Abstract
An effective one-pot strategy was developed for the synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones from pyrazolo[3,4-b]pyridin-6-ones, obtained by reacting 5-aminopyrazoles with 4-arylidene-2-phenyloxazol-5(4H)-ones (azlactones) under solvent-free conditions, through subsequent elimination of a benzamide molecule in a superbasic medium (t-BuOK/DMSO). The fluorescent properties of the synthesized compounds were studied. 4-Arylpyrazolo[3,4-b]pyridin-6-ones luminesce in the region of 409–440 nm with a quantum yield of 0.09–0.23 when irradiated with UV light.
Keywords: 5-aminopyrazole; azlactone; elimination; fluorescence; one-pot synthesis; pyrazolo[3,4-b]pyridin-6-one
Introduction
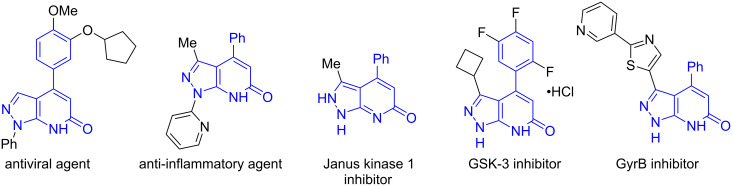
The pyrazolo[3,4-b]pyridine scaffold is present in many biologically active compounds [1–12]. Among them, 4-aryl-substituted derivatives should be distinguished, exhibiting antiviral [13] and anti-inflammatory properties [14], being modulators of estrogen-related receptor alpha [15], JAK1 kinase inhibitor [16], GSK3 [17] and GyrB [8] inhibitors (Figure 1).
Figure 1.
Biologically active 4-arylpyrazolo[3,4-b]pyridin-6-ones.
Despite the high demand, their synthesis methods are few (Scheme 1). To obtain 4-arylpyrazolo[3,4-b]pyridin-6-ones, the only known one-step method is most often used, including the acid-catalyzed condensation of aminopyrazoles with ketoesters [1,16,18] (method A). Its significant disadvantage is the low yields of the target products (11–60%). Yields are also low in two-stage synthesis methods. The first of them is based on the three-component condensation of aminopyrazoles, Meldrum's acid, and aromatic aldehydes, followed by the oxidation of the intermediate with DDQ [13,16,19] (method B). The second one includes the reaction of an aromatic aldehyde with thioglycolic acid and aminopyrazole, followed by the extrusion of sulfur from the resulting thiazepine [20] (method C). The three-stage synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones, involving the preparation of 3-aryl-N-(1H-pyrazol-5-yl)propiolamides (method D), also leads to the formation of the target products with low yields [21]. Therefore, the development of a new effective method for the preparation of 4-arylpyrazolo[3,4-b]pyridin-6-ones is an urgent task.
Scheme 1.
Methods for the synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones.
Results and Discussion
One of the rational approaches to the synthesis of fused pyridine derivatives is based on the domino reaction of enamines with azlactones [22–30]. We have previously reported a plausible mechanism of such reactions [22,25]. 1H-Pyrazol-5-amines also enter into similar transformations with azlactones in various solvents. The yields of tetrahydro-1H-pyrazolo[3,4-b]pyridones 3 obtained by this method vary widely [31–33]. Solvent-free reactions are convenient from both economic and environmental points of view. We obtained tetrahydro-1H-pyrazolo[3,4-b]pyridinone 3a by heating 5-aminopyrazole 1 with azlactone 2a in the absence of solvent at 150 °C in 62% yield (Table 1). For compound 3a, the possibility of benzamide elimination was studied. The benzamide fragment is a poor leaving group; however, in a superbasic medium, we were able to eliminate this group in compound 3a. In order to select optimal synthesis conditions, we heated compound 3a in DMSO at temperatures from 90 to 150 °C for 1.5, 3.5 and 6 h in the presence of KOH or t-BuOK (Table 1).
Table 1.
Optimization of reaction conditionsa.

| |||
|
| |||
| entry | conditions (I) | conditions (II) | yield of 4a (%)b |
|
| |||
| 1 | 150 °C, 40 min, (62%)b | KOH (1 equiv), DMSO, 90 °C, 6 h | traces |
| 2 | KOH (1 equiv), DMSO, 150 °C, 6 h | 58с | |
| 3 | KOH (1.5 equiv), DMSO, 150 °C, 3.5 h | 63 | |
| 4 | t-BuOK (1.5 equiv), DMSO, 150 °C, 1.5 h | 81 | |
| 5d | 150 °C, 40 min then t-BuOK (1.5 equiv), DMSO, 150 °C, 1.5 h | 73 | |
| 6d | DMSO, 150 °C, 2.5 h then t-BuOK (1.5 equiv), 150 °C, 1.5 h | 60 | |
aReaction conditions: 1 (2 mmol), 2a (2 mmol). bIsolated yield after column chromatography. сCompound 3а was additionally isolated in 6% yield. dOne-pot method.
