Abstract
Heat stress in poultry is a serious concern, affecting their health and productivity. To effectively address the issue of heat stress, it is essential to include antioxidant-rich compounds in the poultry diet to ensure the proper functioning of the redox system. Microalgae (Spirulina platensis) are rich in antioxidants and have several health benefits in humans and animals. However, its role in health and production and the underlying mechanism in heat-stressed broilers are poorly understood. This study aimed to determine the effect of microalgae supplementation on the health and production of heat-stressed broilers. Cobb500 day-old chicks (N = 144) were raised in litter floor pens (6 pens/treatment and 8 birds/pen). The treatment groups were: 1) no heat stress (NHS), 2) heat stress (HS), and 3) heat stress + 3% microalgae (HS+MAG). The broilers in the HS+MAG group were fed a diet supplemented with 3% microalgae, whereas NHS and HS groups were fed a standard broiler diet. Broilers in the NHS were raised under standard temperature (20°C–24°C), while HS and HS+MAG broilers were subjected to cyclic heat stress from d 22 to 35 (32°C–33°C for 8 h). Heat stress significantly decreased the final body weight, whereas the supplementation of microalgae increased the final body weight of broilers (P < 0.05). The expressions of ileal antioxidant (GPX3), immune-related (IL4), and tight-junction (CLDN2) genes were increased in microalgae-supplemented broilers compared to heat-stressed broilers (P < 0.05). The ileal villus height to crypt depth ratio was improved in microalgae-supplemented broilers (P < 0.05). In addition, microbial alpha, and beta diversities were higher in the HS+MAG group compared to the HS group (P < 0.05). There was an increase in volatile fatty acid-producing bacteria at the genus level, such as Ruminococcus, Ocillospira, Lactobacillus, Oscillobacter, Flavonifractor, and Colidextribacter in the group that received microalgae supplementation. In conclusion, dietary supplementation of microalgae improved the growth performances of heat-stressed broilers by improving their physiogenomics. Thus, the dietary inclusion of microalgae can potentially mitigate heat stress in broilers.
Key words: broiler, heat stress, microalgae, antioxidant, health
INTRODUCTION
The world's population is growing exponentially, causing a rise in demand for animal-based protein. To meet the meat demand, broiler chickens have been genetically selected for higher feed efficiency and high muscle yield. As a result, these broiler chickens produce a high amount of metabolic body heat and are highly susceptible to heat stress (HS), especially during the summer. Moreover, chickens lack sweat glands and have feather covers, which make them extremely sensitive to heat stress. Exposure to heat stress disrupts the thermoregulatory mechanism of chickens, leading to an imbalance in their physiological redox status. Consequently, heat stress can adversely impact broilers’ health and production. Therefore, the increasing environmental temperature during the summer and amid global warming is an apparent concern of the poultry industry, leading to heat stress and severe economic loss (Wasti et al., 2020). To combat the harmful impact of heat stress on chickens, various nutritional, managemental, and genetic strategies have been implemented (Saeed et al., 2019; Abbas et al., 2022). Mitigating heat stress using managemental and genetic strategies is expensive and time-consuming. Therefore, reducing the impact of heat stress in chickens is more viable by application of nutritional strategy which entails phytochemicals, probiotics, prebiotics, vitamins, and minerals. Phytochemicals, such as antioxidants, antibacterial, antiviral, and antineoplastic, are becoming increasingly popular and have health remedial action in heat-stressed poultry (Kumar et al., 2021). It is of utmost importance to develop a sustainable strategy for the poultry industry to combat heat stress. Thus, exploring new feed supplements with heat stress-alleviating functional properties is critically necessary.
Microalgae (Spirulina platensis) are sustainable sources of energy and are available commercially across the world. Microalgae are filamentous photosynthetic cyanobacteria abundant in protein (∼65%), carbohydrates (∼25%), essential fatty acids (∼18%), vitamins, and minerals (Brito et al., 2020; Pestana et al., 2020). In addition to nutritional abundancy, microalgae are also rich in functional bioactive compounds such as phycobiliproteins (β-phycocyanin and C-phycocyanin), β-carotene, phenolic acid, flavonoid, γ-linolenic acid (Farag et al., 2016; Wu et al., 2016). Microalgae also possess alkaloids, glycosides, tannins, steroids, and saponins (Zeweil et al., 2016). These biomolecules are associated with several health benefits in humans and animals and act as; scavengers of reactive oxygen species (ROS), reactive nitrogen species (RNS), inhibitors of neoplasia, inflammatory mediators, and suppressors of pathogenic bacteria (Dillard and German, 2000). Microalgae supplementation in the diet improves the production of key antioxidant enzymes and confers cellular protection (Mirzaie et al., 2018;Liu et al., 2021; Moustafa et al., 2021). However, the role of microalgae in health and production, along with the underlying mechanism in heat-stressed broilers, is not completely explored. Based on the biochemical properties and health benefits of microalgae, we hypothesized that supplementation of microalgae to heat-stressed broilers' diet might improve growth performances by affecting underlying health-associated mechanisms. Therefore, this study aimed to investigate the effect of microalgae supplementation in broilers’ diets on underlying gut health parameters (expression of antioxidants, heat shock, immune, and tight-junction genes), ileal histomorphometry, volatile fatty acid production, cecal microbiota, and growth performances.
MATERIALS AND METHODS
Experimental Birds, Husbandry, and Diet
The animal experimentation was accomplished at the Magoon Research Station of the College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa. The animal activities were approved by the Institutional Animal Care and Use Committee (Approval No.: 17-2605-6), University of Hawaii at Manoa. Day-old chicks (Cobb500, N = 144, average body weight = 35 g) were raised on litter floor pens (6 replicates/treatment and 8 birds/replicate) in 3 treatment groups: 1) No heat stress fed with basal diet (NHS), 2) Heat stress fed with basal diet (HS), and 3) Heat stress supplemented with 3% microalgae (HS+MAG). Broilers in the NHS were raised in standard condition (18°C–24°C) throughout the experiment, while HS and HS+MAG broilers were subjected to cyclic heat stress using electric heaters (32°C–33°C for 10 h, 8 am–6 pm), which mimics the natural cycle of heat from d 22 to 35. The birds were allowed ad libitum access to fresh feed and water. RH was kept at 50 ± 5%, and a photoperiodic condition of 23L:1D was sustained until the end of the experiment. The pens were arranged into a completely randomized design. The size of each pen was 1 m × 0.61 m, and the stocking density was 0.08 m2/bird. The experimental house had an appropriate ventilation system. Birds were monitored thrice a day to assess their behavioral and health conditions.
The broilers were fed a corn-soybean meal-based (SBM) mash diet in 2 phases (starter and finisher). National Research Council recommended nutritional requirement of commercial broilers was met for the starter (d 0–21) and finisher diet (d 22–35) (NRC, 1994). The energy and protein requirements were adjusted based on the growth stage of the birds (Cobb-Vantress, 2018). The broilers' diet in the HS+MAG supplemented with 3% microalgae, whereas broilers in NHS and HS groups were fed with a standard diet (Table 1). The HS+MAG group received microalgae-supplemented diet throughout the experimental period. Average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were calculated based on the body weight and feed intake data recorded every week. The phytochemical composition of Spirulina is presented in Table S2, and the nutritional and fatty acid profile is in supplementary Table S2.
Table 1.
