Abstract
The aim of this study is to formulate polymeric paclitaxel nanoparticles with various stabilizers to improve solubility, enhance stability, maximize therapeutic efficacy and minimize detrimental toxicities of paclitaxel. In this study, trastuzumab-guided poly lactic-co-glycolic acid (PLGA)-loaded paclitaxel nanoparticles were formulated with pluronic F-127, polyvinyl alcohol (PVA), poloxamer 407, Tween-80, span 20, sodium dodecyl sulfate (SDS), and sodium lauryl sulfate (SLS) at different concentrations (0.5, 1, 1.5 and 2%) using the solvent evaporation method. The nanoparticles were evaluated for physicochemical characteristics and short and long-term stability. The optimum particle size (190 nm ± 12.42 to 350 nm ± 11.1), PDI (0.13 ± 0.02 to 0.2 ± 0.01), surface charge (-19.1mv ± 1.5 to −40.4mv ± 1.6), drug loading (2.43 to 9.5 %) and encapsulation efficiency (greater than 80 %) were obtained with these stabilizers while keeping the polymer concentration, temperature, probe size, amplitude and sonication time constant. The nanoformulations were stably stored at 4 °C. The nanoformulations of paclitaxel with pluronic F-127, polyvinyl alcohol (PVA), and poloxamer 407 were found to be more soluble, stable, uniform in physicochemical properties, and efficient in drug loading and encapsulation for improved therapeutic effects.
Keywords: Stabilizers, Physicochemical parameters, Drug loading, Stability
1. Introduction
Commercially available drugs are associated with poor water solubility, poor stability and limited or no drug selectivity, which requires advancements in drug development technologies. Altering the pharmacological responses by adding functionalities or by manipulating the drug molecule itself is a challenge. Therefore, a drug delivery system in the form of polymeric nanoparticles represents the platform in terms of stability, solubility, bioavailability, selectivity, drug safety and modified drug release (Shkodra-Pula, Grune et al. 2019).
Paclitaxel is among the best antineoplastic agents, but it has some limitations. Most of the current formulations of paclitaxel contain Cremophor EL, which upon I/V administration causes hypersensitivity reactions that lead to high infusion time and precipitate out upon dilution (Win and Feng 2006), (Kalepu and Nekkanti 2015). Paclitaxel has very low oral bioavailability due to the first pass effect by cytochrome P450 and P-glycoprotein (plasma membrane transporter) overexpression in the physiological system (Win and Feng 2006), (Zhao and Feng 2010). Therefore, the need for good aqueous solubility, freedom from side effects and receptor-specific alternate formulations is the demand of current research work.
Over the past few years, great effort has been made to develop stable, cremophor EL-free polymeric paclitaxel nanoformulations, but these formulations still need more consideration due to stability problems. Drug release from these nanoformulations is not consistent and sustained (Schmitt, Trasi et al. 2015). Most of these nanoformulations are under preclinical trials and far from human use. These newer formulations have decreased drug-associated toxicities and the use of pre-medications, but improvements in terms of therapeutic efficacy remain to be achieved.
Stabilizers play an important role in the long-term stability of nanoparticles as well as in preventing aggregation, as nanoparticles may tend to aggregate due to their high surface energy (Tuomela, Hirvonen et al. 2016). They tend to decrease the interfacial tension between the oil and water phases. The type and concentration of surfactant not only determine particle stability but are also important for the encapsulation efficiency, release kinetics, and pharmacokinetic parameters of the nanoparticles. Therefore, different stabilizers were applied to evaluate the effect of these surfactants on the physiochemical properties of nanoparticles and choose the surfactant that results in the optimized nanoparticle formulation (Shkodra-Pula, Grune et al. 2019).
