Abstract
Background
Family caregivers of patients with advanced cancer often have poor quality of life (QOL) and mental health. We examined the effectiveness of interventions offering support for caregivers of patients with advanced cancer on caregiver QOL and mental health outcomes.
Methods
We searched Ovid MEDLINE, EMBASE, Cochrane CENTRAL, and Cumulative Index to Nursing and Allied Health Literature databases from inception through June 2021. Eligible studies reported on randomized controlled trials for adult caregivers of adult patients with advanced cancer. Meta-analysis was conducted for primary outcomes of QOL, physical well-being, mental well-being, anxiety, and depression, from baseline to follow-up of 1-3 months; secondary endpoints were these outcomes at 4-6 months and additional caregiver burden, self-efficacy, family functioning, and bereavement outcomes. Random effects models were used to generate summary standardized mean differences (SMD).
Results
Of 12 193 references identified, 56 articles reporting on 49 trials involving 8554 caregivers were eligible for analysis; 16 (33%) targeted caregivers, 19 (39%) patient–caregiver dyads, and 14 (29%) patients and their families. At 1- to 3-month follow-up, interventions had a statistically significant effect on overall QOL (SMD = 0.24, 95% confidence interval [CI] = 0.10 to 0.39); I2 = 52.0%), mental well-being (SMD = 0.14, 95% CI = 0.02 to 0.25; I2 = 0.0%), anxiety (SMD = 0.27, 95% CI = 0.06 to 0.49; I2 = 74.0%), and depression (SMD = 0.34, 95% CI = 0.16 to 0.52; I2 = 64.4) compared with standard care. In narrative synthesis, interventions demonstrated improvements in caregiver self-efficacy and grief.
Conclusions
Interventions targeting caregivers, dyads, or patients and families led to improvements in caregiver QOL and mental health. These data support the routine provision of interventions to improve well-being in caregivers of patients with advanced cancer.
Family caregivers are relatives or friends who provide unpaid care for patients, assisting with physically, emotionally, and socially demanding tasks (1-3). More than 1 in 10 adults in the United States and Europe are family caregivers, with cancer among the most common diagnoses of care recipients (4,5). Caregivers of patients with advanced cancer (defined in this paper as stage III or greater) are particularly vulnerable compared with those caring for patients with earlier stage cancers, as patients’ increased symptom burden, decreased functional status, and the need for advance care planning and end-of-life discussions lead to increased caregiver demands (1). These caregivers often experience poor quality of life (QOL), a multidimensional construct encompassing physical, emotional, social, financial, and spiritual aspects of well-being (6). In particular, caregivers of patients with advanced cancer may have poor mental health, including depression and anxiety (7-12), as well as physical symptoms including fatigue, sleep disturbance, loss of appetite, and pain (13,14). The tendency of modern medicine to prioritize patient-centered aspects of care including autonomy and confidentiality has led to an unintended neglect of their caregivers, particularly in the advanced cancer setting (15).
Interventions to support caregivers of patients with advanced cancer may be categorized according to their target population of individual caregivers, caregiver-patient dyads, or patients and their families (16-18). Two comprehensive meta-analyses have examined caregiver interventions (19,20)—one in caregivers of patients with cancer mainly at earlier stages (19) and the other in caregivers of patients in the terminal phase of any disease (20)—both were published more than a decade ago. Other meta-analyses were limited to specific psychosocial interventions (21,22) or to interventions in home settings (23) or only assessed impact on caregivers of interventions directed at patients (24-28). Given the heavy burden of caregiving and high levels of distress in caregivers of patients with advanced cancer (15), the objective of the current review was to determine the effect of interventions offering support for caregivers of patients with advanced cancer on caregiver QOL and mental health outcomes.
Methods
The protocol for this systematic review and meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42019136321). This review was conducted and reported in accordance with the Cochrane Handbook for Systematic Reviews (29) and the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement (30).
Identification and selection of studies
With assistance from a health science librarian, we searched the following databases from inception through June 2021: Ovid MEDLINE, EMBASE, Cochrane CENTRAL Register of Controlled Trials, and Cumulative Index to Nursing and Allied Health Literature (CINAHL). MeSH subject headings and specific search terms were used to execute the search (Supplementary Methods 1, available online), which was restricted to clinical trials and English-language publications. Two reviewers (RC and CZ) screened references from retrieved papers and previous systematic reviews to retrieve additional studies not identified by the search strategy. Two of 4 reviewers (RC, SA, EYC, JW) independently evaluated all studies for eligibility using predefined eligibility criteria; discrepancies were resolved by discussion and, if necessary, with the input of a further reviewer (CZ).
Study eligibility criteria
We included studies that reported on randomized controlled trials of interventions for adult (aged 18 years and older) caregivers of adult patients with advanced (stage III or IV) cancer; to reduce heterogeneity, only trials in which all patients had advanced cancer were included. Interventions needed to be either psychoeducational, skills training, counseling, or team-based interventions offering direct or indirect support with caregiving or coping. The interventions could be directed at the caregiver, the patient–caregiver dyad, or the patient and/or his or her family, provided that caregiver outcomes were measured. Interventions specifically designed to target patient–caregiver dyads were classified as dyad interventions, whereas those targeting the patient alone, or targeting the patient and 1 or more family members who were not specified as being caregivers, were classified as directed at the patient and/or his or her family. Studies that assessed complementary therapies (eg, massage) were excluded because they did not meet the definition of a psychoeducational, skills training, counseling, or team-based intervention, and those that assessed interventions targeting only 1 symptom (eg, sleep) were excluded because these interventions were tailored to focus only on that particular symptom rather than on improving overall QOL and mental health. Comparators could be usual care or an active control (Supplementary Methods 2, available online). Studies published only as abstracts were excluded because abstracts often consist of partial or interim data that may change with publication of the final study, and quality of reporting is often poor (31,32). Studies with sample size less than 20 per trial arm were excluded because of greater risk of publication bias and lower trial quality associated with smaller samples (33).
Data extraction and risk of bias assessment
Two of 4 reviewers (RC, JJM, SL, EYC) independently extracted data from the included studies using a standardized, prepiloted data extraction form. The target of the intervention was classified as being the caregiver, the patient–caregiver dyad, or the patient and his or her family (15). Disagreements were resolved by discussion, with input from a further reviewer (CZ), if necessary. Missing data for meta-analysis were requested from study authors up to 2 times; if no response was received after the second request, the study was not included in the meta-analysis. The same reviewers used the Cochrane Risk of Bias Tool 2.0 (34) to assess the risk of bias of the included trials, with disagreements resolved in the same manner. The tool was used to assign each trial a rating of low, high, or some concerns of bias using a standardized method (Supplementary Methods 3, available online) (35). For cluster-randomized trials, the modified Cochrane Risk of Bias Tool 2.0 for cluster-randomized trials was used (36). Publication bias was assessed using funnel plots and Egger tests for all primary outcomes at 1-3 months.
Synthesis and statistical analysis
A narrative synthesis was conducted to describe data for all outcomes reported for each trial. Primary outcomes of overall QOL, QOL subscales of physical and mental well-being, depression, and anxiety were selected a priori for meta-analysis. All of these outcomes were predefined as occurring while the patient was living, to avoid introduction of confounding and heterogeneity due to the impact of the patient’s death on caregivers’ QOL and mental health. Secondary outcomes were described only in the narrative synthesis and comprised caregiver burden (including outcomes of caregiver burden, stress, or strain), caregiver self-efficacy (including self-efficacy, competence, mastery, knowledge, or preparedness), family functioning (including family relationships and family functioning), and bereavement outcomes (including grief and depression after death of the patient).
A meta-analysis was conducted for all primary outcomes; study data were extracted separately by time from baseline to 1-3 months (primary endpoint) and 4-6 months (secondary endpoint) follow-up; these endpoints were chosen because they are commonly used in trials of caregiver interventions and differentiate between short- and longer-term effects (24). Similar to a previous review (24), if a study reported outcomes more than once within the same 1- to 3-month or 4- to 6-month interval, the last time point was used; outcomes reported between these 2 intervals were categorized with the 1- to 3-month interval. For studies with multiple measures assessing the same outcome (eg, 2 measures for QOL), we established a hierarchy for inclusion in the meta-analysis, based on authors’ designation of the measure as the primary outcome, number of items (full measures preferred over abbreviated ones), and validation for use in caregivers. If a study included a brief and more extensive intervention, we conservatively used the brief intervention for the main analysis and conducted a sensitivity analysis using the extensive intervention.
