin inhibits breast cancer growth by blocking the tumor-promoting feedforward loop between cancer cells and macrophages. Mol. Cancer Ther. 15, 1931–1942. 10.1158/1535-7163.MCT-15-0987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J.-Y., Lee J.-K., Jeon Y.-K., Kim C.-W. (2013). Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer 13, 421. 10.1186/1471-2407-13-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S. K., Yang K., Park Y. S., Choi Y. J., Oh S. J., Lee C. W., et al. (2014). Interferon gamma induced by resveratrol analog, HS-1793, reverses the properties of tumor associated macrophages. Int. Immunopharmacol. 22, 303–310. 10.1016/j.intimp.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Ji B., Zhang Z., Guo W., Ma H., Xu B., Mu W., et al. (2018). Isoliquiritigenin blunts osteoarthritis by inhibition of bone resorption and angiogenesis in subchondral bone. Sci. Rep. 8, 1721. 10.1038/s41598-018-19162-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian L., Zhang C., Chen G., Shi X., Qiu Y., Xue Y., et al. (2014). Emodin-mediated cross-linking enhancement for extracellular matrix homeostasis. Biochem. Biophys. Res. Commun. 446, 1022–1028. 10.1016/j.bbrc.2014.03.052 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Zhang H., Wang J., Liu Y., Luo T., Hua H. (2022). Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 15, 34. 10.1186/s13045-022-01252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jnawali H. N., Jeon D., Jeong M. C., Lee E., Jin B., Ryoo S., et al. (2016). Antituberculosis activity of a naturally occurring flavonoid, Isorhamnetin. J. Nat. Prod. 79, 961–969. 10.1021/acs.jnatprod.5b01033 [DOI] [PubMed] [Google Scholar]
- Jung J. W., Park I. H., Cho J. S., Lee H. M. (2013). Naringenin inhibits extracellular matrix production via extracellular signal-regulated kinase pathways in nasal polyp-derived fibroblasts. Phytother. Res. 27, 463–467. 10.1002/ptr.4735 [DOI] [PubMed] [Google Scholar]
- Juurikka K., Butler G. S., Salo T., Nyberg P., Åström P. (2019). The role of MMP8 in cancer: A systematic review. Int. J. Mol. Sci. 20, 4506. 10.3390/ijms20184506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai F., Drain A. P., Weaver V. M. (2019). The extracellular matrix modulates the metastatic journey. Dev. Cell. 49, 332–346. 10.1016/j.devcel.2019.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., Zeisberg M. (2006). Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401. 10.1038/nrc1877 [DOI] [PubMed] [Google Scholar]
- Karamanos N. K., Piperigkou Z., Passi A., Götte M., Rousselle P., Vlodavsky I. (2021). Extracellular matrix-based cancer targeting. Trends Mol. Med. 27, 1000–1013. 10.1016/j.molmed.2021.07.009 [DOI] [PubMed] [Google Scholar]
- Karsdal M. A., Nielsen M. J., Sand J. M., Henriksen K., Genovese F., Bay-Jensen A. C., et al. (2013). Extracellular matrix remodeling: The common denominator in connective tissue diseases. Possibilities for evaluation and current understanding of the matrix as more than a passive architecture, but a key player in tissue failure. Assay. Drug Dev. Technol. 11, 70–92. 10.1089/adt.2012.474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y. Q., Liu C., Hao J. B., Lu L., Lu N. N., Wu Z. K., et al. (2016). Morin inhibits cell proliferation and fibronectin accumulation in rat glomerular mesangial cells cultured under high glucose condition. Biomed. Pharmacother. 84, 622–627. 10.1016/j.biopha.2016.09.088 [DOI] [PubMed] [Google Scholar]
- Kechagia J. Z., Ivaska J., Roca-Cusachs P. (2019). Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell. Biol. 20, 457–473. 10.1038/s41580-019-0134-2 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Plaks V., Werb Z. (2010). Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 141, 52–67. 10.1016/j.cell.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-J., Korm S., Kim W.-S., Kim O.-S., Lee J.-S., Min H.-G., et al. (2014). Nobiletin suppresses MMP-9 expression through modulation of p38 MAPK activity in human dermal fibrobalsts. Biol. Pharm. Bull. 37 (1), 158–163. 10.1248/bpb.b13-00534 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Pham T. H., Bak Y., Ryu H. W., Oh S. R., Yoon D. Y. (2018). Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine 50, 35–42. 10.1016/j.phymed.2018.09.172 [DOI] [PubMed] [Google Scholar]
- Kimura Y., Sumiyoshi M. (2013). Anti-tumor and anti-metastatic actions of wogonin isolated from Scutellaria baicalensis roots through anti-lymphangiogenesis. Phytomedicine 20, 328–336. 10.1016/j.phymed.2012.10.016 [DOI] [PubMed] [Google Scholar]
- Kimura Y., Sumiyoshi M. (2015). Antitumor and antimetastatic actions of dihydroxycoumarins (esculetin or fraxetin) through the inhibition of M2 macrophage differentiation in tumor-associated macrophages and/or G1 arrest in tumor cells. Eur. J. Pharmacol. 746, 115–125. 10.1016/j.ejphar.2014.10.048 [DOI] [PubMed] [Google Scholar]
