Abstract
Complex I (CI) deficiency in mitochondrial oxidative phosphorylation (OXPHOS) is the most common cause of mitochondrial diseases, and limited evidence-based treatment options exist. Although CI provides the most electrons to OXPHOS, complex II (CII) is another entry point of electrons. Enhancement of this pathway may compensate for a loss of CI; however, the effects of boosting CII activity on CI deficiency are unclear at the animal level. 5-Aminolevulinic acid (5-ALA) is a crucial precursor of heme, which is essential for CII, complex III, complex IV (CIV) and cytochrome c activities. Here, we show that feeding a combination of 5-ALA hydrochloride and sodium ferrous citrate (5-ALA-HCl + SFC) increases ATP production and suppresses defective phenotypes in Drosophila with CI deficiency. Knockdown of sicily, a Drosophila homolog of the critical CI assembly protein NDUFAF6, caused CI deficiency, accumulation of lactate and pyruvate and detrimental phenotypes such as abnormal neuromuscular junction development, locomotor dysfunctions and premature death. 5-ALA-HCl + SFC feeding increased ATP levels without recovery of CI activity. The activities of CII and CIV were upregulated, and accumulation of lactate and pyruvate was suppressed. 5-ALA-HCl + SFC feeding improved neuromuscular junction development and locomotor functions in sicily-knockdown flies. These results suggest that 5-ALA-HCl + SFC shifts metabolic programs to cope with CI deficiency.
Bullet outline
5-Aminolevulinic acid (5-ALA-HCl + SFC) increases ATP production in flies with complex I deficiency.
5-ALA-HCl + SFC increases the activities of complexes II and IV.
5-ALA-HCl + SFC corrects metabolic abnormalities and suppresses the detrimental phenotypes caused by complex I deficiency.
Introduction
Mitochondria generate ATP through oxidative phosphorylation (OXPHOS), which involves a series of electron transfer reactions mediated by the electron transfer chain (ETC) comprising complexes I–IV (CI–CIV) (1). An abnormality of the ETC leads to ATP deficiency and causes a group of multisystem disorders called mitochondrial diseases (2). CI, also called nicotinamide adenine dinucleotide (NADH):ubiquinone oxidoreductase, is the most common site for mitochondrial abnormalities, representing as many as one-third of respiratory chain deficiencies (1). CI deficiency causes various clinical symptoms in organs and tissues with high energy demands, such as the brain, the heart, the liver and skeletal muscle. Mitochondrial disorders associated with CI deficiency often lead to severe neurological presentations, including Leber’s hereditary optic neuropathy, mitochondrial encephalopathy, lactic acidosis and stroke-like episodes (MELAS), myoclonic epilepsy with ragged-red fibers (MERRF) and Leigh syndrome. There is no cure for these disorders (2).
CII mediates electron input into the ETC and can function in parallel with CI (3). Although CI oxidizes NADH and provides most electrons that enter the respiratory chain, CII oxidizes succinate from the tricarboxylic acid (TCA) cycle and serves as another entry point of electrons into the ETC (3). CII does not have proton pump activity unlike CI, whereas CIII and CIV pump protons when electrons from CII pass through to create a proton gradient across the inner membrane that drives complex V (CV), the ATP synthase complex (4). It has been reported that cell membrane-permeable prodrugs of succinate increase ATP-linked mitochondrial respiration in CI-deficient cells (5), suggesting that enhancement of CII-linked respiration may rescue ATP deficits caused by CI deficiency. However, the effects of enhancement of CII activity on CI deficiency have not been tested at the animal level.
CII, CIII, CIV and cytochrome c contain heme, which is essential for their activities (6–10). 5-Aminolevulinic acid (5-ALA) is a rate-limiting intermediate of heme synthesis (8,11,12). Treatment of cultured cells with 5-ALA in combination with sodium ferrous citrate (SFC) has been reported to increase the activity of cytochrome c oxidase, expression of CIII, CIV and CV, the oxygen consumption rate, and production of ATP (8–10).
In this study, we used Drosophila as a model system to investigate the effects of 5-ALA hydrochloride and SFC (5-ALA-HCl + SFC) treatment on CI deficiency. Loss of sicily, a Drosophila homolog of NDUFAF6, has been reported to cause CI deficiency and lower ATP levels (13–15). We found that 5-ALA-HCl + SFC feeding increased ATP levels in sicily-knockdown flies. CII, CIII and CIV were upregulated, and lactate and pyruvate accumulation was improved. Detrimental phenotypes caused by CI deficiency, such as abnormal neuromuscular junction (NMJ) development and locomotor dysfunctions, were mitigated. These results suggest that 5-ALA-HCl + SFC enhances resilience against CI deficiency via shifting the metabolic mode.
Results
Sicily knockdown causes a developmental delay and neurological defects
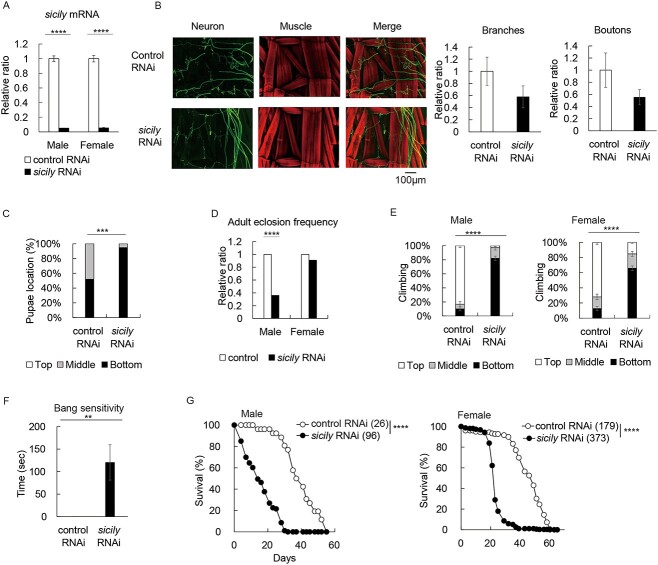
We expressed sicily RNAi using the transgenic binary expression system Gal4/UAS (16). RNAi expression with the ubiquitous driver actin-GAL4 lowered mRNA levels of sicily in both males and females (Fig. 1A). Larvae expressing sicily RNAi developed without gross morphological defects, but muscles of third instar larvae with sicily knockdown were thinner, more fragile and less innervated than those of third instar larvae expressing control RNAi (Fig. 1B). Although control pupae were distributed all over the wall of the vial, pupae with sicily knockdown were found closer to the medium, suggesting that sicily-knockdown larvae could not climb far from the medium (Fig. 1C). Many males with sicily knockdown died before eclosion (Fig. 1D, Male). In contrast, females with sicily knockdown matured normally and survived to the adult stage (Fig. 1D, Female).
