Abstract
Background
There remains a need to identify low-cost interventions to improve coronavirus disease 2019 (COVID-19) outcomes. Vitamin D and zinc play a role in respiratory infections and could hold value as part of therapeutic regimens.
Objectives
To determine the effect of vitamin D or zinc supplementation on recovery from COVID-19.
Methods
We conducted a double-blind, randomly assigned 2 x 2 factorial placebo-controlled trial with 1:1:1:1 allocation ratio, enrolling nonpregnant adults with COVID-19 from hospitals in Mumbai and Pune, India (NCT04641195). Participants (N = 181) were randomly assigned to vitamin D3 (180,000 IU bolus, then 2000 IU daily), zinc (40 mg daily), vitamin D3 and zinc, or placebo, for 8 wk. Participants were followed until 8 wk. The primary outcome was time to resolution of fever, cough, and shortness of breath. Secondary outcomes were duration of individual symptoms; need for assisted ventilation; duration of hospital stay; all-cause mortality; and blood biomarkers, including nutritional, inflammatory, and immunological markers.
Results
We observed no effect of vitamin D or zinc supplementation on time to resolution of all 3 symptoms [vitamin D hazard ratio (HR): 0.92; 95% confidence interval (95% CI): 0.66, 1.30; P = 0.650; zinc HR: 0.94; 95% CI: 0.67, 1.33; P = 0.745)]. Neither vitamin D nor zinc supplementation was associated with secondary outcomes, except for increased endline serum vitamin D with vitamin D supplementation [median (interquartile range) difference between endline and baseline for vitamin D: 5.3 ng/mL (–2.3 to 13.7); for no vitamin D: –1.4 ng/mL (–5.6 to 3.9); P = 0.003]. We observed nonsignificant increases in serum zinc at endline following zinc supplementation. There was no evidence of interaction between vitamin D and zinc supplementation, no effect of either on hypercalcemia, and no adverse events.
Conclusions
Results suggest that neither vitamin D nor zinc supplementation improves COVID-19 treatment outcomes in this population. However, much larger-scale evidence, particularly from populations with vitamin D or zinc deficiency and severe infection, is required to corroborate our findings. This trial was registered at ClinicalTrials.gov and the Clinical Trials Registry of India as NCT04641195 and CTRI/2021/04/032593 respectively.
Keywords: vitamin D, zinc, dietary supplements, COVID-19
Introduction
COVID-19 is an acute respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is characterized by symptoms including cough, shortness of breath, and fever [1,2]. In more severe cases, COVID-19 may lead to outcomes including acute respiratory distress syndrome, septic shock, and mortality [3]. With a global pandemic declared in March 2020 [4], there have been over 754 million confirmed COVID-19 cases and 6.8 million deaths attributed to COVID-19 as of February 5, 2023 [5,6]. Although overall incidence has been declining in recent months, it remains notable, with over 10.5 million new cases and 90,000 related deaths reported between early January and early February 2023 [6]. Monitoring continues for new variants and subvariants of concern, with some, such as the recently-identified XBB subvariant, understood to have enhanced transmissibility, including among individuals with prior infection or vaccination [[7], [8], [9]]. Additionally, approximately 10% of individuals who previously had COVID-19 are estimated to experience post-COVID-19 condition or long COVID, longer-term sequelae continuing beyond 4–12 wk following infection [10]. The global health burden due to COVID-19 thus remains substantial.
Effective measures to prevent SARS-CoV-2 infection and transmission include wearing face masks, hand and respiratory hygiene, vaccination, and testing [[11], [12], [13], [14]]. For infected individuals, recommended treatments continue to evolve based on emerging research. The current WHO living guideline on therapeutics and COVID-19, published on January 13, 2023, includes strong recommendations for nirmatrelvir and ritonavir in nonsevere infection, and corticosteroids, IL-6 receptor blockers and baricitinib in severe and critical infection [3]. Importantly, access to recommended COVID-19 preventative resources and therapeutic drugs may be limited in low- and middle-income settings such as South Asia [[15], [16], [17]]. Given this, there remains a need to explore additional effective and affordable interventions that may be delivered at large scale to improve outcomes among individuals with COVID-19.
Previous research has suggested that vitamin D and zinc may play a role in the development and course of respiratory infections, suggesting their potential utility as part of therapeutic regimens for COVID-19 [[18], [19], [20]]. Vitamin D metabolites influence the expression of key signaling proteins involved in immune response [21,22] and transmembrane proteins targeted by rhinoviruses [23] and have been shown to increase concentrations of the antimicrobial peptide cathelicidin in respiratory epithelial cells [22]. Recent meta-analyses of randomized controlled trials have indicated a reduced risk of acute respiratory tract infections (ARIs) and shorter duration of symptoms among individuals supplemented with vitamin D [18,19]. On the contrary, zinc is required for leukocyte and lymphocyte development and is also involved in immunomodulatory signaling [24]. Zn2+, in combination with pyrithione, has been shown to inhibit SARS-CoV replication in cell cultures [24,25]. Additionally, zinc has been hypothesized to play a role in the expression of ACE-2 [24], a key receptor mediating entry of SARS-CoV-2 into cells [26]. Previous evidence syntheses suggest that zinc supplementation shortens the duration of ARI symptoms [19,20]. However, high-quality evidence directly assessing the potential benefit of vitamin D and zinc supplementation in COVID-19 remains limited and unclear, particularly from lower resource settings [27,28].
It is important to gain a clear understanding of whether vitamin D and zinc supplementation may improve COVID-19 outcomes in low- and middle-income populations such as those in South Asia, where the burdens of vitamin D and zinc deficiency are thought to be notable [29,30], and where such easily accessible supplements may be particularly valuable. In this 2 x 2 factorial randomized controlled trial, we aimed to assess the effect of vitamin D and zinc supplementation on treatment outcomes among individuals with mild-to-moderate COVID-19 in Mumbai and Pune, India.
Methods
Overview, ethics, and reporting
This was a multicenter, double-blind, randomly assigned, placebo-controlled trial with a 2 x 2 factorial design and 1:1:1:1 allocation ratio. The methods of this trial have been outlined [31] and are briefly described below. The trial was approved by the following review boards: Institutional Review Board of the Harvard T.H. Chan School of Public Health (protocol No. IRB20-1425), University Health Network Research Ethics Board (20-5775), Institutional Research Ethics Committee of the Foundation for Medical Research (IREC No. FMR/IREC/C19/02/2020), Institutional Review Board of Saifee Hospital (project No. EC/008/2020) and King Edward Memorial Hospital Research Centre Ethics Committee (KEMHRC ID No. 2027). Permission to undertake the study was also obtained from the Health Ministry’s Screening Committee, Government of India [HMSC (GOI)-2021-0060]. Endorsement from the Drugs Controller General of India was not required as the intervention comprised micronutrient supplementation. This trial followed CONSORT guidelines and was prospectively registered on clincialtrials.gov (NCT04641195) and the clinical trials registry of India (CTRI/2021/04/032593). This study was overseen by an independent data and safety monitoring board, which was responsible for regularly reviewing trial progress and safety and efficacy endpoints [31]. The trial conformed to the principles embodied in the Declaration of Helsinki.
Study setting and time frame
This trial was conducted at 2 sites: KEM Hospital (King Edward Memorial Hospital and Research Centre), Pune, and Saifee Hospital, Mumbai. Both were designated as COVID-19 dedicated hospitals by local municipal corporations. These hospitals were chosen on the basis of existing research collaborations and feasibility of conducting a research trial. Enrolment was conducted from April 2021 and ended in February 2022, and follow-up was completed in August 2022.
Population and eligibility criteria
This study was initially based among hospitalized patients. Inclusion criteria were: age ≥18 y, RT-PCR-confirmed infection with SARS-COV-2, oxygen saturation level ≥90, and written informed consent. Exclusion criteria were: self-reported pregnancy, enrolment in other clinical trials, and daily use of multivitamins for the past 1 mo. With the decline in incidence of COVID-19 in India and to increase generalizability and enroll a wider range and number of symptomatic COVID-19 patients, in June 2021, we: 1) broadened the population to include outpatients, 2) added an inclusion criterion of Rapid Antigen Test-confirmed SARS-COV-2 infection, 3) removed the inclusion criterion oxygen saturation level ≥90, and 4) removed the exclusion criterion recent daily multivitamin use.