- Kimura Y., Sumiyoshi M. (2016). Resveratrol prevents tumor growth and metastasis by inhibiting lymphangiogenesis and M2 macrophage activation and differentiation in tumor-associated macrophages. Nutr. Cancer 68, 667–678. 10.1080/01635581.2016.1158295 [DOI] [PubMed] [Google Scholar]
- Kong R., Wang N., Luo H., Lu J. (2018). Hesperetin mitigates bile duct ligation-induced liver fibrosis by inhibiting extracellular matrix and cell apoptosis via the TGF-β1/smad pathway. Curr. Mol. Med. 18, 15–24. 10.2174/1566524018666180608084947 [DOI] [PubMed] [Google Scholar]
- Kou Y., Ji L., Wang H., Wang W., Zheng H., Zou J., et al. (2017). Connexin 43 upregulation by dioscin inhibits melanoma progression via suppressing malignancy and inducing M1 polarization. Int. J. Cancer 141, 1690–1703. 10.1002/ijc.30872 [DOI] [PubMed] [Google Scholar]
- Kousidou O. C., Mitropoulou T. N., Roussidis A. E., Kletsas D., Theocharis A. D., Karamanos N. K. (2005). Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int. J. Oncol. 26, 1101–1109. 10.3892/ijo.26.4.1101 [DOI] [PubMed] [Google Scholar]
- Kuzet S. E., Gaggioli C. (2016). Fibroblast activation in cancer: When seed fertilizes soil. Cell. Tissue Res. 365, 607–619. 10.1007/s00441-016-2467-x [DOI] [PubMed] [Google Scholar]
- Lee H. S., Jun J. H., Jung E. H., Koo B. A., Kim Y. S. (2014). Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules 19, 12150–12172. 10.3390/molecules190812150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 139, 891–906. 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Pan X. Y., Ma M., Zhao J., Zhao F., Lv Y. P. (2021a). Astragalus polysacharin inhibits hepatocellular carcinoma-like phenotypes in a murine HCC model through repression of M2 polarization of tumour-associated macrophages. Pharm. Biol. 59, 1533–1539. 10.1080/13880209.2021.1991384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Yang D., Zhao Y., Qiu Y., Cao X., Yu Y., et al. (2015a). Inhibitory effects of Isorhamnetin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-2/9. Nutr. Cancer 67, 1191–1200. 10.1080/01635581.2015.1073763 [DOI] [PubMed] [Google Scholar]
- Li C., Zhao Y., Yang D., Yu Y., Guo H., Zhao Z., et al. (2015b). Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell. Biol. 93, 16–27. 10.1139/bcb-2014-0067 [DOI] [PubMed] [Google Scholar]
- Li G., Xing W., Zhang M., Geng F., Yang H., Zhang H., et al. (2018a). Antifibrotic cardioprotection of berberine via downregulating myocardial IGF-1 receptor-regulated MMP-2/MMP-9 expression in diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 315, H802–h813. 10.1152/ajpheart.00093.2018 [DOI] [PubMed] [Google Scholar]
- Li H., Huang N., Zhu W., Wu J., Yang X., Teng W., et al. (2018b). Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 18, 579. 10.1186/s12885-018-4299-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li L., Mei H., Pan G., Wang X., Huang X., et al. (2020a). Antitumor properties of triptolide: Phenotype regulation of macrophage differentiation. Cancer Biol. Ther. 21, 178–188. 10.1080/15384047.2019.1679555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zheng T., Sang S., Lv L. (2014). Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 62, 12152–12158. 10.1021/jf504132x [DOI] [PubMed] [Google Scholar]
- Li Y., Guo F., Huang R., Ma L., Fu P. (2021b). Natural flavonoid pectolinarigenin alleviated kidney fibrosis via inhibiting the activation of TGFβ/SMAD3 and JAK2/STAT3 signaling. Int. Immunopharmacol. 91, 107279. 10.1016/j.intimp.2020.107279 [DOI] [PubMed] [Google Scholar]
- Li Y., Ren R., Wang L., Peng K. (2020b). Eupatilin alleviates airway remodeling via regulating phenotype plasticity of airway smooth muscle cells. Biosci. Rep. 40. 10.1042/BSR20191445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Li L., Zeng J., Gao Y., Chen Y. L., Wang Z. Q., et al. (2012). Inhibitory effect of silibinin on EGFR signal-induced renal cell carcinoma progression via suppression of the EGFR/MMP-9 signaling pathway. Oncol. Rep. 28, 999–1005. 10.3892/or.2012.1874 [DOI] [PubMed] [Google Scholar]
- Lim H., Park H., Kim H. P. (2011). Effects of flavonoids on matrix metalloproteinase-13 expression of interleukin-1β-treated articular chondrocytes and their cellular mechanisms: Inhibition of c-fos/AP-1 and JAK/STAT signaling pathways. J. Pharmacol. Sci. 116, 221–231. 10.1254/jphs.11014fp [DOI] [PubMed] [Google Scholar]
- Lin C. H., Shen M. L., Kao S. T., Wu D. C. (2014). The effect of sesamin on airway fibrosis in vitro and in vivo . Int. Immunopharmacol. 22, 141–150. 10.1016/j.intimp.2014.06.031 [DOI] [PubMed] [Google Scholar]
