Abstract
Dog bite is a common skin injury, which mainly causes structural damage, infection, and psychological trauma. Among these complications, infection by oral flora from animals has a major effect on later treatment. Each animal has a relatively unique oral microbial flora, which has a potential risk of infection and affects the formulation of treatment plans. Although lymph node necrosis is a common disease in the medical field, distant metastatic abscess and lymph node necrosis caused by dog bite are still worthy of early clinical suspicion after a patient presents with a dog bite disease. A high index of suspicion is greatly significant in shortening the patient's hospital stay, promoting wound healing, and reducing psychological trauma of patients.
Keywords: Metastatic abscess, Dog bite, Necrosis of lymph node
Introduction
Dog bite is one of the common types of skin injury. The unique microbial flora of dog mouth has a great impact on the potential risk of infection and wound treatment of patients. This multi-microbial wound environment is composed of a broad mixture of aerobic and anaerobic organisms [1]. The damage caused by dogs to humans can lead to various infections, such as cellulitis [2], abscess formation, infectious arthritis, tenosynovitis, osteomyelitis, and septicemia [3], resulting potentially in death [4], [5]. Additionally, complications include psychological problems, such as pain [6], anxiety [7], and depression [8], caused by soft tissue injury, neurovascular injury, fracture, and dog bite. However, no cases leading to lymph node necrosis have yet been reported in the literature.
Case report
Two months prior to presentation, the patient, a 41-year-old woman, was accidentally bitten by a "wild dog" on the skin at the back of the middle and lower parts of the right leg while walking. After the incident, the patient experienced a localized pain and discomfort associated with minimal bleeding. Later, she went to the District People's Hospital for wound treatment. She was administered with the tetanus antitoxin and rabies vaccine. Subsequently, the patient self-managed the wound without much success with the healing process. Twenty days before admission, no obvious cause of skin redness and swelling were observed on the inner skin of the proximal right thigh of the patient. Initially, the patient did not pay much attention to it. The area of redness and swelling increased to 14 cm × 5 cm, with slight pain and discomfort. Concurrently, the wound site began to ooze a secretion. Therefore, she revisited the District People's Hospital and was administered with topical drugs for disinfection and antibacterial treatment (the specific drug name is unknown). After symptomatic treatment, the volume of the secretion from the wound surface decreased slightly. However, the signs of redness and swelling in the right thigh increased. Subsequently, the patient was treated at our outpatient department. After treatment, the secretion from the wound site ceased. The swelling of her right thigh was slightly reduced, but the skin around the wound site was broken and exudative. For further management, the patient was admitted for treatment of a skin abscess. Since the onset of the disease, no symptoms and signs of appetite loss, sleep disturbance, stool and urine-associated changes, and weight loss were reported.
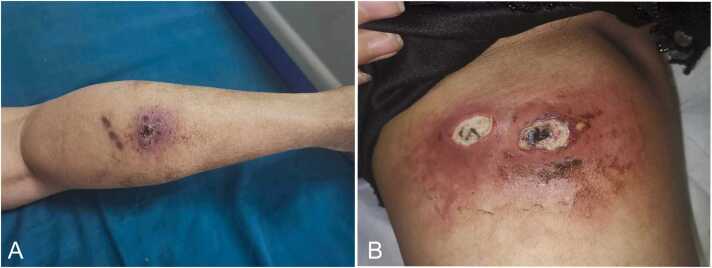
Further laboratory investigations after admission showed that the infection indicators, such as white blood cell count, neutrophils C-reactive protein, and procalcitonin, were normal. Liver function, renal function, electrolyte balance, routine blood parameters, chest radiograph, and electrocardiogram were normal. As shown in Fig. 1A, a physical examination revealed the presence of an irregular skin redness and swelling of size of about 3.0 cm × 2.5 cm at the posterior aspect of the midsection of the right leg. The surface could be covered by an irregular scab shell of about 1.00 cm × 0.8 cm. The adhesion was not tight. After the scab shell was opened, a small amount of necrotic tissue was observed under the scab. A minimal amount of purulent secretion could be observed around the extrusion. A red and swollen skin of size, 14 cm × 6.0 cm, was observed on the inner side of the right thigh. The texture of the local skin was relatively tough. Two oval skin ulcer wounds of about 2.0 cm × 1.0 cm were present (Fig. 1B). The necrotic skin was attached to the wound surface with relative tightness. A light microwave motion could be observed whenever the inner ulcer was squeezed. After the necrotic skin was removed from the wound surface, necrotic tissues with associated minimal purulent secretions in the tissue gaps were observed. From experience, we used Laxef sodium for its anti-infection effect. We further incised and drained the abscess on the right thigh. Culture of the purulent secretion obtained from the wound site revealed the presence of the Staphylococcus squirrel subspecies. The wound healed well after symptomatic treatment by anti-infection and dressing change. The patient was discharged 7 days after the procedure.
