Kim et al. demonstrate that neutrophil extracellular trap histones are the major mediators fueling the pathogenic Th17 inflammation that promotes gum and bone loss in periodontitis.
Abstract
Microbial dysbiosis triggers inflammatory periodontitis. In this issue of JEM, Kim et al. (2023. J. Exp. Med. https://doi.org/10.1084/jem.20221751) demonstrate that neutrophil extracellular trap histones are the major mediators fueling the pathogenic Th17 inflammation that promotes gum and bone loss in periodontitis.
Periodontitis is a prevalent human inflammatory condition that is characterized by pathogenic inflammation that recedes the gums and erodes the bone anchoring the teeth. Periodontitis is associated with an elevated risk for systemic inflammation, suggesting that inflammation at mucosal barriers shares common mechanisms with systemic inflammatory conditions (Irwandi et al., 2022). Hence, understanding the mechanisms that promote periodontitis may offer critical insights for human health.

Insights from Venizelos Papayannopoulos.
Microbial dysbiosis promotes periodontitis by triggering neutrophilic inflammation orchestrated by Th17 cells (Moutsopoulos et al., 2012). Aggressive cases are linked to invasion by specific bacteria such as Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis that are difficult to eradicate, and several studies have documented the benefit of antibiotics (Prakasam et al., 2012). These microbial species induce macrophage and dendritic cell–derived cytokines that expand pathogenic resident memory Th17 cells to recruit neutrophils, resulting in gum tissue and bone erosion (Dutzan et al., 2018; Moutsopoulos et al., 2012). How pathogenic microbes drive inflammatory Th17 responses and the mechanism of tissue destruction remain poorly understood.
Granulocytes such as neutrophils have long been regarded as the tip of the spear of inflammation, executing instructions provided by sentinel cells to destroy pathogens or damage host tissues during chronic disease. Consistently, depletion of neutrophils in experimental periodontitis reduces pathology (Dutzan et al., 2018). Yet, until recently, the ability of neutrophils to shape inflammation had been overseen. Given the capacity of neutrophils to protect against infection or drive pathology, their overall contribution is not always easy to ascertain. Usually, a mismatch between the trigger and the ensuing neutrophil effector mechanism will drive chronic pathology.
Neutrophils fight pathogens either by killing them intracellularly or by releasing antimicrobials attached to large web-like structures called neutrophil extracellular traps (NETs) via a cell death process called NETosis (Papayannopoulos, 2018). NETs are composed of decondensed chromatin and antimicrobial proteins and are deployed predominately against large pathogens such as fungi, parasites, and bacterial biofilms that can’t be cleared intracellularly (Papayannopoulos, 2018; Thanabalasuriar et al., 2019). Dysregulated NETosis can be detrimental, as NETs are a major source of extracellular histones. Histones are cytotoxic to bacteria and host cells but can also promote inflammation at sublethal concentrations by activating macrophages via toll-like receptor 4 (TLR4) to induce cytokines that are important in Th17 responses such as IL-1β and IL-6 (Silvestre-Roig et al., 2019; Tsourouktsoglou et al., 2020). This mechanism is implicated in atherogenesis but can also amplify inflammation during infection (Ioannou et al., 2022; Tsourouktsoglou et al., 2020; Warnatsch et al., 2015). In addition, histones promote Th17 cell differentiation via TLR2 in vitro, but the relevance of this mechanism for immune defence and disease needs further characterization (Wilson et al., 2022). Histone-driven inflammation can be beneficial against peripheral infections, but promotes pathology and immune dysfunction during sepsis and chronic inflammatory conditions (Ioannou et al., 2022; Tsourouktsoglou et al., 2020). Since prior work demonstrated a pathogenic neutrophil role in murine periodontitis, Kim et al. (2023) set out to investigate whether the NET histones provide the missing link between microbial dysbiosis and type 17 inflammation in periodontitis (Kim et al., 2023).
