Abstract
Purpose:
The knowledge used to classify genetic variants is continually evolving, and classification can change based on newly available data. Although up-to-date variant classification is essential for clinical management, reproductive planning, and identifying at-risk family members, there is no consistent practice across laboratories or clinicians on how or under what circumstances to perform variant reinterpretation.
Methods:
We conducted exploratory focus groups (n=142) and surveys (n=1753) with stakeholders involved in the process of variant reinterpretation (laboratory directors, clinical geneticists, genetic counselors, non-genetic providers, and patients/parents) to assess opinions on key issues, including initiation of reinterpretation, variants to report, termination of the responsibility to reinterpret, and concerns about consent, cost, and liability.
Results:
Stakeholders widely agreed that there should be no fixed termination point to the responsibility to reinterpret a previously reported genetic variant. There were significant concerns about liability and lack of agreement about many logistical aspects of variant reinterpretation.
Conclusion:
Our findings suggest a need to (a) develop consensus and (b) create transparency and awareness about the roles and responsibilities of parties involved in variant reinterpretation. These data provide a foundation for developing guidelines on variant reinterpretation which can aid in the development of a low-cost, scalable, and accessible approach.
Introduction
As the cost of genomic sequencing decreases and insurance coverage for patients increases, the volume of clinical genetic testing is growing across indications (1). This has heightened the need for up-to-date interpretation of genetic variants for patients across all ancestral groups. In 2015, the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) issued guidelines to standardize the classification of genetic variants into one of five categories based on their likelihood to cause disease (2). However, because the knowledge used to classify genetic variants is continually evolving, their classification can change over time. This is especially true for variants of uncertain significance (VUS), which lack sufficient evidence to be classified as disease causing or not. Challenges in variant interpretation and VUS disproportionately affect individuals not of European ancestry, largely due to underrepresentation in genomic research and genomic reference databases (3). The higher frequency of VUS in these populations makes results of genetic testing less informative which adds to, rather than reduces, health care inequalities (4–6).
Up-to-date variant interpretation is important to ensure appropriate clinical management, accurately stratify risk for family members, and provide information for reproductive planning. Reclassification of a genetic variant can impact medical decisions for patients and their family, especially regarding risk-reducing procedures/medications and disease screening. Therefore, it is disappointing that surveys of providers in the United States (U.S.), United Kingdom, and Europe have shown significant variability in practice, with reinterpretation and recontact often occurring on an ad hoc basis (7–10). Although the ACMG has published a points-to-consider document on variant reinterpretation, it is primarily to help laboratories develop standard protocols (11). Some have argued there is an ethical duty for reinterpretation and recontact of patients to update genetic results (12), although currently no legal duty to do so exists (13,14). Commonly cited barriers to reinterpretation include a lack of resources (financial, time and infrastructure), concerns about the impact on patients, and variability in patient consent (7,8,15,16).
The topic of variant reinterpretation is complex, highlighting the importance of engaging professionals and patients to provide guidance (15,17). To address this need, we solicited the opinions of key stakeholders about issues related to variant reinterpretation, including whether there should be a responsibility to initiate reinterpretation and, if so, on whom it should fall; the duration of that responsibility; which reinterpretations should be reported and to whom; if and when to obtain patient consent for reinterpretation; and how costs of reinterpretation and recontact should be covered. In this study, variant reinterpretation is defined as variant-level reevaluation which involves reviewing a previously reported genetic variant to determine if there is new information that could lead to a potential reclassification (11).
Methods
To provide an empirical foundation for developing guidance regarding variant reinterpretation, we conducted focus groups and surveys with laboratory directors, clinical geneticists, genetic counselors, non-genetic providers, and adult patients and parents of minor patients.
Focus Groups
Details about recruitment of focus group participants is in the supplemental materials. Sixteen focus groups, each with 6-10 participants, were conducted with geneticists and clinical genetic counselors (2); laboratory directors and genetic counselors (2); non-genetic providers (4); parents of minor patients (5); and patients (3). The goal of the focus groups was to identify key themes related to variant reinterpretation to address in the surveys.
Surveys
Patients/parents were identified from three clinical sites (Columbia University, University of Michigan, and Vanderbilt University). All participants had genetic testing for themselves or their children within the past five years. The parent groups comprised parents whose children were seven years old or younger at the time of testing, with a VUS identified on a molecular panel or exome sequencing (ES), or who had non-diagnostic ES. The patient groups comprised adults who had a VUS identified on a molecular panel. Adults with ES were excluded as intellectual disability is a common indication for adult patients who receive ES.
Non-genetic providers were recruited through a Qualtrics market research panel of U.S. cardiologists, oncologists, and neurologists active in clinical care.
Clinical genetic providers included clinical geneticists and clinical genetic counselors who reported ordering 10 or more genetic tests over the past year. Geneticists were recruited via email to members of the ACMG whose information was listed in the Membership Directory on the ACMG website. Clinical genetic counselors were recruited via email through the Accreditation Council for Genetic Counseling (ACGC) listserv.
Laboratory genetic providers included laboratory directors and laboratory genetic counselors. Laboratory directors were recruited from the list of laboratories in the U.S. conducting molecular testing in the Genetic Testing Registry (GTR). Laboratory genetic counselors were recruited via email through the ACGC listserv.