Polyvinyl alcohol (PVA), a nonionic surfactant, is the most commonly used surfactant for the fabrication of nanoparticles due to the formation of small nanoparticles and inhibition of p-glycoprotein, which results in increased drug accumulation inside cells (Bai, Li et al. 2007). PVA often affects physical properties and cellular uptake by the formation of interconnected networks with polymers at the interface (Aslam, Kalyar et al. 2018). Pluronic acid, a tri-block copolymer (polyethylene oxide-b-polypropylene oxide-b- polyethylene oxide), is a nonionic surfactant that is less toxic to biological membranes and biocompatible. Pluronic F-127 has been shown to modulate efflux pumps such as P-glycoprotein and multidrug resistance-associated proteins, which influence drug pharmacokinetics (BAYINDIR and BADILLI, 2009, Wei et al., 2009). Poloxamer 407 is a cationic, tri-block copolymer containing polyethylene oxide (hydrophilic portion) and poly propylene oxide (hydrophobic portion). The hydrophobic end is anchored to the nanoparticle surface, while the water-loving portion is oriented toward the aqueous medium, forming a hydrophilic layer (Redhead et al., 2001, Stolnik et al., 2001). It is amphiphilic in nature, has bioadhesive properties and increases the solubilization of hydrophobic drugs (Dumortier, Grossiord et al. 2006). Sodium lauryl sulfate is an anionic surfactant used to prepare nanoparticles with a high negative charge, which is important for their stability. As nanoparticles tend to agglomerate, strong repulsive forces prevent particle aggregation (Pongpeerapat et al., 2008, das Neves et al., 2012). In this study, different stabilizers (Pluronic F-127, PVA and Poloxamer 407) at varying concentrations (0.5, 1, 1.5 and 2%) with an optimized polymer (PLGA) concentration were utilized to show their impact on the physicochemical characteristics and stability of the developed nanoformulations. SLS and other anionic surfactants were added to these formulations for optimization of particle size, PDI and ZP. These nanoformulations were used for targeting specific breast cancer receptors for which a negative surface charge is required and negatively charged particles are also more stable.
2. Materials and methods
2.1. Materials
Paclitaxel (≥99.9 % purity) was purchased from Qilu Antibiotic Pharmaceutical Co., Ltd., China. Poly lactic acid coglycolic acid (75:25, Resomer® RG 756H, MW 76000–115000 Da) from Evonik Germany, trastuzumab from Roche Pharmaceuticals UK, Poly Vinyl Alcohol (MW; 30,000–170,000), Poloxamer 407, Pluronic F-127, Sodium lauryl Sulphate (SLS) from Sigma Aldrich Germany, Disodium Hydrogen Phosphate (Na2HPO4), Sodium Chloride (NaCl), Potassium Chloride (KCl), Hydrochloric acid (HCL), Phosphoric acid (H3PO4), methanol, Dichloromethane, mannitol, glucose, sucrose, acetonitrile (purity ≥ 99. 9 %) and other solvents used were of HPLC grade. The water used for solvent preparation was ultra-pure.
2.2. Equipment
A sensitive analytical balance (Shimadzu, Japan), pH/mV meter (Jenway®, UK), vortex mixer (Fisher Scientific®, USA), refrigerated centrifuge (Centurion® Scientific, UK), probe sonicator (Sanyo, UK), magnetic stirrers (Benchmark®, USA), shaking water bath (Korea), zeta sizer (Malvern Zetasizer ZS-90, UK), freeze dryer (TalstarCryodos- 50, USA), UV–visvisible spectrometer (Perkin Elmer Series 200), scanning electron microscope (Jeol, Japan), Mini-PROTEAN® electrophoresis system (BIO-RAD, USA), and microplate reader (Biotek, USA) were used.
2.3. Methodology
2.3.1. Formulation conditions
Different methods have been used for the preparation of nanoparticles, such as solvent evaporation and nanoprecipitation methods. The initial conditions were checked in terms of the method of preparation and solvent for the solubilization of polymer, drug and excipients. Four organic solvents, acetonitrile, dichloromethane, methanol and acetone, were used with different stabilizers while keeping the concentrations of the drug and polymer constant.
