Abstract
Chiral secondary organoboronic esters, when activated with t-butyllithium are shown to undergo efficient stereoretentive transmetalation with either zinc acetate or zinc chloride. This reaction provides chiral secondary alkylzinc reagents that are configurationally stable under practical experimental conditions. The organozinc compounds were found to engage in stereospecific reactions with difluorocarbene, catalytic cross-couplings with palladium-based catalysts, and trifluoromethylation with a copper(III) complex. Mechanistic and computational studies shed light on the inner-workings of the transmetalation event.
Graphical Abstract
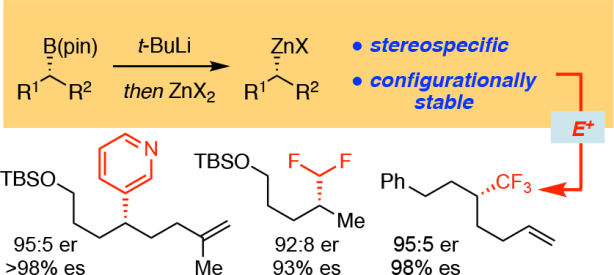
Enantiomerically-enriched alkylboronic esters are useful building blocks in organic synthesis. They are often chemically and configurationally stable, and they can be prepared through a variety of catalytic and non-catalytic methods.1 Moreover, alkylboronic esters undergo stereospecific transformations, allowing them to be used in the construction of a wide array of non-racemic chiral compounds.2 These attractive features notwithstanding, alkylboronic esters are weak nucleophiles and therefore less reactive for transmetalation compared to other organometallic compounds.3 Relative to alkylboronic esters, organozinc reagents are more nucleophilic, yet they are still configurationally stable.4 Pioneering studies by Knochel and coworkers5 have demonstrated that secondary alkylzinc reagents can undergo stereospecific transmetalation to copper and palladium, followed by coupling with electrophiles. Although efficient protocols have been reported for the enantioselective synthesis of α-amino6, α-boryl7 and cyclopropyl-based8 organozinc reagents, preparation of simple enantiomerically-enriched secondary alkylzinc compounds remains challenging.
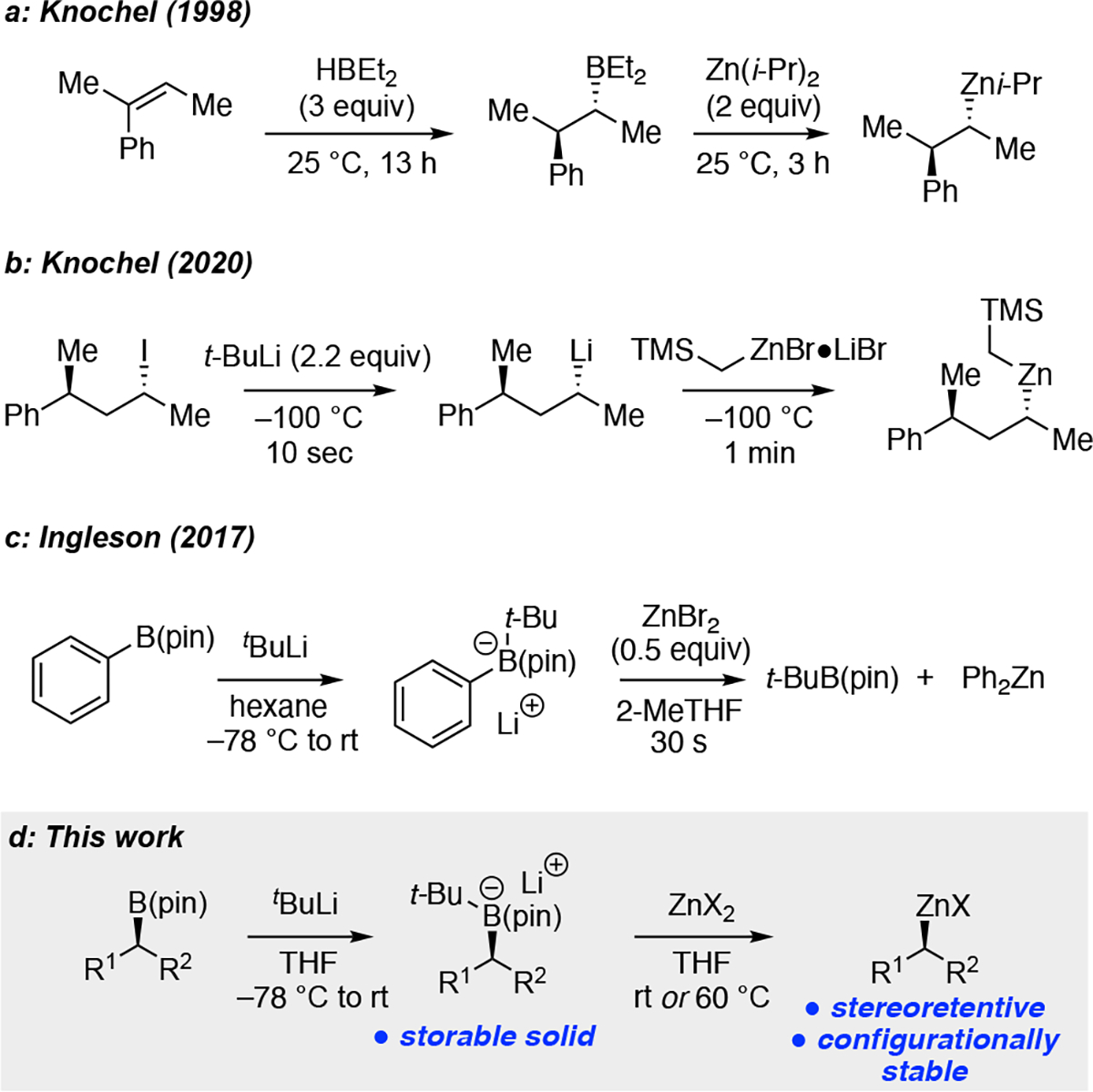
Knochel reported that secondary organoboranes, generated by hydroboration of alkenes, can undergo stereospecific transmetalation with diisopropylzinc thereby providing a route to α-chiral organozinc reagents (Scheme 1a).4a,9 In subsequent studies, it was found that secondary organoboronic esters could be converted to dimethylborane derivatives by treatment with methylmagnesium chloride; reaction with (i-Pr)2Zn then delivers the organozinc compound.10 Unfortunately, this latter process occurs with significant epimerization of the carbon stereo-center, likely as a consequence of metal halide salts present in the reaction mixture. More recently, Knochel reported that stereospecific lithium-iodine exchange, followed by treatment with TMSCH2ZnCl●LiBr generates the corresponding organozinc reagent (Scheme 1b).11 They also demonstrated that the so produced organozinc reagent can be used for stereospecific Negishi cross-coupling reactions. To expand the scope of transformations available to organoboron compounds, we considered direct transmetalation of alkyl-activated alkylboronic esters with zinc salts. Related transmetalations were examined by Ingleson during studies on zinc-catalyzed Suzuki-Miyaura reactions (Scheme 1c).12 They found that reaction of a PhB(pin)●t-BuLi adduct with zinc bromide rapidly furnished diphenylzinc. In this report, we describe an efficient, rapid, and stereospecific transmetalation that furnishes enantiomerically-enriched secondary alkylzinc reagents from readily available pinacol-derived alkylboronic esters (Scheme 1d). We also provide insight into this transformation and demonstrate that the newly generated organozinc reagents can engage in useful stereospecific transformations.