Ingredients and nutrient composition of the experimental diet.
| Starter diet (1–21 d) |
Finisher diet (22–35 d) |
|||
|---|---|---|---|---|
| Ingredients, % | Standard | 3% Microalgae | Standard | 3% Microalgae |
| Corn | 53.67 | 52.83 | 60.84 | 60.5 |
| SBM | 38 | 36 | 31 | 29 |
| Microalgae | 0 | 3 | 0 | 3 |
| Soybean oil | 5 | 4.9 | 5.5 | 5 |
| Limestone | 1.35 | 1.35 | 1.2 | 1.1 |
| Monocalcium phosphate | 0.75 | 0.75 | 0.44 | 0.44 |
| Lysine | 0.18 | 0.13 | 0.1 | 0.04 |
| Methionine | 0.18 | 0.17 | 0.13 | 0.13 |
| Threonine | 0.04 | 0.04 | 0 | 0 |
| NaCl | 0.2 | 0.2 | 0.18 | 0.18 |
| Sodium bicarbonate | 0.12 | 0.12 | 0.1 | 0.1 |
| Vitamin-mineral premix* | 0.5 | 0.5 | 0.5 | 0.5 |
| Total | 100 | 100 | 100 | 100 |
| Calculated nutrient contents, % | ||||
| AMEn, kcal/kg | 3040 | 3044 | 3165 | 3147 |
| CP | 21.47 | 21.46 | 18.54 | 18.56 |
| Ca | 0.91 | 0.91 | 0.77 | 0.74 |
| Total P | 0.71 | 0.71 | 0.61 | 0.62 |
| Available P | 0.45 | 0.45 | 0.37 | 0.37 |
| Lysine | 1.32 | 1.32 | 1.09 | 1.08 |
| Methionine | 0.52 | 0.55 | 0.44 | 0.48 |
| Cysteine | 0.42 | 0.41 | 0.40 | 0.38 |
| Threonine | 0.87 | 0.92 | 0.73 | 0.78 |
| Tryptophan | 0.31 | 0.33 | 0.27 | 0.28 |
| Methionine+cysteine | 0.92 | 0.88 | 0.82 | 0.79 |
| Arginine | 1.55 | 1.48 | 1.35 | 1.28 |
| Valine | 1.18 | 1.13 | 1.05 | 1.00 |
| Isoleucine | 0.90 | 0.95 | 0.78 | 0.84 |
| Leucine | 1.82 | 1.90 | 1.66 | 1.74 |
| NDF | 8.86 | 8.57 | 8.73 | 8.49 |
| CF | 3.84 | 3.68 | 3.51 | 3.36 |
| Na | 0.16 | 0.16 | 0.14 | 0.14 |
| Cl | 0.16 | 0.16 | 0.15 | 0.15 |
| Choline (mg/kg) | 1370.53 | 1310.71 | 1223.82 | 1167.09 |
| Dig lysine % | 1.17 | 1.08 | 0.95 | 0.86 |
| Dig methionine % | 0.48 | 0.46 | 0.40 | 0.39 |
| Dig threonine % | 0.67 | 0.64 | 0.55 | 0.52 |
Includes the following (per kg of diet): vitamin A (trans-retinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 3,000 IU; vitamin E (all-rac-tocopherol-acetate), 30 mg; vitamin B1, 2 mg; vitamin B2, 8 mg; vitamin B6, 4 mg; vitamin B12 (cyanocobalamin), 0.025 mg; vitamin K3 (bisulfate menadione complex), 3 mg; choline (choline chloride), 250 mg; nicotinic acid, 60 mg; pantothenic acid (D-calcium pantothenate), 15 mg; folic acid, 1.5 mg; betaíne anhydrous, 80 mg; D-biotin, 0.15 mg; zinc (ZnO), 80 mg; manganese (MnO), 70 mg iron (FeCO3), 60 mg; copper (CuSO4·5H2O), 8 mg; iodine (KI), 2 mg; selenium (Na2SeO3), 0.2 mg.
Sample Collection
The birds were euthanized using CO2 gas, and ileal tissues were collected (n = 6 birds/treatment; 1 bird/pen) as previously described (Wasti et al., 2021). To isolate total RNA, ileal segments were flushed with distilled water to remove any remaining digesta. Additionally, ileal sections were collected in 10% neutral buffered formalin for histomorphology. The cecal digesta were also collected for volatile fatty acid (VFA) and microbial metagenomics analysis. The samples for gene expression, VFA, and microbiome analysis were snap-frozen in liquid nitrogen and stored at −80°C until further analysis (Sah et al., 2018).
Total RNA Isolation and Real-Time qPCR
Total RNAs were isolated from the ileal tissues (∼100 mg) using TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Then, the total RNA concentration was determined using NanoDrop One (Thermo Fisher Scientific, Madison, WI), and the quality was determined using gel electrophoresis. RNAs were reverse transcribed to synthesize complementary DNA (cDNA) using a High-Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The expressions of the target genes were analyzed using real-time qPCR as previously described (Wasti, 2020). Specific primers were obtained from NCBI Primer-Blast to perform qPCR using PowerUp SYBR Green Master Mix (Applied Biosystems) on QuantStudio 3 real-time PCR system (Applied Biosystems, Foster City, CA). The qPCR plate preparation included PCR master mix, which consisted of 3 µL cDNA, 5 µL SYBR Green, and 1 µL of primers (forward and reverse, 5 µmol), making the final volume of 10 µL. Finally, the target genes were amplified following the standard protocol as previously described (Sah et al., 2018). To select the suitable housekeeping gene for the normalization of target genes, tissue samples were also analyzed with 3 housekeeping genes: glyceraldehyde 3-phosphate dehydrogenase (GAPDH), beta-actin (β-actin), and TATA-box binding protein (TBP), in triplicate. β-Actin was the most stable housekeeping gene in the ileum and was selected to normalize the target gene, and the fold change was calculated using the formula 2−ΔΔCt. The gene primers used in this analysis are listed in supplementary Table S3.
Ileum Histomorphometry
The ileal tissues were fixed in 10% neutral buffered formalin, paraffin-embedded after dehydration in a series of ethanol solutions as previously described (Wasti, 2020). The embedded tissues were sectioned at a thickness of 6 µm and then stained with hematoxylin and eosin (H&E). All the ileal sections were visualized under an Olympus microscope (U-TV0.63XC, Tokyo, Japan). A total of 6 intact, well-oriented villus-crypts units were selected in triplicate (18/sample), and images were taken. Villus height (VH; distance from the tip of the villus to the crypt), crypt depth (CD; distance from the villus base to the submucosa), and the ratio of villus height to crypt depth (VH: CD) were assessed by using Infinity Analyze software (Lumenera Corporation, Ottawa, ON, Canada). The villus surface area (VSA) was measured by using a formula, VSA = 2 × 3.14 × (VW/2) × VH, as previously determined (Sakamoto et al., 2000).
Volatile Fatty Acids Analysis
Approximately 200 mg of cecal digesta were used to determine major VFAs (acetate, propionate, and butyrate) as previously described (Singh et al., 2021). Deionized water (1,100 µL), trimethyl acetic acid (100 µL), and metaphosphoric acid (100 µL) were added to make the final volume of 1,500 µL. Then, samples were vortexed to homogenize and centrifuged at 15,000 rpm at 4°C for 10 min. Supernatants (1,000 µL) were used to analyze VFA using a gas chromatography system (TRACE 1300; Thermo Scientific, Waltham, MA). Helium was used as a carrier gas at a rate of 15.5 mL/min. The run time for the individual sample was set as 17.5 min with a sample injection of 0.5 µL. The initial temperature program was 120°C for 4 min and then increased to 160°C at 4°C/min. The standard stock solution mix containing formic, acetic, propionic, isobutyric, butyric, isovaleric, valeric, isocaproic, hexanoic, and n-caproic acids were used at the rate 0.1, 0.5, 1, 2, 4, 6, 8, 10, 12, and 14 mM. Data were processed on GC using Chromeleon 7.2 software (Thermo Scientific, Waltham, MA).
Enzyme-Linked Immunosorbent Assay (ELISA)
The chicken plasma immunoglobulins, IgA and IgY, were determined using the commercial ELISA Kit (Bethyl Laboratories, Montgomery, TX) following the manufacturer's protocol. Ten standards gradients (1,000, 333, 111, 37.04, 12.35, 4.12, 1.37, 0.456, 0.152, and 0) for IgA and 12 standard gradients (500, 166.67, 55.56, 18.52, 6.17, 2.06, 0.69, 0.23, 0.08, 0.03, 0.009, and 0 ng/mL) for IgY were prepared by serial dilutions. The plasma samples were diluted using Dilution Buffer B; IgA at 1:1,000 and 1:2,000, and IgY at 1:100,000 and 1:200,000. Then, 100 µL of each standard and sample was pipetted in the wells of the ELISA plate in duplicate, precoated with antichicken antibodies. The plate was incubated for an hour at room temperature, followed by washing. Then, 100 µL of Chicken IgA or IgY Detection Antibody was added to each well, incubated for 1 h, and washed again. The colorimetric reaction was catalyzed for 30 min by adding streptavidin-conjugated horseradish peroxidase with TMB substrate. Finally, the reaction was terminated by adding 100 µL of Stop Solution, and the absorbance was measured using a multimode ELISA plate reader (SynergyLX, Biotek, Santa Clara, CA) at 450 nm.