2.3.2. Nanoparticle Formulation
Paclitaxel polymeric nanoparticles were prepared using PLGA as a polymer, polyvinyl alcohol, Pluronic F-127, Poloxamer-407, Tween-80, span 20, sodium dodecyl sulfate and sodium lauryl sulfate as stabilizers, and the method adapted was solvent evaporation. The PLGA concentration was kept constant (10 mg), while stabilizers (0,5, 1, 1.5, 2 %) and drug (1, 2, 3, 4 mg) were used in variable concentrations. Ten milligrams of PLGA (75:25) and paclitaxel (1, 2, 3, 4 mg) were dissolved in 5 mL of ACN. For preparation of the aqueous phase, stabilizers at different concentrations were dissolved in distilled water (10 mL). The organic phase containing the drug and polymer was added dropwise into 10 mL of an aqueous stabilizer solution with constant magnetic stirring. Sonication of the resultant mixture was performed with the help of a probe sonicator (Soni-prep-150, UK) set at 99% power 15 W for 4 min in an ice water bath. The organic solvent was allowed to evaporate overnight by slow magnetic stirring. After the complete removal of the organic solvent, the nanoparticles were centrifuged at 15000 rpm for 30 mins at −8 °C and washed three times with double distilled water (Ali and El-Enin 2021). The nanoparticles were used freshly or freeze dried for further evaluation (Mainardes and Evangelista 2005).
2.3.3. Characterization
2.3.3.1. Dynamic light scattering (DLS)
The size and PDI of the developed nanoformulations were evaluated by DLS (at 90° angle and 25 °C) using a Zetasizer. The nanoformulations were diluted with distilled water wherever needed. The average of three reported values (100 cycles each) was taken using Malvern software and analyzed statistically.
The net charge of particles can affect the circulation half-life, tissue retention, stability or cell entry capabilities and should be well understood to improve the delivery of particles, which is also measured by Zetasizer. Electrophoretic mobility under an electric field is the principal of zeta potential, and at least three readings were recorded for each sample.
The nanoparticles were characterized at three levels: after solvent evaporation (before washing), after washing and after lyophilization. The zetapotential was investigated after washing and lyophilization at 25 °C with three measurements. (Huang, Chen et al. 2007).
2.3.4. Development and validation of the UV–Visible spectrophotometric method
Double beam UV–Visible Spectrophotometer was used to analyze (quantify) paclitaxel and to determine the encapsulation efficiency of nanoformulations. A stock solution (100 µg/mL) of paclitaxel was prepared by dissolving 1 mg of drug in 100 mL of ACN. Working dilutions were prepared (Sanchez-Hachair and Hofmann 2018). Solutions of paclitaxel (1 µg/mL − 30 µg/mL) were prepared and scanned (200 to 400 nm) for the wavelength of maximum absorbance (λ max).
Method linearity was calculated by preparing different dilutions (1–30 µg/mL) of paclitaxel and analyzed in triplicate at the selected wavelength. The average absorbance of each dilution was plotted versus the respective concentration, and the regression coefficient was calculated.
For specificity, the percent recovery method was applied, and the results should not be affected by the presence of placebo or other drug degradation products. A solution containing the drug was prepared from a stock solution at a concentration of 10 µg/mL. Another solution (placebo) was prepared that contained all the excipients used in the preparation of formulations except the active ingredient (Fereja, Seifu et al. 2015). The percent recovery was determined by comparing their results at their selected wavelengths.
The stability of the drug solution (10 µg/mL) was determined by keeping it at room temperature for 72 hrs. In triplicate, the absorbance was measured at the specified time period, and the solution stability was evaluated by comparing the absorbance of the sample solution with that of a freshly prepared solution (Fereja, Seifu et al. 2015).
Evaluation of precision was carried out on repeatability and intermediate precision and was evaluated using a solution of paclitaxel in the concentration range of 10, 20 and 30 µg/mL (in triplicate). For determination of intermediate precision, absorbance was measured (intraday) at 08 hr intervals for 24 hr. For interday precision, absorbance was measured for one week at 24 hr intervals. The obtained results were presented as the mean, standard deviation (±SD), and covariance (%RSD) (Khanage, Mohite et al. 2013).
