Abstract
Purpose:
To examine whether longitudinal exposure to neighborhood socioeconomic vulnerability influences blood pressure changes throughout midlife in a racially, ethnically, and geographically-diverse cohort of women transitioning through menopause.
Methods:
We used longitudinal data on 2,738 women (age 42–52 at baseline) living in six United States cities from The Study of Women’s Health Across the Nation. Residential histories, systolic blood pressures (SBP), and diastolic blood pressures (DBP) were collected annually for ten years. We used longitudinal latent profile analysis to identify patterns of neighborhood socioeconomic vulnerability occurring from 1996–2007 in participant neighborhoods. We used linear mixed-effect models to determine if a woman’s neighborhood profile throughout midlife was associated with blood pressure changes.
Results:
We identified four unique profiles of neighborhood socioeconomic vulnerability – differentiated by residential socioeconomic status, population density, and vacant housing conditions – which remained stable across time. Women residing in the most socioeconomically vulnerable neighborhoods experienced the steepest increase in annual SBP growth by 0.93 mmHg/year (95% CI: 0.65–1.21) across ten-year follow-up.
Conclusions:
Neighborhood socioeconomic vulnerability was significantly associated with accelerated SBP increases throughout midlife among women.
Keywords: neighborhood, blood pressure, midlife, health disparities, hypertension, epidemiology
INTRODUCTION
High blood pressure impacts over 58 million women in the United States (US) and remains a strong and independent predictor of cardiovascular disease (CVD) – the leading cause of death for women [1]. Despite marked improvements in blood pressure (BP) control over the past fifty years, significant racial and ethnic disparities in cardiovascular-related morbidity and mortality persist [2]. Non-Hispanic Black women remain at higher risk of developing hypertension and uncontrolled BP than non-Hispanic White women [3, 4] and continue to have poorer cardiovascular health profiles across the female life-course into later years [5–7]. Moreover, rates of achieving optimal BP control among older adult women have been found to decline with increasing age [1, 8]. Given the heightened sensitivity to environmental stressors experienced across midlife [9, 10], coupled with widening disparities in CVD risk factor burden unaccounted for by individual-level risk factors alone, it remains crucial to consider the influence of upstream neighborhood-level factors which may contribute to differences in cardiovascular health among women into later years.
Aspects of the residential physical, socioeconomic, and built environment have been linked to elevated BP and hypertension in adults [11–13]. Such neighborhood features are hypothesized to influence BP by contributing to poorer health behaviors (e.g., worse diet, physical inactivity) [14, 15], adverse toxicological exposures (e.g., ambient air, water pollution) [16, 17], and chronic psychosocial distress (e.g., depression, anxiety) [18, 19]. Yet these community-based features remain largely structurally determined, and highly patterned by residential racial composition and socioeconomic position [20–26]. As a lasting consequence of structural systemic racism and institutionalized oppression in the US (e.g., segregation, redlining, predatory lending), sustained resource deprivation and risk-enhancing conditions remain disproportionately concentrated within historically and contemporarily marginalized communities [20–26]. Moreover, accumulating evidence suggests that structurally disadvantaged neighborhood environments – characterized by deficits in social capital, economic opportunity, and quality housing – influence BP, above and beyond individual-level factors. However, most work to date utilized crosssectional data [27–35], considered just a single aspect of socioeconomic disadvantage [36–41], or employed time-invariant neighborhood exposure measures [42–45]. As most studies remain limited to just a single time point of residential history data on participants, little work has been able to disentangle the effects of residential re-location/mobility from changes attributable to natural community evolution. Importantly, no studies to date have examined the longitudinal impacts of neighborhood sociodemographic change on BP progression among women throughout midlife. Provided that increased physiological and hormonal changes during midlife place women at higher risk of cardiovascular disturbance and age-related SBP increases [9, 10], it remains critical to investigate whether exposure to neighborhood socioeconomic vulnerability over time may accelerate BP changes among women throughout this sensitive stage of the female life-course, above and beyond individual-level factors. As no studies have explored whether longitudinal neighborhood change profiles influence BP changes among individuals over time [46], the goal of the present study was to investigate the effects of natural community evolution on BP progression in our longitudinal cohort of midlife women (i.e. only using non-mover observations).
Therefore, we used data from the Study of Women’s Health Across the Nation (SWAN) to address these current gaps in existing knowledge. First, we used longitudinal latent profile analysis (LLPA) to identify distinct patterns of neighborhood socioeconomic vulnerability and sociodemographic change occurring among participant neighborhoods in six US cities over the course of a decade (1996–2007). Next, we examined whether exposure to these neighborhood profiles throughout midlife influenced systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels and their annual progression across ten-year follow-up. We hypothesized that (1) women living in the most socioeconomically vulnerable neighborhood profiles consistently throughout midlife and (2) women living in socioeconomically deteriorating neighborhood profiles across midlife would have higher baseline SBP levels and experience faster rates of annual SBP growth across ten-year follow-up.
MATERIALS AND METHODS
Study Population
SWAN is a multi-site, longitudinal cohort study of a racially and ethnically diverse sample of women transitioning through menopause. Briefly, SWAN recruited a total of 3,302 women from seven clinical sites across the US beginning in 1996. Study participants were able to self-identify as either White, Black, Hispanic, Chinese, or Japanese American, and were enrolled from seven sites: Pittsburgh, Pennsylvania; Boston, Massachusetts; Chicago, Illinois; Southeastern Michigan; Newark, New Jersey; Oakland, California; Los Angeles, California. Each study site recruited White women and women of one prespecified racial or ethnic group (i.e., Black, Hispanic, Chinese, or Japanese). Participants were followed for a total of 16 follow-up visits conducted at approximately annual intervals, with 74% of the surviving cohort retained by visit 16. Physical, lifestyle, psychosocial, and hormonal measures were regularly collected at most data collection periods across follow-up, along with medical histories and cardiovascular risk markers. At baseline, those enrolled were between the ages of 42 and 52 years, had their uterus and at least one ovary intact, had at least one menstrual period in the 3 months preceding enrollment, were not pregnant or lactating, and were not taking medications impacting ovarian function. Written informed consent was provided by all participants, and institutional review board approval was obtained at each study site institution. Additional recruitment details are described elsewhere [47].
Residential address histories were collected annually from baseline visit (V00) through exam V11 (2007) for SWAN women who consented to participate in an ancillary air pollution study [48, 49]. The Boston site did not participate in this ancillary study and hence none of their participants (n=450 women) were eligible for inclusion in the current analysis. Addresses were geocoded to the census tract level based upon the 2010 US Census boundary classifications, and linked to each SWAN participant by her unique ID and study visit year. To characterize the social, economic, demographic, and housing conditions among participant neighborhoods across time, we incorporated decennial census estimates available through the Longitudinal Tract Database (Brown University) which normalized data from the 1990, 2000, and 2010 censuses to the 2010 census tract boundaries, allowing for longitudinal comparison across this 1990–2010 period [50, 51]. We linearly interpolated intercensal values to obtain annual estimates for years occurring between the decennial censuses, and linked these time-varying neighborhood characteristics to each SWAN participant based upon her census tract of residence and year.
Analytic Sample
Among the 2,850 SWAN women enrolled at one of the six air pollution ancillary study sites, 2,833 (99.4%) women had available geocoded address data, of whom 2,825 (99.7%) had at least one valid neighborhood exposure measure across our study follow-up period (V00-V10) (Figure S1). Of these eligible 2,825 women with 26,719 observations (from which our analytic samples for the present study were drawn), we excluded 5,672 observations for having missing or implausible/flagged SBP values throughout follow-up; 71 women (1,586 observations) for having missing data on key covariates throughout follow-up; 70 observations from New Jersey site women occurring from V06 onward (due to suspended data collection at the site during V06V08 and V10-V11); and 16 women (2,480 observations) who had reported moving neighborhoods from that visit onward – yielding our final SBP sample of 2,738 women with 16,911 observations. The same exclusion criteria and logic were followed to obtain our final DBP sample of 2,736 women with 16,900 observations. Among women enrolled at one of the six participating sites, compared to those included in the present study, those excluded were more likely to be Black or Hispanic, lower income, unmarried, have less than a high school education, and were slightly more likely to be current smokers and to have depressive symptoms, obesity, hypertension, and diabetes (Table S1).
Exposure: Neighborhood Socioeconomic Vulnerability Profile
We used LLPA to derive distinct profiles of neighborhood socioeconomic vulnerability and sociodemographic change occurring among 1,074 SWAN neighborhoods from 1996–2007. We identified six specific indicators reflecting different neighborhood domains for inclusion into LLPA based upon their importance to population health as identified from prior work [52–54]: percent (%) population with < high school education; % population living in poverty; % vacant housing units; % population unemployed; % foreign-born residents; population density (persons per square mile) (Table S2). We standardized these indicators to each local SWAN site area and year for each SWAN census tract identified across follow-up (by subtracting the local area mean value from the tract-specific value and dividing it by the standard deviation), to best account for important between-site differences (in e.g., cost of living), in alignment with prior work [55–58]. Our indicators thus represented relative values of socioeconomic, housing, demographic characteristics within each local SWAN site area. We next split our neighborhood-level data into even (‘96/’98/’00/’02/’04/’06) and odd (‘97/’99/’01/’03/’05/’07) years, and set the odd-year set aside for cross-validation sensitivity analyses. We entered our even-year indicator z-scores into Mplus to generate latent profiles of neighborhoods based upon shared patterns of neighborhood social, economic, demographic and housing conditions across time. We sequentially estimated 27 unique profile solutions, with the final solution determined based on a combination of empirical criteria, replicability criteria, and overall interpretative validity. First, we considered each model’s Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) (lower values indicating better fit), entropy values (the closest to 1 indicating greatest profile differentiation), and the Vuong-Lo-Mendell-Rubin (VLMR) and Lo-Mendell-Rubin adjusted (LMR) likelihood ratio tests (significant p-value indicating that the k profile solution fit significantly better than the k-1 solution) [59]. To ensure each profile solution converged on a true maximum likelihood solution, we ran an additional second set of models using two times the number of random starts to ensure that the best log-likelihood could still be obtained and solutions replicated [60, 61]. We also used cross-validation to assess the reliability of our derived latent profile solutions – specifically, we considered whether each final solution (derived using even-year data) maintained consistency with its comparable solution derived using odd-year data. Furthermore, we considered the theoretical interpretability of our derived profile solutions, and used additional census indicators belonging to similar domains for post-hoc external validation of our derived latent profiles. After determining our final profile solution, we assigned each SWAN neighborhood to its most likely latent profile of membership. We calculated the mean and median posterior probability of most likely profile membership for our sample of neighborhoods overall, and within each neighborhood profile. Finally, we merged these neighborhood profiles to each SWAN participant by her census tract of residence and visit year on an annual basis.
