Abstract
Streptococcus gallolyticus (SG) is a Gram-positive cocci found as commensal gut flora in animals and humans. SG has emerged as a cause of disease in young poults between 1 and 3 wk of age. SG is associated with septicemia resulting in acute mortality with no premonitory signs in turkeys. Three SG isolates were obtained from clinical field cases of acute septicemia of commercial turkeys and used in three independent experiments. In Experiment 1, embryos were inoculated 25 d of embryogenesis with varying concentrations of SG1, SG2, or SG3. In Experiment 2, day of hatch, poults were inoculated with varying concentrations using different routes of administration of SG1, SG2, or SG3. In Experiment 3, day of hatch, poults were inoculated with only isolate SG1 using different paths. Poults were randomly selected for necropsy on d 8 and d 15 and sampled to collect spleen, heart, and liver for SG on d 21, the remaining poults were necropsied and cultured. Samples were plated on Columbia nalidixic acid and colistin agar (CNA) (40°C, 18–24 h). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) confirmed suspect colonies. Data were analyzed using the chi-square test of independence, testing all possible combinations to determine significance (P < 0.05). Weight data were subjected to ANOVA using JMP with significance (P < 0.05). No differences were found in BW or BWG on d 0, 8, 15, or 22. Splenomegaly, focal heart necrosis, and pericarditis were observed in all groups in experiments 1 through 3. In Experiment 3, only airsacculitis was observed in a negative control in separate isolation (P > 0.05). On d 21 of Experiment 3, increased (P < 0.05) recovery of SG from spleens were observed in co-housed negative controls, as well as poults challenged by oral gavage (P > 0.05 for d 7 and d 14). These results confirm numerous previous studies indicating that SG subsp. pasteurianus is a primary infectious microorganism that causes septicemia in young poults.
Key words: experimental infection, poult, Streptococcus gallolyticus, turkey
INTRODUCTION
Streptococcus gallolyticus (SG), formerly known as Streptococcus bovis, is a Gram-positive, catalase and oxidase-negative bacterium that grows in pairs or chains of cocci (Schlegel et al., 2003). While SG has been implicated in some pathologic illnesses in humans and domestic animals (Beck et al., 2008), the bacteria also play an important role in turkey's health and well-being (Corredoira et al., 2023). SG is a natural inhabitant of the turkey gastrointestinal system, where it plays an important role in establishing a healthy gut microbiome (Crispo et al., 2018). It aids digestion by breaking down complex carbohydrates and improving nutrient absorption, critical for the bird's general growth and well-being (Martínez-Laorden et al., 2023). Moreover, SG is beneficial for turkey immune system development and function. It aids in maintaining the intestinal barrier, inhibiting the entry of pathogenic bacteria and encouraging the creation of antimicrobial compounds, maintaining the birds’ overall health and resistance to illnesses (Siddiqui et al., 2022). Nevertheless, in recent years, SG has been associated with infections in turkeys, including septicemia, endocarditis, airsacculitis, splenomegaly, and hepatomegaly (Figure 1) (Kasamatsu et al., 2022; Oliveira et al., 2022). These health issues can lead to sudden death, generally with no premonitory signs, leading to higher mortality rates and reducing turkey farms’ overall productivity (Droual et al., 1997; Saumya et al., 2014; Schulz et al., 2015). In addition, SG has been linked to human infections, such as septicemia, endocarditis, peritonitis, meningitis, and colon carcinoma. (Gold et al., 2004; Beck et al., 2008; Akahane et al., 2009; Boleij et al., 2010; Dumke et al., 2015; Pasquereau-Kotula et al., 2018; Sitthicharoenchai et al., 2022). The poultry industry's intimate interaction between turkeys and humans enhances the possibility of transmission, emphasizing the need to study this bacterium's function in illness development and prevention (Saumya et al., 2014; Dumke et al., 2015; Budea et al., 2023). Three SG isolates (SG1, SG2, and SG3) were obtained from clinical field cases of acute septicemia of commercial turkeys and were used in three independent experiments. The objectives of the present study were to characterize and evaluate experimental infection of these isolates in ovo and in day of hatch poults with varying concentrations and using different routes of administration.
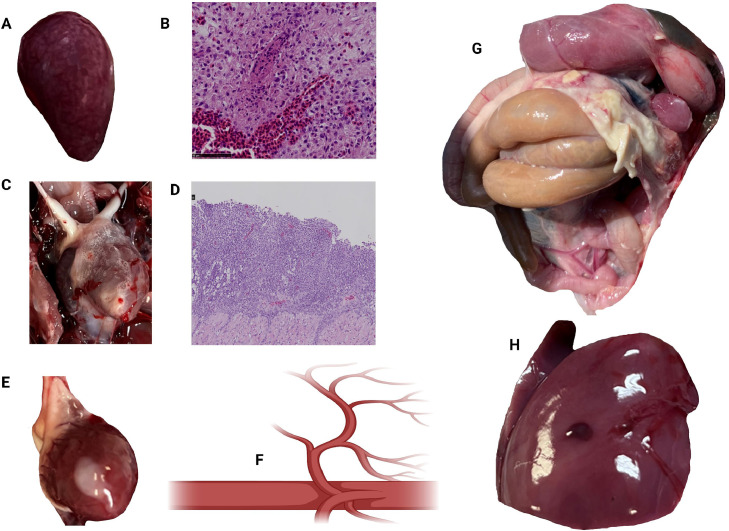
Figure 1.
Lesions of S. gallolyticus. An enlarged spleen (splenomegaly) with mottled appearance, areas of hemorrhage, congestion, and necrosis (A). Histological examination of the spleen showed areas of lymphoid depletion, necrosis, congestion, and infiltration of heterophils and macrophages (B). S. gallolyticus can cause inflammation of the pericardial sac, leading to accumulation of fibrinous or fibrinopurulent exudate within the pericardial space (C). Microscopic examination of the pericardium demonstrated infiltration of inflammatory cells, primarily heterophils and macrophages, along with fibrin deposition, necrosis, and fibrosis of pericardial tissue (D). The heart may appear enlarged and covered with a thick layer of fibrin and necrotic nodule (E). S. gallolyticus is an intracellular bacterium that causes septicemia (F), peritonitis (G), and hepatomegaly with areas of hemorrhage, congestion, and necrosis (H). Histological images stained with hematoxylin and eosin (created with BioRender.com).