Figure 1.
sicily knockdown causes a developmental delay and neurological defects. (A) Expression of sicily RNAi lowered sicily mRNA expression. Homogenates of adult flies with sicily RNAi expression driven by the ubiquitous driver actin-GAL4 were subjected to RT-qPCR. Flies expressing mCherry RNAi were used as a control (mean ± SE, n = 3; ****P < 0.005; unpaired t-test). (B) NMJs of third instar larvae were costained with phalloidin (red) and an anti-HRP antibody (green), which recognizes a neural membrane epitope. Genotypes or conditions as indicated. All scale bars, 100 μm. NMJ growth was assessed by counting the number of branches and boutons at abdominal segment A4, muscle 5 (mean ± SE, n = 4; P = 0.05; unpaired t-test). (C) The distance that larvae traveled to form pupae was assessed by counting the number of pupae formed in the top, middle and bottom areas of the vial wall (mean ± SE, n = 57 or 100; ***P = 0.005; Chi-square test). (D) Egg-to-adult eclosion frequency for progeny flies generated by the cross actin-GAL4/TM3Sb × UAS-sicily RNAi/TM3Ser. Eclosion frequency of actin-GAL4;TM3Ser (control) or actin-GAL4:sicily RNAi (sicily RNAi) expressed as relative ratios (n = 873–1157; ****P < 0.001; Chi-square test). (E) sicily knockdown caused locomotor deficits. Flies were subjected to a climbing assay; they were tapped to the bottom of the vial, and the distance that they climbed in 10 s was measured. Flies were at 12 days after eclosion (mean ± SE, n = 6–29; ****P < 0.001; unpaired t-test). (F) Aged females with sicily knockdown developed shock-induced paralysis. The amount of time taken to recover from mechanical stress (bang sensitivity) is shown for flies of the indicated genotype at 24 days after eclosion (mean ± SE, n = 9 or 11; **P < 0.01; unpaired t-test). (G) Kaplan–Meier survival curves of sicily-knockdown males and females. Numbers in parentheses indicate sample size (number of flies). ****P < 0.001, log-rank test.
Adult males with sicily knockdown had severely impaired locomotor functions (Fig. 1E) and died prematurely (Fig. 1G). Adult females with sicily knockdown also exhibited locomotor defects (Fig. 1E) and premature death (Fig. 1G), and they developed bang sensitivity when they aged (30 days after eclosion, Fig. 1F). Similar results were obtained using another RNAi line (Supplementary Material, Fig. S1).
Sicily knockdown disrupts CI
We analyzed the expression and activities of mitochondrial respiratory complexes. Sicily knockdown did not affect the relative level of mitochondrial DNA (Fig. 2A), suggesting that overall mitochondrial numbers are similar. Real-Time Quantitative Reverse Transcription PCR (qRT-PCR) analyses revealed that sicily knockdown upregulated the CIII genes mt:CytB and mt:COIII, and ATP synthase B in CV (Fig. 2B). In contrast, CIV genes such as COX4L, COX7AL and COX7A2I, and genes encoding the uncoupling proteins UCP4B, UCP4C and UCP5, were downregulated (Fig. 2B).
Figure 2.
Effect of sicily knockdown on mitochondrial respiratory complexes. (A) The numbers of mitochondrial DNA copies in thoraxes dissected from 3-day-old flies expressing sicily RNAi and mCherry RNAi (control RNAi) were assessed by qRT-PCR) and are shown as ratios relative to control RNAi (mean ± SE, n = 3). No significant difference was detected (P > 0.05; unpaired t-test). (B) Heatmap showing fold changes in mRNA levels of the indicated genes (n = 3; *P < 0.05; unpaired t-test). (C) Blue-native PAGE and in-gel activity assay of mitochondria extracted from thoraxes of 3-day-old flies of the indicated genotype and sex. Arrows indicate bands specific to sicily knockdown. Asterisks indicate a possible CI fragment. Numbers in parentheses indicate the band intensities in flies expressing sicily RNAi relative to those in flies expressing control RNAi. (D) Heatmap showing fold changes in mRNA levels of the indicated antioxidant genes (n = 3; *P < 0.05; unpaired t-test).
We performed blue-native PAGE and an in-gel activity assay to analyze each complex. Sicily knockdown dramatically reduced CI levels in both males and females (Fig. 2C). A band was detected with mitochondria from sicily-knockdown flies but not with those from control flies (Fig. 2C, arrow). Considering that sicily encodes a CI assembly factor (17), this band may represent part of CI. The in-gel activity assay revealed that sicily knockdown reduced CI activity in the portion of the gel corresponding to the size of the holoenzyme (Fig. 2C, CI activity). An additional band with a much smaller size was also detected (asterisk). This band may represent an intermediate of CI (17), and its intensity was not dramatically affected by sicily knockdown (Fig. 2C, CI activity). These results indicate that sicily knockdown causes a loss of CI activity.
Expression of CII, CIII, CIV and CV was similar in control and sicily-knockdown flies (Fig. 2C). The activities of these complexes were also similar in control and sicily-knockdown males, whereas the activities of CII and CIV were elevated in females with sicily knockdown (Fig. 2C right). The higher activities of CII and CIV in females may contribute to their viability with CI deficiency.
Electron transport in respiratory complexes produces reactive oxygen species (ROS), which elevates expression of antioxidative stress response genes. Expression of the mitochondrial antioxidant gene SOD2 was upregulated in males with sicily knockdown, whereas many cytoplasmic antioxidant genes (cat, Prx3, Prx4, Prx5, SOD1, SOD3, Trxr-1 and Trxr-2) were downregulated (Fig. 2D), which may reflect lower mitochondrial ETC activity. The antioxidant genes Prx2540–1 and pxd were upregulated in females with sicily knockdown (Fig. 2D), and the protein carbonyl assay indicated that the level of oxidative stress was higher in females than in males with sicily knockdown (Supplementary Material, Fig. S2). These results suggest that the detrimental phenotypes observed upon sicily knockdown are not caused by ROS.
Sicily knockdown causes lactate and pyruvate accumulation
Patients with CI deficiencies often suffer from lactic acidosis, which is characterized by the accumulation of lactate (18–21). Sicily knockdown caused lactate accumulation in males and females (Fig. 3A). Pyruvate also accumulated in males and females with sicily knockdown (Fig. 3B). Glycolytic genes such as Pgi, Pfk, Pyk and HexC were downregulated in flies with sicily knockdown, whereas lactate dehydrogenase (LDH) was upregulated (Fig. 3C), which may be because of a feedback response to accumulated pyruvate and lactate.