Outcomes: Systolic and Diastolic Blood Pressures
Our primary outcomes included SBP (mmHg) and DBP (mmHg) – both measured using a standardized protocol with the participant seated in an upright position after five minutes at rest. Two sequential measurements were taken on the right arm using a standard mercury sphygmomanometer at five-minute intervals (minimum 2-minute rest period between measurements), which were later averaged. Participants had not consumed any caffeinated beverage or smoked within 30 minutes of measurements. If a woman reported anti-hypertensive medication use at the current visit, we added 10 mmHg to her crude SBP level and 5 mmHg to her DBP level to correct for medication effects [62, 63]. Approximately 14% of our sample were taking an anti-hypertensive at baseline, and 32% of women started on anti-hypertensive medication at some point across follow-up.
Covariates
We considered the following time-invariant covariates measured at baseline: study site, baseline age, self-defined race/ethnicity, and education level (high school or less, some college, college or postgraduate). We considered the following time-varying covariates measured via standardized questionnaires: marital status (single, married, separated/widowed/divorced), employment, alcohol use, depressive symptoms, physical activity, smoking status (current, past/never), menopausal status (pre-menopausal, early peri-menopausal, late peri-menopausal, postmenopausal, other), hormone use (current/past, never), and obesity. Presence of depressive symptoms was measured using the Center for Epidemiologic Studies Depression (CES-D) Scale, with a clinical cutoff of 16 or higher indicative of depressive symptomology [64]. Physical activity score was measured based on the modified Kaiser Permanente Health Plan Physical Activity Survey, with greater scores demonstrating higher levels of physical activity in active living, sports/exercise, and household/caregiving domains [65, 66]. As physical activity data were not collected at each follow-up, we carried the last observation forward from the closest prior exam to impute missing values. Menopausal status was defined based upon self-reported bleeding patterns during the prior 12 months prior. Body mass index (kg/m2) was calculated from height and weight and measured using standardized protocols and obesity was defined as having a body mass index ≥ 30 kg/m2 for Black, Hispanic, and White participants, and ≥ 25 kg/m2 for Japanese and Chinese participants [67, 68]. Medical histories (e.g., diabetes status) and residential histories (e.g., moved neighborhood since last recorded visit) were also considered.
Statistical Analysis
We summarized the baseline characteristics of our sample overall, and by latent neighborhood profile of residence. We calculated frequencies and percentages for categorical variables, means and standard deviations (sd) for normally distributed continuous variables, and medians and interquartile ranges for skewed variables. We used linear mixed-effect models (with participant-level random intercepts to account for within-woman correlation) to investigate the relationship between neighborhood socioeconomic vulnerability throughout midlife and longitudinal changes in annual BP levels among women across follow-up. We evaluated whether quadratic and cubic time terms improved model fit, but found just the linear term performed best in all models (and thus was modeled as such throughout analyses). To determine whether the annual rate of BP change differed between neighborhood profiles, we introduced interactions between profile and time – all of which were significant and retained throughout modeling. We estimated mean differences in baseline BP levels, and mean differences in the annual rates of BP change, between neighborhood profiles along with their corresponding 95% confidence intervals (CI). We plotted predicted mean BP levels and their annual rates of progression across ten-years of follow-up for each neighborhood profile. We sequentially adjusted models for individual-level demographics, socioeconomic factors, health behaviors, medical histories, and factors unique to the midlife period. We conducted LLPA using Mplus v.8.6 (Muthén & Muthén, Los Angeles, CA), and used SAS v.9.4 (SAS Institute, Cary, NC) for all other analyses.
Sensitivity Analyses
In sensitivity analyses, we first assessed whether further adjustment for diabetes (as an additional confounder of interest identified from prior work) altered the magnitude or direction of our findings. Next, we used crude BP values as outcomes in modeling and adjusted for any current use of anti-hypertensives as a covariate. We also tested whether the influence of neighborhood profile on BP was modified by local SWAN site area by introducing interaction terms between profile, time, site, and their cross-products. To evaluate the impact of selection bias due to missing data in our study, we also presented models for which we had imputed missing covariate data using its last observation carried forward (LOCF). Finally, we adjusted for self-reported race/ethnicity as a marker of social disadvantage capturing the impacts of racism-related exposures in our primary analyses [21, 22]. Yet given evidence of structural confounding between neighborhood profile and race and ethnicity in our study, we also present results unadjusted for race/ethnicity to assess the sensitivity of our findings to potential positivity violations and avoid off-support inference.
RESULTS
Identification of Latent Neighborhood Profiles