DNA Extraction and Microbial Metagenomics
Cecal microbial DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany), and the concentrations were assessed on NanoDrop One (Thermo Fisher Scientific, Madison, WI). Using this DNA, V3 to V4 hypervariable region of the 16S rRNA gene was amplified following Illumina using primer 5′-CCTACGGGNGGCWGCAG-3′ and 5′-GACTACHVGGGTATCTAATCC-3′ (Klindworth et al., 2013). After quantification and purification, the amplicons were pooled, and pair-end sequenced using an Illumina MiSeq (Illumina, San Diego, CA). The final sequences were analyzed in CLC Genomics Workbench 22.0.2 (Qiagen, Hilden, Germany). The selected reads were clustered as operational taxonomical units (OTUs) with 97% sequence similarity against the reference database of Greengenes v13_8 97%. Then, the alpha diversity was established by Chao 1, Simpson's index, and Shannon entropy and demonstrated on a boxplot. Beta diversity was established by Bray-Curtis, Weighted, and Unweighted UniFrac on principal coordinate analysis.
Statistical Analysis
The data were analyzed using 1-way ANOVA in GraphPad Software (GraphPad Software, San Diego, CA) and RStudio (R version 4. 2. 2, RStudio PBC, Boston, MA). All the results are presented as mean ± SEM. The differences in the mean between treatment groups were assessed using Tukey's post hoc test. The CLC Workbench Genomics module employed a pairwise Kruskal-Wallis test for alpha diversity and a PERMANOVA test for beta diversity. The relationship between differentially enriched microbiota and other parameters was assessed by conducting Spearman rank correlation analysis in GraphPad. The differences were considered statistically significant at P < 0.05.
RESULTS
Growth Performance
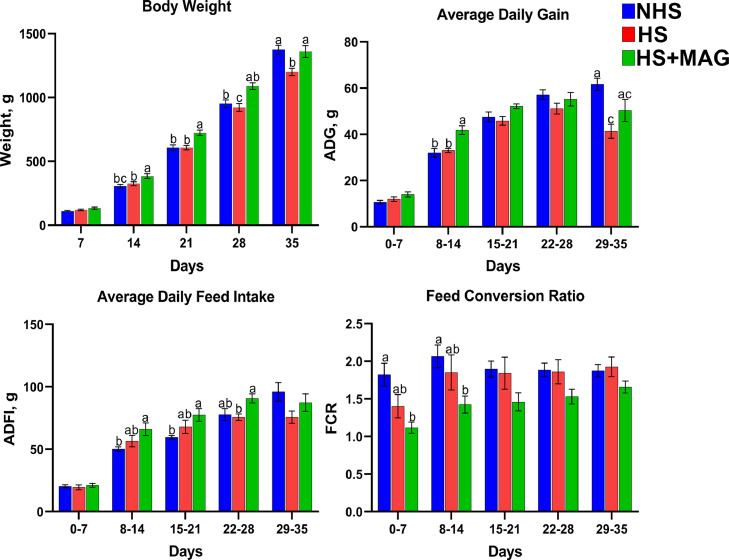
The effects of microalgae supplementation on the growth performances of the heat-stressed broilers are shown in Figure 1. The treatment groups fed with basal diet had similar body weights until d 21, whereas heat-stressed broilers supplemented with microalgae had higher body weights at the concurrent time points (P < 0.05). The exposure to cyclic heat stress significantly decreased the final body weight compared to the control, whereas dietary supplementation of microalgae increased the final body weight of the heat-stressed broilers. Heat stress reduced the ADG, whereas microalgae supplementation improved the ADG throughout the study period. Although insignificant, the trend shows that final ADFI was decreased when the birds were exposed to heat stress, whereas supplementation of microalgae improved the ADFI. The supplementation of microalgae resulted in an improved FCR and became evident when 2 HS groups were compared.
Figure 1.
Effects of microalgae supplementation on the growth performances of the heat-stressed broilers: body weight, average daily gain, average daily feed intake, and feed conversion ratio. Different letters indicate a significant difference between treatments at P < 0.05.
Ileal Gene Expression
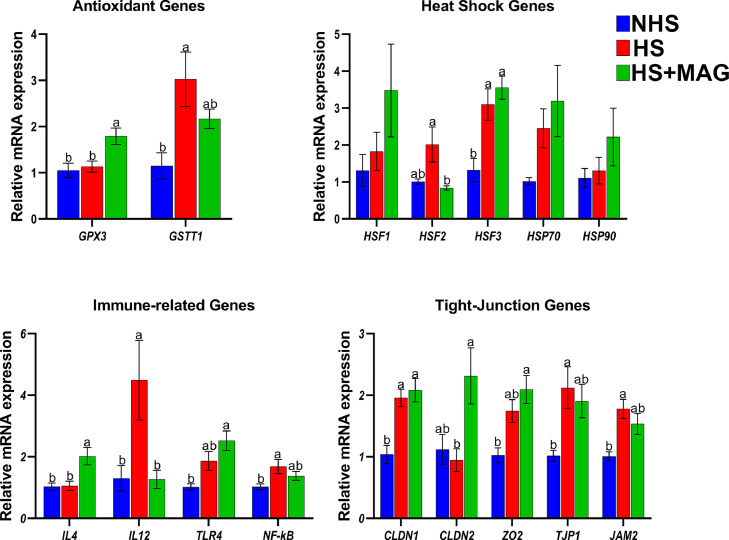
The effects of microalgae supplementation on the expressions of antioxidants, heat shock, immune, and tight-junction genes were analyzed using qPCR. The genes that are expressed differently are presented in Figure 2. The mRNA expression of GPX3 was not changed in the HS group compared to the NHS group, whereas supplementation of microalgae significantly increased its expression in the HS+MAG group (P < 0.05). The mRNA expression of GSTT1 was significantly increased in the HS group than in the NHS group; however, microalgae supplementation decreased its expression more than in the HS group (P < 0.05). Heat stress had no significant effect on the mRNA expression of HSF2 compared to the NHS group, whereas microalgae supplementation decreased its expression (P < 0.05). Similarly, HSF3 mRNA expression was significantly higher (P < 0.05) in the HS and HS+MAG groups, whereas there was no difference between the HS and HS+MAG groups. Other heat shock genes such as HSF1, HSP70, and HSP90 exhibited an increasing trend with microalgae supplementation.
Figure 2.
Effects of microalgae supplementation on the relative mRNA expression of the heat-stressed broilers: antioxidant, heat shock, immune, and tight-junction genes. Different letters indicate a significant difference between treatments at P < 0.05.
Proinflammatory cytokine gene IL4 was not affected by heat stress compared to the control; however, it was significantly upregulated in the microalgae-supplemented group than in the HS group (P < 0.05). The mRNA expression of the IL12 gene was significantly higher in heat-stressed birds than in others (P < 0.05). The expression of TLR4 mRNA showed no significant difference with heat stress, whereas supplementation of microalgae significantly increased its expression compared to the NHS group (P < 0.05). Similarly, the expression of the NF-kB gene was significantly increased after heat stress, whereas supplementation of microalgae had no significant effect (P < 0.05).
The tight-junction gene CLDN1 expression was significantly higher in the HS and HS+MAG than in the NHS group, but there was no difference between HS and HS+MAG groups (P < 0.05). The mRNA expression of CLDN2 was significantly higher (P < 0.05) in the microalgae-supplemented group than in others. Microalgae supplementation increased the expression of ZO2 mRNA in the HS+MAG group than in the NHS; however, there was no apparent change between HS and HS+MAG groups (P < 0.05). The expressions of TJP1 and JAM2 were increased in the birds subjected to heat stress, whereas the microalgae supplementation had no significant effect compared to the HS group but increased than the control group (P < 0.05).
Ileal Histomorphometry
Heat stress did not affect VH, whereas the supplementation of microalgae showed significant improvement in the VH of the HS+MAG group (P < 0.05). Heat stress significantly increased the CD than NHS birds (P < 0.05); however, CD slightly decreased with microalgae supplementation, although insignificant. The VH: CD ratio was significantly decreased in heat-stressed birds, whereas microalgae supplementation significantly improved the VH: CD ratio (P < 0.05). Supplementation of microalgae in heat-stressed birds showed improvement in VSA, but not statistically significant, as shown in Figure 3.
Figure 3.
Effects of microalgae supplementation on ileum histomorphology of the heat-stressed broilers: VH, CD, VH: CD, and VSA. Different letters indicate a significant difference between treatments at P < 0.05.
Production of Volatile Fatty Acids
The production of VFAs such as acetate, propionate, butyrate, and total VFAs was decreased in the cecal digesta of the heat-stressed group, whereas the supplementation of microalgae increased the concentration of acetate and total VFAs, but the changes were insignificant (P > 0.05) as shown in Figure 4.