Study procedures
Potential participants were approached and invited to enroll by trained site hospital staff members when they presented to the site hospitals. Following screening and informed consent, we collected baseline blood samples, sociodemographic data; health and clinical data; dietary measures; and COVID-19 symptoms, treatment, and vaccination information. Participants were then randomly assigned to 1 of 4 groups allocating them to 8-wk supplementation with either: 1) vitamin D3 (180,000 IU single bolus in the form of 3 tablets of 60,000 IU, then 2000 IU daily), 2) zinc (40 mg daily), 3) vitamin D3 and zinc, or 4) placebo. We chose this dose of vitamin D3 as similar bolus and daily doses have been shown to safely and quickly boost and sustain vitamin D concentrations in previous studies, including among individuals with COVID-19 [28,[32], [33], [34]]. The 40 mg daily dose of zinc was considered to be high enough to examine efficacy and within tolerable upper intake limits [35], and similar doses have also been examined in the context of COVID-19 [36,37]. To ensure blinding of participants and staff, each participant received 3 bolus tablets (either vitamin D3 or placebo) to be consumed under supervision of site hospital staff and a supplement bottle containing the full appropriate assigned regimen (1 tablet/d for 8 wk for all groups). Active and placebo tablets were indistinguishable, and supplement containers (envelopes for bolus doses and bottles for daily doses) were prelabeled with codes (see Randomization and blinding below). Following this, inpatient participants were directly observed taking their supplements daily by the site hospital staff, and participants who left the hospital (outpatients or inpatient participants who were discharged) were reminded to take their supplements during regular telephone follow-ups.
Following the baseline visit, participants were followed either daily in person (if in hospital) or every 3 d via telephone (upon leaving the hospital) for 8 wk to record information on COVID-19 symptoms, supplement compliance, and any adverse events. For hospitalized participants, we additionally collected information on clinical measures, including vital signs, prescribed medications and supplements, complications, and the need for assisted ventilation. At 8 wk, an endline visit was conducted in the hospital or at another private and convenient location. This consisted of a clinical and physical examination, blood sample collection, and data collection, including clinical information, other medication or supplement use, compliance (including pill count), and COVID-19 symptoms and updates to vaccination status. Participants could also provide a subset of information via telephone if an in-person visit were not possible. A final telephone follow-up was then completed at 12 wk, where we assessed COVID-19 symptoms and any updates to vaccination status.
All informed consent and data collection procedures were undertaken in an appropriate local language (Marathi, Hindi, or English). All data were collected on standardized electronic forms [38] and uploaded directly to a secure server. All participants were provided care and treatment in line with Indian National Guidelines at the time [[39], [40], [41]] and were encouraged to visit the hospital at any time for medical attention if they felt unwell.
Randomization and blinding
Randomization was in blocks of 20 and stratified by site. The randomization list was prepared by an independent statistician and assigned each participant’s randomization identifier to a regimen code. Supplement containers were labeled with these codes to maintain blinding. The actual regimen was known only to the supplement manufacturer (Excellamed Laboratories Private Limited) and to no other research staff or investigators and was accessible to the data and safety monitoring board in a sealed envelope. Unblinding was undertaken only after blinded primary analyses were completed.
Outcomes
The primary outcome was time to resolution of all of fever, cough, and shortness of breath. These symptoms have been most commonly reported among individuals with COVID-19, including in India, and assessed as part of previous trials on vitamin D and zinc supplementation for ARIs [2,19,42]. At baseline and at each follow-up, participants were asked whether they had experienced each symptom that day and how many days in total they had experienced the symptom. Information collected at all follow-ups was then used to identify the timepoint of resolution and calculate the duration of symptoms.
Secondary outcomes were the duration of individual symptoms, need for assisted ventilation, duration of hospital stay (among inpatients), all-cause mortality, and blood biomarker concentrations. Blood biomarkers were measured both at baseline and endline (8 wk) and included nutritional measures (serum 25(OH)D and calcium, and zinc in whole blood) and immunological and inflammatory markers [serum ferritin, CRP, lactate dehydrogenase and plasma D-dimer (a protein product of fibrin degradation, that has been linked to inflammation and indicated as a biomarker of severity for COVID-19 in previous research)] [43], IL-6, angiopoietin-2, soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), IgG and IgM]. Sample analysis was undertaken at nationally accredited laboratories (nutritional biomarkers all measured in a single laboratory using validated methods, ferritin, CRP, D-dimer, and lactate dehydrogenase) or at the Foundation for Medical Research (IL-6, angiopoietin-2, sTREM-1, IgG, and IgM). Because of the technical issues with sample assay kits, sTREM-1 could be measured only for a subset of participants (see Supplementary Methods).
Sample size calculation
Sample size calculations used methodology for survival times. Based on an assumption of time to recovery of 22.2 d and 5% loss to follow-up, we estimated that enrolment of 700 participants would provide ≥80% power to detect a moderate (25–30%) effect of either vitamin D or zinc supplementation, given a maximum 30% true effect of the other treatment.
Statistical analysis
Primary analysis was by intent-to-treat, comparing vitamin D supplementation with no vitamin D and zinc supplementation with no zinc. The primary outcome was analyzed using Cox regression, and secondary outcomes were analyzed using Cox regression (duration of individual symptoms and of hospital stay), Poisson regression (need for assisted ventilation and hypercalcemia), and Wilcoxon rank sum tests (all blood biomarkers at endline, and difference between endline and baseline); primary analyses were unadjusted. We performed additional pre-specified analyses to assess the robustness of results. First, we adjusted models examining clinical outcomes for site and key baseline characteristics BMI (kg/m2), pre-existing conditions, COVID-19 medications at baseline, supplement intake at baseline, number of days participant had experienced any symptom at baseline, age, and vaccination status at baseline). We similarly examined biomarkers in linear regression models adjusted for the same variables, and also for days since last vaccine dose, the COVID-19 wave in India (second wave or third wave) during which the participant was enrolled, days between baseline and endline assessment, and baseline biomarker concentrations where the outcome was endline biomarker concentrations. Following this, we examined associations in models restricted to participants with ≥90% compliance. Finally, we examined interaction between vitamin D and zinc supplementation by stratifying models and using likelihood ratio tests comparing models with and without interaction terms, and examined interactions between vitamin D or zinc supplementation and key characteristics, including sex, age, and BMI, in a similar manner. In further post hoc analyses, we also examined whether results for vitamin D supplementation varied by baseline vitamin D status (vitamin D <20 ng/mL compared with ≥20 ng/mL) using similar methods. All analyses were undertaken using Stata 16 (StataCorp).
Patient and public involvement
Patients and the public were not involved in the design of this study.
Results
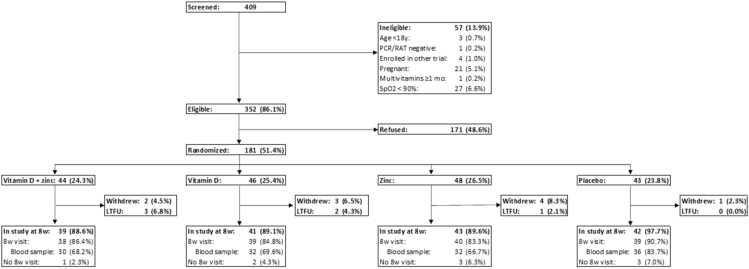
This study ended before meeting its enrolment target of 700 participants because of a progressive decrease in COVID-19 cases at sites and across India. In all, a total of 409 individuals were screened during the course of the trial, of which 352 (86.1%) met eligibility criteria (Figure 1). Of these, 181 (51.4%) consented to participate in the study and were randomly assigned, 44 (24.3%) to vitamin D and zinc, 46 (25.4%) to vitamin D, 48 (26.5%) to zinc, and 43 (23.8%) to placebo. Overall, 10 (5.5%) participants withdrew, and 6 (3.3%) were lost to follow-up between baseline and the 8-wk endline follow-up. Of the 165 (91.2%) remaining participants at endline, 9 (5.0%) participants did not provide data for the endline follow-up. Thus, a total of 156 (86.2%) participants had data collected at endline, with 130 (71.8%) providing blood samples.
FIGURE 1.
Study enrolment and follow-up. LTFU, lost to follow-up; RAT, rapid antigen test; SpO2, oxygen saturation.
