Abstract
Exosomes isolated from potato (Solanum tuberosum) exhibit the biophysical characteristics of exosomes observed in mammalian cells and microorganisms, as determined by dynamic light scattering analysis and transmission electron microscopy. In the present study, it was shown that potato exosomes (ExoPs) can penetrate keratinocyte HaCaT cells, as determined by confocal microscopy and flow cytometry. In addition, ExoPs can suppress the expression of the collagen-destroying enzymes MMP1, 2 and 9, and the inflammatory cytokines IL6 and TNF-α, while inducing the expression of glutathione S-transferase α 4, a cellular detoxifying enzyme, as revealed by reverse transcription-quantitative PCR. Furthermore, ExoPs promote HaCaT cell proliferation, exhibit in vitro antioxidant activity against the free radical 2,2-diphenyl-β-picrylhydrazyl, and protect cells from hydrogen peroxide-induced cytotoxicity. ExoPs can also minimize the induction of photodamage initiated by ultraviolet B (UVB) irradiation, and have the tendency to cure the photodamage already incurred on cells by UVB irradiation. ExoPs also prevent collagen degradation as observed in the culture media of UVB-irradiated HaCaT cells. Collectively, ExoPs may protect and ameliorate photodamage in keratinocyte HaCaT cells.
Keywords: ExoP, photoaging, antioxidant, skin inflammation, UVB damage, Solanum tuberosum
Introduction
Despite the health benefits of sun exposure; notably, cutaneous production of vitamin D is dependent on solar UVB exposure (1), chronic sun exposure is one of the most significant environmental factors that can accelerate extrinsic photoaging, which is characterized by sunburn cell formation, the elevation of collagenases, specifically MMP1, 2 and 9 (2,3), the fragmentation of collagen and elastic fibers, wrinkle formation, and the generation of inflammatory responses (4) and even skin cancer (5).
Repetitive UVB irradiation leads to activation of cytokine and growth factor receptors on the cell surface by reactive oxygen species, the AP-1 transcription factor, MMPs and elastases, which result in the degradation of collagen and elastin, culminating in the formation of skin wrinkles (6). Collagen proteins are abundant in extracellular matrices (ECMs) and provide cellular integrity, but are susceptible to digestion by several MMPs, such as MMP 1, 2 and 9 (7) Skin collagen proteins show the highest decrease following UV-initiated photodamage. Collagen degradation can lead to skin wrinkling (8,9), and therefore the development of safe inhibitors for the activity or transcription of MMPs can be applied to prevent skin wrinkling or restore its elasticity (10,11).
Several synthetic and natural small molecules have been used to improve skin quality and reduce wrinkle appearance; for example, α hydroxy acids provide a chemical peeling effect by removing dead skin cells, and promoting collagen and hyaluronic acid synthesis, to effectively reduce skin wrinkles and improve skin appearance (12,13). Several types of retinoids, which are derivatives of vitamin A, including retinol, have also been widely regarded as anti-aging cosmetic ingredients to reduce face wrinkles and hyperpigmentation. Retinol reduces the expression or activities of MMP1, 2, 7 and 9 (14). Although these molecules have commonly been used to reduce skin wrinkles and improve skin appearance by enhancing procollagen synthesis and antioxidant activity, they show various side effects, including mild skin irritation, redness, itching and long-term toxicities (11,15).
Natural plants and marine algae are rich in the antioxidant phytochemicals polyphenols, such as flavonoids, which exert protective activities against inflammation, allergic reactions, cancer, viral infections and oxidative damage (15,16). Therefore, plant extracts and components purified from plants have been actively investigated as sources of skin care products, and their biological activities have been investigated in detail (17). In 2018, the most popular botanical species used in skin care products included natural fat from Butyrospermum parkii, known as shea butter, Glycine soja (wild soybean) and Vitis vinifera L. (grapes). Notably, DNA protection and modulation of enzyme activities were the most common working mechanisms for the in vitro and in vivo anti-aging effects of the aforementioned botanical species (18). Aside from molecular anti-aging activities, other variables, such as cell-penetrating ability, determine the final use of botanicals as anti-aging ingredients. For example, epigallocatechin-3-gallate (EGCG), which originated from green tea, is one of the most thoroughly studied polyphenolic compounds showing beneficial effects against cancer, diabetes and cardiovascular diseases in cell culture and animal model settings (19). Even though EGCG also shows effective anti-aging activity, its use is limited due to poor absorption (20). Therefore, the development of new anti-aging cosmetic ingredients from natural sources with proven function, including skin-penetrating ability and few side effects, is one of top priorities in the cosmetic industry (21,22).
Exosomes are a type of extracellular vesicle released from cells of possibly all forms of life, ranging from microorganisms to higher eukaryotes (23). Characterized as a membrane vesicle composed of lipid bilayers with a diameter of 30–150 nm that encapsulate proteins, RNA, DNA and metabolites, exosomes mediate communication between cells (5). For example, mRNA in exosomes can be delivered to and expressed in recipient cells, functioning as exosomal shuttle RNA (24), whereas microRNA (miRNA) can be transported via exosomes to exert a gene-silencing effect, leading to the corresponding phenotypical changes in the receiving cells (25).
Advances in exosome research show that exosome-like vesicles derived from citrus can provide antioxidant activity in human cells (26); they have been shown to inhibit the proliferation of a p53-inactivated colorectal cancer cell line in a concentration-dependent manner (27). Plant cells produce exosomes or similar nanoparticles, called plant-derived exosome-like nanoparticles (ELNs), plant-derived extracellular vesicles (28) or edible plant-derived ELNs (29). Since exosomes derived from fruits and vegetables are edible nanoparticles that can be scaled up in production with relative ease and at a relatively low cost, and are more biocompatible and biodegradable, there is a high possibility that they can be developed as safe and useful cell-free therapeutic agents (30) There is also an industrial trend to bring food ingredients into cosmetics and personal care products, with the elucidation of biological activities of such ingredients currently under way (31). Therefore, the trend to apply plant-derived exosomes and extracellular vesicles to human use will only grow over time.
In the present report, exosomes from fruits and vegetables were screened for their abilities to suppress the expression of MMP1, 2, and 9 genes. Potato exosomes (ExoPs) were then selected to test cell-penetrating, anti-oxidant, collagen-promoting and anti-photoaging activities.