Scheme 1. Stereospecific Preparation of α-Chiral Organozinc Reagents.
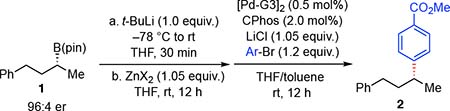
Recently, we developed several Cu-catalyzed stereospecific transformations of organoboronic esters.13 These reactions are believed to occur by a facile boron-to-copper transmetalation, followed by coupling with electrophiles. The success of this process relied on alkyllithium activation of otherwise unreactive alkyl boronic esters. It was of interest to determine whether similar boronic ester activation might obtain in other transmetalation processes. In spite of encouraging precedent from Falck14 with s-BuLi-activated primary boronic esters, preliminary experiments in Pd-catalyzed Suzuki-Miyaura cross-coupling of secondary boronic ester 1 with t-BuLi activation failed to deliver product (Table 1, entry 1). On the other hand, it was found that treating the tert-butyllithium-activated boronic ester with zinc salts lead to efficient consumption of the boron “ate” complex as determined by 11B NMR (70–98% conversion, 12 h at rt), and presumably generated the corresponding organozinc reagent. Subsequent addition of a palladium catalyst15 and an aryl halide electrophile furnished the Negishi cross-coupling product in 46–82% yield. Of note, the enantiospecificity of the reaction was found to be dependent on the identity of the zinc salt and the reaction time: zinc triflate and zinc acetate provided the product with high stereospecificity (Table 1, entries 5 and 6), while the reaction with zinc pivalate only provided high specificity if the transmetalation was conducted for short reaction periods (compare entries 7 and 8).
Table 1.
B-to-Zn Transmetalation and Negishi Coupling.
| ||||
|---|---|---|---|---|
| entrya | conditions | conversion (%) | yield (%)b | er (es %)c |
| 1 | w/o ZnX2 | <5 | <5 | - |
| 2 | ZnCl2 | 92 | 60 | 21:79 (63) |
| 3 | ZnBr2 | 70 | 49 | 79:71 (46) |
| 4 | Znl2 | 91 | 46 | 48:52 (4) |
| 5 | Zn(OTf)2 | >98 | 79 | 93:7 (93) |
| 6 | Zn(OAc)2 | >98 | 82 | 95:5 (98) |
| 7 | Zn(OPiv)2 | >98 | 67 | 82:18 (70) |
| 8d | Zn(OPiv)2 | >98 | 72 | 95:5 (98) |
Reactions were carried out with 0.10 mmol of 1.
Yields are determined by 1H NMR using tetrachloroethane as internal standard.
Enantiomeric ratio was measured by chiral SFC analysis and have an error of ±1%.
Step b conducted for 1 h.
Racemization in the couplings above might occur at any stage of the multistep sequence. To directly probe the stereospecificity of the boron-to-zinc exchange step, we examined the transmetalation of isotopically-labeled organoboronates 3-syn and 3-anti by in situ NMR spectroscopy (Figure 1).16 11B NMR was used to first learn about conditions needed to effect transmetalation of 3-syn (Figure 1a–c). Addition of t-butyllithium to 3-syn (δ 35 ppm) in THF furnished the boron “ate” complex (δ 10.4 ppm), cleanly (Figure 1a). Subsequent addition of zinc acetate (Figure 1b) resulted in consumption of the ate complex, as determined by reduction in the 10.4 ppm resonance, and formation of both t-BuB(pin) (δ 35.9 ppm) and a compound consistent with a three-coordinate borinic ester (δ 55.8 ppm).17 Heating the reaction mixture to 60 °C for 4 hours (Figure 1c), led to complete conversion of both the ate complex and the putative borinic ester to t-BuB(pin) and, presumably, the corresponding organozinc transmetalation product.