Baseline characteristics of the study population by treatment arm are presented in Table 1. Participants were split generally evenly across treatment arms. About 80% of participants each were enrolled at KEM Hospital, were inpatients, and were aged over 30 y. Close to half of all participants had graduate or postgraduate education, and <1 tenth had primary education or less. Over half were overweight or obese, and about one-third had a pre-existing chronic condition. COVID-19 medication use and supplement use at baseline were generally low (<5% except for remdesivir), and participants had experienced COVID-19 symptoms (including but not limited to fever, cough, and shortness of breath) for approximately 3 d before enrolment. At least half of participants had received 1 or more doses of a COVID-19 vaccine before enrolment (Table 1). Overall, at baseline, 47.4% of participants had total serum vitamin D <20 ng/mL and 12.4% had serum zinc <407 μg/dL (Supplemental Table 1). For both comparisons (vitamin D compared with no vitamin D, zinc compared with no zinc), we observed notable differences by arm in the distribution of age, education level, BMI, pre-existing conditions, remdesivir use, and vaccination status (Table 1).
TABLE 1.
Baseline characteristics of randomly assigned participants
|
n (%) or median (IQR) |
||||
|---|---|---|---|---|
| Vitamin D | No vitamin D | Zinc | No zinc | |
| N | 90 | 91 | 92 | 89 |
| Hospital, n (%) | ||||
| KEM | 72 (80.0) | 73 (80.2) | 75 (81.5) | 70 (78.7) |
| Saifee | 18 (20.0) | 18 (19.8) | 17 (18.5) | 19 (21.3) |
| Women, n (%) | 43 (47.8) | 45 (49.5) | 45 (48.9) | 43 (48.3) |
| Age, y, n (%) | ||||
| 18–29 | 20 (22.2) | 10 (11.0) | 16 (17.4) | 14 (15.7) |
| 30–44 | 23 (25.6) | 32 (35.2) | 19 (20.7) | 36 (40.4) |
| 45–59 | 28 (31.1) | 23 (25.3) | 25 (27.2) | 26 (29.2) |
| 60+ | 19 (21.1) | 26 (28.6) | 32 (34.8) | 13 (14.6) |
| Education | ||||
| Illiterate, no formal, primary | 8 (8.9) | 7 (7.7) | 8 (8.7) | 7 (7.9) |
| Secondary | 23 (25.6) | 31 (34.1) | 31 (33.7) | 23 (25.8) |
| Graduate | 34 (37.8) | 31 (34.1) | 30 (32.6) | 35 (39.3) |
| Postgraduate | 24 (26.7) | 21 (23.1) | 21 (22.8) | 24 (7.0) |
| Missing | 1 (1.1) | 1 (1.1) | 2 (2.2) | 0 (0.0) |
| BMI, n (%) | ||||
| <18.5 | 4 (4.4) | 2 (2.2) | 2 (2.2) | 4 (4.5) |
| 18.5–24.9 | 26 (28.9) | 37 (40.7) | 38 (41.3) | 25 (28.1) |
| 25–29.9 | 32 (35.6) | 24 (26.4) | 24 (26.1) | 32 (36.0) |
| 30+ | 28 (31.1) | 28 (30.8) | 28 (30.4) | 28 (31.5) |
| Pre-existing conditions, n (%) | ||||
| Diabetes | 12 (13.3) | 26 (28.6) | 21 (22.8) | 17 (19.1) |
| Hypertension | 16 (17.8) | 29 (31.9) | 26 (28.3) | 19 (21.3) |
| Heart disease | 6 (6.7) | 8 (8.8) | 5 (5.4) | 9 (10.1) |
| Asthma | 5 (5.6) | 2 (2.2) | 4 (4.3) | 3 (3.4) |
| Other conditions | 3 (3.3) | 6 (6.6) | 6 (6.5) | 3 (3.4) |
| Any pre-existing condition, n (%) | 29 (32.2) | 43 (47.3) | 38 (41.3) | 34 (38.2) |
| Use of medications for COVID-19, n (%) | ||||
| Favipiravir | 2 (2.2) | 1 (1.1) | 2 (2.2) | 1 (1.1) |
| Remdesivir | 14 (15.6) | 8 (8.8) | 13 (14.1) | 9 (10.1) |
| Dexamethasone | 1 (1.1) | 0 (0.0) | 1 (1.1) | 0 (0.0) |
| Antibody treatment | 3 (3.3) | 5 (5.5) | 4 (4.3) | 4 (4.5) |
| Molnupiravir | 3 (3.3) | 2 (2.2) | 2 (2.2) | 3 (3.4) |
| Anticoagulants | 1 (1.1) | 0 (0.0) | 1 (1.1) | 0 (0.0) |
| Other medications | 0 (0.0) | 1 (1.1) | 0 (0.0) | 1 (1.1) |
| Use of supplements at baseline, n (%) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 1 (1.1) |
| Inpatients, n (%) | 73 (81.1) | 75 (82.4) | 73 (79.3) | 75 (84.3) |
| Number of days with symptoms, median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 2 (2–4) |
| Vaccination status at baseline, n (%) | ||||
| No vaccination | 16 (17.8) | 20 (22.0) | 16 (17.4) | 20 (22.5) |
| 1 dose | 12 (13.3) | 12 (13.2) | 13 (14.1) | 11 (12.4) |
| 2 or 3 doses | 44 (48.9) | 50 (55.0) | 47 (51.1) | 47 (52.8) |
| Missing | 18 (20.0) | 9 (9.9) | 16 (17.4) | 11 (12.4) |
| Days since last vaccination at baseline, n (%) available | 56 (62.2) | 62 (68.1) | 60 (65.2) | 58 (65.2) |
| Median (IQR) days since the last vaccination | 149.5 (63.5–231.5) | 120.5 (45–205) | 136 (50–216) | 120.5 (56–203) |
| >30 d since the last vaccine dose at baseline | 49 (87.5) | 52 (83.8) | 53 (88.3) | 48 (82.8) |
| Received vaccine dose during follow-up, n (%) | ||||
| Yes | 9 (12.5) | 13 (15.9) | 9 (11.8) | 13 (16.7) |
| No | 63 (67.5) | 69 (74.2) | 67 (70.8) | 65 (70.9) |
| Missing | 18 (20.0) | 9 (9.9) | 16 (17.4) | 11 (12.4) |
| Vaccine type, n (%) | ||||
| Covishield (AstraZeneca) | 60 (66.7) | 71 (78.2) | 64 (69.6) | 67 (75.3) |
| Covaxin (Bharat Biotech) | 9 (10.0) | 9 (9.9) | 9 (9.8) | 9 (10.1) |
| Missing | 21 (23.3) | 11 (12.1) | 19 (20.7) | 13 (14.6) |
| COVID-19 wave, n (%) | ||||
| Wave 2 | 58 (64.4) | 62 (68.1) | 59 (64.1) | 61 (68.5) |
| Wave 3 | 32 (35.6) | 29 (31.9) | 33 (35.9) | 28 (31.5) |
| Days between baseline and endline contact, n (%) available | 80 (88.9) | 84 (92.3) | 81 (88.0) | 83 (93.3) |
| Median (IQR) days between baseline and endline | 86.5 (69.5–135) | 97.5 (73.5–136.5) | 94 (71–137) | 94 (70–133) |
Other conditions: neurologic conditions, endocrine disorders, chronic renal disease, prior gestational diabetes mellitus, tuberculosis, cancer, chronic obstructive pulmonary disease, HIV, reproductive conditions, and alcohol use disorder. Other COVID-19 medications: ibuprofen+paracetamol.
No participants used tocilizumab or hydroxychloroquine as treatment for COVID-19.Wave 2 spanned from the beginning of the study (April 2021) to December 15th, 2021. Wave 3 spanned from December 16th until the end of enrolment (the first participant enrolled in Wave 3 was on December 21st, 2021).
BMI, body mass index; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; IQR, interquartile range; KEM, King Edward Memorial.