Because measures to evaluate each outcome varied among trials, summary statistics were reported as standardized mean differences (SMDs) for each trial, corrected for scale directionality when necessary, and calculated using a Hedges adjusted G estimator to correct for small sample bias (37). SMDs of 0.2, 0.5, and 0.8 represent small, moderate, and large effects, respectively (38). To account for statistical heterogeneity of treatment effects across trials, random effects (Dersimonian and Laird) models were used to generate summary SMDs. The Hartung-Knapp adjustment was used for confidence intervals and statistical tests (39). The proportion of the total between-study variance in the treatment effects attributable to between-study heterogeneity (and not sampling variability) was documented using the I2 statistic (40). Heterogeneity was also assessed using the between-study variance of the treatment effect () and the Cochrane Q statistic. Subgroup analyses were conducted to compare pooled results of trials by intervention and by risk of bias. StataBE 17.0 (StataCorp) was used for all analyses; all statistical tests were 2-tailed, with a P value less than .05 considered statistically significant.
Results
Study characteristics
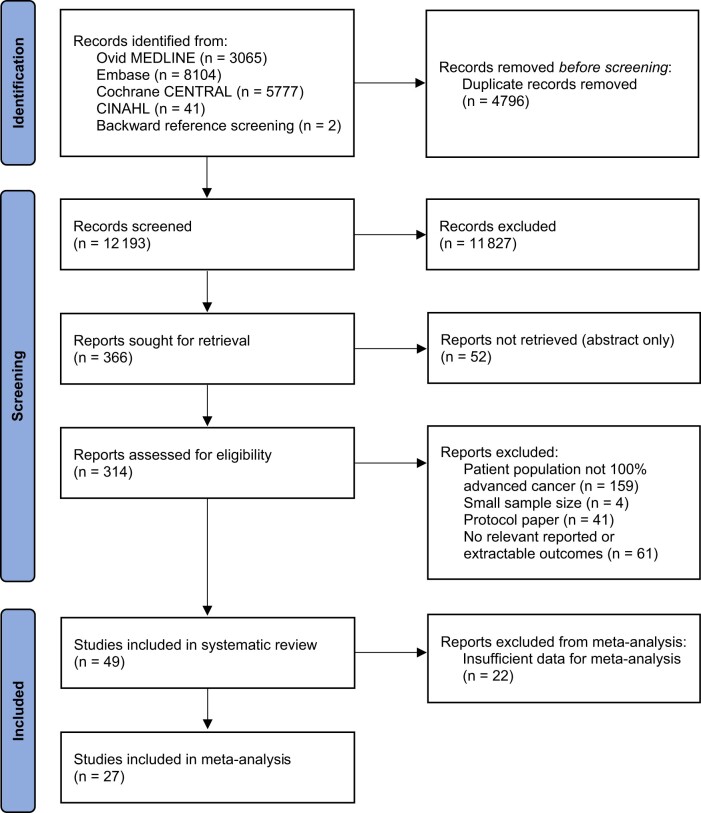
A total of 12 193 unique references were screened and 314 full-text articles were assessed; of these, 56 articles (41-96) reporting on outcomes of 49 trials for 8554 caregivers were ultimately included (Figure 1). Of the 49 included trials, 34 (69%) included caregivers of patients with mixed solid tumor malignancies, 6 (12%) only lung cancer (42,59-62,69,72,81), 1 (2%) breast cancer (74), 1 (2%) pancreatic cancer (94), 1 (2%) high-grade glioma (43), 2 (4%) gastrointestinal cancers (73,76), and 4 (8%) hematological malignancies (44,49,80,84). A total of 32 (65%) trials were conducted in an outpatient setting (42-45,53-57,59-77,81-83,85,87,88,91,92,94), 5 (10%) in a home setting (46-48,51,78,79,86), 4 (8%) in an inpatient setting (41,80,84,96), and 8 (16%) in mixed settings (49,50,52,58,89,90,93,95). Of the trials, 28 were conducted in the United States (44,45,49-51,53,55,59-68,71-77,83-86,89,92,94,95); 4 each in Australia (46-48,52,87,88) and China (69,80,81,96); 3 in Canada (42,70,91); 2 each in the United Kingdom (54,82), Netherlands (43,90), and Denmark (58,78,79); and 1 each in Colombia (41), Jordan (56), Korea (57), and Norway (93). Seven trials were at low risk of bias (42,44,60-62,65,66,84,85,91), 8 had some concerns for bias (47,48,55,59,63,64,74,76,77,83), and 34 were at high risk of bias (41,43,45,46,49-54,56-58,67-73,75,78-82,86-90,92-96) (Supplementary Figure 1, available online). There was concern for publication bias for the outcome of overall QOL (P = .009) (Supplementary Figure 2, available online).
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses flow diagram. CINAHL = Cumulative Index to Nursing and Allied Health Literature.
A total of 16 (33%) trials reported on interventions directed at caregivers (41-57), 19 (39%) on interventions directed at the patient–caregiver dyad (58-81), and 14 (29%) on interventions directed at patients and/or their families (83-96). Most trials directed at caregivers evaluated psychoeducational or problem-solving interventions (13 of 16, 81%) (41-43,45-50,52,53,55-57); most directed at dyads evaluated counseling or therapy interventions (18 of 19, 95%) (58,59,63-67,69-81); and most directed at patients evaluated palliative care team interventions (10 of 14, 71%) (83-89,91,93,94,96). A total of 42 (86%) trials compared 1 intervention to usual care (42-46,49,50,52-56,58,59,63-72,74,75,77-96), 2 (4%) compared 2 interventions to usual care (47,48,51), and 5 (10%) compared 2 or more interventions without a usual care arm (41,57,60-62,73,76). For most trials reporting on proportion of spousal caregivers, more than 50% of participants were patients’ spouses (31 of 36, 86%) (42,44-49,52-55,58,59,63-66,68-73,75-77,83-85,90-94); for most reporting on caregiver sex, more than 60% were female caregivers (30 of 42, 71%) (41-51,54,55,57-59,65-69,73,76,78,79,83-86,90-93); and for most conducted in the United States and western Europe reporting on race, more than 70% of caregivers were White or Caucasian (15 of 18, 83%) (44,45,54,55,65-67,71-73,75,76,84,85,92,94). Other aspects of the study design and outcomes are reported in Supplementary Tables 1-3 (available online).