The survey was developed by a working group of three medical geneticists; a physician-ethicist; three genetic counselors; and one medical sociologist. Survey topics were based on the results of the focus groups, as well as input from the study’s economics, legal, and ethics working groups and expert advisory panel. The survey differed slightly for each stakeholder group, but all versions contained a common core set of questions (Supplemental Appendix 1).
Survey pre-testing was conducted using facilitated brainstorming with a sample of 17 individuals including members of each group. Qualtrics, which hosted the survey, was also engaged to provide further feedback following which surveys were refined to improve clarity and focus. Participants received a $25 gift card.
Data analyses were conducted using SAS software, version 9.4 (18). Respondents were collapsed into four groups: patients/parents, non-genetic providers, clinical genetic providers (geneticists and clinical genetic counselors), and laboratory genetic providers (laboratory directors and genetic counselors). Data from the six groups are in supplementary materials. Chi-squared tests were used to explore differences in the frequency of responses across groups. For questions with more than two responses, one response was selected, usually the most frequent, and the frequency of this response was compared to all other responses to create a 4x2 analysis. When the full comparison demonstrated significance, pairwise comparisons were undertaken. A significance level of <.0001 was selected to partially correct for multiple testing. No statistical analysis was done for “select all that apply” questions.
Results
Focus Groups Responses
There were 142 participants in the focus groups: 72 patients/parents, 32 non-genetic providers, 18 laboratory genetic providers, and 20 clinical genetics providers. Themes that emerged from the focus groups are summarized in Table S1 and informed the development of the survey.
Survey Responses
Demographics
A total of 1753 surveys were completed. Recruitment was capped for non-genetic providers (n=300), clinical genetic counselors (n=496), and laboratory genetic counselors (n=128), and therefore response rates could not be determined. Response rates were 46% for patients/parents (n=651), 30% for geneticists (n=118), and 16% for laboratory directors (n=60). For analysis, respondents were categorized as patients/parents (n=651), non-genetic providers (n=300), clinical genetic providers (n=614), and laboratory genetic providers (n=188).
Detailed demographic data are shown in Table 1. Further details are in Tables S2, S3, and S4. Most geneticists practice in general genetics or pediatrics, while most clinical genetic counselors practice in oncology. Most laboratory directors work in a non-profit hospital laboratory, while most laboratory genetic counselors work in a for-profit laboratory. Non-genetic providers were equally split among oncology, neurology, and cardiology. Most patients and parents had at least a college degree and private health insurance.
Table 1.
Survey sample demographics by stakeholder group.
| Patient/Parent (n=651) n (%) |
Non-Genetic Provider (n=300) n (%) |
Clinical Genetic Provider (n=614) n (%) |
Laboratory Genetic Provider (n=188) n (%) |
||
|---|---|---|---|---|---|
| Gender | Female | 592 (91%) | 57 (19%) | 525 (86%) | 151 (80%) |
| Male | 52 (8%) | 232 (77%) | 84 (14%) | 31 (16%) | |
| Non Binary | 2 (0%) | 0 (0%) | 0 (0%) | 2 (1%) | |
| Prefer not to answer | 5 (1%) | 11 (4%) | 5 (1%) | 4 (2%) | |
|
| |||||
| Age | 18-24 | 18 (3%) | 0 (0%) | 8 (1%) | 0 (0%) |
| 25-34 | 136 (21%) | 16 (5%) | 282 (46%) | 59 (31%) | |
| 35-44 | 239 (37%) | 70 (23%) | 148 (24%) | 68 (36%) | |
| 45-54 | 105 (16%) | 84 (28%) | 88 (14%) | 35 (19%) | |
| 55-64 | 90 (14%) | 87 (29%) | 60 (10%) | 14 (7%) | |
| 65-74 | 56 (9%) | 41 (14%) | 23 (4%) | 11 (6%) | |
| 75+ | 7 (1%) | 2 (1%) | 5 (1%) | 1 (1%) | |
|
| |||||
| Race | American Indian or Alaska Native | 1 (0%) | 2 (1%) | 0 (0%) | 1 (1%) |
| Asian | 24 (4%) | 72 (24%) | 36 (6%) | 23 (12%) | |
| Black, African American, or African | 42 (6%) | 3 (1%) | 5 (1%) | 0 (0%) | |
| Hispanic, Latino, or Spanish | 37 (6%) | 13 (4%) | 6 (1%) | 5 (3%) | |
| Middle Eastern or North African | 5 (1%) | 16 (5%) | 9 (1%) | 3 (2%) | |
| Native Hawaiian or other Pacific Islander | 0 (0%) | 1 (0%) | 0 (0%) | 0 (0%) | |
| White | 497 (76%) | 156 (52%) | 534 (87%) | 142 (76%) | |
| More than one | 25 (4%) | 3 (1%) | 13 (2%) | 6 (3%) | |
| None of the above | 2 (0%) | 4 (1%) | 1 (0%) | 0 (0%) | |
| Prefer not to answer | 18 (3%) | 30 (10%) | 10 (2%) | 8 (4%) | |
Initiation of Reinterpretation
There was no clear consensus across stakeholders regarding who should initiate the process of reinterpretation (Figure 1, 4x2 chi-square p-value <.0001 and S1). The laboratory was most often selected by the clinical (59%) and laboratory (39%) genetic providers. The laboratory was a less common choice among non-genetic providers (24%) and parents/patients (11%) who more frequently chose the ordering provider (34% and 33% respectively) or specialist (36% and 35% respectively). Across all groups, patient/parent and primary care provider were rarely selected.