MATERIALS AND METHODS
Streptococcus Gallolyticus Isolates
Three SG isolates were obtained and reported from clinical field cases cultured from whole spleens at the Veterinary Diagnostic Laboratory (VDL, University of Arkansas Division of Agriculture, Fayetteville, AR). Identification was confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) serviced through the Oklahoma Animal Disease Diagnostic Laboratory (OADDL, Stillwater, OK). Nucleic acid sequencing was performed at Oklahoma State University Protein and DNA Core Facility (Stillwater, OK). Isolates were chosen for the present experiments based on geographical disparities (Arkansas and Missouri) and possible virulence. Samples were preserved at −80°C in a 30% glycerol and brain heart infusion broth (BHI, Remel, Catalog No, R060264, Lenexa, KS) mixture to preserve cell viability. For the challenge, 1 mL of SG1, SG2, or SG3 was added to 25 mL BHI and incubated aerobically at 37°C for 18 to 24 h. Postincubation, bacteria were washed with sterile 0.9% saline by centrifugation at 2,103 × g for 15 min at 4°C and resuspended in saline. Stock concentration was determined with a microplate dilution method after 10-fold serial dilutions were plated on Columbia nalidixic acid and colistin agar (CNA, Catalog No. 212104, disc dispenser (BD), Sparks, MD). Plates were incubated aerobically at 37°C for 24 h to enumerate total SG cfu. Isolates SG1, SG2, and SG3 were each serially diluted to reach 102, 104, and 106 cfu/mL. Each challenge concentration was confirmed with the microplate dilution method.
Experimental Design for Experiments 1, 2, and 3
Experiment 1
Fertile eggs (n = 470) from the Nicholas genetic line (Aviagen, Lewisburg, WV) were obtained from a commercial hatchery and used for Experiments 1 and 2. Embryos were incubated at the University of Arkansas poultry research experimental farm using a Jamesway PS500 Multi-Stage Controller incubator and candled for viability at 13 and 25 d of embryogenesis (DOE). On 25 DOE, all embryos were placed into styrofoam hatching cabinets (Hovabator, Catalog No. 1602N, G.Q.F. Manufacturing Co., Savannah, GA) until the day of hatch (DOH). In Experiment 1, embryos were separated into 10 different experimental groups. Group 1 was the sham negative control injected with saline. Groups 2, 3, and 4 received isolate SG1 at 102, 104, or 106 cfu/0.2 mL, respectively. Groups 5, 6, and 7 received isolate SG2 at 102, 104, or 106 cfu/0.2 mL, respectively. Groups 8, 9, and 10 were inoculated with isolate SG3 at 102, 104, or 106 cfu/0.2 mL, respectively. Before injection on 25 DOE, in groups 1 through 10 (n = 30 embryos/hatcher), an 18-gauge needle punctured a hole into the air cell of 300 viable embryos. Each embryo was challenged with 0.2 mL of respective inoculum via amnion injection using a blunt-tipped needle and then placed into their corresponding hatching cabinets. Hatchers were set to 37.5°C with humidity supplied according to commercial standards (Smith, 2000; Aviagen Turkeys, 2021). Poults from groups 1 through 10 were wing banded and weighed before being placed into battery cages. BW and BWG were evaluated on d 0, 8, 15, and 22. On d 8 and 15, 2 birds per cage per group were selected for sampling to enumerate the cfu of SG from the spleen. Birds were euthanized by CO2 inhalation. This process was performed on d 22 with the remaining poults available in each treatment group. For both Experiments 1 and 2, a subset of 10 poults from the negative control group was selected for sampling to confirm control count at zero time.
Experiment 2
In Experiment 2, turkey poults at DOH were distributed into 9 experimental groups to evaluate different routes of SG1 challenge administration as follows: Group 1: unchallenged control, Group 2: intratracheal (IT) at 104, Group 3: intraperitoneal (IP) at 104, Group 4: oral gavage 104, Group 5: IV at 104, Group 6: IT at 106, Group 7: IP at 106, Group 8: oral gavage 106, and Group 9: IV at 106. Poults (n = 12/group) were administered at DOH 0.2 mL of 2 doses of isolate SG1 (104 or 106 cfu/0.2 mL). A 25-gauge 5/8″ needle was used for IP and IV administration. A curved, round-ball-tipped needle was used for IT and oral gavage. Each wire-bottom cage (24 in × 24 inches) contained an individual feeder and water line—the environmental conditions simulated commercial turkey production settings (Aviagen Turkeys, 2019). A gradual reduction in temperature was used from 35°C to 26°C from DOH to 22 d of age. Birds were fed turkey starter diets ad libitum. BW and BWG were evaluated on d 0, 8, 15, and 22. On d 8 and 15, 2 per cage per group were randomly selected for sampling to enumerate cfu of SG1 from the spleen. This process was performed on d 22 with the remaining poults available in each treatment group.
Experiment 3
In this trial, DOH female turkey poults (n = 320) from the Nicholas genetic line (Aviagen) were obtained from a commercial hatchery and used for Experiment 3. A subset of 20 birds was sampled upon arrival to confirm they were disease-free. The liver, heart, and spleen were collected into 25 mL of tryptic soy broth (TSB, Catalog No. 22092, Sigma, St. Louis, MO) and incubated overnight at 37°C. The following day, a sterile cotton swab was used to collect the sample from each tube to swab onto CNA, streaking for isolation with a sterile loop. Plates were incubated in 5% CO2 at 37°C for 18 to 24 h. The remainder of the birds was wing banded and placed into 5 groups allocated by weight. The treatments included Group 1: negative control in a separate isolation room, Group 2: negative control in the same isolation room as the challenged birds, Group 3: IT-challenged, Group 4: oral gavage challenged, and Group 5: aerosol challenged. The challenge dose for IT and aerosol administration was approximately 102 cfu/0.2 mL of SG1. The challenge dose for oral gavage was about 103 cfu/0.2 mL of SG1. Each wire-bottom cage (24 in × 24 inches) contained a feeder and water line. Each treatment group had 5 replicates with 12 poults/cage—the environmental conditions simulated commercial turkey production settings (Aviagen Turkeys, 2019). A gradual reduction in temperature was set from 35°C to 26°C from DOH to 21 d of age. Birds were fed turkey starter diets ad libitum. Body weight and weight gain were evaluated on d 0, 8, 15, and 22. On d 7 and 14, 3 birds per cage (n = 15/treatment) were randomly selected for sampling to evaluate the incidence of SG. On d 21, all remaining birds were processed to assess the incidence of SG1.
All animal handling procedures in Experiments 1, 2, and 3 complied with the Institutional Animal Care and Use Committee at the University of Arkansas, Fayetteville, under protocol #22057.