Quality of life
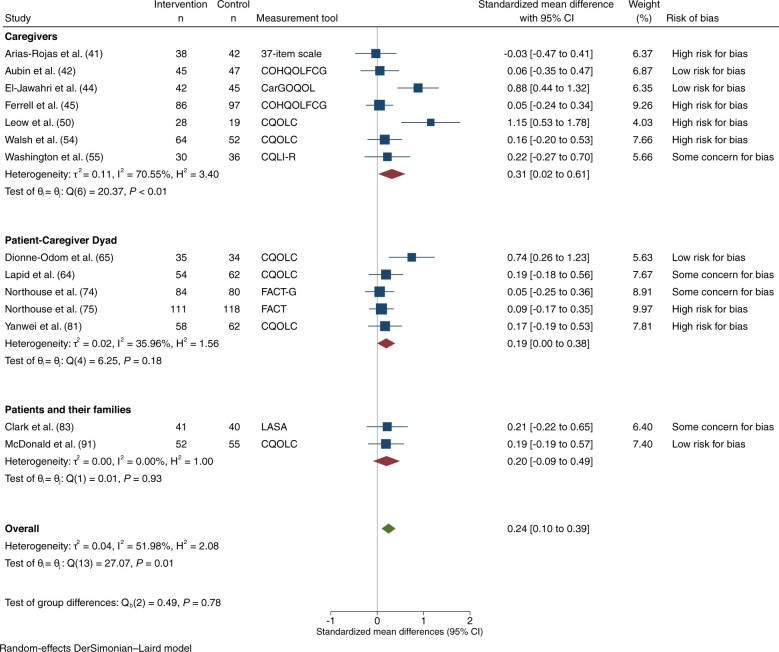
QOL and/or its individual domains were assessed in 23 trials; 16 assessed overall QOL, 10 physical well-being, 8 mental well-being, and 6 other QOL subscales. A total of 16 trials assessed overall QOL: 5 at low risk of bias (42,44,65,66,84,91), 3 with some concerns (55,63,64,83), and 8 at high risk (41,45,50,51,54,56,57,81). Ten were directed at caregivers (41,42,44,45,50,51,54-57), 3 at the patient–caregiver dyad (64-66,81), and 3 at patients and/or their families (83,84,91). Fourteen trials involving 2264 participants had data that could be pooled for meta-analysis of overall QOL: 14 at 1-3 months (41,42,44,45,50,54,55,63-66,74,75,81,83,91) and 4 at 4- to 6-month follow-up (42,74,75,91). Of those measuring QOL at 1-3 months, 4 were at low risk (42,44,65,66,91), 4 had some concerns (55,63,64,74,83), and 6 at high risk of bias (41,45,50,54,55,75,81). There was a statistically significant effect on QOL at 1-3 months (SMD = 0.24, 95% [confidence interval] CI = 0.10 to 0.39; I2 = 52.0%; Figure 2), albeit with high risk of publication bias (Supplementary Figure 2, available online). There were no differences among subgroups by intervention target or by risk of bias. Among the 4 trials evaluating QOL at 4-6 months, there was no statistically significant improvement in QOL (Supplementary Figure 3.1, available online). Sensitivity analyses substituting the extensive for the brief intervention in 1 study (75) yielded similar conclusions.
Figure 2.
Overall quality of life, at 1-3 months. CI = confidence interval; CarGOQOL = Caregiver Oncology Quality of Life Questionnaire; COHQOLFCG = City of Hope Quality of Life Family Caregiver Version; CQLI-R = Caregiver Quality of Life Index-Revised; CQOLC = Caregiver Quality of Life Index-Cancer; FACT = Functional Assessment of Cancer Therapy; FACT-G = Functional Assessment of Cancer Therapy-General; LASA = linear analog self-assessment items.
Physical well-being
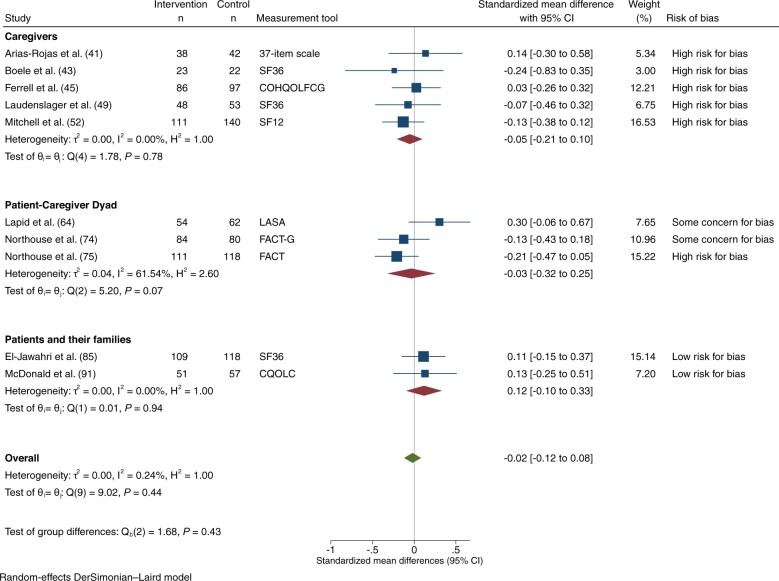
Physical well-being was reported in 11 trials: 2 at low risk of bias (85,91), 2 with some concerns (63,64,74), and 7 at high risk (41,45,49,52,58,71,75). Four were directed at caregivers (41,45,49,52), 5 at the patient–caregiver dyad (58,63,64,71,74,75), and 2 at patients and/or their families (85,91). A total of 11 trials involving 2226 participants had data on physical well-being that could be extracted and pooled for meta-analysis: 10 trials at 1-3 months (41,43,45,49,52,63,64,74,75,85,91) and 7 at 4-6 months (43,52,58,74,75,85,91). For those assessing physical well-being at 1-3 months, risk of bias was rated low for 2 trials (85,91), some concerns for 2 (63,64,74), and high for 6 (41,43,45,49,52,75). There was no statistically significant effect on physical well-being at 1-3 months (SMD = -0.02, 95% CI = -0.12 to 0.08; I2 = 0.2%; Figure 3), with no differences among subgroups by intervention target or by risk of bias. At 4-6 months, there was no effect on physical well-being (Supplementary Figure 3.2, available online). Results were similar in sensitivity analyses substituting the extensive for the brief intervention in 1 study (75).
Figure 3.
Physical well-being, at 1-3 months. COHQOLFCG = City of Hope Quality of Life Family Caregiver Version; CQOLC = Caregiver Quality of Life Index-Cancer; FACT = Functional Assessment of Cancer Therapy; FACT-G = Functional Assessment of Cancer Therapy-General; LASA = linear analog self-assessment items; SF12 = 12-Item Short-Form Health Survey; SF36 = 36-Item Short-Form Health Survey.
Mental well-being
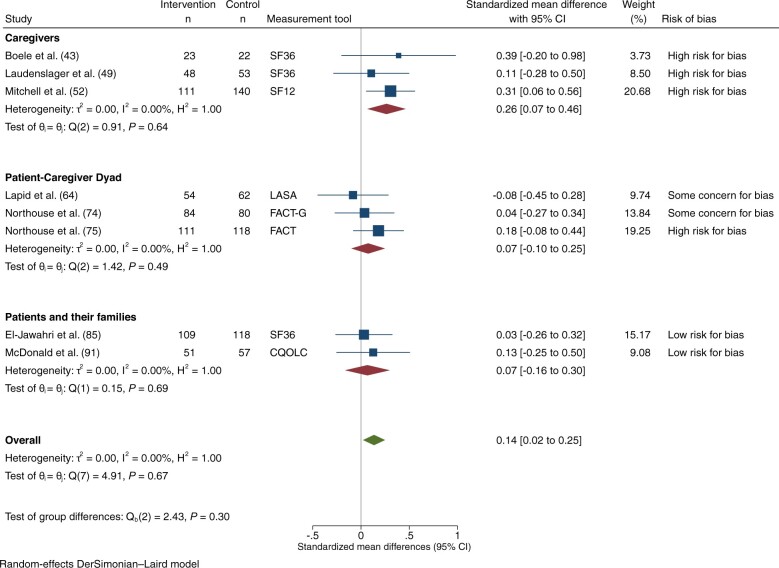
Mental well-being was reported in 9 trials: 2 at low risk of bias (85,91), 2 with some concerns (63,64,74), and 5 at high risk (43,49,52,58,75). Nine trials, reporting on 1906 participants, had extractable data on mental well-being that could be pooled for meta-analysis (43,49,52,58,63,64,74,75,85,91): 8 at 1-3 months (43,49,52,63,64,74,75,85,91) and 7 at 4-6 months (43,52,58,74,75,85,91). Among those assessing mental well-being at 1-3 months, 2 were at low risk (85,91), 2 had some concerns (63,64,74), and 4 were at high risk of bias (43,49,52,75). There was a statistically significant effect on mental well-being at 1-3 months (SMD = 0.14, 95% CI = 0.02 to 0.25; I2 = 0.0%; Figure 4), with no statistically significant differences among subgroups by intervention target or by risk of bias. At 4-6 months, there was no statistically significant effect on mental well-being (Supplementary Figure 3.3, available online). Results were similar in a sensitivity analysis substituting the extensive for the brief intervention in 1 study (75).
Figure 4.
Mental well-being, at 1-3 months. CI = confidence interval; CQOLC = Caregiver Quality of Life Index-Cancer; FACT = Functional Assessment of Cancer Therapy; FACT-G = Functional Assessment of Cancer Therapy-General; LASA = linear analog self-assessment items; SF12 = 12-Item Short-Form Health Survey; SF36 = 36-Item Short-Form Health Survey.