Figure 1. Initiation of reinterpretation.
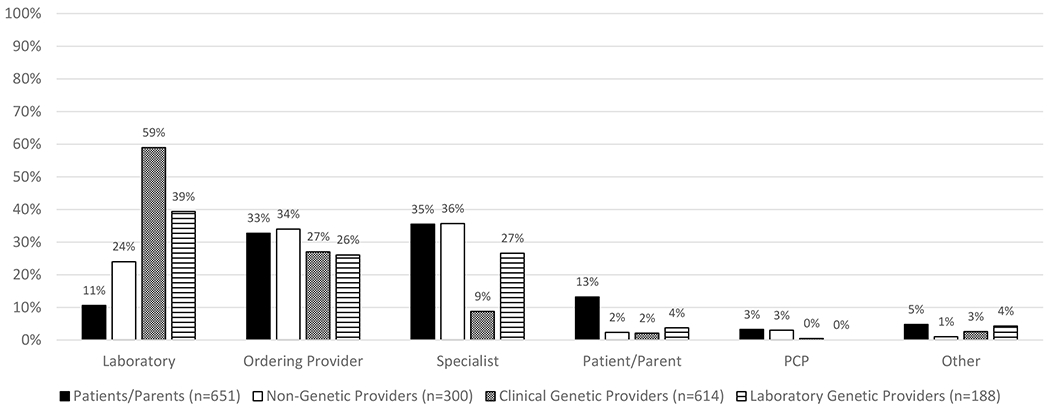
Responses to a multiple-choice question, “Who should initiate reinterpretation? Who should be the primary party to decide that reinterpretation should be done to see if there is new information that may change the results of the initial genetic testing?” Comparison of the choice of laboratory to all other options showed an overall p-value of <.0001 (chi-square analysis). Clinical and laboratory genetic providers selected laboratory more frequently than the other groups (59% and 39% respectively, chi-square analysis, pairwise comparison with laboratory as the reference compared to each other and to patients/parents, p-value<.0001). The choice of laboratory was less common among non-genetic providers (24%, chi-square analysis, pairwise comparison with laboratory as the reference for non-genetic providers compared to clinical genetic providers and to patients/parents, p-value <.0001) and patients/parents (11%).
Write-in responses (n=58) reflected a desire for the process of initiating reinterpretation to involve multiple parties or for a clinical genetics provider to initiate the process.
Triggering Events
Participants were asked what should trigger the process of reinterpretation and could select multiple responses (Figure S2). The most frequently selected responses among clinical and laboratory genetic providers was “a certain time interval since the previous interpretation” (94% and 90% respectively), followed by “clinician request due to a new phenotype” (87% and 86% respectively) or “clinician request due to a time interval that has passed” (82% and 83% respectively), whereas “availability of new treatment options based on the specific gene” was selected least (56% and 39% respectively).
Write-in responses (n=31) included requests by the patient/parent, familial or variant segregation studies that may impact interpretation, and technological updates in methods for sequence data analysis.
What Should Be Reinterpreted and Reported
Regarding which previously reported variants should be reinterpreted, with the option to choose more than one, nearly 100% of clinical and laboratory genetic providers selected VUS (Figure S3a). The least endorsed option was benign variants. When these groups were broken down further, the laboratory directors chose reinterpretation of likely pathogenic and likely benign variants (68% and 50% respectively) less frequently than the laboratory genetic counselors (87% and 61% respectively), clinical genetic counselors (87% and 69% respectively), and geneticists (79% and 65% respectively, Figure S3b).
Stakeholders were asked what types of reinterpretations should be reported and couldchoose more than one (Figure S4a). Patients/parents (68%), clinical genetic providers (66%), and laboratory genetic providers (65%) selected “any new result” most frequently. Non-genetic providers selected this option least (37%), whereas their most selected response was “new results that could change the patient’s management” (56%). When the laboratory genetic providers were broken down further, the laboratory directors selected “any new result” less (45%) than the laboratory genetic counselors (75%) and selected “new results that could change the patient’s management” more (53%) than the laboratory genetic counselors (23%, Figure S4b).
Write-in responses (n=19) included specific changes in variant classifications (e.g., any change to or from a VUS).
Notably, when patients/parents were asked how reinterpretation would affect their confidence in genetic testing, 83% responded that it would have a positive impact (Figure S5).
Consent for Reinterpretation
There was disagreement across stakeholders on whether consent should be obtained for reinterpretation (Figure 2a, 4x2 chi-square p-value <.0001 and S6a). The majority of clinical (82%) and laboratory (73%) genetic providers responded that consent should not be required prior to reinterpretation, whereas slightly less than half of patients/parents (42%) and non-genetic providers (49%) said it should not be required (pairwise comparisons between clinical genetic providers and laboratory genetic providers to patients/parents and non-genetic providers, p-values <.0001).