Figure 4.
Effects of microalgae supplementation on cecal volatile fatty acids production of heat-stressed broilers: acetate, propionate, butyrate, and total VFAs.
Plasma Immunoglobulins
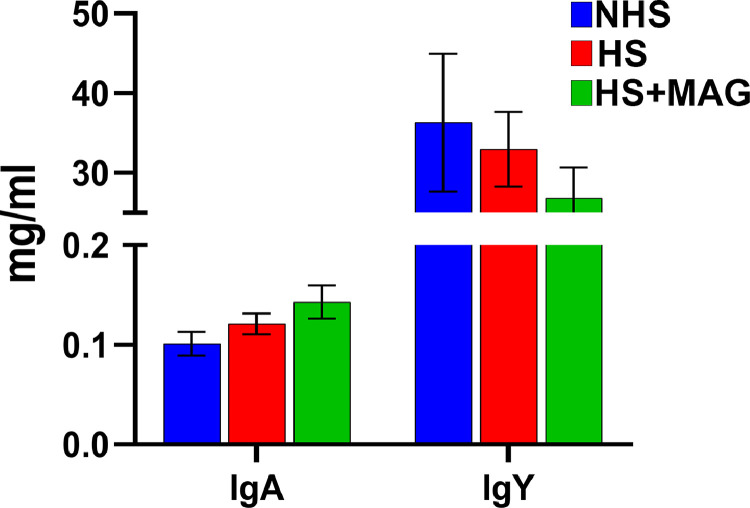
As shown in Figure 5, microalgae supplementation had no significant effects on the plasma immunoglobulin (IgA and IgY) concentration (P > 0.05). However, microalgae supplementation tended to increase plasma IgA in heat-stressed birds.
Figure 5.
Effects of microalgae supplementation on plasma IgA and IgY of the heat-stressed broilers.
Microbial Alpha and Beta Diversity
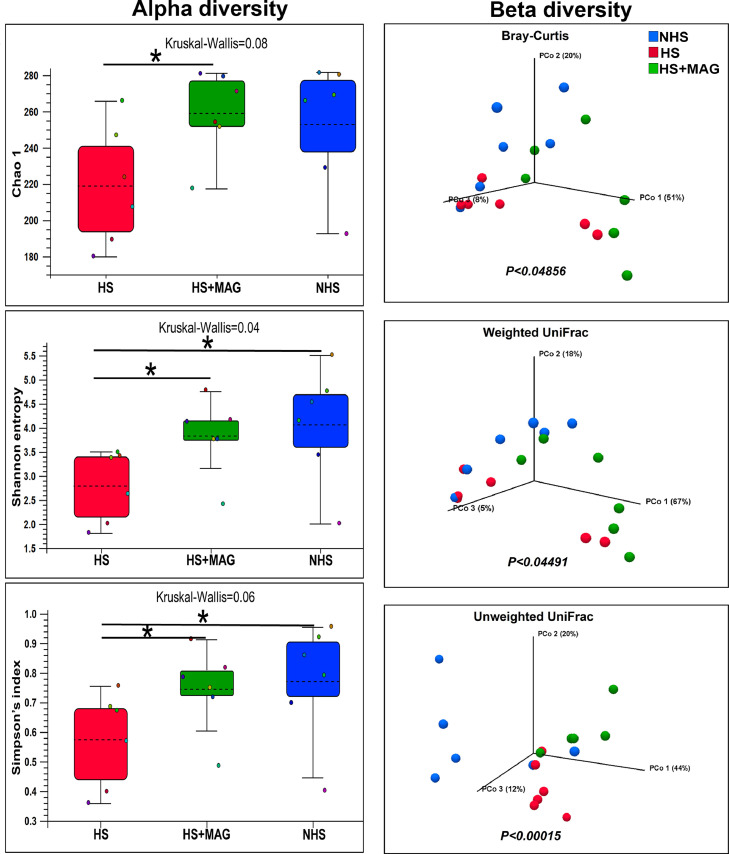
Alpha diversity refers to the number and abundance of microbial species within a treatment measured by Chao 1, Shannon entropy, and Simpson's index at P < 0.05. The Chao1 index estimates the species’ richness (i.e., the number of species present) in a treatment. Simpson's index measures microbial richness or evenness, while Shannon entropy measures species richness and community's evenness in a sample or within a treatment. Chao 1, Shannon entropy, and Simpson's index were significantly increased in the microalgae-supplemented group relative to the HS group (Figure 6).
Figure 6.
Effects of microalgae supplementation on microbial alpha diversity: Chao 1, Shannon entropy, Simpson's index, and beta diversity: Bray-Curtis, Weighted, and Unweighted UniFrac, in heat-stressed broilers. The effect of treatment was statistically different at P < 0.05.
Beta diversity estimates the degree of dissimilarity in microbial community composition between different environments/treatments. In our study, the principal component analysis (PCoA) showed a significant difference in the Bray-Curtis (P = 0.04856), Weighted (P = 0.04491), and Unweighted (P = 0.00015) UniFrac of microbial composition as shown in Figure 6.
Microbial Enrichments
The microbial composition was determined at phylum, class, order, family, and genus level (Figure 7). The most dominant phyla include Firmicutes and Proteobacteria across treatment groups. The abundance of Firmicutes and Proteobacteria varied in the NHS (95 and 5%, respectively), HS (81 and 19%, respectively), and HS+MAG (62 and 38%, respectively) groups.
Figure 7.
Relative abundance of microbiota at phylum, class, order, family, and genus level.
The cecal microbial composition, at class level, was dominated by Clostridia, Gamma-Proteobacteria, and Bacilli, and their composition varied in NHS (92, 5, and 3%, respectively), HS (76, 20, and 4%, respectively), and HS+MAG (61, 37, and 2%, respectively) groups.
The Clostridiales, Enterobacteriales, and Turicibacterales were the most abundant orders, and they were present at different proportions in NHS (92, 5, and 3%, respectively), HS (78, 14, and 3%, respectively), and HS+MAG (61, 37, and 2%, respectively) groups.
The Enterobacteriaceae, Lachnospiraceae, and Ruminococcaceae were most abundant in bacterial families and present in various proportions in NHS (4, 26, and 20%, respectively), HS (19, 16, and 9%, respectively), and HS+MAG (37, 15, and 23%, respectively) groups.
The Oscillospira, Ruminococcus, and Turicibacter were the most abundant genera, and they were present at different proportions in NHS (46, 17, and 12%, respectively), HS (30, 18, and 20%, respectively), and HS+MAG (58, 20, and 5%, respectively) groups.
Microbial Correlation With Measured Parameters
The Spearman's rank correlation between the microbial genus and measured parameters was significantly changed (Table 2). The expression of antioxidant genes (GPX3) positively correlated with Lactobacillus, whereas PRDX1, CAT, and SOD2 were negatively correlated with Ruminococcus, Lachnoclostridium, Flavonifractor, and Anaerotruncus. Similarly, the heat shock genes HSF3, HSP70, and HSP90 were negatively correlated with Romboutsia and Lachnospiraceae_unclultured genus. The genes related to immunity, such as TLR4, IL1β, AvBD4, IL4, and NF-kB were negatively correlated with Romboutsia, Blautia, Lachnospiraceae_unclultured genus, Lachnoclostridium, and Eisenbergiella. The genes NF-kB and IL4 were also positively correlated with Flavonifractor and Oscillibacter, respectively. The expressions of tight-junction-related genes (ZO2, CLDN1, CLDN2, and OCLN) were negatively correlated with Romboutsia, Christensenellaceae R-7 group, and Lachnoclostridium. There was a negative association between SGLT1 and Lactobacillus, and Anaerotruncus. The appetite-related intestinal genes; GLP1R, GLP2R, and Galanin also negatively correlated with Ruminococcus, Blautia, Flavonifractor, and Oscillibacter. The body weight was negatively correlated with Turicibacter, and VH: CD ratio was positively correlated with Clostridia UCG-014.
Table 2.