LLPA modeling results and fit statistics for the derived 2–7 latent profile solutions are presented in Table 1. AIC and BIC declined progressively across models as the number of latent profiles estimated increased. Entropy remained considerably high across all models, with values ranging from 0.97–0.99 indicating significant profile differentiation. All models converged and solutions were replicated using two times the number of random starts, aside from the seven-profile model. The VLMRT and adjusted-LMRT p-values indicated that the three-profile solution was significantly better than the two-profile solution (p=0.0086), but that the four-profile solution did no better than the three-profile solution (p=0.4561). However, the four-profile solution revealed an additional, uniquely distinct profile pattern which had been obscured in the three-profile solution. We also found marked consistency in the magnitude, direction, and patterning of class indicators between the four-profile solutions derived using even and odd-year data. The fiveprofile solutions lacked replicability between even and odd-year data sets. Therefore, we selected the four-profile solution as our final model for its interpretability, parsimony, replicability, and favorable fit statistics.
Table 1.
Longitudinal latent profile analysis results: neighborhood profile enumeration, model fit statistics, and final profile solutions: the Study of Women’s Health Across the Nation, United States (n=1,074 census tracts).
| LLPA Model Run | Log-likelihood | AIC | BIC | aBIC | Entropy | Latent Profile Counts | VLMRT p-value | LMRT p-value |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 2 Profile Solution | −43525.517 | 87269.034 | 87811.761 | 87465.556 | 0.989 | 1: 813, 2: 261 | 0.0024 | 0.0025 |
| 3 Profile Solution | −40406.648 | 81105.295 | 81832.25 | 81368.526 | 0.975 | 1: 394, 2: 462, 3: 218 | 0.0086 | 0.0089 |
| 4 Profile Solution | −38468.558 | 77303.116 | 78214.299 | 77633.056 | 0.970 | 1: 356, 2: 105, 3: 407, 4: 206 | 0.4561 | 0.4574 |
| 5 Profile Solution | −36611.43 | 73662.86 | 74758.272 | 74059.509 | 0.978 | 1: 297, 2: 111, 3: 392, 4: 187, 5: 87 | 0.7609 | 0.7611 |
| 6 Profile Solution | −34972.275 | 70458.549 | 71738.19 | 70921.908 | 0.982 | 1: 279, 2: 366, 3: 104, 4: 211, 5: 73, 6: 41 | 0.1865 | 0.1866 |
| 7 Profile Solution | −33934.976 | 68457.953 | 69921.821 | 68988.02 | 0.980 | 1: 272, 2: 285, 3: 201, 4: 128, 5: 98, 6: 38, 7: 52 | 0.3846 | 0.3854 |
Abbreviations: VLMRT, Vuong-Lo-Mendell-Rubin likelihood ratio test; LMRT, Lo-Mendell-Rubin adjusted likelihood ratio test; AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion; aBIC, adjusted Bayesian Information Criterion; LLPA, longitudinal latent profile analysis;
Characteristics of Latent Neighborhood Profiles
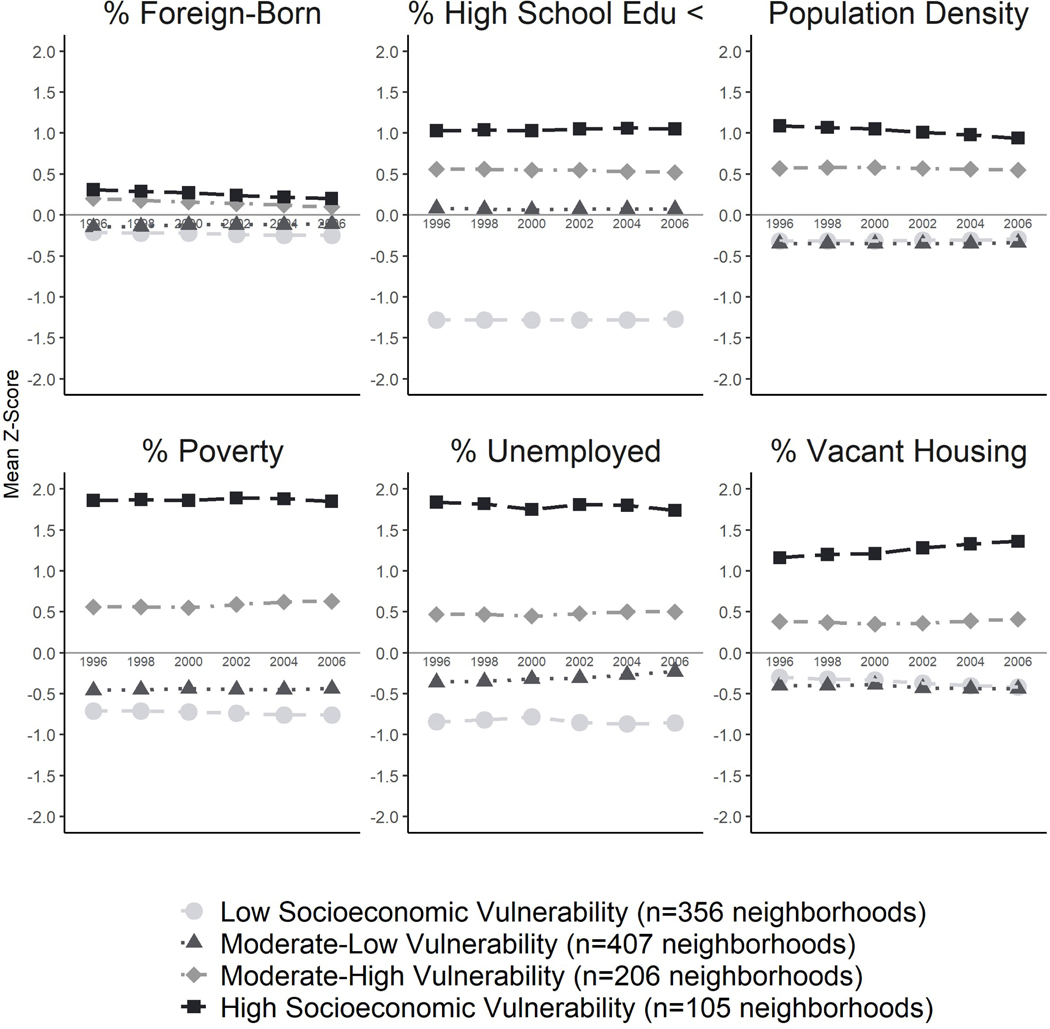
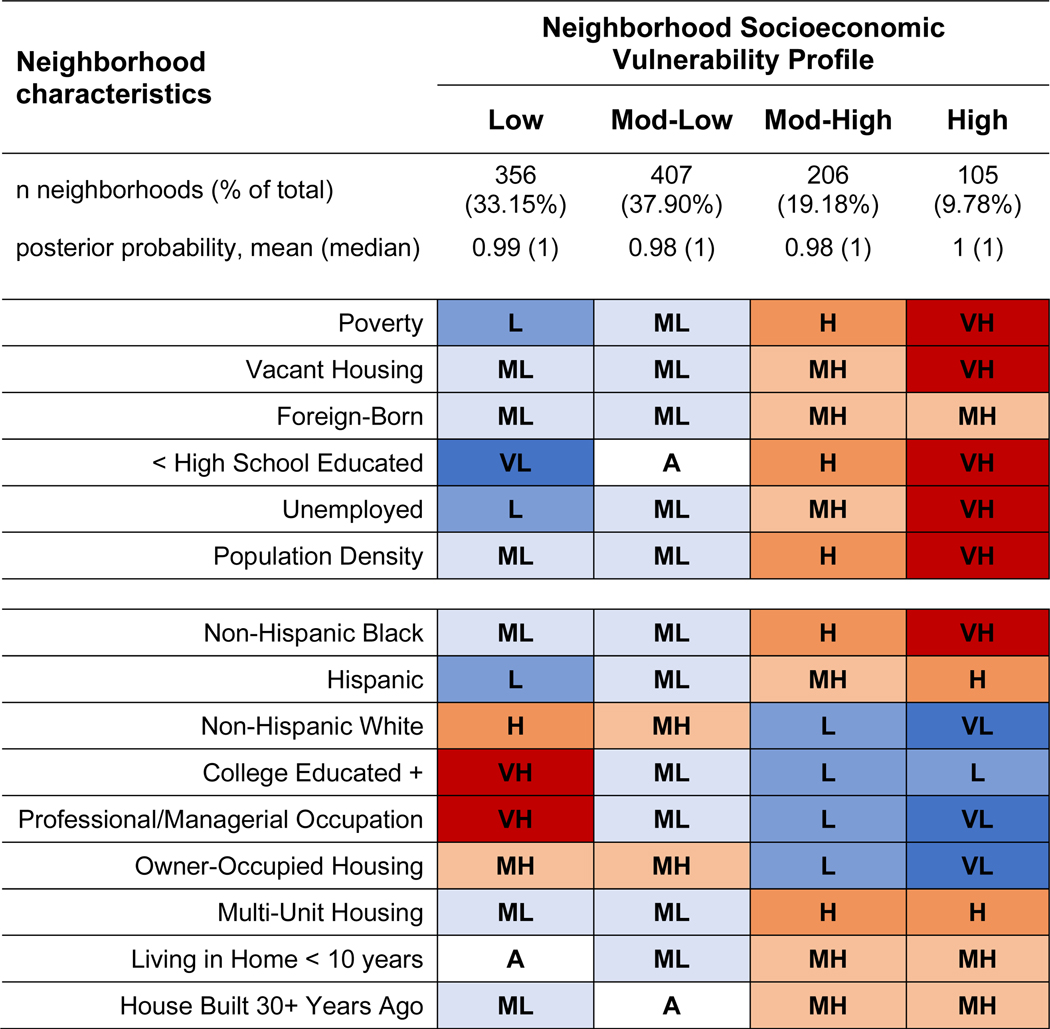
This final solution revealed the following four neighborhood profiles – all of which remained stable across time: Low Socioeconomic Vulnerability (n=356 neighborhoods; 33% of total), characterized by very high levels of SES (e.g., education, employment, income), lower levels of vacant housing, and lower population density; Moderate-Low Vulnerability (n=407; 38%) had moderate-high SES, lower vacant housing, and lower population density; Moderate-High Vulnerability (n=206; 19%) had moderate-low SES, higher vacant housing, and high population density; High Socioeconomic Vulnerability (n=105; 10%) had very low SES, very high vacant housing, and very high population density (Figure 1). Comparable census indicators tracked expectedly alongside our LLPA input variables across profiles, as Moderate-High and HighVulnerability neighborhoods also had significantly fewer college educated residents, residents in managerial/professional occupations, and owner-occupied housing, along with significantly higher levels of residents living in older housing units and in multi-unit housing structures (Figure 2). Neighborhood socioeconomic vulnerability remained strongly patterned by area-level racial/ethnic composition, such that majority non-Hispanic White neighborhoods had significantly more favorable social, economic, and housing conditions compared to majority non-Hispanic Black or Hispanic neighborhoods.
Figure 1.
Longitudinal latent profile characteristics of neighborhoods over time, by census indicator variable: the Study of Women’s Health Across the Nation, United States, 1996–2007 (n=1,074 census tracts).
Figure 2.
Latent profile characteristics of SWAN neighborhoods at year 2000, by census indicator variablesa: the Study of Women’s Health Across the Nation, United States (n=1,074 census tracts).
Abbreviations: VL, Very Low; L, Low; ML, Moderately Low; A, Average; MH, Moderately High; H, High; VH, Very High;
aLevels, indicating degree of presence in neighborhood, correspond to the following indicator z-score values: Very Low (z <= −1); Low (−1 < z <= −0.5); Moderately Low (−0.5 < z <= −0.1); Average (−0.1 < z < 0.1); Moderately High (0.1 <= z < 0.5); High (0.5 <= z < 1); Very High (z >= 1)
Sample Characteristics Across Neighborhood Profiles
Our sample consisted of 2,738 women with a mean (sd) age of 46.0 (2.7) years at baseline (Table 2). Of those, 46.0% were White, 25.4% were Black, 10.2% were Japanese, 9.5% were Hispanic, and 8.9% were Chinese. At baseline, mean SBP and DBP were 117.9 mmHg (sd=17.2) and 75.2 mmHg (sd=10.7), respectively. Forty percent of our sample (n=1082 women) lived in Low-Vulnerability neighborhoods at baseline, followed by Moderate-Low (32.0%), ModerateHigh (21.4%), and High-Vulnerability (6.9%) neighborhood residents. Mean SBP was lowest among women living in Low-Vulnerability tracts – with each increasing profile having progressively higher baseline SBP. A greater proportion of Low-Vulnerability participants were White, while the majority of Moderate-High and High-Vulnerability neighborhood residents were either Hispanic or Black. Relative to women living in Moderate-Low and LowVulnerability neighborhoods, Moderate-High and High-Vulnerability neighborhood residents were more likely to be less educated, current smokers, physically inactive, and to have depressive symptoms, obesity, and diabetes.
Table 2.