Streptococcus Gallolyticus Recovery
In Experiments 1 and 2, spleen samples were collected (n = 2 poults/cage) on d 8 and 15. After collection, each spleen sample was homogenized and diluted with saline (1:4 weight/volume) and 10-fold serial dilutions were plated on CNA. On d 22, all remaining birds were sampled for incidence of SG using the method mentioned above. Plates were incubated aerobically at 37°C for 24 h to enumerate total SG colony-forming units. For Experiment 3, samples were taken from the spleen, heart, and liver with sterile cotton swabs and swabbed onto CNA, streaking for isolation. Plates were incubated in 5% CO2 at 40°C for 18 to 24 h. The focus of this experiment was the qualitative presence or absence of SG, with plates being evaluated based on colony morphology.
Streptococcus Gallolyticus Identification
Isolates were subcultured for isolation from original CNA plates onto fresh agar incubated at 37°C under aerobic conditions. A single isolated colony was picked from each sample with an inoculation loop, transferred to a tryptic soy agar slant (TSA, Catalog No. R064860, Remel, Lenexa, KS), and incubated at 37°C under aerobic conditions. MALDI-TOF MS identified slants serviced through OADDL (Stillwater, OK). Nucleic acid sequencing was performed at Oklahoma State University Protein and DNA Core Facility (Stillwater, OK).
Antibiogram
Each isolate cultured from the spleen of cases submitted to the VDL (Fayetteville, AR) was individually grown under aerobic conditions at 37°C in 4 mL of BHI broth to achieve turbidity of 0.5 MacFarland. A cotton swab was submerged into the inoculated BHI broth and swabbed onto Mueller Hinton agar with 5% sheep blood (Catalog No. R01622, Remel, Lenexa, KS) to achieve a uniform lawn. The Mueller Hinton plate was then tamped with a spring-loaded antibiotic disc dispenser (BD, Catalog No. 260640, Sparks, MD) containing 12 different antibiotics: clindamycin (CC), 2 μg/disc; erythromycin (E), 15 μg/disc; florfenicol (FFC), 30 μg/disc; gentamycin (GM), 10 μg/disc; neomycin (N), 30 μg/disc; penicillin (P), 10 μg/disc; sulfadiazine (SD), 0.25 μg/disc; spectinomycin (SPT), 100 μg/disc; sulfamethoxazole/trimethoprim (SXT), 23.75/1.25 μg/disc; oxytetracycline (T), 30 μg/disc; tetracycline (TE), 30 μg/disc; ceftiofur (XNL), 30 μg/disc; (BD, Sparks, MD). The plate was incubated overnight at 37°C under aerobic conditions. After approximately 18 h, the zone of inhibition for each antibiotic was determined using a caliper, rounding to the nearest millimeter. Susceptibility was determined according to the American Standard of the Clinical and Laboratory Standards Institute (Hudzicki, 2009).
Histopathology
Tissue samples from the spleen and heart displaying focal necrosis were aseptically collected and fixed in 10% neutral-buffered formalin. Tissues were processed at Texas A&M Veterinary Medical Diagnostic Laboratory, where they were embedded in paraffin, sectioned (4–5 μm thick), and stained with hematoxylin and eosin.
Statistical Analysis
Each poult was considered the experimental unit for bacterial enumeration, SG incidence, and presentation of macroscopic lesions. Data were subjected to analysis of variance (ANOVA) as a completely randomized design using the General Linear Models procedure of the Statistical Analysis System (SAS, 2002). Significant differences among the means were determined by Duncan's range test for log10 cfu/g at P < 0.05. The SG incidence and macroscopic lesion data were expressed as positive samples or lesions/total poults evaluated using a chi-square test of independence to determine the significant difference with 1° of freedom, testing all possible combinations to determine the significance (P < 0.05).
RESULTS
In Experiments 1 and 2, fertile eggs, the results of SG enumeration and incidence on CNA from spleens of poults challenged in ovo (Experiment 1) and at DOH (Experiment 2) from spleens of poults challenged with SG at DOH are summarized in Table 1. Interestingly in Experiment 1, at d 15, turkey poults challenged with 102 cfu SG2 showed a significant increase in SG cfu isolated from spleen lesions compared to SG2 at higher doses, SG1 at any dose, or SG3 106 cfu. However, SG3 challenged at 102 cfu showed higher colonization, followed by 106 cfu SG1 compared with the rest of the groups. No differences were observed in SG recovery on d 8 between experimental groups in Experiment 1. A similar trend was observed in the incidence of SG (Table 1). In Experiment 2, no differences were observed in SG recovery at d 8 between experimental groups; however, turkey poults that received an oral challenge with SG1 at 106 cfu had significantly higher colonization compared with oral 104 cfu SG1 challenge, intravenous 104 cfu SG1 challenge, or negative control poults. Nevertheless, at d 22, turkey poults that received 106 cfu SG1 by oral gavage showed a significant increase compared with negative control poults and was also confirmed by the incidence of SG isolated from spleen (Table 1).
Table 1.
Log10 cfu/g† and incidence‡ of Streptococcus gallolyticus (SG) from spleens in Columbia nalidixic acid and colistin agar (CNA) of poults challenged in ovo (Experiment 1) or at day of hatch (Experiment 2).