Fig. 1.
Patient's physical examination on initial admission. 1 A: A physical examination revealed the presence of an irregular skin redness and swelling of size of about 3.0 cm × 2.5 cm at the posterior aspect of the midsection of the right leg. The surface could be covered by an irregular scab shell of about 1.00 cm × 0.8 cm. 1B: A red and swollen skin of size, 14 cm × 6.0 cm, was observed on the inner side of the right thigh. The texture of the local skin was relatively tough. Two oval skin ulcer wounds of about 2.0 cm × 1.0 cm were present.
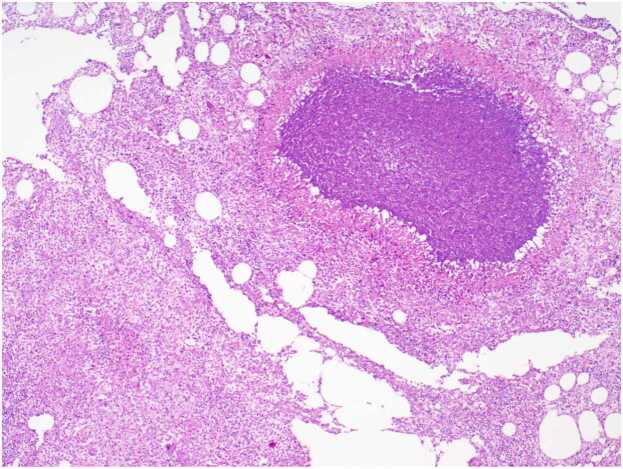
Three days after discharge, the patient found a mass of about 4 cm × 4 cm in size on the medial side of the right thigh (Fig. 2). The mass was associated with tenderness, no wave motion and skin temperature rise. Therefore, she revisited the hospital. Upon admission, various laboratory investigations showed no abnormalities in liver function, renal function, electrolyte balance, and routine blood parameters. An ultrasound examination showed a fluid accumulation under the right thigh root incision. The ranges of two dark areas under the right thigh root incision were 3.0 cm × 1.9 cm × 1.0 cm and 4.0 cm × 3.5 cm × 1.6 cm (Fig. 3). We performed a local puncture and extracted 25 ml of clear yellowish liquid (Fig. 4). No positive findings were obtained from acid-fast staining, fungal culture, and bacterial culture. Thereafter, the mass on the proximal end of the right thigh was resected again. During the operation, the skin, fat, and superficial fascia layers were cut to locate the two liquid dark areas shown by ultrasonography. The liquids in both areas were yellow and clear, and they communicated with each other through the sinus. Two swollen lymph nodes were found above the liquid dark area. After blunt separation, two dark and necrotic lymph nodes with sizes of 3.0 cm × 2.0 cm and 0.5 cm × 0.5 cm were found (Fig. 5). We completely excised the lymph nodes, ligated lymph vessels, and reduced tension sutured after hemostasis. Postoperative pathological examination showed that the right inguinal lymph node had undergone a caseous necrotizing granulomatous inflammation. A test for nucleic acids of Mycobacterium tuberculosis was negative (Fig. 6). After the operation, the patient were treated by anti-infection, pressure bandaging, and dressing change. She was discharged afterwards.