Baseline characteristics of study sample, overall and by latent neighborhood profile of residence: the Study of Women’s Health Across the Nation, United States, 1996 (n=2,738 women).
| Variable | Overall (n=2738) | Neighborhood Socioeconomic Vulnerability Profile |
|||
|---|---|---|---|---|---|
| Low (n=1082) | Moderate-Low (n=877) | Moderate-High (n=587) | High (n=190) | ||
|
| |||||
| Race/ethnicity, n (%) | |||||
| Black | 694 (25.4) | 198 (18.3) | 217 (24.7) | 220 (37.5) | 59 (31.1) |
| White | 1260 (46.0) | 595 (55.0) | 468 (53.4) | 164 (27.9) | 31 (16.3) |
| Chinese | 244 (8.9) | 84 (7.7) | 108 (12.3) | 32 (5.5) | 20 (10.5) |
| Hispanic | 261 (9.5) | 5 (0.5) | 17 (1.9) | 159 (27.1) | 80 (42.1) |
| Japanese | 279 (10.2) | 200 (18.5) | 67 (7.6) | 12 (2.0) | 0 (0) |
| Educational attainment, n (%) | |||||
| High school or less | 708 (25.9) | 120 (11.1) | 232 (26.5) | 253 (43.1) | 103 (54.2) |
| Some college | 899 (32.8) | 316 (29.2) | 324 (36.9) | 200 (34.1) | 59 (31.1) |
| College or post-graduate | 1131 (41.3) | 646 (59.7) | 321 (36.6) | 134 (22.8) | 28 (14.7) |
| Marital status, n (%) | |||||
| Single | 313 (11.5) | 87 (8.1) | 96 (11.0) | 90 (15.5) | 39 (20.7) |
| Currently married/Living as married | 1869 (68.8) | 817 (76.0) | 596 (68.4) | 350 (60.2) | 105 (55.9) |
| Separated/Widowed/Divorced | 536 (19.7) | 171 (15.9) | 180 (20.6) | 141 (24.3) | 44 (23.4) |
| Employed, n (%) | 2184 (79.8) | 883 (81.6) | 733 (83.6) | 431 (73.4) | 135 (71.1) |
| CES-D score >= 16, n (%) | 676 (24.7) | 198 (18.3) | 217 (24.7) | 192 (32.8) | 69 (36.3) |
| Current smoker, n (%) | 486 (17.8) | 144 (13.3) | 161 (18.4) | 139 (23.7) | 42 (22.1) |
| Alcohol use, n (%) | |||||
| 2+ drinks / week | 543 (19.9) | 307 (28.5) | 138 (15.7) | 74 (12.6) | 24 (12.6) |
| 1 drink / month - 2+ drinks / week | 772 (28.3) | 293 (27.2) | 220 (25.1) | 202 (34.5) | 55 (29.0) |
| <1 drink / month | 1418 (51.9) | 478 (44.3) | 519 (59.2) | 310 (52.9) | 111 (58.4) |
| Obese, n (%) | 953 (35.2) | 273 (25.6) | 348 (40.0) | 246 (42.3) | 85 (45.2) |
| Hypertensive, n (%) | 884 (32.3) | 306 (28.3) | 280 (32.0) | 226 (38.5) | 71 (37.4) |
| Diabetic, n (%) | 114 (4.2) | 25 (2.3) | 37 (4.2) | 36 (6.1) | 15 (7.9) |
| Menopausal status, n (%) | |||||
| Premenopausal | 1483 (54.3) | 594 (55.2) | 486 (55.5) | 307 (52.5) | 96 (50.8) |
| Early perimenopausal | 1242 (45.5) | 482 (44.8) | 390 (44.5) | 275 (47.0) | 93 (49.2) |
| Unknown due to hormone therapy use | 4 (0.2) | 1 (0.1) | 0 (0) | 3 (0.5) | 0 (0) |
| Age at baseline (years), mean (sd) | 45.8 (2.7) | 46.0 (2.7) | 45.7 (2.7) | 45.6 (2.7) | 45.8 (2.7) |
| Anti-hypertensive use, n (%) | 380 (13.9) | 112 (10.4) | 134 (15.3) | 99 (16.9) | 34 (17.9) |
| Systolic blood pressure (mmHg), mean (sd) | 117.9 (17.2) | 115.6 (16.5) | 116.9 (16.5) | 122.4 (19.1) | 122.0 (15.3) |
| Diastolic blood pressure (mmHg), mean (sd) | 75.2 (10.7) | 74.6 (10.0) | 74.1 (10.9) | 77.0 (11.5) | 77.7 (9.7) |
| Physical activity score, mean (sd) | 7.6 (1.8) | 8.0 (1.8) | 7.5 (1.7) | 7.3 (1.8) | 7.1 (1.8) |
Abbreviations: CESD, Center for Epidemiologic Studies of Depression Scale; sd, standard deviation; Note: n=2 women in our sample were missing neighborhood information at baseline (but had complete data at subsequent visits) who are not reflected in row totals.
Associations Between Neighborhood Socioeconomic Vulnerability and Blood Pressure
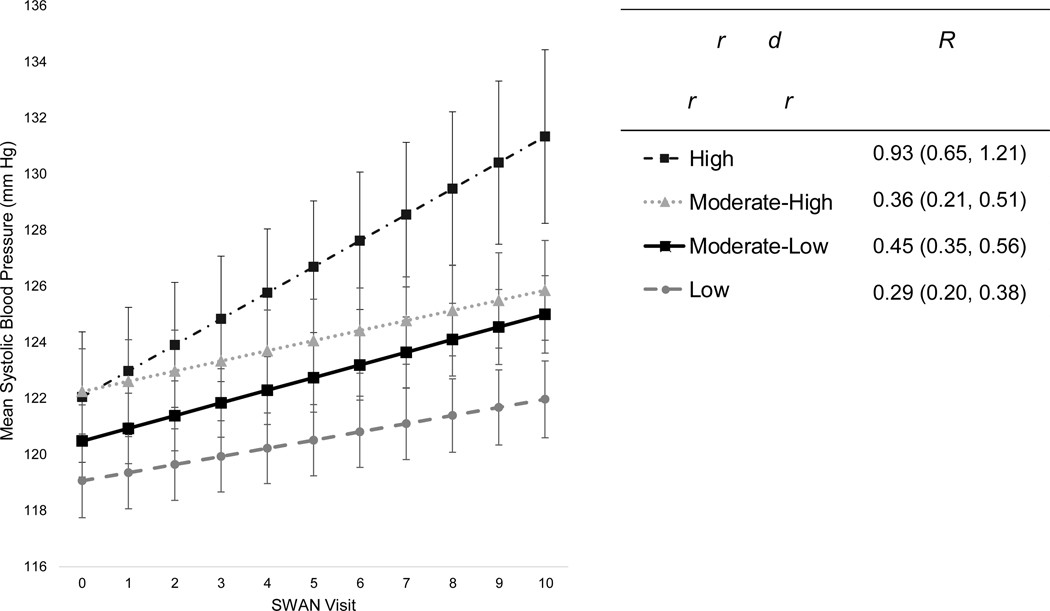
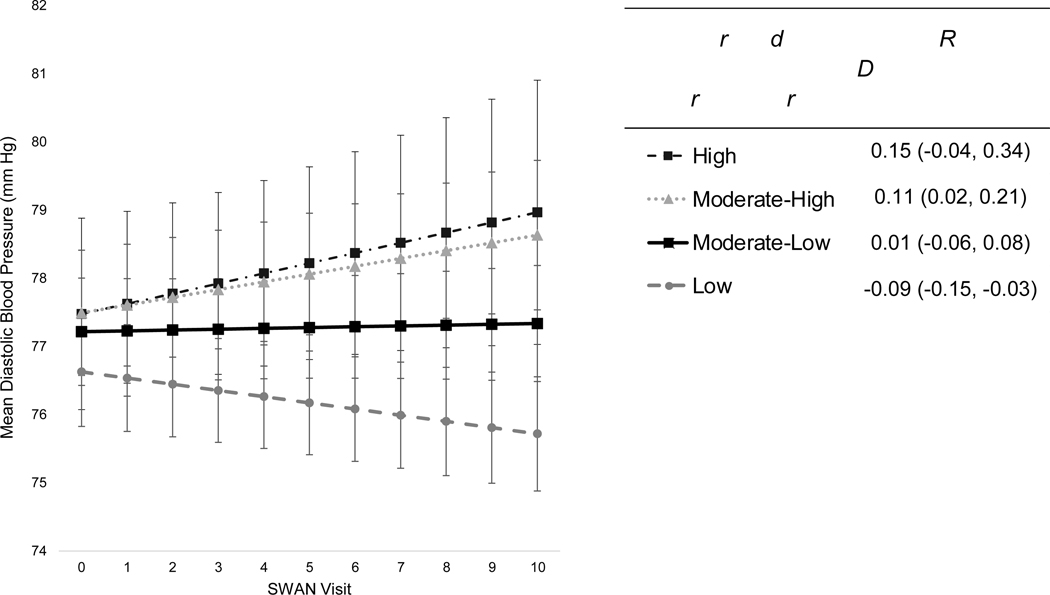
In minimally-adjusted models (controlling only for baseline age and site), lower neighborhood socioeconomic vulnerability was associated with higher baseline SBP (Table 3). Further accounting for socioeconomic and menopause-associated factors reduced the magnitude, but not the significance, of baseline SBP differences somewhat. Relationships persisted upon further controlling for depressive symptoms, alcohol use, current smoking, physical activity, and obesity. In fully adjusted models, residents of Moderate-Low, Moderate-High, and High-Vulnerability communities had 1.42 mmHg higher (95% CI: −0.03, 2.87), 3.19 mmHg higher (95% CI: 1.33, 5.06), and 3.00 mmHg higher (95% CI: 0.34, 5.65) baseline SBP levels, respectively, compared to women living in Low-Vulnerability neighborhoods. While SBP increased with time across all neighborhood profiles, SBP differences widened throughout follow-up between the most socioeconomically vulnerable residents and the remaining three groups. Specifically, greater neighborhood socioeconomic vulnerability was associated with steeper increases in SBP, even after full covariate adjustment. Residents of Moderate-Low and High-Vulnerability neighborhoods throughout midlife experienced 0.16 mmHg/year faster (95% CI: 0.04, 0.28) and 0.64 mmHg/year faster (95% CI: 0.35, 0.93) annual SBP increase, respectively, compared to women living in Low-Vulnerability communities. Residents of the most socioeconomically vulnerable neighborhoods throughout midlife experienced the fastest acceleration in annual SBP increases at 0.93 mmHg/year (95% CI: 0.65, 1.21), whereas residents of the least socioeconomically vulnerable communities experienced the slowest rate of SBP change at just 0.29 mmHg/year (95% CI: 0.20, 0.38), across ten-years of follow-up (Figure 3). Rates of SBP change for Moderate-Low and Moderate-High residents were 0.45 mmHg/year (95% CI: 0.35, 0.56) and 0.36 mmHg/year (95% CI: 0.21, 0.51), respectively. By final ten-year follow-up, SBP had risen an estimated 2.91 mmHg (95% CI: 1.97, 3.85) absolutely among LowVulnerability residents, while SBP increased by 9.29 mmHg (95% CI: 6.46, 12.13) absolutely among High-Vulnerability residents (Table S3). Moreover, SBP differences became increasingly more pronounced between women residing in High-Vulnerability and Low-Vulnerability communities throughout midlife, as predicted SBP levels were 122.1 mmHg (95% CI: 119.7, 124.4) vs 119.1 mmHg (95% CI: 117.8, 120.4) for the two groups at baseline, which increased to 131.4 mmHg (95% CI: 128.3, 134.5) vs 122.0 mmHg (95% CI: 120.6, 123.4) between the groups by final ten-year follow-up. Neighborhood profiles were not associated with baseline DBP after covariate adjustment (Table 4), and annual changes in DBP were observed only among select groups across follow-up. Annual rates of DBP change for Moderate-High and Low-Vulnerability residents were 0.11 mmHg/year (95% CI: 0.02, 0.21) and −0.09 mmHg/year (95% CI: −0.15, −0.03), respectively, while Moderate-Low and High-Vulnerability residents experienced no change in DBP across time (Figure 4; Table S4).