Anxiety
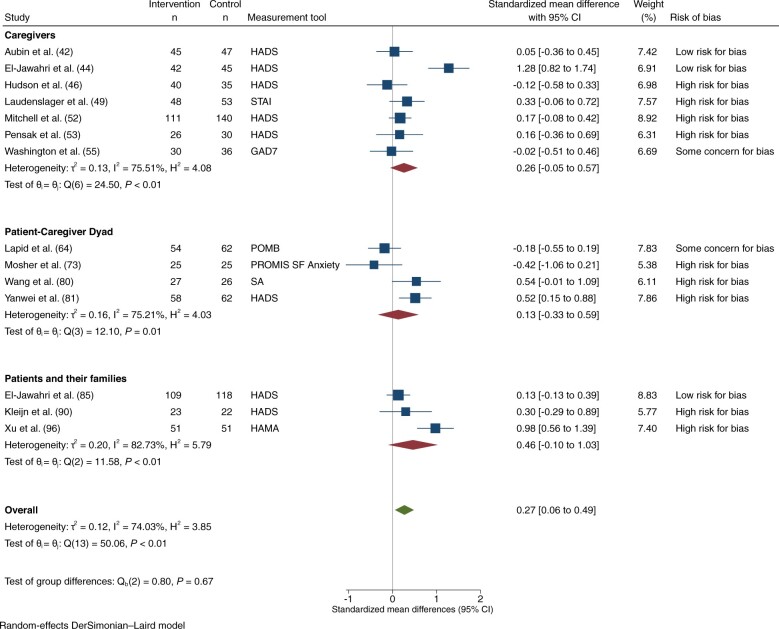
Anxiety was assessed in 21 trials: 4 were at low risk of bias (42,44,84,85), 3 had some concerns (55,59,63,64), and 14 were at high risk (46,49,52,53,57,58,73,80-82,86,90,94,96). Eight trials had interventions directed at caregivers (42,44,46,49,52,53,55,57), 6 at the patient–caregiver dyad (58,59,63,64,73,80,81), and 7 at patients and/or their families (82,84-86,90,94,96). A total of 15 trials with 1874 participants had extractable data on anxiety for meta-analysis: 14 at 1-3 months (42,44,46,49,52,53,55,63,64,73,80,81,85,90,96) and 4 at 4-6 months (42,52,58,85). Among trials reporting on anxiety at 1-3 months, 3 were at low risk of bias (42,44,85), 2 had some concerns (55,63,64), and 9 were at high risk of bias (46,49,52,53,73,80,81,90,96). There was a statistically significant effect on anxiety at 1-3 months (SMD = 0.27, 95% CI = 0.06 to 0.49; I2 = 74.0%; Figure 5). There were no statistically significant differences among subgroups by intervention target or by risk of bias. Among the 4 studies reporting on anxiety at 4-6 months, there was no statistically significant effect on anxiety (Supplementary Figure 3.4, available online).
Figure 5.
Anxiety, at 1-3 months. CI = confidence interval; HADS = Hospital Anxiety and Depression Scale; HAMA = Hamilton Anxiety Scale; GAD7 = General Anxiety Disorder-7; POMB = Profile of Mood States-B; PROMIS SF Anxiety = 6-Item Patient Reported Outcomes Measurement Information System Short-Form Anxiety Measure; SA = Social Anxiety Scale; STAI = State-Trait Anxiety Inventory.
Depression
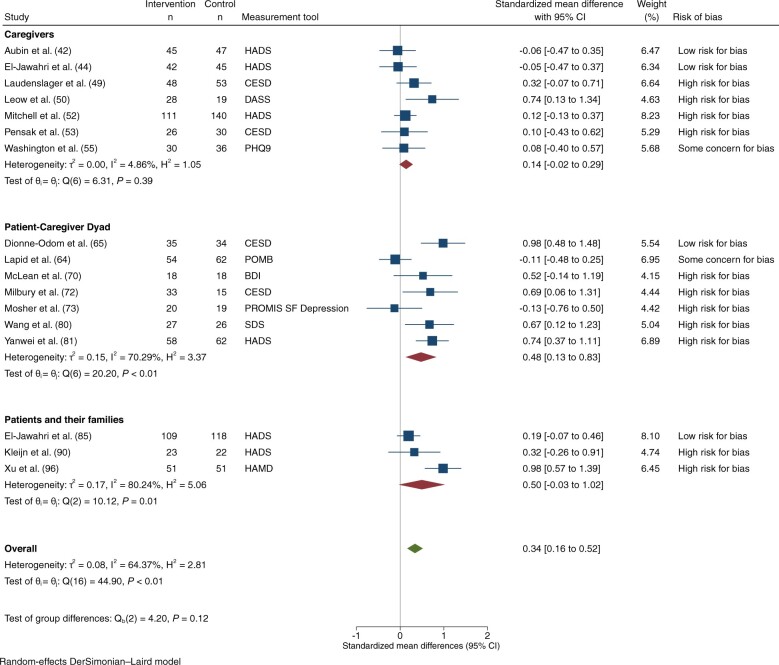
Depression was assessed in 27 trials: 4 at low risk of bias (42,44,65,66,84), 4 with some concerns (55,59,63,64,77), and 19 at high risk (49,50,52,53,57,58,70,72,73,80-82,86-90,94-96). Interventions were directed at caregivers in 8 trials (42,44,49,50,52,53,55,57), patient–caregiver dyads in 10 (58,59,63-66,70,72,73,77,80,81), and patients and/or their families in 9 (82,84,86-90,94-96). Eighteen studies involving 2087 participants had extractable data on depression for meta-analysis: 17 at 1-3 months (42,44,49,50,52,53,55,63-66,70,72,73,80,81,85,90,96) and 4 at 4-6 months (42,52,58,85). Among trials reporting at 1-3 months, 4 were at low risk of bias (42,44,65,66,85), 2 at some concerns (55,63,64), and 11 at high risk (49,50,52,53,70,72,73,80,81,90,96). There was a statistically significant effect on depression at 1-3 months (SMD = 0.34, 95% CI = 0.16 to 0.52; I2 = 64.4%; Figure 6). Although the difference between subgroups was not statistically significant (P = .12), interventions directed at patient–caregiver dyads tended to be associated with the greatest improvement in depression (SMD = 0.48, 95% CI = 0.13 to 0.83; I2 = 70.3). There was a similar trend for interventions directed at patients and/or their families, but there were only 3 trials in this subgroup and results were not statistically significant. Most trials were at high risk of bias, with this larger group also tending to have the greatest improvement in depression (P = .03). At 4-6 months, there was no statistically significant effect on depression (Supplementary Figure 3.5, available online).
Figure 6.
Depression, at 1-3 Months. BDI = Beck Depression Inventory; CESD = Center for Epidemiologic Studies-Depression; CI = confidence interval; DASS = Depression Anxiety Stress Scale; HADS = Hospital Anxiety and Depression Scale; HAMD = Hamilton Depression Scale; PHQ-9 = Patient Health Questionnaire-9; POMB = Profile of Mood States-B; PROMIS SF Depression = 6-Item Patient Reported Outcomes Measurement Information System Short-Form Depression Measure; SDS = Self-rating Depression Scale.
Other outcomes
Caregiver burden was reported in 20 trials: 3 at low risk of bias (44,60-62,65,66), 3 with some concerns (59,77,83), and 14 at high risk (45,49-51,53,54,67,70,73,90,92,94-96). Seven reported on interventions directed at caregivers (44,45,49-51,53,54), 7 at the patient–caregiver dyad (59-62,65-67,70,73,77), and 6 at patients and/or their families (83,90,92,94-96). Statistically significant results favoring the intervention were reported for 8 trials of which 2 were at low risk of bias (44,65,66), 2 were directed at caregivers (44,49), 5 at dyads (59,65,66,70,73), and 1 at patients and/or their families (96). No differences were noted between trial groups for the other 24 endpoints related to caregiver burden.
Caregiver self-efficacy was reported in 15 trials (42-48,50,51,59-62,67,73,75-77): 3 at low risk of bias (42,44,60-62), 4 with some concerns (47,48,59,76,77), and 8 at high risk (43,45,46,50,51,67,73,75). Eight trials studied interventions directed at caregivers (42-48,50,51), and 7 studied interventions directed at the patient–caregiver dyad (59-62,67,73,75-77). Statistically significant results favoring the intervention were reported for 9 trials of which 2 were at low risk of bias (42,44); 5 were directed at caregivers (42-45,50), and 4 at dyads (59,67,75,77).