2.3.5. Freeze drying
Freeze drying of the nanoformulations was performed using mannitol (1% & 2%) as a cryoprotectant. Samples were frozen first and then freeze dried at −45 °C for 24 hrs at 0.05 mbar. After freeze-drying, the samples were reconstituted by slowly injecting distilled water into the inside wall of the vial, and then it was maintained for 10 min to ensure proper cake wetting. After this period of time, the samples were gently shaken in a ZX Classic vortex mixer for 3 min to completely homogenize the samples. After reconstitution, samples were physico-chemically characterized. To check the stability of the nanoformulations as well as the validity of the process, the difference in the physicochemical characteristics before and after drying was calculated.
2.3.6. Stability studies
The stability of nanoparticles is defined in terms of their core composition, size, zeta potential, shape/morphology and surface chemistry. The stability of nanoparticles is an important parameter because at the nanoscale, both the surface-to-volume ratio and surface energy increase. The size, zeta potential, morphology and composition of nanoparticles are important parameters for physical targeting or drug delivery to specific areas. The stability studies were carried out at 4 °C and 25 °C in closed glass vials for 6 months.
3. Results and discussion
3.1. Formulation conditions
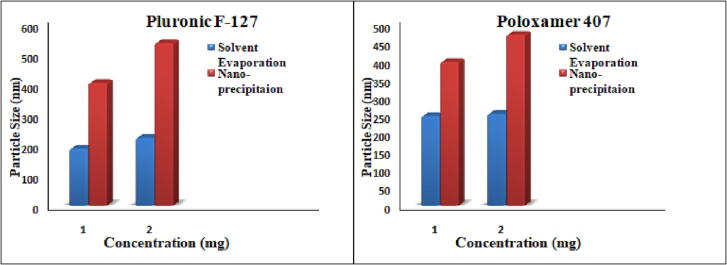
Different methods have been used for the preparation of nanoparticles, such as solvent evaporation and nanoprecipitation methods. On the basis of the small particle size and monodisperse particles, the solvent evaporation method was selected for formulation development, as shown in Table 1 and Fig. 1. This method is used most commonly and produces uniform sized nanoparticles.
Table 1.
Optimization of the method of preparation of nanoformulations.
| No | Code | D:P (mg) | Stabilizer 1% (10 mL) | Preparation method | P.Size (nm) | Z.P (mv) | PDI | E.E (%) |
|---|---|---|---|---|---|---|---|---|
| 01 | PTX 05 | 1:10 | Pluronic F-127 | Solvent evaporation | 190 ± 13.2 | −8.1 ± 1.8 | 0.2 ± 0.03 | 71 |
| 02 | PTX 06 | 2:10 | 226 ± 11.6 | −9 ± 0.7 | 0.16 ± 0.01 | 96 | ||
| 03 | PTX 07 | 1:10 | Nano-precipitation | 409 ± 10.4 | −6.1 ± 1.2 | 0.59 ± 0.1 | 73 | |
| 04 | PTX 08 | 2:10 | 542 ± 13.8 | −5.8 ± 0.1 | 01 | 52 | ||
| 05 | PTX 21 | 1:10 | Poloxamer 407 | Solvent evaporation | 249 ± 9.7 | −5.6 ± 0.21 | 0.19 ± 0.01 | 83 |
| 06 | PTX 22 | 2:10 | 255 ± 14.2 | −6.9 ± 0.11 | 0.3 ± 0.02 | 67 | ||
| 07 | PTX 23 | 1:10 | Nano-precipitation | 399 ± 12.4 | −3.09 ± 1.4 | 0.46 ± 0.04 | 69 | |
| 08 | PTX 24 | 2:10 | 475 ± 10.5 | −5.22 ± 0.8 | 0.91 ± 0.03 | 75 |
Organic solvent: ACN (5 mL), D: Paclitaxel, P: PLGA.
Fig. 1.
Effect of the method of preparation on particle size.