Figure 1.

Probing reactivity and stereospecificity of B-to-Zn transmetalation by in situ 11B and 13C NMR. (a) 11B NMR spectrum acquired after treatment of 3-syn with t-BuLi. (b) 11B NMR spectrum when 3-syn●t-BuLi was treated with Zn(OAc)2 at rt. (c) 11B NMR spectrum upon heating 3-syn●t-BuLi/Zn(OAc)2 mixture to 60 °C for 4 h. (d) 13C NMR analysis of 3-anti. (e) 13C NMR spectrum 3-anti●t-BuLi. (f) 13C Spectrum obtained upon reaction of t-BuLi●3-anti with Zn(OAc)2 at 60 °C for 4 h, followed by dilution with DMSO. (g) Summary of the efficiency and stereospecificity of transmetalations as determined by 13C NMR with labeled substrates.
To determine the stereospecificity of the transmetalation, the reaction sequence above was analyzed by in situ 13C NMR spectroscopy (Figure 1d–f). As shown in Figure 1d, the stereoisomer ratio of the reaction intermediates is readily measured by integrating the 13C NMR resonance for the labeled carbon, and this ratio reflects the stereospecificity of each transformation, as well as the configurational stability each intermediate. Analysis of the reaction sequence showed epimerization-free conversion of the organoboronic ester (Figure 1d) to the derived ate complex (Figure 1e), however, subsequent addition of zinc acetate provided a mixture exhibiting several resonances (not shown), presumably because the alkylzinc reagents are not homogenous and likely oligomeric.18 Addition of DMSO (50% volume) to this solution resulted in a homogenous mixture and cleaner spectra (Figure 1f). While a few minor resonances can be observed at the end of the sequence, the resonance corresponding to 4-syn could be assigned by its independent preparation from 3-syn and the data shows a high level of stereospecificity for the overall process. As summarized in Figure 1g (see SI for complete spectral data), reactions employing either zinc acetate or zinc chloride were observed to be stereospecific which suggests that racemization observed in Table 1 (i.e. entry 2) likely arises during the zinc-to-palladium transmetalation.19 Additionally, the observations in Figure 1 suggest that the alkylzinc species themselves are configurationally stable, even at 60 °C for several hours.
With robust conditions for the boron-to-zinc transmetalation identified, the synthetic utility of this process was investigated. Stereospecific coupling of unactivated alkylboronic esters to C(sp2) electrophiles is an important process.20 Available reactions include transition-metal-free alkenylation and arylation21 employing alkenyl or aryllithium reagents. In addition, direct stereospecific Suzuki-Miyaura cross-coupling between non-activated aliphatic secondary organoboron reagents and aryl halides have been reported by Biscoe and Sigman22 (trifluoroborates), and Burke and coworkers23 (boronic acids). Despite these advances, the current scope of these processes has been primarily explored with sterically unhindered alkylboron species (usually α-methyl substituted) and non-heterocyclic arene or alkene coupling partners. To complement these existing methods, enantiomerically-enriched organozinc reagents prepared from boron-to-zinc transmetalation, were examined as coupling partners for stereospecific Negishi cross-coupling reactions (Figure 2). Using Buchwald’s Pd-G3 complex in conjunction with CPhos,15 efficient coupling reactions were observed (Figure 2, 50–76% yield, 93 – 98% es). Challenging heterocyclic electrophiles can be employed in this reaction when PhCPhos is used as the ligand. As observed in the production of 14 and 16, alkenyl halides can also be used as electrophiles. The absolute configuration of compound 10 was assigned to be (S) by comparing its optical rotation values with the reported data,24 which suggests this overall reaction is a stereoretentive process.
Figure 2.
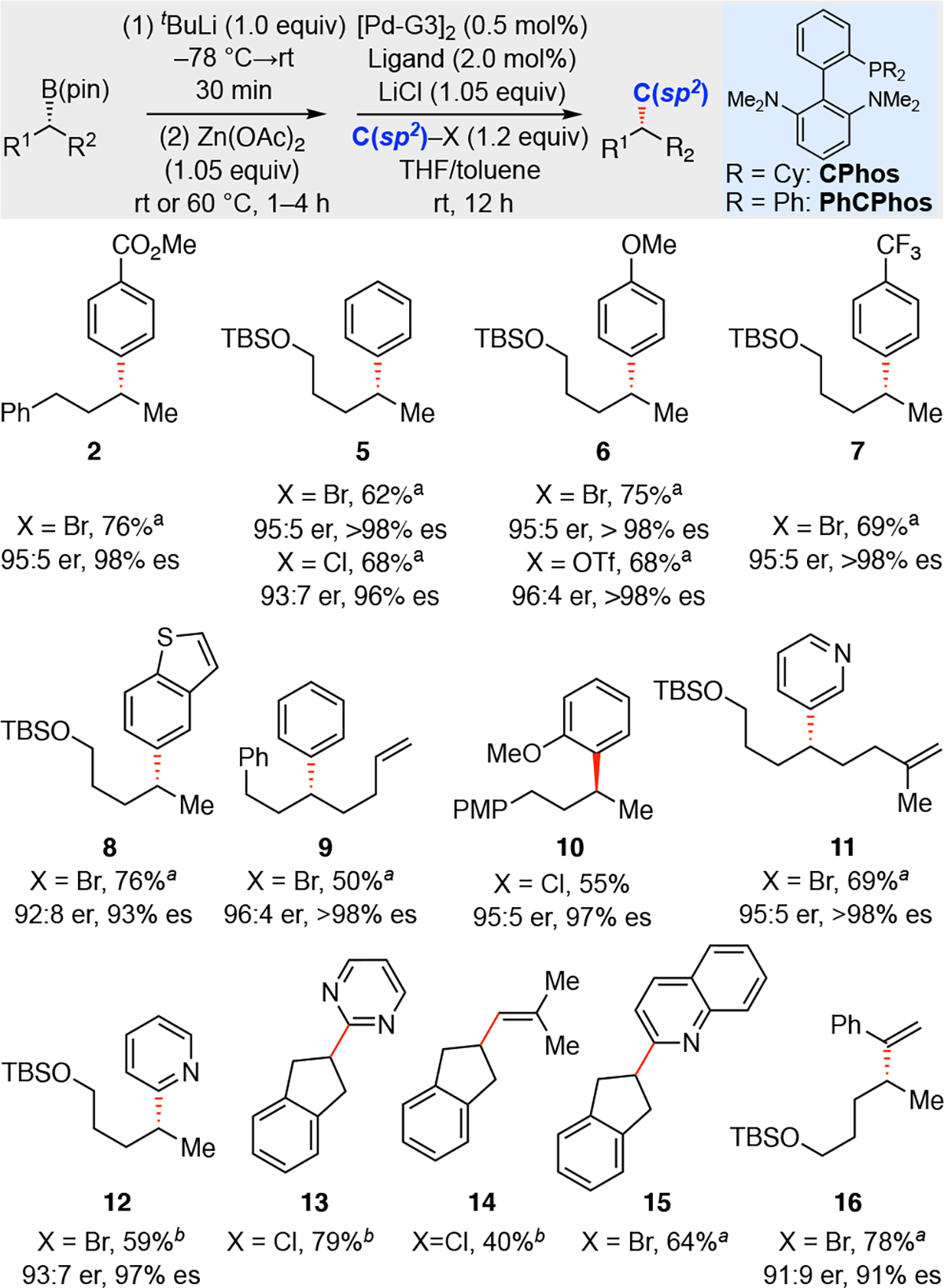