Table 3.
Mean differences (95% confidence intervals) in baseline systolic blood pressure levels (mmHg) and annual rates of change (mmHg/year) between latent neighborhood profiles throughout midlifea (n=2,738 women; n=16,911 observations): the Study of Women’s Health Across the Nation, United States (1996–2007).
| Neighborhood Socioeconomic Vulnerability Profile | Mean Differences (95% CI) |
||
|---|---|---|---|
| Model 1b | Model 2c | Model 3d | |
|
| |||
| Baseline SBP level (mmHg) | |||
| Low Vulnerability | (ref) | (ref) | (ref) |
| Moderate-Low | 3.68 (2.17, 5.19) | 1.91 (0.41, 3.41) | 1.42 (−0.03, 2.87) |
| Moderate-High | 8.06 (6.18, 9.94) | 3.64 (1.71, 5.57) | 3.19 (1.33, 5.06) |
| High Vulnerability | 9.15 (6.45, 11.84) | 3.98 (1.23, 6.73) | 3.00 (0.34, 5.65) |
| Annual rate of SBP change (mmHg/year) | |||
| Low Vulnerability | (ref) | (ref) | (ref) |
| Moderate-Low | 0.17 (0.05, 0.29) | 0.17 (0.05, 0.29) | 0.16 (0.04, 0.28) |
| Moderate-High | 0.08 (−0.07, 0.24) | 0.07 (−0.08, 0.23) | 0.07 (−0.09, 0.23) |
| High Vulnerability | 0.59 (0.30, 0.88) | 0.60 (0.31, 0.89) | 0.64 (0.35, 0.93) |
Abbreviations: SBP, systolic blood pressure; CI, confidence interval;
Mean differences (95% CI) as estimated from linear mixed-effect models, with the Low Vulnerability profile serving as the referent group for all comparisons.
Model 1 adjusted for baseline age and study site.
Model 2 adjusted for Model 1 + race/ethnicity, education level, marital status, employment, ever use of hormone therapy, and menopausal status.
Model 3 adjusted for Model 2 + alcohol use, current smoking, depressive symptoms, physical activity score, and obesity.
Figure 3.
Trajectories of systolic blood pressure change (mmHg) per latent neighborhood profile of residence throughout midlifea (n=2,738 women; n=16,911 observations): the Study of Women’s Health Across the Nation, United States (1996–2007).
Abbreviations: SBP, systolic blood pressure; CI, confidence interval;
aPredicted mean SBP levels (mmHg), and annual rates of SBP change (95% CI), by latent neighborhood profile of residence throughout midlife, as estimated from linear mixed-effect models adjusted for baseline age, race/ethnicity, site, education level, marital status, employment status, alcohol use, current smoking, depressive symptoms, physical activity score, menopausal status, ever use of hormone therapy, and obesity. Note: SWAN visits V00-V10 were conducted at approximately yearly intervals starting in 1996 at baseline (V00) through ten-year follow-up (V10).
Table 4.
Mean differences (95% confidence intervals) in baseline diastolic blood pressure levels (mmHg) and annual rates of change (mmHg/year) between latent neighborhood profiles throughout midlifea (n=2,736 women, n=16,900 observations): the Study of Women’s Health Across the Nation, United States (1996–2007).
| Neighborhood Socioeconomic Vulnerability Profile | Mean Differences (95% CI) |
||
|---|---|---|---|
| Model 1b | Model 2c | Model 3d | |
|
| |||
| Baseline DBP level (mmHg) | |||
| Low Vulnerability | (ref) | (ref) | (ref) |
| Moderate-Low | 1.51 (0.63, 2.39) | 0.79 (−0.09, 1.68) | 0.59 (−0.28, 1.46) |
| Moderate-High | 2.95 (1.85, 4.05) | 1.05 (−0.10, 2.19) | 0.87 (−0.25, 1.98) |
| High Vulnerability | 3.74 (2.17, 5.32) | 1.32 (−0.32, 2.95) | 0.85 (−0.74, 2.44) |
| Annual rate of DBP change (mmHg/year) | |||
| Low Vulnerability | (ref) | (ref) | (ref) |
| Moderate-Low | 0.11 (0.03, 0.19) | 0.11 (0.03, 0.19) | 0.10 (0.02, 0.18) |
| Moderate-High | 0.21 (0.11, 0.31) | 0.21 (0.10, 0.31) | 0.21 (0.10, 0.31) |
| High Vulnerability | 0.21 (0.02, 0.40) | 0.22 (0.03, 0.41) | 0.24 (0.05, 0.43) |
Abbreviations: DBP, diastolic blood pressure; CI, confidence interval
Mean differences (95% CI) as estimated from linear mixed-effect models, with the Low Vulnerability profile serving as the referent group for all comparisons.
Model 1 adjusted for baseline age and study site.
Model 2 adjusted for Model 1 + race/ethnicity, education level, marital status, employment, ever use of hormone therapy, and menopausal status.
Model 3 adjusted for Model 2 + alcohol use, current smoking, depressive symptoms, physical activity score, and obesity.
Figure 4.
Trajectories of diastolic blood pressure change (mmHg) per latent neighborhood profile of residence throughout midlifea (n=2,736 women, n=16,900 observations): the Study of Women’s Health Across the Nation, United States (1996–2007).
Abbreviations: DBP, diastolic blood pressure; CI, confidence interval;
aPredicted mean DBP levels (mmHg), and annual rates of DBP change (95% CI), by latent neighborhood profile of residence throughout midlife, as estimated from linear mixed-effect models adjusted for baseline age, race/ethnicity, site, education level, marital status, employment status, alcohol use, current smoking, depressive symptoms, physical activity score, menopausal status, ever use of hormone therapy, and obesity. Note: SWAN visits V00-V10 were conducted at approximately yearly intervals starting in 1996 at baseline (V00) through ten-year follow-up (V10).
Sensitivity Analyses
Modeling results for SBP and DBP remained largely unchanged after adjustment for diabetes (Table S5). We found only a slight reduction in the magnitude of baseline BP differences between neighborhood profiles when using crude BP values and adjusting for anti-hypertensive use. Results were comparable in models using neighborhood profiles derived from odd-year data. We found no significant interactions nor empirical evidence of effect modification by site in either SBP or DBP models. Models using LOCF-covariate data produced similar results to those reported herein. Only baseline differences in SBP were magnified in models unadjusted for race/ethnicity. In a set of exploratory secondary analyses also using observations from movers, we observed no differences in future BP levels associated with moving to a more (or less) socioeconomically vulnerable profile during midlife, nor with the number of changes made between different neighborhood profiles across midlife (data not shown).
DISCUSSION
Neighborhood social, economic, and housing conditions have been found to influence BP, yet studies utilizing longitudinal data, samples of midlife women, annually collected residential histories, and time-varying neighborhood measures remain limited. In our racially, ethnically, and geographically-diverse cohort of women transitioning through menopause, we found that residents of the most socioeconomically vulnerable communities throughout midlife (characterized by lower SES, greater vacant housing, higher population density, and more non-Hispanic Black and Hispanic residents) experienced fastest acceleration in annual SBP growth across ten-year follow-up. Relationships persisted after robust adjustment for individual-level demographics, SES, health behaviors, medical histories, and factors unique to the female midlife period.
To our knowledge, this is the first study to use longitudinal latent profile analysis to characterize distinct patterns of neighborhood change and examine their impact on individual-level health outcomes [46]. We identified four unique profiles of neighborhood socioeconomic vulnerability – differentiated by levels of neighborhood SES, housing conditions, population density, and racial/ethnic composition – all of which remained temporally stable across time. Neighborhood profiles were largely differentiated from one another by quantitative differences in socioeconomic (e.g., education, income, employment) and demographic (e.g., non-Hispanic Black, non-Hispanic White, Hispanic residents) factors, and to a lesser degree by differences in housing conditions and population density. We found consistently gradated declines in neighborhood socioeconomic status with each subsequent profile moving from the Low-Vulnerability to the High-Vulnerability class. Differences in population density and housing conditions largely separated the Moderate-Low and Low-Vulnerability profiles from the Moderate-High and High-Vulnerability profiles. Importantly, we found that High-Vulnerability neighborhoods had the highest rates of poverty and unemployment, vacant housing, population density, and non-Hispanic Black and Hispanic residents. Moreover, we observed a strong, racialized patterning in neighborhood socioeconomic vulnerability by area-level racial/ethnic composition. Neighborhoods with the greatest proportions of non-Hispanic Black and Hispanic residents had the greatest socioeconomic vulnerability, while majority non-Hispanic White neighborhoods remained the least vulnerable. Our findings thus corroborate existing evidence of a disproportionate burden of socioeconomic disadvantage in the US being largely concentrated within marginalized communities – as a lasting consequence of historical and contemporary structural racism perpetuating inequities over time as a root cause [20–26]. Furthermore, our LLPA measures reflect the consequences of structural racism within society, and are not necessarily measures of the structurally racist policies themselves [69]. This may help to explain why certain domains remain stronger and more consistent indicators than others, which we observed in our own work. Ultimately, our results suggest that neighborhood economic hardship and residential segregation may play significant roles in differentiating neighborhoods from one another and influencing SBP trajectories among women as they age.
