Abstract
Background
Distal radius fractures (DRFs) are the most frequent first-ever osteoporotic fragility fractures. However, most patients are treated only for fractures and not for osteoporosis. Therefore, we investigated early osteoporosis intervention using zoledronic acid.
Methods
This prospective study enrolled 30 patients aged 50 years or older who had no history of fragility fractures or osteoporosis treatment and who underwent surgical treatment for DRFs. Patients whose lumbar spine or femur bone mineral density (BMD) values were less than 80% of the young adult mean (YAM) were treated with a 5-mg intravenous infusion of zoledronic acid. Lumbar spine and femur YAM BMD values, TRACP-5b and PINP were statistically evaluated using the paired t-test. The relationship between adverse effects, age, body mass index (BMI), and creatinine clearance (CCr) was statistically examined using Mann-Whitney's U test. The incidence of the bone fusion and secondary fractures within the 60-months postoperative period were assessed.
Results
The mean lumbar spine and femur YAM BMD values before treatment were 76.1 ± 13.1% and 70.7 ± 8.5%. This indicates osteopenia in both locations. These values differed significantly between the pre-treatment period and each subsequent period. Five patients with a target YAM BMD value over 80% within 60 months after treatment were observed. The TRACP-5b and PINP values differed significantly between the pre-treatment period and each subsequent period. Adverse drug reactions were observed in 12 patients (40%). Age, BMI, and CCr did not show statistically significant differences in the occurrence of adverse effects. Bone fusion was confirmed at a mean of 3.6 months postoperatively. Secondary fractures were observed in 3 patients within 60 months after treatment.
Conclusion
DRFs occur at a younger age than other fragility fractures, and it is important to intervene aggressively with osteoporosis treatment to prevent secondary fractures.
Level of evidence
Level V.
Keywords: Zoledronic, Distal radius fracture, Osteoporosis, Bone mineral density, Young adult mean
1. Introduction
Distal radius fractures (DRFs) are the most frequent first-ever osteoporotic fragility fractures. However, patients with DRFs are generally treated only for fractures and not for osteoporosis. Therefore, among patients with DRFs at our hospital, we investigated the status of osteoporosis intervention and provided early, aggressive osteoporosis treatment with zoledronic acid. We investigated the rate of osteoporosis treatment, levels of bone turnover markers (BTMs), bone mineral density (BMD), postoperative bone healing periods, and the incidences of side effects and secondary fractures.
2. Material and methods
The study protocol was approved by the Ethics Committee of Toho University Ohashi Medical Center (approval number: H22016_H17033). All patients provided written informed consent. We introduced the treatment protocol shown in Table 1. Sixty-five patients aged 50 years or older received surgical treatment for DRFs and had no history of fragility fractures or osteoporosis treatment, and 40 of these patients underwent dual-energy X-ray absorptiometry (DXA). Thirty patients (2 male, 28 female) who met the following criteria were enrolled in this prospective study: lumbar spine (L2–L4) or femur BMD on DXA <80% of the young adult mean (YAM), no history of fragility fractures or osteoporosis treatment, no severe renal impairment (creatinine clearance (CCR) > 30 mL/min), no hypocalcemia (serum Ca >8.6 mg/dL), and no pregnancy or possible pregnancy.
Table 1.
Study follow chart.
Patients were treated with a 5-mg intravenous infusion of zoledronic acid and oral vitamin D preparations for 1 week after the operation. BMD evaluation and blood sampling were performed at an outpatient clinic 1 week postoperatively and every 6 months postoperatively. Lumbar spine and femur YAM BND values, tartrate-resistant acid phosphatase 5b (TRACP-5b) values, and serum procollagen type I N-terminal propeptide (PINP) values were measured before and 6, 12, 18, 24, 30, 36, 42, 48, 54, and 60 months after zoledronic acid administration. Values of lumbar spine and femur YAM BMD, TRACP-5b, and PINP were statistically evaluated before zoledronic acid administration and at each measurement time point using the paired t-test. The relationships between adverse effects, age, body mass index (BMI) values, and CCR were statistically examined using Mann-Whitney's U test. SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. p < 0.01 was considered statistically significant. Simple X-rays were performed every month until 1 year postoperatively and every 6 months thereafter. Finally, the following were assessed in 42 patients aged 50 years or older who sustained DRFs in the year before protocol initiation: the rate of BMD testing before the protocol was introduced, the intervention rates before and after the protocol, and the incidence of secondary fractures within the 60-month postoperative period.