Material and methods
Preparation of exosomes from fruits and vegetables, and characterization of ExoPs
Vegetables [potato and radish (Raphanus sativus)] and fruits [pear (Pyrus pyrifolia) and citrus (Citrus sinensis)] bought from grocery stores were washed with distilled water, and 1X PBS (pH 7.4), towel-dried and juices were extracted using a commercial juice extractor with minimal heat generation during the extraction process. After centrifugation at 6,000 × g for 1 h at 4°C, 1 l cleared extract was obtained from 2 kg unprocessed potatoes. Cleared extract was further fractionated by centrifugation at 10,000 × g for 1 h at 4°C, and 39,000 × g for 1 h at 4°C, in succession, followed by filtration through 0.8 µm-cellulose nitrate membrane filters. As a last step, exosomes were pelleted by centrifugation at 120,000 × g for 2 h at 4°C. Exosome pellets were resuspended in 1X PBS (pH 7.4). Purified exosomes were quantified by the amounts of proteins in exosomes using Pierce™ Coomassie (Bradford) Protein Assay Kit (cat. no. 23200; Thermo Fisher Scientific, Inc.). The measurement of exosome size by dynamic light scattering (DLS) and ζ potential of purified ExoPs was performed with 0.1 mg exosome in 1 ml of 1X PBS using ζ-potential and a particle size analyzer (ELSZ-2000; Otsuka Electronics Co., Ltd.). DLS analyses were repeated >10 times, each with independent exosome purification from independent batches of potatoes to corroborate the observations. To observe the morphology of purified exosomes using transmission electron microscopy (TEM; JEOL Ltd.), exosome samples in 0.1 mg/ml PBS were dropped onto Formvar/Carbon Supported Copper Grids (cat. no. TEM-FCF300CU50; MilliporeSigma) support and were stained with 1% uranyl acetate for 15 sec at room temperature, washed with distilled water twice, air dried, and were observed using TEM (JEM-1011; JEOL Ltd.).
Preparation of bacterial exosomes (ExoBACs) from Escherichia coli strain DH5α, potato protein extract (Ext) and potato alcohol extract (AE)
For the isolation of ExoBACs, DH5α (cat. no. 9057; Takara Bio Inc.) was grown in Luria-Bertani broth (1% tryptone, 0.5% yeast extract, 1% NaCl) at 37°C with agitation at 200 rpm. After centrifugation at 6,000 × g for 1 h at 4°C, the supernatant was further fractionated by centrifugation at 10,000 × g for 1 h at 4°C, and 39,000 × g for 1 h at 4°C, in succession, followed by filtration through 0.8 µm-cellulose nitrate membrane filters. Finally, exosomes were pelleted by centrifugation at 120,000 × g for 2 h at 4°C. Exosome pellets were resuspended in PBS (pH 7.4). For Ext, potato juices were extracted using a commercial juice extractor. After centrifugation at 6,000 × g for 1 h at 4°C, total proteins were quantified using the Pierce™ Coomassie (Bradford) Protein Assay Kit (Thermo Fisher Scientific, Inc.). For potato AE, 200 µl Ext was mixed with 400 µl 99% ethanol (MilliporeSigma), vortexed and mixed in a shaking incubator at room temperature for 20 min at 300 rpm in a closed-cap microcentrifuge tube. Alcohols in the tube were evaporated and 1X PBS was added. The alcohol concentration in the HaCaT cell treatment was <0.01% of the culture media.
Exosome tracking
A total of 300 µg isolated ExoPs in 1X PBS (pH 7.4) were mixed with 1 µl ExoGlow™ Protein EV Labeling Kit (Green; cat. no. EXOGP300A-1; System Biosciences, LLC) to a total volume of 500 µl, and were incubated at 37°C with agitation at 350 rpm for 20 min. Free fluorescent dyes from the ExoGlow Protein EV Labeling Kit were cleared of the labeled exosomes by ExoQuick-TC, which was included in the ExoGlow Protein EV Labeling Kit, or Amicon® Ultra-15 centrifugal filter units (cat. no. UFC900308; MilliporeSigma). Labeled exosomes were resuspended in 1X PBS. A total of 1×105 HaCaT cells were treated with fluorescent dyes only, or with 50 and 100 µg/ml labeled exosomes, and were incubated in Dulbecco's Modified Eagle's Medium-high glucose (DMEM; cat. no. 11965092; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS; MilliporeSigma), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C and 5% CO2 for 4 h. A confocal microscope (LSM 980 with Airyscan 2; Zeiss AG) was used to capture the image of labeled exosomes.
Cell culture, exosome treatment and cell viability assays
Keratinocyte HaCaT cells (cat. no. 300493; CLS Cell Lines Service GmbH) were maintained in DMEM at a density of 5×103 cells/well in a 96-well culture plate, with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C and 5% CO2. For the cell proliferation assay, ExoPs at a final concentration of 25, 50, 100, 150 and 200 µg/ml, or ascorbic acid (AA; cat. no. BP461; MilliporeSigma) at a final concentration of 1, 3, 5 and 100 µg/ml, were added to the cells, and incubated at 37°C and 5% CO2. AA- and exosome-treated HaCaT cells were further incubated for 24 or 48 h before Cell Proliferation Reagent WST-1 (cat. no. 5015944001; MilliporeSigma) was added. Formazan formation was detected at 420 nm in a microplate reader.
Exosome effect before and after UVB irradiation of HaCaT cells
First, HaCaT cells maintained in DMEM with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin were seeded in a 6-well culture plate at a density of 5×103 cells/well and were then irradiated with 15 mJ/cm2 UVB using a UVP Crosslinker (cat. no. 849-95-0615-04; Analytik Jena GmbH). Subsequently, cells were cultured for 24 h at 37°C and 5% CO2, before treatment with either ExoPs (50 µg/ml) in PBS or PBS, and cells were then cultured for an additional 24 h at 37°C and 5% CO2. In the second experiment, HaCaT cells were pre-treated with either ExoPs (50 µg/ml) in PBS or PBS for 24 h at 37°C and 5% CO2. Cells were then irradiated with 15 mJ/cm2 UVB and cultured for an additional 24 h at 37°C and 5% CO2. During the irradiation step, cells were washed with 1X PBS twice, and were then incubated in 1X PBS for irradiation for a span of 5 sec, and the 1X PBS was replaced with DMEM for further incubation.
Reverse transcription-PCR (RT-PCR)
Total RNA was isolated from untreated HaCaT cells, HaCaT cells treated with exosomes isolated from vegetables and fruits, HaCaT cells treated with ExoPs in combination with UVB irradiation, and HaCaT cells treated with Ext, AE and ExoBACs in combination with UVB irradiation using the RNeasy Mini Kit (cat. no. 74104; Qiagen GmbH). A total of 1 µg isolated total RNA was incubated with 0.5 µg oligo-dT primer (Bionics Co., Ltd.) in a total volume of 13.4 µl at 65°C for 10 min. The mixture was further incubated, following the addition of SuperScript™ II Reverse Transcriptase (cat. no. 18064022; Invitrogen; Thermo Fisher Scientific, Inc.), reaction buffer, DTT and dNTPs in a final volume of 20 µl at 42°C for 90 min. For qPCR, AccuPower® GoldHotstart Taq PCR PreMix & Master Mix was used (cat. no. K-2621; Bioneer). The MiniAmp Plus (cat. No. A37835; Thermo Fisher Scientific, Inc.) was used for gene-specific amplifications in a 50 µl reaction volume at 95°C for 5 min, followed by 30 cycles at 94°C for 30 sec, 48°C for 30 sec and 72°C for 60 sec, followed by a final incubation at 72°C for 10 min. GAPDH was used as a reference gene for normalization on 1% agarose gel electrophoresis. Agarose gels were stained with MIDORIGreen Xtra (Nippon Genetics Europe GmbH) and were visualized using a gel imager (AZURE 200; Azure Biosystems, Inc.). Densities of gel bands were obtained using ImageJ (version 2; National Institute of Health). Oligonucleotide primers used for the PCR are listed in Table I.