Stereospecific Negishi coupling of enantiomerically enriched organozinc reagents. Reactions were carried out with 0.20 mmol of alkylboronic ester. Yields are of purified materials. Enantiomeric ratio was measured by chiral SFC analysis and have an error of ±1%. aCPhos was used as ligand. bPhCPhos was used as ligand.
Racemic organozinc reagents are known to react with difluorocarbene and deliver a wide range of useful fluorinated compounds.25 It was of interest to determine if enantiomerically-enriched organozinc compounds as prepared above, might engage in this process in a stereospecific fashion. As shown in Figure 3, with a modification of conditions developed by Dilman and coworkers, the organozinc acetate and chloride intermediates were found to react with in situ-generated difluorocarbene, to deliver a difluoromethylzinc intermediate; iodinolysis then produced difluoromethyliodo derivatives with only slight erosion of enantiomeric purity. Study of the reaction conditions showed that the use of zinc pivalate in the transmetalation resulted in increased yield of the overall process (see Supporting Information), likely by enhancing the stability of the difluoromethyl zinc intermediate. With these conditions, various secondary organoboronic esters were examined in this reaction, and found to delivered the product in good yield and good enantiospecificity. Other than trapping the difluoromethyl zinc reagent with iodine to provide the difluoroiodomethyl derivative, NBS or HOAc can also be used as trapping reagent, delivering bromodifluoromethylation (27) and difluoromethylation26 products (29–32), respectively. Furthermore, the difluoromethyl zinc intermediate can undergo a copper-catalyzed allylation to give difluoroalkylation product 28. Of note, zinc chloride can also be used for this reaction (17, 20 and 24).
Figure 3.

Reaction of difluoromethyl carbene with enantiomerically enriched organozinc reagents. Reactions were carried out with 0.20 mmol of alkylboronic ester. Yields are of purified materials. Enantiomeric ratio was measured by chiral SFC or HPLC analysis and have an error of ±1%. aObtained with NBS. bObtained with 10% CuI, 20% 1,10-phenanthroline, allylbromide. cObtained with acetic acid/lithium bromide.
Stereospecific trifluoromethylation of organoboronic esters is an important objective.27 However, our attempts to access these compounds by trapping the above-described difluoromethyl zinc intermediate with electrophilic fluorine reagents (e.g. Selectfluor) were unsuccessful, and only bromination product was observed (presumably, the electrophilic fluorination reagent oxidizes residual bromide to a competent electrophile). To address this problem, we considered direct trifluoromethylation of the organozinc reagent. Liu has established trifluoromethylation of primary alkylzinc and cyclohexylzinc reagents by transmetalation/reductive elimination with bench stable PyCu(III)(CF3)3.28 In our hands, this process proceeded smoothly and delivered enantiomerically enriched CF3-containing products (Figure 4). The use of nonpolar hexane as solvent for the trifluoromethylation reaction provided the best results despite the poor solubility of starting materials in this system (see Supporting Information). Both primary and secondary organoboronic esters can be used as starting materials for the transformation, however, sterically demanding or β-aryl substituted alkylboronic esters are not suitable for this reaction. In both cases, significant amount of alkene side product were generated, likely due to the β-hydrogen elimination from the corresponding alkyl copper(III) intermediate. NOESY NMR experiment with compound 36 indicated that the trifluoromethyl group remains to be at the syn position after the transformation, suggesting this process is also a stereoretentive transformation.
Figure 4.