Figure 2. Consent for reinterpretation.
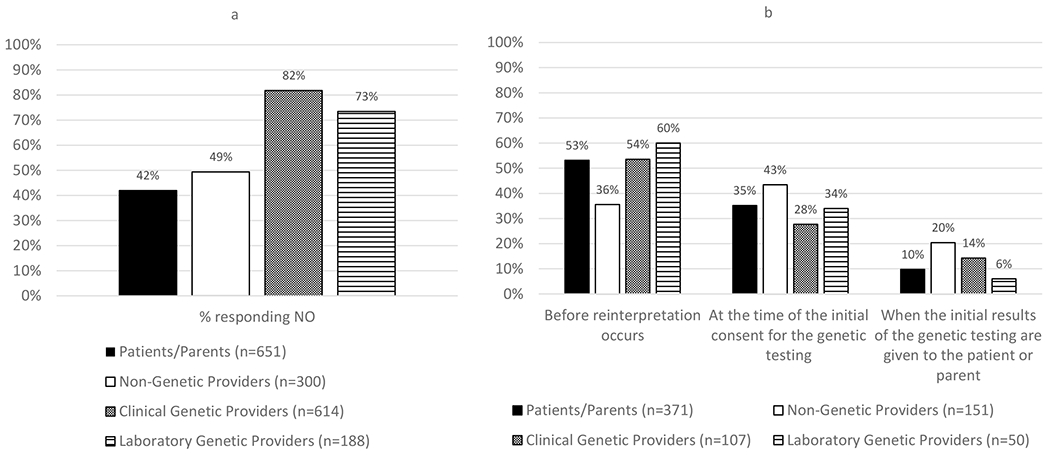
(a) Responses to the question, “Should consent be required prior to reinterpretation?” Pairwise comparisons between the patients/parents compared to clinical genetic providers or laboratory genetic providers AND between the non-genetic providers compared to clinical genetic providers or laboratory genetic providers all had a p-value of <.0001 (chi-square analysis). (b) Response from those who responded yes (n=692, 39% of the total sample) to the multiple-choice question of “At what point should consent for reinterpretation be obtained?” The option of “other” was available but was removed from the figure.
Among those responding that consent should be required (n=692, 39% of the total sample), views about when consent should be obtained were similar (Figure 2b and S6b). Most patients/parents (53%), clinical genetic providers (54%) and laboratory genetic providers (60%) said that consent should be obtained immediately before reinterpretation occurs, while non-genetic providers selected this option less frequently (36%). Non-genetic providers most often indicated that consent should be obtained at the time of the initial consent (43%), which was selected less often by patients/parents (35%), clinical genetic providers (28%), and laboratory genetic providers (34%).
Write-in responses (n=13) most often indicated that consent should be obtained and reaffirmed at multiple points.
Duration of Responsibility
A majority of respondents in each group endorsed no fixed termination point to a laboratory’s responsibility to reinterpret a previously reported variant, though the frequency of this endorsement differed (Figure 3a, 4 x 2 chi square p-value <.0001 and S7a). Clinical genetic providers selected this option the most (85%), followed by patients/parents (80%), whereas non-genetic providers selected this option the least (62%, pairwise comparisons between non-genetic providers to clinical genetic providers and patients/parents, p-values <.0001). Laboratory genetic providers were intermediate (74%).
Figure 3. Duration of responsibility for reinterpretation.
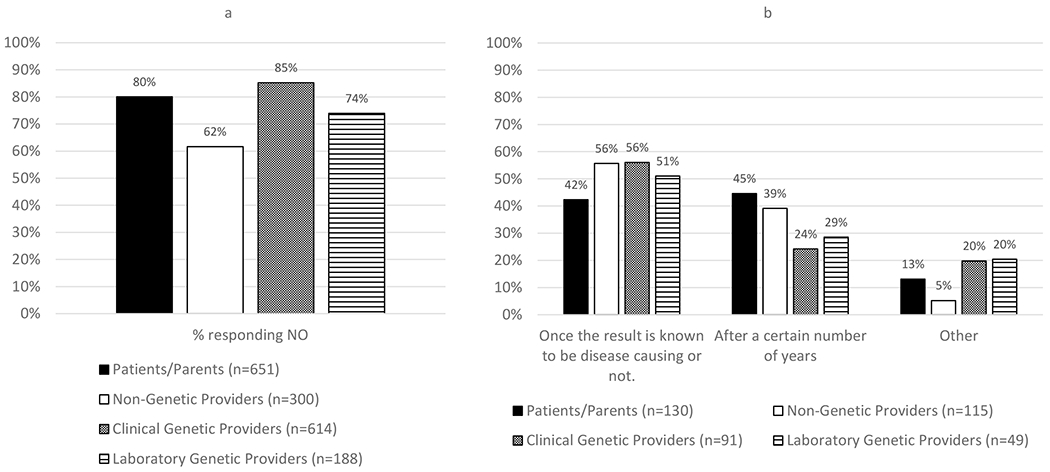
(a) Responses to the question, “Should there be a point after a variant is reported when a lab should no longer be expected to reinterpret it?” While majority of respondents in all groups endorsed no end to a laboratory’s responsibility to reinterpret a previously reported variant, non-genetic providers (62%) were less frequent in this response than patients/parents and clinical genetic providers (80% and 85% respectively, chi-square analysis, pairwise comparison for non-genetic providers compared to clinical genetic providers and patients/parents, p-value <.0001). (b) Response from those who responded yes (n=385, 22% of the total sample) to the select all that apply question “What should that point be?”