Figure 3.
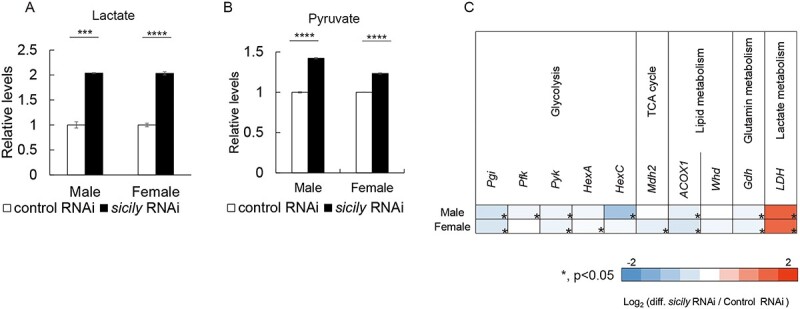
sicily knockdown causes lactate and pyruvate accumulation. (A-B) Relative abundance of lactate (A) and pyruvate (B) in flies of the indicated genotype and sex (mean ± SE, n = 3–4; ***P < 0.005, ****P < 0.0001; unpaired t-test). (C) Heatmap showing fold changes in mRNA levels of metabolic genes (n = 3; *P < 0.05; unpaired t-test).
Genes encoding ACOX1, the first enzyme in the fatty acid β-oxidation pathway, and Gdh, which catalyzes the reversible conversion of glutamate to α-ketoglutarate using NAD(P)+, were downregulated in sicily-knockdown flies (Fig. 3C). These pathways provide acetyl CoA and alpha-ketoglutarate for the TCA cycle, and therefore their downregulation may also contribute to the lower metabolism in sicily-knockdown flies.
5-ALA-HCl + SFC increases the activities of CII and CIV and ATP levels in sicily-knockdown flies
To test the effect of 5-ALA-HCl + SFC treatment on mitochondria of sicily-knockdown flies, we raised these flies on media containing 5-ALA-HCl + SFC. 5-ALA-HCl + SFC feeding significantly increased the ATP levels in males and females with sicily knockdown (Fig. 4A). 5-ALA-HCl + SFC feeding did not affect mitochondrial DNA copy numbers (Fig. 4B), indicating that it does not increase the number of mitochondria. 5-ALA-HCl + SFC feeding upregulated the CIII genes mt:CytB and mt:COIII in both males and females with sicily knockdown (Fig. 4C). In males with sicily knockdown, CIV genes such as COX4L and COX7A2I, and genes encoding the uncoupling proteins UCP4B, UCP4C and UCP5, were also upregulated (Fig. 4C).
Figure 4.
5-ALA-HCl + SFC feeding increases ATP levels without CI. (A) Quantitation of the ATP levels in thoraxes of sicily-knockdown flies fed food containing the indicated concentrations of 5-ALA-HCl + SFC. Flies were 3 days old. ATP levels are normalized to protein levels and shown as ratios relative to the control (mean ± SD, n = 3; ***P < 0.005, ****P = 0.001; unpaired t-test). (B) The number of mitochondrial copies was determined by qRT-PCR. The numbers of mitochondrial copies are shown as ratios relative to the control (mean ± SE, n = 3). No significant difference was detected (P > 0.05, unpaired t-test). (C) Heatmap showing fold changes in mRNA levels of the indicated genes (n = 3; *P < 0.05, unpaired t-test). (D) Blue-native PAGE and an in-gel activity assay of mitochondria extracted from thoraxes of flies fed food containing the indicated concentrations of 5-ALA-HCl + SFC. Flies were at 3 days after eclosion. Numbers in parentheses indicate the band intensities in flies expressing sicily RNAi relative to those in flies expressing control RNAi.
Blue-native PAGE and an in-gel activity assay revealed that the expression and activity of CI were still lacking in sicily-knockdown flies fed 5-ALA-HCl + SFC (Fig. 4D). Interestingly, 5-ALA-HCl + SFC feeding increased the activities of CII and CIV in males with sicily knockdown (Fig. 4D). Considering that females were more resistant to loss of CI than males (Fig. 1), and that CII and CIV activities were higher in females than in males with sicily knockdown (Fig. 2C), these results suggest that elevated expression of CIII genes and CII and CIV activities mitigates the detrimental phenotypes of sicily-knockdown flies.
5-ALA-HCl + SFC suppresses lactate and pyruvate accumulation caused by sicily knockdown
5-ALA-HCl + SFC feeding reduced lactate and pyruvate accumulation in males and females with sicily knockdown (Fig. 5A and B). It did not affect expression of the glycolytic genes LDH, ACOX1 and Gdh in females with sicily knockdown, whereas LDH and Gdh were upregulated, and ACOX1 was downregulated further, in males with sicily knockdown (Fig. 5C). Expression of several antioxidative stress genes was downregulated. The level of oxidative stress was similar in sicily-knockdown males fed 5-ALA-HCl + SFC and increased in sicily-knockdown females fed 5-ALA-HCl + SFC (Supplementary Material, Fig. S3), indicating that ROS levels do not correlate with improvement of the detrimental phenotypes. These results suggest that 5-ALA-HCl + SFC mitigates the metabolic abnormalities caused by CI deficiency.
Figure 5.
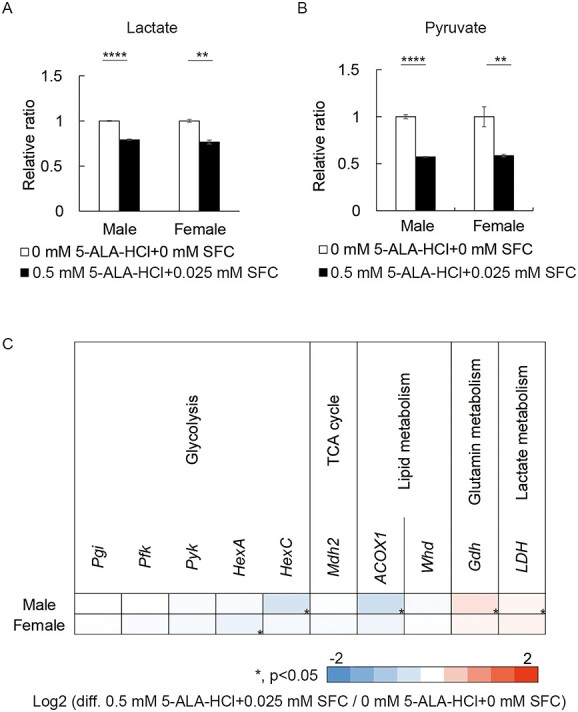
5-ALA-HCl + SFC suppresses lactate and pyruvate accumulation caused by sicily knockdown. (A-B) Relative abundance of lactate (A) and pyruvate (B) in sicily-knockdown flies fed food containing the indicated concentrations of 5-ALA-HCl + SFC (mean ± SE, n = 3–4; **P < 0.01, ****P < 0.0001; unpaired t-test). (C) Heatmap showing fold changes in mRNA levels of metabolic genes (n = 3; *P < 0.05; unpaired t-test).