- Lin C. W., Chen P. N., Chen M. K., Yang W. E., Tang C. H., Yang S. F., et al. (2013). Kaempferol reduces matrix metalloproteinase-2 expression by down-regulating ERK1/2 and the activator protein-1 signaling pathways in oral cancer cells. PLoS One 8, e80883. 10.1371/journal.pone.0080883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Lin C., Fu C., Lu H., Jin H., Chen Q., et al. (2020). The protective effect of Ellagic acid (EA) in osteoarthritis: An in vitro and in vivo study. Biomed. Pharmacother. 125, 109845. 10.1016/j.biopha.2020.109845 [DOI] [PubMed] [Google Scholar]
- Lipponen P., Aaltomaa S., Tammi R., Tammi M., Agren U., Kosma V. M. (2001). High stromal hyaluronan level is associated with poor differentiation and metastasis in prostate cancer. Eur. J. Cancer 37, 849–856. 10.1016/s0959-8049(00)00448-2 [DOI] [PubMed] [Google Scholar]
- Liu H., Liu A., Shi C., Li B. (2016). Curcumin suppresses transforming growth factor-β1-induced cardiac fibroblast differentiation via inhibition of Smad-2 and p38 MAPK signaling pathways. Exp. Ther. Med. 11, 998–1004. 10.3892/etm.2016.2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liao S., Diop-Frimpong B., Chen W., Goel S., Naxerova K., et al. (2012). TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl. Acad. Sci. U. S. A. 109, 16618–16623. 10.1073/pnas.1117610109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Luo F., Ding C., Albeituni S., Hu X., Ma Y., et al. (2015). Dectin-1 activation by a natural product beta-glucan converts immunosuppressive macrophages into an M1-like phenotype. J. Immunol. 195, 5055–5065. 10.4049/jimmunol.1501158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Hodge J., Wang J., Wang Y., Wang L., Singh U., et al. (2020). Emodin reduces breast cancer lung metastasis by suppressing macrophage-induced breast cancer cell epithelial-mesenchymal transition and cancer stem cell formation. Theranostics 10, 8365–8381. 10.7150/thno.45395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Xu L., Wang C., Chen K., Xia Y., Li J., et al. (2019). Alleviation of hepatic fibrosis and autophagy via inhibition of transforming growth factor-β1/Smads pathway through shikonin. J. Gastroenterol. Hepatol. 34, 263–276. 10.1111/jgh.14299 [DOI] [PubMed] [Google Scholar]
- Lu M. K., Chen P. H., Shih Y. W., Chang Y. T., Huang E. T., Liu C. R., et al. (2010a). alpha-Chaconine inhibits angiogenesis in vitro by reducing matrix metalloproteinase-2. Biol. Pharm. Bull. 33, 622–630. 10.1248/bpb.33.622 [DOI] [PubMed] [Google Scholar]
- Lu M. K., Shih Y. W., Chang Chien T. T., Fang L. H., Huang H. C., Chen P. S. (2010b). α-Solanine inhibits human melanoma cell migration and invasion by reducing matrix metalloproteinase-2/9 activities. Biol. Pharm. Bull. 33, 1685–1691. 10.1248/bpb.33.1685 [DOI] [PubMed] [Google Scholar]
- Lu P., Takai K., Weaver V. M., Werb Z. (2011). Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 3, a005058. 10.1101/cshperspect.a005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Lu N., Li C., Li F., Zhao K., Lin B., et al. (2012). Oroxylin A inhibits matrix metalloproteinase-2/9 expression and activation by up-regulating tissue inhibitor of metalloproteinase-2 and suppressing the ERK1/2 signaling pathway. Toxicol. Lett. 209, 211–220. 10.1016/j.toxlet.2011.12.022 [DOI] [PubMed] [Google Scholar]
- Ma J. Q., Sun Y. Z., Ming Q. L., Tian Z. K., Yang H. X., Liu C. M. (2019). Ampelopsin attenuates carbon tetrachloride-induced mouse liver fibrosis and hepatic stellate cell activation associated with the SIRT1/TGF-β1/Smad3 and autophagy pathway. Int. Immunopharmacol. 77, 105984. 10.1016/j.intimp.2019.105984 [DOI] [PubMed] [Google Scholar]
- Maccario C., Savio M., Ferraro D., Bianchi L., Pizzala R., Pretali L., et al. (2012). The resveratrol analog 4,4'-dihydroxy-trans-stilbene suppresses transformation in normal mouse fibroblasts and inhibits proliferation and invasion of human breast cancer cells. Carcinogenesis 33, 2172–2180. 10.1093/carcin/bgs244 [DOI] [PubMed] [Google Scholar]
- Mahalanobish S., Saha S., Dutta S., Sil P. C. (2020). Matrix metalloproteinase: An upcoming therapeutic approach for idiopathic pulmonary fibrosis. Pharmacol. Res. 152, 104591. 10.1016/j.phrs.2019.104591 [DOI] [PubMed] [Google Scholar]