For those who answered that there should be an end to this responsibility (n=385, 22% of the total sample), responses were similar regarding what should lead to termination (Figure 3b and S7b). Most non-genetic providers (56%), clinical genetic providers (56%), and laboratory genetic providers (51%) responded that responsibility to reinterpret should end once the variant has been determined to be disease-causing or not. For the patients/parents, the most frequently selected response was after a certain number of years (45%). When asked to indicate what the duration should be, the average across groups ranged from 7 to 12 years.
Write-in responses (n=51) included when a new technology replaces the test that was done and once the patient is deceased.
Cost of Reinterpretation
The majority of providers responded that the cost of reinterpretation should be included in the initial cost of testing rather than as an additional cost, either at the time of the initial testing or reinterpretation (Figure S8, 4x2 chi-square, p-value <.0001). Clinical genetic providers (82%) were more likely to hold this opinion compared to laboratory genetic providers (58%) and non-genetic providers (53%, pairwise comparisons between clinical genetic providers and both other groups, p-values <.0001).
Write-in responses (n=42) included that cost responsibility depends on who is initiating reinterpretation and that there should be a combination of insurance coverage and a fee charged to patients, depending on the type of test and frequency of requested reinterpretation.
When patients/parents were asked if they would be willing to pay out of pocket for reinterpretation, only 23% were unwilling to pay under any circumstances, whereas 45% would be willing to pay if reinterpretation was recommended by their provider and 43% would be if there were changes in their/their child’s health (Figure S9).
Liability for Reinterpretation and Recontact
Across all provider groups, 70% or more of respondents expressed some degree of concern about liability, for duties related both to reinterpretation and recontact (Figure 4, 4x2 chi-square p-value <.0001 and S10). For liability related to recontact, laboratory genetic providers (86%) expressed more concern than clinical genetic providers (75%) and non-genetic providers (70%, Figure 4b, pairwise comparisons between laboratory genetic providers to both other groups, p-values <.0001).
Figure 4. Liability for reinterpretation and recontact.
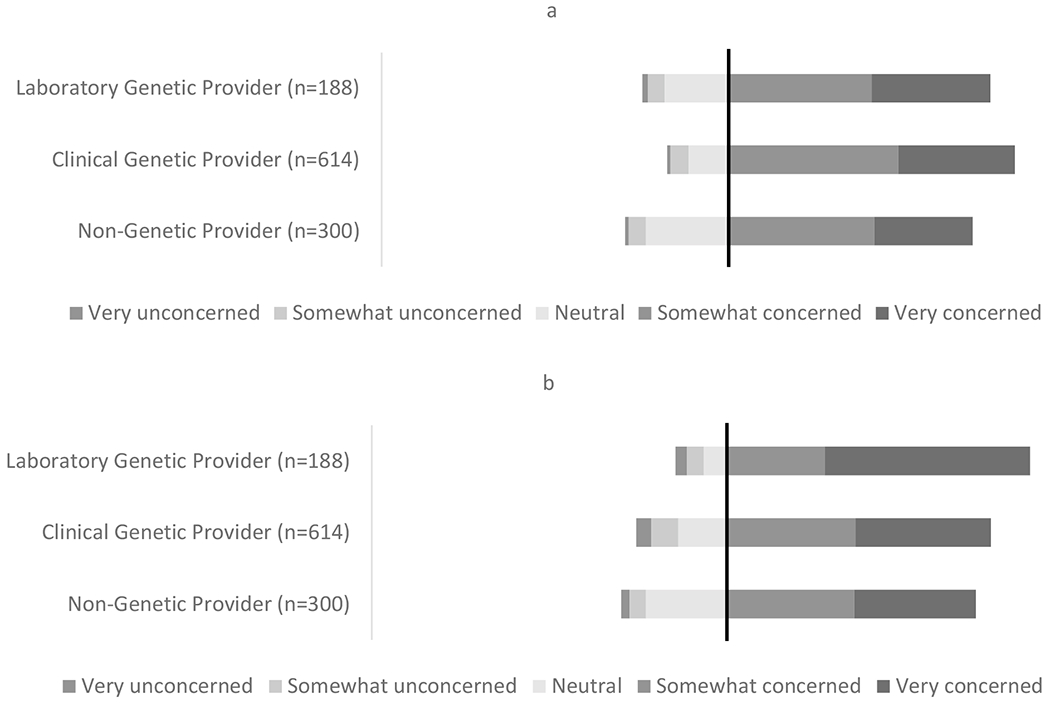
The black line marks the point of neutrality, where everything to the right indicates concern and everything to the left indicates neutrality or lack of concern. (a) Responses to the multiple-choice question, “How concerned or unconcerned would you be about legal liability if you had a duty to decide when reinterpretation of a patient’s genetic testing should be done?” (b) Responses to the multiple-choice question, “How concerned or unconcerned would you be about legal liability if you or your lab had a duty to recontact patients with reinterpreted results from their genetic testing?” For concern about recontact, we compared responses that expressed some level of concern to all other responses. For pairwise comparisons between the laboratory genetic providers compared to clinical genetic providers or non-genetic providers, the p-value was <.0001 (chi-square analysis).