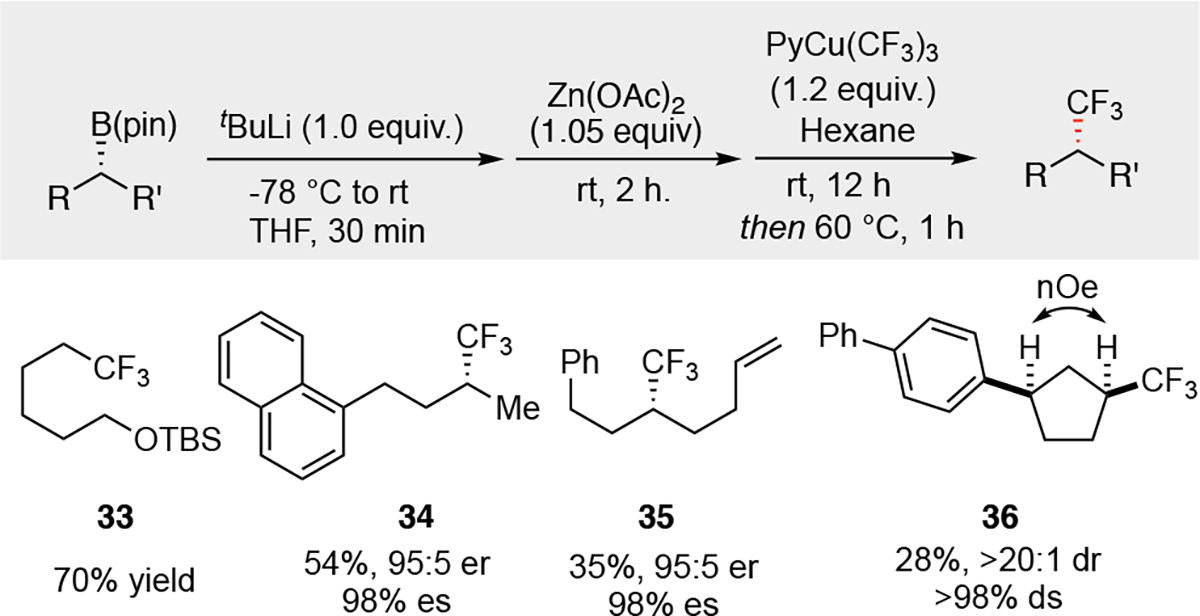
Stereospecific trifluoromethylation of enantiomerically enriched organozinc. aReactions were carried out with 0.20 mmol of alkylboronic ester. bYields are of purified materials. cEnantiomeric ratio was measured by chiral SFC analysis and have an error of ±1%.
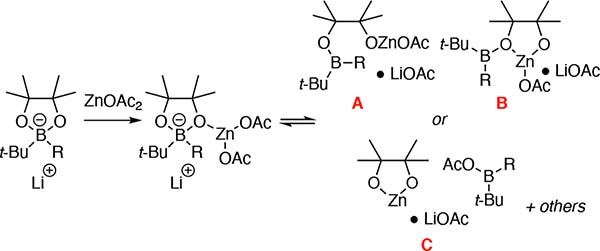
To gain insight into the mechanism for boron-to-zinc transmetalation, we conducted several experiments. Based on comparison to known compounds17, the transient 11B NMR resonance observed at 55 ppm (Figure 1b) during transmetalation with Zn(OAc)2 might be an intervening borinic ester, acyloxyborane17b, or a related compound. One plausible route to such-compounds would arise from dissociation of a pinacolato oxygen atom from boron upon addition of the zinc salt. Such a process finds precedent in studies from Aggarwal29, and might provide either ring-opened borinic esters or derived ensembles (i.e. A–C, Figure 5a).
Figure 5.
Plausible species involved in transmetalation of R–B(pin)●t-BuLi and Zn(OAc)2.
To probe the intermediacy of species such as A–C, related structures were examined. In one experiment, pinacol was deprotonated with one equivalent of n-BuLi30 and then treated with Zn(OPiv)2 (Figure 6a). The presumed ligand-exchange complex was then treated with dicyclohexyborane, whereupon gas evolution was observed and a compound immediately formed that exhibited an 11B NMR resonance at 55 ppm (proposed to be A). Over time, the 55 ppm resonance diminished and a signal at 35 ppm appeared, suggesting that the initial species is competent for transmetalation. Interestingly, a signal at 10 ppm corresponding to a four-coordinate boron complex (B or related) appeared transiently during the reaction. In an alternate experiment (Figure 6b), Et2B(OPiv)17b was prepared; its resonance at 55 ppm was quickly consumed (62% conversion at 30 minutes) and replaced with a resonance at 35 ppm upon treatment with lithium pinacolate and zinc pivalate. While this experiment suggests that an acyloxyborane may be a participant in transmetalations, we note that the 11B NMR resonance of this species is much sharper than that observed in Figure 1. Moreover, a transient 55 ppm resonance, with a similar lineshape as in Figure 1, appears during transmetalations with ZnCl2 (see Supporting Information). Collectively, these experiments suggest that three-coordinate borinic esters are plausible intermediates during the course of transmetalation, but they do not rigorously establish whether these species engage in transmetalation themselves, or whether they are off-cycle progenitors of reactive four-coordinate boronates.
Figure 6.