Family functioning was reported in 7 trials (47,48,69,70,76,80,81,87,88): 4 with some concerns for bias (47,48,69,70,76) and 3 at high risk (80,81,87,88). One trial reported on an intervention directed at caregivers (47,48), 5 at patient–caregiver dyads (69,70,76,80,81), and 1 at patients and/or their families (87,88). Three trials reported improved family functioning related to the intervention; all 3 were at high risk of bias and directed at dyads (69,70,80).
Bereavement outcomes of depression or grief were reported in 5 trials (54,65,66,87-89,93): 1 at low risk (65,66) and 4 at high risk of bias (54,87-89,93). One reported on an intervention directed at caregivers (54), 1 at the patient–caregiver dyad (65,66), and 3 at patients and/or their families (87-89,93). Two studies reported improved grief (89,93), and none improved depression postbereavement.
Discussion
In this systematic review and meta-analysis of 49 randomized controlled trials, interventions for caregivers resulted in improvements at 1-3 months in caregiver QOL, mental well-being, anxiety, and depression but not in physical well-being. The longer-term impact of these interventions is uncertain because of the lack of statistically significant findings at 4-6 months in the few studies with outcome data at this endpoint. In the narrative synthesis, interventions led to improvements in caregiver self-efficacy, with mixed findings for caregiver burden, family functioning, and bereavement outcomes.
To our knowledge, this is the first meta-analysis that reports specifically on studies of interventions for caregivers of patients with advanced cancer; our results provide substantive evidence for the benefit of interventions for this vulnerable population. The last large meta-analysis of interventions for family caregivers of patients with cancer was in 2010 and focused mostly on those caring for patients at earlier stages of cancer (19). Statistically significant effects were observed for caregiver burden, coping, and self-efficacy, as well as for a combined outcome of anxiety, distress, and mood but not for depression or physical QOL; risk of bias assessment was not performed. Of note, depression was low at baseline, which might have been due to the inclusion mainly of patients at earlier disease stages. Subsequent reviews summarized results for recent trials but were unable to make definitive conclusions because of lack of meta-analysis and risk of bias assessment (15,97) or because of restrictive inclusion criteria yielding small samples (21-23). Our review adds to these results by providing a comprehensive meta-analysis including 29 trials (41-44,49,52,53,57,67-69,72,73,76,77,80-90,92,93,95,96) that were not included in recent publications (21-23). In addition, our meta-analysis focuses specifically on trials of interventions for caregivers of patients with advanced cancer, who are in particular need of support due to having worse QOL, mental health, anxiety, and depression than those caring for patients at earlier stages of the disease trajectory (6,15). Moreover, we have evaluated the risk of bias of each trial and included subgroup analyses according to the target of the intervention.
Our meta-analysis demonstrated a modest effect from caregiver interventions on overall QOL and mental well-being but not on physical well-being. Although many trials used QOL measures developed and validated for caregivers, these measures lack items relevant to the advanced cancer setting, which might have attenuated the effect in this population (98). Mental well-being was measured mostly using subscales of QOL measures designed for general populations or for patients and included items on social functioning and vitality in addition to mental health items (99,100). Development and validation of caregiver QOL measures specifically for the advanced cancer setting would be a valuable contribution to this area of research. The lack of impact on physical well-being may be related to the relatively good physical well-being of many caregivers compared with general population samples (6,10). As well, our review explicitly focused on psychoeducational, skills training, counseling, or team-based interventions focusing on caregiving or coping; most of these interventions had a psychoeducational and/or psychotherapeutic focus. Interventions with a focus on individual symptoms such as sleep might be more likely to improve physical well-being, but these were excluded because of being outside the focus of the current review.
The effects of caregiver interventions on outcomes of depression and anxiety are noteworthy because these are important mental health conditions for which the prevalence in advanced care settings is as great or greater among caregivers than among the patients they care for (9,15,101). High levels of depression and anxiety in this population may reflect the considerable symptom burden and care needs of patients with advanced disease, lack of preparation of caregivers for their role, and grief due to current and anticipated relational losses (8,102-104). Of note, improvements in self-efficacy were reported for most interventions in this review, which might have played a part in alleviating anxiety and depression (11).
Results of the subgroup analyses according to intervention target demonstrated no statistically significant subgroup effects. The most substantial subgroup differences were observed for depression for which interventions directed at patient–caregiver dyads (mainly using counseling or therapy interventions) had the greatest effect. Relational factors that have been associated with depression in caregivers include a spousal patient–caregiver relationship and family or spousal conflict (8,11,105). Of the 6 trials targeting dyads with data meta-analyzed for depression that provided patient–caregiver relationship status (63-66,70,72,73,80), 5 (>70%) included mainly spouses or partners (63-66,70,72,73) and 1 included only couples with marital difficulties (70). As well, physical and emotional symptom distress are common in patients with advanced cancer and are associated with increased depression in their caregivers (11,15). Addressing concerns of the patient and caregiver simultaneously may contribute to alleviation of depression in caregivers receiving dyad interventions. To further assess the impact of intervention target and factors that may contribute to this impact, trials are needed that directly compare interventions targeting patients, caregivers, or both and that conduct analyses to explore factors that mediate or moderate the effects of different interventions.
This review has limitations. Most trials had less than 100 participants and were at high risk of bias, and standardized mean differences were small. Although we were strict in applying risk of bias criteria and ratings should be considered conservative, there are areas that could be improved for future trials. For many trials, bias was due to missing outcome data, as observed in previous reviews that assessed patient outcomes in advanced cancer (24,25). Availability of data from 95% of participants, as recommended by the Cochrane Collaboration, is rare for patients with advanced cancer and their caregivers because of high levels of distress and burden (34). Nevertheless, few studies used analysis methods that corrected for bias or conducted sensitivity analyses. Similar to previous reviews in advanced cancer or palliative care settings, bias in measurement of outcome data was noted for all studies, because outcomes were participant reported and it is not possible to blind participants receiving behavioral interventions (24,35). Although almost all trials included in our meta-analysis used validated measures, none were validated specifically for caregivers of adults with advanced cancer, and degree of adherence to the intervention (or lack thereof) was often not reported. In addition, there was diversity among studies in intervention design, measurement and reporting of outcomes, and countries and their health-care systems, which may have contributed to the high heterogeneity in several analyses. Most trials either did not report on participants’ race or ethnicity or included predominantly White or Caucasian caregivers. Additional trials with diverse samples are needed to provide conclusions with wider generalizability.
In this systematic review and meta-analysis, caregiver interventions resulted in improvements in QOL and mental health outcomes for caregivers of patients with advanced cancer. Further trials with large samples and longer follow-up are needed to substantiate these data and to delineate which interventions are most effective. To reduce risk of bias, investigators planning future studies should prespecify their analysis plan and register their trial prior to commencing recruitment; adhere to guidelines such as the Template for Intervention Description and Replication checklist to describe their intervention and adherence by participants (106); use measures validated for caregivers when these are available; and make a plan for handling missing data, including sensitivity analyses to demonstrate that data are little changed under a range of assumptions. Particular attention should be paid to diversity of participants. Although no specific intervention can be recommended over another at this time, cancer centers should ensure that interventions for caregivers are available; these could be in the form of psychoeducational or problem-solving interventions, couple-based counseling, or referral to an interdisciplinary palliative care team. Oncologists providing care for patients with advanced cancer should routinely ask about caregiver well-being and offer referral to available services.
Supplementary Material
Acknowledgements
The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Contributor Information
Ronald Chow, Department of Supportive Care, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Jean J Mathews, Division of Palliative Medicine, Department of Medicine and Department of Oncology, Queen’s University, Kingston, ON, Canada.
Emily YiQin Cheng, Department of Supportive Care, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada.
Samantha Lo, Department of Supportive Care, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Joanne Wong, Department of Supportive Care, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Sorayya Alam, Palliative Medicine, Sobell House, Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
Breffni Hannon, Department of Supportive Care, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Department of Medicine, University Health Network, Toronto, ON, Canada.