While prior work on residential advantage and BP has been somewhat inconsistent, this is likely due, at least in part, to variation in how neighborhood disadvantage has been operationalized. Several studies considered just a single aspect of socioeconomic vulnerability (e.g., poverty rate, unemployment, housing tenure) [36–41, 45, 70–73], while others derived composite indices from multiple indicators using variable-centered approaches (e.g., summary scores, principal components analysis, factor analysis) [30–35, 42–44]. Among work involving just a single aspect, studies considering neighborhood economic hardship and residential segregation demonstrated largely positive associations with BP [37, 39, 70–72], while evidence regarding housing conditions or residential density remains largely mixed [36, 38, 40, 41, 73]. Studies utilizing composite indices were most common, and largely documented positive relationships between residential disadvantage and BP, cross-sectionally [30–35] and prospectively [42–44]. Few studies have used latent variable modeling or hierarchical clustering methods to derive neighborhood typologies based upon a range of indicators from multiple domains and study their impact on BP. Two such studies in a single cohort of older adults in North Carolina documented higher rates of hypertension among participants residing in urban, low SES communities [27] and in rural, lower SES, majority Black communities [29]. Similar findings were observed in a metropolitan-based study of Parisian adults, with residents of urban, low social standing communities having the highest SBP cross-sectionally [28]. Despite being conducted in a single geographically-localized area, these studies remain largely consistent with a nationwide analysis of US adults finding significantly poorer cardiovascular health profiles among residents of lowincome, majority non-Hispanic Black communities across US locales [74].
Consistent with prior cross-sectional work [27–29], our study demonstrated an inverse relationship been neighborhood socioeconomic vulnerability and SBP at a single time point. Women living in more structurally disadvantaged, lower SES, urban, majority non-Hispanic Black and Hispanic neighborhoods had higher baseline SBP – though no relationships were found with DBP. Still, most comparable evidence remains cross-sectional, and no studies have examined prospective associations between neighborhood profiles and BP. Therefore, our study extends previous work by investigating whether longitudinal exposure to neighborhood socioeconomic vulnerability throughout midlife influences BP changes across ten-year followup. We found that neighborhood socioeconomic vulnerability profiles were significantly associated with longitudinal SBP changes throughout midlife, after full covariate adjustment. Residents of neighborhoods characterized by lower SES, higher population density, greater vacant housing, and more non-Hispanic Black and Hispanic residents had significantly higher SBP levels across ten-year follow-up. Despite methodological differences in measurement of neighborhood vulnerability across studies, our findings align with prospective work linking lower neighborhood SES to incident hypertension and BP change [42–45]. Neighborhood profiles were generally not as related to baseline DBP or levels across time in our study, possibly due to the different pathologies underlying age-related increases in SBP, but decreases in DBP, impacting women into later life [75]. Importantly, our study found that women living in the most socioeconomically vulnerable communities consistently throughout midlife experienced the fastest rate of annual SBP growth at 0.93 mmHg/year (95% CI: 0.65–1.21) across the decade. Our results suggest that longitudinal exposures to adverse social, economic, and housing conditions may accumulate across midlife and accelerate SBP increases with age across the decade. These findings broadly corroborate existing observational evidence linking long-term cumulative neighborhood poverty to greater subclinical CVD burden among women in later years [76, 77]. Another longitudinal study found that adults who had consistently lived in poor neighborhoods throughout adolescence had 52% higher odds of hypertension in early adulthood (compared to those who never lived in a poor neighborhood) [39]. While not specific to midlife, such findings align with our own that residents of High-Vulnerability neighborhoods throughout midlife had significantly higher baseline SBP and steeper annualized SBP increases across ten years (relative to consistently Low-Vulnerability residents).
To our knowledge, no studies have explored whether longitudinal neighborhood change profiles influence BP changes among individuals over time [46]. Given this dearth in knowledge, the goal of the present study was to investigate the effects of natural community evolution on BP progression in our longitudinal cohort of midlife women (i.e., only using non-mover observations). Still, there is limited evidence suggesting that moving to a more/less advantaged community impacts degree of weight gain [78, 79] and BP change [39, 72]. Moreover, residential mobility may serve as the greater driver of neighborhood change effects when residential re-location is considered alongside natural community evolution in a single measure [72]. Therefore, in a set of exploratory analyses we included both movers and non-movers to assess whether exposure to a given neighborhood profile or movement between different profiles across midlife (V00-V11) were predictive of future BP levels in later years (V12). Approximately 20% of our sample moved at least once across our study period, and among movers, 25% experienced an increase in socioeconomic vulnerability, while 33% experienced a decline in socioeconomic vulnerability sometime throughout follow-up. Ultimately, we found no differences in future SBP or DBP associated with moving to a more (or less) socioeconomically vulnerable profile during midlife, nor with the number of changes made between different neighborhood profiles across midlife. However, our cohort was less residentially mobile than others, which may be attributable to their slightly older age (being late midlife and into early older age) and not being retired.
Our results identify a significant disparity in age-related SBP progression between residents of the most and the least socioeconomically vulnerable neighborhoods throughout midlife. SBP differences became larger and more pronounced between the two groups over time, as annual SBP growth remained slowest for Low-Vulnerability residents and fastest among High-Vulnerability residents across follow-up. Moreover, among those living in the most socioeconomically vulnerable communities, SBP rose from an estimated 122 mmHg (95% CI: 120, 124) at study start (mean age 42–52) to 131 mmHg (95% CI: 128, 134) by study close (aged 52–62) – corresponding to an absolute increase of 9.3 mmHg (95% CI: 6.5–12.1) over the ten-year follow-up. Given that each 1 mmHg increase in SBP has been linked to 2% increased risk of coronary heart disease mortality [80, 81], our results suggesting that SBP rose annually by 0.93 mmHg/year (95% CI: 0.65–1.21) for ten years in the most socioeconomically vulnerable neighborhoods has significant public health implications. Our findings support the notion that age-related acceleration in SBP growth may be partially explained by differences in midlife residential socioeconomic vulnerability above and beyond traditional risk factors, and suggest that consistent midlife neighborhood hardship may play a significant role in accelerating SBP increases among women as they age. Still, the specific mechanisms underlying this relationship remain unclear. Deprivation of community-based health-promoting resources, and sustained exposure to psychological adversity and distress, may contribute to the adoption of unhealthy coping methods (e.g., smoking, alcohol use), worse health behaviors (e.g., physical inactivity, poor diet), and to poorer physical (e.g., obesity, diabetes) and mental (e.g., depression, anxiety) wellbeing – all of which may accelerate SBP increases among midlife women. We attempted to control for many of these factors in our analyses, suggesting that the association between neighborhood socioeconomic vulnerability and BP may not be explained by personal risk factor burden alone. Potentially operating through alternative pathways, there is also evidence linking more proximal aspects of the neighborhood environment – such as crime [82, 83], pollution [16, 17], and built environment features [14, 15] – to elevated BP. Moreover, the prolonged secretion of inflammatory and stress cytokines (and their associated contribution to systemic allostatic system dysregulation) remains an important pathway worth exploration in future work [18, 19], as does investigation into which specific factors may provide resilience and buffer against the harmful impacts of structural racism and community disadvantage on BP changes among women as they age.
Our study had several strengths, including a longitudinal design with ten-year follow-up of a geographically, racially, and ethnically diverse sample of midlife women, annually collected residential histories and objective BP measurements, and appreciable control for sociodemographic, behavioral, and biomedical confounders. Furthermore, we incorporated time-varying indicators spanning multiple neighborhood domains, and used LLPA to generate latent neighborhood groupings which may better characterize the complex multi-dimensional identities of communities reflected over time. Our study also had limitations. First, we relied upon census tracts to proxy neighborhoods, which may not reflect meaningful community boundaries nor demarcate local effects in real life. Thus, future studies are needed to explore whether neighborhood profile creation (and its associated BP effects) remain consistent when measured at smaller spatial scales (e.g., census block group). Second, our study primarily captures neighborhoods located within larger urban areas, so our results may not be generalizable to more rural settings or to American Indian populations. Third, latent profile analysis is a probabilistic-based categorization approach, and thus subject to misclassification of profile assignment. However, the mean posterior probability of most likely profile membership remained considerably high across all neighborhood groups (ranging from 0.98–1.00) – indicating very high confidence in the predictive accuracy of latent profile assignment. Our final profile solution also demonstrated high reliability over time in cross-validation analyses, and comparable census indicators tracked expectedly alongside our LLPA input variables across profiles (suggesting high external construct validity too) (Figure S2; Table S6). Fourth, we were unable to account for the number of years spent living within a given neighborhood at study start – which may conflate the impacts of midlife and early life exposures to neighborhood vulnerability as a result. Fifth, we utilized relative values of socioeconomic, housing, demographic conditions within each local SWAN site area in our LLPA. These values may represent very different absolute values between communities, and absolute wealth (not just relative) may serve as more important predictor of community vulnerability. As a result, future studies should explore use of absolute values and city-specific LLPA analyses to assess the impact of neighborhood typologies on BP levels in varying US communities and localized areas across time. Sixth, many SWAN women already had high BP at baseline, and selection issues both upon recruitment (e.g., left truncation) and in follow-up (e.g, loss to follow up, missing data) may have contributed to an underestimation of our true associations of interest, as a result. Seventh, we observed a relatively small number of high vulnerability neighborhoods (and women living in these neighborhoods over time), and our findings may not be generalizable to neighborhoods (and women) living outside of our local SWAN site regions. Eighth, our method for accounting for anti-hypertensive medication use (by adding a constant of 10 mmHg to crude SBP levels and 5 mmHg to crude DBP levels) may have introduced bias and unduly influenced our results. While there is no perfect solution to correct for these medication effects to date, our sensitivity analyses revealed only slight reductions in the magnitude of baseline differences in BP between groups when using crude BP values and adjusting for anti-hypertensive use as a covariate. Differences in the rates of BP change remained largely similar in magnitude using either approach to account for medication effects. Finally, our finding of solely stable neighborhood profiles across time was contrary to our hypothesis that socioeconomically improving and socioeconomically deteriorating classes would emerge. However, our study period might not have been long enough to document substantive shifts in neighborhood conditions throughout follow-up (with only three periods of observed decennial census data from which to interpolate intervening values from as well). Alternatively, our findings may instead reflect a more prosperous and stable period in the US. Specifically, our study period concludes in 2007, which is right before the 2008 financial crisis and economic crash occurred in the US. In this case, major temporal changes may have been observed in our socioeconomic indicators starting in 2008 and 2009 – years which were not included in our study period – thereby perhaps helping to explain our temporally stable profiles over time.