The best yield of 4-phenylpyrazolo[3,4-b]pyridin-6-one 4а (81%) was achieved at 150 °C in DMSO containing 1.5 equiv of t-BuOK for 1.5 h. Obviously, the preparation of 4-phenylpyrazolo[3,4-b]pyridin-6-one 4а could be carried out as one-pot synthesis, without isolation of the intermediate dihydro derivative 3а. In this case, the solvent (DMSO) could be added at the stage of obtaining dihydro derivative 3a or introduced into the reaction together with t-BuOK. We have explored both variants. When intermediate 3a was obtained under solvent-free conditions followed by the addition of t-BuOK in DMSO, the yield of pyrazolo[3,4-b]pyridin-6-one 4a was higher (73%, Table 1, entry 5) than when performing the reaction in a solvent (60%, Table 1, entry 6). Therefore, this procedure was used for the synthesis of compounds 4b–i, 9a, 10a. The yields of pyrazolo[3,4-b]pyridin-6-ones 4a–i, 9a, 10a obtained by this method are in the range of 55–75% (Scheme 2).
Scheme 2.
One-pot synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones 4a–i, 9a, and 10a.
It should be noted that for compounds containing an electron-donating substituent in the C-4 position, such as 4-methoxyphenyl- (4c), 3,4-dimethoxyphenyl- (4d), 3,4,5-trimethoxyphenyl- (4e), 2-furyl- (4h) and 2-thienyl- (4i), the product yields are reduced to 55–60% (Scheme 2).
All the compounds obtained are colorless crystalline substances. When dissolved, they produce colorless solutions exhibiting distinct fluorescent properties with blue emission when exposed to UV light. We recorded absorption and fluorescence spectra of ethanolic solutions of compounds 4a–i, 9a, and 10a. The emission and absorption spectra of all the compounds differ slightly from each other. Their spectral parameters are presented in Table 2.
Table 2.
Data of absorption and fluorescence spectra of compounds 4a–i, 9a, and 10a.a
| Compound | UV–vis | Photoluminescence | ||||
| maxλabs, nm | ε, 103, M–1·cm–1 (λ, nm) |
λex, nm | maxλem, nm | Stokes shift, nm; eV |
Quantum yield Φflb | |
|
| ||||||
| 4a | 260; 302 | 30.3 ± 0.7 (260) |
300; 320 | 419 | 117; 1.15 | 0.22 ± 0.01 |
| 4b | 260; 302 | 38.3 ± 0.7 (260) |
300; 320 | 428 | 126; 1.21 | 0.23 ± 0.01 |
| 4c | 262; 302 | 22.2 ± 0.8 (262) |
300; 320 | 409 | 107; 1.07 | 0.16 ± 0.01 |
| 4d | 260; 301 | 35.1 ± 0.9 (260) |
300; 320 | 414 | 113; 1.12 | 0.15 ± 0.01 |
| 4e | 262; 301 | 22.7 ± 0.9 (262) |
300; 320 | 416 | 115; 1.14 | 0.18 ± 0.01 |
| 4f | 260; 302 | 27.6 ± 0.8 (260) |
300; 320 | 415 | 113; 1.12 | 0.20 ± 0.01 |
| 4g | 261; 300 | 41.5 ± 0.9 (261) |
300; 320 | 411 | 111; 1.12 | 0.20 ± 0.01 |
| 4h | 265; 305 | 32.4 ± 1.0 (265) |
300; 310 | 421 | 116; 1.12 | 0.23 ± 0.01 |
| 4i | 263; 301 | 26.2 ± 0.8 (263) |
300; 310 | 431 | 130; 1.24 | 0.09 ± 0.00 |
| 9a | 259; 303 | 40.0 ± 0.9 (261) |
305 | 433 | 130; 1.23 | 0.19 ± 0.01 |
| 10a | 261; 288 | 34.9 ± 0.5 (259) |
290 | 440 | 152; 1.49 | 0.11 ± 0.01 |
aIn EtOH solution, c = 1.0·10−5 mol·L−1. bQuantum yield determined relative to quinine sulfate standard in 0.5 M H2SO4 (Фf = 0.546).
In the UV spectra of ethanolic solutions of compounds 4a–i, 9a, and 10a, a band with a maximum at 260–265 nm is observed, which has a shoulder at 300–305 nm. These signals seem to correspond to π–π* and n–π* transitions. In the luminescence spectra of compounds 4a–i, 9a, and 10a, there is one broadened band with an emission maximum at 409–440 nm (Figure 2). Their diluted alcohol solutions luminesce with a quantum yield of 0.09–0.23. Pyrazolo[3,4-b]pyridinones 4a–i, 9a, and 10a are characterized by an abnormally high Stokes shift (107–152 nm, 1.07–1.49 eV, Table 2). Such luminophores, which are colorless in daylight but become colored when irradiated with UV light, are used in forensics, in protection against forgery of banknotes, securities, and other important documents [34].