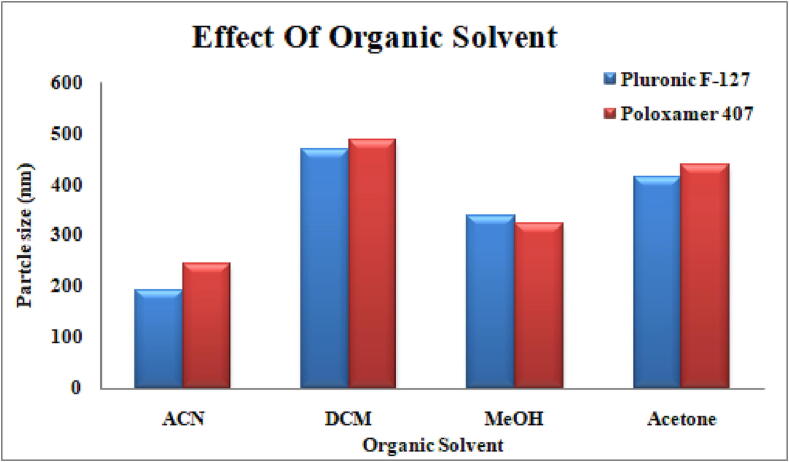
Four organic solvents, acetonitrile, dichloromethane, methanol and acetone, were used with different stabilizers, keeping the concentration of drug and polymer constant by the solvent evaporation method. Acetonitrile was selected as an organic solvent for the preparation of nanoformulations on the basis of its small particle size and monodisperse nanoparticles with high encapsulation efficiency and negative zeta potential, as shown in Table 2 and Fig. 2.
Table 2.
Organic solvents used for preparation of the nanoformulation.
| No | Code | D:P (mg) | Stabilizer 1% (10 mL) | Organic solvent (5 mL) | P.size (nm) | Z.P (mv) | PDI | E.E (%) |
|---|---|---|---|---|---|---|---|---|
| 01 | PTX 33 | 1:10 | Pluronic F-127 | ACN | 192 ± 11.1 | −8.7 ± 0.2 | 0.2 ± 0.01 | 75 |
| 02 | PTX 34 | 1:10 | DCM | 468 ± 11.6 | 2.4 ± 0.7 | 0.34 ± 0.01 | 72 | |
| 03 | PTX 35 | 1:10 | MeOH | 339 ± 8.2 | −1.2 ± 0.8 | 0.22 ± 0.03 | 33 | |
| 04 | PTX 36 | 1:10 | Acetone | 415 ± 9.1 | 3.3 ± 0.1 | 0.45 ± 0.2 | 42 | |
| 05 | PTX 45 | 1:10 | Poloxamer 407 | ACN | 244 ± 10.1 | −5.9 ± 0.11 | 0.12 ± 0.02 | 83 |
| 06 | PTX 46 | 1:10 | DCM | 486 ± 8.7 | 3.1 ± 0.13 | 0.19 ± 0.01 | 66 | |
| 07 | PTX 47 | 1:10 | MeOH | 324 ± 11.0 | −2.3 ± 1.6 | 0.6 ± 0.01 | 61 | |
| 08 | PTX 48 | 1:10 | Acetone | 439 ± 12.2 | 1.9 ± 1.2 | 0.27 ± 0.6 | 53 |
D: Paclitaxel, P: PLGA.
Fig. 2.
Effect of organic solvents on particle size.
3.2. Nanoformulations with different stabilizers
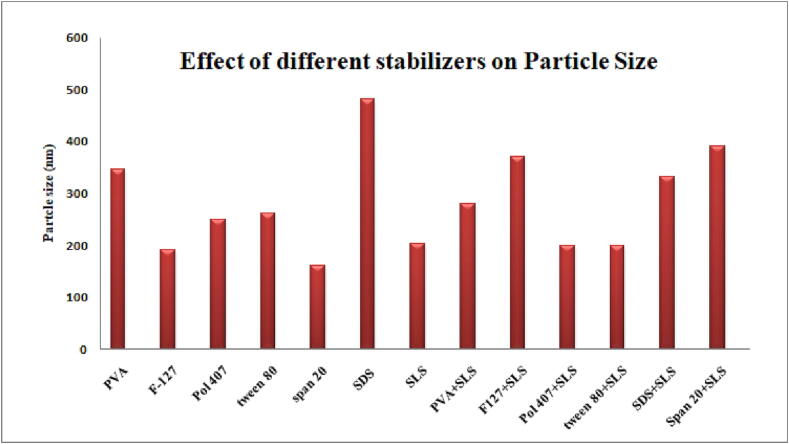
Different stabilizing agents were used alone and in combination to evaluate their effect on particle size, polydispersity index, surface charge and encapsulation efficiency. More than 150 nanoformulations were prepared for optimization of the stabilizing agent. Formulations prepared with Pluronic F-127 + SLS and Poloxamer 407 + SLS were selected for further characterization studies due to their small particle size, negative surface charge, round morphology and high encapsulation efficiency.
