Abstract
Mesonephric-like adenocarcinomas are newly classified rare neoplasms of the upper genital tract. They share identical features with mesonephric adenocarcinomas, with the exception of location. There is ongoing discussion on whether mesonephric-like adenocarcinomas arise from mesonephric remnants or are of Müllerian origin.
A 65-year-old woman (G2P1) presented with pelvic pain. Transvaginal ultrasound revealed multiple fibroids, and a robotic total laparoscopic hysterectomy with bilateral salpingo-oophorectomy was planned. Intraoperatively, a complex mass with cystic and solid components was found on the left ovary, which also adhered to the rectosigmoid colon. Pathologic reports documented mesonephric-like adenocarcinoma that appeared to be arising in association with an endometrioid adenofibroma. This case is notable due to the patient's unique background of in-utero exposure to diethylstilbestrol (DES) and multiple gynecological malignancies within her mother, as well as the associated endometrioid adenofibroma. This case contributes evidence to two seemingly opposing theories of mesonephric-like adenocarcinoma histogenesis: the mesonephric remnant theory (supported by the patient's DES exposure), and the Müllerian theory (supported by the associated endometrioid adenofibroma).
Keywords: Mesonephric-like adenocarcinoma, Mesonephric adenocarcinoma, Case report, DES, Endometrioid adenofibroma
Highlights
-
•
Mesonephric-like adenocarcinoma is a rare neoplasm of the upper genital tract.
-
•
Originally thought to be of mesonephric remnant origin, recent evidence has pointed towards a Müllerian histogenesis.
-
•
It is unknown if genetics and/or environmental exposures are risk factors for developing mesonephric-like adenocarcinoma.
1. Introduction
Mesonephric-like adenocarcinomas are newly classified rare neoplasms of the upper genital tract. They are so named because of their many features identical to mesonephric adenocarcinomas, including histological characterization and immunohistochemical staining patterns. They differ only by location, with the upper genital tract being a theoretically unlikely area for mesonephric remnants. As there are so few cases in the literature, there is an ongoing discussion on whether mesonephric-like adenocarcinomas are derived from mesonephric remnants or Müllerian structures due to the frequency of co-occurrence with known Müllerian neoplasms. The case presented is of a mesonephric-like adenocarcinoma in a patient with the unique risk factors of diethylstilbestrol (DES) exposure in-utero and a family history of multiple gynecological cancers. This case is further notable due to the adenocarcinoma's association with an endometrioid adenofibroma.
2. Case Presentation
A 65-year-old woman, gravida 2 para 1011 (G2P1), presented with a 9-month history of pelvic pain. Gynecological medical history was significant for DES exposure in-utero, and family history included maternal cases of both uterine and ovarian carcinomas. Workup included a transvaginal ultrasound scan, which revealed multiple uterine fibroids with the largest measuring 2.3 cm × 2.4 cm × 3.2 cm. The left ovary was not visualized, and all other visualized structures demonstrated no abnormalities. Given the patient's symptoms highly suggestive of being caused by the uterine fibroids, her lack of desire for fertility and uterine preservation, and her condition being suitable for surgery, she opted to undergo a robotic total laparoscopic hysterectomy with bilateral salpingo-oophorectomy. Intraoperatively, an enlarged left ovary was attached to the rectosigmoid colon and had produced a complex mass with cystic and solid components. Pathology reports following the procedure documented an unspecified adenocarcinoma predominantly involving the left ovary with peritoneal washings positive for malignant cells.