Table I.
Oligonucleotide primers used for PCR.
| Gene | PCR product size, bp | Sequence, 5′-3′ |
|---|---|---|
| GAPDH | 380 | ATTCCATGGCACCGTCAAGG |
| TGATGGCATGGACTGTGGTC | ||
| IL6 | 343 | CCAGTACCCCCAGGAGAAGA |
| CAGCTCTGGCTTGTTCCTCA | ||
| TNF-α | 373 | GTGACAAGCCTGTAGCCCAT |
| CTGAGTCGGTCACCCTTCTC | ||
| MMP1 | 396 | GGTGTGAGTCCAAACAAGGTG |
| CCTTGCCTATCCAGGGTGAC | ||
| MMP2 | 330 | GCCCCCAAAACGGACAAAG |
| CCAGACTTGGAAGGCACGAG | ||
| MMP9 | 396 | TCTATGGTCCTCGCCCTGAA |
| GCTCCTCAAAGACCGAGTCC | ||
| Glutathione S-transferase α 4 | 332 | GAGGGGACACTGGATCTGCT |
| GGAGGCTTCTTCTTGCTGCC |
Hydrogen peroxide (H2O2) scavenging activity of ExoPs
In a 96-well plate, HaCaT cells were plated at a density of 5×103 cells/well in 100 µl DMEM, together with 100 U/ml penicillin and 100 µg/ml streptomycin, and were cultured at 37°C and 5% CO2. After washing the cells twice with 1X DPBS (pH 7.0), cells were mock-treated with 1X DPBS (pH 7.0) or treated with ExoPs (50–150 µg/ml) in 1X DPBS (pH 7.0) for 30 min at 37°C and 5% CO2. Afterwards, H2O2 (cat. No. 4158-4405; Daejung Chemicals & Metals Co., Ltd) at a final concentration of 200 µM was added to each well, with the exception of the well containing control HaCaT cells, and cells were incubated for 3 h at 37°C and 5% CO2 before the addition of Cell Proliferation Reagent WST-1 (cat. No. 5015944001; MilliporeSigma).
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity of ExoPs
To assess the antioxidant activity of ExoPs against the free radical DPPH, OxiTec™ DPPH Antioxidant Assay Kit (Colorimetric; cat. No. BO-DPH-200; Biomax.Ltd) was used according to the manufacturer's instructions. AA was used as a reference. Briefly, 1, 2, 4 mg/ml ExoPs in 20 µl 1X Dulbecco's-PBS (DPBS; pH 7.0) were mixed with 80 µl 1X assay buffer and 100 µl DPPH working solution. The reaction mixture was incubated at room temperature in the dark for 30 min and absorbance was measured at 517 nm. Inhibition rate was calculated as follows: (Control-Sample) ×100/(Control).
Flow cytometric analysis of penetration of ExoPs into HaCaT cells
Membrane proteins of ExoPs were labeled with ExoGlow-Protein EV Labeling Kit (Green; cat. no. EXOGP300A-1; Systems Biosciences, LLC), and cleared of free dyes with Amicon Ultra-15 Centrifugal Filter Units (cat. no. UFC900308; Merck). HaCaT cells were cultured in a 12-well plate at a density of 1×105 cells/well, and were mock-treated with 1X DPBS, treated with free labeling dyes from the ExoGlow Protein EV Labeling Kit or fluorescence-labeled ExoPs for 24 h at 37°C and 5% CO2. Cells were recovered following trypsin-EDTA treatment and washed twice with 1X DPBS. Washed cells were resuspended in BD Pharmingen™ Stain Buffer (BSA) (cat. no. 554657; BD Biosciences), assessed using FACS Canto II (BD Biosciences) and analyzed with BD FACSDiva (version 8.0; BD Biosciences). Free dye in this analysis refers to the reaction of ExoGlow-Protein EV Labeling Kit without exosome substrate, incubated and cleared by Amicon Ultra-15 centrifugal filter units.
Effect of ExoPs on the synthesis of soluble collagen in UVB-irradiated HaCaT cells
HaCaT cells were cultured in duplicate, each in 100 µl DMEM, 10% FBS, and 1% penicillin and streptomycin in a 96-well plate at a density of 5×103 cells/well maintained at 37°C and 5% CO2. A total of 6 h after cell plating, HaCaT cells were washed twice with 1X DPBS and mock-treated with 1X DPBS or treated with ExoPs (50–150 µg/ml) in 1X DPBS (pH 7.0) for 30 min at 37°C and 5% CO2. A total of 24 h after treatment, cells were irradiated with 15 mJ/cm2 UVB and cultured for an additional 24 h. The amount of secreted soluble collagens was estimated using Soluble Collagen Assay Kit (cat. no. ab241015; Abcam) in the cell culture media.
Graph creation and statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 software (Dotmatics). The data are presented as the mean ± SD from three independent experiments and were analyzed using the unpaired Student's t-test (for two groups) or one-way ANOVA with Tukey's honestly significant difference post-hoc test (for three or more groups). Graphs were created using GraphPad Prism 8.0 software or the ggpubr package (32) of R 4.3.1 for Windows and RStudio (Version: 2023.06.0+421; http://www.rstudio.com/). P<0.05 was considered to indicate a statistically significant difference.