3. Results
The study flow chart and baseline characteristics of the patients are shown in Table 1, Table 2. Of 65 patients aged 50 years or older who underwent surgery for DRFs after the introduction of the protocol, BMD testing was performed in 40 patients (intervention rate: 61.5%); of these, 30 patients who had a YAM value below 80% and who met the treatment criteria were treated with zoledronic acid. The mean age of the 30 patients was 74.2 ± 9.7 years, the mean BMI was 21.3 ± 3.2 kg/m2, and the mean CCR was 65.2 ± 18.5 mL/min. BMD evaluations and blood testing were performed in the following numbers of patients at each time point: 30 at the start of treatment, 30 at 6 months, 30 at 12 months, 29 at 18 months, 28 at 24 months, 27 at 30 months, 26 at 36 months, 18 at 42 months, 15 at 48 months, 11 at 54 months, and 11 at 60 months. Thirty patients received the first dose of zoledronic acid, 30 received the second, 28 received the third, 26 received the fourth, 15 received the fifth, and 11 received the sixth. Only 3 of the 42 patients with DRFs in the year before protocol initiation (7.1% intervention rate) had BMD testing performed in our department before the protocol was introduced.
Table 2.
Baseline characteristics of the patients.
| Variable | Before administration |
|---|---|
| Sex | |
| Female(n) | 28 |
| Male(n) | 2 |
| Age | 74.2 ± 9.7 |
| Body Mass Index (kg/m2) | 21.3 ± 3.2 |
| Creatinine clearance (mL/min) | 65.2 ± 18.5 |
| Young Adult Mean (%) | |
| Lumbar | 76.1 ± 13.1 |
| Femur | 70.7 ± 8.5 |
| Bone turnover markers | |
| TRACP-5b (mU/dL) | 531.3 ± 127.9 |
| PINP (ng/mL) | 68.7 ± 29.4 |
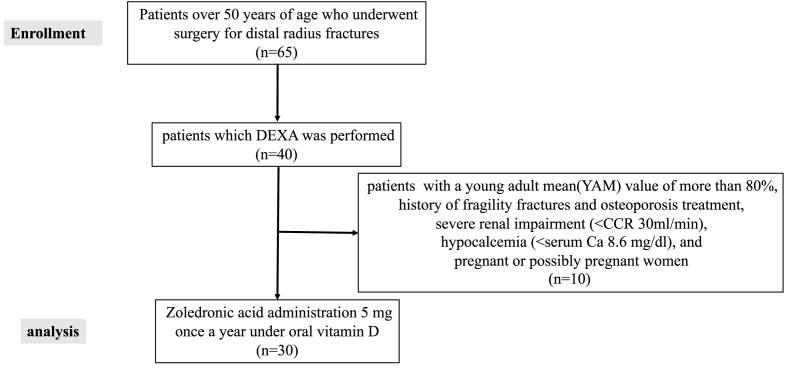
The changes in lumbar spine and femur YAM BMD values over time are shown in Fig. 1, Fig. 2. The mean lumbar spine YAM BMD values were 76.1 ± 13.1% before treatment, 79.1 ± 12.7% after 6 months, 80.0 ± 12.7% after 12 months, 82.1 ± 12.1% after 18 months, 81.7 ± 12.8% after 24 months, 83.0 ± 13.6% after 30 months, 83.0 ± 14.8% after 36 months, 82.8 ± 14.6% after 42 months, 81.7 ± 15.2% after 48 months, 85.4 ± 15.1% after 54 months, and 85.7 ± 14.7% after 60 months.
Fig. 1.
Young adult mean (Lumbar spine).
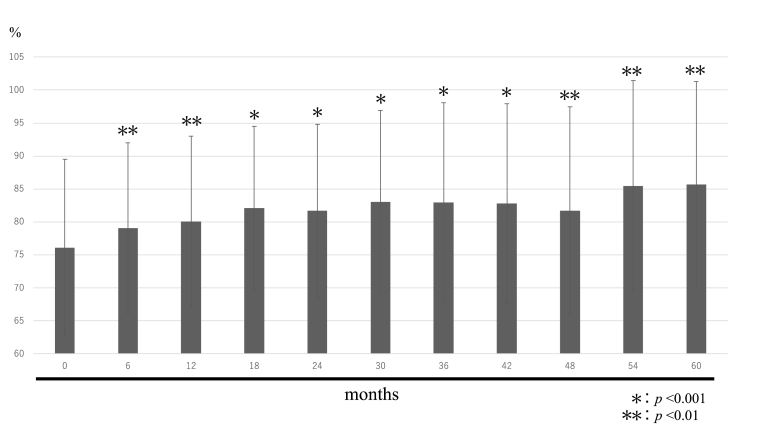
Fig. 2.
Young adult mean (Femur).
The mean femur YAM BMD values were 70.7 ± 8.5% before treatment, 71.7 ± 8.4% after 6 months, 72.2 ± 8.8% after 12 months, 72.4 ± 9.0% after 18 months, 72.4 ± 8.5% after 24 months, 72.5 ± 8.4% after 30 months, 74.7 ± 8.2% after 36 months, 75.2 ± 6.7% after 42 months, 74.5 ± 6.6% after 48 months, 73.2 ± 5.9% after 54 months, and 73.5 ± 6.1% after 60 months.
The lumbar spine and femur YAM BMD values differed significantly between the pre-treatment period and each subsequent period. Lumbar spine and femur YAM BMD values exceeded the target YAM BMD value of 80% at the last observation in 5 cases.
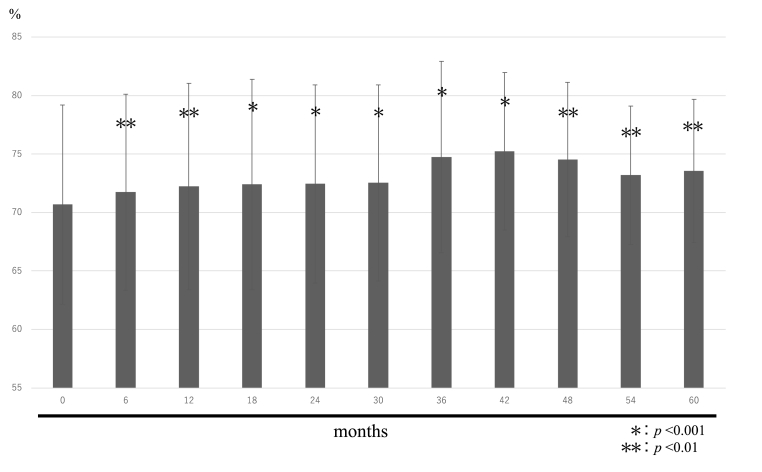
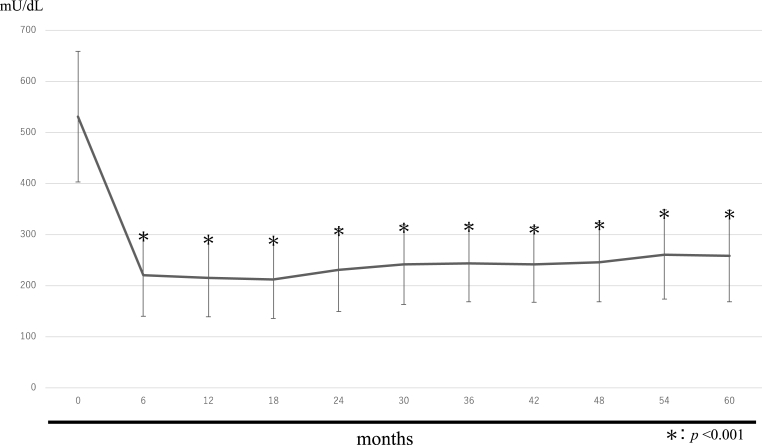
The changes in TRACP-5b and PINP levels over time are shown in Fig. 3, Fig. 4. The mean TRACP-5b values were 531.3 ± 127.9 mU/dL before treatment, 220.1 ± 80.0 mU/dL after 6 months, 214.8 ± 76.6 mU/dL after 12 months, 212.2 ± 76.7 mU/dL after 18 months, 230.3 ± 81.4 mU/dL after 24 months, 240.8 ± 77.6 mU/dL after 30 months, 243.8 ± 76.1 mU/dL after 36 months, 240.9 ± 74.2 mU/dL after 42 months, 246.0 ± 78.1 mU/dL after 48 months, 260.6 ± 87.7 mU/dL after 54 months, and 257.7 ± 89.6 mU/dL after 60 months.
Fig. 3.
TRACP-5b.
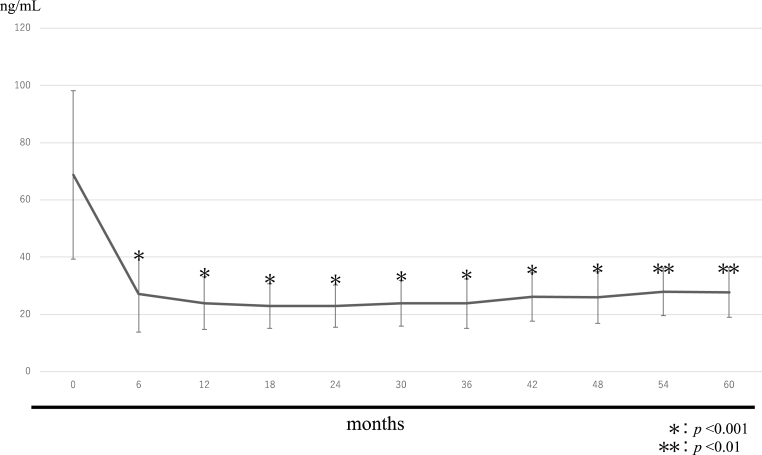
Fig. 4.
PINP.
The mean PINP values were 68.7 ± 29.4 ng/mL before treatment, 27.1 ± 13.2 ng/mL after 6 months, 23.9 ± 9.1 ng/mL after 12 months, 22.8 ± 7.8 ng/mL after 18 months, 22.9 ± 7.4 ng/mL after 24 months, 23.9 ± 7.9 ng/mL after 30 months, 23.8 ± 8.6 ng/mL after 36 months, 26.1 ± 8.4 ng/mL after 42 months, 25.9 ± 9.0 ng/mL after 48 months, 27.8 ± 8.3 ng/mL after 54 months, and 27.6 ± 8.7 ng/mL after 60 months.
Six months after treatment, TRACP-5b and PINP levels were both approximately 50% lower than at baseline, indicating a significant decrease. Thereafter they remained almost at a plateau, with a slight decrease.
Adverse drug reactions were observed in 12 patients (40%): fever in 8 patients (one patient ≥38 °C), general malaise in 1 patient, and arthralgia in 3 patients. The mean ages of patients with or without side effects were 71.8 ± 11.8 years and 75.8 ± 7.4 years, respectively. The mean BMI values of patients with or without side effects were 20.7 ± 2.6 kg/m2 and 21.7 ± 3.4 g/m2, respectively. The mean CCR values of patients with or without side effects were 62.9 ± 16.5 mL/min and 66.7 ± 18.4 mL/min, respectively. There were no statistically significant differences between the 2 groups (Table 3).
Table 3.
Comparison of acute phase reaction and each item.
| APR (-) group (n = 18) | APR (+) group (n = 12) |
p-value (Mann-Whitney U test) | |
|---|---|---|---|
| Age | 75.8 ± 7.4 | 71.8 ± 11.8 | 0.27 |
| BMI(kg/m2) | 21.7 ± 3.4 | 20.7 ± 2.6 | 0.51 |
| CCR(ml/min) | 66.7 ± 18.4 | 62.9 ± 16.5 | 0.67 |
APR: acute phase reaction.
BMI: body mass index.
CCR: creatinine clearance.
Bone fusion was confirmed by simple X-rays at an average of 3.6 months postoperatively in all patients and there were no cases of pseudoarthrosis or prolonged fusion. Secondary fractures were observed in 3 patients within 60 months after treatment.
4. Discussion
Sontag et al.1 reported that the most frequent site of first-ever osteoporotic fractures is the distal radius, higher even than the vertebrae. After DRFs occur, however, only about 16% of patients are prescribed osteoporosis medications by orthopedic surgeons.2 The risk of secondary fracture is significantly increased after DRFs,3 and treatment of osteoporosis has been reported to be effective in preventing secondary fractures for 3 years after the initial fracture.4 In addition, osteoporotic DRFs are associated with poor scores on the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire 1 year postoperatively.5 Preventing initial fragility fractures may be key to reducing the incidence of subsequent secondary fractures, which in turn would extend healthy life expectancy.
Fragility fractures caused by osteoporosis are known to impair activities of daily living and quality of life, and to increase mortality. The 2019 Japanese Comprehensive Survey of Living Conditions showed that fractures and falls were the fourth most common cause (12.5%) of nursing care needs, and there is concern about the fracture-related burden on nursing care. In a survey of the caregivers of patients with osteoporotic fractures, Soen et al.6 reported that 68.3% of caregivers changed their employment status to provide care. The study also found that 81.6% of caregivers were employed, that in a given week they missed 27.4% of their work time due to caregiving, and that only 49.3% of their time was spent at work, which translates to a loss of 43,317 Japanese yen per week when converted to monetary value. The authors proposed that when evaluating the impact of fragility fractures, the substantial human and financial burden of family members’ care for osteoporotic fracture patients might be alleviated by osteoporosis management and support systems. DRFs are more common in older people, and we believe that the financial burden on their caregivers will be reduced by administering zoledronic acid only once yearly.
DRFs are the most common fragility fractures. All patients in this study experienced DRFs as first-ever fragility fractures, and we examined the osteoporosis treatment interventions they received. The mean BMD before treatment was 76.1 ± 13.1% in the lumbar spine and 70.7 ± 8.5% in the femur. This indicates osteopenia in both locations, and in this study we were able to initiate early treatment for osteoporosis by evaluating BMD.
The usefulness of bisphosphonates for osteopenia was demonstrated in a study by Reid et al.7 They showed that zoledronate administration every 18 months for 6 years reduced the risk of fragility fractures (both vertebral and nonvertebral) in older women with hip BMD indicating osteopenia, defined by a bone-density T score of −1.0 to −2.5.
It is very important to treat osteopenia, and therefore treating only patients with osteoporosis has a minimal effect on the total number of fractures.8
In this study, 5 patients who received osteoporosis treatment at an early stage had YAM BMD values that exceeded the target of 80% at the last observation. Since DRFs occur at a relatively young age compared to other fragility fractures, BMD is maintained in many cases and the target YAM value can often be reached. As a result, bisphosphonates are considered to be the preferred osteoporosis drugs for DRF patients.
Adverse reactions occurred in 12 patients (40%) in this study. Acute-phase reactions after zoledronic acid administration include transient fever, myalgia, arthralgia, chills, headache, and influenza-like symptoms within 3 days after administration. High risk is associated with the first administration of zoledronic acid, lack of experience with bisphosphonates, younger age, and low serum 25(OH)D.9 Acute-phase reactions can be alleviated by prophylactic administration of antipyretic analgesics.10 Our patients had no history of treatment for osteoporosis; because initial bisphosphonate administration carries a risk of acute reactions, acetaminophen was administered prophylactically at the time of initial administration. A previous study reported that rapid intravenous zoledronate infusion increased the risk of developing acute renal failure,11 and the drug was administered intravenously over 30 min. Although neither BMI nor CCR was significantly associated with the occurrence of side effects, both were lower in patients who experienced side effects than in those who did not. Particular attention should be paid to patients with low BMI or CCR, since they are at increased risk of side effects that could require giving antipyretic analgesics on the day of zoledronic acid infusion or lengthening its administration time. A disadvantage of zoledronic acid is that it is more likely to cause acute reactions than other bisphosphonates, but it is important to carefully explain to patients that most of the side effects are minor and that they can be treated safely.
TRACP-5b is a BTM of osteoclasts, and high TRACP-5b levels indicate increased bone resorption. PINP is produced during the early stage of osteoblast differentiation; it is an excellent marker of bone formation that reflects bone metabolic turnover, and is widely used in clinical practice.12 Changes in BTMs are observed within a few weeks after the start of treatment with bisphosphonates, and these early changes predict long-term BMD responses.13, 14, 15 Diez-Perez et al.14 proposed that levels of PINP and C-telopeptide of type I collagen assessed 3 months after the start of therapy could be used to screen for the treatment effect of oral bisphosphonate therapy.
This study showed that 6 months after treatment, both the TRACP-5b and PINP levels were approximately 50% lower than at baseline, and remained at a plateau for 60 months afterward. The results of the Horizon-Pivotal Fracture Trial (PFT) study by Black16,17 showed a similar trend in PINP levels. The changes in these BTMs upon osteopenia treatment in this study suggest that bone metabolism was improved soon after bisphosphonate administration, and that this improvement was maintained. In addition, PINP levels did not fall below the lower limit of normal in any patients after treatment, suggesting that bone metabolism was improved without excessively suppressing bone formation.
In this study, the cooperation of the medical staff enabled us to increase the intervention rate from 7.1% to 61.5%. A multidisciplinary osteoporosis (fracture) liaison service that sought to improve treatment adherence, the rate of bone density testing, and the initiation of osteoporosis treatment was reported to reduce the incidence of recurrent fractures.18
In addition, in order to raise awareness of the importance of osteoporosis, a new management fee for secondary fracture prevention and continuation was implemented in Japan with the revision of medical fees in 2022. This management fee has been in place at our hospital since August 2022. However, this fee only applies to patients with proximal femur fractures, and we hope that this indication will be expanded in the future. In particular, osteoporosis treatment focusing on DRFs, which are usually the first fragility fractures to occur, is key to preventing secondary fractures.
Due to concerns about prolonged bone healing, orthopedic surgeons hesitate to administer osteoporosis treatment after fractures, especially bisphosphonates, which may prevent early intervention for osteoporosis. However, it has been reported that bone healing following DRFs is independent of bisphosphonate administration.19 In this study, bone healing was confirmed by simple X-rays in all patients treated with zoledronic acid at an average of 3.6 months after surgery, suggesting that osteoporosis treatment should be an active intervention in the treatment of fragility fractures.
This study has several limitations. The study does not have a control group and the study period was short, the patient enrollment criteria were strict, and the sample size was small. To mitigate these issues, future studies should evaluate long-term follow-up in consecutive patients. In addition, the vast majority of patients were women. Thus, caution is needed when comparing our findings to those of other studies with different sex distributions.
5. Conclusion
All patients in this study experienced DRFs as first-ever fragility fractures, and we examined the osteoporosis treatment using zoledronic acid. The mean BMD before treatment was 76.1 ± 13.1% in the lumbar spine and 70.7 ± 8.5% in the femur. This indicates osteopenia in both locations, and in this study we were able to initiate early treatment for osteoporosis. Five patients with a target YAM BMD value over 80% within 60 months after treatment were observed. Bone fusion was confirmed at a mean of 3.6 months postoperatively. Secondary fractures were observed in 3 patients within 60 months after treatment. DRFs occur at a younger age than other fragility fractures, and it is important to intervene aggressively with osteoporosis treatment to prevent secondary fractures.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution statement
Dr. Shu Yoshizawa: Obtaining informed consent, selecting eligible patients, collecting the data, analyzed the data - Wrote and edited the paper. Dr. Takanori Shintaku: Obtaining informed consent, selecting eligible patients, collecting the data. Dr. Hideaki Ishii: Obtaining informed consent, selecting eligible patients, collecting the data. Dr. Misato Sakamoto: Obtaining informed consent, selecting eligible patients, collecting the data. Prof. Yoshiro Musha: Conception of the study, managing personal information. Prof. Hiroyasu Ikegami: Researched the idea. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
None.
Contributor Information
Shu Yoshizawa, Email: shuu.yoshizawa@med.toho-u.ac.jp.
Takanori Shintaku, Email: t41444078y@yahoo.co.jp.
Hideaki Ishii, Email: h.ishii.1414@gmail.com.
Misato Sakamoto, Email: misato.ia0215@gmail.com.
Yoshiro Musha, Email: yoshiro2006musha@yahoo.co.jp.
Hiroyasu Ikegami, Email: hiroyasu.ikegami@med.toho-u.ac.jp.
References
- 1.Sontag A., Krege J.H. First fractures among postmenopausal women with osteoporosis. J Bone Miner Metabol. 2010 Jul;28(4):485–488. doi: 10.1007/s00774-009-0144-9. [DOI] [PubMed] [Google Scholar]
- 2.Iba K., Dohke T., Takada J., et al. Improvement in the rate of inadequate pharmaceutical treatment by orthopaedic surgeons for the prevention of a second fracture over the last 10 years. J Orthop Sci. 2018 Jan;23(1):127–131. doi: 10.1016/j.jos.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Robinson C.M., Royds M., Abraham A., McQueen M.M., Court-Brown C.M., Christie J. Refractures in patients at least forty-five years old. A prospective analysis of twenty-two thousand and sixty patients. J Bone Joint Surg Am. 2002 Sep;84(9):1528–1533. doi: 10.2106/00004623-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Fitspatrick S.K., Casemyr N.E., Zurakowski D., Day C.S., Rozental T.D. The effect of osteoporosis on outcomes of operatively treated distal radius fractures. J Hand Surg Am. 2012 Oct;37(10):2027–2034. doi: 10.1016/j.jhsa.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Bawa H.S., Weick J., Dirschl D.R. Anti-osteoporotic therapy after fragility fracture lowers rate of subsequent fracture: analysis of a large population sample. J Bone Joint Surg Am. 2015 Oct 7;97(19):1555–1562. doi: 10.2106/JBJS.N.01275. [DOI] [PubMed] [Google Scholar]
- 6.Soen S., Usuba K., Crawford B., Adachi K. Family caregiver burden of patients with osteoporotic fracture in Japan. J Bone Miner Metabol. 2021 Jul;39(4):612–622. doi: 10.1007/s00774-020-01197-9. [DOI] [PubMed] [Google Scholar]
- 7.Reid I.R., Horne A.M., Mihov B., et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med. 2018 Dec 20;379(25):2407–2416. doi: 10.1056/NEJMoa1808082. [DOI] [PubMed] [Google Scholar]
- 8.Siris E.S., Chen Y.T., Abbott T.A., et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004 May 24;164(10):1108–1112. doi: 10.1001/archinte.164.10.1108. [DOI] [PubMed] [Google Scholar]
- 9.Reid I.R., Gamble G.D., Mesenbrink P., Lakatos P., Black D.M. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metabol. 2010 Sep;95(9):4380–4387. doi: 10.1210/jc.2010-0597. [DOI] [PubMed] [Google Scholar]
- 10.Watts N.B., Bilezikian J.P., Camacho P.M., et al. American association of clinical endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010 Nov-Dec;16(Suppl 3):1–37. doi: 10.4158/ep.16.s3.1. Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid I.R., Brown J.P., Burckhardt P., et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002 Feb 28;346(9):653–661. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 12.Eastell R., Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017 Nov;5(11):908–923. doi: 10.1016/S2213-8587(17)30184-5. [DOI] [PubMed] [Google Scholar]
- 13.Chen P., Satterwhite J.H., Licata A.A., et al. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005 Jun;20(6):962–970. doi: 10.1359/JBMR.050105. [DOI] [PubMed] [Google Scholar]
- 14.Diez-Perez A., Naylor K.E., Abrahamsen B., et al. International osteoporosis foundation and European calcified tissue society working group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int. 2017 Mar;28(3):767–774. doi: 10.1007/s00198-017-3906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujimoto M., Chen P., Miyauchi A., Sowa H., Krege J.H. PINP as an aid for monitoring patients treated with teriparatide. Bone. 2011 Apr 1;48(4):798–803. doi: 10.1016/j.bone.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Black D.M., Reid I.R., Boonen S., et al. The effect of 3 versus 6 Years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-pivotal fracture trial (PFT) J Bone Miner Res. 2012 Feb;27(2):243–254. doi: 10.1002/jbmr.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black D.M., Reid I.R., Cauley J.A., et al. The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT) J Bone Miner Res. 2015 May;30(5):934–944. doi: 10.1002/jbmr.2442. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama A., Major G., Holliday E., Attia J., Bogduk N. Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int. 2016 Mar;27(3):873–879. doi: 10.1007/s00198-015-3443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoji K.E., Earp B.E., Rozental T.D. The effect of bisphosphonates on the clinical and radiographic outcomes of distal radius fractures in women. J Hand Surg Am. 2018 Feb;43(2):115–122. doi: 10.1016/j.jhsa.2017.09.006. [DOI] [PubMed] [Google Scholar]