Figure 2.
Normalized absorption and fluorescence spectra of solutions of compounds 4a–i, 9a, and 10a in EtOH.
Conclusion
In summary, we developed a simple one-pot synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones, based on the solvent-free reaction of the available starting compounds 5-aminopyrazoles 1, 5, 6 and azlactones 2a–i, followed by heating the resulting intermediate in DMSO in the presence of t-BuOK. Photophysical properties of the obtained compounds were studied.
Supporting Information
Experimental procedures, characterization data, and 1H and 13C NMR spectra for all new compounds.
This article is part of the thematic issue "Catalytic multi-step domino and one-pot reactions".
Funding Statement
This work was supported by the Russian Science Foundation (grant No. 22-13-00356).
References
- 1.Cross J B, Zhang J, Yang Q, Mesleh M F, Romero J A C, Wang B, Bevan D, Poutsiaka K M, Epie F, Moy T, et al. ACS Med Chem Lett. 2016;7(4):374–378. doi: 10.1021/acsmedchemlett.5b00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo D, Guo Z, Zhao X, Wu L, Liu X, Zhang Y, Zhang Y, Deng Z, Qu X, Cui S, et al. Eur J Med Chem. 2022;227:113923. doi: 10.1016/j.ejmech.2021.113923. [DOI] [PubMed] [Google Scholar]
- 3.Tucker T J, Sisko J T, Tynebor R M, Williams T M, Felock P J, Flynn J A, Lai M-T, Liang Y, McGaughey G, Liu M, et al. J Med Chem. 2008;51(20):6503–6511. doi: 10.1021/jm800856c. [DOI] [PubMed] [Google Scholar]
- 4.Hamblin J N, Angell T D R, Ballantine S P, Cook C M, Cooper A W J, Dawson J, Delves C J, Jones P S, Lindvall M, Lucas F S, et al. Bioorg Med Chem Lett. 2008;18:4237–4241. doi: 10.1016/j.bmcl.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 5.Barghash R F, Eldehna W M, Kovalová M, Vojáčková V, Kryštof V, Abdel-Aziz H A. Eur J Med Chem. 2022;227:113952. doi: 10.1016/j.ejmech.2021.113952. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro J L S, Soares J C A V, Portapilla G B, Providello M V, Lima C H S, Muri E M F, de Albuquerque S, Dias L R S. Bioorg Med Chem. 2021;29:115855. doi: 10.1016/j.bmc.2020.115855. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P K, Singh K, Kumar S, Kumar P, Dhawan S N, Lal S, Ulbrich H, Dannhardt G. Med Chem Res. 2011;20:239–244. doi: 10.1007/s00044-010-9312-7. [DOI] [Google Scholar]
- 8.Mesleh M F, Cross J B, Zhang J, Kahmann J, Andersen O A, Barker J, Cheng R K, Felicetti B, Wood M, Hadfield A T, et al. Bioorg Med Chem Lett. 2016;26:1314–1318. doi: 10.1016/j.bmcl.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Lu Y, Mao F, Li X, Zheng X, Wang M, Xu Q, Zhu J, Li J. J Med Chem. 2017;60:5099–5119. doi: 10.1021/acs.jmedchem.7b00468. [DOI] [PubMed] [Google Scholar]
- 10.Wager T T, inventor. Pyrazolo[3,4-c]pyridines as gsk-3 inhibitors. WO2005000303А1. PCT Pat. Appl. 2005 Jan 6;
- 11.Behnke D, Cotesta S, Hintermann S, Fendt M, Gee C E, Jacobson L H, Laue G, Meyer A, Wagner T, Badiger S, et al. Bioorg Med Chem Lett. 2015;25:5555–5560. doi: 10.1016/j.bmcl.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 12.Choi P J, Lu G-L, Sutherland H S, Giddens A C, Franzblau S G, Cooper C B, Denny W A, Palmer B D. Tetrahedron Lett. 2022;90:153611. doi: 10.1016/j.tetlet.2021.153611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plemper R K, Lee E, Vernachio J, Bourque E, inventors. Bicyclic fused pyrazole derivatives for the treatment of rsv. WO2017196982А1. PCT Pat. Appl. 2017 Nov 16;