- Maller O., Hansen K. C., Lyons T. R., Acerbi I., Weaver V. M., Prekeris R., et al. (2013). Collagen architecture in pregnancy-induced protection from breast cancer. J. Cell. Sci. 126, 4108–4110. 10.1242/jcs.121590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S., Li J., Fan W., Chai H., Liu Z., Gao W. (2015). Inhibition of pulmonary adenoma in diethylnitrosamine-induced rats by Rhizoma paridis saponins. J. Steroid Biochem. Mol. Biol. 154, 62–67. 10.1016/j.jsbmb.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Mao X., Zhang X., Zheng X., Chen Y., Xuan Z., Huang P. (2021). Curcumin suppresses LGR5(+) colorectal cancer stem cells by inducing autophagy and via repressing TFAP2A-mediated ECM pathway. J. Nat. Med. 75, 590–601. 10.1007/s11418-021-01505-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M. M., Hubbell J. A. (2010). The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. Faseb J. 24, 4711–4721. 10.1096/fj.09-151282 [DOI] [PubMed] [Google Scholar]
- Mathy-Hartert M., Jacquemond-Collet I., Priem F., Sanchez C., Lambert C., Henrotin Y. (2009). Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm. Res. 58, 899–908. 10.1007/s00011-009-0063-1 [DOI] [PubMed] [Google Scholar]
- Meng X. M., Nikolic-Paterson D. J., Lan H. Y. (2016). TGF-Β: The master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338. 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- Mokbel K., Wazir U., Mokbel K. (2019). Chemoprevention of prostate cancer by natural agents: Evidence from molecular and epidemiological studies. Anticancer Res. 39, 5231–5259. 10.21873/anticanres.13720 [DOI] [PubMed] [Google Scholar]
- Mott J. D., Werb Z. (2004). Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell. Biol. 16, 558–564. 10.1016/j.ceb.2004.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi M., Farhood B., Mortezaee K. (2019). Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 120, 2782–2790. 10.1002/jcb.27681 [DOI] [PubMed] [Google Scholar]
- Nguyen-Ngoc K. V., Cheung K. J., Brenot A., Shamir E. R., Gray R. S., Hines W. C., et al. (2012). ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc. Natl. Acad. Sci. U. S. A. 109, E2595–E2604. 10.1073/pnas.1212834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny K., Castro J. P., Hugo M., Braune S., Weber D., Pignitter M., et al. (2018). Oxidants produced by methylglyoxal-modified collagen trigger ER stress and apoptosis in skin fibroblasts. Free Radic. Biol. Med. 120, 102–113. 10.1016/j.freeradbiomed.2018.03.022 [DOI] [PubMed] [Google Scholar]
- Orsolic N., Kunstic M., Kukolj M., Odeh D., Ancic D. (2020). Natural phenolic acid, product of the honey bee, for the control of oxidative stress, peritoneal angiogenesis, and tumor growth in mice. Molecules 25, 5583. 10.3390/molecules25235583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadas A., Arauz G., Cicala A., Wiesner J., Asimakopoulos F. (2020). Versican and versican-matrikines in cancer progression, inflammation, and immunity. J. Histochem Cytochem 68, 871–885. 10.1369/0022155420937098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek M. J., Dufort C. C., Rossier O., Bainer R., Mouw J. K., Godula K., et al. (2014). The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325. 10.1038/nature13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup M. W., Mouw J. K., Weaver V. M. (2014). The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243–1253. 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorget A., Berthet V., Luis J., Moghrabi A., Annabi B., Beliveau R. (2003). Medulloblastoma cell invasion is inhibited by green tea (-)epigallocatechin-3-gallate. J. Cell. Biochem. 90, 745–755. 10.1002/jcb.10667 [DOI] [PubMed] [Google Scholar]
- Posey J. A., Khazaeli M. B., Delgrosso A., Saleh M. N., Lin C. Y., Huse W., et al. (2001). A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti alpha v beta 3) antibody in patients with metastatic cancer. Cancer Biother. Radiopharm. 16, 125–132. 10.1089/108497801300189218 [DOI] [PubMed] [Google Scholar]
- Pothongsrisit S., Arunrungvichian K., Hayakawa Y., Sritularak B., Mangmool S., Pongrakhananon V. (2021). Erianthridin suppresses non-small-cell lung cancer cell metastasis through inhibition of Akt/mTOR/p70(S6K) signaling pathway. Sci. Rep. 11, 6618. 10.1038/s41598-021-85675-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiney C., Billard C., Mirshahi P., Fourneron J. D., Kolb J. P. (2006). Hyperforin inhibits MMP-9 secretion by B-CLL cells and microtubule formation by endothelial cells. Leukemia 20, 583–589. 10.1038/sj.leu.2404134 [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S., Salza R. (2014). Matricryptins and matrikines: Biologically active fragments of the extracellular matrix. Exp. Dermatol 23, 457–463. 10.1111/exd.12435 [DOI] [PubMed] [Google Scholar]
- Ricart A. D., Tolcher A. W., Liu G., Holen K., Schwartz G., Albertini M., et al. (2008). Volociximab, a chimeric monoclonal antibody that specifically binds alpha5beta1 integrin: A phase I, pharmacokinetic, and biological correlative study. Clin. Cancer Res. 14, 7924–7929. 10.1158/1078-0432.CCR-08-0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P., Junker K., Franz M., Berndt A., Geyer C., Gajda M., et al. (2008). IIICS de novo glycosylated fibronectin as a marker for invasiveness in urothelial carcinoma of the urinary bladder (UBC). J. Cancer Res. Clin. Oncol. 134, 1059–1065. 10.1007/s00432-008-0390-6 [DOI] [PubMed] [Google Scholar]