| Group | CNA | Day 8 | CNA | Day 15 | CNA | Day 22 |
|---|---|---|---|---|---|---|
| Enumeration of SG, Experiment 1 | Log10 cfu/g | Incidence of SG | Log10 cfu/g | Incidence of SG | Log10 cfu/g | Incidence of SG |
| 1. NC | 2.64 ± 0.954 | 3/4 (75.0) | 0.00 ± 0.000c | 0/4 (0) | 0.52 ± 0.348 bc | 2/11 (18.18) b |
| 2. SG1 102 | 2.51 ± 0.840 | 3/4 (75.0) | 0.00 ± 0.000 c | 0/4 (0) | 0.00 ± 0.000 c | 0/4 (0) b |
| 3. SG1 104 | 2.10 ± 0.703 | 3/4 (75.0) | 0.68 ± 0.675 bc | 1/4 (25.0) | 0.45 ± 0.303 bc | 2/12 (16.66) b |
| 4. SG1 106 | 2.42 ± 0.962 | 4/6 (66.66) | 2.78 ± 0.605 bc | 5/6 (83.33) | 2.11 ± 0.536 ab | 6/9 (66.66) a |
| 5. SG2 102 | 1.27 ± 1.272 | 1/2 (50.0) | 5.50 ± 1.500 a | 2/2 (100.0) | 0.92 ± 0.925 bc | 1/4 (25.0) b |
| 6. SG2 104 | 2.93 ± 0.982 | 3/4 (75.0) | 1.62 ± 0.940 bc | 2/4 (50.0) | 0.90 ± 0.900bc | 1/3 (33.33) b |
| 7. SG2 106 | 3.22 ± 0.114 | 4/4 (100.0) | 2.00 ± 2.000 bc | 1/2 (50.0) | 0.54 ± 0.540 bc | 1/5 (20.0) b |
| 8. SG3 102 | 3.33 ± 0.293 | 4/4 (100.0) | 3.30 ± 0.575ab | 2/2 (100.0) | 3.03 ± 0.333a | 3/3 (100.0) a |
| 9. SG3 104 | 0.98 ± 0.976 | 1/4 (25.0) | 0.00 ± 0.000 c | 0/2 (0) | ND | 0/0 (0) b |
| 10. SG3 106 | 3.37 ± 0.090 | 4/4 (100.0) | 1.04 ± 1.044 bc | 1/4 (25.0) | 0.60 ± 0.600 bc | 1/5 (20.0) b |
| Enumeration of SG, Experiment 2 | Log10 cfu/g | Incidence of SG | Log10 cfu/g | Incidence of SG | Log10 cfu/g | Incidence of SG |
| 1. NC | 2.64 ± 0.954 | 3/4 (75.0) | 0.00 ± 0.000 b | 0/4 (0) | 0.52 ± 0.348 b | 2/11 (18.18) b |
| 2. Intratracheal SG1 104 | 3.29 ± 0.111 | 1/2 (50.0) | 3.00 ± 0.301 ab | 2/2 (100.0) | 1.68 ± 0.607 ab | 4/7 (57.14) a |
| 3. Intraperitoneal SG1 104 | 1.74 ± 1.739 | 1/2 (50.0) | 3.20 ± 0.199 ab | 2/2 (100.0) | 1.75 ± 0.633 ab | 4/7 (57.14) a |
| 4. Oral SG1 104 | 2.70 ± 0.000 | 0/2 (0) | 0.00 ± 0.000 b | 0/2 (0) | 1.76 ± 0.521 ab | 5/8 (62.5) a |
| 5. Intravenous SG1 104 | 1.50 ± 1.500 | 2/2 (100.0) | 0.00 ± 0.000 b | 0/2 (0) | 0.77 ± 0.498 ab | 2/7 (28.57) ab |
| 6. Intratracheal SG1 106 | 1.35 ± 1.349 | 0/2 (0) | 1.85 ± 1.849 ab | 1/2 (50.0) | 1.65 ± 0.763 ab | 3/6 (50.0) a |
| 7. Intraperitoneal SG1 106 | 1.35 ± 1.349 | 1/2 (50.0) | 2.94 ± 2.938 ab | 1/2 (50.0) | 1.70 ± 0.608 ab | 5/7 (71.42) a |
| 8. Oral SG1 106 | 4.55 ± 1.849 | 1/2 (50.0) | 3.94 ± 0.239 a | 2/2 (100.0) | 2.57 ± 0.550 a | 4/6 (66.66) a |
| 9. Intravenous SG1 106 | 1.27 ± 1.272 | 1/2 (50.0) | 1.50 ± 1.500 ab | 1/2 (50.0) | 2.15 ± 0.714 ab | 4/6 (66.66) a |
Values within columns with different superscripts differ significantly (P < 0.05) using chi-square analysis with 1 degree of freedom. NC: negative control.
Data expressed in Log10 cfu/g of tissue. Samples were collected at d 8, 15, and 22 postchallenge. Data expressed as mean ± SE.
Data expressed as number of poults with lesions/total sampled poults (%).
Isolates of SG recovered in 8- to 22-day-old poults from spleen lesions in all 3 experiments on CNA were subjected to identification by MALDI-TOF MS and 16S rDNA sequence analyses, as previously described. With no exception, all isolates recovered from spleen lesions were identified as SG by MADI-TOF MS. Moreover, 16S rDNA sequence analyses confirmed that all strains were SG subsp. pasteurianus (data not shown).
Table 2 shows the results of the incidence of SG from spleens in Columbia nalidixic acid and colistin agar (CNA) of poults challenged at day of hatch in Experiment 3. At d 8 and 15 of age, no significant differences in the recovery of SG were observed between experimental groups. However, on d 22, a substantial increase in the recovery of SG was observed in the group that received an oral gavage SG1 103. Interestingly, the negative control group, which was kept in the same isolation room as the challenge groups also had a high incidence of SG recovery compared with groups challenge by intratracheal or aerosol inoculation (Table 2).
Table 2.
Incidence of Streptococcus gallolyticus (SG) from spleens in Columbia nalidixic acid and colistin agar (CNA) of poults challenged at day of hatch in Experiment 3.
| Group | CNA day 8 | CNA day 15 | CNA day 22 |
|---|---|---|---|
| Incidence of SG, Experiment 3‡ | |||
| 1. NC* | 0/15 (0) | 0/15 (0) | 1/27 (3.70) b |
| 2. NC# | 0/15 (0) | 0/15 (0) | 7/30 (23.33) a |
| 3. Intratracheal SG1 102 | 0/15 (0) | 2/15 (13.33) | 3/29 (10.34) b |
| 4. Oral gavage SG1 103 | 2/15 (13.33) | 0/15 (0) | 6/28 (21.42) a |
| 5. Aerosol SG1 102 | 0/15 (0) | 0/15 (0) | 3/29 (10.34) b |
Values within columns with different superscripts differ significantly (P < 0.05) using chi-square analysis with 1 degree of freedom. NC: negative control.
NC Group 1 was kept in a separate isolation room from challenge groups.
Data expressed as number of poults with lesions/total sampled poults (%).
NC Group 2 was kept in the same isolation room as the challenge group.
Table 3 shows the results of the macroscopic lesions from 8- to 22-day-old poults challenged in ovo with SG in Experiment 1 or DOH in Experiments 2 and 3. In Experiment 1, embryos challenged with isolate SG2 at a concentration of 102 cfu showed a significant (P < 0.05) increase in the incidence of lesions in the spleen (splenomegaly) and heart (focal heart necrosis) compared with SG1 or SG3 and their respective challenge doses. However, embryos challenged with SG3 at 102 cfu or 106 cfu presented a significant increase in pericarditis. No significant differences in airsacculitis or yolk sac retention were observed in embryos challenged with any of the 3 SG isolates at any of the challenge doses (Table 3). In Experiment 2, poults challenged by the IT route with SG1 at 106 cfu exhibited a significant increase in focal heart lesions compared with the rest of the challenge routes and doses (Table 3). In Experiment 3, poults in the negative control group kept in a separate isolation room from SG challenge groups showed no macroscopic lesions. However, all experimental groups, including the negative control group, observed significant splenomegaly lesions compared with the negative control poults kept in a different isolation room. No differences in focal heart necrosis lesions, pericarditis, yolk sac retention, or peritonitis were observed (Table 3).