At baseline, 133 participants had fever, cough, or shortness of breath (TABLE 2, TABLE 3). The median (95% CI) time to resolution of all 3 symptoms among participants in the vitamin D and no vitamin D groups was 3 (2–5) d and 3 (2–4) d, with no evidence of effect of vitamin D supplementation compared with no supplementation on time to resolution (HR: 0.92; 95% CI: 0.66, 1.30; P = 0.650; adjusted HR: 1.09; 95% CI: 0.72, 1.65; P = 0.673) (Table 2; Supplemental Table 3). Similarly, the median (95% CI) time to resolution of all 3 symptoms in the zinc and no zinc groups was 3 (2–4) d and 3 (2–4) d, with no association between zinc supplementation and time to resolution (HR: 0.94; 95% CI: 0.67, 1.33; P = 0.745; adjusted HR: 0.91; 95% CI: 0.61, 1.34; P = 0.630) (Table 3; Supplemental Table 3). Neither vitamin D nor zinc supplementation affected duration or time to recovery from individual symptoms, duration of hospital stay among inpatients, and need for assisted ventilation (TABLE 2, TABLE 3; Supplemental Table 3). No deaths were recorded during follow-up, so the effect of supplementation on all-cause mortality could not be assessed.
TABLE 2.
Effect of vitamin D supplementation compared with no vitamin D on primary and secondary outcomes
| Vitamin D | No vitamin D | HR or risk ratio1 (95% CI) | P value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Fever, cough, or shortness of breath at baseline, n (%) | ||||
| No | 20 (22.2) | 27 (29.7) | ||
| Yes | 70 (77.8) | 63 (69.2) | ||
| Missing | 0 (0.0) | 1 (1.1) | ||
| Days to resolution, median (95% CI) (N = 132) | 3 (2-5) | 3 (2-4) | 0.92 (0.66, 1.30) | 0.650 |
| Secondary outcomes | ||||
| Fever at baseline, n (%) | ||||
| No | 45 (50.0) | 54 (59.3) | ||
| Yes | 45 (50.0) | 37 (40.7) | ||
| Days to resolution, median (95% CI) (N = 81) | 2 (2–2) | 2 (2–2) | 1.02 (0.65, 1.59) | 0.938 |
| Cough at baseline, n (%) | ||||
| No | 36 (40.0) | 35 (38.5) | ||
| Yes | 54 (60.0) | 56 (61.5) | ||
| Days to resolution, median (95% CI) (N = 109) | 3 (2–6) | 3 (2–4) | 0.88 (0.60, 1.28) | 0.498 |
| Shortness of breath at baseline, n (%) | ||||
| No | 77 (85.6) | 80 (87.9) | ||
| Yes | 13 (14.4) | 10 (11.0) | ||
| Missing | 0 (0.0) | 1 (1.1) | ||
| Days to resolution, median (95% CI) (N = 23) | 3 (2–5) | 2 (1–2) | 0.78 (0.33, 1.84) | 0.570 |
| Days to discharge, median (95% CI) (N = 147) | 6 (5–7) | 5 (4–6) | 0.82 (0.59, 1.14) | 0.236 |
| Need for assisted ventilation1 (N = 148), n (%) | ||||
| No | 70 (95.9) | 71 (94.7) | ||
| Yes | 2 (2.7) | 2 (2.7) | ||
| Missing | 1 (1.4) | 2 (2.7) | 1.00 (0.73, 1.38) | 0.998 |
Estimates based on univariable Cox or Poisson regression models.
P for the test of proportional hazards assumption (global test) >0.05 for all outcomes for which Cox regression models were performed.
Risk ratio calculated for the need for assisted ventilation; HR calculated for all other outcomes.
TABLE 3.
Effect of zinc supplementation compared with no zinc on primary and secondary outcomes
| Zinc | No zinc | HR or risk ratio1 (95% CI) | P value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Fever, cough, or shortness of breath at baseline, n (%) | ||||
| No | 25 (27.2) | 22 (24.7) | ||
| Yes | 66 (71.7) | 67 (75.3) | ||
| Missing | 1 (1.1) | 0 (0.0) | ||
| Days to resolution, median (95% CI) (N = 132) | 3 (2–4) | 3 (2–4) | 0.94 (0.67, 1.33) | 0.745 |
| Secondary outcomes | ||||
| Fever at baseline, n (%) | ||||
| No | 55 (59.8) | 44 (49.4) | ||
| Yes | 37 (40.2) | 45 (50.6) | ||
| Days to resolution, median (95% CI) (N = 81) | 2 (2–3) | 2 (1–2) | 0.89 (0.57, 1.40) | 0.616 |
| Cough at baseline, n (%) | ||||
| No | 38 (41.3) | 33 (37.1) | ||
| Yes | 54 (58.7) | 56 (62.9) | ||
| Days to resolution, median (95% CI) (N = 109) | 3 (2–5) | 3 (2–5) | 0.96 (0.66, 1.41) | 0.843 |
| Shortness of breath at baseline, n (%) | ||||
| No | 76 (82.6) | 81 (91.0) | ||
| Yes | 15 (16.3) | 8 (9.0) | ||
| Missing | 1 (1.1) | 0 (0.0) | ||
| Days to resolution, median (95% CI) (N = 23) | 2 (2–4) | 2 (1–.) | 0.61 (0.25, 1.52) | 0.291 |
| Days to discharge, median (95% CI) (N = 147) | 6 (5–7) | 6 (5–6) | 0.83 (0.59, 1.15) | 0.261 |
| Need for assisted ventilation1 (N = 148), n (%) | ||||
| No | 68 (93.2) | 73 (97.3) | ||
| Yes | 3 (4.1) | 1 (1.3) | ||
| Missing | 2 (2.7) | 1 (1.3) | 1.03 (0.75, 1.42) | 0.864 |
Estimates based on univariable Cox or Poisson regression models.
P for test of proportional hazards assumption (global test) >0.05 for all outcomes for which Cox regression models were performed.
Risk ratio calculated for need for assisted ventilation; HR calculated for all other outcomes.
Among blood biomarkers, we observed no clear effect of vitamin D or zinc supplementation apart from an increase in serum vitamin D concentrations with vitamin D supplementation [median (interquartile range)] difference between endline and baseline in vitamin D group: 5.3 ng/mL (–2.3 to 13.7); in no vitamin D group: –1.4 ng/mL [(–5.6 to 3.9); P = 0.003]. We also observed nonsignificant increases in serum zinc concentrations at endline among participants supplemented with zinc [median (IQR)] difference between endline and baseline in zinc group: 59.2 μg/dL (–60.0 to 161.3); in no zinc group: 4.3 μg/dL [(–81.9 to 146.0); P = 0.353] (Table 4). At baseline, 51.2% and 43.6% of participants in the vitamin D and no vitamin D groups had serum vitamin D <20 ng/mL, whereas, at endline, the corresponding proportions were 31.6% and 42.9% (P = 0.457 at endline), respectively. Additionally, 13.3% and 11.5% of participants in the zinc and no zinc groups had serum zinc <407 μg/dL, whereas corresponding endline proportions were 6.9% and 10.6% (P = 0.328 at endline), respectively (Supplemental Table 1).
TABLE 4.