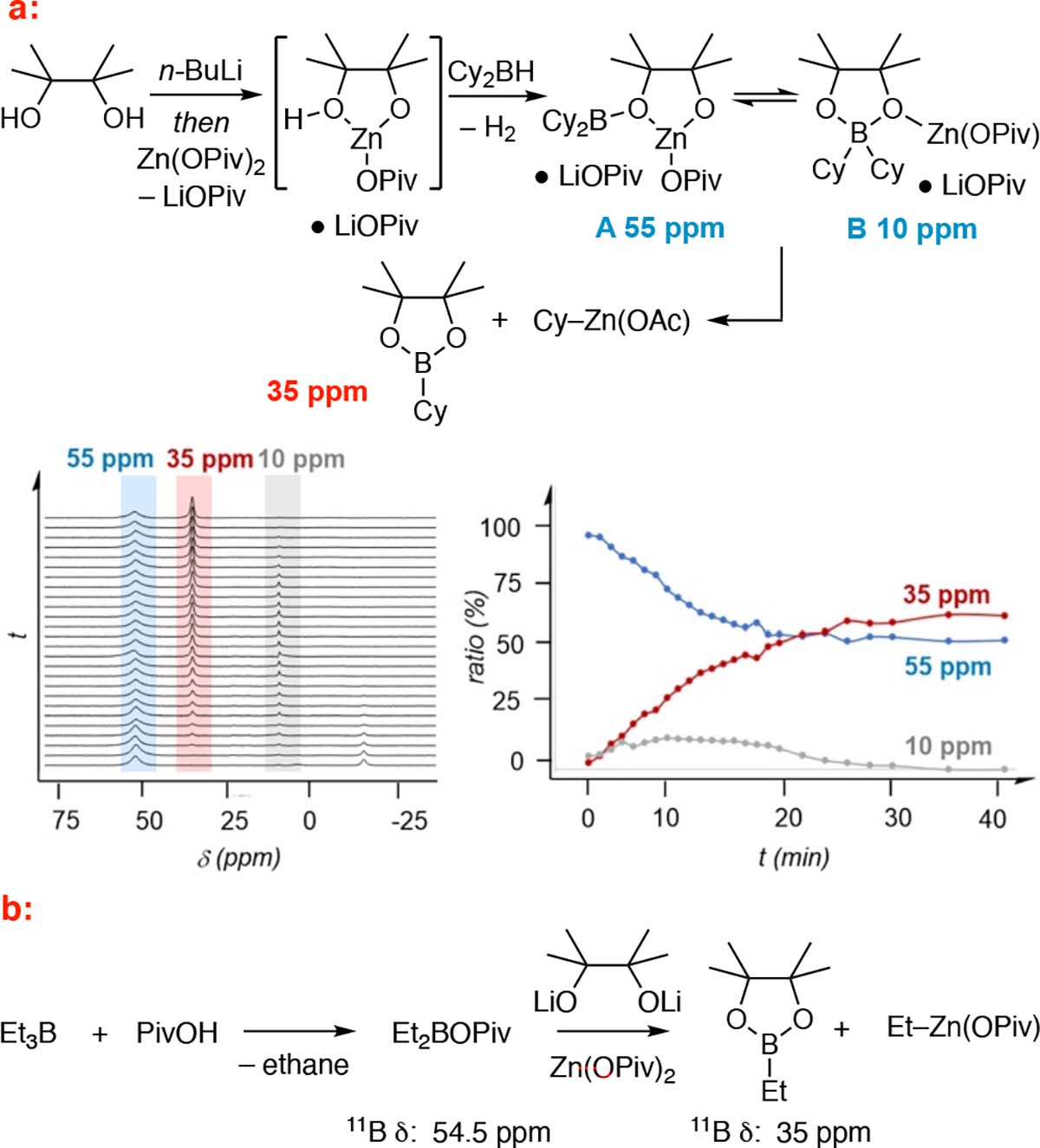
Identification of plausible intermediates in boron-to-zinc transmetalation. (a) Synthesis of putative borinic ester A from a zinc half-pinacolate and Cy2BH and analysis of the formation of Cy–B(pin) and Cy–ZnOAc by 11B NMR (t=0 at addition of Cy2BH). (b) Synthesis and transmetalation of an acyloxyborane.
To learn whether transmetalation is more likely from four-coordinate or three-coordinate organoboron compounds, DFT calculations were conducted for both zinc carboxylate and zinc chloride processes. While transmetalation from three-coordinate boron species was higher in energy, transmetalation of four-coordinate boronates appeared readily accessible (Figure 7, see SI for complete details) thereby suggesting this may be the operative pathway.
Figure 7.

DFT calculated energy surface of boron-to-zinc transmetalation and GIAO-based 11B chemical shifts
In summary, we have reported a stereospecific boron-to-zinc transmetalation and show the organozinc compounds can undergo useful stereospecific transformations. Preliminary mechanistic investigations suggest that stereospecific transfer of the alkyl group to zinc occurs from four-coordinate boronate complexes. We expect that this process may be useful for preparation of enantiomerically enriched organozinc reagents and also extend the application scope of alkylboronic esters.
Supplementary Material
Acknowledgements
This research was supported by instrumentation grants from NSF MRI award CHE2117246, and NIH HEI-S10 award 1S10OD026910. The authors thank Dr. Bo Li and Dr. Thusitha Jayasundera of Boston College for assistance with x-ray structure analysis and NMR spectroscopy, respectively.
Funding Sources
This work was supported by a grant from the NIH (NIGMS R35GM127140 to J.P.M.)
Footnotes
The authors declare no financial conflicts of interest.