Table 3.
Macroscopic lesions from 8- to 22-day-old poults challenged with Streptococcus gallolyticus (SG) in ovo in Experiment 1 or day of hatch in Experiments 2 and 3.
| Group | Splenomegaly | Focal heart necrosis | Pericarditis | Airsacculitis | Yolk sac retention |
|---|---|---|---|---|---|
| Experiment 1† | |||||
| 1. NC | 0/19 (0) b | 0/19 (0) b | 0/19 (0) b | 0/19 (0) | 1/19 (5.26) |
| 2. SG1 102 | 0/12 (0) b | 0/12 (0) b | 0/12 (0) b | 2/12 (16.66) | 1/12 (8.33) |
| 3. SG1 104 | 0/20 (0) b | 0/20 (0) b | 0/20 (0) b | 1/20 (5.0) | 1/20 (5.0) |
| 4. SG1 106 | 2/20 (10.0) b | 0/20 (0) b | 2/20 (10.0) b | 1/20 (5.0) | 2/20 (10.0) |
| 5. SG2 102 | 2/8 (25.0) a | 2/8 (25.0) a | 0/8 (0) b | 0/8 (0) | 1/8 (12.5) |
| 6. SG2 104 | 1/11 (9.09) b | 0/11 (0) b | 2/11 (18.18) b | 0/11 (0) | 1/11 (9.09) |
| 7. SG2 106 | 0/11 (0) b | 0/11 (0) b | 1/11 (9.09) b | 1/11 (9.09) | 0/11 (0) |
| 8. SG3 102 | 0/9 (0) b | 0/9 (0) b | 3/9 (33.33) a | 1/9 (11.11) | 0/9 (0) |
| 9. SG3 104 | 1/6 (16.66) b | 0/6 (0) b | 0/6 (0) b | 0/6 (0) | 0/6 (0) |
| 10. SG3 106 | 1/13 (7.69) b | 2/13 (15.38) b | 3/13 (23.07) a | 0/13 (0) | 0/13 (0) |
| Experiment 2‡ | |||||
| 1. NC | 0/19 (0) | 0/19 (0) b | 0/19 (0) | 0/19 (0) | 1/19 (5.26) |
| 2. Intratracheal SG1 104 | 0/11 (0) | 0/11 (0) b | 1/11 (9.09) | 0/11 (0) | 0/11 (0) |
| 3. Intraperitoneal SG1 104 | 0/11 (0) | 1/11 (9.09) b | 1/11 (9.09) | 0/11 (0) | 0/11 (0) |
| 4. Oral SG1 104 | 0/12 (0) | 1/12 (8.33) b | 1/12 (8.33) | 0/12 (0) | 0/12 (0) |
| 5. Intravenous SG1 104 | 1/11 (9.09) | 0/11 (0) b | 0/11 (0) | 0/11 (0) | 0/11 (0) |
| 6. Intratracheal SG1 106 | 0/10 (0) | 3/10 (30.0) a | 1/10 (10.0) | 0/10 (0) | 0/10 (0) |
| 7. Intraperitoneal SG1 106 | 1/11 (9.09) | 1/11 (9.09) b | 0/11 (0) | 0/11 (0) | 1/11 (9.09) |
| 8. Oral SG1 106 | 0/10 (0) | 1/10 (10.0) b | 1/10 (10.0) | 0/10 (0) | 0/10 (0) |
| 9. Intravenous SG1 106 | 1/10 (10.0) | 1/10 (10.0) b | 0/10 (0) | 0/10 (0) | 1/10 (10.0) |
| Experiment 3§ | |||||
| 1. NC * | 0/57 (0) b | 2/57 (3.50) | 1/57 (1.75) | 0/57 (0) | 0/57 (0) |
| 2. NC # | 4/60 (6.66) a | 1/60 (1.66) | 2/60 (3.33) | 0/60 (0) | 0/60 (0) |
| 3. Intratracheal SG1 102 | 4/59 (6.77) a | 3/59 (5.08) | 2/59 (3.38) | 0/59 (0) | 1/59 (1.69) |
| 4. Oral gavage SG1 103 | 4/58 (6.89) a | 1/58 (1.72) | 0/58 (0) | 0/58 (0) | 0/58 (0) |
| 5. Aerosol SG1 102 | 8/59 (13.55) a | 3/59 (5.08) | 0/59 (0) | 0/59 (0) | 0/59 (0) |
Data expressed as number of poults with lesions/total sampling poults (%).
Values within columns with different superscripts differ significantly (P < 0.05) using chi-square analysis with 1 degree of freedom. NC: negative control.
NC Group 1 was kept in a separate isolate room from challenge groups.
Turkey poult from Experiment 1 in groups 2 through 10 were challenged in ovo at 25 DOE with three different isolates of SG at various doses ranging 102 to 106 cfu/0.2 mL.
Turkey poults from Experiment 2 in Groups 2 to 9 were challenged with isolate SG1 at DOH via intratracheal, oral gavage, intraperitoneal, or intravenous at either 104 or 106 cfu/0.2 mL.
Turkey poults from Experiment 3 were challenged with isolate SG1 at DOH via intratracheal, oral gavage, or aerosol at either 102 or 103 cfu/0.2 mL.
NC Group 2 was kept in the same isolation room as the challenge groups.
No significant differences were observed in BW and BWG on d 0, 8, 15, and 22 for any of the 3 experiments (body weight and body wight gain data not shown). In the experiment, 3 deaths occurred: 1 on d 3 from SG3 106, 1 on d 7 in SG1 104, and 1 on d 12 from SG3 104, with SG being cultured from the yolk sac in all cases. In Experiment 2, 3 deaths occurred: 1 in IP route 106 on d 3, 1 in IT route 106 on d 7, and 1 in IT route 104 on d 13, with SG being cultured from the yolk sac in all cases. Two deaths occurred in Experiment 3 from poults challenged via oral gavage and aerosol on d 7 and 12, respectively, with SG isolated from the liver, heart, and spleen.