Gary Rodin, Department of Supportive Care, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada; Centre for Mental Health, University Health Network, Toronto, ON, Canada.
Rinat Nissim, Department of Supportive Care, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Centre for Mental Health, University Health Network, Toronto, ON, Canada.
Sarah Hales, Department of Supportive Care, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Centre for Mental Health, University Health Network, Toronto, ON, Canada.
Dio Kavalieratos, Division of Palliative Medicine, Department of Family and Preventive Medicine, Emory University, Atlanta, GA, USA.
Kieran L Quinn, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada; Division of General Internal Medicine and Palliative Care, Department of Medicine, Sinai Health System, Temmy Latner Centre for Palliative Care, Toronto, ON, Canada.
George Tomlinson, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Department of Medicine, University Health Network, Toronto, ON, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada.
Camilla Zimmermann, Department of Supportive Care, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; Department of Medicine, University Health Network, Toronto, ON, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Author contributions
Ronald Chow, MS (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing—original draft; Writing—review & editing), Jean J. Mathews, MBBS, MD (Investigation; Writing—review & editing), Emily YiQin Cheng, MD (Investigation; Writing—review & editing), Samantha Lo, MSc (Investigation; Validation; Writing—review & editing), Joanne Wong, MSc (Investigation; Writing—review & editing), Sorayya Alam, MBChB (Conceptualization; Investigation; Methodology; Writing—review & editing), Breffni Hannon, MBChB, MSc (Conceptualization; Methodology; Writing—review & editing), Gary Rodin, MD (Investigation; Writing—review & editing), Rinat Nissim, PhD (Conceptualization; Methodology; Writing—review & editing), Sarah Hales, MD (Conceptualization; Methodology; Writing—review & editing), Dio Kavalieratos, PhD (Conceptualization; Methodology; Writing—review & editing), Kieran L. Quinn, MD, PhD (Conceptualization; Methodology; Writing—review & editing), George Tomlinson, PhD (Formal analysis; Writing—review & editing), and Camilla Zimmermann, MD PhD (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Validation; Writing—original draft; Writing—review & editing).
Funding
This work was funded by the Canadian Institutes of Health Research (grant number 152996 to CZ). Dr Zimmermann is supported by the Harold and Shirley Lederman Chair in Psychosocial Oncology and Palliative Care, a joint Chair among the University of Toronto, Princess Margaret Cancer Centre/University Health Network and the Princess Margaret Cancer Foundation. The researchers are independent from the funders, and all authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
We declare no conflicts of interests.
References
- 1. Girgis A, Lambert S, Johnson C, Waller A, Currow D. Physical, psychosocial, relationship, and economic burden of caring for people with cancer: a review. J Oncol Pract. 2013;9(4):197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biegel DES, Schulz R. Family Caregiving in Chronic Illness: Alzheimer’s Disease, Cancer, Heart Disease, Mental Illness, and Stroke. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- 3. Kent EE, Rowland JH, Northouse L, et al. Caring for caregivers and patients: research and clinical priorities for informal cancer caregiving. Cancer. 2016;122(13):1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK Carers. State of Caring 2021. 2021. https://www.pslhub.org/learn/patient-safety-in-health-and-care/social-care/carers-uk-report-state-of-caring-2021-a-snapshot-of-unpaid-care-in-the-uk-3-november-2021-r5546/. Accessed March 27, 2023.
- 5. National Alliance for Caregiving (NAC) and the AARP Public Policy Institute. Caregiving in the U.S. Research Report. 2020. https://www.caregiving.org/wp-content/uploads/2021/01/full-report-caregiving-in-the-united-states-01-21.pdf. Accessed March 27, 2023.
- 6. Wadhwa D, Burman D, Swami N, Rodin G, Lo C, Zimmermann C. Quality of life and mental health in caregivers of outpatients with advanced cancer. Psychooncology. 2013;22(2):403-410. [DOI] [PubMed] [Google Scholar]
- 7. Burridge LH, Barnett AG, Clavarino AM. The impact of perceived stage of cancer on carers’ anxiety and depression during the patients’ final year of life. Psychooncology. 2009;18(6):615-623. [DOI] [PubMed] [Google Scholar]
- 8. Dumont S, Turgeon J, Allard P, Gagnon P, Charbonneau C, Vézina L. Caring for a loved one with advanced cancer: determinants of psychological distress in family caregivers. J Palliat Med. 2006;9(4):912-921. [DOI] [PubMed] [Google Scholar]
- 9. Nipp RD, El-Jawahri A, Fishbein JN, et al. Factors associated with depression and anxiety symptoms in family caregivers of patients with incurable cancer. Ann Oncol. 2016;27(8):1607-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaffer KM, Jacobs JM, Nipp RD, et al. Mental and physical health correlates among family caregivers of patients with newly-diagnosed incurable cancer: a hierarchical linear regression analysis. Support Care Cancer. 2017;25(3):965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang ST, Chang WC, Chen JS, et al. Course and predictors of depressive symptoms among family caregivers of terminally ill cancer patients until their death. Psychooncology. 2013;22(6):1312-1318. [DOI] [PubMed] [Google Scholar]
- 12. Williams AL, McCorkle R. Cancer family caregivers during the palliative, hospice, and bereavement phases: a review of the descriptive psychosocial literature. Palliat Support Care. 2011;9(3):315-325. [DOI] [PubMed] [Google Scholar]
- 13. Stenberg U, Ruland CM, Miaskowski C. Review of the literature on the effects of caring for a patient with cancer. Psychooncology. 2010;19(10):1013-1025. [DOI] [PubMed] [Google Scholar]
- 14. Kim Y, Carver CS, Shaffer KM, Gansler T, Cannady RS. Cancer caregiving predicts physical impairments: roles of earlier caregiving stress and being a spousal caregiver. Cancer. 2015;121(2):302-310. [DOI] [PubMed] [Google Scholar]
- 15. Alam S, Hannon B, Zimmermann C. Palliative care for family caregivers. J Clin Oncol 2020;38(9):926-936. [DOI] [PubMed] [Google Scholar]
- 16. Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psychooncology. 2013;22(8):1688-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Q, Loke AY. A systematic review of spousal couple-based intervention studies for couples coping with cancer: direction for the development of interventions. Psychooncology. 2014;23(7):731-739. [DOI] [PubMed] [Google Scholar]
- 18. Teixeira RJ, Applebaum AJ, Bhatia S, Brandao T. The impact of coping strategies of cancer caregivers on psychophysiological outcomes: an integrative review. Psychol Res Behav Manag. 2018;11:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Northouse LL, Katapodi MC, Song L, Zhang L, Mood DW. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA Cancer J Clin. 2010;60(5):317-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Candy B, Jones L, Drake R, Leurent B, King M. Interventions for supporting informal caregivers of patients in the terminal phase of a disease. Cochrane Database Syst Rev. 2011;(6):CD007617. [DOI] [PubMed] [Google Scholar]
- 21. Treanor CJ, Santin O, Prue G, et al. Psychosocial interventions for informal caregivers of people living with cancer. Cochrane Database Syst Rev. 2019;6(6):CD009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JZJ, Chen H-C, Lee JX, Klainin-Yobas P. Effects of psychosocial interventions on psychological outcomes among caregivers of advanced cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2021;29(12):7237-7248. [DOI] [PubMed] [Google Scholar]
- 23. Ahn S, Romo RD, Campbell CL. A systematic review of interventions for family caregivers who care for patients with advanced cancer at home. Patient Educ Couns. 2020;103(8):1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA. 2016;316(20):2104-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I. Effectiveness of specialized palliative care: a systematic review. JAMA. 2008;299(14):1698-1709. [DOI] [PubMed] [Google Scholar]
- 26. Bajwah S, Oluyase AO, Yi D, et al. The effectiveness and cost‐effectiveness of hospital‐based specialist palliative care for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2020;9(9):CD012780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fulton JJ, LeBlanc TW, Cutson TM, et al. Integrated outpatient palliative care for patients with advanced cancer: a systematic review and meta-analysis. Palliat Med 2019;33(2):123-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost‐effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013;2013(6):CD007760., [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane; 2022. www.training.cochrane.org/handbook. [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berwanger O, Ribeiro RA, Finkelsztejn A, et al. The quality of reporting of trial abstracts is suboptimal: survey of major general medical journals. J Clin Epidemiol. 2009;62(4):387-392. [DOI] [PubMed] [Google Scholar]
- 32. Sivendran S, Newport K, Horst M, Albert A, Galsky MD. Reporting quality of abstracts in phase III clinical trials of systemic therapy in metastatic solid malignancies. Trials. 2015;16:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 35. Quinn KL, Shurrab M, Gitau K, et al. Association of receipt of palliative care interventions with health care use, quality of life, and symptom burden among adults with chronic noncancer illness: a systematic review and meta-analysis. JAMA. 2020;324(14):1439-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eldridge S, Campbell MK, Campbell MJ, et al. RoB 2 for cluster-randomized trials. 2021. https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-trials. Accessed March 27, 2023.