5-ALA-HCl + SFC rescues premature death and neurological dysfunction caused by sicily knockdown
We tested the effect of 5-ALA-HCl + SFC treatment on the detrimental phenotypes of sicily-knockdown flies. 5-ALA-HCl + SFC feeding increased the numbers of branches and boutons in the muscles of third instar larvae with sicily knockdown (Fig. 6A). Sicily-knockdown larvae climbed higher to form pupae in 5-ALA-HCl + SFC-containing vials than in 5-ALA-HCl + SFC-lacking vials, suggesting that their locomotor functions are improved (Supplementary Material, Fig. S4). 5-ALA-HCl + SFC feeding improved the eclosion rate in males with sicily knockdown (Fig. 6B) and significantly suppressed locomotor defects in males and females (Fig. 6C) and bang sensitivity in females (Fig. 6D). In addition, 5-ALA-HCl + SFC feeding extended the lifespan of females with sicily knockdown (Fig. 6E).
Figure 6.

5-ALA-HCl + SFC feeding rescues developmental delays, premature death and neurological dysfunction caused by sicily knockdown. (A) Third instar larvae were costained with phalloidin (red) and an anti-HRP antibody (green), which recognizes a neural membrane epitope. Concentrations of 5-ALA-HCl + SFC in food are as indicated. NMJ growth was assessed by counting the numbers of branches and boutons at abdominal segment A4, muscle 6/7 (mean ± SE, n = 4; **P = 0.01, ***P = 0.005; unpaired t-test). (B) Egg-to-adult eclosion frequency of flies with the indicated concentrations of 5-ALA-HCl + SFC in food (n = 74–115; **P < 0.01; Chi-square test and Holm–Sidak post hoc test). (C) Locomotor functions of flies fed food containing the indicated concentrations of 5-ALA-HCl + SFC were assessed by a climbing assay. Flies were tapped to the bottom of the vial, and the number of flies that reached the top, middle and bottom third of the vial in 20 s was counted. Flies were at 9 days after eclosion (mean ± SE, n = 4–29; *P < 0.05, ****P < 0.0001; one-way ANOVA followed by Dunnett’s test). (D) Amount of time taken to recover from mechanical stress (bang sensitivity) by females fed food containing the indicated concentrations of 5-ALA-HCl + SFC. Flies were at 21 days after eclosion (mean ± SE, n = 11–26; *P < 0.05, **P < 0.01; one-way ANOVA followed by Dunnett’s test). (E) Survival curves of sicily-knockdown males and females raised on food containing the indicated concentrations of 5-ALA-HCl + SFC. Numbers in parentheses indicate sample size (number of flies); *P < 0.05; log-rank test and Holm–Sidak post hoc test. (F–H) 5-ALA-HCl + SFC feeding after eclosion suppressed detrimental phenotypes caused by sicily knockdown. Flies were raised on food lacking 5-ALA-HCl + SFC until eclosion and then transferred to food containing the indicated concentrations of 5-ALA-HCl + SFC. (F) Climbing assay with flies fed food containing the indicated concentrations of 5-ALA-HCl + SFC. Flies were at 12 days after eclosion (mean ± SE, n = 10–22; **P < 0.01, ****P < 0.001; one-way ANOVA followed by Dunnett’s test). (G) Amount of time taken to recover from mechanical stress by females fed food containing the indicated concentrations of 5-ALA-HCl + SFC. Flies were at 30 days after eclosion (mean ± SE, n = 13–30; **P < 0.01; one-way ANOVA followed by Dunnett’s test). (H) Survival curves of sicily-knockdown males and females maintained on food containing the indicated concentrations of 5-ALA-HCl + SFC after eclosion. Numbers in parentheses indicate sample size (number of flies); *P < 0.05, ***P < 0.005; log-rank test and Holm–Sidak post hoc test.
We tested whether 5-ALA-HCl + SFC feeding at the adult stage has protective effects in sicily-knockdown flies. Sicily-knockdown flies were raised in regular food, and eclosed flies were transferred to food containing 5-ALA-HCl + SFC. Sicily-knockdown flies fed 5-ALA-HCl + SFC after eclosion exhibited improved locomotor functions (Fig. 6F). In addition, 5-ALA-HCl + SFC feeding at the adult stage rescued bang sensitivity of females (Fig. 6G) and extended the lifespan of males (Fig. 6H). These results suggest that some of the defective phenotypes caused by CI deficiency can be mitigated after the developmental period.
Discussion
In this study, we found that 5-ALA-HCl + SFC mitigated detrimental phenotypes caused by CI deficiency in flies. Knockdown of sicily, the Drosophila homolog of NDUFAF6, caused abnormal NMJ development, locomotor defects and premature death, with accumulation of pyruvate and lactate (Figs 1–3). 5-ALA-HCl + SFC feeding improved NMJ development and locomotor functioning in sicily-knockdown flies (Fig. 6). Although CI activity was still lacking, ATP levels were increased, and metabolic abnormalities such as accumulation of pyruvate and lactate were suppressed, in sicily-knockdown flies fed 5-ALA-HCl + SFC (Fig. 4A and B). Detrimental phenotypes were negatively correlated with the mRNA levels of genes encoding CIII and activities of CII and CIV (Figs 2,4, and5). Metabolic bypassing of mitochondrial CI has been reported in cultured tissues (5,22) but has not been reported at the animal level. This study, for the first time, showed that 5-ALA-HCl + SFC promotes bypassing of CI.
sicily knockdown caused more severe phenotypes in males than in females (Fig. 1). Although the knockdown efficiency was similar in males and females (Fig. 1A, sicily RNAi), and CI activity was lost in both males and females with sicily RNAi expression (Fig. 2C), sicily knockdown was partially lethal in males but not in females (Fig. 1). A similar result was obtained with another RNAi line (Fig. S1), indicating that the sex-specific lethality is not attributable to an off-target effect or transgene insertion. Interestingly, sicily knockdown upregulated CIII genes and increased CII and CIV activities only in females (Fig. 2B and C). 5-ALA-HCl + SFC feeding, which suppressed abnormal NMJ development and locomotor defects, also upregulated CIII genes and increased CII and CIV activities in males (Fig. 4C and D). These results suggest that enhancement of CII, CIII and CIV mediates resilience against CI deficiency, and females may be more resilient to sicily knockdown because of their ability to enhance CII, CIII and CIV (Fig. 7).