Results
ExoPs suppress the expression of MMPs and inflammatory cytokine genes in keratinocyte HaCaT cells
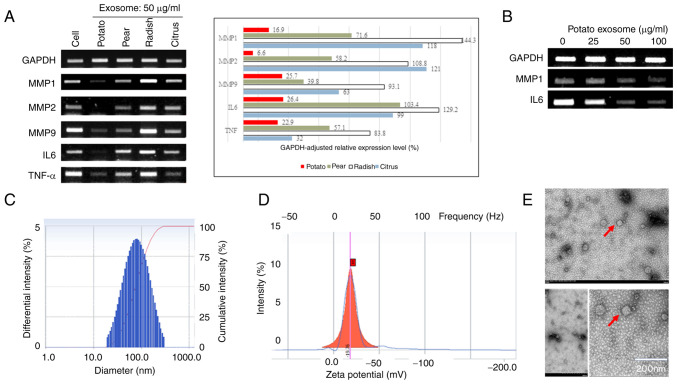
The effects of isolated exosomes on the expression of MMPs and inflammatory cytokine genes in the keratinocyte cell line HaCaT were investigated by RT-PCR (Fig. 1A). ExoPs downregulated the expression of MMP1, 2 and 9. Exosomes from pear also markedly suppressed the expression of MMPs, even though to a lower level compared with ExoP treatment. By contrast, exosomes isolated from radish did not seem to affect the expression of any of the investigated genes. The citrus exosomes only slightly suppressed MMP9, without affecting the expression of either MMP1 or 2. The effect of exosomes on the expression of inflammatory cytokines in HaCaT cells was also investigated. The expression levels of the proinflammatory cytokines TNF-α and IL6 were reduced in ExoP-treated HaCaT cells, and to a lesser extent in pear exosome-treated cells, whereas exosomes from radish and citrus did not induce changes in IL6 expression. On the other hand, exosomes from citrus specifically suppressed the expression of TNF-α without showing any influence on the expression of either MMPs or IL6. The effect of ExoPs on the suppression of MMP1 and IL6 in HaCaT cells was then tested in an ExoP concentration-dependent manner. ExoPs showed concentration-dependent suppression of MMP1 and IL6 without affecting the expression of the housekeeping gene GAPDH (Fig. 1B) in HaCaT cells. These results suggested that ExoPs and pear exosomes potentially have anti-aging and anti-inflammatory capabilities. Due to higher efficiency in MMP suppression, the relative ease in isolation and higher yield, the present study focused on ExoPs to investigate their anti-photoaging capability.
Figure 1.
Characterization of ExoPs. (A) Screening of exosomes from fruits and vegetables for the inhibitory activities on the expression of MMPs and cytokine genes in keratinocyte HaCaT cells investigated by RT-PCR. PCR bands were semi-quantified using ImageJ and normalized to GAPDH. (B) Concentration-dependent effect of ExoP on the expression of MMP1 and IL6 in HaCaT cells, as measured by RT-PCR. (C) Determination of size distribution of ExoPs using the dynamic light scattering principle and a particle size analyzer. (D) Measurement of ζ potential for ExoPs. (E) Transmission electron microscopy of ExoPs revealed double-layered oval shapes, a common morphology also observed among exosomes isolated from microorganisms and higher eukaryotic cells. Scale bar, 200 nm. ExoPs, potato exosomes; RT-PCR, reverse transcription-PCR.
Characterization of ExoPs
DLS analyses showed that the diameter range of ExoPs was 50–250 nm, with an average diameter of 60 nm (Fig. 1C). The peak of DLS was at 90 nm, with 80% of all intensities being distributed at 80–120 nm. A ζ potential of −19 mV for ExoPs in 1X PBS was obtained (Fig. 1D), indicating non-aggregating, relatively stable particles in solution (33). ExoPs displayed oval-shaped, double-layered morphology under TEM (Fig. 1E).
Uptake of ExoPs by HaCaT cells
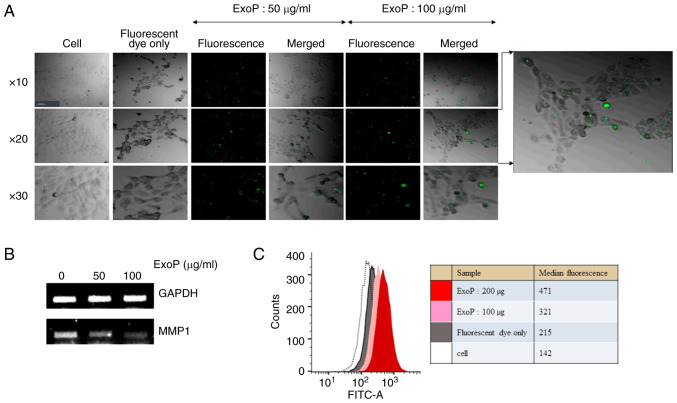
To prove HaCaT cell penetration by ExoPs, green fluorescence-labeled ExoPs were incubated with HaCaT cells and were observed under a confocal microscope. Whereas the HaCaT cells treated with fluorescence dye only did not show any signals, cells treated with fluorescence-labeled ExoPs displayed green dots representing ExoPs (Fig. 2A). These results suggested that ExoPs are acquired by HaCaT cells. To determine the association between the penetration of ExoPs into HaCaT cells and their effect on the expression of MMPs, a duplicate set of HaCaT cells was treated with fluorescence-labeled ExoPs following the same procedure as for confocal microscopy, total RNA was isolated for cDNA synthesis, and MMP1 RT-PCR was repeated. Again, it was shown that ExoPs downregulated the expression levels of MMP1 in HaCaT cells in a concentration-dependent manner (Fig. 2B). Entry of ExoPs into HaCaT cells was investigated by an independent flow cytometric analysis (Fig. 2C). Compared with unlabeled cells and free dyes, the fluorescent peaks following treatment with either 100 or 200 µg ExoPs show the concentration-dependent entry of ExoPs into HaCaT cells. These results suggested that ExoPs may enter HaCaT cells and contribute to the suppression of MMP1 gene expression, thus affecting the expression of other MMPs and cytokine genes (Fig. 1A).
Figure 2.
Cell penetration by ExoPs. (A) Confocal microscopy images of HaCaT cells, HaCaT cells treated with fluorescent dyes only, and HaCaT cells treated with either 50 or 100 µg/ml fluorescence-labeled ExoPs are shown. Magnification, ×10, ×20 and ×40. (B) Confirmation of MMP1 suppression following ExoP treatment. (C) Flow cytometric analyses of HaCaT cell penetration by ExoPs. Labeled ExoPs were incubated with HaCaT cells for 24 h and were analyzed by a flow cytometer. The median intensities are shown. ExoPs, potato exosomes.