Spearman correlation between different parameters and most abundant microbial species.
| Variables | Differential microbiota | P | r |
|---|---|---|---|
| GPX3 | Lactobacillus | 0.0422 | 0.4832 |
| SGLT1 | Lactobacillus | 0.0260 | −0.5229 |
| PRDX1 | Ruminococcus | 0.0389 | −0.4902 |
| GLP1R | Ruminococcus | 0.0052 | −0.6285 |
| HSF3 | Romboutsia | 0.0007 | −0.7214 |
| HSP70 | Romboutsia | 0.0186 | −0.5480 |
| ZO2 | Romboutsia | 0.0162 | −0.5576 |
| CLDN1 | Romboutsia | 0.0024 | −0.6698 |
| TLR4 | Romboutsia | 0.0001 | −0.7785 |
| CASP3 | Romboutsia | 0.0090 | −0.5996 |
| IL1B | Romboutsia | 0.0180 | −0.5521 |
| AvBD4 | Blautia | 0.0270 | −0.5191 |
| GLP2R | Blautia | 0.0440 | −0.4799 |
| HSF3 | Lachnospiraceae_unclultured genus | 0.0027 | −0.6636 |
| HSP70 | Lachnospiraceae_unclultured genus | 0.0186 | −0.5480 |
| HSP90 | Lachnospiraceae_unclultured genus | 0.0335 | −0.5026 |
| TLR-4 | Lachnospiraceae_unclultured genus | 0.0057 | −0.6236 |
| ZO2 | Lachnoclostridium | 0.0219 | −0.5359 |
| NF-kB | Lachnoclostridium | 0.0500 | −0.4654 |
| IL-4 | Lachnoclostridium | 0.0182 | −0.5493 |
| OCLN | Lachnoclostridium | 0.0100 | −0.5913 |
| CADHERIN | Lachnoclostridium | 0.0350 | −0.4997 |
| CAT | Lachnoclostridium | 0.0250 | −0.5273 |
| GLP2R | Flavonifractor | 0.0080 | −0.6071 |
| NF-kB | Flavonifractor | 0.0241 | 0.5287 |
| SOD2 | Flavonifractor | 0.0360 | 0.4977 |
| SGLT1 | Anaerotruncus | 0.0230 | −0.5323 |
| SOD2 | Anaerotruncus | 0.0460 | −0.4755 |
| Galanin | Oscillibacter | 0.0500 | −0.0918 |
| IL-4 | Oscillibacter | 0.0322 | 0.5059 |
| CLDN2 | Christensenellaceae R-7 group | 0.0004 | 0.7482 |
| IL-4 | Eisenbergiella | 0.0024 | −0.6680 |
| Body weight | Turicibacter | 0.0399 | −0.0627 |
| VH: CD | Clostridia UCG-014 | 0.0388 | 0.4904 |
The correlations are statistically significant at P < 0.005.
DISCUSSION
Broiler chickens are a great source of animal-sourced protein for human consumption. However, high environmental temperatures negatively impact broilers' health and production, resulting in severe economic loss in the broilers industry (Lara and Rostagno, 2013; Nawab et al., 2018; Humam et al., 2019). Therefore, it is crucial to mitigate heat stress in broilers using sustainable strategies. This study found that cyclic heat stress decreased the final body weight, ADG, and ADFI while increasing FCR in broilers. However, dietary microalgae supplementation increased final body weight, ADG, and ADFI, and improved FCR. Further, this study revealed that the dietary supplementation of microalgae improved the redox system, intestinal integrity, and gut microbiome of heat-stressed broilers. This positive outcome of the study suggests that microalgae could be a beneficial supplement in broilers for combating the negative effects of heat stress.
Broilers that experience heat stress are prone to oxidative stress, as their endogenous antioxidant system cannot effectively eliminate cellular free radicals (Wasti et al., 2020). To understand the mechanism by which microalgae supplementation improved the redox system, we analyzed the expressions of several antioxidant-related genes in the ileum. The expression of the GPX3 was improved in heat-stressed broilers supplemented with microalgae, which is attributed to the antioxidant properties of microalgae. The increased expression of the GSTT1 in the heat-stressed group indicates the acclimatization of broilers to chronic heat stress. Free radicals, ROS/RNS, are normally maintained at physiological levels. However, heat stress causes excessive generation of free radicals and disrupts the redox dynamic leading to oxidative damage to proteins, lipids, and nucleic acids (Arnaud et al., 2002). These free radicals are scavenged by the integrated action of antioxidant genes, including superoxide dismutase, glutathione peroxidase, and catalase. Superoxide dismutase is considered the forefront enzyme as it catalyzes the superoxides to H2O2, which are neutralized to H2O and O2 by glutathione peroxides and catalases (Surai, 2016; Surai et al., 2018). Glutathione S-transferases, including GSTT1, inhibit the Jun N-terminal kinase and confers protection against H2O-induced cellular damage (Sheehan et al., 2001). Therefore, heat stress damages intestinal epithelium by inducing oxidative stress, compromising intestinal integrity and nutrient absorption, which was improved by microalgae supplementation.
Heat shock proteins (HSPs) are produced in response to oxidative stress and act as molecular chaperones in protecting the cells from oxidative damage. Reactive oxygen species produced due to oxidative stress interact with cellular proteins and impair their functions. Thus, HSPs are produced as a downstream mechanism to facilitate protein scaffolding and prevent protein aggregation (Sikora and Grzesiuk, 2007). Transcriptional factors such as heat shock factors (HSF1-4) bind to heat shock regulatory elements to increase the biosynthesis of heat shock proteins (Fujimoto and Nakai, 2010). This study demonstrated that microalgae supplementation improved the production of HSP70, HSP90, HSF1, and HSF3. In avian species, HSF1 and HSF3 are key transcriptional regulators for producing substantial heat shock proteins (Inouye et al., 2003; Cedraz et al., 2017). HSF1 is believed to activate at medium thermal stress, whereas HSF3 is activated at chronic thermal stress (Cedraz et al., 2017). With the minimal role of HSF2 in heat stress, it is responsible for the developmental process and activated during spermatogenesis, embryogenesis, and neurogenesis (Pirkkala et al., 2001). Therefore, supplementation of microalgae in heat-stressed broilers showed a promising result by increasing the expression of protective heat shock proteins.
The immune status of a flock is an indispensable factor in poultry production as it protects birds from endogenous and exogenous pathogens. Heat stress compromises immunity by disturbing the proper development of the immune organs and reducing the immunocompetence of birds to pathogens (Chegini et al., 2018; Hirakawa et al., 2020). Once pattern recognition receptors recognize the notorious substances, the TLR4-NF-kB signaling pathway leads to the production of proinflammatory cytokines (Shibata et al., 2011; Tang et al., 2021). Cytokines are immune regulatory proteins secreted by a wide variety of cells that are important in stimulating the immune response in birds. Heat stress alters the expression of these immune markers, weakens the immune system, and makes it harder for the body to fight off infections. However, a significant upregulation of the IL12 gene in the heat-stressed birds symbolizes the acclimatization of birds to chronic heat stress. IL12 activates natural killer cells and induces the production of interferon-ƴ (Balu et al., 2011). In this study, the mRNA expressions of immune genes such as TLR4, NF-kB, IL4, and IL12 were increased in the birds supplemented with microalgae. The increased expressions of these genes are associated with enhanced immunity in microalgae-supplemented birds related to the immunostimulatory effect of microalgae.
Intact intestinal mucosal integrity is paramount for the efficient absorption of nutrients and for preventing paracellular diffusion of notorious antigens. The tight-junction proteins are dynamic structures formed by the interaction of multimeric proteins (Aijaz et al., 2007), which create a seal between adjacent epithelial cells (Farquhar and Palade, 1963). The junctional transmembrane proteins, occludins (OCLN), claudins (CLDN), junctional adhesion molecule (JAM), and tricelluin interact with intracellular scaffold protein called zona occludens (ZO), which anchors with the actin cytoskeleton (Lee, 2015). However, heat stress damages the gut epithelial lining and disrupts the barrier function. However, heat stress-induced oxidative damage can impair the digestion and absorption of nutrients in poultry (Mishra and Jha, 2019). In this study, CLDN1, CLDN2, ZO2, TJP1, and JAM2 expressions were increased in the heat-stressed birds supplemented with microalgae. The upregulation of tight-junction genes indicates the adaptation of the birds to chronic heat stress. The increased expression of tight-junction genes with microalgae supplementation is ascribed to the protective roles of bioactive compounds. Small intestines are accountable for digestion and absorption of nutrients and, ultimately, the final growth.