The results of the antibiogram profiles of SG subsp. pasteurianus isolates from clinical cases of turkey poults used in Experiments 1 and 2 are summarized in Table 4. Isolate 1 of SG was susceptible to all antibiotics tested but showed an intermediate profile for sulfamethoxazole/trimethoprim. Isolates SG2 and SG3 showed medium antibiotic resistance to sulfamethoxazole/trimethoprim and neomycin but were sensitive to all remaining antibiotics tested (Table 4).
Table 4.
Antibiogram profiles of Streptococcus gallolyticus (SG) subsp. pasteurianus isolates from clinical cases of turkey poults used in Experiments 1 and 2*.
| Antibiotic1 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate | Source | Age (wk) | CC | E | FFC | GM | N | P | SD | SPT | SXT | T | TE | XNL |
| SG1 | Spleen | 3 | S | S | S | S | S | S | S | S | I | S | S | S |
| SG2 | Spleen | 1.5 | S | S | S | S | I | S | S | S | I | S | S | S |
| SG3 | Spleen | 2.2 | S | S | S | S | I | S | S | S | I | S | S | S |
Antibiogram profiles were determined using the Kirby–Bauer disk diffusion method.
CC: clindamycin, 2 μg/disc; E: erythromycin, 15 μg/disc; FFC: florfenicol, 30 μg/disc; GM: gentamycin, 10 μg/disc; N: neomycin, 30 μg/disc; P: penicillin, 10 μg/disc; SD: sulfadiazine, 0.25 μg/disc; SPT: spectinomycin, 100 μg/disc; SXT: sulfamethoxazole/trimethoprim, 23.75/1.25 μg/disc 23.75/1.25 μg/disc; T: oxytetracycline, 30 μg/disc; TE: tetracycline, 30 μg/disc; XNL: ceftiofur, 30 μg/disc. S: sensitive, I: intermediate.
All three isolates were used in Experiment 1. SG1 was used in Experiment 2. Sensitivity based on recommendations determined by the American standard of the Clinical and Laboratory Standards Institute.
Figure 1 shows representative lesions found in necropsy. Splenomegaly with a mottled appearance and areas of hemorrhages, congestion, and necrosis were observed. Histological examination of the spleen showed lymphoid depletion, necrosis, congestion, and infiltration of heterophils and macrophages. SG can cause inflammation of the pericardial sac (pericarditis), accumulating fibrinous or fibrinopurulent exudate within the pericardial space. The heart also appeared enlarged and covered with a thick layer of fibrin and necrotic nodules. Microscopic examination of the pericardium demonstrated infiltration of inflammatory cells, primarily heterophils and macrophages, along with fibrin deposition, necrosis, and fibrosis of the pericardial tissue.
DISCUSSION
Streptococcus gallolyticus is known to cause various infections in animals, including endocarditis, septicemia, hepatitis, myositis, and meningitis (Boleij et al., 2010; Motamedi et al., 2023). In turkeys, SG has been associated with respiratory diseases, septicemia, and bacterial endocarditis (Saumya et al., 2014). The pathogenesis of SG in turkeys may involve multiple virulence factors, including a capsule composed of polysaccharides (Sitthicharoenchai et al., 2022), adhesins (Lichtl-Häfele, 2022; Arya et al., 2023), hyaluronidase enzyme that can break down hyaluronic acid and a major component of the extracellular matrix (Suvarna and Mahon, 2022), pore-forming hemolysin toxins that can lyse erythrocytes and other host cells (Zeng et al., 2022), biofilm formation that involves the attachment of bacterial cells to a surface and production of an extracellular matrix that encapsulates the cells to protect them from environmental stressors (Bruggeling, 2023), and intracellular survival (Kumar et al., 2022).
In the present study, experimental infection with all 3 strains of SG resulted in splenomegaly, marbled spleen, and pericarditis in a dose-dependent fashion by in ovo challenge at 25 DOE (Experiment 1). In Experiments 2 and 3, turkey poults challenged at DOH by different routes exhibited a similar trend in splenomegaly, focal heart nodules, and pericarditis, as observed in Experiment 1. Bacteria from splenic samples on CNA indicated that SG caused the recent outbreak of a similar septicemic disease in commercial turkeys. All isolates were identified as SG subsp. pasteurianus by MALDI-TOF MS and confirmed by 16S rRNA identification sequencing, confirming Kochs's postulates. The SG isolates showed low pathogenicity for turkey poults when challenged in ovo or by different inoculation routes. Nonetheless, the pathological changes were consistent, with the spleen and heart displaying multifocal necrosis and pericarditis.
Recovery of SG and lesions observed in negative controls in Experiments 1 and 2 led us to hypothesize that SG was horizontally transmitted. The SG challenged birds were housed in the same room as the negative controls and perhaps became infected by airborne dust particles or through interaction between the cages. In 2015, Dumke et al. (2015) investigated the incidence of SG in fecal samples from hens, and a grower was diagnosed with infective endocarditis. SG was isolated from feces and dust particles. Multilocus sequence typing of recovered SG revealed blood from the grower was the same as his laying hens, showing a potential transmission from animals to humans. Schulz et al. (2015) tested feces from organic turkey flocks aged 4 to 18 wk to see occurrence and information, finding that turkeys shed SG at a median concentration of 105 cfu/g. The potential of dust particles containing SG caused us to separate a negative control group in Experiment 3 to test this hypothesis. Only 1 poult, out of the 27, tested positive for SG, leading us to believe introduction into the separate isolation room was due to fomites from failed biosecurity or colonization in poults that were at undetectable levels. The environment may play a role in transmitting this bacterium, as Droual et al. (1997) noted with repeated outbreaks in turkey farms, some of which were in close contact with pigeons. Moreover, Hogg and Pearson (2009) hypothesized bedding may also play a transmission part, as observed with ducklings.
In 1995, De Herdt et al. suggested that the intracellular multiplication of S. bovis in pigeons could be involved in the virulence of the studied strain. Intracellular multiplication has been reported to play a role in the virulence of some bacterial species, including Streptococcus spp. In the case of SG, a pathogen that can infect humans and animals, intracellular multiplication may contribute to its virulence in commercial turkeys. Several investigators have reported histopathology results of coccoid bacteria within the cytoplasm of macrophages from splenic lesions (De Herdt et al., 1995; Barnett et al., 2008).