Only 5% of clinical genetic providers responded that a duty related to reinterpretation would be a deterrent to ordering genetic testing, while 17% were uncertain (Figure S11). More non-genetic providers (24%) responded that such a duty would be a deterrent, while 32% were uncertain (2x2 chi-square p-value <.0001).
Service Alerts
When providers were asked about their interest in a service to alert them to new information about genetic variants, over 80% expressed interest in such a service (Figure S12). When asked what types of information they would want to be alerted about, the most common responses from the clinical and laboratory genetic providers were “variant reclassification after a ClinGen working group review” (81% and 83% respectively), followed by “new publications that include the variant” (65% and 69% respectively) and “any ClinVar entry discordant with the previous interpretation” (64% and 61% respectively, Figure S13).
Write-in responses (n=15) included that an alert is dependent on the specific gene or variant.
Patients/parents were asked about their interest in such a service, and each respondent was randomly assigned a cost for this service per year of either $25, $50 or $100 (Figure S14). Low levels of interest were reported at all price points (21%, 26%, 27% respectively). There were no significant differences in interest based on price.
Discussion
We compared the perspectives of stakeholders involved in genetic variant reinterpretation to provide data to inform the development of recommendations about variant reinterpretation. We assessed opinions on many of the key questions about variant reinterpretation using data generated from focus groups and surveys of laboratory directors, clinical geneticists, genetic counselors, and non-genetic providers in specialties that frequently utilize genetic testing, as well as patients and parents of patients. Given that currently non-European patients have a higher frequency of VUS and therefore are more likely to benefit from systematic variant reinterpretation, such recommendations would likely be helpful in addressing current inequities in genetic test interpretation.
One of the most striking findings is that across all stakeholders, most respondents endorsed an unlimited duration for the responsibility to reinterpret a previously reported genetic variant. This may reflect an underlying belief in an ethical duty to reinterpret previously reported genetic variants or, among those less familiar with practices in genetic testing laboratories, a perception that this is already being done and therefore should be continued. However, there currently is no consistency across clinical practices or genetic laboratories in how frequently or under what circumstances to initiate reinterpretation. Genetic laboratories commonly perform reinterpretation reactively, following a clinician request or when a previously classified variant is seen in a new patient in their lab, usually after a minimum period of time has elapsed since the variant was last assessed (10,15). Clinical genetic providers also typically initiate variant reinterpretation reactively when patients follow up after a certain period of time or if there are significant changes to their medical or family history (16). Although patients benefit most from an up-to-date interpretation of genetic variants, they also have the least knowledge and expertise to know when to request reinterpretation. Further, the lack of consistency in practices relating to variant reinterpretation make it challenging for patients to understand their role in the process.
Most providers expressed concern about legal liability associated with a duty to initiate reinterpretation and recontact patients about reinterpreted results. Clinical genetic providers were most concerned about liability related to initiating the process of reinterpretation. This may be related to challenges they foresee in systematically tracking all patients to determine if reinterpretation is necessary and to concerns that patients are often lost to follow up after initial testing. Clinicians are unlikely to have access to the informatic tools to monitor public databases efficiently for updated interpretations. Laboratory genetic providers were concerned about liability related to recontact. This may be because the laboratory rarely communicates with the patient directly but rather through the ordering provider and generally does not have patients’ contact information. Although no courts have yet imposed liability on clinicians or laboratories for failure to reinterpret a genetic variant, there are no clear standards for responsibilities related to variant reinterpretation (13,19).
Tension exists between the desire of providers and patients/parents to have the most up-to-date information to inform medical management and the practical and logistical challenges this entails. Furthermore, the cost of implementation at scale is unknown, with concerns that it could be substantial. One approach to address the cost of variant reinterpretation, endorsed by most providers surveyed, is for the laboratory to build it into the cost of the initial test. Since many people who have genetic testing will not require reinterpretation, the cost per person for reinterpretation would likely be low and therefore not significantly impact the overall cost of testing. Building the cost of reinterpretation into the initial test poses fewer logistical challenges than billing for reinterpretation separately at the time of initial testing or when reinterpretation occurs. In addition, each laboratory could set rules that dictate the circumstances of reinterpretation and providers could counsel their patients accordingly. The potential business implications of this model are complex and further discussion with laboratory directors, administrators, and payers is needed. Currently, there is no CPT code for reinterpretation of a single variant, and laboratories are not billing for this service. A systematic approach to variant reinterpretation may allow the field to gather data about cost and value to advocate for reimbursement by payers (20,21). Only a minority of patients/parents surveyed responded that they would be unwilling to pay out of pocket for reinterpretation under any circumstances. Many were willing to pay if there were a change in their/their child’s health or if their provider recommended reinterpretation. Therefore, a solution should also include the option for patients/parents to pay for additional reinterpretation in situations in which they or their provider think reinterpretation could be informative. Systematic approaches to variant reinterpretation will have to account for the associated costs and how to cover them.