Effect of ExoPs on HaCaT cell viability
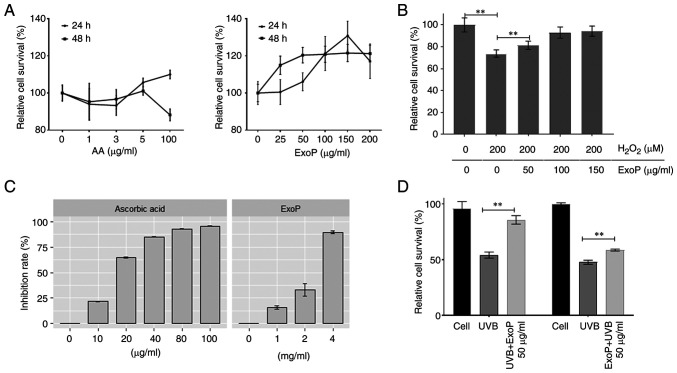
To investigate whether ExoPs have any effect on HaCaT cell viability, cells were treated with an increasing concentration of ExoPs before WST-1 assays were performed 24 h posttreatment. As a control, treatment of HaCaT cells with AA did not alter cell viability at either 5 or 100 µg/ml for 24 h, but decreased cell viability was observed after 48 h of AA treatment from 5 to 100 µg/ml (Fig. 3A), consistent with a previously published result (34). By contrast, ExoPs promoted HaCaT cell proliferation in a concentration-dependent manner (up to 200 µg/ml; Fig. 3A). However, by 48 h posttreatment, the exosome effect on HaCaT cell proliferation was stalled, but treated cells still maintained 20% higher relative cell survival compared with untreated cells. These results indicated that ExoPs can promote keratinocyte cell viability, and no cytotoxicity was observed in HaCaT cells after treatment with 200 µg/ml ExoPs for 48 h.
Figure 3.
Effects of ExoPs on HaCaT cell viability. (A) Relative viability of HaCaT cells treated with ExoPs, as measured by WST-1 assay. Counts of untreated cells over treated cells are represented as %. AA was used as a reference. (B) H2O2-scavenging activities of ExoPs. H2O2 was used to treat HaCaT cells, followed by the addition of an increasing concentration of ExoPs. (C) Free radical-scavenging activity of ExoPs was investigated using DPPH free radical with AA as a standard antioxidant. ExoPs (range, 0–4 mg/ml) were incubated with DPPH at room temperature in the dark for 30 min, before absorbance was measured at 517 nm by spectrophotometry. (D) Effects of ExoPs on UVB irradiation on HaCaT cells. Cells were treated with ExoPs either before UVB irradiation (pre-treatment) or following UVB irradiation (post-treatment). Data are shown as the mean ± standard deviation. **P<0.01. ExoPs, potato exosomes; AA, ascorbic acid; H2O2, hydrogen peroxide; DPPH, 1,1-diphenyl-2-picryl-hydrazyl; UVB, ultraviolet B.
Antioxidant activities of ExoPs
Addition of excessive H2O2 to cultured cells can mimic environmental oxidative stress, which is considered the main mechanism for oxidative damage (35). When HaCaT cells were treated with 200 µM H2O2, proliferation was reduced to 73% of untreated cells (Fig. 3B). When HaCaT cells were treated with a final concentration of either 50, 100 or 150 µg/ml ExoPs for 30 min prior to H2O2 addition, ExoPs prevented H2O2-initiated reductions in cell viability in a concentration-dependent manner, rescuing the population of treated cells to 81, 92 and 94% of untreated cells. To investigate the direct antioxidant activity of ExoPs, a colorimetric DPPH inhibition assay was used (Fig. 3C). When the violet DPPH radical is reduced by antioxidant molecules, it turns into a yellowish color, and the colorimetric changes are detected at 517 nm. The changes in absorbance are interpreted as DPPH inhibition or scavenging activity (36). AA, used as a reference, showed a concentration-dependent DPPH inhibition (Fig. 3C). ExoPs also exhibited a concentration-dependent DPPH-scavenging activity (Fig. 3C).
Effect of ExoPs on HaCaT cells exposed to UVB irradiation
The relationship between ExoP treatment and UVB-initiated photodamage was investigated in HaCaT cells. When HaCaT cells were irradiated with 15 mJ/cm2 UVB, and further cultured for 24 h, a ~55% reduction in cell viability was detected (Fig. 3D). When HaCaT cells were pretreated with ExoPs, and then irradiated with UVB, the reduction in cell viability was only 10%, indicating that pretreatment of HaCaT cells with ExoPs may minimize UVB damage to cells (Fig. 3D). When HaCaT cells were first irradiated with UVB, and then incubated with ExoPs for an additional 24 h, cell viability loss was reduced to 40% (Fig. 3D). This finding revealed that ExoPs can rescue even pre-UVB-exposed cells, albeit to a lesser degree compared with pretreatment with ExoPs. Taken together, these results suggested that ExoPs not only minimize UVB-initiated photodamage in HaCaT cells, but also have the potential to rescue cells from photodamage already incurred by UVB irradiation.
Gene expression changes induced by ExoPs in keratinocyte HaCaT cells in relation to photodamage incurred by UVB irradiation
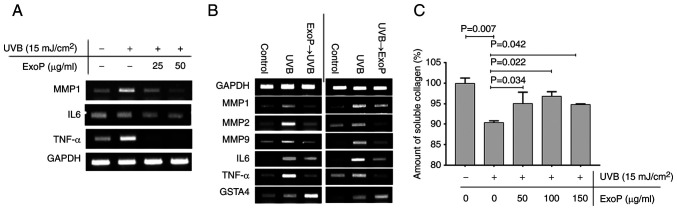
To further investigate the relationship between UVB-initiated photodamage in HaCaT cells and the preventive and recovering effects of ExoPs at a molecular level, the treatment of UVB-irradiated HaCaT cells with an increasing concentration of ExoPs was investigated, and changes in gene expression levels were monitored using RT-PCR. UVB irradiation induced the expression of MMP1, IL6 and TNF-α without altering the expression of GAPDH in HaCaT cells (Fig. 4A). The elevated expression levels of MMP1 and IL6 induced by UVB irradiation were gradually downregulated following treatment of UVB-irradiated HaCaT cells with ExoPs in a concentration-dependent manner. This result suggested that ExoPs may contribute to the suppression of the aforementioned genes. In particular, TNF-α was highly induced by UVB irradiation, but was markedly suppressed by ExoPs at 25 µg/ml.
Figure 4.
Mechanism of prevention and amelioration of UVB photodamage by ExoPs (A) Reverse transcription-PCR validation of concentration-dependent suppression of UVB-induced MMP1, IL6 and TNF-α by ExoPs in keratinocyte HaCaT cells. (B) PCR analyses of the expression levels of MMPs, cytokine genes and GSTA4, when HaCaT cells were treated with ExoPs prior to UVB irradiation and following UVB irradiation. (C) Effect of ExoPs on collagen synthesis in HaCaT cells pre-irradiated with UVB. Data are shown as the mean ± standard deviation. UVB, ultraviolet B; ExoPs, potato exosomes; GSTA4, glutathione S-transferase α 4.