Nutritional, inflammatory, and immunologic biomarkers at baseline and endline, and differences between baseline and endline, across vitamin D and zinc treatment supplementation groups
| Vitamin D |
No vitamin D |
P value | Zinc |
No zinc |
P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | |||
| Baseline | ||||||||||
| Nutritional biomarkers | ||||||||||
| Calcium, mg/dL | 88 | 8.8 (8.4–9.3) | 86 | 8.9 (8.3–9.3) | 88 | 8.8 (8.4–9.4) | 86 | 8.9 (8.4–9.2) | ||
| Zinc, ug/dL | 88 | 469.4 (422.3–574.7) | 89 | 522.9 (458.6–596.1) | 90 | 496.8 (441.9–594.9) | 87 | 492.7 (448.3–576.6) | ||
| Vitamin D, ng/mL | 88 | 18.2 (10.6–30.0) | 85 | 23.5 (11.4–29.3) | 87 | 23.1 (10.7–33.4) | 86 | 17.8 (11.0–27.6) | ||
| Inflammatory and immunologic biomarkers | ||||||||||
| CRP, mg/L | 86 | 12.7 (5.0–35.5) | 90 | 13.8 (5.4–32.0) | 92 | 13.1 (5.0–39.6) | 84 | 13.7 (5.8–32.7) | ||
| LDH, IU/L | 77 | 240.0 (200.0–304.7) | 77 | 249.0 (199.0–325.0) | 79 | 233.0 (188.6–319.7) | 75 | 249.0 (203.0–321.0) | ||
| D-dimer, ng/ml | 80 | 402.2 (300.5–577.0) | 88 | 451.0 (288.0–756.8) | 89 | 443.0 (321.3–645.8) | 79 | 381.8 (274.4–729.0) | ||
| Ferritin, ng/ml | 79 | 119.8 (54.3–305.0) | 81 | 164.0 (103.0–350.0) | 85 | 161.5 (61.0–346.8) | 75 | 150.8 (52.0–288.0) | ||
| IL-6, pg/ml | 89 | 5.0 (2.7–10.5) | 89 | 5.1 (3.0–10.1) | 91 | 5.3 (3.0–11.1) | 87 | 4.7 (2.3–9.5) | ||
| Ang-2, pg/ml | 88 | 1140.4 (850.6–1594.2) | 90 | 1233.3 (847.8–1714.1) | 90 | 1250.4 (871.8–1778.6) | 88 | 1135.6 (845.1–1522.1) | ||
| sTREM-1, pg/ml | 22 | 150.2 (70.2–174.7) | 26 | 143.5 (26.9–188.6) | 22 | 150.2 (84.6–174.7) | 26 | 137.6 (26.5–226.2) | ||
| IgG, U/mL | 88 | 5.3 (0.4–81.3) | 89 | 7.5 (1.0–94.0) | 90 | 5.6 (0.9–113.9) | 87 | 8.1 (0.4–46.2) | ||
| IgM, U/mL | 88 | 2.8 (1.0–8.2) | 89 | 3.1 (1.1–6.4) | 90 | 3.1 (1.1–7.1) | 87 | 2.9 (1.0–6.6) | ||
| Endline | ||||||||||
| Nutritional biomarkers | ||||||||||
| Calcium, mg/dL | 59 | 9.4 (9.2–9.7) | 65 | 9.5 (9.1–9.8) | 0.998 | 59 | 9.4 (9.2–9.7) | 65 | 9.5 (9.2–9.8) | 0.371 |
| Zinc, ug/dL | 58 | 533.0 (457.5–619.8) | 66 | 534.4 (476.9–611.2) | 0.754 | 58 | 552.1 (463.3–655.2) | 66 | 517.7 (457.6–595.8) | 0.117 |
| Vitamin D, ng/mL | 57 | 26.7 (16.5–32.2) | 63 | 22.0 (14.6–27.7) | 0.034 | 57 | 24.1 (16.1–29.0) | 63 | 22.4 (14.1–31.8) | 0.665 |
| Inflammatory and immunologic biomarkers | ||||||||||
| CRP, mg/L | 60 | 2.3 (0.9 to 5.0) | 66 | 2.2 (1.1 to 5.0) | 0.813 | 58 | 1.8 (0.8 to 5.0) | 68 | 2.4 (1.2 to 5.0) | 0.227 |
| LDH, IU/L | 59 | 194.0 (152.0 to 255.0) | 66 | 206.5 (170.8 to 248.0) | 0.405 | 57 | 203.0 (169.0 to 236.8) | 68 | 199.3 (162.1 to 257.0) | 0.909 |
| D-dimer, ng/ml | 55 | 260.7 (191.1 to 342.0) | 65 | 319.5 (225.3 to 402.2) | 0.042 | 54 | 283.5 (205.1 to 368.6) | 66 | 288.0 (205.1 to 383.1) | 0.918 |
| Ferritin, ng/ml | 57 | 41.2 (16.0 to 91.0) | 65 | 50.0 (22.0 to 110.0) | 0.567 | 57 | 58.9 (23.3 to 111.1) | 65 | 30.0 (15.3 to 107.0) | 0.095 |
| IL-6, pg/ml | 53 | 2.7 (0.9 to 5.1) | 61 | 2.6 (1.5 to 5.2) | 0.643 | 53 | 2.3 (1.1 to 4.3) | 61 | 3.2 (1.4 to 5.5) | 0.100 |
| Ang-2, pg/ml | 53 | 1165.0 (760.3 to 1519.8) | 61 | 1006.4 (763.0 to 1581.7) | 0.438 | 53 | 1132.2 (791.3 to 1695.3) | 61 | 992.8 (745.4 to 1478.9) | 0.347 |
| sTREM-1, pg/ml | 22 | 99.9 (80.3 to 129.4) | 26 | 125.4 (93.2 to 174.3) | 0.142 | 22 | 110.7 (83.9 to 129.4) | 26 | 113.3 (92.1 to 216.5) | 0.385 |
| IgG, U/mL | 52 | 30.4 (10.3 to 74.7) | 60 | 47.2 (15.4 to 100.2) | 0.357 | 52 | 47.2 (14.5 to 92.6) | 60 | 30.9 (10.0 to 86.3) | 0.510 |
| IgM, U/mL | 52 | 3.1 (1.5 to 6.7) | 60 | 2.7 (0.8 to 7.1) | 0.704 | 52 | 3.4 (1.5 to 7.3) | 60 | 2.5 (0.8 to 7.1) | 0.536 |
| Difference | ||||||||||
| Nutritional biomarkers | ||||||||||
| Calcium (mg/dL) | 58 | 0.5 (0.1 to 1.0) | 62 | 0.7 (0.1 to 1.1) | 0.533 | 58 | 0.6 (0.1 to 1.1) | 62 | 0.5 (0.1 to 1.1) | 0.852 |
| Zinc, ug/dL | 57 | 46.1 (–30.8 to 147.8) | 65 | –12.4 (–107.8 to 139.7) | 0.132 | 58 | 59.2 (–60.0 to 161.3) | 64 | 4.3 (–81.9 to 146.0) | 0.353 |
| Vitamin D, ng/mL | 56 | 5.3 (–2.3 to 13.7) | 60 | –1.4 (–5.6 to 3.9) | 0.003 | 56 | –0.6 (–4.9 to 7.9) | 60 | 1.4 (–4.6 to 11.4) | 0.315 |
| Inflammatory and immunologic biomarkers | ||||||||||
| CRP (mg/L) | 57 | –8.2 (–28.2 to –1.3) | 65 | –10.6 (–32.4 to –1.6) | 0.415 | 58 | –8.7 (–33.1 to –1.3) | 64 | –10.3 (–28.9 to –1.5) | 0.802 |
| LDH (IU/L) | 49 | –32.0 (–113.7 to 25.0) | 57 | –35.0 (–108.0 to 11.0) | 0.575 | 49 | –30.0 (–110.0 to 13.0) | 57 | –46.0 (–114.0 to 14.0) | 0.759 |
| D-dimer (ng/ml) | 49 | –106.9 (–386.9 to –35.5) | 63 | –90.4 (–377.1 to –8.8) | 0.790 | 53 | –106.9 (–377.1 to –35.5) | 59 | –89.6 (–418.3 to 3.1) | 0.652 |
| Ferritin (ng/ml) | 50 | –36.7 (–139.0 to –4.0) | 58 | –99.8 (–260.0 to –35.4) | 0.003 | 52 | –66.0 (–179.1 to –8.3) | 56 | –75.8 (–154.6 to –17.5) | 0.537 |
| IL-6 (pg/ml) | 53 | –1.4 (–6.0 to 0.1) | 59 | –0.9 (–4.7 to 0.5) | 0.859 | 53 | –1.8 (–6.0 to –0.2) | 59 | –0.8 (–4.7 to 0.8) | 0.246 |
| Ang-2 (pg/ml) | 52 | 80.4 (–347.3 to 360.7) | 60 | –17.9 (–534.3 to 307.2) | 0.342 | 52 | 127.2 (–206.0 to 435.1) | 60 | –54.9 (–557.8 to 250.7) | 0.046 |
| sTREM-1 (pg/ml) | 22 | –42.3 (–69.3 to 28.4) | 26 | –23.6 (–50.0 to 33.9) | 0.408 | 22 | –43.0 (–69.3 to –1.5) | 26 | –13.5 (–65.5 to 51.4) | 0.379 |
| IgG (U/mL) | 52 | 8.2 (–8.2 to 31.5) | 59 | 5.9 (–12.4 to 35.3) | 0.675 | 52 | 12.0 (–14.3 to 43.1) | 59 | 5.8 (–10.4 to 28.2) | 0.658 |
| IgM (U/mL) | 52 | 0.3 (–1.1 to 2.6) | 59 | 0.0 (–2.5 to 2.9) | 0.741 | 52 | 0.0 (–1.6 to 2.8) | 59 | 0.0 (–2.1 to 3.2) | 0.953 |
P values based on Wilcoxon rank sum tests.
Ang-2, angiopoietin-2; CRP, C-reactive protein; Ig, immunoglobulin; IL, interleukin; LDH, lactate dehydrogenase; sTREM-1, soluble triggering receptor expressed on myeloid cells-1.