- 14.Uchikawa O, Mitsui K, Asakawa A, Morimoto S, Yamamoto M, Kimura H, Moriya T, Mizuno M, inventors. Condensed pyrazole derivatives, process for producing the same and use thereof. US2003187014A1. U.S. Patent. 2003 Oct 2;
- 15.Lemmers J G H, Deretey E, Klomp J P G, Cals J M G B, Oubrie A, inventors. Estrogen-related receptor alpha (ERRα) modulators. WO2021001453A1. PCT Pat. Appl. 2021 Jan 7;
- 16.Hansen B B, Jepsen T H, Larsen M, Sindet R, Vifian T, Burhardt M N, Larsen J, Seitzberg J G, Carnerup M A, Jerre A, et al. J Med Chem. 2020;63:7008–7032. doi: 10.1021/acs.jmedchem.0c00359. [DOI] [PubMed] [Google Scholar]
- 17.Wager T, inventor. GSK-3 inhibitors. US2005026946A1. U.S. Patent. 2005 Feb 3;
- 18.Ratajczyk J D, Swett L R. J Heterocycl Chem. 1975;12:517–522. doi: 10.1002/jhet.5570120315. [DOI] [Google Scholar]
- 19.Quiroga J, Hormaza A, Insuasty B, Márquez M. J Heterocycl Chem. 1998;35:409–412. doi: 10.1002/jhet.5570350225. [DOI] [Google Scholar]
- 20.Swett L R, Ratajczyk J D, Nordeen C W, Aynilian G H. J Heterocycl Chem. 1975;12:1137–1142. doi: 10.1002/jhet.5570120611. [DOI] [Google Scholar]
- 21.Minami S, Tomita M, Kawaguchi K. Chem Pharm Bull. 1972;20:1716–1728. doi: 10.1248/cpb.20.1716. [DOI] [PubMed] [Google Scholar]
- 22.Shuvalov V Y, Samsonenko A L, Rozhkova Y S, Morozov V V, Shklyaev Y V, Fisyuk A S. ChemistrySelect. 2021;6:11265–11269. doi: 10.1002/slct.202103028. [DOI] [Google Scholar]
- 23.Shuvalov V Yu, Chernenko S A, Shatsauskas A L, Samsonenko A L, Dmitriev M V, Fisyuk A S. Chem Heterocycl Compd. 2021;57:764–771. doi: 10.1007/s10593-021-02980-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuvalov V Y, Rozhkova Y S, Plekhanova I V, Kostyuchenko A S, Shklyaev Y V, Fisyuk A S. Chem Heterocycl Compd. 2022;58:7–14. doi: 10.1007/s10593-022-03050-5. [DOI] [Google Scholar]
- 25.Shuvalov V Yu, Fisyuk A S. Synthesis. 2023;55:1267–1273. doi: 10.1055/a-1993-3714. [DOI] [Google Scholar]
- 26.Cunha S, dos Santos Filho R F, Saraiva K H, Azevedo-Santos A V, Menezes D. Tetrahedron Lett. 2013;54:3366–3370. doi: 10.1016/j.tetlet.2013.04.055. [DOI] [Google Scholar]
- 27.Chen X, Zhu D, Wang X, Yan S, Lin J. Tetrahedron. 2013;69:9224–9236. doi: 10.1016/j.tet.2013.08.052. [DOI] [Google Scholar]
- 28.Vanden Eynde J J, Labuche N, Van Haverbeke Y. Synth Commun. 1997;27:3683–3690. doi: 10.1080/00397919708007288. [DOI] [Google Scholar]
- 29.Worayuthakarn R, Nealmongkol P, Ruchirawat S, Thasana N. Tetrahedron. 2012;68:2864–2875. doi: 10.1016/j.tet.2012.01.094. [DOI] [Google Scholar]
- 30.Liu X-Q, Liu Y-Q, Shao X-S, Xu Z-P, Xu X-Y, Li Z. Chin Chem Lett. 2016;27(1):7–10. doi: 10.1016/j.cclet.2015.10.002. [DOI] [Google Scholar]
- 31.Shi F, Zhang J, Tu S, Jia R, Zhang Y, Jiang B, Jiang H. J Heterocycl Chem. 2007;44:1013–1017. doi: 10.1002/jhet.5570440506. [DOI] [Google Scholar]
- 32.Kim H S, Hammill J T, Scott D C, Chen Y, Min J, Rector J, Singh B, Schulman B A, Guy R K. J Med Chem. 2019;62:8429–8442. doi: 10.1021/acs.jmedchem.9b00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H S, Hammill J T, Scott D C, Chen Y, Rice A L, Pistel W, Singh B, Schulman B A, Guy R K. J Med Chem. 2021;64:5850–5862. doi: 10.1021/acs.jmedchem.1c00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulyankin E B, Bogza Y P, Kostyuchenko A S, Chernenko S A, Samsonenko A L, Shatsauskas A L, Yurpalov V L, Fisyuk A S. Synlett. 2021;32:790–794. doi: 10.1055/a-1392-2209. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, characterization data, and 1H and 13C NMR spectra for all new compounds.