- Salama A. a. A., Allam R. M. (2021). Promising targets of chrysin and daidzein in colorectal cancer: Amphiregulin, CXCL1, and MMP-9. Eur. J. Pharmacol. 892, 173763. 10.1016/j.ejphar.2020.173763 [DOI] [PubMed] [Google Scholar]
- Saw S., Aiken A., Fang H., Mckee T. D., Bregant S., Sanchez O., et al. (2019). Metalloprotease inhibitor TIMP proteins control FGF-2 bioavailability and regulate skeletal growth. J. Cell. Biol. 218, 3134–3152. 10.1083/jcb.201906059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaus A., Bauer J., Sleeman J. P. (2014). Sugars in the microenvironment: The sticky problem of HA turnover in tumors. Cancer Metastasis Rev. 33, 1059–1079. 10.1007/s10555-014-9532-2 [DOI] [PubMed] [Google Scholar]
- Senthebane D. A., Rowe A., Thomford N. E., Shipanga H., Munro D., Mazeedi M., et al. (2017). The role of tumor microenvironment in chemoresistance: To survive, keep your enemies closer. Int. J. Mol. Sci. 18, 1586. 10.3390/ijms18071586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaei M., Csaki C., Nebrich S., Mobasheri A. (2008). Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 76, 1426–1439. 10.1016/j.bcp.2008.05.029 [DOI] [PubMed] [Google Scholar]
- Shen K. H., Hung J. H., Chang C. W., Weng Y. T., Wu M. J., Chen P. S. (2017). Solasodine inhibits invasion of human lung cancer cell through downregulation of miR-21 and MMPs expression. Chem. Biol. Interact. 268, 129–135. 10.1016/j.cbi.2017.03.005 [DOI] [PubMed] [Google Scholar]
- Shi M. D., Shih Y. W., Lee Y. S., Cheng Y. F., Tsai L. Y. (2013). Suppression of 12-O-tetradecanoylphorbol-13-acetate-induced MCF-7 breast adenocarcinoma cells invasion/migration by α-tomatine through activating PKCα/ERK/NF-κB-dependent MMP-2/MMP-9 expressions. Cell. Biochem. Biophys. 66, 161–174. 10.1007/s12013-012-9465-8 [DOI] [PubMed] [Google Scholar]
- Shiri S., Alizadeh A. M., Baradaran B., Farhanghi B., Shanehbandi D., Khodayari S., et al. (2015). Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac J. Cancer Prev. 16, 3917–3922. 10.7314/apjcp.2015.16.9.3917 [DOI] [PubMed] [Google Scholar]
- Somchit N., Kimseng R., Dhar R., Hiransai P., Changtam C., Suksamrarn A., et al. (2018). Curcumin pyrazole blocks lipopolysaccharide-induced inflammation via suppression of JNK activation in RAW 264.7 macrophages. Asian Pac J. Allergy Immunol. 36 (3), 184–190. 10.12932/AP-130417-0073 [DOI] [PubMed] [Google Scholar]
- Su X., Esser A. K., Amend S. R., Xiang J., Xu Y., Ross M. H., et al. (2016). Antagonizing integrin β3 increases immunosuppression in cancer. Cancer Res. 76, 3484–3495. 10.1158/0008-5472.CAN-15-2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J., Kim D. H., Surh Y. J. (2018). Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch. Biochem. Biophys. 643, 62–71. 10.1016/j.abb.2018.02.011 [DOI] [PubMed] [Google Scholar]
- Sullivan W. J., Mullen P. J., Schmid E. W., Flores A., Momcilovic M., Sharpley M. S., et al. (2018). Extracellular matrix remodeling regulates glucose metabolism through TXNIP destabilization. Cell. 175, 117–132.e21. 10.1016/j.cell.2018.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Lu N., Ling Y., Gao Y., Chen Y., Wang L., et al. (2009). Oroxylin A suppresses invasion through down-regulating the expression of matrix metalloproteinase-2/9 in MDA-MB-435 human breast cancer cells. Eur. J. Pharmacol. 603, 22–28. 10.1016/j.ejphar.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R. L., Laversanne M., Soerjomataram I., Jemal A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Swindell W. R., Bojanowski K., Chaudhuri R. K. (2020). A zingerone analog, acetyl zingerone, bolsters matrisome synthesis, inhibits matrix metallopeptidases, and represses IL-17a target gene expression. J. Invest. Dermatol 140, 602–614.e15. 10.1016/j.jid.2019.07.715 [DOI] [PubMed] [Google Scholar]
- Tajaldini M., Saeedi M., Amiriani T., Amiriani A. H., Sedighi S., Mohammad Zadeh F., et al. (2022). Cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs); where do they stand in tumorigenesis and how they can change the face of cancer therapy? Eur. J. Pharmacol. 928, 175087. 10.1016/j.ejphar.2022.175087 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Shinto O., Yashiro M., Yamazoe S., Iwauchi T., Muguruma K., et al. (2010). Transforming growth factor â signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol. Rep. 24, 1637–1643. 10.3892/or_00001028 [DOI] [PubMed] [Google Scholar]