The process of informed consent also needs to be considered. Although the majority of genetic providers responded that consent should not be required prior to reinterpretation, most patients/parents and non-genetic providers disagreed. This may reflect the fact that genetic providers are more aware of the limitations of our current knowledge in genetics and the potential for variant reinterpretation than patients/parents and non-genetic providers. Of the respondents who said consent should be required for reinterpretation, most patients/parents, clinical genetic providers, and laboratory genetic providers thought this should be obtained at the time reinterpretation occurs. This approach seems to present the most logistical challenges. A simpler approach could include it as part of the initial consent process, ensuring patients/parents are aware of the potential for reinterpretation, the circumstances under which it may occur, and the steps that would follow a reinterpretation, including recontact. It would also present an opportunity to remind patients/parents of their responsibility to maintain up-to-date contact information with their provider. However, in considering the logistics, it becomes clear that the origin point of initiation of reinterpretation impacts the complexity of the steps that follow. For example, if a provider initiates reinterpretation after seeing a patient for an evaluation, there is an opportunity to obtain informed consent, establish a plan for recontact, and discuss potential billing concerns. On the other hand, if the laboratory initiates reinterpretation, there are more potential complications in contacting the ordering provider and the patient. The patient/parent may also be concerned, both because they are receiving information they likely did not anticipate and perhaps also being billed for this service. In clinical genetic testing, consent is often tied to billing since patients/parents sign paperwork indicating their consent for the testing itself as well as an agreement regarding their financial responsibilities. If costs of reinterpretation were built into the cost of the initial test, this might alleviate some of the concern about an unanticipated bill and perhaps enable a more streamlined process for consent for reinterpretation within the initial consent process.
When clinical and laboratory genetic providers were asked what events should trigger reinterpretation of a previously reported variant, the least endorsed response was “new treatment becomes available for disorders related to this gene.” However, when asked what types of reinterpretations should be reported, a frequent response was “new results that could change the patient’s management.” As more treatments for genetic disorders become available and as evidence-based standards of care are developed, the need for up-to-date variant classification to ensure appropriate surveillance and treatment will increase.
This study focused on variant-level reinterpretation of previously reported genetic variants and did not address case-level reanalysis, which involves review of all the variants in an exome or genome(11). For non-diagnostic exome/genome sequencing, the diagnostic yield increases over time due to technological advances, improvements in bioinformatic tools, and identification of new disease genes (22). The issues surrounding case-level reanalysis of exome/genome sequencing are different than for variant-level reinterpretation since the reanalysis process is more time-consuming and costlier, requiring reassessment of the patient’s phenotype, updated bioinformatics pipelines, and reconsideration of multiple variants (23). Another common clinical situation not addressed here is reproductive testing. It is routine for laboratories not to report VUS from expanded carrier screening; neither the provider nor the patient can initiate the process of reinterpretation for a variant they do not know exists (24). Moreover, the clinician-patient relationship in reproductive testing may be limited to the duration of the pregnancy, unless another pregnancy ensues. Although many of the issues discussed in this study are relevant to these additional situations, their unique characteristics may require solutions tailored to each context.
Many challenges surround variant reinterpretation, among them a need to develop guidelines, including scope, timing, and process of informed consent. Laboratories, clinicians, and payers need to consider billing and reimbursement mechanisms to pay for the additional work required. Consideration must be given to the increasing role of non-genetic providers in ordering genetic testing. It will be important to maintain transparency about variant reinterpretation practices, ensuring that all parties are aware of the possibility of reinterpretation, the circumstances under which this may occur, and the role they each play in that process. Availability of a low-cost, scalable, and accessible infrastructure will likely be necessary to support systematic variant reinterpretation. Attention to infrastructure, transparency and consistency may ease some concerns about liability and support implementation in an equitable manner.
Supplementary Material
Acknowledgements
We thank all of the study participants for their contribution. This work was supported by a grant (R01HG010365) from the National Human Genome Research Institute (NHGRI). We acknowledge the contributions of the ethical, legal, and economic working groups as well as the expert advisory panel supported by this grant. We thank the Irving Institute for Clinical and Translational Research at Columbia University Irving Medical Center for its assistance (NCATS grant number UL1TR001873) and the Center for Excellence in ELSI Research (NIH grant number RM1HG007257). We thank the masters in biotechnology students and medical student, Abigayle Dolmseth, at Columbia University for their work on this project.
Footnotes
Ethics Declaration
This study was approved by the WIRB-Copernicus Group, Columbia University Irving Medical Center, University of Michigan, and Vanderbilt University institutional review boards. As required by the IRB, all invited participants reviewed the e-consent language prior to proceeding with the focus group/survey.
Conflicts of Interest
W.K.C. is on the Regeneron Genetics Center Scientific Advisory Board. The other authors declare no conflict of interest.