Molecular mechanisms underlying prevention and salvage of UVB damage by ExoPs
ExoPs not only prevented HaCaT cells from UVB damage, but also alleviated UVB damage that had already occurred to a certain extent (Fig. 3D). The effects of ExoPs on HaCaT cells against UVB-induced photodamage were next investigated at a molecular level. First, HaCaT cells were irradiated with UVB, ExoPs were added to the culture media, and the cells were incubated under culture conditions for another 24 h. UVB irradiation induced the expression of MMP1, 2 and 9, and the inflammatory cytokine genes IL6 and TNF-α. UVB irradiation also induced the expression of glutathione S-transferase α 4 (GSTA4), which acts as a cellular defense mechanism against lipid peroxidation (Fig. 4B). Treatment with ExoPs reduced the UVB-induced gene expression levels of MMPs, IL6 and TNF-α. By contrast, the expression of GSTA4 was further augmented in HaCaT cells following treatment with ExoPs. These results revealed that treatment of HaCaT cells with ExoPs following UVB irradiation selectively suppressed the expression of genes whose protein products contribute to the destruction of collagen proteins and to the induction of inflammation, but at the same time induced the expression of GSTA4, whose role is to protect the cells against oxidative damage, providing the cells with double-layered protection against UVB-induced photodamage. Therefore, posttreatment of HaCaT cells with ExoPs provides an ameliorative effect against UVB-induced photodamage. When ExoPs were used to treat cells prior to UVB irradiation, they limited the expression of MMPs and inflammatory cytokine genes from reaching the level induced by UVB alone, and at the same time further increased the expression levels of the cell-protecting GSTA4 (Fig. 4B). These data indicated that pretreatment of HaCaT cells with ExoPs may provide preventive effects against UVB-induced photodamage. Combined, these data point to the possibility that ExoPs influence the expression levels of MMPs, inflammatory cytokine genes and GSTA4, leading to a preventive and ameliorative effect on the keratinocyte cell line HaCaT from UVB-induced photodamage.
Prevention of UVB-mediated collagen destruction by ExoPs in HaCaT cells
Since suppression of MMP genes in HaCaT cells by ExoPs implies that the ExoPs might exert a positive effect on collagen synthesis, the changes in the amount of soluble collagen secreted into the media following UVB irradiation of HaCaT cells was directly measured and compared with that of HaCaT cells pre-treated with ExoPs prior to UVB irradiation. UVB irradiation of HaCaT cells at 15 mJ/cm2 reduced the amount of soluble collagen by 10% compared with untreated HaCaT cells (Fig. 4C). By contrast, pretreatment with 150 µg/ml ExoPs reduced the amount of soluble collagen by 5% compared with untreated HaCaT cells, minimizing or preventing the collagen destruction (Fig. 4C). Taken together, ExoPs readily penetrate the cellular membrane and prevent the destruction of collagen proteins in response to UVB photodamage by modulating the expression of cellular genes, including MMPs, inflammatory cytokines and host defense genes.
Regulation of target gene transcription is specific to ExoPs
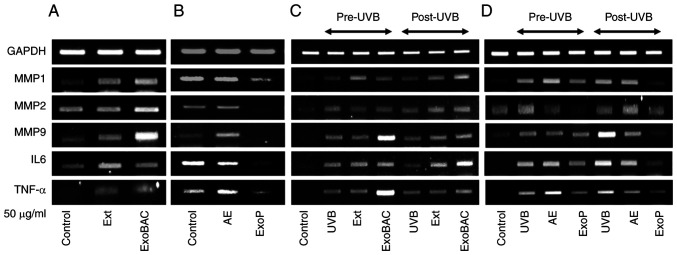
To ensure that the regulation of photodamage-related gene transcription is due to the specific activities of ExoPs, other components of potato extracts were investigated for their effect on HaCaT cells. Whereas ExoPs significantly suppressed the expression of MMP1, 2 and 9, IL6 and TNF-α, Ext, potato AE and ExoBACs did not have a suppressing effect on the same set of genes (Fig. 5A and B). Ext was shown to increase the expression levels of MMP1 and 9, and those of the inflammatory cytokines IL6 and TNF-α (Fig. 5A). ExoBACs markedly promoted the expression of MMP1, 2 and 9, IL6 and TNF-α (Fig. 5A). AE had no influence on the expression of the investigated genes, with the exception of an increase in the expression MMP9 (Fig. 5B). Also, since ExoPs showed a notable effect on preventing and ameliorating the effect of UVB-induced photodamage on HaCaT cells by downregulating the transcription of MMP1, 2 and 9, IL-6 and TNF-α (Fig. 4B), the same set of experiments was repeated to enable comparisons with the activities of Ext, AE and ExoBACs. Again, compared with the significant induction of MMP1, 2 and 9, IL6 and TNF-α transcription by UVB irradiation and the minimization of this induction by pretreatment of ExoPs (Fig. 5D), Ext, ExoBACs (Fig. 5C) and AE (Fig. 5D) had no visible effect on the activated genes. ExoBACs further elevated the UVB-activated transcription of MMP9 and TNF-α. Downregulation of MMP1, 2 and 9, IL6 and TNF-α by posttreatment of UVB-activated HaCaT cells with ExoPs were again validated post-UVB irradiation (Fig. 5C), whereas Ext and ExoBACs post-UVB (Fig. 5C), and AE post-UVB (Fig. 5D) did not show marked effects. Compared with the cell viability-promoting effect of ExoP at 25 and 50 µg/ml (Fig. S1A), and at ≤200 µg/ml for 48 h (Fig. S1B), Ext did not have any effect on HaCaT cell viability. By contrast, AE markedly suppressed HaCaT cell viability (Fig. S1A and B). In addition, whereas ExoPs exhibited preventive and rescuing activities on HaCaT cells against UVB photodamage, Ext, AE (Fig. S2) and ExoBACs (Fig. S3) did not display such activities.
Figure 5.
Regulation of target gene transcription is specific to ExoPs compared with Ext, AE and ExoBACs. (A) RT-PCR analyses of the effect of Ext and ExoBACs on the expression of MMP1, 2 and 9, IL6 and TNF-α in HaCaT cells. (B) RT-PCR analyses of the effect of AE and ExoPs on the expression of the same set of genes in HaCaT cells. (C) RT-PCR analyses of the expression of MMP1, 2 and 9, IL6 and TNF-α, when the Ext and ExoBACs were used for the treatment of HaCaT cells pre-UVB and post-UVB irradiation. (D) RT-PCR analyses of the expression of MMP1, 2 and 9, IL6 and TNF-α, when the AE and ExoPs were used for the treatment of HaCaT cells pre-UVB and post-UVB irradiation. Ext refers to the total protein extract of potatoes, which was diluted to the final cell treatment concentration, equivalent to 50 µg/ml in starting protein amount and ≤0.1% of alcohol. Ext, potato protein extract; AE, alcohol extract; ExoBACs, bacterial exosomes; RT-PCR, reverse transcription-PCR; ExoPs, potato exosomes; Con, untreated control HaCaT cells.
Discussion
The present study revealed that ExoPs possess biological functions related to the suppression of MMPs, which are major contributors to the destruction of ECM components, including fibrillar collagens, and can lead to the formation of wrinkles.