- Tariq M., Zhang J., Liang G., Ding L., He Q., Yang B. (2017). Macrophage polarization: Anti-cancer strategies to target tumor-associated macrophage in breast cancer. J. Cell. Biochem. 118, 2484–2501. 10.1002/jcb.25895 [DOI] [PubMed] [Google Scholar]
- Terra M., Oberkampf M., Fayolle C., Rosenbaum P., Guillerey C., Dadaglio G., et al. (2018). Tumor-derived TGFβ alters the ability of plasmacytoid dendritic cells to respond to innate immune signaling. Cancer Res. 78, 3014–3026. 10.1158/0008-5472.CAN-17-2719 [DOI] [PubMed] [Google Scholar]
- Thongchot S., Ferraresi A., Vidoni C., Loilome W., Yongvanit P., Namwat N., et al. (2018). Resveratrol interrupts the pro-invasive communication between cancer associated fibroblasts and cholangiocarcinoma cells. Cancer Lett. 430, 160–171. 10.1016/j.canlet.2018.05.031 [DOI] [PubMed] [Google Scholar]
- Ting H., Deep G., Kumar S., Jain A. K., Agarwal C., Agarwal R. (2016). Beneficial effects of the naturally occurring flavonoid silibinin on the prostate cancer microenvironment: Role of monocyte chemotactic protein-1 and immune cell recruitment. Carcinogenesis 37, 589–599. 10.1093/carcin/bgw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting H. J., Deep G., Jain A. K., Cimic A., Sirintrapun J., Romero L. M., et al. (2015). Silibinin prevents prostate cancer cell-mediated differentiation of naïve fibroblasts into cancer-associated fibroblast phenotype by targeting TGF β2. Mol. Carcinog. 54, 730–741. 10.1002/mc.22135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Trivedi R., Lin S. Y. (2022). Tumor microenvironment: Barrier or opportunity towards effective cancer therapy. J. Biomed. Sci. 29, 83. 10.1186/s12929-022-00866-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J. J., Chen J. H., Chen C. H., Chung J. G., Hsu F. T. (2020). Apoptosis induction and ERK/NF-κB inactivation are associated with magnolol-inhibited tumor progression in hepatocellular carcinoma in vivo . Environ. Toxicol. 35, 167–175. 10.1002/tox.22853 [DOI] [PubMed] [Google Scholar]
- Tyagi A., Singh R. P., Ramasamy K., Raina K., Redente E. F., Dwyer-Nield L. D., et al. (2009). Growth inhibition and regression of lung tumors by silibinin: Modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3. Cancer Prev. Res. (Phila) 2, 74–83. 10.1158/1940-6207.CAPR-08-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanakakis G. N., Giatagana E. M., Berdiaki A., Spyridaki I., Hida K., Neagu M., et al. (2021). The role of IGF/IGF-IR-Signaling and extracellular matrix effectors in bone sarcoma pathogenesis. Cancers (Basel) 13, 2478. 10.3390/cancers13102478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshenyuk T. G., Landesman E. S., Khoutorova E., Hart A. D., Gardner J. D. (2011). Induction of cardiac fibroblast lysyl oxidase by TGF-β1 requires PI3K/Akt, Smad3, and MAPK signaling. Cytokine 55, 90–97. 10.1016/j.cyto.2011.03.024 [DOI] [PubMed] [Google Scholar]
- Walker C., Mojares E., Del Río Hernández A. (2018). Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci. 19, 3028. 10.3390/ijms19103028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li Y., Chen H., Zhang J., Zhang J., Qin T., et al. (2017a). Inhibition of CYP4A by a novel flavonoid FLA-16 prolongs survival and normalizes tumor vasculature in glioma. Cancer Lett. 402, 131–141. 10.1016/j.canlet.2017.05.030 [DOI] [PubMed] [Google Scholar]
- Wang F., Fan K., Zhao Y., Xie M. L. (2021). Apigenin attenuates TGF-β1-stimulated cardiac fibroblast differentiation and extracellular matrix production by targeting miR-155-5p/c-Ski/Smad pathway. J. Ethnopharmacol. 265, 113195. 10.1016/j.jep.2020.113195 [DOI] [PubMed] [Google Scholar]
- Wang J., Huang S. (2018). Fisetin inhibits the growth and migration in the A549 human lung cancer cell line via the ERK1/2 pathway. Exp. Ther. Med. 15, 2667–2673. 10.3892/etm.2017.5666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N. P., Wang Z. F., Tootle S., Philip T., Zhao Z. Q. (2012). Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br. J. Pharmacol. 167, 1550–1562. 10.1111/j.1476-5381.2012.02109.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wang N., Huang X., Yang B., Zheng Y., Zhang J., et al. (2020). Baohuoside i suppresses breast cancer metastasis by downregulating the tumor-associated macrophages/C-X-C motif chemokine ligand 1 pathway. Phytomedicine 78, 153331. 10.1016/j.phymed.2020.153331 [DOI] [PubMed] [Google Scholar]
- Wang Y., Huang Z. Q., Wang C. Q., Wang L. S., Meng S., Zhang Y. C., et al. (2011). Artemisinin inhibits extracellular matrix metalloproteinase inducer (EMMPRIN) and matrix metalloproteinase-9 expression via a protein kinase Cδ/p38/extracellular signal-regulated kinase pathway in phorbol myristate acetate-induced THP-1 macrophages: Artemisinin inhibits inflammation. Clin. Exp. Pharmacol. Physiol. 38, 11–18. 10.1111/j.1440-1681.2010.05454.x [DOI] [PubMed] [Google Scholar]