No adverse events were observed in this study, and neither vitamin D nor zinc supplementation was associated with risk of hypercalcemia (Supplemental Table 2). In additional analyses, results remained unchanged when adjusting for site and other key characteristics (Supplementary Table 3, data not shown for biomarkers). Of participants with available information (N = 156), 127 (81.4%) consumed ≥90% of the supplements (Supplemental Table 4). Restricting analyses to these participants did not materially change results (Supplemental Tables 5–7). There was no evidence of interaction between vitamin D and zinc supplementation (Supplemental Tables 8 and 9, data not shown for change in blood biomarker concentrations between baseline and endline), and no evidence of interaction between either of the supplements and key characteristics including age, sex and BMI (data not shown). In post hoc sensitivity analyses, we observed no clear significant differences in the effect of vitamin D supplementation by baseline vitamin D status (<20 ng/mL compared with ≥20 ng/mL), although there was some indication of potential protective effects on time to discharge among those with baseline serum vitamin D <20 ng/mL (Supplemental Table 10).
Discussion
Given previous evidence surrounding the role of vitamin D and zinc in risk of and recovery from ARIs [[18], [19], [20]] and potential underlying mechanisms [[21], [22], [23], [24], [25]], it is plausible that supplementation with these micronutrients could improve COVID-19 outcomes. However, in this 2 x 2 factorial double-blind, randomly assigned placebo-controlled trial based in India, we observed no effect of vitamin D or zinc supplementation on time to resolution of key COVID-19 symptoms. Furthermore, neither vitamin D nor zinc supplementation affected other key parameters of treatment outcome, including need for assisted ventilation, duration of hospital stay among inpatients, and inflammatory and immune biomarkers. Our results do not support the use of vitamin D or zinc supplements as part of therapeutic regimens for COVID-19. However, our results are based on a small sample size and a specific population, and further experimental evidence to corroborate these findings would be valuable.
Previous experimental studies examining the effect of vitamin D supplementation in the context of COVID-19 have been mainly based in upper-middle-income or higher-income countries [28,34,[44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55]], with just 1 study identified here based on a South Asian population [44]. From the few studies examining risk of SARS-CoV-2 infection, evidence has been inconsistent regarding the potential benefit of vitamin D supplementation [[52], [53], [54], [55]]. Additionally, studies assessing the effect of vitamin D supplementation on individuals with COVID-19 have been mostly based among small samples (N < 300), with mixed evidence regarding impact [28,34,[44], [45], [46], [47], [48], [49], [50], [51],56,57]. Differences in the results of these studies may be because of factors including severity of COVID-19 at baseline, severity of vitamin D deficiency at baseline, and the dose and duration of supplementation. To note, studies identified here that indicated a positive impact of vitamin D supplementation on a range of clinical outcomes were based among participants with varying severity of COVID-19 [[44], [45], [46], [47], [48], [49]]. However, importantly, of those studies that indicated a positive effect of supplementation among individuals with COVID-19 [[44], [45], [46], [47], [48], [49]], most were based exclusively among participants with low vitamin D concentrations (<30 ng/mL), assessing the effect of daily supplementation (at least initially) with doses ranging from 5000–60,000 IU compared with placebo or lower dose supplementation, and duration ranging from 7 d to ≤6 wk [[44], [45], [46], [47], [48]]. One study was based among participants aged ≥65 y and found a protective effect of supplementation with a single 400,000 IU dose compared with a 50,000 IU dose of vitamin D3 on 14-d mortality [49].
Among studies identified here that did not restrict to COVID-19 patients with low baseline vitamin D concentrations or other high-risk characteristics [28,34,50,51,57], results regarding the impact of vitamin D supplementation were less definitive. This included 3 studies among inpatients with mild, moderate, and severe COVID-19, which found no effect of a single high dose of vitamin D3 (100,000–500,000 IU) compared with placebo or no supplementation on clinical outcomes including duration of hospital stay, risk of intensive care unit admission, risk of mortality [28,34,50,57], and multiple biomarkers including IL-6 [50]. Additionally, 2 small studies (N ≤ 76) reported weak to no evidence of effect of ≥7 and ≤14 d supplementation with calcifediol and calcitriol on similar clinical outcomes, respectively [51,56]. Data from our study are in line with this evidence, suggesting that vitamin D supplementation in a COVID-19 patient population with vitamin D deficiency prevalence of about 50% and a range of underlying risk characteristics does not improve outcomes. However, exact comparison of results is limited, given the differences in study population characteristics and regimens.
Evidence from previous trials regarding the effect of zinc on COVID-19 risk and outcomes is more limited [27,36,37,[58], [59], [60], [61]]. Although all studies identified here examined daily or twice-daily supplementation, they differed with respect to the dose (range: 15–80 mg) and duration (range: 5 d to 6 wk) of zinc administered and outcomes studied [27,36,37,[58], [59], [60], [61]]. None of the identified studies exclusively enrolled populations with low baseline zinc concentrations [27,36,37,[58], [59], [60], [61]]. Among studies examining the effect of zinc supplementation on risk of COVID-19, there was no clear evidence of impact [37,58], in line with previous evidence on zinc supplementation to prevent ARIs [19]. In studies assessing zinc administered as part of therapeutic regimens for COVID-19, all based on relatively small sample sizes, evidence was inconsistent [27,36,[59], [60], [61]]. One United States-based placebo-controlled factorial trial suggested no effect of daily supplementation with 50 mg zinc for 10 d, with or without ascorbic acid, on clinical outcomes, including median days to reach 50% reduction in fever, cough, shortness of breath, and fatigue; hospitalization; and death [36]. Another Egypt-based trial suggested no effect of adding zinc (50 mg daily for 5 d) to a treatment regimen with hydroxychloroquine on recovery within 28 d, need for mechanical ventilation, or mortality [61]. On the contrary, a study based among inpatients and outpatients with COVID-19 in Tunisia indicated protective effects of 15-d supplementation with 25 mg zinc compared with placebo on mortality, intensive care unit admission, duration of symptoms, and length of hospital stay [27]. Additionally, a cluster-randomized trial based in Singapore indicated increased IgG concentrations and conversion to neutralizing antibody positivity among men supplemented with zinc (80 mg) and vitamin C daily for 6 wk compared with those supplemented with vitamin C only [59]. To our knowledge, ours is the first study based in South Asia, adding to this evidence base on zinc supplementation within the context of COVID-19.
An important consideration that may explain our results is the small sample size of the study. We were unable to reach the target of 700 participants because of a substantial decline in case numbers as the study began enrolment – ultimately enrolling just about 25% of this target. This may, to a certain extent, explain the lack of effect observed in this study. However, we note that small sample size was a limitation common to multiple other identified previous studies above [28,36] – some of which did observe positive effects of vitamin D and zinc supplementation on COVID-19 outcomes [27,[44], [45], [46], [47], [48], [49],59,60]. Additionally, these studies were based on participants with a range of disease severities, including those with milder symptoms, similar to our study population [27,[44], [45], [46], [47], [48], [49],59,60]. Furthermore, at endline, most participants in the current study had consumed most of the supplements, with resultant increases in serum vitamin D and zinc in the supplemented groups. This indicates that other factors may also at least partly explain the absence of effect observed in the current study, including the baseline concentrations of vitamin D and zinc deficiency in our study population and the dose and duration of supplementation. As noted earlier, most studies suggesting an effect of vitamin D supplementation were based specifically on participants with low baseline vitamin D concentrations and provided higher daily doses of vitamin D to participants than in the current study, although for shorter periods of time and with no initial bolus dose [[44], [45], [46], [47], [48]]. On the contrary, the 2 studies indicating some positive effects of zinc supplementation were not limited to participants with baseline zinc deficiency and examined shorter durations of supplementation with doses that were both higher and lower than in this study [27,59]. In our post hoc analyses stratifying by baseline vitamin D concentrations, we generally found no clear evidence of greater impact of vitamin D supplementation among those with low (<20 ng/mL) vitamin D status – although notably, we were extremely limited by small sample sizes. Additional large-scale studies would be valuable to explore with greater statistical resolution the possible influence of these factors, particularly vitamin D and zinc deficiency at baseline, on the impact of supplementation.