Sodium lauryl sulfate (SLS) was used in combination with Pluronic F-127 and Poloxamer 407 at different concentrations using PLGA (75:25, Resomer® RG 756 S, MW 76000–115000 Da). The nanoparticles obtained were small in size (190 ± 12.42 to 350 ± 11.1), had a negative zeta potential (-19.1 ± 1.5 to –40.4 ± 1.6) and had a high encapsulation efficiency (greater than75 %), as shown in Table 3 and Fig. 3.
Table 3.
Stabilizers used for preparation of the nanoformulation.
| NO | Code | D:P (mg) | Stabilizer (10 mL) | Stabilizer (%) | Size (nm) | PDI | Z.P(mv) | E. E (%) |
|---|---|---|---|---|---|---|---|---|
| 01 | PTX 01 | 1:10 | PVA | 1:0 | 347 ± 10.3 | 0.6 ± 0.01 | −8.1 ± 1.8 | 55 |
| 02 | PTX 05 | 1:10 | Pluronic F-127 | 1:0 | 190 ± 13.2 | 0.2 ± 0.03 | −8.1 ± 1.8 | 71 |
| 03 | PTX 21 | 1:10 | Poloxamer 407 | 1:0 | 249 ± 9.7 | 0.19 ± 0.01 | −5.6 ± 0.21 | 83 |
| 04 | PTX 124 | 1:10 | Tween 80 | 1:0 | 261 ± 12.2 | 0. ± 0.04 | −7.39 ± 0.1 | 41 |
| 05 | PTX 126 | 1:10 | Span 20 | 1:0 | 160 ± 11.9 | 0.9 ± 0.2 | 0.6 ± 1.1 | 25 |
| 06 | PTX 131 | 1:10 | SDS | 1:0 | 482 ± 7.1 | 0.9 ± 0.01 | −51.6 ± 0.1 | 24 |
| 07 | PTX 142 | 1:10 | SLS | 1:0 | 203 ± 7.3 | 0.4 ± 0.06 | −40.8 ± 1.4 | 56 |
| 08 | PTX 66 | 1:10 | PVAa + SLSb | 1:0.05 | 279 ± 13.1 | 0.8 ± 0.04 | −4.16 ± 0.11 | 68 |
| 09 | PTX 88 | 1:10 | Pluronic F-127a + SLSb | 1:0.05 | 371 ± 22.61 | 0.7 ± 0.02 | −29.77 ± 0.2 | 76 |
| 10 | PTX 104 | 1:10 | Poloxamer 407a + SLSb | 1:0.05 | 199 ± 21.80 | 0.4 ± 0.01 | −26.85 ± 0.03 | 77 |
| 11 | PTX 120 | 1:10 | Tween 80a + SLSb | 1:0.05 | 198 ± 14.6 | 0.4 ± 0.23 | −43.2 ± 1.01 | 41 |
| 12 | PTX 133 | 1:10 | SDSa + SLSb | 1:0.05 | 333 ± 15.5 | 0.5 ± 0.05 | −50.1 ± 0.21 | 35 |
| 13 | PTX 137 | 1:10 | Span 20a + SLSb | 1:0.05 | 391 ± 9.7 | 0.9 ± 0.06 | −60.2 ± 0.09 | 66 |
5 mL, b 5 mL, D: Paclitaxel, P: PLGA.
Fig. 3.
Effect of different stabilizers on particle size.