In addition to the underlying health markers, the intestinal mucosal microstructures are extremely important for efficient nutrient absorption and comprise finger-like projections known as villi. Villi are further composed of minuscule structures called microvilli. Villi increase the small intestine's surface area, which is essential for nutrient absorption (Noy et al., 2001). However, many factors can impact intestinal health, altering the villi length and thus reducing the absorption potential of the small intestine, such as heat stress (Burkholder et al., 2008). This study showed that ileal villi height increased, and crypt depth decreased with microalgae supplementation in heat-stressed birds. Consequently, microalgae supplementation significantly increased the ratio of villi height to crypt depth, which is fundamental for efficient nutrient absorption. Better absorption of nutrients leads to better growth of a bird.
The birds' performance also depends on the cecal volatile fatty acids (acetate, propionate, and butyrate). VFAs regulate gut development, barrier integrity, and immunity of birds (Kihara and Sakata, 1997; Yang et al., 2020). VFAs maintain a suitable milieu in the gut, influence the growth of epithelial cells, stimulate mineral absorption, maintain pH, and limit the invasion of pathogenic microorganisms (Walugembe et al., 2015). Most importantly, VFAs produce energy through glycolysis, and gluconeogenesis, and act as major fuel for colonocytes (Jha et al., 2019). In addition, VFA, mainly butyrate helps to minimize the pathogenic bacterial colonization of the intestine (Jha and Mishra, 2021). This study did not find remarkable changes in the cecal VFAs; however, acetate and total VFA showed an increasing trend in microalgae supplemented group.
Plasma immunoglobulins are the potential markers of immune status in birds. In this study, microalgae supplementation did not impact plasma IgA and IgY across the treatment groups. In the previous study, Spirulina supplementation in heat-stressed broilers did not affect plasma IgA and IgY immunoglobulin levels (Elbaz et al., 2022). Chickens are the only avian species with 3 major antibody classes: IgA, IgM, and IgY. Environmental factors such as heat stress can affect the birds' immune status and circulating antibody titers. However, there is a dearth of scientific evidence, and need more investigations to explain the baseline mechanism of heat stress on the immune physiology of birds.
The poultry gut is acclimatized for harboring complex microbial communities, including beneficial bacteria and pathogens (Barnes, 1979). As the chicks are hatched, their guts are colonized by various microorganisms and form an intricate relationship with the host. The gut microbiota has a vital role in gut development and maturation, fermenting polysaccharides, producing energy in the form of amino acids and VFAs, and immune establishment (Oakley and Kogut, 2016). Microbiota has bidirectional interplay with several organs affecting their physiology, such as the microbiota-gut-brain axis (Mullaney et al., 2022) and microbiota-gut-liver axis (Compare et al., 2012). However, heat stress disrupts the cecal microbial homeostasis leading to comorbidity and compromised growth performance. This study revealed that dietary supplementation of microalgae improved the microbial richness and diversity in heat-stressed broilers. Beta diversity determined with Weighted and Unweighted UniFrac showed a significant difference in microbial metagenomics. The changes in the bacterial diversities may be ascribed to the microalgal richness of polysaccharides acting as prebiotics and bioactive compounds. However, further investigations are required to explain the underlying reasons that may be associated with the effect of microalgal components on cecal microbiota.
Unlike other food animals, the anatomic peculiarity of the poultry gut (with a shorter intestinal tract and faster digesta transit) has a selective effect on microbial diversity (Pan and Yu, 2014). The cecum is the primary fermentation site harboring complex microbiota in poultry. These microbes affect intestinal villi morphology and potentially affect gut physiology by altering enzyme activities, tight junctions, and immune response. However, microbial species and their role are yet to be understood. In this study, OTU alignment in CLC Workbench revealed that Firmicutes, Proteobacteria, Actinobacteria, and Tenericutes were major phyla across the treatment groups making Firmicutes the most dominant phylum. This finding was consistent with the results of several previous studies (Sohail et al., 2015; Xiao et al., 2017; Wasti et al., 2021). Firmicutes include many genera of bacteria beneficial for gut health (Mancabelli et al., 2016).
The major microbial genera across the different groups were Oscillospira, Ruminococcus, Turicibacter, Coprococcus, Blautia, Dorea, and Anaerotruncus. Oscilliospira is associated with producing volatile fatty acids, enhancing mucin production, maintaining intestinal integrity, and reducing tissue damage (Duncan et al., 2002). The exposure of birds to heat stress reduced the Oscillospira population, which is consistent with the finding of previous studies (Shi et al., 2019). However, the dietary supplementation of microalgae increased the abundance of Oscillospira. Ruminococcus is one of the dominant bacteria with a dietary fiber fermenting role in producing volatile fatty acids such as butyrate, which is crucial in reducing inflammation and maintaining gut epithelial health (Lakshmanan et al., 2022). Moreover, Ruminococcus is also associated with the production of neurometabolites such as serotonin, norepinephrine, and N-acetyl aspartate, potentially affecting brain function and behavior (Mudd et al., 2017; Lukić et al., 2019). In the present study, the abundance of other genera of bacteria, Turicibacter, Coprococcus, Blautia, Dorea, and Anaerotruncus was increased in the heat-stressed birds and decreased in the microalgae-supplemented groups. Turicibacter influences the body's fat composition by affecting the bile acids and lipid metabolisms (Lynch et al., 2022). In addition, studies have shown that other genera of bacteria, such as Coprococcus, Blautia, Dorea, and Anaerotruncus are linked with the maintenance of gut microbial homeostasis, generation of crucial VFAs and vitamin B-complexes, and host immunity (Liu et al., 2021; Nogal et al., 2021; Palmnäs-Bédard et al., 2022). However, the role of most of the bacteria present in the birds’ ceca is not clear. Knowing and understanding the nature and effect of those bacteria on the bird's gut health can have further implications in the poultry industry.
Microbiome and host interaction have a significant influence on several biological processes. Gut microbial richness with beneficial bacteria can result in a useful impact; on the other hand, gut dysbiosis can be inimical, resulting in an undesirable effect on the health and performance of a bird (Singh et al., 2021). Though microbiota's impact on birds’ health has not been clearly illustrated, a correlation study can estimate the nexus between gut microbiota and several health parameters. The correlation study exhibited that several genes were significantly correlated with cecal microbiota. The expression of ileal GPX3 was positively correlated. In contrast, sodium-dependent glucose transporter 1 (SGLT1) was negatively correlated with Lactobacillus. These bacteria are important in maintaining a healthy gut through intestinal homeostasis, fermentation, and immune modulation (Leal et al., 2023). Ruminococcus negatively correlated with peroxidoredoxin 1 (PRDX1) and glucagon-like peptide 1 receptor (GLP1R). Several genes (HSF3, HSP70, ZO2, CLDN1, TLR4, CASP3, and IL1ß) were negatively correlated with Romboutsia and are assumed to have a potential role in the regulation of lipid and cholesterol metabolism (Yin et al., 2023). Metagenomic analysis correlated Lachnoclostridium with several beneficial roles in intestinal health, such as acetate production (Nogal et al., 2021) and also associated with atherosclerosis in mice (Cai et al., 2022). Lachnoclostridium was negatively correlated with ZO2, NF-kB, IL4, OCLN, CAT, and Cadherin. Flavonifractors regulate inflammation in obese mice and are linked with the metabolism of flavonoids such as catechins and quercetin (Mikami et al., 2020; Rodriguez-Castaño et al., 2020). Flavonifractor showed a negative correlation with GLP2R and a positive correlation with NF-kB and SOD2. Anerotruncus, negatively correlated with SGLT1 and SOD2 genes, are the bacteria that stimulate the regulatory T-cells and decrease inflammation in mouse models. Thus, these bacteria may play a significant role in immune modulation, improving birds’ performances.
In conclusion, the dietary supplementation of microalgae to heat-stressed birds improved the final body weight, intestinal histomorphology, and expressions of antioxidant, immune-related, and tight-junction genes. Moreover, microbial diversity and beneficial bacteria were highly enriched in the microalgae-supplemented group. These findings suggest that microalgae can potentially be used as a natural and effective dietary supplement to mitigate heat stress in broilers, thereby improving the health and production performance of broiler chickens.
ACKNOWLEDGMENTS
The study was supported by a Start-up grant from the College of Tropical Agriculture and Human Resources, the University of Hawaii at Manoa, and USDA Multistate (2052R) to B. M. These organizations were not involved in any animal experimental practice and manuscript preparation. The authors also sincerely thank Socorro Tauyan for helping in animal experimentation and Dr. Mohammad Arif for providing the facilities for microbiome analysis.
Data Availability: The metagenomics sequence data used in this study have been submitted to the NCBI database (accession no: PRJNA961296).