Figure 7.
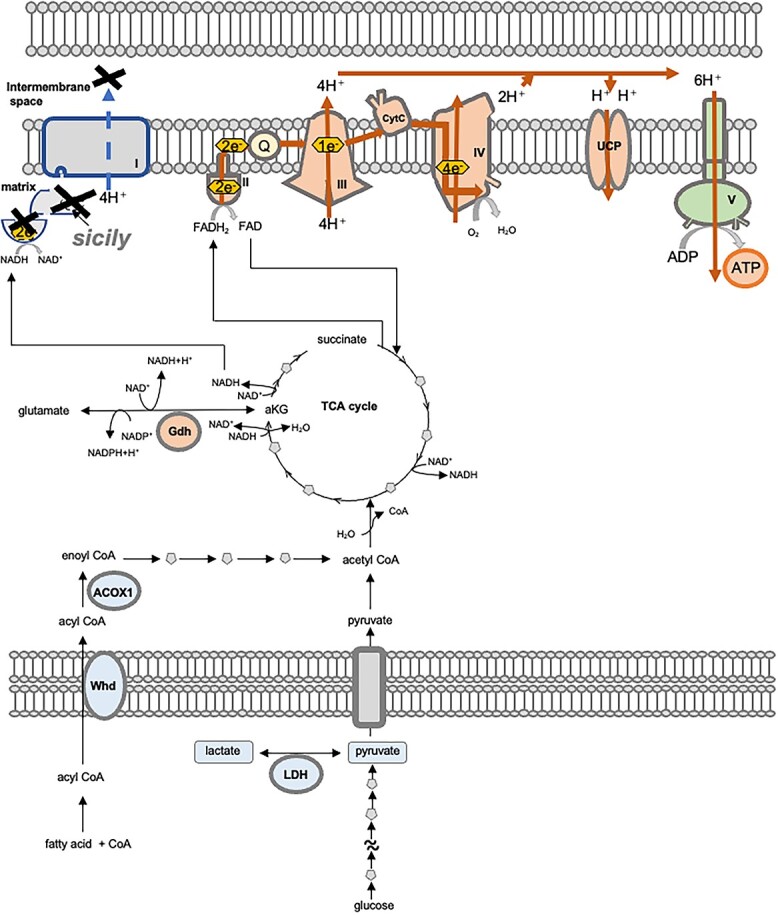
Possible mechanisms by which 5-ALA-HCl + SFC increases ATP production without CI. The components upregulated and downregulated by 5-ALA-HCl + SFC are colored orange and blue, respectively. Sicily knockdown causes loss of CI, whereas upregulation of CII compensates for the lack of electron transfer in CI. Upregulation of CIII and CIV increases the number of protons pumped into the IMS. 5-ALA-HCl + SFC may also affect other ATP-synthesizing reactions, including glycolysis, the TCA cycle and β-oxidation, which in combination may counteract the accumulation of lactate and pyruvate and increase ATP synthesis.
In addition to CII, CIII and CIV, our results suggest that 5-ALA-HCl + SFC affects multiple ATP-synthesizing reactions, including glycolysis, the TCA cycle, OXPHOS, β-oxidation and other routes for oxidation of NADH such as the glutamate dehydrogenase pathway (Figs 4 and 5). 5-ALA stimulates the synthesis of porphyrins and heme (8,11,12). Heme regulates various cellular processes associated with oxygen by controlling the activities of enzymes, signal transducers and transcriptional regulators (23). 5-ALA is also metabolized to protoporphyrin IX, which affects gene expression by interacting with DNA or RNA sequences that fold into non-canonical four-stranded nucleic acid structures known as G-quadruplexes (24–26). 5-ALA-HCl + SFC may shift the cellular metabolic mode to cope with the lack of CI via orchestrated changes in multiple pathways (Fig. 7).
In summary, our results revealed that 5-ALA-HCl + SFC bypasses CI deficiency and promotes ATP production, and thus corrects metabolic abnormalities and mitigates detrimental phenotypes in Drosophila. Further investigation would enhance our understanding of metabolic regulation and the pathogenesis of mitochondrial diseases.
Materials and Methods
Chemicals
5-ALA hydrochloride (neo-ALA Co. Ltd, Tokyo, Japan) and SFC (Komatsuya Corporation, Osaka, Japan) were provided by SBI Pharmaceuticals Co., Ltd. Alexa 488 Goat Anti-Horseradish Peroxidase (Jackson Immuno Research), Rhodamin-Phalloidin (Merck/Sigma-Aldrich, Germany), NativePAGE™ Running Buffer and NativePAGE™ Cathode Buffer Additive (Thermo Fisher Scientific, USA) were purchased.
Fly stocks and husbandry
Flies were maintained in standard cornmeal media (10% glucose, 0.7% agar, 9% cornmeal, 4% yeast extract, 0.3% propionic acid and 0.1% n-butyl p-hydroxybenzoate) at 25°C under light–dark cycles of 12:12 h. The flies were transferred to fresh food vials for every 2–3 days. Actin-GAL4 (Act5C-GAL4, #3954), UAS-mCherry RNAi (#35785) and UAS-sicily RNAi (#55332) are obtained from Bloomington Drosophila Stock Center. UAS-sicily RNAi (#103029) is from VDRC.
5-ALA-HCl + SFC feeding
Flies were raised on regular cornmeal. After eclosion, male flies were maintained on regular cornmeal food mixed with 5-ALA-HCl + SFC at the indicated concentration. Food vials were changed every 2–3 days.
Muscle and NMJs staining
The muscles of third instar larvae were dissected in HL3.1 buffer (70 mM NaCl, 5 mM KCl, 0.2 mM CaCl2, 20 mM MgCl2, 10 mM NaHCO3, 5 mM trehalose and 5 mM HEPES) and fixed in 4% PFA for 10 min. Samples were incubated in PBS with 0.05% Triton X-100 (PBST) containing 5% normal goat serum (Jackson Immuno Research) for 30 min, then incubated with PBST containing 2 μg·ml−1 phalloidin/tetramethylrhodamine B isothiocyanate peptide (Sigma-Aldrich) and 3 μg·ml−1 Alexa 488 goat anti-horseradish peroxidase (Jackson Immuno Research) at 4°C for overnight. The samples were washed three times in 0.1% PBST and mounted in VectaShield mounting medium (Vector Laboratories). Images were captured using a fluorescence microscope (Keyence, BZ-X710).