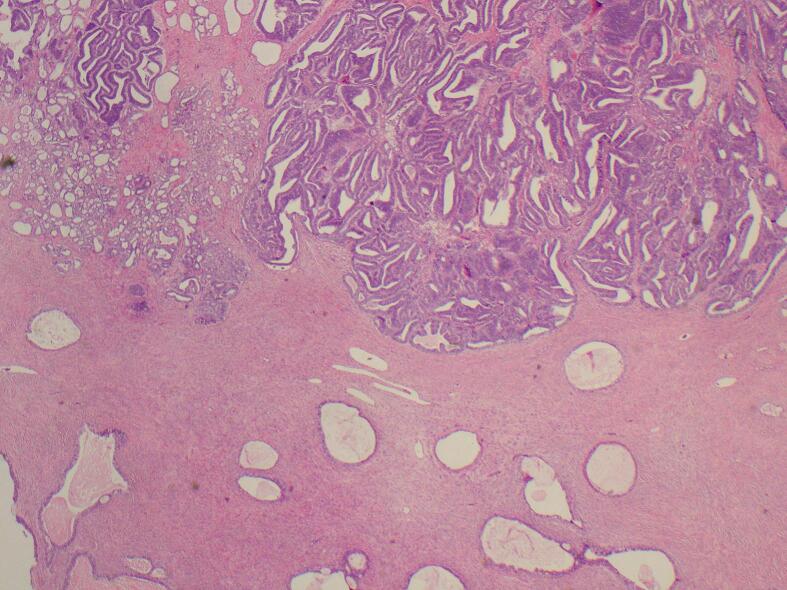
Confirmatory pathology reports noted an ovarian mesonephric-like adenocarcinoma (MLA), 5.3 cm in greatest dimension, and arising in association with endometrioid adenofibroma (Fig. 1, Fig. 2, Fig. 3). The neoplastic cells had vesicular, higher-grade nuclei with areas that were hyper-stratified. In some areas, these neoplastic cells were lined by tubular glands, suggestive of endometrioid carcinoma. However, other areas were more suggestive of MLA by small glands and tubules lined by neoplastic polygonal cells with clear cytoplasm and eosinophilic secretions. There was no evidence of involvement of the bilateral fallopian tubes, right ovary, ectocervix and endocervical tissue, or endometrium. There were additional findings of adenomyosis and endometriosis involving adnexal tissue. Immunohistochemical staining performed on the carcinoma tissue was positive for CK8/18, CK7, GATA3, Vimentin, TTF1, and P16. There were very few cells positive for p40. Immunohistochemical staining was negative for CK20, CDX 2, ER, PR, Napsin A, D2–40, WT1, inhibin, CD10, and SF-1. P53 demonstrated a wild-type pattern. Though immunohistochemical staining for EMA was initially deemed patchy positive, repeat testing was negative in the carcinoma tissue but positive in the adjacent adenofibroma. Peritoneal washings revealed malignant epithelial cells in tight clusters, consistent with adenocarcinoma, negative for ER and weakly patchy positive for PAX8.
Fig. 1.
Mesonephric-like adenocarcinoma. A mesonephric component has tubular glands lined by polygonal cells w/ clear cytoplasm, some w/ pink material within the lumina. The second component has nuclear stratification, cell crowding, & abundant mitotic figures. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Endometrioid adenofibroma with benign endometrioid-like glands.
Fig. 3.
The mesonephric-like adenocarcinoma with both histologic components is shown alongside the associated endometrioid adenofibroma.
The patient underwent computed tomography of the thorax, abdomen, and pelvis (CT TAP), which was negative for additional visible masses but positive for small lung nodules. These nodules were subsequently biopsied, revealing benign pleural and lung tissue negative for malignancy, essentially ruling out metastases. CA-125 tumor marker levels were assessed at this time, which measured 23.3 U/mL.
On the recommendation of a multidisciplinary tumor board, the patient underwent subsequent staging surgery. Intraoperatively, frozen sections of a vaginal cuff nodule and a bladder peritoneum nodule were sent for analysis by surgical pathology. The left remnant infundibulopelvic ligament with a peritoneal nodule, a left pelvic sidewall nodule, omentum, and an additional bladder peritoneal nodule were also removed. Histopathology revealed foci of adenocarcinoma within the vaginal cuff nodule, bladder peritoneal nodules, and left remnant infundibulopelvic ligament. The omentum and left pelvic sidewall nodule were read as benign. At this time, the cancer was staged as FIGO classification IIIC. However, classification was later modified to IIB as the disease was confined to the pelvis only.