ASSOCIATED CONTENT
Supporting Information
Procedures, characterization, and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).(a) Collins BSL; Wilson CM; Myers EL; Aggarwal VK Asymmetric Synthesis of Secondary and Tertiary Boronic Esters. Angew. Chem. Int. Ed. 2017, 56, 11700–11733. [DOI] [PubMed] [Google Scholar]; (b) Hu J; Ferger M; Shi Z; Marder TB Recent advances in asymmetric borylation by transition metal catalysis. Chem. Soc. Rev. 2021, 50, 13129–13188. [DOI] [PubMed] [Google Scholar]
- (2).Sandford C; Aggarwal VK Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 2017, 53, 5481–5494. [DOI] [PubMed] [Google Scholar]
- (3).Wang C–Y; Derosa J; Biscoe MR Configurationally stable, enantioenriched organometallic nucleophiles in stereospecific Pd-catalyzed cross-coupling reactions: an alternative approach to asymmetric synthesis. Chem. Sci. 2015, 6, 5105–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Boudier A; Darcel C; Flachsmann F; Micouin L; Oestreich M; Knochel P Stereoselective Preparation and Reactions of Configurationally Defined Dialkylzinc Compounds. Chem. Eur. J. 2000, 6, 2748–2761. [DOI] [PubMed] [Google Scholar]; (b) Duddu R; Eckhardt M; Furlong M; Knoess HP; Berger S; Knochel P Preparation and reactivity of chiral β-amido-alkylzinc iodides and related configurationally stable zinc organometallics. Tetrahedron 1994, 50, 2415–2432. [Google Scholar]; (c) Guijarro A; Rieke RD Study of the Configuration Stability of the Carbon – Zinc Bond, Direct Measurement of Enantiomeric Ratios, and Tentative Assignment of the Absolute Configuration in Secondary Organozinc Halides. Angew. Chem. Int. Ed. 2000, 39, 1475–1479. [DOI] [PubMed] [Google Scholar]; (d) Klein S; Marek I; Normant JF Carbolithiation of Cinnamyldialkylamines - Stereochemistry of the Li to Zn Transmetalation and Configurational Stability of Benzylic Organozinc Halides. J. Org. Chem. 1994, 59, 2925–2936. [Google Scholar]
- (5).Boudier A; Bromm LO; Lotz M; Knochel P New Applications of Polyfunctional Organometallic Compounds in Organic Synthesis. Angew. Chem. Int. Ed. 2000, 39, 4414–4435. [PubMed] [Google Scholar]
- (6).(a) Beng TK; Gawley RE Application of Catalytic Dynamic Resolution of N-Boc-2-lithiopiperidine to the Asymmetric Synthesis of 2-Aryl and 2-Vinyl Piperidines. Org. Lett. 2011, 13, 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Campos KR; Klapars A; Waldman JH; Dormer PG; Chen C.-y. Enantioselective, Palladium-Catalyzed α-Arylation of N-Boc-pyrrolidine. J. Am. Chem. Soc. 2006, 128, 3538–3539. [DOI] [PubMed] [Google Scholar]
- (7).Zhang C; Hu W; Lovinger GJ; Jin J; Chen J; Morken JP Enantiomerically Enriched α-Borylzinc Reagents by Nickel-Catalyzed Carbozincation of Vinylboronic Esters. J. Am. Chem. Soc. 2021, 143, 14189–14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Krämer K; Leong P; Lautens M Enantioselective Palladium-Catalyzed Carbozincation of Cyclopropenes. Org. Lett. 2011, 13, 819–821. [DOI] [PubMed] [Google Scholar]; (b) Müller DS; Marek I Asymmetric Copper-Catalyzed Carbozincation of Cyclopropenes en Route to the Formation of Diastereo- and Enantiomerically Enriched Polysubstituted Cyclopropanes. J. Am. Chem. Soc. 2015, 137, 15414–15417. [DOI] [PubMed] [Google Scholar]
- (9).Boudier A; Flachsmann F; Knochel P Stereoselective Preparation and Reaction of Chiral Secondary Cycloalkyl- and Alkyl-Zinc Reagents. Synlett 1998, 1998, 1438–1440. [Google Scholar]
- (10).Hupe E; Marek I; Knochel P Diastereoselective Reduction of Alkenylboronic Esters as a New Method for Controlling the Stereochemistry of up to Three Adjacent Centers in Cyclic and Acyclic Molecules. Org. Lett. 2002, 4, 2861–2863. [DOI] [PubMed] [Google Scholar]
- (11).Skotnitzki J; Kremsmair A; Keefer D; Gong Y; de VivieRiedle R; Knochel P Stereoselective Csp3–Csp2 Cross-Couplings of Chiral Secondary Alkylzinc Reagents with Alkenyl and Aryl Halides. Angew. Chem. Int. Ed. 2020, 59, 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Procter RJ; Dunsford JJ; Rushworth PJ; Hulcoop DG; Layfield RA; Ingleson MJ A Zinc Catalyzed C(sp3)–C(sp2) Suzuki–Miyaura Cross-Coupling Reaction Mediated by Aryl-Zincates. Chem. Eur. J. 2017, 23, 15889–15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Xu N; Kong Z; Wang JZ; Lovinger GJ; Morken JP Copper-Catalyzed Coupling of Alkyl Vicinal Bis(boronic Esters) to an Array of Electrophiles. J. Am. Chem. Soc. 2022, 144, 17815–17823. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Xu N; Liang H; Morken JP Copper-Catalyzed Stereospecific Transformations of Alkylboronic Esters. J. Am. Chem. Soc. 2022, 144, 11546–11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Zou G; Falck JR Suzuki–Miyaura cross-coupling of lithium n-alkylborates. Tetrahedron Lett. 2001, 42, 5817–5819. [Google Scholar]