Climbing assay
The climbing assay was performed as previously described (27). Flies were placed in an empty plastic vial (2.5 cm in diameter × 10 cm in length). The vial was gently tapped to knock the flies to the bottom, and the number of flies reached to the top, middle and bottom areas of the vials in 20 s was counted. Experiments were repeated 10 times, and the mean percentage of flies in each area and standard deviations was calculated. Experiments were repeated with independent cohorts more than three times, and a representative result was shown.
Bang-sensitivity
About 9–30 flies were placed in an empty plastic vial (2.5 cm in diameter × 10 cm in length). Flies were tapped down to the bottom, and the mean recovery time and standard deviations were calculated.
Lifespan analysis
Flies were placed in the food vials on their sides at 25°C under conditions of a 12:12-h light and dark cycle, and transferred to fresh food vials every 2–3 days, and the number of dead flies was counted each time.
Blue-native PAGE
Blue-native PAGE to analyze mitochondrial respiratory chain protein was carried out as described with a modification (28,29). Thorax from 54–100 flies was homogenized in 1 ml of chilled mitochondrial isolation medium [250 mM sucrose, 10 mM Tris–HCl (pH 7.4), 0.15 mM MgCl2]. The samples were centrifuged twice for 15 min at 600g at 4°C to remove debris. The supernatant was centrifuged again for 5 min at 13 000g at 4°C to collect mitochondrial fraction as the pellet. Mitochondrial protein levels were determined using a bicinchoninic acid (BCA) assay. For blue-native PAGE, The NativePAGE Novex Bis-Tris Gel System (Life Technologies) was used according to the manufacturer’s protocol. About 18–96 μg mitochondrial protein was solubilized in sample buffer (50 mM NaCl, 20 mM Tris–HCl (pH 7.4), 1% Triton X-100). After centrifugation for 5 min at 14 000 rpm at 4°C, the supernatants were collected and mixed with 10× loading dye solution (5% Coomassie Blue G, 1 M aminohexanoic acid, 100 mM Bis-Tris). The separated on 3–12% NativePAGE gels.
In-gel activity assay
In-gel activity assay to analyze mitochondrial respiratory chain activity was carried out as described previously with a modification (30). Mitochondrial fractions were subjected to blue-native PAGE. For CI activity, the gel was incubated in complex I activity substrate [2 mM Tris–HCl (pH 7.4), 0.01% (w/v) NADH, 0.025% (w/v) Nitrotetorazolium Bule chloride (NTB)] for 15 min at room temperature. The gel was then transferred to 10% acetic acid to stop reaction. For CII activity, the gel was incubated in complex II activity substrate [5 mM Tris–HCl (pH 7.4), 20 mM sodium succinate, 0.025% (w/v) NTB, 200 μM phenazine methosulphate] for 40 min at 37°C. The gel was then transferred to 10% acetic acid to stop reaction. For CIV, the gel was incubated with complex IV activity substrate [0.05% (w/v) diaminobenzidine, 0.01% (w/v) cytochrome c, 45 mM phosphate buffer (pH 7.4)] for overnight at room temperature. For CV activity, the gel was incubated with complex V activity substrate [35 mM Tris, 270 mM glycine, 14 mM MgSO4, 10 mM ATP, 0.2% (w/v) Pb(NO3)2] for overnight at room temperature. The gel was transferred to 50% of methanol to stop the reaction.
High-resolution clear native-PAGE
High-resolution Clear Native-PAGE to analyze mitochondrial respiratory chain protein was carried out as described with a modification (31). Thorax from 59 to 100 flies was homogenized in 1 ml of chilled mitochondrial isolation medium [250 mM sucrose, 10 mM Tris–HCl (pH 7.4), 0.15 mM MgCl2]. The samples were centrifuged twice for 15 min at 600g at 4°C to remove debris. The supernatant was centrifuged again for 5 min at 13 000g at 4°C to collect mitochondria as pellet. Mitochondrial protein levels were determined using a BCA assay. Mitochondrial fractions were incubated in sample buffer [50 mM NaCl, 50 mM imidazole/HCl, 2 mM 6-aminohexanoic acid, 1 mM EDTA (pH 7.0), DDM (2.5 g/g protein) and digitonin (4 g/g protein)] for 10 min on ice. After centrifugation for 15 min at 10 000g at 4°C, the supernatants were mixed with 10× loading dye solution (50% glycerol, 0.1% Ponceau S) and separated on 3–12% NativePAGE Novex Bis-Tris Gel System (Life Technologies). The gels were stained with colloidal blue staining kit (Thermo Fisher Scientific).
Pyruvate assay
Thoraxes from the 35 flies were homogenized in pyruvate assay buffer (Pyruvate Assay kit, Abcam), and pyruvate concentration was measured by using the Pyruvate Assay kit (Abcam) according to the manufacturer’s manual.
Lactate assay
Thoraxes from the 40 flies were homogenized in lactate assay buffer [L-Lactate Assay kit (Abcam)], and lactate concentration was measured by using the L-Lactate Assay kit (Abcam) according to the manufacturer’s manual.
ATP assay
Thoraxes from the 20 flies were homogenized in CellTiter-Glo® buffer [Celltiter-Glo® Luminescent Cell Viability Assay (Promega))], and ATP was measured by using the Celltiter-Glo® Luminescent Cell Viability Assay (Promega) according to the manufacturer’s manual. Protein levels were measured with the BCA Protein Assay kit (Thermo Fisher Scientific).
Oxyblot (protein carbonyl assay kit)
Thoraxes from the 20 flies were homogenized in PBS (pH. 7.2), and oxidative stress levels were measured by using the Protein Carbonyl Assay kit (Abcam) according to the manufacturer’s manual. Protein levels were measured with the BCA Protein Assay kit (Thermo Fisher Scientific). A total of 85 μg of mitochondrial protein was spotted to polyvinylidene difluoride membranes, blocked with 5% skim milk in PBS-T and incubated with DNP antibody followed by HRP goat anti-rabbit secondary antibody. Immunolabeled proteins were analyzed by using a chemiluminescence kit (ImmunoStar LD) and a lumino-image analyzer (ChemiDoc MP System, Bio-Rad Laboratories).
mtDNA/nDNA ratio
The total DNA of the 20 thoraxes was purified according to the protocol provided with the QIAamp DNA Mini kit (QIAGEN). The expression level of each gene was measured using the PowerUp SYBR Green Master Mix (Life Technologies) and the StepOnePlus Real-Time PCR System (Life Technologies). The average value of levels of mito:COI and mito:COIII was used as mitochondrial DNA, and the average value of levels of sicily and Act5C was used to quantify nuclear DNA. Primers were designed using DRSC FlyPrimerBank (32). Primer sequences are shown in Supplementary Material, Table S1.
qRT-PCR
The total RNA of the 20 thoraxes was purified according to the protocol provided with the RNAeasy Plus Universal Mini kit (QIAGEN). Copy DNA (cDNA) was synthesized from 2.0 μg of total RNA using the High Capacity RNA-to-cDNA kit (Life Technologies). The expression level of each gene was measured using the PowerUp SYBR Green Master Mix (Life Technologies) and the StepOnePlus Real-Time PCR System (Life Technologies). The expression of genes of interest was standardized relative to Act57B. Primers were designed using DRSC FlyPrimerBank (33). Primer sequences are listed in Supplementary Material, Table S1.