Important strengths of this study include its randomly assigned, double-blind design, limiting potential confounding, and minimizing bias because of the knowledge of supplement administered. Additionally, the factorial design of the study enabled the efficient examination of 2 potentially valuable interventions for COVID-19. To our knowledge, this is one of the first studies examining the effect of vitamin D and zinc supplementation among individuals with COVID-19 in India and, indeed, South Asia. This is key, given the dearth of population-specific evidence to inform treatment recommendations in this region. We measured a comprehensive set of clinical and other indices, including key inflammatory and immunological biomarkers, with regular follow-up of participants. Although there were some participant withdrawals and losses to follow up by endline, most occurred after the median duration of symptom resolution, allowing us to assess the primary outcome for most individuals with these data. Key limitations include the small sample size of the study, with resultant decreased statistical power to detect associations, as discussed above. We also did not account for possible differences in COVID-19 treatments across groups during the study. On average, endline measurements occurred later than the targeted 8-wk point and later than the median observed resolution of symptoms, which may have limited our ability to capture important effects of supplementation on relevant biomarkers. Importantly, adjustment for length of time between baseline and endline did not substantially change estimates of effect for biomarker outcomes, suggesting that inferences regarding the effect of supplementation at around 8 wk were robust.
In all, in this study, we observed no effect of vitamin D and zinc supplementation on time to resolution of key symptoms and other clinical and related outcomes among individuals with COVID-19 in India. The results of the current study do not suggest that these supplements would be beneficial to improve outcomes in this and similar populations. However, given the small size and specific population of this study, larger-scale evidence, particularly based on populations with specific baseline characteristics such as baseline vitamin D or zinc deficiency, is required to corroborate these findings.
In summary, in a randomly assigned factorial trial in a small population in India, neither vitamin D nor zinc supplementation appeared to improve COVID-19 treatment outcomes. Large-scale data are needed to corroborate findings.
Funding
This trial is supported by the Canadian Institutes of Health Research, Operating Grant: COVID-19 Rapid Research Funding Opportunity – Therapeutics, application number: 447092, and the Canada Research Chair program (to KCK). SB was supported by the National Institutes of Health (grant D43 TW010543). The funding bodies had no role in study design and procedures or the decision to submit manuscripts for publication.
Author contributions
The authors’ responsibilities were as follows – WWF, KCK, YD, NM: conceptualized the project and designed the study along with SB, KKS, ECH, YM, and UP; YD, NM, PDC, GG, KKS, YM, SS: were involved in data acquisition, and study monitoring along with KCK, WWF, UP; MW: provided statistical expertise; UP: analyzed the data and drafted the manuscript, and all authors: read and approved the final manuscript.
Conflict of Interest
The authors report no conflicts of interest.
Data availability
Data from this study are currently not available to be shared due to regulatory restrictions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cdnut.2023.101971.
Contributor Information
Uttara Partap, Email: upartap@hsph.harvard.edu.
Wafaie W. Fawzi, Email: mina@hsph.harvard.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Menni C., Valdes A.M., Polidori L., Antonelli M., Penamakuri S., Nogal A., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar N., Shahul Hameed S.K., Babu G.R., Venkataswamy M.M., Dinesh P., Kumar Bg P., et al. Descriptive epidemiology of SARS-CoV-2 infection in Karnataka state, South India: transmission dynamics of symptomatic vs. asymptomatic infections. EClinicalmedicine. 2021;32 doi: 10.1016/j.eclinm.2020.100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; Geneva: Sep 16 2022. Therapeutics and COVID-19: living guideline. [PubMed] [Google Scholar]
- 4.WHO . 2022. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Internet] Available from: [Google Scholar]
- 5.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. COVID-19 weekly epidemiological update: Edition 129. Geneva: World Health Organization; Published Feb 8, 2023.
- 7.Uraki R., Ito M., Furusawa Y., Yamayoshi S., Iwatsuki-Horimoto K., Adachi E., et al. Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB, Lancet. Infect. Dis. 2023;23(1):30–32. doi: 10.1016/S1473-3099(22)00816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai M., Ito M., Kiso M., Yamayoshi S., Uraki R., Fukushi S., et al. Efficacy of antiviral agents against omicron subvariants BQ.1.1 and XBB. N. Engl. J. Med. 2023;388(1):89–91. doi: 10.1056/NEJMc2214302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurhade C., Zou J., Xia H., Liu M., Chang H.C., Ren P., et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med. 2023;29(2):344–347. doi: 10.1038/s41591-022-02162-x. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian A., Nirantharakumar K., Hughes S., Myles P., Williams T., Gokhale K.M., et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022;28(8):1706–1714. doi: 10.1038/s41591-022-01909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y., Ma N., Witt C., Rapp S., Wild P.S., Andreae M.O., et al. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science. 2021;372(6549):1439–1443. doi: 10.1126/science.abg6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . World Health Organization; 2022. Advice for the public on COVID-19.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public [Internet] Available from: [Google Scholar]
- 13.WHO . 2022. COVID-19 vaccines advice.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice [Internet] Available from: [Google Scholar]
- 14.Rannan-Eliya R.P., Wijemunige N., Gunawardana J.R.N.A., Amarasinghe S.N., Sivagnanam I., Fonseka S., et al. Increased intensity of PCR testing reduced COVID-19 transmission within countries during the first pandemic wave. Health Aff (Millwood). 2021;40(1):70–81. doi: 10.1377/hlthaff.2020.01409. [DOI] [PubMed] [Google Scholar]
- 15.Maxwell D., Sanders K.C., Sabot O., Hachem A., Llanos-Cuentas A., Olotu A., et al. COVID-19 therapeutics for low- and middle-income countries: a review of candidate agents with potential for near-term use and impact. Am. J. Trop. Med. Hyg. 2021;105(3):584–595. doi: 10.4269/ajtmh.21-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batista C., Hotez P., Amor Y.B., Kim J.H., Kaslow D., Lall B., et al. The silent and dangerous inequity around access to COVID-19 testing: A call to action. EClinicalmedicine. 2022;43 doi: 10.1016/j.eclinm.2021.101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boro E., Stoll B. Barriers to COVID-19 health products in low-and middle-income countries during the COVID-19 pandemic: A rapid systematic review and evidence synthesis. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.928065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolliffe D.A., Camargo C.A., Sluyter J.D., Aglipay M., Aloia J.F., Ganmaa D., et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276–292. doi: 10.1016/S2213-8587(21)00051-6. [DOI] [PubMed] [Google Scholar]
- 19.Abioye A.I., Bromage S., Fawzi W. Effect of micronutrient supplements on influenza and other respiratory tract infections among adults: a systematic review and meta-analysis. BMJ Glob. Health. 2021;6(1) doi: 10.1136/bmjgh-2020-003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemilä H., Fitzgerald J.T., Petrus E.J., Prasad A. Zinc acetate lozenges may improve the recovery rate of common cold patients: an individual patient data meta-analysis. Open Forum Infect. Dis. 2017;4(2):ofx059. doi: 10.1093/ofid/ofx059. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7(6):4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telcian A.G., Zdrenghea M.T., Edwards M.R., Laza-Stanca V., Mallia P., Johnston S.L., et al. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res. 2017;137:93–101. doi: 10.1016/j.antiviral.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Greiller C.L., Suri R., Jolliffe D.A., Kebadze T., Hirsman A.G., Griffiths C.J., et al. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid. Biochem. Mol. Biol. 2019;187:152–159. doi: 10.1016/j.jsbmb.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., et al. Zinc and respiratory tract infections: perspectives for COVID-19. Int. J. Mol. Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and Arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell. Biol. 2022;23(1):3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben Abdallah S., Mhalla Y., Trabelsi I., Sekma A., Youssef R., Bel Haj Ali K., et al. Twice-daily oral zinc in the treatment of patients with coronavirus disease 2019: A randomized double-blind controlled trial. Clin. Infect. Dis. 2023;76(2):185–191. doi: 10.1093/cid/ciac807. [DOI] [PubMed] [Google Scholar]
- 28.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA. 2021;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra P., Srivastava R., Misra A., Kant S., Kardam P., Vikram N.K. Vitamin D status of adult females residing in Ballabgarh health and demographic surveillance system: A community-based study. Indian J. Public Health. 2017;61(3):194–198. doi: 10.4103/ijph.IJPH_176_16. [DOI] [PubMed] [Google Scholar]
- 30.Institute for Health Metrics and Evaluation . 2014. GBD compare.https://www.healthdata.org/data-visualization/gbd-compare [Internet] Available from: [Google Scholar]
- 31.Sharma K.K., Partap U., Mistry N., Marathe Y., Wang M., Shaikh S., et al. Randomised trial to determine the effect of vitamin D and zinc supplementation for improving treatment outcomes among patients with COVID-19 in India: trial protocol. BMJ Open. 2022;12(8) doi: 10.1136/bmjopen-2022-061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Y., Stallmann-Jorgensen I.S., Pollock N.K., Harris R.A., Keeton D., Huang Y., et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J. Clin. Endocrinol. Metab. 2010;95(10):4584–4591. doi: 10.1210/jc.2010-0606. [DOI] [PubMed] [Google Scholar]
- 33.Kearns M.D., Alvarez J.A., Tangpricha V. Large. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr. Pract. 2014;20(4):341–351. doi: 10.4158/EP13265.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mariani J., Antonietti L., Tajer C., Ferder L., Inserra F., Sanchez Cunto M., et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: multicentre randomized controlled clinical trial. PLoS ONE. 2022;17(5) doi: 10.1371/journal.pone.0267918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Institute of Medicine (US) Panel on Micronutrients . National Academies Press. US; Washington, (DC): 2001. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc [Internet]http://www.ncbi.nlm.nih.gov/books/NBK222310/ Available from: [PubMed] [Google Scholar]
- 36.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seet R.C.S., Quek A.M.L., Ooi D.S.Q., Sengupta S., Lakshminarasappa S.R., Koo C.Y., et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial. Int. J. Infect. Dis. 2021;106:314–322. doi: 10.1016/j.ijid.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ODK. Collect Data Anywhere [Internet]. Accessed December 17, 2021. Available from: https://getodk.org.