CONCLUSIONS
In our racially, ethnically, and geographically-diverse cohort of 2,738 women transitioning through menopause, those living in the least socioeconomically vulnerable communities throughout midlife experienced the fastest acceleration in annual SBP growth across ten-year follow-up. Our findings support the hypothesis that longitudinal exposure to neighborhood socioeconomic vulnerability during midlife accelerates SBP increases among women throughout this sensitive stage of the female life-course. Our work emphasizes the value of targeting socioeconomically vulnerable communities for place-based interventions, community-based participatory outreach, engagement in evidence-based revitalization efforts, and policy-work to stimulate socioeconomic opportunity and growth as potential avenues to reduce inequities in hypertension burden throughout midlife, and disparities in CVD morbidity and mortality among women into later years.
Supplementary Material
Neighborhood SES has been found to influence systolic blood pressure (SBP).
We used longitudinal latent profile analysis to derive neighborhood change classes.
Neighborhood socioeconomic vulnerability was linked to accelerated SBP increases.
Acknowledgements
Clinical Centers:
University of Michigan, Ann Arbor - Carrie Karvonen-Gutierrez, PI 2021 - present, Siobán Harlow, PI 2011 - 2021, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA - Sherri-Ann Burnett-Bowie, PI 2020 - Present; Joel Finkelstein, PI 1999 - 2020; Robert Neer, PI 1994 - 1999; Rush University, Rush University Medical Center, Chicago, IL - Imke Janssen, PI 2020 - Present; Howard Kravitz, PI 2009 - 2020; Lynda Powell, PI 1994 - 2009; University of California, Davis/Kaiser - Elaine Waetjen and Monique Hedderson, PIs 2020 - Present; Ellen Gold, PI 1994 - 2020; University of California, Los Angeles - Arun Karlamangla, PI 2020 - Present; Gail Greendale, PI 1994 - 2020; Albert Einstein College of Medicine, Bronx, NY - Carol Derby, PI 2011 - present, Rachel Wildman, PI 2010 - 2011; Nanette Santoro, PI 2004 - 2010; University of Medicine and Dentistry - New Jersey Medical School, Newark - Gerson Weiss, PI 1994 - 2004; and the University of Pittsburgh, Pittsburgh, PA - Rebecca Thurston, PI 2020 - Present; Karen Matthews, PI 1994 - 2020.
NIH Program Office:
National Institute on Aging, Bethesda, MD - Rosaly Correa-de-Araujo 2020 - present; Chhanda Dutta 2016- present; Winifred Rossi 2012-2016; Sherry Sherman 1994 - 2012; Marcia Ory 1994 - 2001; National Institute of Nursing Research, Bethesda, MD - Program Officers.
Central Laboratory:
University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center:
University of Pittsburgh, Pittsburgh, PA - Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 - 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 - 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Funding:
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Funding (removed from Anonymized manuscript for blinded review but should be put back into the Funding section when appropriate):
During completion of this work, Mary Schiff was supported as a Predoctoral Trainee in the Cardiovascular Epidemiology Training Program (T32 HL083825, PI: Sekikawa) at the University of Pittsburgh Graduate School of Public Health.
Footnotes
Declaration of competing interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
SWAN provides access to public use datasets that include data from SWAN screening, the baseline visit and follow-up visits (https://agingresearchbiobank.nia.nih.gov/). To preserve participant confidentiality, some, but not all, of the data used for this manuscript are contained in the public use datasets. A link to the public use datasets is also located on the SWAN web site: http://www.swanstudy.org/swanresearch/data-access/. Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu.
REFERENCES
- 1.Wenger NK, et al. , Hypertension Across a Woman’s Life Cycle. J Am Coll Cardiol, 2018. 71(16): p. 1797–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virani SS, et al. , Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation, 2020: p. CIR0000000000000757. [DOI] [PubMed] [Google Scholar]
- 3.Kramer H, et al. , Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA). Am J Hypertens, 2004. 17(10): p. 963–70. [DOI] [PubMed] [Google Scholar]
- 4.Carnethon MR, et al. , Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association. Circulation, 2017. 136(21): p. e393–e423. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, et al. , Ethnic variation in hypertension among premenopausal and perimenopausal women: Study of Women’s Health Across the Nation. Hypertension, 2005. 46(4): p. 689–95. [DOI] [PubMed] [Google Scholar]
- 6.Mujahid MS, et al. , Neighborhoods and racial/ethnic differences in ideal cardiovascular health (the Multi-Ethnic Study of Atherosclerosis). Health Place, 2017. 44: p. 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas SJ, et al. , Cumulative Incidence of Hypertension by 55 Years of Age in Blacks and Whites: The CARDIA Study. J Am Heart Assoc, 2018. 7(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pimenta E, Hypertension in women. Hypertension Research, 2012. 35(2): p. 148–152. [DOI] [PubMed] [Google Scholar]
- 9.El Khoudary SR, et al. , The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause, 2019. 26(10): p. 1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews KA., et al., Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? Journal of the American College of Cardiology, 2009. 54(25): p. 2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser P, et al. , Neighborhood Environments and Incident Hypertension in the MultiEthnic Study of Atherosclerosis. Am J Epidemiol, 2016. 183(11): p. 988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leal C and Chaix B, The influence of geographic life environments on cardiometabolic risk factors: a systematic review, a methodological assessment and a research agenda. Obes Rev, 2011. 12(3): p. 217–30. [DOI] [PubMed] [Google Scholar]
- 13.Mujahid MS, et al. , Neighborhood characteristics and hypertension. Epidemiology, 2008. 19(4): p. 590–8. [DOI] [PubMed] [Google Scholar]
- 14.Morland K, Wing S, and Diez Roux A, The contextual effect of the local food environment on residents’ diets: the atherosclerosis risk in communities study. Am J Public Health, 2002. 92(11): p. 1761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon-Larsen P, et al. , Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics, 2006. 117(2): p. 417–24. [DOI] [PubMed] [Google Scholar]
- 16.Chi GC, et al. , Individual and Neighborhood Socioeconomic Status and the Association between Air Pollution and Cardiovascular Disease. Environ Health Perspect, 2016. 124(12): p. 1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erqou S, et al. , Particulate Matter Air Pollution and Racial Differences in Cardiovascular Disease Risk. Arterioscler Thromb Vasc Biol, 2018. 38(4): p. 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird CE, et al. , Neighbourhood socioeconomic status and biological ‘wear and tear’ in a nationally representative sample of US adults. J Epidemiol Community Health, 2010. 64(10): p. 860–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazmi A, et al. , Cross-sectional and longitudinal associations of neighborhood characteristics with inflammatory markers: findings from the multi-ethnic study of atherosclerosis. Health Place, 2010. 16(6): p. 1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford CL and Airhihenbuwa CO, The public health critical race methodology: praxis for antiracism research. Soc Sci Med, 2010. 71(8): p. 1390–8. [DOI] [PubMed] [Google Scholar]
- 21.Ford CL and Airhihenbuwa CO, Critical race theory, race equity, and public health: toward antiracism praxis. American journal of public health, 2010. 100(S1): p. S30–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones CP, Levels of racism: a theoretic framework and a gardener’s tale. American journal of public health, 2000. 90(8): p. 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey ZD, et al. , Structural racism and health inequities in the USA: evidence and interventions. Lancet, 2017. 389(10077): p. 1453–1463. [DOI] [PubMed] [Google Scholar]
- 24.Williams DR and Collins C, Racial residential segregation: a fundamental cause of racial disparities in health. Public health reports (Washington, D.C. : 1974), 2001. 116(5): p. 404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osypuk TL., et al., Quantifying Separate and Unequal: Racial-Ethnic Distributions of Neighborhood Poverty in Metropolitan America. Urban affairs review (Thousand Oaks, Calif.), 2009. 45(1): p. 25–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quillian L, Segregation and poverty concentration: The role of three segregations. American Sociological Review, 2012. 77(3): p. 354–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirowsky JE, et al. , A novel approach for measuring residential socioeconomic factors associated with cardiovascular and metabolic health. J Expo Sci Environ Epidemiol, 2017. 27(3): p. 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Hulst A, et al. , A typology of neighborhoods and blood pressure in the RECORD Cohort Study. J Hypertens, 2012. 30(7): p. 1336–46. [DOI] [PubMed] [Google Scholar]
- 29.Weaver AM, et al. , Associations between neighborhood socioeconomic cluster and hypertension, diabetes, myocardial infarction, and coronary artery disease within a cohort of cardiac catheterization patients. Am Heart J, 2022. 243: p. 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buys DR, et al. , Association between neighborhood disadvantage and hypertension prevalence, awareness, treatment, and control in older adults: results from the University of Alabama at Birmingham Study of Aging. Am J Public Health, 2015. 105(6): p. 1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diez-Roux AV, et al. , Neighborhood environments and coronary heart disease: a multilevel analysis. American journal of epidemiology, 1997. 146(1): p. 48–63. [DOI] [PubMed] [Google Scholar]