- Wang Z. F., Ma D. G., Zhu Z., Mu Y. P., Yang Y. Y., Feng L., et al. (2017b). Astragaloside IV inhibits pathological functions of gastric cancer-associated fibroblasts. World J. Gastroenterol. 23, 8512–8525. 10.3748/wjg.v23.i48.8512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B., Zhang Y., Tang L., Ji Y., Yan C., Zhang X. (2019). Protective effects of quercetin against inflammation and oxidative stress in a rabbit model of knee osteoarthritis. Drug Dev. Res. 80, 360–367. 10.1002/ddr.21510 [DOI] [PubMed] [Google Scholar]
- Wei K., Dai J., Wang Z., Pei Y., Chen Y., Ding Y., et al. (2020). Oxymatrine suppresses IL-1β-induced degradation of the nucleus pulposus cell and extracellular matrix through the TLR4/NF-κB signaling pathway. Exp. Biol. Med. (Maywood) 245, 532–541. 10.1177/1535370219900773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Jian P., Erjiong H., Qihan Z. (2021). Ginkgetin alleviates high glucose-evoked mesangial cell oxidative stress injury, inflammation, and extracellular matrix (ECM) deposition in an AMPK/mTOR-mediated autophagy axis. Chem. Biol. Drug Des. 98, 620–630. 10.1111/cbdd.13915 [DOI] [PubMed] [Google Scholar]
- Wells J. M., Gaggar A., Blalock J. E. (2015). MMP generated matrikines. Matrix Biol. 44-46, 122–129. 10.1016/j.matbio.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatcott C. J., Han H., Posner R. G., Hostetter G., Von Hoff D. D. (2011). Targeting the tumor microenvironment in cancer: Why hyaluronidase deserves a second look. Cancer Discov. 1, 291–296. 10.1158/2159-8290.CD-11-0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J., Abisoye-Ogunniyan A., Metcalf K. J., Werb Z. (2020). Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120. 10.1038/s41467-020-18794-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Friedl P. (2011). Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell. Biol. 21, 736–744. 10.1016/j.tcb.2011.09.006 [DOI] [PubMed] [Google Scholar]
- Woo J. H., Ahn J. H., Jang D. S., Lee K. T., Choi J. H. (2017). Effect of kumatakenin isolated from cloves on the apoptosis of cancer cells and the alternative activation of tumor-associated macrophages. J. Agric. Food Chem. 65, 7893–7899. 10.1021/acs.jafc.7b01543 [DOI] [PubMed] [Google Scholar]
- Wu C. J., Chou Y. C., Cheng Y. W., Hsiao C. J., Wang C. H., Wang H. Y., et al. (2011). Aristolochic acid downregulates monocytic matrix metalloproteinase-9 by inhibiting nuclear factor-κB activation. Chem. Biol. Interact. 192, 209–219. 10.1016/j.cbi.2011.03.012 [DOI] [PubMed] [Google Scholar]
- Wu J., Qian Y., Chen C., Feng F., Pan L., Yang L., et al. (2021). Hesperetin exhibits anti-inflammatory effects on chondrocytes via the AMPK pathway to attenuate anterior cruciate ligament transection-induced osteoarthritis. Front. Pharmacol. 12, 735087. 10.3389/fphar.2021.735087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Chen G.-L., Li Y.-J., Chen Y., Lin F.-Z. (2015). Paeoniflorin inhibits macrophage-mediated lung cancer metastasis. Chin. J. Nat. Med. 13, 925–932. 10.1016/S1875-5364(15)30098-4 [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Hsu W. J., Wu L. H., Liou H. P., Pangilinan C. R., Tyan Y. C., et al. (2020). Hinokitiol reduces tumor metastasis by inhibiting heparanase via extracellular signal-regulated kinase and protein kinase B pathway. Int. J. Med. Sci. 17, 403–413. 10.7150/ijms.41177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D., Hu W., Ye S. T., Tan Y. S. (2018). Isoliquiritigenin alleviated the Ang II-induced hypertensive renal injury through suppressing inflammation cytokines and oxidative stress-induced apoptosis via Nrf2 and NF-κB pathways. Biochem. Biophys. Res. Commun. 506, 161–168. 10.1016/j.bbrc.2018.09.013 [DOI] [PubMed] [Google Scholar]
- Xiong J., Yan L., Zou C., Wang K., Chen M., Xu B., et al. (2021). Integrins regulate stemness in solid tumor: An emerging therapeutic target. J. Hematol. Oncol. 14, 177. 10.1186/s13045-021-01192-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Zhao L. J., Liao T., Li Z. C., Wang L. L., Lin P. Y., et al. (2022). Ononin ameliorates inflammation and cartilage degradation in rat chondrocytes with IL-1β-induced osteoarthritis by downregulating the MAPK and NF-κB pathways. BMC Complement. Med. Ther. 22, 25. 10.1186/s12906-022-03504-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Guo L., Bu H., Wang H. (2019). Daphnetin inhibits high glucose-induced extracellular matrix accumulation, oxidative stress and inflammation in human glomerular mesangial cells. J. Pharmacol. Sci. 139, 91–97. 10.1016/j.jphs.2018.11.013 [DOI] [PubMed] [Google Scholar]