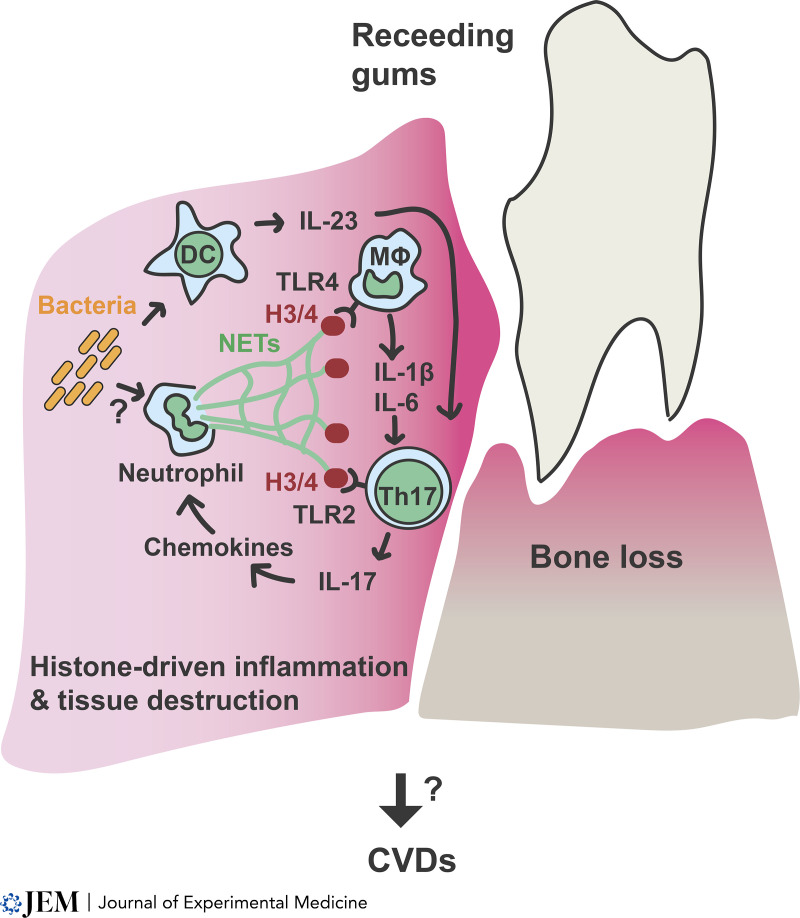
Model of NET histone–driven inflammation in periodontitis. Pathogenic bacteria are likely the triggers of NETosis. NET formation leads to the release of NET-associated histones that promote Th17 differentiation directly via TLR2 signaling. In addition, histones induce cytokines in macrophages that promote Th17 proliferation and survival. Th17 cells instruct the epithelium to upregulate neutrophil chemokines that recruit more neutrophils and amplify inflammation via a possible feedback loop. Persistent periodontitis inflammation may also exert its impact systemically on the vasculature by releasing NET histones or downstream cytokines into the circulation or by sustaining pathogenic Th17 cells that disseminate and act systemically.
The authors first confirmed the induction of a sustained neutrophilic inflammatory program in gingival tissues using RNA sequencing analysis in a mouse model of experimental ligature-induced periodontitis (LIP). Neutrophil immunodepletion led to a threefold reduction in bone loss, confirming that neutrophils play an active role in bone destruction. Next, the authors interrogated the role of NETs in the LIP model. Staining of gingival tissues with NET-specific antibodies uncovered extensive NET deposition. Moreover, degradation of NETs by systemic administration of the endonuclease DNase I reduced bone loss by 30% and the inflammatory transcriptional signature while limiting osteoclast proliferation on the surface of damaged bones. Similar results were obtained in mice deficient in neutrophil elastase (NE), a protease required for NET formation, or by using an NE inhibitor (Papayannopoulos, 2018). A reduction in bone loss was also observed in mice deficient in protein arginine deiminase 4 (PAD4) or by using a broad PAD inhibitor. PAD4 reduces the charge of histones by converting arginine residues to citrulline. This modification occurs specifically during NETosis and is required for chromatin decondensation in response to calcium flux inducing stimuli. Although PAD4 is dispensable for NETosis in response to microbial and damage-derived stimuli, it potentiates the proinflammatory capacity of histones by boosting their interaction with TLR4 (Tsourouktsoglou et al., 2020). Consistently, staining for Ly6G, a neutrophil cell surface receptor that associates with NETs, confirmed the presence of uncitrullinated NETs in gingival tissues from PAD4-deficient mice, accompanied by reduced inflammation and bone loss. Interestingly, PAD4 deficiency did not reduce neutrophil infiltration which is usually coupled via a feedback loop to NET-mediated inflammation.