Statistics
Statistics were done with GraphPad Prism9 (GraphPad Prism ver.9.10 for Windows, GraphPad Software, San Diego California USA). Differences were assessed using unpaired t-test, one-way ANOVA followed by Dunnett’s test or log-rank test and Holm–Sidak post hoc test. P-values < 0.05 were considered statistically significant.
Acknowledgements
Authors thank Drs Yoshihito Kishita, Akira Otake and Hiroko Harashima for instruction of mitochondrial activity assay, and Drs Masaru Shimura and Tohru Tanaka for insightful discussion. The authors also thank the Harvard Transgenic RNAi project (TRiP) [NIH/NIGMS R01-GM084947 (32)], the Bloomington Stock Center, and The Vienna Drosophila Resource Center (VDRC).
Conflict of Interest statement. N.N., T.I., K.T. and M.I. are employees of SBI Pharmaceuticals Co., Ltd No other conflict of interest.
Funding
This work was supported in part by a research award from the Japan Foundation for Aging and Health (to K.A.) and a Grant-in-Aid for Scientific Research on Challenging Research (Exploratory) [JSPS KAKENHI Grant number 19 K21593] (to K.A.), and SBI Pharmaceuticals Co., Ltd (to K. A.). 5-ALA hydrochloride and SFC were provided by SBI Pharmaceuticals Co., Ltd.
Authors’ contributions
N.N. and K.A. designed the experiments. N.N., M.N., K. S., A.A., T.S. and K.A. performed the experiments. N.N., K.S. and K.A. analyzed the data. N.N., M.N., T.I, K.T, M.I. and K.A. wrote the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article or available from the lead contact upon request.
Supplementary Material
Contributor Information
Naoko Nozawa, Department of Biological Sciences, Graduate School of Science, Tokyo Metropolitan University, Tokyo 192-0397, Japan; Division of Pharmaceutical Research, SBI Pharmaceuticals Co., Ltd, Tokyo 106-6020, Japan.
Marie Noguchi, Department of Biological Sciences, Graduate School of Science, Tokyo Metropolitan University, Tokyo 192-0397, Japan.
Kanako Shinno, Department of Biological Sciences, Graduate School of Science, Tokyo Metropolitan University, Tokyo 192-0397, Japan.
Taro Saito, Department of Biological Sciences, Graduate School of Science, Tokyo Metropolitan University, Tokyo 192-0397, Japan; Department of Biological Sciences, School of Science, Tokyo Metropolitan University, Tokyo 192-0397, Japan.
Akiko Asada, Department of Biological Sciences, Graduate School of Science, Tokyo Metropolitan University, Tokyo 192-0397, Japan; Department of Biological Sciences, School of Science, Tokyo Metropolitan University, Tokyo 192-0397, Japan.
Takuya Ishii, Division of Pharmaceutical Research, SBI Pharmaceuticals Co., Ltd, Tokyo 106-6020, Japan; Medical- Engineering Collaboration and Innovation Office, National Cancer Center Hospital East, 6-5-1 Kashinoha, Kashiwa, Chiba 277-8577, Japan.
Kiwamu Takahashi, Division of Pharmaceutical Research, SBI Pharmaceuticals Co., Ltd, Tokyo 106-6020, Japan.
Masahiro Ishizuka, Division of Pharmaceutical Research, SBI Pharmaceuticals Co., Ltd, Tokyo 106-6020, Japan.
Kanae Ando, Department of Biological Sciences, Graduate School of Science, Tokyo Metropolitan University, Tokyo 192-0397, Japan; Department of Biological Sciences, School of Science, Tokyo Metropolitan University, Tokyo 192-0397, Japan.
References
- 1. Murayama, K., Shimura, M., Liu, Z., Okazaki, Y. and Ohtake, A. (2019) Recent topics: the diagnosis, molecular genesis, and treatment of mitochondrial diseases. J. Hum. Genet., 64, 113–125. [DOI] [PubMed] [Google Scholar]
- 2. Gorman, G.S., Chinnery, P.F., DiMauro, S., Hirano, M., Koga, Y., McFarland, R., Suomalainen, A., Thorburn, D.R., Zeviani, M. and Turnbull, D.M. (2016) Mitochondrial diseases. Nat. Rev. Dis. Primers, 2, 16080. [DOI] [PubMed] [Google Scholar]
- 3. Nolfi-Donegan, D., Braganza, A. and Shiva, S. (2020) Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol., 37, 101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cogliati, S., Enriquez, J.A. and Scorrano, L. (2016) Mitochondrial cristae: where beauty meets functionality. Trends Biochem. Sci., 41, 261–273. [DOI] [PubMed] [Google Scholar]
- 5. Ehinger, J.K., Piel, S., Ford, R., Karlsson, M., Sjovall, F., Frostner, E.A., Morota, S., Taylor, R.W., Turnbull, D.M., Cornell, C. et al. (2016) Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat. Commun., 7, 12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atamna, H., Killilea, D.W., Killilea, A.N. and Ames, B.N. (2002) Heme deficiency may be a factor in the mitochondrial and neuronal decay of aging. Proc. Natl. Acad. Sci. U. S. A., 99, 14807–14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atamna, H., Liu, J. and Ames, B.N. (2001) Heme deficiency selectively interrupts assembly of mitochondrial complex IV in human fibroblasts: revelance to aging. J. Biol. Chem., 276, 48410–48416. [DOI] [PubMed] [Google Scholar]
- 8. Shimura, M., Nozawa, N., Ogawa-Tominaga, M., Fushimi, T., Tajika, M., Ichimoto, K., Matsunaga, A., Tsuruoka, T., Kishita, Y., Ishii, T. et al. (2019) Effects of 5-aminolevulinic acid and sodium ferrous citrate on fibroblasts from individuals with mitochondrial diseases. Sci. Rep., 9, 10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogura, S., Maruyama, K., Hagiya, Y., Sugiyama, Y., Tsuchiya, K., Takahashi, K., Abe, F., Tabata, K., Okura, I., Nakajima, M. et al. (2011) The effect of 5-aminolevulinic acid on cytochrome c oxidase activity in mouse liver. BMC Res Notes, 4, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ota, U., Hara, T., Nakagawa, H., Tsuru, E., Tsuda, M., Kamiya, A., Kuroda, Y., Kitajima, Y., Koda, A., Ishizuka, M. et al. (2017) 5-aminolevulinic acid combined with ferrous ion reduces adiposity and improves glucose tolerance in diet-induced obese mice via enhancing mitochondrial function. BMC Pharmacol. Toxicol., 18, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mingone, C.J., Gupte, S.A., Chow, J.L., Ahmad, M., Abraham, N.G. and Wolin, M.S. (2006) Protoporphyrin IX generation from delta-aminolevulinic acid elicits pulmonary artery relaxation and soluble guanylate cyclase activation. Am. J. Physiol. Lung Cell Mol. Physiol., 291, L337–L344. [DOI] [PubMed] [Google Scholar]