Inhibition of MMP transcription by ExoPs is less likely to be an event caused by contaminating factors that were cofractionated with ExoPs because, as determined by RT-PCR analysis, only ExoPs and exosomes from pears showed suppressing activity on MMP transcription, whereas exosomes from radish did not show comparable activity to suppress the transcription of MMP1, 2 and 9. Even pear exosomes showed distinction compared with ExoPs, in that their ability to suppress MMP1 and 2, IL6 and TNF-α was between that of ExoPs and radish exosomes. Pear exosomes were very effective in MMP9 suppression. On the other hand, citrus exosomes were effective only in the suppression of inflammatory TNF-α transcription, but not in the suppression of MMP1, 2 and 9, and IL6.
Even though it was shown in the current study that ExoPs showed the best ability to suppress the transcription of MMP1, 2 and 9, and proinflammatory cytokines IL6 and TNF-α, the exact mechanism underlying transcriptional suppression is not currently clear. Potatoes are known to contain various bioactive compounds; for example, AE has been shown to notably increase the expression of type I collagen mRNA and the secretion of collagen from human dermal fibroblasts (37). Potatoes are also rich in phenolic acids containing chlorogenic and caffeic acids, which are known as strong inhibitors of MMP9 (38). Therefore, it is possible that dietary polyphenols, possibly encapsulated in ExoPs, readily traverse the cellular membrane to contribute to the signal transduction pathways that lead to changes in gene expression levels in HaCaT cells in the current study. Excessive UV irradiation, as well as improper and overuse of cosmetic products, can lead to the disruption of the skin barrier and cause skin inflammation. Cells react to oxidative damage by elevating the production of antioxidant enzymes, including GSTs. GSTA4 belongs to the α class of cytoplasmic, soluble GSTs and functions as a phase II detoxifying enzyme against oxidative damage. GSTA4 is adaptive to oxidative stress in its induction and provides cells protection against oxidative stress (39).
The data in this report can be summarized that purified ExoPs readily penetrate HaCaT cells, they show no signs of cytotoxicity, promote cell proliferation, decrease the synthesis of collagen-degrading MMPs, increase the production of detoxifying GSTA4, and, following cell pretreatment, they protect cells against UVB-induced damage by limiting the synthesis of the inflammatory cytokines IL6 and TNF-α. Furthermore, ExoPs minimize UVB-induced damage by downregulating the transcription of IL6 and TNF-α, upon treatment of UVB-irradiated cells. ExoPs have the potential to decrease the appearance of wrinkles and therefore fit the description of natural and safe cosmeceuticals, which are combinations of a drug and a cosmetic (39). Further studies employing 2D and 3D cell cultures would further validate the effect of the photo-protective role of ExoPs, and clinical evaluation would ensure the safety of cosmetics containing ExoPs as the main ingredient.
To the best of our knowledge, the present study is the first to report on the isolation and investigation of the activity of exosomes from Solanum tuberosum. Therefore, several steps were taken to ensure that high-quality ExoPs were obtained. Potatoes were purchased from NH Nonghyup Hanaro Mart, a national specialty chain store for agricultural and aquatic products, where a quality control is assured on a regular basis for freshness, residual pesticides and radioactivity. Sprouting or green potatoes were scarce and were eliminated from the processing to prevent unintended contamination of glycoalkaloids, including solanine. In the subsequent processing of potatoes, extensive cleaning and peeling was performed to eliminate any possible contamination of ExoPs with soil bacteria. Even with this speedy process, precautions were taken when necessary to minimize the exposure of extracts to light and air by storing the extracts during the intermediate stages in vacuum-sealed containers in the dark. Future inspection of purified ExoPs by multiomics, especially metabolomics analysis, will reveal more about their contents.
Undergoing work to set the condition for mid- to long-term storage, large-scale production of ExoPs and multiomics analyses of ExoPs would reveal more about the nature of ExoPs, and the feasibility of developing these ExoPs into ingredients of restorative and safe anti-aging cosmetic products. Based on the data showing that ExoPs can prevent and ameliorate UVB damage with their anti-inflammatory effects, it is feasible to apply ExoPs or sunscreen cosmetics containing ExoPs for use before or after UVB exposure. Why and how ExoPs downregulate MMPs and cytokine genes, while upregulating host protective genes, such as GSTA4, are unknown, and likely involve the combination of activities of exosome contents. Future studies on the components of ExoPs, and global gene expression analyses of keratinocyte and fibroblast cells treated with ExoPs, would help to elucidate the underlying molecular pathways.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- DLS
dynamic light scattering
- DPPH
2,2-diphenyl-β-picrylhydrazyl
- GSTA4
glutathione S-transferase α 4
- H2O2
hydrogen peroxide
- ExoP
potato exosome
- Ext
potato protein extract
- AE
alcohol extract
- ExoBAC
bacterial exosome
Funding Statement
The current study was funded by Nextab, Inc.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YL carried out data curation, formal analysis, validation and visualization, participated in investigation, developing methodology, and writing of the manuscript. DJ contributed to the cell culture and RT-PCR parts of the investigation. YJ conceptualized the study. HC conceptualized the study and wrote the manuscript. SY conceptualized the study, provided resources, carried out supervision, acquired funding, participated in the investigation, carried out project administration, and participated in writing, reviewing and editing the manuscript. YL and SY confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Note that a patent for skin improvement compositions based on the potato-derived exosomes has been submitted by authors YL, DJ and SY, which is awaiting approval.