- 37. Higgins JPT, Li T, Deeks JJ, eds. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane; 2022. www.training.cochrane.org/handbook. [Google Scholar]
- 38. Meurer WJ, Tolles J. Logistic regression diagnostics: understanding how well a model predicts outcomes. JAMA. 2017;317(10):1068-1069. [DOI] [PubMed] [Google Scholar]
- 39. IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arias-Rojas M, Carreño-Moreno S, Arias-Quiroz N. The “PalliActive Caregivers” intervention for caregivers of patients with cancer in palliative care: a feasibility pilot study. J Hosp Palliat Nurs. 2020;22(6):495-503. [DOI] [PubMed] [Google Scholar]
- 42. Aubin M, Vézina L, Verreault R, et al. A randomized clinical trial assessing a pragmatic intervention to improve supportive care for family caregivers of patients with lung cancer. Palliat Support Care. 2021;19(2):146-153. [DOI] [PubMed] [Google Scholar]
- 43. Boele FW, Hoeben W, Hilverda K, et al. Enhancing quality of life and mastery of informal caregivers of high-grade glioma patients: a randomized controlled trial. J Neurooncol. 2013;111(3):303-311. [DOI] [PubMed] [Google Scholar]
- 44. El-Jawahri A, Jacobs JM, Nelson AM, et al. Multimodal psychosocial intervention for family caregivers of patients undergoing hematopoietic stem cell transplantation: a randomized clinical trial. Cancer. 2020;126(8):1758-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferrell B, Kravits K, Borneman T, Pal SK, Lee J. A support intervention for family caregivers of advanced cancer patients. J Adv Pract Oncol. 2019;10(5):444-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hudson PL, Aranda S, Hayman-White K. A psycho-educational intervention for family caregivers of patients receiving palliative care: a randomized controlled trial. J Pain Symptom Manage. 2005;30(4):329-341. [DOI] [PubMed] [Google Scholar]
- 47. Hudson P, Trauer T, Kelly B, et al. Reducing the psychological distress of family caregivers of home-based palliative care patients: short-term effects from a randomised controlled trial. Psycho-Oncology. 2013;22(9):1987-1993. [DOI] [PubMed] [Google Scholar]
- 48. Hudson P, Trauer T, Kelly B, et al. Reducing the psychological distress of family caregivers of home based palliative care patients: longer term effects from a randomised controlled trial. Psycho-Oncology. 2015;24(1):19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Laudenslager ML, Simoneau TL, Kilbourn K, et al. A randomized control trial of a psychosocial intervention for caregivers of allogeneic hematopoietic stem cell transplant patients: effects on distress. Bone Marrow Transplant. 2015;50(8):1110-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leow M, Chan S, Chan M. A pilot randomized, controlled trial of the effectiveness of a psychoeducational intervention on family caregivers of patients with advanced cancer. Oncol Nurs Forum. 2015;42(2):E63-E72. [DOI] [PubMed] [Google Scholar]
- 51. McMillan SC, Small BJ, Weitzner M, et al. Impact of coping skills intervention with family caregivers of hospice patients with cancer: a randomized clinical trial. Cancer. 2006;106(1):214-222. [DOI] [PubMed] [Google Scholar]
- 52. Mitchell GK, Girgis A, Jiwa M, Sibbritt D, Burridge LH, Senior HE. Providing general practice needs-based care for carers of people with advanced cancer: a randomised controlled trial. Br J Gen Pract. 2013;63(615):e683-e690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pensak NA, Carr AL, Jones J, et al. A pilot study of mobilized intervention to help caregivers of oncology patients manage distress. Psycho-Oncology. 2021;30(4):520-528. [DOI] [PubMed] [Google Scholar]
- 54. Walsh K, Jones L, Tookman A, et al. Reducing emotional distress in people caring for patients receiving specialist palliative care. Randomised trial. Br J Psychiatry. 2007;190:142-147. [DOI] [PubMed] [Google Scholar]
- 55. Washington KA-O, Demiris G, Parker Oliver DA-O, Albright DL, Craig KW, Tatum P. Delivering problem-solving therapy to family caregivers of people with cancer: a feasibility study in outpatient palliative care. Psycho-Oncology. 2018;27(10):2494-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bani Younis MK, Al-Rawashdeh A, Alnjadat RM. The effect of palliative care intervention program on the quality of life among Jordanian caregivers of cancer patients. Home Health Care Serv Q. 2019;38(4):286-296. [DOI] [PubMed] [Google Scholar]
- 57. Yun YH, Lee M, Park S, et al. Use of a decision aid to help caregivers discuss terminal disease status with a family member with cancer: a randomized controlled trial. J Clin Oncol. 2011;29(36):4811-4819. [DOI] [PubMed] [Google Scholar]
- 58. Ammari ABH, Hendriksen C, Rydahl-Hansen S. Results from the family and coping oriented palliative homecare intervention study (FamCope)–a randomized controlled trial. J Psychosoc Oncol. 2018;36(5):557-581. [DOI] [PubMed] [Google Scholar]
- 59. Badr H, Smith CB, Goldstein NE, Gomez JE, Redd WH. Dyadic psychosocial intervention for advanced lung cancer patients and their family caregivers: results of a randomized pilot trial. Cancer. 2015;121(1):150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chih MY, DuBenske LL, Hawkins RP, et al. Communicating advanced cancer patients’ symptoms via the Internet: a pooled analysis of two randomized trials examining caregiver preparedness, physical burden, and negative mood. Palliat Med. 2013;27(6):533-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. DuBenske LL, Gustafson DH, Namkoong K, et al. CHESS improves cancer caregivers’ burden and mood: results of an eHealth RCT. Health Psychol. 2014;33(10):1261-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Namkoong K, DuBenske LL, Shaw BR, et al. Creating a bond between caregivers online: effect on caregivers’ coping strategies. J Health Commun. 2012;17(2):125-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Clark MM, Rummans TA, Atherton PJ, et al. Randomized controlled trial of maintaining quality of life during radiotherapy for advanced cancer. Cancer. 2013;119(4):880-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lapid MI, Atherton PJ, Kung S, et al. Cancer caregiver quality of life: need for targeted intervention. Psychooncology. 2016;25(12):1400-1407. [DOI] [PubMed] [Google Scholar]
- 65. Dionne-Odom JN, Azuero A, Lyons KD, et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1446-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dionne-Odom JN, Azuero A, Lyons KD, et al. Family caregiver depressive symptom and grief outcomes from the ENABLE III randomized controlled trial. J Pain Symptom Manage. 2016;52(3):378-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Keefe FJ, Ahles TA, Sutton L, et al. Partner-guided cancer pain management at the end of life: a preliminary study. J Pain Symptom Manage. 2005;29(3):263-272. [DOI] [PubMed] [Google Scholar]
- 68. Kubo A, Kurtovich E, McGinnis M, et al. Pilot pragmatic randomized trial of mHealth mindfulness-based intervention for advanced cancer patients and their informal caregivers. Psychooncology. 2020. doi: 10.1002/pon.5557. [DOI] [PubMed] [Google Scholar]
- 69. Li Y, Ling L, Zhanyu P. Effect of wellness education on quality of life of patients with non-small cell lung cancer treated with first-line icotinib and on their family caregivers. Integr Cancer Ther. 2019;18:1534735419842373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McLean LM, Walton T, Rodin G, Esplen MJ, Jones JM. A couple-based intervention for patients and caregivers facing end-stage cancer: outcomes of a randomized controlled trial. Psychooncology. 2013;22(1):28-38. [DOI] [PubMed] [Google Scholar]