DISCLOSURES
The authors declare that they have no competing interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2023.102958.
Appendix. Supplementary materials
REFERENCES
- Abbas G., Arshad M., Tanveer A.J., Jabbar M.A., AL-Taey D.K.A., Mahmood A., Khan M.A., Imran M.S., Khan A.A., Konca Y., Sultan Z., Qureshi R.A.M., Iqbal A., Amad F., Ashraf M., Asif M., Abbas S., Mahmood R., Abbas H., mohyuddin S.G., jiang M.Y. Combating heat stress in laying hens a review. Pak. J. Sci. 2022;73:633. [Google Scholar]
- Aijaz S., Sanchez-Heras E., Balda M.S., Matter K. Regulation of tight junction assembly and epithelial morphogenesis by the heat shock protein Apg-2. BMC Cell Biol. 2007;8:49. doi: 10.1186/1471-2121-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud C., Joyeux M., Garrel C., Godin-Ribuot D., Demenge P., Ribuot C. Free-radical production triggered by hyperthermia contributes to heat stress-induced cardioprotection in isolated rat hearts. Br. J. Pharmacol. 2002;135:1776–1782. doi: 10.1038/sj.bjp.0704619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu S., Rothwell L., Kaiser P. Production and characterisation of monoclonal antibodies specific for chicken interleukin-12. Vet. Immunol. Immunopathol. 2011;140:140–146. doi: 10.1016/j.vetimm.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Barnes E.M. The intestinal microflora of poultry and game birds during life and after storage. Address of the president of the Society for Applied Bacteriology delivered at a meeting of the society on 10 January 1979. J. Appl. Bacteriol. 1979;46:407–419. doi: 10.1111/j.1365-2672.1979.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Brito A.d.F., Silva A.S., de Oliveira C.V.C., de Souza A.A., Ferreira P.B., de Souza I.L.L., da Cunha Araujo L.C., da Silva Félix G., de Souza Sampaio R., Tavares R.L. Spirulina platensis prevents oxidative stress and inflammation promoted by strength training in rats: dose-response relation study. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-63272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder K.M., Thompson K.L., Einstein M.E., Applegate T.J., Patterson J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult. Sci. 2008;87:1734–1741. doi: 10.3382/ps.2008-00107. [DOI] [PubMed] [Google Scholar]
- Cai Y.-Y., Huang F.-Q., Lao X., Lu Y., Gao X., Alolga R.N., Yin K., Zhou X., Wang Y., Liu B., Shang J., Qi L.-W., Li J. Integrated metagenomics identifies a crucial role for trimethylamine producing Lachnoclostridium in promoting atherosclerosis. Npj Biofilms Microbiom. 2022;8:1–12. doi: 10.1038/s41522-022-00273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedraz H., Gromboni J.G.G., Junior A.A.P.G., Filho R.V.F., Souza T.M., de Oliveira E.R., de Oliveira E.B., do Nascimento C.S., Meneghetti C., Wenceslau A.A. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186083. e0186083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chegini S., Kiani A., Rokni H. Alleviation of thermal and overcrowding stress in finishing broilers by dietary propolis supplementation. Ital. J. Anim. Sci. 2018;17:377–385. [Google Scholar]
- Cobb-Vantress. 2018. Broiler Performance & Nutrition Supplement. Accessed Aug. 2023. https://www.cobb-vantress.com/assets/5a88f2e793/Broiler-Performance-Nutrition-Supplement.pdf.
- Compare D., Coccoli P., Rocco A., Nardone O.M., De Maria S., Cartenì M., Nardone G. Gut–liver axis: the impact of gut microbiota on non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2012;22:471–476. doi: 10.1016/j.numecd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Dillard C.J., German J.B. Phytochemicals: nutraceuticals and human health. J. Sci. Food Agric. 2000;80:1744–1756. [Google Scholar]
- Duncan S.H., Barcenilla A., Stewart C.S., Pryde S.E., Flint H.J. Acetate utilization and butyryl coenzyme a (CoA): acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A.M., Ahmed A.M.H., Abdel-Maqsoud A., Badran A.M.M., Abdel-Moneim A.-M.E. Potential ameliorative role of Spirulina platensis in powdered or extract forms against cyclic heat stress in broiler chickens. Environ. Sci. Pollut. Res. 2022;29:45578–45588. doi: 10.1007/s11356-022-19115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M., Alagawany M., El-Hack M., Dhama K. Nutritional and healthical aspects of Spirulina (arthrospira) for poultry, animals and human. Int. J. Pharmacol. 2016;12:36–51. [Google Scholar]
- Farquhar M.G., Palade G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M., Nakai A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010;277:4112–4125. doi: 10.1111/j.1742-4658.2010.07827.x. [DOI] [PubMed] [Google Scholar]
- Hirakawa R., Nurjanah S., Furukawa K., Murai A., Kikusato M., Nochi T., Toyomizu M. Heat stress causes immune abnormalities via massive damage to effect proliferation and differentiation of lymphocytes in broiler chickens. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humam A.M., Loh T.C., Foo H.L., Samsudin A.A., Mustapha N.M., Zulkifli I., Izuddin W.I. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals. 2019;9:644. doi: 10.3390/ani9090644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Katsuki K., Izu H., Fujimoto M., Sugahara K., Yamada S., Shinkai Y., Oka Y., Katoh Y., Nakai A. Activation of heat shock genes is not necessary for protection by heat shock transcription factor 1 against cell death due to a single exposure to high temperatures. Mol. Cell. Biol. 2003;23:5882–5895. doi: 10.1128/MCB.23.16.5882-5895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Fouhse J.M., Tiwari U.P., Li L., Willing B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 2019;6 doi: 10.3389/fvets.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Mishra P. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: a review. J. Anim. Sci. Biotechnol. 2021;12:51. doi: 10.1186/s40104-021-00576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M., Sakata T. Fermentation of dietary carbohydrates to short-chain fatty acids by gut microbes and its influence on intestinal morphology of a detritivorous teleost tilapia (Oreochromis niloticus) Comp. Biochem. Physiol. A Physiol. 1997;118:1201–1207. [Google Scholar]
- Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Ratwan P., Dahiya S.P., Nehra A.K. Climate change and heat stress: Impact on production, reproduction and growth performance of poultry and its mitigation using genetic strategies. J. Therm. Biol. 2021;97 doi: 10.1016/j.jtherbio.2021.102867. [DOI] [PubMed] [Google Scholar]
- Lakshmanan A.P., Al Zaidan S., Bangarusamy D.K., Al-Shamari S., Elhag W., Terranegra A. Increased relative abundance of ruminoccocus is associated with reduced cardiovascular risk in an obese population. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.849005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal K., Truong L., Maga E., King A. Lactobacillus (L. plantarum & L. rhamnosus) and Saccharomyces (S. cerevisiae): effects on performance, biochemical parameters, ammonium ion in manure, and digestibility of broiler chickens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest. Res. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Mao B., Gu J., Wu J., Cui S., Wang G., Zhao J., Zhang H., Chen W. Blautia—a new functional genus with potential probiotic properties? Gut Microb. 2021;13 doi: 10.1080/19490976.2021.1875796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukić I., Getselter D., Ziv O., Oron O., Reuveni E., Koren O., Elliott E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry. 2019;9:1–16. doi: 10.1038/s41398-019-0466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch, J. B., E. L. Gonzalez, K. Choy, K. F. Faull, T. Jewell, A. Arellano, J. Liang, K. B. Yu, J. Paramo, and E. Y. Hsiao. 2022. Turicibacter modifies host bile acids and lipids in a strain-specific manner. Accessed Mar. 2023. https://www.biorxiv.org/content/10.1101/2022.0.27.497673v1. [DOI] [PMC free article] [PubMed]
- Mancabelli L., Ferrario C., Milani C., Mangifesta M., Turroni F., Duranti S., Lugli G.A., Viappiani A., Ossiprandi M.C., van Sinderen D., Ventura M. Insights into the biodiversity of the gut microbiota of broiler chickens. Environ. Microbiol. 2016;18:4727–4738. doi: 10.1111/1462-2920.13363. [DOI] [PubMed] [Google Scholar]
- Mikami A., Ogita T., Namai F., Shigemori S., Sato T., Shimosato T. Oral administration of Flavonifractor plautii attenuates inflammatory responses in obese adipose tissue. Mol. Biol. Rep. 2020;47:6717–6725. doi: 10.1007/s11033-020-05727-6. [DOI] [PubMed] [Google Scholar]