The patient began a course of six chemotherapy sessions with carboplatin/paclitaxel every three weeks with concurrent CA-125 level monitoring. At the completion of the six chemotherapy sessions, she underwent a repeat CT TAP, revealing no definite new sites of disease. CA-125 levels were downtrending with each subsequent treatment, from 23.3 U/mL at her initial visit to 13.4 U/mL at her final chemotherapy session. Currently, the patient remains with no evidence of disease recurrence. Follow-up with a repeat CT TAP and serial CA-125 levels is planned for 3 months following the final chemotherapy session.
3. Discussion
Embryologically, the Müllerian (paramesonephric) and Wolffian (mesonephric) ducts are both present, symmetrical, and undifferentiated until week 9 of development. In genetically XY embryos, Leydig-cell derived testosterone drives the masculinization of the internal and external genitalia, with the mesonephric duct differentiating into the epididymis, vas deferens, and seminal vesicles. Simultaneously, Sertoli-cell derived anti-Müllerian hormone induces regression of the Müllerian duct. In genetically XX embryos, the absence of anti-Müllerian hormone allows the Müllerian ducts to develop into the fallopian tubes, uterus, and upper third of the vagina. The lack of adequate amounts of testosterone results in the regression of the mesonephric duct [1].
Mesonephric adenocarcinoma (MA) is thought to derive from remnants of the mesonephric duct that did not fully regress during early development. They occur most often in the lateral cervix and vagina due to the anatomical association of mesonephric remnants and hyperplasia [2,3]. Mesonephric-like adenocarcinomas share many features with mesonephric adenocarcinomas. MAs and MLAs are generally morphologically identical, with a variety of different architectures identified, such as papillary, ductal, and tubular with eosinophilic secretions. Likewise, they share almost identical immunohistochemical features, with frequent positivity for CD10, GATA3, Calrenin, and TTF-1 but negativity for ER and PR. The main feature differentiating an MLA from an MA is location in the upper genital tract. In addition, MAs are often entirely within the cervical wall or myometrium, while MLAs are located predominantly in the endometrium with potential subsequent invasion into the myometrium [4].
Ovarian MLAs are extremely rare. As of 2020, only 11 cases had been published, according to an English-language database search. As of today, the number is still less than 20 [2,[5], [6], [7], [8], [9]]. Of these cases, none reported a history of patient in-utero exposure to DES. The association between in-utero DES exposure and development of clear cell carcinoma of the vagina and cervix is well recognized [10]. Though few in number, there are cases where a link is proposed between DES exposure and the development of ovarian cancers in second- and third-generation daughters along a maternal line [11,12]. However, there are no reports of a mesonephric subtype. An association between DES exposure and retention of mesonephric duct material was observed in a murine study. Prominent dilated mesonephric remnants and ovarian cysts with epithelium that appeared to be derived from mesonephric remnants were observed in DES-exposed mice in a disproportionate number compared with controls [13]. In a follow-up study, a statistically significant number of women with DES exposure were found to have paraovarian cysts of probable mesonephric origin with abnormal histologic features [14]. The body of research on prenatal DES exposure and the development of ovarian cancer and/or mesonephric retention abnormalities is too sparse to have a definitive conclusion. It cannot definitively be said whether this patient's ovarian MLA was in any part a consequence of her in-utero DES exposure, nor can a possible association be ruled out given the evidence of DES-induced major disruption of female reproductive tract development.