- (15).(a) Han C; Buchwald SL Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides. J. Am. Chem. Soc. 2009, 131, 7532–7533. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang Y; Niedermann K; Han C; Buchwald SL Highly Selective Palladium-Catalyzed Cross-Coupling of Secondary Alkylzinc Reagents with Heteroaryl Halides. Org. Lett. 2014, 16, 4638–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).For related isotopically-labeled stereochemical probes, see: Evans MA; Morken JP Isotopically Chiral Probes for in Situ High-Throughput Asymmetric Reaction Analysis. J. Am. Chem. Soc. 2002, 124, 9020–9021. [DOI] [PubMed] [Google Scholar]
- (17).(a) Wrackmeyer B Nuclear Magnetic Resonance Spectroscopy of Boron Compounds Containing Two-, Three- and Four-Coordinate Boron. Annu. Rep. NMR Spectrosc 1988, 20, 61–203. [Google Scholar]; (b) Yalpani M; Boese R; Seevogel K; Koster R Monomeric and Dimeric Acyloxydialkylboranes. J. Chem. Soc., Dalton Trans. 1993, 47–50. [Google Scholar]
- (18).Grala A; Wolska-Pietkiewicz M; Wojewódzka A; Dabergut M; Justyniak I; Lewiński J Structural Diversity of Ethylzinc Carboxylates. Organometallics 2015, 34, 4959–4964. [Google Scholar]
- (19).Thaler T; Haag B; Gavryushin A; Schober K; Hartmann E; Gschwind RM; Zipse H; Mayer P; Knochel P Highly diastereoselective Csp3–Csp2 Negishi cross-coupling with 1,2-, 1,3- and 1,4-substituted cycloalkylzinc compounds. Nat. Chem. 2010, 2, 125–130. [DOI] [PubMed] [Google Scholar]
- (20).Ma X; Murray B; Biscoe MR Stereoselectivity in Pd-catalysed cross-coupling reactions of enantioenriched nucleophiles. Nat. Rev. Chem. 2020, 4, 584–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) Armstrong RJ; Aggarwal VK 50 Years of Zweifel Olefination: A Transition-Metal-Free Coupling. Synthesis 2017, 49, 3323–3336. [Google Scholar]; (b) Wang H; Jing C; Noble A; Aggarwal VK Stereospecific 1,2-Migrations of Boronate Complexes Induced by Electrophiles. Angew. Chem. Int. Ed. 2020, 59, 16859–16872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).(a) Li L; Zhao S; Joshi-Pangu A; Diane M; Biscoe MR Stereospecific Pd-Catalyzed Cross-Coupling Reactions of Secondary Alkylboron Nucleophiles and Aryl Chlorides. J. Am. Chem. Soc. 2014, 136, 14027–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao S; Gensch T; Murray B; Niemeyer ZL; Sigman MS; Biscoe MR Enantiodivergent Pd-catalyzed C-C bond formation enabled through ligand parameterization. Science 2018, 362, 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Lehmann JW; Crouch IT; Blair DJ; Trobe M; Wang P; Li J; Burke MD Axial shielding of Pd(II) complexes enables perfect stereoretention in Suzuki-Miyaura cross-coupling of Csp3 boronic acids. Nat. Commun. 2019, 10, 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Bigler R; Aggarwal VK ortho-Directing Chromium Arene Complexes as Efficient Mediators for Enantiospecific C(sp2)–C(sp3) Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2018, 57, 1082–1086. [DOI] [PubMed] [Google Scholar]
- (25).(a) Dilman AD; Levin VV Difluorocarbene as a Building Block for Consecutive Bond-Forming Reactions. Acc. Chem. Res. 2018, 51, 1272–1280. [DOI] [PubMed] [Google Scholar]; (b) Levin VV; Zemtsov AA; Struchkova MI; Dilman AD Reactions of Difluorocarbene with Organozinc Reagents. Org. Lett. 2013, 15, 917–919. [DOI] [PubMed] [Google Scholar]
- (26).Fasano V; Winter N; Noble A; Aggarwal VK Divergent, Stereospecific Mono- and Difluoromethylation of Boronic Esters. Angew. Chem. Int. Ed. 2020, 59, 8502–8506. [DOI] [PubMed] [Google Scholar]
- (27).Inoue M; Sumii Y; Shibata N Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Paeth M; Tyndall SB; Chen L-Y; Hong J-C; Carson WP; Liu X; Sun X; Liu J; Yang K; Hale EM; Tierney DL; Liu B; Cao Z; Cheng M-J; Goddard WA; Liu W Csp3–Csp3 Bond-Forming Reductive Elimination from Well-Defined Copper(III) Complexes. J. Am. Chem. Soc. 2019, 141, 3153–3159. [DOI] [PubMed] [Google Scholar]
- (29).Chen JLY; Scott HK; Hesse MJ; Willis CL; Aggarwal VK Highly Diastereo- and Enantioselective Allylboration of Aldehydes using α-Substituted Allyl/Crotyl Pinacol Boronic Esters via in Situ Generated Borinic Esters. J. Am. Chem. Soc. 2013, 135, 5316–5319. [DOI] [PubMed] [Google Scholar]
- (30).(a) Nather C; John A; Ruppert K; Bock H Bis(N,N-dimethylformamide)(pyrocatecholato-O,O’)lithium. Acta Crystallogr. Sect. C 1996, 52, 1166–1168. [Google Scholar]; (b) Mamak M; Zavalij PY; Whittingham MS Layered Structure of Lithium Ethylene Glycolate, Li(OCH2CH2OH). Acta Crystallogr. Sect. C 1998, 54, 937–939 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.