- 32.Dubowitz T, et al. , The Women’s Health Initiative: The food environment, neighborhood socioeconomic status, BMI, and blood pressure. Obesity (Silver Spring), 2012. 20(4): p. 862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keita AD, et al. , Associations of neighborhood area level deprivation with the metabolic syndrome and inflammation among middle- and older- age adults. BMC Public Health, 2014. 14: p. 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le-Scherban F, et al. , Identifying neighborhood characteristics associated with diabetes and hypertension control in an urban African-American population using geo-linked electronic health records. Prev Med Rep, 2019. 15: p. 100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, et al. , Association between neighbourhood deprivation and hypertension in a USwide Cohort. J Epidemiol Community Health, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christine PJ, et al. , Exposure to Neighborhood Foreclosures and Changes in Cardiometabolic Health: Results From MESA. Am J Epidemiol, 2017. 185(2): p. 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coulon SM, et al. , Multilevel Associations of Neighborhood Poverty, Crime, and Satisfaction With Blood Pressure in African-American Adults. Am J Hypertens, 2016. 29(1): p. 90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cozier YC, et al. , Relation between neighborhood median housing value and hypertension risk among black women in the United States. Am J Public Health, 2007. 97(4): p. 718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippert AM, et al. , Associations of Continuity and Change in Early Neighborhood Poverty With Adult Cardiometabolic Biomarkers in the United States: Results From the National Longitudinal Study of Adolescent to Adult Health, 1995–2008. Am J Epidemiol, 2017. 185(9): p. 765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers EC, et al. , Relationship between area mortgage foreclosures, homeownership, and cardiovascular disease risk factors: The Hispanic Community Health Study/Study of Latinos. BMC Public Health, 2019. 19(1): p. 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirwa K., et al., Residential proximity to major roadways and prevalent hypertension among postmenopausal women: results from the Women’s Health Initiative San Diego Cohort. J Am Heart Assoc, 2014. 3(5): p. e000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claudel SE, et al. , Association between neighborhood-level socioeconomic deprivation and incident hypertension: A longitudinal analysis of data from the Dallas heart study. Am Heart J, 2018. 204: p. 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDoom MM, et al. , Late life socioeconomic status and hypertension in an aging cohort: the Atherosclerosis Risk in Communities Study. J Hypertens, 2018. 36(6): p. 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savin KL, et al. , Social and built neighborhood environments and blood pressure 6 years later: Results from the Hispanic Community Health Study/Study of Latinos and the SOL CASAS ancillary study. Soc Sci Med, 2022. 292: p. 114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shahu A, et al. , Disparities in Socioeconomic Context and Association With Blood Pressure Control and Cardiovascular Outcomes in ALLHAT. J Am Heart Assoc, 2019. 8(15): p. e012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lekkas P, et al. , Finite mixture models in neighbourhoods-to-health research: A systematic review. Health Place, 2019. 59: p. 102140. [DOI] [PubMed] [Google Scholar]
- 47.Sowers M, et al. , SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathology. Menopause Biology and Pathobiology, 2000: p. 175–88. [Google Scholar]
- 48.Green R, et al. , Long- and Short-term Exposure to Air Pollution and Inflammatory/Hemostatic Markers in Midlife Women. Epidemiology, 2016. 27(2): p. 21120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostro B, et al. , Chronic PM2.5 exposure and inflammation: determining sensitive subgroups in mid-life women. Environ Res, 2014. 132: p. 168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Logan JR, Xu Z, and Stults B, Interpolating U.S. Decennial Census Tract Data from as Early as 1970 to 2010: A Longtitudinal Tract Database. Prof Geogr, 2014. 66(3): p. 412420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Logan JR, Stults BD, and Xu Z, Validating Population Estimates for Harmonized Census Tract Data, 2000–2010. Ann Am Assoc Geogr, 2016. 106(5): p. 1013–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Messer LC, et al. , The development of a standardized neighborhood deprivation index. J Urban Health, 2006. 83(6): p. 1041–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh GK, Area deprivation and widening inequalities in US mortality, 1969–1998. American journal of public health, 2003. 93(7): p. 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bilal U, et al. , Neighborhood social and economic change and diabetes incidence: The HeartHealthyHoods study. Health Place, 2019. 58: p. 102149. [DOI] [PubMed] [Google Scholar]
- 55.Delmelle EC, Differentiating pathways of neighborhood change in 50 U.S. metropolitan areas. Environment and Planning A: Economy and Space, 2017. 49(10): p. 2402–2424. [Google Scholar]
- 56.Richardson AS, et al. , Neighborhood socioeconomic status and food environment: a 20year longitudinal latent class analysis among CARDIA participants. Health & place, 2014. 30: p. 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delmelle EC, Five decades of neighborhood classifications and their transitions: A comparison of four US cities, 1970–2010. Applied Geography, 2015. 57: p. 1–11. [Google Scholar]
- 58.Ling C and Delmelle EC, Classifying multidimensional trajectories of neighbourhood change: a self-organizing map and k-means approach. Annals of GIS, 2016. 22(3): p. 173186. [Google Scholar]
- 59.Collins LM and Lanza ST, Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. Vol. 718. 2009: John Wiley & Sons. [Google Scholar]
- 60.Muthén B and Muthén BO, Statistical analysis with latent variables. Vol. 123. 2009: Wiley; New York. [Google Scholar]
- 61.Asparouhov T and Muthén B, Using Mplus TECH11 and TECH14 to test the number of latent classes. Mplus web notes, 2012. 14(22): p. 1–17. [Google Scholar]
- 62.Ji H, et al. , Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol, 2020. 5(3): p. 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tobin MD, et al. , Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med, 2005. 24(19): p. 2911–35. [DOI] [PubMed] [Google Scholar]
- 64.Boyd JH., et al., Screening for depression in a community sample. Understanding the discrepancies between depression symptom and diagnostic scales. Arch Gen Psychiatry, 1982. 39(10): p. 1195–200. [DOI] [PubMed] [Google Scholar]
- 65.Baecke JA, Burema J, and Frijters JE, A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr, 1982. 36(5): p. 93642. [DOI] [PubMed] [Google Scholar]
- 66.Ainsworth BE, et al. , Evaluation of the kaiser physical activity survey in women. Med Sci Sports Exerc, 2000. 32(7): p. 1327–38. [DOI] [PubMed] [Google Scholar]
- 67.Razak F, et al. , Defining obesity cut points in a multiethnic population. Circulation, 2007. 115(16): p. 2111–8. [DOI] [PubMed] [Google Scholar]
- 68.Hutchins F, et al. , The effect of gestational weight gain across reproductive history on maternal body mass index in midlife: the study of women’s health across the nation. Journal of Women’s Health, 2020. 29(2): p. 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gee GC and Hicken MT, Structural Racism: The Rules and Relations of Inequity. Ethn Dis, 2021. 31(Suppl 1): p. 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barber S, et al. , At the intersection of place, race, and health in Brazil: Residential segregation and cardio-metabolic risk factors in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Soc Sci Med, 2018. 199: p. 67–76. [DOI] [PubMed] [Google Scholar]
- 71.Bravo MA, Batch BC, and Miranda ML, Residential Racial Isolation and Spatial Patterning of Hypertension in Durham, North Carolina. Prev Chronic Dis, 2019. 16: p. E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kershaw KN, et al. , Association of Changes in Neighborhood-Level Racial Residential Segregation With Changes in Blood Pressure Among Black Adults: The CARDIA Study. JAMA Intern Med, 2017. 177(7): p. 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarkar C, et al. , Liveable residential space, residential density, and hypertension in Hong Kong: A population-based cohort study. PLoS Med, 2021. 18(11): p. e1003824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tabb LP, et al. , Spatially varying racial inequities in cardiovascular health and the contribution of individual- and neighborhood-level characteristics across the United States: The REasons for geographic and racial differences in stroke (REGARDS) study. Spatial and Spatio-temporal Epidemiology, 2022. 40: p. 100473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izzo JL Jr. and Shykoff BE, Arterial stiffness: clinical relevance, measurement, and treatment. Rev Cardiovasc Med, 2001. 2(1): p. 29–34, 37–40. [PubMed] [Google Scholar]
- 76.Murray ET, et al. , Trajectories of neighborhood poverty and associations with subclinical atherosclerosis and associated risk factors: the multi-ethnic study of atherosclerosis. Am J Epidemiol, 2010. 171(10): p. 1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemelin ET, et al. , Life-course socioeconomic positions and subclinical atherosclerosis in the multi-ethnic study of atherosclerosis. Soc Sci Med, 2009. 68(3): p. 444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leonard T., et al., Do neighborhoods matter differently for movers and non-movers? Analysis of weight gain in the longitudinal dallas heart study. Health Place, 2017. 44: p. 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Powell-Wiley TM, et al. , Change in Neighborhood Socioeconomic Status and Weight Gain: Dallas Heart Study. Am J Prev Med, 2015. 49(1): p. 72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stamler J, Stamler R, and Neaton JD, Blood Pressure, Systolic and Diastolic, and Cardiovascular Risks: US Population Data. Archives of Internal Medicine, 1993. 153(5): p. 598–615. [DOI] [PubMed] [Google Scholar]
- 81.Ragland DR and Brand RJ, Coronary heart disease mortality in the Western Collaborative Group Study. Follow-up experience of 22 years. Am J Epidemiol, 1988. 127(3): p. 462–75. [DOI] [PubMed] [Google Scholar]
- 82.Tung EL, et al. , Association of Rising Violent Crime with Blood Pressure and Cardiovascular Risk: Longitudinal Evidence from Chicago, 2014–2016. Am J Hypertens, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tung EL, et al. , Police-Recorded Crime and Disparities in Obesity and Blood Pressure Status in Chicago. J Am Heart Assoc, 2018. 7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
SWAN provides access to public use datasets that include data from SWAN screening, the baseline visit and follow-up visits (https://agingresearchbiobank.nia.nih.gov/). To preserve participant confidentiality, some, but not all, of the data used for this manuscript are contained in the public use datasets. A link to the public use datasets is also located on the SWAN web site: http://www.swanstudy.org/swanresearch/data-access/. Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu.