Abstract
This study developed a novel, sensitive and selective LC-MS/MS method for the concurrent determination of DCB and VTX in rat plasma using encorafenib as internal standard (IS). To identify DCB, VTX, and IS, the positive multiple reaction monitoring (MRM) mode was used. Chromatographic separation was carried out using a reversed-phase Agilent Eclipse plus C18 column (100 mm × 2.1 mm, 3.5 µm) and an isocratic mobile phase made up of water with 0.1% formic acid and acetonitrile (50:50, v/v, pH 3.2) at a flow rate of 0.30 mL/min for 3.0 min. Prior to analysis, the DCB and VTX with the IS were extracted from plasma using the solid-phase extraction (SPE) method. High recovery rates for DCB, VTX and IS were achieved using the C18 cartridge without interference from plasma endogenous. The developed method was validated as per the FDA guidelines over a linear concentration range in rat plasma from 5–3000 and 5–1000 ng/mL for DCB and VTX, respectively with r2 ≥ 0.998. For both drugs, the lower limits of detection (LLOD) were 2.0 ng/mL. After the HLOQ sample was injected, less than 20% of the LLOQ of DCB, VTX, and less than 5% of the IS carry-over in the blank sample was attained. The overall recoveries of DCB and VTX from rat plasma were in the range of 90.68–97.56%, and the mean RSD of accuracy and precision results was ≤6.84%. For the first time, the newly developed approach was effectively used in a pharmacokinetic study on the simultaneous oral administration of DCB and VTX in rats that received 15.0 mg/kg of DCB and 100.0 mg/kg of VTX.
Keywords: LC–MS/MS, Acute myeloid leukemia, Decitabine, Venetoclax, Rat plasma, Pharmacokinetics
1. Introduction
An excessive growth of immature white blood cells (myeloblasts) in the bone marrow and in circulation, which has a negative impact on the hematopoiesis process, distinguishes acute myeloid leukemia (AML), a hematologic cancer (Totiger et al., 2023). About 80% of cases of leukemia in adults are AML, which is the most prevalent kind (Cancer Stat Facts, 2023). AML is a complicated and heterogeneous tumor that advances quickly and, if untreated, can be fatal within weeks or months (Ediriwickrema et al., 2022, Dohner et al., 2015, McGrattan et al., 2007, Lazarus et al., 1989). Hematological cancers are now commonly treated with small-molecule inhibitor-based targeted anticancer treatments. Specifically for the treatment of AML and chronic lymphocytic leukemia (CLL), Venclexta a novel, orally bioavailable small-molecule inhibitor for selective targeting of B-cell lymphoma-2 (BCL-2), demonstrates increased efficacy and superior safety profiles (Eyre et al., 2020, Juarez-Salcedo et al., 2019, Konopleva et al., 2016). Venetoclax is a promising agent for the treatment of B-cell malignancies due to its special qualities, particularly its high selectivity for the BCL-2 protein and decreased hematological toxicity when compared to other medications in its class (Deeks, 2016). A DNA methyltransferase inhibitor, decitabine has anti-cancer properties that work by preventing DNA methylation, which causes DNA hypomethylation and causes gene re-expression and cellular differentiation (Contieri et al., 2020, Wei et al., 2021, DiNardo et al., 2019, Ball et al., 2020, Azizi et al., 2020). It has been broadly utilized in clinic for the treatment of AML, CLL and myelodysplastic syndrome (Contieri et al., 2020). The US-FDA approved venetoclax with decitabine as an effective treatment in November 2018 for newly diagnosed AML patients (FDA Approves, 2018). In May 2019, the treatment was prolonged to all adult patients with CLL or small lymphocytic leukemia (SLL) (Drugs, 2019).
Few mass spectrometry-based methods, including liquid chromatography with tandem mass spectrometry (LC-MS/MS) have been published for the quantification of individually DCB (Xu et al., 2012, Liu et al., 2006) and VTX (Yang et al., 2022) or in combination with other anti-cancer agents (Reddy et al., 2021, Sai Prudhvi et al., 2021, Zhang et al., 2013). Bioanalytical method for determination of DCB and VTX in biological samples, have not yet been reported. The goal of this work was to create a fast and accurate analytical approach for the simultaneous quantitation of DCB and VTX in rat plasma with the application to a pharmacokinetic investigation following SPE. In this study rats received 15.0 mg/kg of DCB and 100.0 mg/kg of VTX.
2. Experimental
2.1. Chemicals and reagents
Analytical-grade supplies were used for all reagents and solvents. Med. Chem. Express (USA) provided the reference standards for DCB (99.0%), VTX (99.0%), and encorafenib (ENF, internal standard, IS, 98.7%). HPLC-grade acetonitrile, methanol and formic acid were procured from Sigma-Aldrich (NJ, USA). HPLC-grade water was obtained from an in-house Milli-Q plus purification system procured from the Millipore Company (Millipore, USA). Rat plasma devoid of drugs was obtained from the King Saud University College of Pharmacy's Animal Care Center.
2.2. LC-MS/MS conditions
For chromatographic separation, an Acquity water UPLC with model code (UPH) and serial number (H10UPH) was utilized, and for mass analysis of eluted analytes peaks, an Acquity TQD MS with model code (TQD) and serial number (QBB1203). Liquid chromatographic analytical parameters that involved separated DCB, VTX and ENF (IS), including stationary phase nature, mobile phase composition and pH were optimized. DCB, VTX and IS were separated on a Eclipse plus C18 column (100 mm × 2.1 mm, 3.5 µm; Agilent Technologies, Palo Alto, CA, USA) in isocratic mode. The mobile phase composed of 50% acetonitrile and 50% aqueous solution (0.1% formic acid in water) was utilized. The pH of the solution containing 0.1% formic acid was attuned to 3.2. Membrane filters (0.22 µm) from Chrom Tech (Kent, UK) were used to filter the solvents. The sample injection volume was 5.0 µL and total elution time was 3.0 min.
Triple quadrupole mass analyzer (TQD MS) mass spectrometry parameters were optimized to provide good separation of DCB, VTX, and ENF with good sensitivity. TQD MS in positive mode (ESI+) was used to estimate the DCB, VTX, and ENF. Utilizing IntelliStart® software, the tuning parameters for DCB, VTX, and ENF were modified manually in combined mode (fluidics and LC) to improve chromatographic peak properties including signal intensity and selectivity. At 350 °C, nitrogen (650 L/H) was employed as a drying gas. Cone gas flow was maintained at 100L/H. The fragmentation cell's collision gas was argon (0.14 mL/min). To improve the selectivity and sensitivity of the developed approach, the estimation of DCB, VTX, and ENF was done using the multiple reaction monitoring (MRM) mass analyzer mode. The cone voltages for DCB, VTX and ENF were set as 8 (V), 50 (V) and 54 (V), respectively. Capillary voltage, extractor voltage, and RF lens were set at 4 (kV), 3.0 (V), and 0.1 (V), respectively. A 150 °C source temperature was chosen.
2.3. Preparation of stock, standard, calibrators and quality control samples
DCB, VTX, and ENF were freely soluble in dimethyl sulfoxide (DMSO); accordingly, the first stocks were prepared for each of these compounds in DMSO at a concentration of 1 mg/mL and stored at −20 °C. Using ultrapure water, working solutions of DCB and VTX in concentrations of 0.05 µg/mL, 0.5 µg/mL, 5.0 µg/mL, and 20.0 µg/mL, were prepared from the stock solutions. ENF (IS) working solution was prepared in DMSO and diluted with ultrapure water to 40.0 µg/mL. Prior to analysis, the plasma samples were thawed at room temperature. The intermediate solutions were used to create calibrators in blank rat plasma at doses of 5.0, 15.0, 50.0, 100.0, 150.0, 200.0, 300.0, 400.0, 500.0, 900.0, 1500.0, 2400.0, and 3000.0 ng/mL for DCB and 5.0, 10.0, 15.0, 50.0, 80.0, 100.0, 150.0, 200.0, 250.0, 300.0, 400.0, 500.0, 800.0, and 1000.0 ng/mL for VTX. By spiking the appropriate volume of the intermediate solutions with blank rat plasma, three levels; low (LQC), medium (MQC), and high (HQC) for each analyte quality control sample were prepared, with concentrations of 15.0, 1400.0, and 2300.0 ng/mL for DCB, and 15.0, 500.0, and 800.0 ng/mL for VTX.
2.4. Sample preparation
A 50.0 µL aliquot of plasma was placed in 2.0 mL disposable polypropylene micro centrifuge tubes together with 50.0 µL of the IS solution (2.0 µg/mL). Each tube was vortexed for 30 s after being diluted to 750.0 µL with ultrapure water. A vacuum manifold (VacElute, Harbor City, CA, USA) was used to attach a C18 cartridge solid-phase extraction, which was preconditioned with 2 × 500.0 µL of methanol and 2 × 500.0 µL of deionized water. The cartridges were carefully observed to prevent drying. After loading the cartridges with the blank and plasma sample, a vacuum was applied to achieve a flow rate of 0.5 mL/min. The cartridges were rinsed twice, first with 500.0 µL of a 0.05 M hydrochloric acid solution containing 5% methanol and then by 500.0 µL methanol. The cartridges were dried for 3.0 min at 15 psi of vacuum. 2 × 100.0 µL of ammonium hydroxide in methanol (5:95, v/v) were used to elute DCB and VTX. The eluates were evaporated to dryness under nitrogen at 35 °C and then reconstituted in 2 × 100.0 µL of 1.0% DMSO in water by vortex mixing (1 min). 5.0 µL of the resultant solution was then injected into the LC-MS/MS system after being transferred to autosampler vials.
2.5. Pre-study validation
The developed LC-MS/MS approach has been validated according to US-FDA bioanalytical method validation guidelines (US-FDA Guidelines, 2018). Determining method selectivity and carry-over, precision and accuracy, extraction recovery, dilution integrity, and matrix effect, were the evaluated validation parameters in the rat plasma. The least squares statistical method was used to derive the calibration curve equations (y = mx + b). The linear fit was verified using the coefficient of determination (r2) value, which showed linearity in the 5–3000 ng/mL range for DCB and the 5–1000 ng/mL range for VTX.
In blank rat plasma from six different batches, endogenous interference at the retention times of DCB, VTX, and IS was investigated to check method selectivity. It was possible to evaluate intra-day and inter-day accuracy and precision by analyzing a calibration curve (in triplicate) and spiked plasma samples at the lower limit (LLOQ), in addition to three different QC levels (L-M-H), on three different days, (n = 6). For DCB, the levels were tested at 5.0 ng/mL (LLOQ), 15.0 ng/mL (LQC), 1400.0 ng/mL (MQC), and 2300.0 ng/mL (HQC), whereas for VTX, the levels were tested at 5.0 ng/mL (LLOQ), 15.0 ng/mL (LQC), 500.0 ng/mL (MQC), and 800.0 ng/mL (HQC). The RSD must meet the LLOQ's acceptance requirements and should be less than 20%. The RSD for all other concentrations must be less than 15% (US-FDA Guidelines, 2018).
Carry-over was tested by injecting a blank sample without IS following the HLOQ containing the DCB and VTX and IS to ensure that it had no impact on the precision of the study samples. This procedure was carried out six times. Less than 20% of the LLOQ of each drug and less than 5% of the IS should be the maximum allowed for the detected response.
By contrasting the results of extracted plasma samples at three levels (LQC, MQC, and HQC) with those obtained by direct injection of the same amount of the two analytes in the standard solutions in triplicate, the recoveries of DCB and VTX were evaluated. Over the concentration range, the recovery must be constant and reproducible. Additionally, the extraction recovery of the IS at the same concentration level of the method was calculated.
Plasma samples were diluted twice and four times with blank plasma after being spiked with the HLOQ for DCB (4500.0 ng/mL) and VTX (1500.0 ng/mL). To determine whether dilution has an impact on accuracy and precision, the final concentration was compared to the nominal concentration. Precision and accuracy should be within 15% (US-FDA Guidelines, 2018).
During method validation, it is important to evaluate the matrix factor (MF) between various batches of sample matrices. For DCB, VTX, and the IS, the matrix factor (MF) was determined in six different batches of blank plasma. The ratio between the peak area in the presence of a blank spiked with analytes following solid phase extraction and the lack of matrix (pure analytes solution) was used to determine the MF. The ratio of the analytes' MF to the IS MF must be within 15% of RSD to be considered “IS normalized MF.”
After subjecting the QC samples at QCL, QCM, and QCH to various storage conditions (temperature and time), the stability of DCB and VTX was evaluated. The applied conditions included short-term stability for 24 h at room temperature and for 24 h at 10 °C in an autosampler. After 30 days of QC storage at −80 °C, long-term stability was evaluated. Three cycles of freezing and thawing were used to test freeze and thaw stability in comparison to freshly manufactured QCs. All sample accuracies should be ±15% to be considered as stable.
2.6. Pharmacokinetic study
A pharmacokinetic (PK) investigation was carried out in six Wistar healthy male rats (180–220 g) in order to assess the capability of the developed method to determine DCB and VTX concentration in vivo samples. The Institutional Research Ethics Committee (REC) at King Saud University, which has the ethics reference number (SE-19–109), approved all experimental procedures. Before the experiment was directed, rats were acclimatized for 7 days to laboratory environments. Dietary was restrictions for 12 h before to the experiment, although water was freely available. On the day of the experiments, rats were treated with a single oral dose of DCB (15.0 mg/kg) (Xu et al., 2012) and VTX (100.0 mg/kg) (European, 2012) dissolved in 1% DMSO/saline.
At the following time intervals: zero (before administration), 0.15, 0.5, 1, 2, 4, 6, 8, 12, 18 and 24 h, blood samples (300.0 µL) were collected into tubes containing ethylenediamine tetra-acetic acid dipotassium (EDTA K2) (anticoagulant). The samples were immediately centrifuged for 10.0 min at 4 °C at 3500 rpm. The resulting plasma was kept at −80 °C until analysis. The sample was prepared using the same extraction technique that was discussed under the calibration standards preparation. Using the PK Solver Add-In software, the PK parameters were calculated by fitting the data to a noncompartmental analysis (NCA) model (Zhang et al., 2010).
3. Results
3.1. Optimization of chromatographic conditions and MS detections
The multiple reaction monitoring (MRM) mass analyzer was used in the positive mode for the estimation of DCB, VTX, and ENF in order to increase the developed approach's selectivity and sensitivity. DCB peak was quantified using MRM mode transitions from 229 → 113 (CV: 8 and CE: 10) and 229 → 98.97 (CV: 8 and CE: 22) (Fig. 1A and Table 1).VTX peak was quantified using MRM mode transitions from 868 → 177 (CV: 50 and CE: 38) and 868 → 321 (CV: 50 and CE: 44) (Fig. 1B and Table 1). MRM mass transitions for ENF were 540 → 359 (CV: 54 and CE: 46) and 540 → 116 (CV: 54 and CE: 44) (Fig. 1C and Table 1). To get the best separation and the maximum signal for DCB, VTX, and ENF, the chromatographic parameters, including the types of stationary phases, the nature of the mobile phase, and its composition, were changed through numerous trials. In an isocratic mode, several mobile phase mixtures of ammonium format buffer, ammonium acetate buffer, 0.1% acetic acid, 0.1% formic acid, and 0.1% trifluoracetic acid in water were evaluated for peak shape, peak area, response, and analysis time. Higher pH values caused peak tailing and prolonged elution times, so the 0.1% formic acid solution in the aqueous mobile phase had its pH reduced to 3.2. Also, the effects of a chosen mobile phase with various methanol or acetonitrile percentages (10–90%) and water each mixed with 0.1% formic acid were studied. The separation and retention times of DCB, VTX and IS were significantly influenced by the percentage of acetonitrile in the mobile phase. Long running times were observed by a decreasing acetonitrile percentage, while overlapping peaks and poor separation were caused by an increasing percentage of acetonitrile. Due to the resolution of the chromatographic peaks being decreased at higher ACN concentrations and elution durations being prolonged at lower ACN concentrations, the optimal mobile phase was composed of 50% 0.1% formic acid in water and 50% ACN. With an improved signal-to-noise ratio, the optimized mobile phase of 0.1% formic acid in water (50%) and 50% acetonitrile was found to be appropriate for the chromatographic separation of the analytes at a flow rate of 0.3 mL/min.
Fig. 1.
Multiple reaction monitoring (MRM) mass spectra and the expected fragmentation pathway of A) decitabine, B) venetoclax, and C) encorafenib (IS).
Table 1.
LC-MS/MS optimized parameters for the determination of decitabine, venetoclax and IS.
| Drug | Retention time (min) | Ion mode | Precursor (m/z) | Quantification trace (m/z) | Qualification trace (m/z) | Cone Voltage (V) | Collision energy (CE, eV) |
|---|---|---|---|---|---|---|---|
| DCB | 0.98 | +ve | 229.03 | 113.03 | 98.94 | 8 | 10/22 |
| VTX | 2.44 | +ve | 868.28 | 177.05 | 321.13 | 50 | 38/44 |
| ENF (IS) | 1.48 | +ve | 540.10 | 359.10 | 116.00 | 54 | 46/44 |
For chromatographic separation, various stationary phases, both polar and non-polar, were tested using various column packs, with different dimensions such as hydrophilic interaction liquid chromatography (HILIC) DIOL column (100 × 2.1 mm,1.7 µm, Fortis™ technologies ltd.; USA), pentafluorophenyl (PFP) column (100 × 2.0 mm, 3 µm, Phenomenex®; USA), polar imidazole column (50 × 2.1 mm, 1.8 µm, Sepax technologies; USA), and biphenyl column (50 × 2.1 mm, 2.7 µm, Raptor™; USA), but such stationary phases were unable to retain or separate DCB, VTX, or ENF; however, good results were achieved using Eclipse plus C18 column (100 mm × 2.1 mm, 3.5 µm; Agilent Technologies Palo Alto, CA, USA). As well as, we investigate the use of different internal standards, such as pemigatinib, binimetinib, sulpride encorafenib, repaglinide and chloroquine, however, these internal standards either produced poor peaks or lead to overlapping with DCB and VTX. Encorafenib was selected as the method's IS because it has chemical similarities to DCB and VTX, well separates from both drugs with a short run time (1.5 min), and exhibits good extraction recovery and performance characteristics.
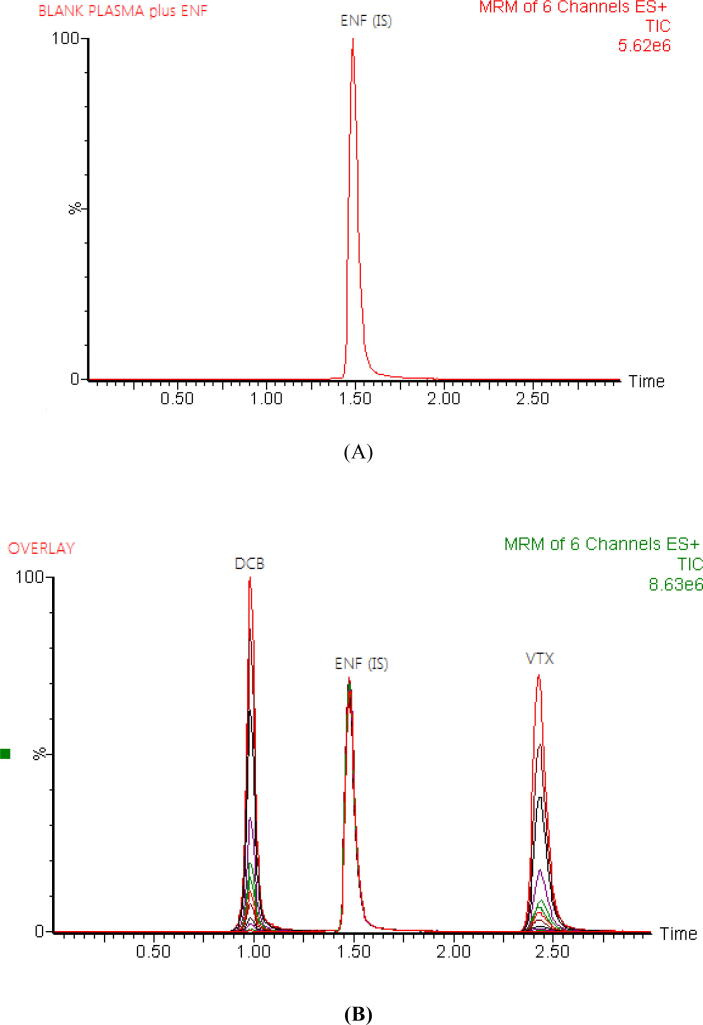
The SPE method was an effective protein precipitation extraction procedure in the current study, resulting in high recoveries of the DCB, VTX, and IS from rat plasma with less solvent consumption, less time spent, and low background noise (Hefnawy et al., 2021). For plasma sample cleanup, five different SPE cartridges including Water oasis HLB, C18, C8, C2, CN and were investigated. Numerous solvents' eluting capacities toward DCB, VTX, and IS were investigated. Only ammonium hydroxide in methanol (5:95, v/v) rather than a mixture of methanol with water or acetonitrile was able to disrupt all types of interactions in the case of DCB, VTX, and IS and thus to extract them from the C18 sorbent. With the C18 cartridge, high recoveries and clear chromatograms for DCB, VTX, and IS were attained. The recoveries ranged from 90.68 to 94.99 and 93.15 to 97.56 for DCB and VTX in the rat plasma, respectively. Chromatographic separation of DCB, VTX and IS was achieved with good separation over a run time of 3.0 min (Fig. 2).
Fig. 2.
Representative total ion chromatograms for blank rat plasma spiked with encorafenib (IS) at a concentration of 2000 ng/mL (A) and overlays of the LC–MS/MS analysis of decitabine (DCB), venetoclax (VTX) at concentrations of 5–3000, 5–1000 ng/ml, respectively, and encorafenib (ENF) at a concentration of 2000 ng/mL (B).
3.2. In-study validation
The developed LC-MS/MS method was completely validated in accordance with US-FDA Guidelines for the Validation of bioanalytical methods (US-FDA Guidelines, 2018). The evaluated validation characteristics in the rat plasma included method linearity and range, selectivity, precision and accuracy, carry-over, extraction recovery, matrix effect, dilution integrity, and stability.
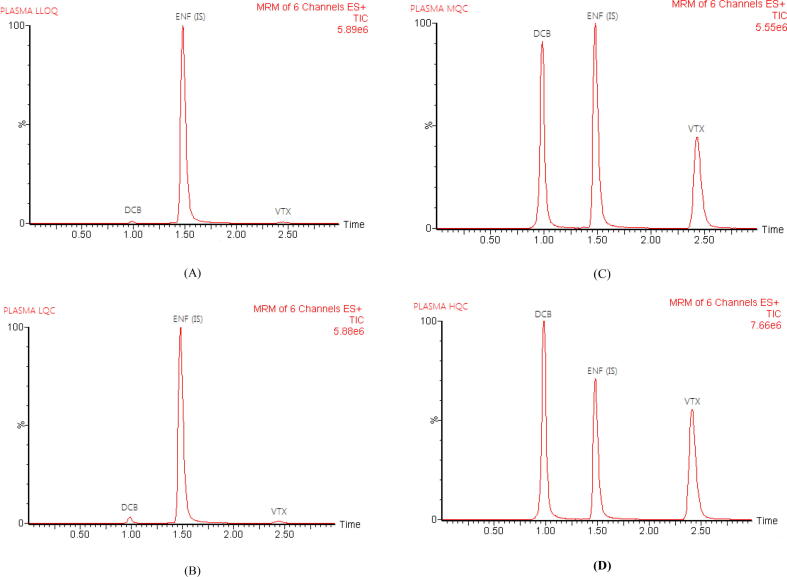
Analyzing plasma samples that had been spiked with LLOQ, QCM, and QCH as well as blank plasma samples showed that there were no interferences at the retention times of DCB, VTX, and IS, proving the developed assay's selectivity. Fig. 3 displays typical total ion chromatograms. After injection of the HLOQ sample, the carry-over in the blank sample for DCB and VTX was less than 20% of LLOQ, while the response for IS was less than 5% (US-FDA Guidelines, 2018).
Fig. 3.
Representative total ion chromatograms of rat plasma spiked with LLOQ (A), LOQ (B), MQC (C), and HQC (D); for decitabine (DCB), venetoclax (VTX) and encorafenib (ENF, IS).
The developed approach was found to have a linear range for DCB concentrations between 5 and 3000 ng/mL and VTX concentrations between 5 and 1000 ng/mL in the plasma matrix. Table 2 contains a list of the linear regression of DCB and VTX attained throughout the method validation. The regression equations attained by least squared regression for the DCB was y = 0.0046x + 0.0072, and for the VTX was y = 0.0015x + 0.0028, where y is the peak area ratio of D/IS and × is the concentration (ng/mL). The results established the linearity and reproducibility of the developed method. The LLOD of DCB and VTX in rat plasma, which was 2 ng/mL, demonstrated the efficacy of the established assay for the measurement of trace quantities of DCB and VTX in plasma.
Table 2.
Regression parameters to determine decitabine (DCB) and venetoclax (VTX) using the developed LC-MS/MS method.
| Parameters | DCB | VTX |
|---|---|---|
| Concentration range (ng/mL) | 5–3000 | 5–1000 |
| Intercept (a) | 7.29 × 10−2 | 2.88 × 10−2 |
| Slope (b) | 4.65 × 10−3 | 1.55 × 10−3 |
| Coefficient of determination (r2) | 0.999 | 0.998 |
| SY/Na | 3.92 × 10−3 | 2.94 × 10-3 |
| Sab | 1.30 × 10−3 | 1.03 × 10−3 |
| Sbc | 1.30 × 10−4 | 2.64 × 10−4 |
| LLOQ (ng/mL) | 5.0 | 5.0 |
| LLOD (ng/mL) | 2.0 | 2.0 |
SD of the residual.
SD of the intercept.
SD of the slope.
Table 3 displays the results of the DCB and VTX determination's accuracy and precision. Six replicates of the four concentrations of QC samples (LLOQ, LQC, MQC, and HQC) were employed in order to evaluate the intra- and inter-assay precision and accuracy. The values for intra-day and inter-day precision and accuracy were 2.00–6.84% and 90.27–102.78% for DCB and 2.41–6.50% and 93.23–100.67% for VTX, respectively; these values satisfy the guidelines' acceptance criteria (LLOQ within 20% and the other QCs within 15%) (US-FDA Guidelines, 2018).
Table 3.
The accuracy and precision data for the determination of decitabine (DCB) and venetoclax (VTX) in rat plasma.
| Analyte | Concentration (ng/mL) | Within-run |
Between-run |
|||
|---|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |||
| DCB | LLOQ | 5.0 | 94.60 | 6.84 | 93.62 | 5.24 |
| QCL | 15.0 | 90.27 | 5.29 | 91.68 | 4.95 | |
| QCM | 1400.0 | 94.79 | 3.37 | 94.47 | 3.18 | |
| QCH | 2300.0 | 102.78 | 2.23 | 97.46 | 3.75 | |
| VTX | LLOQ | 5.0 | 93.23 | 4.54 | 93.43 | 4.26 |
| QCL | 15.0 | 94.07 | 6.50 | 94.47 | 6.06 | |
| QCM | 500.0 | 95.66 | 4.17 | 95.35 | 2.41 | |
| QCH | 800.0 | 100.60 | 5.22 | 100.67 | 4.46 | |
| n | 6 | 18 | ||||
After SPE sample preparation, DCB and VTX were extracted from the plasma matrix and examined at three QC levels (QCL, QCM, QCH) in six replicates. The mean percent recoveries were 92.38% and 94.66%, respectively. For all of the tested samples shown in Table 4 the mean% recovery of IS was not less than 98.34%.
Table 4.
Extraction recovery for the analysis of decitabine (DCB) and venetoclax (VTX) and IS in rat plasma by the developed LC-MS/MS method.
| Nominal concentration (ng/mL) |
Decitabine |
Venetoclax |
IS | ||||
|---|---|---|---|---|---|---|---|
| 15.0 | 1400.0 | 2300.0 | 15.0 | 500.0 | 800.0 | 2000.0 | |
| Mean a | 13.60 | 1329.86 | 2103.81 | 13.99 | 487.81 | 745.26 | 1966.96 |
| RSD | 0.47 | 1.88 | 1.64 | 0.76 | 1.19 | 0.35 | 1.12 |
| Recovery (%) | 90.68 | 94.99 | 91.47 | 93.28 | 97.56 | 93.15 | 98.34 |
| Mean recovery (%) | 92.38 | 94.66 | 98.34 | ||||
Mean of six measurements.
The peak area in the presence of matrix components was divided by the peak area in the neat standard solution of the analyte to determine the matrix factor (MF) for the DCB, VTX, and IS samples (QCL, QCM, and QCH). By dividing the MF of the analyte by the MF of the IS, the IS normalized MF is calculated. The matrix's six batches had an RSD of IS-normalized MF that was less than 15%. For DCB, it was 0.75, 0.48 and 0.33 for QCL, QCM and QCH, respectively. For VTX, it was 0.51, 1.00 and 0.41 for QCL.QCM and QCH, respectively, demonstrating that any plasma enrichment or ion suppression was insignificant.
Six replicates of plasma samples spiked with high quantities of each drug beyond the linear range were processed and analyzed using dilution factors 2 and 4 to test the precision of the developed assay after dilution. The dilution integrity values of DCB and VTX were 92.83 ± 1.41 and 97.05 ± 1.09 at 2:1 dilutions, and 94.13 ± 1.06 and 95.26 ± 0.95 at 1:4 dilutions, respectively (Table 5). This approved the minimal effect of dilution on the outcomes of the method.
Table 5.
Evaluation of the dilution integrity of decitabine and venetoclax in rat plasma.
| Analyte | Spiked Conc. (ng/ mL) |
Dilution fold | Mean recovery (%) ± RSD a |
|---|---|---|---|
| Decitabine | 4500.0 | 1:2 | 92.83 ± 1.41 |
| 1:4 | 94.13 ± 1.06 | ||
| Venetoclax | 1500.0 | 1:2 | 97.05 ± 1.09 |
| 1:4 | 95.26 ± 0.95 |
Mean recovery (%) ± RSD of six determinations.
Through the study of three QC samples (QCL, QCM, and QCH) of each drug following the application of the various storage conditions, the stability of DCB and VTX was investigated. The various parameters were tested for 24 h at ambient temperature, 24 h in an autosampler at 10 °C, three cycles of freezing and thawing after being stored at −80 °C, and 30 days at −80 °C. As presented in Table 6, the calculated accuracies were within the range of 93.35–103.12% for DCB and the range of 91.91–105.13% for VTX of the nominal concentrations which lies within the acceptable range (US-FDA Guidelines, 2018).
Table 6.
Stability results for decitabine (DCB) and venetoclax (VTX) in rat plasma at different conditions.
| Analyte | Concentration (ng/mL) |
Short term stability at room temperature (24 h) |
Auto sampler stability at −10 °C (24 h) |
Freeze and thaw stability at −80 °C (3 cycles) |
Long term stability at −80 °C (30 days) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | |||
| Decitabine | QCL | 15.0 | 97.28 | 5.69 | 95.04 | 2.77 | 98.55 | 4.25 | 96.02 | 5.28 |
| QCM | 1400.0 | 95.87 | 3.47 | 103.12 | 4.37 | 93.81 | 6.48 | 94.42 | 1.88 | |
| QCH | 2300.0 | 93.35 | 8.14 | 95.83 | 0.51 | 98.73 | 3.18 | 95.40 | 2.90 | |
| Venetoclax | QCL | 15.0 | 99.15 | 2.90 | 97.20 | 3.16 | 91.91 | 4.83 | 94.46 | 2.94 |
| QCM | 500.0 | 95.74 | 3.68 | 94.62 | 1.08 | 93.16 | 3.79 | 105.13 | 5.14 | |
| QCH | 800.0 | 102.02 | 7.34 | 93.79 | 5.25 | 103.94 | 1.57 | 95.37 | 1.53 | |
| n | 3 | 3 | 3 | 3 | ||||||
3.3. Application to pharmacokinetic study
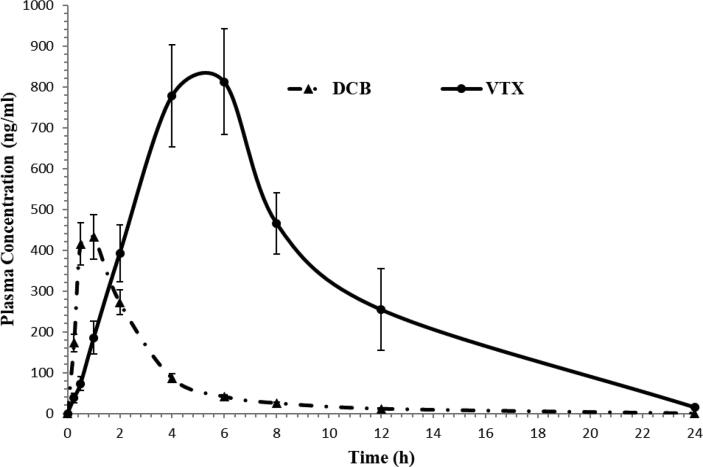
Following oral administration of 15.0 mg/kg DCB and 100.0 mg/kg VTX to six Wistar healthy male rats in a fasting condition, the validated assay was successfully used to assess DCB and VTX in rat plasma for a pharmacokinetic study. As far as we are aware, this study is the first to use the LC-MS/MS technique to a pharmacokinetic study and to quantify the quantities of DCB and VTX in rat plasma. Fig. 4 show the normal MRM chromatograms of rat plasma at 1.0 h for DCB and at 6.0 h for VTX following oral treatment. The established assay has reportedly been proven to be adequate for a good resolution for DCB, VTX, and IS. Fig. 5 shows the mean plasma concentration–time profiles of DCB and VTX, and Table 7 summarizes the pharmacokinetic parameters from non-compartment model analysis.
Fig. 4.
Typical multiple reaction monitoring (MRM) chromatograms for in vivo rat plasma sample 1.0 h after oral administration of 15.0 mg/kg decitabine (A) and 6.0 h after oral administration 100.0 mg/kg enetoclax (B) with ENF (IS).
Fig. 5.
Mean plasma concentration–time profile of decitabine and venetoclax in rats after a single oral dose of 15.0 mg/kg decitabine (DCB) and 100.0 mg/kg venetoclax (VTX) (n = 6, mean ± SD).
Table 7.
The pharmacokinetic parameters of decitabine and venetoclax in rat plasma after oral administration of 15 mg/kg decitabine and 100 mg/kg venetoclax (n = 6, mean ± SD).
| Parsmeter | Unit | Decitabine | Venetoclax |
|---|---|---|---|
| AUC0-ta | ng/mL.h | 1287.40 ± 151.47 | 7469.69 ± 1355.19 |
| AUC0-∞b | ng/mL.h | 1343.61 ± 151.47 | 7539.22 ± 1366.34 |
| Cmaxc | ng/mL | 432.27 ± 54.43 | 812.13 ± 129.40 |
| Tmaxd | h | 1 | 6 |
| Cl/Fe | ng/mL.h | 11.28 ± 1.33 | 13.56 ± 2.24 |
| t1/2kelf | h | 3.32 ± 0.16 | 3.17 ± 0.03 |
| MRT0-∞g | h | 3.16 ± 0.04 | 7.53 ± 0.50 |
* Data are presented as mean ± SD.
Area under the curve up to the last sampling time.
Area under the curve extrapolated to infinity.
Maximum plasma concentration.
Time taken to reach the maximum plasma concentration.
Total clearance of drug from plasma after oral administration.
Half-life in elimination phase.
Mean residence time.
4. Discussion
There is an urge need to developed a simple bioanalytical method with full validation of the DCB and VTX using LC-MS/MS technique have a several advantages; high limit of detection (2.0 ng/mL), small volume of plasma (50.0 µL), effective solid phase extraction procedure (C18 cartridge) and short run time (3.0 min). The new developed validated method has a wide linear range, and five-fold lower concentration in the limit of quantification than other bioanalytical methods (Liu et al., 2006, Reddy et al., 2021).
The selection of an appropriate stationary phase for DCB and VTX separation was the first challenge in this study. Several mobile phase compositions were investigated in an isocratic mode regarding peak shape, peak area, response, analysis time to achieve baseline separation of DCB and VTX. With an improved signal-to-noise ratio, the optimized mobile phase of 0.1% formic acid in water (50%) and 50% acetonitrile was found to be appropriate for the chromatographic separation of the DCB, VTX and IS in rat plasma at a flow rate of 0.3 mL/min.
We believe that this newly established method is the first study to be used with the requisite accuracy and precision for monitoring the pharmacokinetic study of DCB and VTX in rats given 15 mg/kg of decitabine and 100 mg/kg of venetoclax, respectively. The maximum plasma concentration (Cmax) for DCB and VTX was 432.27 ± 54.43 and 812.13 ± 129.40 ng/mL; respectively. The AUC0-∞ for DCB and VTX was found to be 1343.61 ± 151.47 and 7539.22 ± 1366.34 ng/mL.h, respectively. These values obtained in the current assay for DCB and VTX are in close concurrence with the outcomes from other reports (Xu et al., 2012, Liu et al., 2006, Reddy et al., 2021). For Tmax, and AUC0-∞ parameters, it has been seen that the values acquired in the current investigation for VTX not a long way from different study (Reddy et al., 2021). On the other hands, the elimination half-life (t1/2kel), CL, and mean residence time (MRT0-∞) parameters for DCB is near to that represented in published paper (Liu et al., 2006).
5. Conclusions
A sensitive and fast fully validated LC-MS/MS bioanalytical method for the simultaneous determination of DCB and VTX in rat plasma after solid-phase extraction procedure has been developed. The newly developed approach was successfully applied for the first time, to a pharmacokinetic study on concurrent oral administration of DCB and VTX in rats received 15.0 mg/kg of DCB and 100.0 mg/kg of VTX. The developed method stands out for its high extraction recovery and absence of matrix interference. Findings further demonstrated the high sensitivity of the developed assay, which allowed for effective routine tests in pharmacokinetic investigations with a low detection limit of 2.0 ng/mL and a run time of 3.0 min.
Author contributions
All authors contributed to data analysis and drafting and revising the article gave final approval of the version to be published, and agreed to be accountable for all aspects of this work.
Availability of data and material
All data and material are available upon reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation “Ministry of Education” in Saudi Arabia for funding this research work through project number IFKSUOR3-374-1.
Footnotes
Peer review under responsibility of King Saud University.
References
- Azizi A., Ediriwickrema A., Dutta R. Venetoclax and hypomethylating agent therapy in high risk myelodysplastic syndromes: a retrospective evaluation of a real-world experience. Leuk Lymphoma. 2020;61:2700–2707. doi: 10.1080/10428194.2020.1775214. [DOI] [PubMed] [Google Scholar]
- Ball B.J., Famulare C.A., Stein E.M. Venetoclax and hypomethylating agents (HMAs) induce high response rates in MDS, including patients after HMA therapy failure. Blood Adv. 2020;4:2866–2870. doi: 10.1182/bloodadvances.2020001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Stat Facts: Leukemia — Acute Myeloid Leukemia (AML), 2023. https://www.ncbi.nlm.nih.gov/books/NBK507875/#:∼:text=Acute%20myeloid%20leukemia%20(AML)%20is,erythropoiesis%20and%20bone%20marrow%20failure. (Accessed on 10 May 2023).
- Contieri B., Duarte B., Lazarini M. Updates on DNA methylation modifiers in acute myeloid leukemia. Ann. Hematol. 2020;99:693–701. doi: 10.1007/s00277-020-03938-2. [DOI] [PubMed] [Google Scholar]
- Deeks E.D. Venetoclax: First global approval. Drugs. 2016;76:979–987. doi: 10.1007/s40265-016-0596-x. [DOI] [PubMed] [Google Scholar]
- DiNardo C.D., Pratz K., Pullarkat V. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohner H., Weisdorf D.J., Bloomfield C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- Drugs.com. 2019. Venclexta Approval History. https://www.drugs.com/history/venclexta.html. (Accessed on 10 May 2023).