- 71. Meyers FJ, Carducci M, Loscalzo M, Linder J, Greasby T, Beckett LA. Effects of a problem-solving intervention (COPE) on quality of life for patients with advanced cancer on clinical trials and their caregivers: simultaneous care educational intervention (SCEI): linking palliation and clinical trials. J Palliat Med. 2011;14(4):465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Milbury K, Li Y, Durrani S, et al. A mindfulness-based intervention as a supportive care strategy for patients with metastatic non-small cell lung cancer and their spouses: results of a three-arm pilot randomized controlled trial. Oncologist. 2020;25(11):e1794-e1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mosher CE, Secinti E, Johns SA, et al. Examining the effect of peer helping in a coping skills intervention: a randomized controlled trial for advanced gastrointestinal cancer patients and their family caregivers. Qual Life Res. 2018;27(2):515-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Northouse L, Kershaw T, Mood D, Schafenacker A. Effects of a family intervention on the quality of life of women with recurrent breast cancer and their family caregivers. Psychooncology. 2005;14(6):478-491. [DOI] [PubMed] [Google Scholar]
- 75. Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a brief and extensive dyadic intervention for advanced cancer patients and their family caregivers. Psychooncology. 2013;22(3):555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Porter LS, Keefe FJ, Baucom DH, Olsen M, Zafar SY, Uronis H. A randomized pilot trial of a videoconference couples communication intervention for advanced GI cancer. Psychooncology. 2017;26(7):1027-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sherwood PR, Given BA, Given CW, Sikorskii A, You M, Prince J. The impact of a problem-solving intervention on increasing caregiver assistance and improving caregiver health. Support Care Cancer. 2012;20(9):1937-1947. [DOI] [PubMed] [Google Scholar]
- 78. von Heymann-Horan AB, Puggaard LB, Nissen KG, et al. Dyadic psychological intervention for patients with cancer and caregivers in home-based specialized palliative care: the Domus model. Palliat Support Care. 2018;16(2):189-197. [DOI] [PubMed] [Google Scholar]
- 79. Heymann‐Horan A, Bidstrup PE, Johansen C, et al. Dyadic coping in specialized palliative care intervention for patients with advanced cancer and their caregivers: effects and mediation in a randomized controlled trial. Psychooncology. 2019;28(2):264-270. [DOI] [PubMed] [Google Scholar]
- 80. Wang C, Chen J, Wang Y, et al. Effects of family participatory dignity therapy on the psychological well-being and family function of patients with haematologic malignancies and their family caregivers: a randomised controlled trial. Int J Nurs Stud. 2021;118:103922. [DOI] [PubMed] [Google Scholar]
- 81. Yanwei L, Minghui F, Manman Q, Zhuchun Y, Dongying L, Zhanyu P. Influence of wellness education on first-line icotinib hydrochloride patients with stage IV non-small cell lung cancer and their family caregivers. Curr Probl Cancer. 2018;42(3):358-366. [DOI] [PubMed] [Google Scholar]
- 82. Addington-Hall JM, MacDonald LD, Anderson HR, et al. Randomised controlled trial of effects of coordinating care for terminally ill cancer patients. BMJ. 1992;305(6865):1317-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Clark MM, Rummans TA, Sloan JA, et al. Quality of life of caregivers of patients with advanced-stage cancer. Am J Hosp Palliat Care. 2006;23(3):185-191. [DOI] [PubMed] [Google Scholar]
- 84. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. El-Jawahri A, Greer JA, Pirl WF, et al. Effects of early integrated palliative care on caregivers of patients with lung and gastrointestinal cancer: a randomized clinical trial. Oncologist. 2017;22(12):1528-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kane RL, Klein SJ, Bernstein L, Rothenberg R, Wales J. Hospice role in alleviating the emotional stress of terminal patients and their families. Med Care. 1985;23(3):189-197. [DOI] [PubMed] [Google Scholar]
- 87. Kissane DW, McKenzie M, Bloch S, Moskowitz C, McKenzie DP, O’Neill I. Family focused grief therapy: a randomized, controlled trial in palliative care and bereavement. Am J Psychiatry. 2006;163(7):1208-1218. [DOI] [PubMed] [Google Scholar]
- 88. Kissane D, Lichtenthal WG, Zaider T. Family care before and after bereavement. Omega (Westport). 2008;56(1):21-32. [DOI] [PubMed] [Google Scholar]
- 89. Kissane DW, Zaider TI, Li Y, et al. Randomized controlled trial of family therapy in advanced cancer continued into bereavement. J Clin Oncol. 2016;34(16):1921-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kleijn G, Lissenberg-Witte BI, Bohlmeijer ET, et al. A randomized controlled trial on the efficacy of life review therapy targeting incurably ill cancer patients: do their informal caregivers benefit? Support Care Cancer. 2021;29(3):1257-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McDonald J, Swami N, Hannon B, et al. Impact of early palliative care on caregivers of patients with advanced cancer: cluster randomised trial. Ann Oncol. 2017;28(1):163-168. [DOI] [PubMed] [Google Scholar]
- 92. O’Hara RE, Hull JG, Lyons KD, et al. Impact on caregiver burden of a patient-focused palliative care intervention for patients with advanced cancer. Palliat Support Care. 2010;8(4):395-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ringdal GI, Jordhoy MS, Ringdal K, Kaasa S. The first year of grief and bereavement in close family members to individuals who have died of cancer. Palliat Med. 2001;15(2):91-105. [DOI] [PubMed] [Google Scholar]
- 94. Schenker Y, Bahary N, Claxton R, et al. A pilot trial of early specialty palliative care for patients with advanced pancreatic cancer: challenges encountered and lessons learned. J Palliat Med. 2018;21(1):28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Steel JL, Geller DA, Kim KH, et al. Web-based collaborative care intervention to manage cancer-related symptoms in the palliative care setting. Cancer. 2016;122(8):1270-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xu M, Zhou W, Yang L, Liu G, Chen L. Effect of palliative care on the anxiety, depression and sleep quality in primary caregivers of elderly patients with terminal cancer. Am J Transl Res. 2021;13(4):3738-3744. [PMC free article] [PubMed] [Google Scholar]
- 97. Ferrell B, Wittenberg E. A review of family caregiving intervention trials in oncology. CA Cancer J Clin. 2017;67(4):318-325. [DOI] [PubMed] [Google Scholar]
- 98. McDonald J, Swami N, Pope A, et al. Caregiver quality of life in advanced cancer: qualitative results from a trial of early palliative care. Palliat Med 2018;32(1):69-78. [DOI] [PubMed] [Google Scholar]
- 99. Ware JE Jr, Kosinski M, Gandek B, et al. The factor structure of the SF-36 health survey in 10 countries: results from the IQOLA project. J Clin Epidemiol. 1998;51(11):1159-1165. [DOI] [PubMed] [Google Scholar]
- 100. Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570-579. [DOI] [PubMed] [Google Scholar]
- 101. Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G. Hidden morbidity in cancer: spouse caregivers. J Clin Oncol. 2007;25(30):4829-4834. [DOI] [PubMed] [Google Scholar]
- 102. Coelho A, de Brito M, Teixeira P, Frade P, Barros L, Barbosa A. Family caregivers’ anticipatory grief: a conceptual framework for understanding its multiple challenges. Qual Health Res 2020;30(5):693-703. [DOI] [PubMed] [Google Scholar]
- 103. Mohammed S, Swami N, Pope A, et al. “I didn’t want to be in charge and yet I was”: bereaved caregivers’ accounts of providing home care for family members with advanced cancer. Psychooncology. 2018;27(4):1229-1236. [DOI] [PubMed] [Google Scholar]
- 104. Seow H, Barbera L, Sutradhar R, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29(9):1151-1158. [DOI] [PubMed] [Google Scholar]
- 105. Siminoff LA, Wilson-Genderson M, Baker S. Jr Depressive symptoms in lung cancer patients and their family caregivers and the influence of family environment. Psychooncology. 2010;19(12):1285-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.