Contrary to a DES-exposure risk-factor hypothesis, which would imply a mesonephric duct derivation of MLA, several recent studies hypothesize that MLA is actually of Müllerian duct origin. This is largely due to its frequent association with other Müllerian neoplasms. In one review, 3 of 5 MLA cases had coexisting endometriosis on the same ovary [15]. Other cases have described simultaneous MLA and endometrioid carcinomas [16]. In a review, McCluggage et al. found coexisting Müllerian-derived lesions in 8 of 11 cases of MLA [17]. Multiple cases have been associated with serous borderline tumors [2,18]. Ishida et al. reported a case of ovarian MLA that potentially arose from an endometrioid adenofibroma, a rare Müllerian-derived tumor in itself, with the addition of a synchronous uterine MLA [8,19]. The case discussed herein, similarly, was associated with an endometrioid adenofibroma, bolstering the theory for a Müllerian origin of MLA.
Finally, it would be remiss to ignore the patient's strong family history of gynecological malignancies. Of the published literature reviewed, there were no reported cases of MLA associated with a familial lineage of ovarian carcinoma of any type. This patient described a history of both ovarian and endometrial cancers in her mother. Unfortunately, this history was self-reported with no records for review, so the specific subtype of these malignancies remains unknown. As a plethora of other hereditary mutations strongly associated with ovarian cancers are well known, it is plausible that the development of MLA could have a genetic component [20].
4. Conclusion
This is a case of MLA in a patient with multiple unique potential risk factors. The patient's history of DES exposure in-utero is a novel contribution to the collection of known MLA cases. This case is only the second in the literature to have been reported as arising in association with an endometrioid adenofibroma, and the first to be associated with a strong family history of gynecological malignancies. The most recent opinions in MLA literature are that MLAs are likely of Müllerian origin, and this case's association with another rare Müllerian neoplasm further that claim. Continuing research is needed to conclude whether DES exposure and genetics played a role in this case, or if they were just two incredible coincidences.
Acknowledgments
Contributors
Jillian Linck contributed to undertaking the literature review, interpreting the data, and writing and editing the manuscript.
Wanda Torres, MD, FACOG, contributed to patient care, conception of the case report, acquiring the data, and editing of the article.
Both authors approved the final submitted manuscript.
Funding
The authors did not receive any funding from an external source for the publication of this case report.
Patient consent
Consent was obtained from the patient to publish the clinical details and the images included in this report.
Provenance and peer review
This article was not commissioned and was peer reviewed.
Acknowledgments
Acknowledgements
The authors would like to thank Michael J. Licata, MD, AB Pathology, for providing the images and histopathological examinations included in this case report.
Conflict of interest statement
The authors declare that they have no conflict of interest regarding the publication of this case report.
References
- 1.Sajjad Y. Development of the genital ducts and external genitalia in the early human embryo. J. Obstet. Gynaecol. Res. Oct 2010;36(5):929–937. doi: 10.1111/j.1447-0756.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 2.Dundr P., Gregova M., Nemejcova K., et al. Ovarian mesonephric-like adenocarcinoma arising in serous borderline tumor: a case report with complex morphological and molecular analysis. Diagn. Pathol. Jul 21 2020;15(1):91. doi: 10.1186/s13000-020-01012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kezlarian B., Muller S., Werneck Krauss Silva V., et al. Cytologic features of upper gynecologic tract adenocarcinomas exhibiting mesonephric-like differentiation. Cancer Cytopathol. Aug 2019;127(8):521–528. doi: 10.1002/cncy.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howitt B.E., Nucci M.R. Mesonephric proliferations of the female genital tract. Pathology. Feb 2018;50(2):141–150. doi: 10.1016/j.pathol.2017.11.084. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q., Shen Y., Xie C. Mesonephric-like adenocarcinoma of the ovary: A case report and a review of the literature. Medicine (Baltimore) Nov 25 2020;99(48) doi: 10.1097/MD.0000000000023450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakshmanan A., S A., Ramakrishnan V., Srinivasan A. An incidental ovarian endometrioid adenofibroma with sertoliform tubules in a patient with endometrial malignant mixed mullerian tumor. Indian J. Obstet. Gynecol. Res. 2023;10(1):108–110. [Google Scholar]