- Ediriwickrema A., Gentles A.J., Majeti R. Single cell genomics in AML: Extending the frontiers of AML research. Blood. 2022;141:345–355. doi: 10.1182/blood.2021014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency, venetoclax Assessment report, EMA/725631/2016 Committee for Medicinal Products for Human Use (CHMP), 13 October 2016. https://www.ema.europa.eu/en/documents/assessment-report/venclyxto-epar-public-assessment-report_en.pdf (Accessed on 10 May 2023).
- Eyre T.A., Roeker L.E., Fox C.P., Gohill S.H., Walewska R., Walter H.S., Forconi F., Broom A., Arumainathan A., Brander D.M. The efficacy and safety of venetoclax therapy in elderly patients with relapsed, refractory chronic lymphocytic leukaemia. Br. J. Haematol. 2020;188:918–923. doi: 10.1111/bjh.16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA approves venetoclax in combination for AML in adults | FDA https://www.fda.gov/drugs/fda-approves-venetoclax-combination-aml-adults. (Accessed on 10 May 2023).
- Hefnawy M., Al-Majed A., Alrabiah H., Algrain N., Mohammed M., Bin Jardand Y. Rapid and sensitive LC-MS/MS method for the enantioanalysis of verapamil in rat plasma using superficially porous silicaisopropyl-cyclofructan 6 chiral stationary phase after SPE: Application to a stereoselective pharmacokinetic study. J. Pharm. Biomed. Anal. 2021;201 doi: 10.1016/j.jpba.2021.114108. [DOI] [PubMed] [Google Scholar]
- Juarez-Salcedo L.M., Desai V., Dalia S. Venetoclax: evidence to date and clinical potential. Drugs Context. 2019;8 doi: 10.7573/dic.212574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M., Pollyea D.A., Potluri J., Chyla B., Hogdal L., Busman T., McKeegan E., Salem A.H., Zhu M., Ricker J.L. Efficacy and biological correlates of response in a phase II study of Venetoclax monotherapy in patients with acute Myelogenous Leukemia. Cancer Discov. 2016;6:1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus H.M., Vogler W.R., Burns C.P., Winton E.F. High-dose cytosine arabinoside and daunorubicin as primary therapy in elderly patients with acute myelogenous leukemia. A phase I-II study of the Southeastern Cancer Study Group. Cancer. 1989;63:1055–1059. doi: 10.1002/1097-0142(19890315)63:6<1055::aid-cncr2820630602>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Liu Z., Marcucci G., Byrd J.C., Grever M., Xiao J., Chan K.K. Characterization of decomposition products and preclinical and low dose clinical pharmacokinetics of decitabine (5-aza-2'-deoxycytidine) by a new liquid chromatography/tandem mass spectrometry quantification method. Rapid Commun Mass Spectrom. 2006;20:1117–1126. doi: 10.1002/rcm.2423. [DOI] [PubMed] [Google Scholar]
- McGrattan P., Humphreys M., Hull D., McMullin M.F. Transformation of cytogenetically normal chronic myelomonocytic leukaemia to an acute myeloid leukaemia and the emergence of a novel +13, +15 double trisomy resulting in an adverse outcome. Ulster Med. J. 2007;76:131–135. [PMC free article] [PubMed] [Google Scholar]
- Reddy A., Jadav T., Sahu A.K., Sengupta P. LC–MS/MS bioanalytical method for quantification of binimetinib and venetoclax, and their pharmacokinetic interaction. Bioanalysis. 2021;14:75–86. doi: 10.4155/bio-2021-0207. [DOI] [PubMed] [Google Scholar]
- Sai Prudhvi N., Venkateswarlu B.S., Kumudhavalli M.V., Muruganantham V. Bio-analytical method development and validation for the simultaneous estimation of Decitabine and Cedazuridine in human plasma using LC-MS/MS. Int. J. App. Pharm. 2021;13:257–262. [Google Scholar]
- Totiger T.M., Ghoshal A., Zabroski J., Sondhi A., Bucha S., Jahn J., Feng Y., Taylor J. Targeted therapy development in acute myeloid Leukemia. Biomedicines. 2023;11:641–652. doi: 10.3390/biomedicines11020641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US-FDA, Bioanalytical method validation guidance for industry. 2018. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (Accessed on 10 May 2023).
- Wei Y., Xiong X., Li, Lu W., He X., Jin X., Sun R., Lyu H., Yuan T., Sun T., Zhao M. Low-dose decitabine plus venetoclax is safe and effective as post-transplant maintenance therapy for high-risk acute myeloid leukemia and myelodysplastic syndrome. Cancer Sci. 2021;112:3636–3644. doi: 10.1111/cas.15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Lv S., Qiao M., Fu Y., Jiang X., Jin Y., Li C., Yuan B. Development and validation of a liquid chromatography-tandem mass spectrometry method for quantification of decitabine in rat plasma. J. Chromatogr. B Anal. Tech. Biomed. Life Sci. 2012;15, 899:81–85. doi: 10.1016/j.jchromb.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Yang X., Mei C., He X., He L., Lu X., Tong H., Lou Y. Quantification of venetoclax for therapeutic drug monitoring in chinese acute myeloid Leukemia patients by a validated UPLC-MS/MS method. Molecules. 2022;28(27):1607–1619. doi: 10.3390/molecules27051607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Huo M., Zhoua J., Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010;99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Sun J., Gao Y., Kong Y., Xu Y., Jia W., Liao C., Zhang P., Lian H., Han X. An HPLC-MS/MS method for simultaneous determination of decitabine and its valyl prodrug valdecitabine in rat plasma. J Chromatogr. B Anal. Tech. Biomed Life Sci. 2013;1917–918:78–83. doi: 10.1016/j.jchromb.2012.12.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are available upon reasonable request.