- 39.AIIMS/ICMR National Task Force/Joint Monitoring Group. Clinical guidelines for management of adult COVID-19 patients: revised on 14/01/2022 [Internet]. New Delhi: Ministry of Health and Family Welfare, Government of India. Accessed February 28, 2022. Available from: https://www.icmr.gov.in/pdf/covid/techdoc/archive/COVID_Clinical_Management_14012022.pdf
- 40.MOHFW, GOI . Ministry of Health and Family Welfare, Government of India; New Delhi: 2021 May. Clinical management protocol for COVID-19 (in adults). Version 6.https://www.mohfw.gov.in/pdf/UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf 24.05.21 [Internet] Available from: [Google Scholar]
- 41.MOHFW, GOI, Revised Discharge Policy for COVID-19: updated on 9th January 2022 [internet]. New Delhi: Ministry of Health and Family Welfare, Government of India. Accessed February 28, 2022. Available from: https://www.mohfw.gov.in/pdf/RevisedDischargePolicyforCOVID19updatedon9thJanuary2022.pdf.
- 42.Laxminarayan R., Mohan B.C., Vinay T.G., Kumar K.V.A., Wahl B., Lewnard J.A. SARS-CoV-2 infection and mortality during the first epidemic wave in Madurai, south India: a prospective, active surveillance study. Lancet. Infect. Dis. 2021;21(12):1665–1676. doi: 10.1016/S1473-3099(21)00393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tóth K., Fresilli S., Paoli N., Maiucci G., Salvioni M., Kotani Y., et al. D-dimer levels in non-COVID-19 ARDS and COVID-19 ARDS patients: A systematic review with meta-analysis. PLoS ONE. 2023;18(2) doi: 10.1371/journal.pone.0277000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rastogi A., Bhansali A., Khare N., Suri V., Yaddanapudi N., Sachdeva N., et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad. Med. J. 2022;98(1156):87–90. doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 45.Torres M., Casado G., Vigón L., Rodríguez-Mora S., Mateos E., Ramos-Martín F., et al. Changes in the immune response against SARS-CoV-2 in individuals with severe COVID-19 treated with high dose of vitamin D, Biomed. Pharmacother. 2022;150 doi: 10.1016/j.biopha.2022.112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabico S., Enani M.A., Sheshah E., Aljohani N.J., Aldisi D.A., Alotaibi N.H., et al. Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients. 2021;13(7) doi: 10.3390/nu13072170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maghbooli Z., Sahraian M.A., Jamalimoghadamsiahkali S., Asadi A., Zarei A., Zendehdel A., et al. Treatment with 25-hydroxyvitamin D3 (calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in hospitalized patients with COVID-19: A pilot multicenter, randomized, placebo-controlled, double-blinded clinical trial. Endocr. Pract. 2021;27(12):1242–1251. doi: 10.1016/j.eprac.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Niet S., Trémège M., Coffiner M., Rousseau A.F., Calmes D., Frix A.N., et al. Positive effects of vitamin D supplementation in patients hospitalized for COVID-19: A randomized, double-blind, placebo-controlled trial. Nutrients. 2022;14(15) doi: 10.3390/nu14153048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annweiler C., Beaudenon M., Gautier J., Simon R., Dubée V., Gonsard J., et al. Covid-19 and high-dose vitamin D supplementation TRIAL in high-risk older patients (COVIT-TRIAL): study protocol for a randomized controlled trial. Trials. 2020;21(1):1031. doi: 10.1186/s13063-020-04928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandes A.L., Murai I.H., Reis B.Z., Sales L.P., Santos M.D., Pinto A.J., et al. Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19. Am. J. Clin. Nutr. 2022;115(3):790–798. doi: 10.1093/ajcn/nqab426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elamir Y.M., Amir H., Lim S., Rana Y.P., Lopez C.G., Feliciano N.V., et al. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone. 2022;154 doi: 10.1016/j.bone.2021.116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villasis-Keever M.A., López-Alarcón M.G., Miranda-Novales G., Zurita-Cruz J.N., Barrada-Vázquez A.S., González-Ibarra J., et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. A randomized clinical trial. Arch. Med. Res. 2022;53(4):423–430. doi: 10.1016/j.arcmed.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jolliffe D.A., Holt H., Greenig M., Talaei M., Perdek N., Pfeffer P., et al. Effect of a test-and-treat approach to vitamin D supplementation on risk of all cause acute respiratory tract infection and Covid-19: phase 3 randomised controlled trial (CORONAVIT) BMJ. 2022;378 doi: 10.1136/bmj-2022-071230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunvoll S.H., Nygaard A.B., Ellingjord-Dale M., Holland P., Istre M.S., Kalleberg K.T., et al. Prevention of Covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trial. BMJ. 2022;378 doi: 10.1136/bmj-2022-071245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karonova T.L., Chernikova A.T., Golovatyuk K.A., Bykova E.S., Grant W.B., Kalinina OV O.V., et al. Vitamin D intake may reduce SARS-CoV-2 infection morbidity in health care workers. Nutrients. 2022;14(3) doi: 10.3390/nu14030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcalá Díaz J.F., López Miranda J., Bouillon R., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid. Biochem. Mol. Biol. 2020;203 doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannata-Andía J.B., Díaz-Sottolano A., Fernández P., Palomo-Antequera C., Herrero-Puente P., Mouzo R., et al. A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D-a randomised multicentre international clinical trial. BMC Med. 2022;20(1):83. doi: 10.1186/s12916-022-02290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stambouli N., Driss A., Gargouri F., Bahrini K., Arfaoui B., Abid R., et al. COVID-19 prophylaxis with doxycycline and zinc in health care workers: a prospective, randomized, double-blind clinical trial. Int. J. Infect. Dis. 2022;122:553–558. doi: 10.1016/j.ijid.2022.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quek A.M.L., Ooi D.S.Q., Teng O., Chan C.Y., Ng G.J.L., Ng M.Y., et al. Zinc and vitamin C intake increases spike and neutralising antibody production following SARS-CoV-2 infection. Clin. Transl. Med. 2022;12(2) doi: 10.1002/ctm2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdelmaksoud A.A., Ghweil A.A., Hassan M.H., Rashad A., Khodeary A., Aref Z.F., et al. Olfactory disturbances as presenting manifestation among Egyptian patients with COVID-19: possible role of zinc. Biol. Trace. Elem. Res. 2021;199(11):4101–4108. doi: 10.1007/s12011-020-02546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abd-Elsalam S., Soliman S., Esmail E.S., Khalaf M., Mostafa E.F., Medhat M.A., et al. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: a randomized, multicenter trial. Biol. Trace. Elem. Res. 2021;199(10):3642–3646. doi: 10.1007/s12011-020-02512-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are currently not available to be shared due to regulatory restrictions.