Septicemia, endocarditis, and geriatric colorectal cancer are linked to SG. Due to its intracellular proliferation in multiple cell tissues, SG can form biofilms, express specific pili to colonize host tissues and produce a bacteriocin that eliminates commensal bacteria in the mouse colon (Teh et al., 2019). Other studies have also confirmed that both high- and low-virulence strains can adhere to the intestinal mucosa, ensuring that adhesion to enterocytes and intracellular cell proliferation are key factors in the pathogenesis of SG (Kimpe et al., 2003; Abranches et al., 2011; Kumar et al., 2022). Interestingly, the induction of inflammation by different SG strains was associated with their intracellular survivability in red blood cells and macrophages (Grimm et al., 2017). To better understand this potential mechanism, it is important to consider the general pathogenesis of Streptococcus infections and the specifics of SG infection in turkeys, as demonstrated in humans previously.
While there is limited research on the role of intracellular multiplication in the virulence of SG in turkeys specifically, it is plausible that this mechanism contributes to the pathogenesis of the infection. Further studies are needed to elucidate the exact mechanisms and factors involved in the virulence of SG in turkeys, including the potential role of intracellular multiplication. Understanding these mechanisms could inform the development of targeted prevention and treatment strategies for this infection in turkeys and other susceptible species.
Acute mortality was observed in all 3 experiments, with poults exhibiting systemic infections within 13 d and SG being recovered from multiple organs, including the yolk sac, liver, spleen, and air sac. Mortality in Experiments 1 and 2 was only measured with acute death due to hatching issues. However, mortality was observed as seen in commercial productions within 2 to 3 wk of age (Saumya et al., 2014).
In conclusion, the current study's findings indicated that all 3 SG strains infected in turkeys could induce septicemia. The capacity of SG subsp. pasteurianus to reproduce intracellularly may be connected to its pathogenicity in turkey poults. As a result, the etiology of this bacterium deserves further investigation. In addition, studies evaluating this pathogen under stress settings may provide more information about this emerging disease.
ACKNOWLEDGMENTS
This project was funded by USDA Animal Health Awards (FY2021 & FY2022), and by USDA-NIFA. Sustainable Agriculture Systems, grant no. 2019-69012-29905. Title of Project: Empowering U.S. Broiler Production for Transformation and Sustainability.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Abranches J., Miller J.H., Martinez A.R., Simpson-Haidaris P.J., Burne R.A., Lemos J.A. The collagen-binding protein Cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect. Immun. 2011;79:2277–2284. doi: 10.1128/IAI.00767-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahane T., Takahashi K., Matsumoto T., Kawakami Y. A case of peritonitis due to Streptococcus gallolyticus subsp. pasteurianus. Kansenshogaku Zasshi. 2009;83:56–59. doi: 10.11150/kansenshogakuzasshi.83.56. [DOI] [PubMed] [Google Scholar]
- Arya J., Sharma D., Kumar D., Jakhar R., Khichi A., Dangi M., Chhillar A.K. Comparative genome analysis of Streptococcus strains to identify virulent genes causing neonatal meningitis. Infect. Genet. Evol. 2023;107 doi: 10.1016/j.meegid.2022.105398. [DOI] [PubMed] [Google Scholar]
- Aviagen Turkeys. 2019. Management guidelines for breeding turkeys. Accessed Aug. 2002.https://www.aviagenturkeys.us/uploads/2022/01/17/HA25_V2_Management%20Guidelines%20for%20Turkey%20Hatcheries_US.pdf
- Aviagen Turkeys. 2021. Management guidelines for turkey hatcheries. Accessed Aug. 2022.https://www.aviagenturkeys.com/uploads/2021/03/02/HA27_V1_Management%20Guidelines%20for%20Turkey%20Hatcheries_UK.pdf.
- Barnett J., Ainsworth H., Boon J.D., Twomey D.F. Streptococcus gallolyticus subsp. pasteurianus septicaemia in goslings. Vet. J. 2008;176:251–253. doi: 10.1016/j.tvjl.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Beck M., Frodl R., Funke G. Comprehensive study of strains previously designated Streptococcus bovis consecutively isolated from human blood cultures and emended description of Streptococcus gallolyticus and Streptococcus infantarius subsp. coli. J. Clin. Microbiol. 2008;46:2966–2972. doi: 10.1128/JCM.00078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleij A., Roelofs R., Schaeps R.M., Schülin T., Glaser P., Swinkels D.W., Kato I., Tjalsma H. Increased exposure to bacterial antigen RpL7/L12 in early stage colorectal cancer patients. Cancer. 2010;116:4014–4022. doi: 10.1002/cncr.25212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggeling C.E. Univ. Radboud; Netherlands: 2023. Bacterial Biofilms in Early Colon Carcinogenesis: A Study in Lynch Syndrome and Ulcerative Colitis. PhD Dissertation. [Google Scholar]
- Budea C.M., Pricop M., Mot I.C., Horhat F.G., Hemaswini K., Akshay R., Negrean R.A., Oprisoni A.L., Citu C., Bumbu B.A., Adi A., Khan I., Mavrea A., Bogdan I., Bota A.V., Fericean R.M., Marincu I. The assessment of antimicrobial resistance in gram-negative and gram-positive infective endocarditis: a multicentric retrospective analysis. Medicina. 2023;59:457. doi: 10.3390/medicina59030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corredoira J., Grau I., Garcia-Rodriguez J.F., Romay E., Cuervo G., Berbel D., Ayuso B., García-Pais M.J., Rabuñal R., García-Garrote F., Alonso M.P., Pallrés R. Species and biotypes of Streptococcus bovis causing infective endocarditis. Enferm. Infecc. Microbiol. Clin. 2023;41:215–220. doi: 10.1016/j.eimce.2021.08.017. [DOI] [PubMed] [Google Scholar]
- Crispo M., Shivaprasad H.L., Cooper G.L., Bickford A.A, Stoute S.T. Streptococcosis in commercial and noncommercial avian species in California: 95 cases (2000–2017) Avian Dis. 2018;62:152–162. doi: 10.1637/11765-103117-Reg.1. [DOI] [PubMed] [Google Scholar]
- De Herdt P., Ducatelle R., Lepoudre C., Charlier G., Nauwynck H. An epidemic of fatal hepatic necrosis of viral origin in racing pigeons (Columba livia) Avian Pathol. 1995;24:475–483. doi: 10.1080/03079459508419087. [DOI] [PubMed] [Google Scholar]
- Droual R., Ghazikhanian G.Y., Shivaprasad H.L., Barr B.C., Bland M.B. Streptococcus bovis infection in turkey poults. Avian Pathol. 1997;26:433–439. doi: 10.1080/03079459708419225. [DOI] [PubMed] [Google Scholar]
- Dumke J., Hinse D., Vollmer T., Schulz J., Knabbe C., Dreier J. Potential transmission pathways of Streptococcus gallolyticus subsp. gallolyticus. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.S., Bayar S., Salem R.R. Association of Streptococcus bovis bacteremia with colonic neoplasia and extracolonic malignancy. Arch. Surg. 2004;139:760–765. doi: 10.1001/archsurg.139.7.760. [DOI] [PubMed] [Google Scholar]
- Grimm I., Weinstock M., Birschmann I., Dreier J., Knabbe C., Vollmer T. Strain-dependent interactions of Streptococcus gallolyticus subsp. gallolyticus with human blood cells. BMC Microbiol. 2017;17:210. doi: 10.1186/s12866-017-1116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R., Pearson A. Streptococcus gallolyticus subspecies gallolyticus infection in ducklings. Vet. Rec. 2009;165:297–298. doi: 10.1136/vetrec.165.10.297-a. [DOI] [PubMed] [Google Scholar]
- Hudzicki, J. 2009. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. American Society for Microbiology. Accessed Mar. 2023http://www.microbelibrary.org/component/resource/laboratory-test/3189-kiby-bauer-disk-diffusion-susceptibility-test-protocol.