- Yang S. L., Kuo F. H., Chen P. N., Hsieh Y. H., Yu N. Y., Yang W. E., et al. (2017). Andrographolide suppresses the migratory ability of human glioblastoma multiforme cells by targeting ERK1/2-mediated matrix metalloproteinase-2 expression. Oncotarget 8, 105860–105872. 10.18632/oncotarget.22407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Zhang H., Yang H., Zhang J., Wang J., Luo T., et al. (2021). SEPHS1 promotes SMAD2/3/4 expression and hepatocellular carcinoma cells invasion. Exp. Hematol. Oncol. 10, 17. 10.1186/s40164-021-00212-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W., Ba Q., Li X., Li H., Zhang S., Yuan Y., et al. (2017). A natural CCR2 antagonist relieves tumor-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer. EBioMedicine 22, 58–67. 10.1016/j.ebiom.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelken B. O., Balci T., Susluer S. Y., Kayabasi C., Avci C. B., Kirmizibayrak P. B., et al. (2017). The effect of tomatine on metastasis related matrix metalloproteinase (MMP) activities in breast cancer cell model. Gene 627, 408–411. 10.1016/j.gene.2017.06.054 [DOI] [PubMed] [Google Scholar]
- Yuzhalin A. E., Lim S. Y., Kutikhin A. G., Gordon-Weeks A. N. (2018). Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim. Biophys. Acta Rev. Cancer 1870, 207–228. 10.1016/j.bbcan.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Zeisberg M., Khurana M., Rao V. H., Cosgrove D., Rougier J. P., Werner M. C., et al. (2006). Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 3, e100. 10.1371/journal.pmed.0030100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Du Q., Zhang Z., Ma J., Han L., Wang Y., et al. (2020). Curcumin promotes cancer-associated fibroblasts apoptosis via ROS-mediated endoplasmic reticulum stress. Arch. Biochem. Biophys. 694, 108613. 10.1016/j.abb.2020.108613 [DOI] [PubMed] [Google Scholar]
- Zhai W. Y., Jia C. P., Zhao H., Xu Y. S. (2011). Procyanidins inhibit tumor angiogenesis by crosslinking extracellular matrix. Chin. J. Cancer Res. 23, 99–106. 10.1007/s11670-011-0099-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Ma J., Lu Y., Ni G. X., Ni C. Y., Zhang X. J., et al. (2012). Acupuncture combined with curcumin attenuates carbon tetrachloride-induced hepatic fibrosis in rats. Acupunct. Med. 30, 132–138. 10.1136/acupmed-2011-010116 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Huang H., Zheng F., Liu H., Qiu F., Chen Y., et al. (2020). Resveratrol exerts antitumor effects by downregulating CD8(+)CD122(+) Tregs in murine hepatocellular carcinoma. Oncoimmunology 9, 1829346. 10.1080/2162402X.2020.1829346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Guo Y., Zhang S., Zhang Y., Wang Y., Ni W., et al. (2013). Curcumin modulates cannabinoid receptors in liver fibrosis in vivo and inhibits extracellular matrix expression in hepatic stellate cells by suppressing cannabinoid receptor type-1 in vitro . Eur. J. Pharmacol. 721, 133–140. 10.1016/j.ejphar.2013.09.042 [DOI] [PubMed] [Google Scholar]
- Zhao K., Wei L., Hui H., Dai Q., You Q. D., Guo Q. L., et al. (2014). Wogonin suppresses melanoma cell B16-F10 invasion and migration by inhibiting Ras-medicated pathways. PLoS One 9, e106458. 10.1371/journal.pone.0106458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Cai P., Zhang N., Wu T., Sun A., Jia G. (2022). Inhibitory effects of polyphenols from black chokeberry on advanced glycation end-products (AGEs) formation. Food Chem. 392, 133295. 10.1016/j.foodchem.2022.133295 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Shao Q., Zhu H., Xu H., Long W., Yu B., et al. (2018). Resveratrol ameliorates Lewis lung carcinoma-bearing mice development, decreases granulocytic myeloid-derived suppressor cell accumulation and impairs its suppressive ability. Cancer Sci. 109, 2677–2686. 10.1111/cas.13720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Li D., Li J., Wang B., Zhang L., Yuan X., et al. (2019). Optimization of the process for purifying icariin from Herba Epimedii by macroporous resin and the regulatory role of icariin in the tumor immune microenvironment. Biomed. Pharmacother. 118, 109275. 10.1016/j.biopha.2019.109275 [DOI] [PubMed] [Google Scholar]
- Zhou J., Ueda K., Zhao J., Sparrow J. R. (2015). Correlations between photodegradation of bisretinoid constituents of retina and dicarbonyl adduct deposition. J. Biol. Chem. 290, 27215–27227. 10.1074/jbc.M115.680363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. H., Ji C. D., Xiao H. L., Zhao H. B., Cui Y. H., Bian X. W. (2017). Reorganized collagen in the tumor microenvironment of gastric cancer and its association with prognosis. J. Cancer 8, 1466–1476. 10.7150/jca.18466 [DOI] [PMC free article] [PubMed] [Google Scholar]