Histones are the major citrullinated NET proteins. Thus, the authors probed the proinflammatory role of histones directly, using blocking antibodies targeting histones H3 and H4, or citrullinated histone H3. Histone targeting decreased bone loss and resulted in extensive reduction in IL-6 and IL-17 mRNA in gingival tissues. IL-17 induces neutrophil chemokines and is produced by type 17 cells, such as Th17 cells that require macrophage-derived IL-1β and IL-6, for their survival and proliferation. Hence, NET histones had a major impact on both macrophage activation and Th17 cell differentiation. To confirm the impact of NETs on gingival type 17 cells, the authors treated LIP-challenged mice with DNase I and measured total IL-17 producing CD45+ and CD4+ cells using an IL-17 reporter mouse strain. NET degradation reduced both CD4+ Th17 cells and other type 17 cells. In addition to inducing IL-1β and IL-6, histones can promote Th17 cell differentiation directly in a TLR2-dependent manner. Interestingly, IL-1R is dispensable, and only IL-6 is required for Th17 cell expansion in murine LIP (Dutzan et al., 2018). The reduction in IL-6 observed in mice treated with citrullinated histone H3–blocking antibodies suggests that NET histones potentiate type 17 inflammation indirectly by acting on macrophages and directly by promoting Th17 cell differentiation. The contribution of these pathways could be further dissected using cell-specific ablations of TLR2 and TLR4.
Given the potential for NETs to control microbial populations, the authors also probed the impact that the degradation of NETs had on the oral microbiome. DNase I treatment did not alter the gingival microbial load and diversity, indicating that the reduction of inflammation was not caused by bacterial alterations. Consistent with the murine model studies, NET-specific components such as citrullinated and carbamylated histones and cell-free DNA were elevated in gingival fluids and the blood serum of patients with severe periodontitis compared to healthy controls. These NET markers also correlated with disease severity in healthy donors and patients. Hence, a pathway for amplifying inflammation that was first identified as a driver of sterile inflammatory diseases is broadly relevant in non-sterile mucosal and systemic inflammation.
These findings paint a picture of a pathogenic inflammatory response that results from the dysregulated release of NET histones that does little to resolve the insult and instead promotes chronic pathology. NET accumulation in large concentrations can also have an anti-inflammatory effect through the degradation of chemokines by NET-bound proteases (Papayannopoulos, 2018). In murine gout models, NETs promote inflammation early and suppress it later as they aggregate. This regulatory phase was not observed in periodontitis but could be relevant in histone-blocking experiments where histone neutralization may tip the balance in favor of the anti-inflammatory properties of NET proteases.
A critical question in this paradigm is what triggers NET formation in the gums. The evidence points towards specific pathogenic bacterial species such as A. actinomycetemcomitans that aggregate and form biofilms as these virulence mechanisms trigger NETosis (Hirschfeld et al., 2015). A well-described in vivo paradigm stems from studies of ocular Pseudomonas aeruginosa infection, where biofilm promotes a NET barrier that enables the pathogen to evade phagocytosis while keeping the infection confined to the periphery (Thanabalasuriar et al., 2019). Hence, by reducing biofilm formation, antibiotics could help control NETosis and inflammation. While some studies demonstrated the benefit of antibiotic use in periodontitis, their efficacy is not widely accepted (Prakasam et al., 2012). The correlation between NET markers and disease severity suggests that severe patients who tend to be positive for pathogenic microbes would benefit the most from antibiotics or anti-NET therapies. NET markers could serve as a valuable tool to screen for severe patients who would benefit from antibiotic treatment. A systematic correlation analysis between NET markers and microbiomes in mild and severe periodontitis patients could offer important insights into the link between oral dysbiosis and NETosis.
In a related question, Kim et al. (2023) found that NET degradation did not shape the gingival microbiome in the murine LIP model. However, this may not fully reflect on the situation in patients, given that additional pathogenic bacteria may be present that can be affected by NETs. As NETs upregulate bacterial virulence mechanisms such as biofilm formation, controlling NET formation or potentiating NET clearance in patients may have added benefits of positively influencing the oral microbiome (Thanabalasuriar et al., 2019). However, it is worth noting that aggressive periodontitis and oral fungal infections are prevalent in individuals with deficiencies in neutrophil antimicrobial enzymes that are required for NET formation, such as NE and myeloperoxidase (Papayannopoulos, 2018; Sørensen et al., 2014). This suggests that periodontitis may also persist in NET-deficient individuals, and thus prolonged pharmacological interference with NET formation could increase the risk for fungal infection or exacerbate periodontitis. Therefore, the outcome of NET-targeting interventions will depend on whether the periodontitis associated with Papillon Lefèvre syndrome is linked to NET deficiency or to NET-independent functions of NE. The complexity of these scenarios suggests that therapies with histone-blocking antibodies that target specific NET components are likely to be more promising anti-inflammatory therapeutic avenues.