Data Availability
Data and materials are available upon request.
References
- 1.Phillips KA, Deverka PA, Hooker GW, Douglas MP. Genetic test availability and spending: Where are we now? Where are we going? Health Aff. 2018;37(5):710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popejoy AB, Fullerton SM. Genomics is failing on diversity. Vol. 538, Nature. 2016. p. 161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caswell-Jin JL, Gupta T, Hall E, Petrovchich IM, Mills MA, Kingham KE, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20(2):234–9. [DOI] [PubMed] [Google Scholar]
- 5.Landry LG, Rehm HL. Association of racial/ethnic categories with the ability of genetic tests to detect a cause of cardiomyopathy. JAMA Cardiol. 2018;3(4):341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndugga-Kabuye MK, Issaka RB. Inequities in multi-gene hereditary cancer testing: lower diagnostic yield and higher VUS rate in individuals who identify as Hispanic, African or Asian and Pacific Islander as compared to European. Fam Cancer. 2019;18(4):465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrieri D, Dheensa S, Doheny S, Clarke AJ, Turnpenny PD, Lucassen AM, et al. Recontacting in clinical practice: An investigation of the views of healthcare professionals and clinical scientists in the United Kingdom. Eur J Hum Genet. 2017;25(3):275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirchia F, Carrieri D, Dheensa S, Benjamin C, Kayserili H, Cordier C, et al. Recontacting or not recontacting? A survey of current practices in clinical genetics centres in Europe. Eur J Hum Genet. 2018;26(7):946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dheensa S, Carrieri D, Kelly S, Clarke A, Doheny S, Turnpenny P, et al. A ‘joint venture’ model of recontacting in clinical genomics: challenges for responsible implementation. Eur J Med Genet. 2017;60(7):403–9. [DOI] [PubMed] [Google Scholar]
- 10.Chisholm C, Daoud H, Ghani M, Mettler G, McGowan-Jordan J, Sinclair-Bourque L, et al. Reinterpretation of sequence variants: One diagnostic laboratory’s experience, and the need for standard guidelines. Genet Med. 2018;20(3):365–8. [DOI] [PubMed] [Google Scholar]
- 11.Deignan JL, Chung WK, Kearney HM, Monaghan KG, Rehder CW, Chao EC. Points to consider in the reevaluation and reanalysis of genomic test results: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2019;21(6):1267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appelbaum PS, Parens E, Berger SM, Chung WK, Burke W. Is there a duty to reinterpret genetic data? The ethical dimensions. Genet Med. 2020;22(3):633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clayton EW, Appelbaum PS, Chung WK, Marchant GE, Roberts JL, Evans BJ. Does the law require reinterpretation and return of revised genomic results? Genet Med. 2021;23(5):833–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts JL, Foulkes AL. William & Mary Law Review Genetic Duties. 2021;62(1). [PMC free article] [PubMed] [Google Scholar]
- 15.El Mecky J, Johansson L, Plantinga M, Fenwick A, Lucassen A, Dijkhuizen T, et al. Reinterpretation, reclassification, and its downstream effects: Challenges for clinical laboratory geneticists. BMC Med Genomics. 2019;12(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vears DF, Sénécal K, Borry P. Genetic health professionals’ experiences with initiating reanalysis of genomic sequence data. Fam Cancer. 2020;19(3):273–80. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell C, Ploem C, Retèl V, Gevers S, Hennekam R. Experts reflecting on the duty to recontact patients and research participants; why professionals should take the lead in developing guidelines. Eur J Med Genet. 2020;63(2):103642. [DOI] [PubMed] [Google Scholar]
- 18.SAS Institute Inc. 2014.
- 19.Marchant G, Barnes M, Evans JP, LeRoy B, Wolf SM. From Genetics to Genomics: Facing the Liability Implications in Clinical Care. J Law, Med Ethics. 2020;48(1):11–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veenstra DL, Rowe JW, Pagán JA, Brown HS, Schneider JE, Gupta A, et al. Reimbursement for genetic variant reinterpretation: Five questions payers should ask. Am J Manag Care. 2021;27(10):E336–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagán JA, Brown HS, Rowe J, Schneider JE, Veenstra DL, Gupta A, et al. Genetic Variant Reinterpretation: Economic and Population Health Management Challenges. Popul Health Manag. 2021;24(3):310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji J, Leung ML, Baker S, Deignan JL, Santani A. Clinical Exome Reanalysis: Current Practice and Beyond. Mol Diagnosis Ther. 2021;25(5):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson AJ, Tan NB, Spurdle AB, Metke-Jimenez A, Sullivan C, Waddell N. Re-analysis of genomic data: An overview of the mechanisms and complexities of clinical adoption. Genet Med [Internet]. 2022;24(4):798–810. Available from: 10.1016/j.gim.2021.12.011 [DOI] [PubMed] [Google Scholar]
- 24.Edwards JG, Feldman G, Goldberg J, Gregg AR, Norton ME, Rose NC, et al. Expanded carrier screening in reproductive medicine-points to consider. Obstet Gynecol. 2015;125(3):653–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials are available upon request.