References
- 1.Alfredsson L, Armstrong BK, Butterfield DA, Chowdhury R, de Gruijl FR, Feelisch M, Garland CF, Hart PH, Hoel DG, Jacobsen R, et al. Insufficient sun exposure has become a real public health problem. Int J Environ Res Public Health. 2020;17:5014. doi: 10.3390/ijerph17145014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim HS, Song JH, Youn UJ, Hyun JW, Jeong WS, Lee MY, Choi HJ, Lee HK, Chae S. Inhibition of UVB-induced wrinkle formation and MMP-9 expression by mangiferin isolated from Anemarrhena asphodeloides. Eur J Pharmacol. 2012;689:38–44. doi: 10.1016/j.ejphar.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 4.Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241–251. doi: 10.1002/path.2098. [DOI] [PubMed] [Google Scholar]
- 5.Gonzaga ER. Role of UV light in photodamage, skin aging, and skin cancer: Importance of photoprotection. Am J Clin Dermatol. 2009;10((Suppl 1)):S19–S24. doi: 10.2165/0128071-200910001-00004. [DOI] [PubMed] [Google Scholar]
- 6.Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–720. doi: 10.1016/S1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 7.Panwar P, Butler GS, Jamroz A, Azizi P, Overall CM, Brömme D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018;65:30–44. doi: 10.1016/j.matbio.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Kang S, Fisher GJ, Voorhees JJ. Photoaging and topical tretinoin: Therapy, pathogenesis, and prevention. Arch Dermatol. 1997;133:1280–1284. doi: 10.1001/archderm.1997.03890460104012. [DOI] [PubMed] [Google Scholar]
- 9.Al-Atif H. Collagen supplements for aging and wrinkles: A paradigm shift in the fields of dermatology and cosmetics. Dermatol Pract Concept. 2022;12:e2022018. doi: 10.5826/dpc.1201a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae JY, Choi JS, Kang SW, Lee YJ, Park J, Kang YH. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp Dermatol. 2010;19:e182–e190. doi: 10.1111/j.1600-0625.2009.01044.x. [DOI] [PubMed] [Google Scholar]
- 11.Jung YA, Lee JY, Lee P, Shin HS, Kim JE. Inhibition of solar UV-induced matrix metalloproteinase (MMP)-1 expression by non-enzymatic softening cherry blossom (prunus yedoensis) extract. Plants (Basel) 2021;10:1016. doi: 10.3390/plants10051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein EF, Lee J, Brown DB, Yu R, Van Scott E. Glycolic acid treatment increases type I collagen mRNA and hyaluronic acid content of human skin. Dermatol Surg. 2001;27:429–433. doi: 10.1097/00042728-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Sunder S. Relevant topical skin care products for prevention and treatment of aging skin. Facial Plast Surg Clin North Am. 2019;27:413–418. doi: 10.1016/j.fsc.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Park EY, Wilder ET, Lane MA. Retinol inhibits the invasion of retinoic acid-resistant colon cancer cells in vitro and decreases matrix metalloproteinase mRNA, protein, and activity levels. Nutr Cancer. 2007;57:66–77. doi: 10.1080/01635580701268238. [DOI] [PubMed] [Google Scholar]
- 15.David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273–288. doi: 10.1007/BF03259940. [DOI] [PubMed] [Google Scholar]
- 16.Wang HD, Chen CC, Huynh P, Chang JS. Exploring the potential of using algae in cosmetics. Bioresour Technol. 2015;184:355–362. doi: 10.1016/j.biortech.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Rona C, Vailati F, Berardesca E. The cosmetic treatment of wrinkles. J Cosmet Dermatol. 2004;3:26–34. doi: 10.1111/j.1473-2130.2004.00054.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira MS, Magalhães MC, Oliveira R, Sousa-Lobo JM, Almeida IF. Trends in the use of botanicals in anti-aging cosmetics. Molecules. 2021;26:3584. doi: 10.3390/molecules26123584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan N, Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. 2018;11:39. doi: 10.3390/nu11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avadhani KS, Manikkath J, Tiwari M, Chandrasekhar M, Godavarthi A, Vidya SM, Hariharapura RC, Kalthur G, Udupa N, Mutalik S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017;24:61–74. doi: 10.1080/10717544.2016.1228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed IA, Mikail MA, Zamakshshari N, Abdullah AH. Natural anti-aging skincare: Role and potential. Biogerontology. 2020;21:293–310. doi: 10.1007/s10522-020-09865-z. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Hyun CG. Natural products for cosmetic applications. Molecules. 2023;28:534. doi: 10.3390/molecules28020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osaki M, Okada F. Exosomes and their role in cancer progression. Yonago Acta Med. 2019;62:182–190. doi: 10.33160/yam.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 25.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldini N, Torreggiani E, Roncuzzi L, Perut F, Zini N, Avnet S. Exosome-like nanovesicles isolated from citrus limon L. Exert antioxidative effect. Curr Pharm Biotechnol. 2018;19:877–885. doi: 10.2174/1389201019666181017115755. [DOI] [PubMed] [Google Scholar]
- 27.Takakura H, Nakao T, Narita T, Horinaka M, Nakao-Ise Y, Yamamoto T, Iizumi Y, Watanabe M, Sowa Y, Oda K, et al. Citrus limonL.-Derived nanovesicles show an inhibitory effect on cell growth in p53-Inactivated colorectal cancer cells via the macropinocytosis pathway. Biomedicines. 2022;10:1352. doi: 10.3390/biomedicines10061352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urzì O, Gasparro R, Ganji NR, Alessandro R, Raimondo S. Plant-RNA in extracellular vesicles: The secret of cross-Kingdom communication. Membranes (Basel) 2022;12:352. doi: 10.3390/membranes12040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao J, Feng S, Wang X, Long K, Luo Y, Wang Y, Ma J, Tang Q, Jin L, Li X, Li M. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186. doi: 10.7717/peerj.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemati M, Singh B, Mir RA, Nemati M, Babaei A, Ahmadi M, Rasmi Y, Golezani AG, Rezaie J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun Signal. 2022;20:69. doi: 10.1186/s12964-022-00889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faria-Silva C, Ascenso A, Costa AM, Marto J, Carvalheiro M, Ribeiro HM, Simões S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci Technol. 2020;95:21–32. doi: 10.1016/j.tifs.2019.11.015. [DOI] [Google Scholar]
- 32.Kassambara A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. https://CRANR-project.org/package=ggpubr. R package version 0.2. 2020 [Google Scholar]
- 33.Darwish MSA, Al-Harbi LM, Bakry A. Synthesis of magnetite nanoparticles coated with polyvinyl alcohol for hyperthermia application. J Therm Anal Calorim. 2022;147:11921–11930. doi: 10.1007/s10973-022-11393-6. [DOI] [Google Scholar]
- 34.Savini I, D'Angelo I, Ranalli M, Melino G, Avigliano L. Ascorbic acid maintenance in HaCaT cells prevents radical formation and apoptosis by UV-B. Free Radic Biol Med. 1999;26:1172–1180. doi: 10.1016/S0891-5849(98)00311-6. [DOI] [PubMed] [Google Scholar]
- 35.Ransy C, Vaz C, Lombès A, Bouillaud F. Use of H2O2 to cause oxidative stress, the catalase issue. Int J Mol Sci. 2020;21:9149. doi: 10.3390/ijms21239149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai CE, Lin LH. DPPH scavenging capacity of extracts from Camellia seed dregs using polyol compounds as solvents. Heliyon. 2019;5:e02315. doi: 10.1016/j.heliyon.2019.e02315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suto M, Masutomi H, Ishihara K, Masaki H. A potato peel extract stimulates type I collagen synthesis via Akt and ERK signaling in normal human dermal fibroblasts. Biol Pharm Bull. 2019;42:1510–1516. doi: 10.1248/bpb.b19-00193. [DOI] [PubMed] [Google Scholar]
- 38.Jin UH, Lee JY, Kang SK, Kim JK, Park WH, Kim JG, Moon SK, Kim CH. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: Isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 39.Milam EC, Rieder EA. An approach to cosmeceuticals. J Drugs Dermatol. 2016;15:452–456. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.