- 12. Abe, K., Ikeda, M., Ide, T., Tadokoro, T., Miyamoto, H.D., Furusawa, S., Tsutsui, Y., Miyake, R., Ishimaru, K., Watanabe, M. et al. (2022) Doxorubicin causes ferroptosis and cardiotoxicity by intercalating into mitochondrial DNA and disrupting Alas1-dependent heme synthesis. Sci. Signal, 15, eabn8017. [DOI] [PubMed] [Google Scholar]
- 13. Zhang, K., Li, Z., Jaiswal, M., Bayat, V., Xiong, B., Sandoval, H., Charng, W.L., David, G., Haueter, C., Yamamoto, S. et al. (2013) The C8ORF38 homologue Sicily is a cytosolic chaperone for a mitochondrial complex I subunit. J. Cell Biol., 200, 807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu, L., Zhang, K., Sandoval, H., Yamamoto, S., Jaiswal, M., Sanz, E., Li, Z., Hui, J., Graham, B.H., Quintana, A. et al. (2015) Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell, 160, 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaiswal, M., Haelterman, N.A., Sandoval, H., Xiong, B., Donti, T., Kalsotra, A., Yamamoto, S., Cooper, T.A., Graham, B.H. and Bellen, H.J. (2015) Impaired mitochondrial energy production causes light-induced photoreceptor degeneration independent of oxidative stress. PLoS Biol, 13, e1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brand, A.H. and Perrimon, N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- 17. Garcia, C.J., Khajeh, J., Coulanges, E., Chen, E.I. and Owusu-Ansah, E. (2017) Regulation of mitochondrial complex I biogenesis in Drosophila flight muscles. Cell Rep., 20, 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stacpoole, P.W. (1997) Lactic acidosis and other mitochondrial disorders. Metab. Clin. Exp., 46, 306–321. [DOI] [PubMed] [Google Scholar]
- 19. Robinson, B.H. (2006) Lactic acidemia and mitochondrial disease. Mol. Genet. Metab., 89, 3–13. [DOI] [PubMed] [Google Scholar]
- 20. Schubert Baldo, M. and Vilarinho, L. (2020) Molecular basis of Leigh syndrome: a current look. Orphanet J. Rare Dis., 15, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rahman, S. (2020) Mitochondrial disease in children. J. Intern. Med., 287, 609–633. [DOI] [PubMed] [Google Scholar]
- 22. Nilsson, A., Bjornson, E., Flockhart, M., Larsen, F.J. and Nielsen, J. (2019) Complex I is bypassed during high intensity exercise. Nat. Commun., 10, 5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mense, S.M. and Zhang, L. (2006) Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res., 16, 681–692. [DOI] [PubMed] [Google Scholar]
- 24. Balasubramanian, S., Hurley, L.H. and Neidle, S. (2011) Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat. Rev. Drug Discov., 10, 261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shioda, N., Yabuki, Y., Yamaguchi, K., Onozato, M., Li, Y., Kurosawa, K., Tanabe, H., Okamoto, N., Era, T., Sugiyama, H. et al. (2018) Targeting G-quadruplex DNA as cognitive function therapy for ATR-X syndrome. Nat. Med., 24, 802–813. [DOI] [PubMed] [Google Scholar]
- 26. Asamitsu, S., Yabuki, Y., Ikenoshita, S., Kawakubo, K., Kawasaki, M., Usuki, S., Nakayama, Y., Adachi, K., Kugoh, H., Ishii, K. et al. (2021) CGG repeat RNA G-quadruplexes interact with FMRpolyG to cause neuronal dysfunction in fragile X-related tremor/ataxia syndrome. Sci. Adv., 7, eabd9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iijima-Ando, K., Hearn, S.A., Shenton, C., Gatt, A., Zhao, L. and Iijima, K. (2009) Mitochondrial mislocalization underlies Abeta42-induced neuronal dysfunction in a Drosophila model of Alzheimer's disease. PLoS One, 4, e8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pareek, G., Thomas, R.E., Vincow, E.S., Morris, D.R. and Pallanck, L.J. (2018) Lon protease inactivation in Drosophila causes unfolded protein stress and inhibition of mitochondrial translation. Cell Death Discov., 4, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kohda, M., Tokuzawa, Y., Kishita, Y., Nyuzuki, H., Moriyama, Y., Mizuno, Y., Hirata, T., Yatsuka, Y., Yamashita-Sugahara, Y., Nakachi, Y. et al. (2016) A comprehensive genomic analysis reveals the genetic landscape of mitochondrial respiratory chain complex deficiencies. PLoS Genet., 12, e1005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jha, P., Wang, X. and Auwerx, J. (2016) Analysis of mitochondrial respiratory chain supercomplexes using blue native polyacrylamide gel electrophoresis (BN-PAGE). Curr Protoc Mouse Biol, 6, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wittig, I., Karas, M. and Schagger, H. (2007) High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol. Cell. Proteomics, 6, 1215–1225. [DOI] [PubMed] [Google Scholar]
- 32. Hu, Y., Comjean, A., Rodiger, J., Liu, Y., Gao, Y., Chung, V., Zirin, J., Perrimon, N. and Mohr, S.E. (2021) FlyRNAi.Org-the database of the Drosophila RNAi screening center and transgenic RNAi project: 2021 update. Nucleic Acids Res., 49, D908–D915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu, Y., Sopko, R., Foos, M., Kelley, C., Flockhart, I., Ammeux, N., Wang, X., Perkins, L., Perrimon, N. and Mohr, S.E. (2013) FlyPrimerBank: an online database for Drosophila melanogaster gene expression analysis and knockdown evaluation of RNAi reagents. G3, 3, 1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article or available from the lead contact upon request.