Aberrant NET release may also be accompanied by defects in NET clearance to further exacerbate NET accumulation in periodontitis. Such NET clearance deficiencies appear in young healthy individuals with low-grade inflammation that exhibit normal levels of DNase activity (Aramburu et al., 2022). A decade later, these donors exhibited vascular dysfunction, an early sign of cardiovascular disease (CVD) development that is prominent in periodontitis (D’Aiuto et al., 2018). It is therefore plausible that low-grade inflammation caused by periodontitis may interfere with NET clearance, or that pre-existing NET clearance defects may increase the risk for periodontitis.
One may also speculate on whether histones induce inflammation via their lethal or sublethal properties in periodontitis. At concentrations above 1 μM, histones and nucleosomes kill cells rapidly. This cytotoxicity is thought be proinflammatory and destabilizes atheroma fibrous caps (Silvestre-Roig et al., 2019). However, lethal histone concentrations do not induce cytokine production in monocytes, as they kill cells before they are able to produce cytokines (Tsourouktsoglou et al., 2020). The inflammatory component of histone pathology is therefore likely to be mediated by sublethal histone-mediated signaling, but histone cytotoxicity may be responsible for direct tissue destruction.
These studies have further implications for systemic disease. The strong link between periodontitis and CVDs is not only supported epidemiologically but also functionally, as periodontitis debridement reduces vascular dysfunction (D’Aiuto et al., 2018). The findings by Kim et al. (2023) reveal that atherosclerosis and periodontitis share common underlying pathological mechanisms. This common thread may help explain how periodontitis treatments influence the development of CVDs, but further research is needed to understand the exact molecular links (D’Aiuto et al., 2018; Irwandi et al., 2022). The shared pathologenic mechanism suggests that histone-targeting treatments that prove effective against periodontitis could have therapeutic value in CVD prevention and treatment. Periodontitis clinical trials are simpler and cheaper to conduct and may provide important proof of concept information in humans for any NET-targeting treatment.
References
- Aramburu, I.V., et al. 2022. Immunity. 10.1016/j.immuni.2022.11.007 [DOI] [Google Scholar]
- D’Aiuto, F., et al. 2018. Lancet Diabetes Endocrinol. 10.1016/S2213-8587(18)30038-X [DOI] [Google Scholar]
- Dutzan, N., et al. 2018. Sci. Transl. Med. 10.1126/scitranslmed.aat0797 [DOI] [Google Scholar]
- Hirschfeld, J., et al. 2015. Int. J. Med. Microbiol. 10.1016/j.ijmm.2015.04.002 [DOI] [PubMed] [Google Scholar]
- Ioannou, M., et al. 2022. Nat. Commun. 10.1038/s41467-022-32320-1 [DOI] [Google Scholar]
- Irwandi, R.A., et al. 2022. Front. Immunol. 10.3389/fimmu.2022.915081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T.S., et al. 2023. J. Exp. Med. 10.1084/jem.20221751 [DOI] [Google Scholar]
- Moutsopoulos, N.M., et al. 2012. J. Autoimmun. 10.1016/j.jaut.2012.03.003 [DOI] [Google Scholar]
- Papayannopoulos, V. 2018. Nat. Rev. Immunol. 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- Prakasam, A., et al. 2012. J. Pharm. Bioallied Sci. 10.4103/0975-7406.100226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre-Roig, C., et al. 2019. Nature. 10.1038/s41586-019-1167-6 [DOI] [Google Scholar]
- Sørensen, O.E., et al. 2014. J. Clin. Invest. 10.1172/JCI76009 [DOI] [Google Scholar]
- Thanabalasuriar, A., et al. 2019. Cell Host Microbe. 10.1016/j.chom.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsourouktsoglou, T.D., et al. 2020. Cell Rep. 10.1016/j.celrep.2020.107602 [DOI] [PubMed] [Google Scholar]
- Warnatsch, A., et al. 2015. Science. 10.1126/science.aaa8064 [DOI] [Google Scholar]
- Wilson, A.S., et al. 2022. Nat. Commun. 10.1038/s41467-022-28172-4 [DOI] [Google Scholar]