Fig. 2.
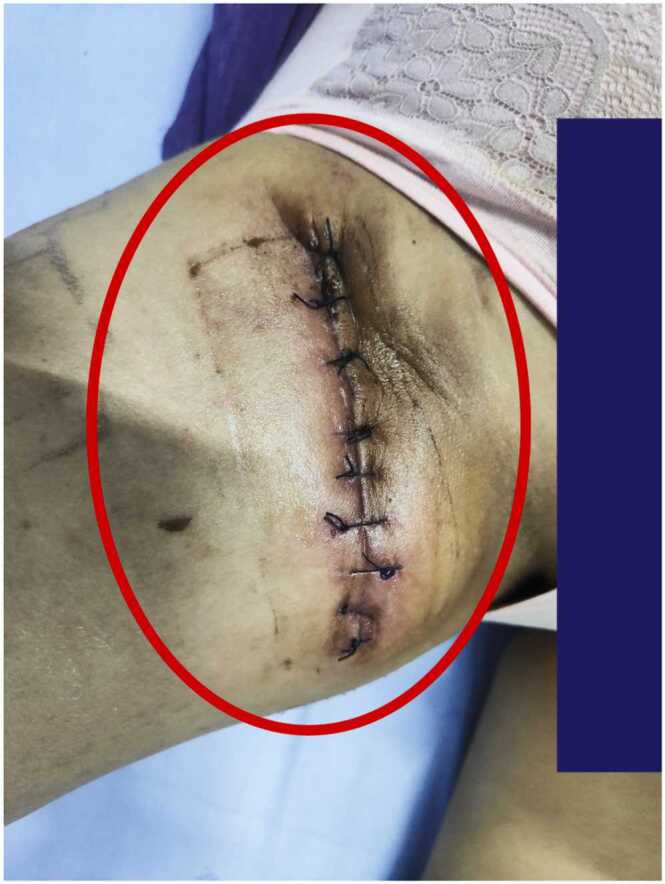
Changes in the patient's condition 3 days after discharge. Three days after discharge, the patient found a mass of about 4 cm × 4 cm in size on the medial side of the right thigh.
Fig. 3.
Ultrasound results of the right thigh. An ultrasound examination showed a fluid accumulation under the right thigh root incision. The ranges of two dark areas under the right thigh root incision were 3.0 cm × 1.9 cm × 1.0 cm and 4.0 cm × 3.5 cm × 1.6 cm.
Fig. 4.
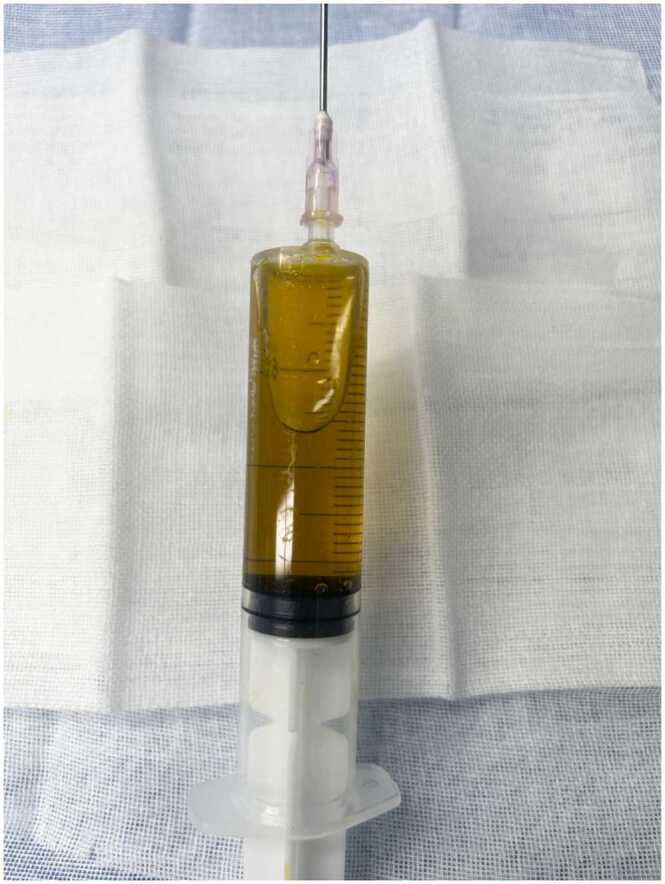
Local puncture results. We performed a local puncture and extracted 25 ml of clear yellowish liquid.