- 7.Xu J., Park K.J., Rehrauer W.M., Weisman P.S. Mesonephric-like adenocarcinoma of the ovary with squamoid morular metaplasia, aberrant beta-catenin expression, and concurrent FGFR2 and CTNNB1 mutations: a case report. Virchows Arch. Mar 1 2023 doi: 10.1007/s00428-023-03522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishida K., Ashihara T., So M., Minamiguchi S., Matsumura N., Nonogaki T. Synchronous ovarian and uterine mesonephric-like carcinoma that potentially arose from endometrioid adenofibroma: A case report. J. Obstet. Gynaecol. Res. Mar 2023;49(3):1052–1056. doi: 10.1111/jog.15539. [DOI] [PubMed] [Google Scholar]
- 9.Restaino S., Pellecchia G., Tulisso A., et al. Mesonephric-like adenocarcinomas a rare tumor: the importance of diagnosis. Int. J. Environ. Res. Public Health. Nov 4 2022;19(21) doi: 10.3390/ijerph192114451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst A.L., Ulfelder H., Poskanzer D.C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. Apr 15 1971;284(15):878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 11.Dasanu C.A., Herzog T.J. Clear cell adenocarcinoma of the ovary associated with in utero diethylstilbestrol exposure: case report and clinical overview. Medscape J. Med. 2009;11(1):6. [PMC free article] [PubMed] [Google Scholar]
- 12.Titus-Ernstoff L., Troisi R., Hatch E.E., et al. Offspring of women exposed in utero to diethylstilbestrol (DES): a preliminary report of benign and malignant pathology in the third generation. Epidemiology. Mar 2008;19(2):251–257. doi: 10.1097/EDE.0b013e318163152a. [DOI] [PubMed] [Google Scholar]
- 13.Newbold R.R., Bullock B.C., Mc Lachlan J.A. Exposure to diethylstilbestrol during pregnancy permanently alters the ovary and oviduct. Biol. Reprod. Apr 1983;28(3):735–744. doi: 10.1095/biolreprod28.3.735. [DOI] [PubMed] [Google Scholar]
- 14.Haney A.F., Newbold R.R., Fetter B.F., McLachlan J.A. Paraovarian cysts associated with prenatal diethylstilbestrol exposure. Comparison of the human with a mouse model. Am. J. Pathol. Sep 1986;124(3):405–411. [PMC free article] [PubMed] [Google Scholar]
- 15.McFarland M., Quick C.M., McCluggage W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology. Jun 2016;68(7):1013–1020. doi: 10.1111/his.12895. [DOI] [PubMed] [Google Scholar]
- 16.Yano M., Shintani D., Katoh T., et al. Coexistence of endometrial mesonephric-like adenocarcinoma and endometrioid carcinoma suggests a Mullerian duct lineage: a case report. Diagn. Pathol. Jun 7 2019;14(1):54. doi: 10.1186/s13000-019-0830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCluggage W.G., Vosmikova H., Laco J. Ovarian combined low-grade serous and mesonephric-like adenocarcinoma: further evidence for a Mullerian origin of mesonephric-like adenocarcinoma. Int. J. Gynecol. Pathol. Jan 2020;39(1):84–92. doi: 10.1097/PGP.0000000000000573. [DOI] [PubMed] [Google Scholar]
- 18.Chapel D.B., Joseph N.M., Krausz T., Lastra R.R. An ovarian adenocarcinoma with combined low-grade serous and mesonephric morphologies suggests a Mullerian origin for some mesonephric carcinomas. Int. J. Gynecol. Pathol. Sep 2018;37(5):448–459. doi: 10.1097/PGP.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 19.Bettaieb I., Mekni A., Bellil K., et al. Endometrial adenofibroma: a rare entity. Arch. Gynecol. Obstet. Mar 2007;275(3):191–193. doi: 10.1007/s00404-006-0196-1. [DOI] [PubMed] [Google Scholar]
- 20.Toss A., Tomasello C., Razzaboni E., et al. Hereditary ovarian cancer: not only BRCA 1 and 2 genes. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/341723. [DOI] [PMC free article] [PubMed] [Google Scholar]