- Mirzaie S., Zirak-Khattab F., Hosseini S.A., Donyaei-Darian H. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian-Australas. J. Anim. Sci. 2018;31:556. doi: 10.5713/ajas.17.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 2019;6 doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa E.S., Alsanie W.F., Gaber A., Kamel N.N., Alaqil A.A., Abbas A.O. Blue-green algae (Spirulina platensis) alleviates the negative impact of heat stress on broiler production performance and redox status. Animals. 2021;11 doi: 10.3390/ani11051243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd A.T., Berding K., Wang M., Donovan S.M., Dilger R.N. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microb. 2017;8:589–600. doi: 10.1080/19490976.2017.1353849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney J.A., Roy N.C., Halliday C., Young W., Altermann E., Kruger M.C., Dilger R.N., McNabb W.C. Effects of early postnatal life nutritional interventions on immune-microbiome interactions in the gastrointestinal tract and implications for brain development and function. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.960492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawab A., Ibtisham F., Li G., Kieser B., Wu J., Liu W., Zhao Y., Nawab Y., Li K., Xiao M., An L. Heat stress in poultry production: mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018;78:131–139. doi: 10.1016/j.jtherbio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Nogal A., Louca P., Zhang X., Wells P.M., Steves C.J., Spector T.D., Falchi M., Valdes A.M., Menni C. Circulating levels of the short-chain fatty acid acetate mediate the effect of the gut microbiome on visceral fat. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.711359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy Y., Geyra A., Sklan D. The effect of early feeding on growth and small intestinal development in the posthatch poult. Poult. Sci. 2001;80:912–919. doi: 10.1093/ps/80.7.912. [DOI] [PubMed] [Google Scholar]
- NRC . Nutrient Requirements of Poultry: Ninth Revised Edition. National Academies Press; Washington, DC: 1994. [Google Scholar]
- Oakley B.B., Kogut M.H. Spatial and temporal changes in the broiler chicken cecal and fecal microbiomes and correlations of bacterial taxa with cytokine gene expression. Front. Vet. Sci. 2016;3 doi: 10.3389/fvets.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmnäs-Bédard M.S., Costabile G., Vetrani C., Åberg S., Hjalmarsson Y., Dicksved J., Riccardi G., Landberg R. The human gut microbiota and glucose metabolism: a scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022;116:862–874. doi: 10.1093/ajcn/nqac217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microb. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestana J.M., Puerta B., Santos H., Madeira M.S., Alfaia C.M., Lopes P.A., Pinto R.M.A., Lemos J.P.C., Fontes C.M.G.A., Lordelo M.M., Prates J.a.M. Impact of dietary incorporation of Spirulina (Arthrospira platensis) and exogenous enzymes on broiler performance, carcass traits, and meat quality. Poult. Sci. 2020;99:2519–2532. doi: 10.1016/j.psj.2019.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkkala, L., P. Nykanen, and L. Sistonen. 2001. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. Accessed May 2023. https://faseb.onlinelibrary.wiley.com/doi/10.1096/fj00-0294rev. [DOI] [PubMed]
- Rodriguez-Castaño G.P., Rey F.E., Caro-Quintero A., Acosta-González A. Gut-derived Flavonifractor species variants are differentially enriched during in vitro incubation with quercetin. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227724. e0227724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Abbas G., Alagawany M., Kamboh A.A., Abd El-Hack M.E., Khafaga A.F., Chao S. Heat stress management in poultry farms: a comprehensive overview. J. Therm. Biol. 2019;84:414–425. doi: 10.1016/j.jtherbio.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Sah N., Kuehu D.L., Khadka V.S., Deng Y., Peplowska K., Jha R., Mishra B. RNA sequencing-based analysis of the laying hen uterus revealed the novel genes and biological pathways involved in the eggshell biomineralization. Sci. Rep. 2018;8:16853. doi: 10.1038/s41598-018-35203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Hirose H., Onizuka A., Hayashi M., Futamura N., Kawamura Y., Ezaki T. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- Sheehan D., Meade G., Foley V.M., Dowd C.A. Structure, function, and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Bai L., Qu Q., Zhou S., Yang M., Guo S., Li Q., Liu C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019;98:2405–2413. doi: 10.3382/ps/pez026. [DOI] [PubMed] [Google Scholar]
- Shibata T., Motoi Y., Tanimura N., Yamakawa N., Akashi-Takamura S., Miyake K. Intracellular TLR4/MD-2 in macrophages senses Gram-negative bacteria and induces a unique set of LPS-dependent genes. Int. Immunol. 2011;23:503–510. doi: 10.1093/intimm/dxr044. [DOI] [PubMed] [Google Scholar]
- Sikora A., Grzesiuk E. Heat shock response in gastrointestinal tract. J. Physiol. Pharmacol. 2007;58(Suppl. 3):43–62. [PubMed] [Google Scholar]
- Singh A.K., Mandal R.K., Bedford M.R., Jha R. Xylanase improves growth performance, enhances cecal short-chain fatty acids production, and increases the relative abundance of fiber fermenting cecal microbiota in broilers. Anim. Feed Sci. Technol. 2021;277 [Google Scholar]
- Sohail M.U., Hume M.E., Byrd J.A., Nisbet D.J., Shabbir M.Z., Ijaz A., Rehman H. Molecular analysis of the caecal and tracheal microbiome of heat-stressed broilers supplemented with prebiotic and probiotic. Avian Pathol. 2015;44:67–74. doi: 10.1080/03079457.2015.1004622. [DOI] [PubMed] [Google Scholar]
- Surai P. Antioxidant systems in poultry biology: superoxide dismutase. Anim. Nutr. 2016;1:8. [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I. Glutathione peroxidases in poultry biology: part 1. Classification and mechanisms of action. Worlds Poult. Sci. J. 2018;74:185–198. [Google Scholar]
- Tang L.-P., Liu Y.-L., Ding K.-N., Hou X.-J., Qin J.-J., Zhang Y.-A., Liu H.-X., Shen X.-L., He Y.-M. Chai Hu oral liquid enhances the immune functions of both spleen and bursa of Fabricius in heat-stressed broilers through strengthening TLR4-TBK1 signaling pathway. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walugembe M., Hsieh J.C.F., Koszewski N.J., Lamont S.J., Persia M.E., Rothschild M.F. Effects of dietary fiber on cecal short-chain fatty acid and cecal microbiota of broiler and laying-hen chicks. Poult. Sci. 2015;94:2351–2359. doi: 10.3382/ps/pev242. [DOI] [PubMed] [Google Scholar]
- Wasti, S. 2020. Mitigation of heat stress in poultry using dried plum or alpha-lipoic acid supplement. ProQuest Diss. Theses. Accessed Oct. 2022. https://www.proquest.com/pqdtlocal1005836/docview/2429012576/abstract/A9939A483DAB4233PQ/1.
- Wasti S., Sah N., Mishra B. Impact of heat stress on poultry health and performances, and potential mitigation strategies. Animals. 2020;10:1266. doi: 10.3390/ani10081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasti S., Sah N., Singh A.K., Lee C.N., Jha R., Mishra B. Dietary supplementation of dried plum: a novel strategy to mitigate heat stress in broiler chickens. J. Anim. Sci. Biotechnol. 2021;12:58. doi: 10.1186/s40104-021-00571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Liu L., Miron A., Klímová B., Wan D., Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch. Toxicol. 2016;90:1817–1840. doi: 10.1007/s00204-016-1744-5. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Xiang Y., Zhou W., Chen J., Li K., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- Yang W., Yu T., Huang X., Bilotta A.J., Xu L., Lu Y., Sun J., Pan F., Zhou J., Zhang W., Yao S., Maynard C.L., Singh N., Dann S.M., Liu Z., Cong Y. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020;11:4457. doi: 10.1038/s41467-020-18262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Huang J., Guo X., Xia J., Hu M. Romboutsia lituseburensis JCM1404 supplementation ameliorated endothelial function via gut microbiota modulation and lipid metabolisms alterations in obese rats. FEMS Microbiol. Lett. 2023;370:fnad016. doi: 10.1093/femsle/fnad016. [DOI] [PubMed] [Google Scholar]
- Zeweil H., Abaza I.M., Zahran S.M., Ahmed M.H., AboulEla H.M., Saad A.A. Effect of Spirulina platensis as dietary supplement on some biological traits for chickens under heat stress condition. Asian J. Biomed. Pharm. Sci. 2016;6:8–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.