Fig. 5.
Condition of the patient's operating area during the operation. 5 A: During the operation, the skin, fat, and superficial fascia layers were cut to locate the two liquid dark areas shown by ultrasonography. The liquids in both areas were yellow and clear, and they communicated with each other through the sinus. Two swollen lymph nodes were found above the liquid dark area. 5B/5 C: After blunt separation, two dark and necrotic lymph nodes with sizes of 3.0 cm × 2.0 cm and 0.5 cm × 0.5 cm were found.
Fig. 6.
Pathological examination results after surgery. Postoperative pathological examination showed that the right inguinal lymph node had undergone a caseous necrotizing granulomatous inflammation.
Discussion and review of the literature
The main pathophysiological mechanism underlying a dog bite is direct inoculation of bacterial flora from the dog's mouth and throat into the injury site [9]. Additionally, the normal human skin bacterial flora may become a pathogenic factor in a wound resulting from a dog bite. In this case, culture of pus from the wound site after disinfection during the initial admission showed the presence of Staphylococcus squirrel subspecies. Staphylococcus strains have been isolated from small animals, especially eastern grey squirrel, southern flying squirrel, and opossum. Additionally, these strains have been isolated from raccoons, dogs, sheep, humans, and the environment. The study found that Staphylococcus squirrel often lives in cats and dogs, usually in their oral and nasal mucosa [10], [11], [12]. The main therapeutic factor of infection may be associated with the direct inoculation of Staphylococcus squirrel from the dog's mouth into the wound site. Our experience from this case confirms the necessity of open and repeated debridement of early wounds from dog bite.
Acute metastatic abscess is a localized pus accumulation in tissues, organs, or body cavities caused by necrosis and liquefaction of diseased tissues due to the transfer of pathogenic bacteria from distant primary infection sources through blood flow and lymphatic vessels during acute infection. Our findings suggest that the patient may have been bitten by a dog on the right leg, causing Staphylococcus squirrel in the dog's mouth to invade and infect the wound. The bacteria likely metastasized along the lymphatic vessel to the ipsilateral thigh, resulting in the formation of an abscess at the root of the ipsilateral thigh. Based on the culture and sensitivity test on the pathogenic bacteria, sensitive antibiotics were used for anti-infection, surgery, postoperative dressing change, and other symptomatic treatments. As a result, good therapeutic effects were achieved.
However, the physical examination of the patient 2 months after the dog bite did not reveal any enlargement of the ipsilateral inguinal lymph nodes. From the patient’s medical history, the patient felt a mass in the ipsilateral groin about 15 days after the initial operation and reported at the hospital. A color ultrasound of the groin showed a fluid accumulation under the right thigh root incision. The physical examination could reveal two subcutaneous tumors in the groin, which were about 2.0 cm × 2.0 cm and 1.0 cm × 1.0 cm in size, with poor mobility. We extracted 25 ml of clear yellowish liquid after a puncture. No positive results were obtained from acid fast staining, fungal culture, and bacterial culture. These clinical manifestations, tests, and examinations suggest that the lymphatic metastasis of dog bite not only leads to the formation of abscess in the ipsilateral thigh but also leads to delayed necrosis of the ipsilateral lymph nodes. This may be related to poor nutrition and immunosuppression caused by anxiety from experiencing a dog bite.