- Kasamatsu A., Fukushima K., Horiuchi M., Sekiya N. Streptococcus gallolyticus subspecies pasteurianus bacteremia accompanied by acute pancreatitis. J. Infect. Chemother. 2022;28:1663–1666. doi: 10.1016/j.jiac.2022.08.009. [DOI] [PubMed] [Google Scholar]
- Kimpe A., Decostere A., Hermans K., Mast J., Haesebrouck E. Association of Streptococcus gallolyticus strains of high and low virulence with the intestinal tract of pigeons. Avian Dis. 2003;47:559–565. doi: 10.1637/6081. [DOI] [PubMed] [Google Scholar]
- Kumar R., Taylor J.C., Jain A., Jung S.Y., Garza V., Xu Y. Modulation of the extracellular matrix by Streptococcus gallolyticus subsp. gallolyticus and importance in cell proliferation. PLoS Pathog. 2022;18 doi: 10.1371/journal.ppat.1010894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtl-Häfele J. Univ. Ulm; Germany: 2022. Design of a Molecular Method to Detect Streptococcus Gallolyticus in Human Stools: Prevalence of the Bacteria in Healthy and Colorectal Conditions. PhD Dissertation. [Google Scholar]
- Martínez-Laorden A., Arraiz-Fernández C., González-Fandos E. Microbiological quality and safety of fresh turkey meat at retail level, including the presence of ESBL-producing Enterobacteriaceae and methicillin-resistant S. aureus. Foods. 2023;12:1274. doi: 10.3390/foods12061274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi H., Ari M.M., Shahlaei M., Moradi S., Farhadikia P., Alvandi A., Abiri R. Designing multi-epitope vaccine against important colorectal cancer (CRC) associated pathogens based on immunoinformatics approach. BMC Bioinf. 2023;24:65. doi: 10.1186/s12859-023-05197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A.R., de Castro M.F., Pimentel S.P., de Carvalho T.P., Santana C.H., Santos D.D.O., Tinoco H.P., Coelho C.M., Pessanha A.T., da Paixão T.A., Santos R.L. Streptococcus pasteurianus-induced valvular endocarditis and sepsis in a puerperal emperor tamarin (Saguinus imperator) J. Med. Primatol. 2022;51:388–391. doi: 10.1111/jmp.12587. [DOI] [PubMed] [Google Scholar]
- Pasquereau-Kotula E., Martins M., Aymeric L., Dramis S. Significance of Streptococcus gallolyticus subsp gallolyticus association with colorectal cancer. Front. Microbiol. 2018;9:614. doi: 10.3389/fmicb.2018.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. 2nd ed. SAS Institute Inc.; Cary, NC: 2002. SAS/STAT User's Guide. [Google Scholar]
- Saumya D., Wijetunge S., Dunn P., Wallner-Pendleton E., Lintner V., Matthews T., Pierre T., Kariyawasam S. Acute septicemia caused by Streptococcus gallolyticus subsp. pasteurianus in turkey poults. Avian Dis. 2014;58:318–322. doi: 10.1637/10617-071813-Case.1. [DOI] [PubMed] [Google Scholar]
- Schlegel L., Grimont F., Ageron E., Grimont P.A.D., Bouvet A. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int. J. Syst. Evol. Microbiol. 2003;3:631–645. doi: 10.1099/ijs.0.02361-0. [DOI] [PubMed] [Google Scholar]
- Schulz J., Dumke J., Hinse D., Dreier J., Habig C., Kemper N. Organic turkey flocks: a reservoir of Streptococcus gallolyticus subspecies gallolyticus. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui R., Boghossian A., Alharbi A.M., Alfahemi H., Khan N.A. The pivotal role of the gut microbiome in colorectal cancer. Biology. 2022;11:1642. doi: 10.3390/biology11111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitthicharoenchai P., Burrough E.R., Arruda B.L., Sahin O., Dos Santos J.G., Magstadt D.R., Piñeyro P.E., Schwartz K.J., Rahe M.C. Streptococcus gallolyticus and bacterial endocarditis in swine, United States, 2015–2020. Emerg. Infect. Dis. 2022;28:192–195. doi: 10.3201/eid2801.210998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T. 2000. Care and Incubation of Hatching Eggs. Mississippi State University Extension Service. Accessed Aug. 2022. https://docplayer.net/23957919-Care-and-incubation-of-hatching-eggs.html
- Suvarna K., Mahon C.R. In: Pages 224–346 in Textbook of Diagnostic Microbiology. Mahon C.R., Lehman D.C., editors. Elsevier; St. Louis, MO: 2022. Streptococcus, Enterococcus, and other catalase-negative, gram-positive cocci. [Google Scholar]
- Teh W.K., Dramsi S., Tolker-Nielsen T., Yang L., Givskov M. Increased intracellular cyclic di-AMP levels sensitize Streptococcus gallolyticus subsp. gallolyticus to osmotic stress and reduce biofilm formation and adherence on intestinal cells. J. Bacteriol. 2019;201:e00597–e00618. doi: 10.1128/JB.00597-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Wang Y., Fan L., Yang N., Pan J., Han Y., Wang X., Li Q., Guo G., Zheng J., Zeng W. Novel Streptococcus uberis sequence types causing bovine subclinical mastitis in Hainan, China. J. Appl. Microbiol. 2022;132:1666–1674. doi: 10.1111/jam.15235. [DOI] [PubMed] [Google Scholar]