In summary, the key to the treatment of dog bite is early thorough debridement, selection of reasonable antibiotics, improvement of tetanus immunoglobulin, rabies vaccination, and strengthening of nutritional support. A culture of wound microorganisms is necessary in choosing a sensitive and effective antibiotics. Subsequently, clinicians should be aware of the risk of a possible formation of distant metastatic abscesses and lymph node necrosis on the same side.
CRediT authorship contribution statement
Lan Liu and Xiaobing Li participated in interpretation and manuscript writing. Chao Yang, Bo Jiang, Xiaochuan He and Aibing Xiong participated in the preparation of figures. Hong Yan was involved in designing the work, drafting the manuscript, critically revising important content, and approving the final version for submission.
Declaration of Competing Interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Acknowledgements
We are grateful to the Affiliated Hospital of Southwest Medical University for her support of our work.
References
- 1.Stefanopoulos P.K., Tarantzopoulou A.D. Facial bite wounds: management update. Int J Oral Maxillofac Surg. 2005;34(5):464–472. doi: 10.1016/j.ijom.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Murphy J., Qaisi M. Management of human and animal bites. Oral Maxillofac Surg Clin North Am. 2021;33(3):373–380. doi: 10.1016/j.coms.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Carlson P., Kontiainen S., Anttila P., Eerola E. Septicemia caused by Neisseria weaveri. Clin Infect Dis. 1997;24(4):739. doi: 10.1093/clind/24.4.739. [DOI] [PubMed] [Google Scholar]
- 4.Langley R.L. Human fatalities resulting from dog attacks in the United States, 1979-2005. Wilderness Environ Med. 2009;20(1):19–25. doi: 10.1580/08-WEME-OR-213.1. [DOI] [PubMed] [Google Scholar]
- 5.Byard R.W., Langlois N.E.I. Variable mechanisms of dog-related deaths. Am J Forensic Med Pathol. 2020;41(4):287–290. doi: 10.1097/PAF.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 6.De Keuster T., Lamoureux J., Kahn A. Epidemiology of dog bites: a Belgian experience of canine behaviour and public health concerns. Vet J. 2006;172(3):482–487. doi: 10.1016/j.tvjl.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon J., Hoopes J., Epp T. Scoping decades of dog evidence: a scoping review of dog bite-related sequelae. Can J Public Health. 2019;110(3):364–375. doi: 10.17269/s41997-018-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali S.S., Ali S.S. Dog bite injuries to the face: A narrative review of the literature. World J Otorhinolaryngol Head Neck Surg. 2022;8(3):239–244. doi: 10.1016/j.wjorl.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinha T. Cellulitis and Bacteremia due to Neisseria weaveri following a dog bite. IDCases. 2018;12:56–57. doi: 10.1016/j.idcr.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox H.U., Hoskins J.D., Newman S.S., Turnwald G.H., Foil C.S., Roy A.F., et al. Distribution of staphylococcal species on clinically healthy cats. Am J Vet Res. 1985;46(9):1824–1828. [PubMed] [Google Scholar]
- 11.Han J.I., Yang C.H., Park H.M. Prevalence and risk factors of Staphylococcus spp. carriage among dogs and their owners: A cross-sectional study. Vet J. 2016;212:15–21. doi: 10.1016/j.tvjl.2015.10.059. [DOI] [PubMed] [Google Scholar]
- 12.Muniz I.M., Penna B., Lilenbaum W. Treating animal bites: susceptibility of Staphylococci from oral mucosa of cats. Zoonoses Public Health. 2013;60(7):504–509. doi: 10.1111/zph.12027. [DOI] [PubMed] [Google Scholar]