3.3. Characterization
3.3.1. Dynamic light scattering technique
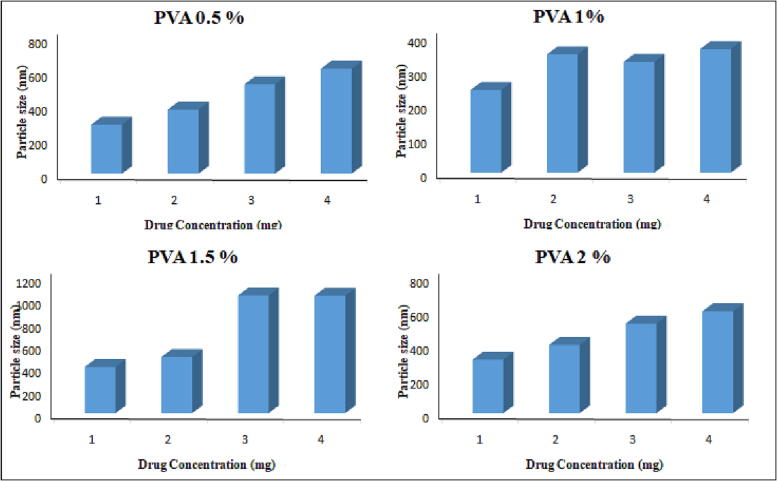
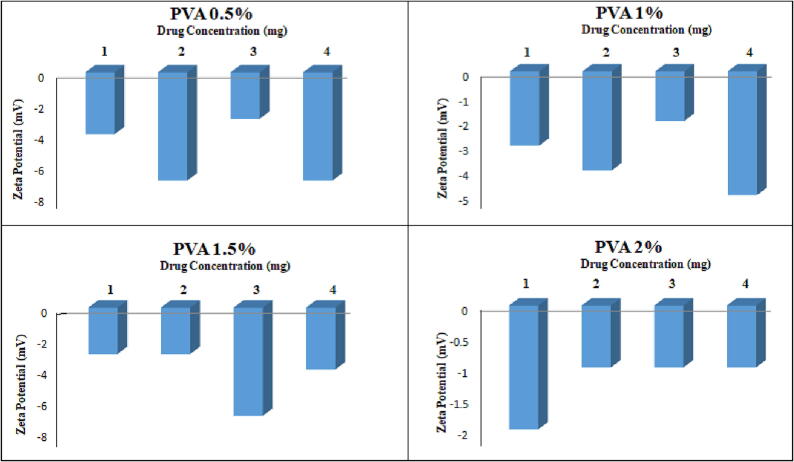
The particle size was within the range of 189 ± 12.42 to 620 ± 14.47 nm for 0.5 %, 244 ± 22.61 to 365 ± 44.0 nm for 1 %, 408 ± 11.8 to 1042 ± 23.9 nm for 1.5 % and 315 ± 18.45 to 601 ± 43.21 nm for 2 % PVA. The zeta potential values ranged from −1.1 ± 0.32 to −7.75 ± 1.3 mV as the drug concentration was increased from 1 mg to 4 mg, respectively, as given in Table 4 and Fig. 4, Fig. 5. The particle size increased with increasing drug content from 1 to 4 mg, with a minimum value of 1 mg and a maximum value of 4 mg. The PDI is almost in the range with a maximum value of 0.05, while the maximum zeta potential is −7 ± 0.5, which is not in the required range as far as our study is concerned. The encapsulation efficiency is very high in this particular case, i.e., from 78 to 98%.
Table 4.
Formulation of paclitaxel with PLGA, 0.05% SLS and PVA.
| NO | Code | D:P (mg) | Size (nm) | PDI | Z.P (mv) | Amount Encapsulate (mg/mL) | (%) E.E | (%) Drug Loading |
|---|---|---|---|---|---|---|---|---|
| PVA (0.5%) | ||||||||
| 01 | PTX 67 | 1:10 | 289 ± 0.12 | 0.3 ± 0.01 | −4 ± 0.5 | 0.81 | 81% | 8.1 |
| 02 | PTX 68 | 2:10 | 378 ± 22.11 | 0.9 ± 0.04 | −7 ± 0.3 | 1.1 | 55% | 11 |
| 03 | PTX 69 | 3:10 | 528 ± 12.3 | 1.0 ± 0.05 | −3 ± 0.4 | 2.94 | 98% | 29.4 |
| 04 | PTX 70 | 4:10 | 620 ± 11.1 | 0.3 ± 0.01 | −7 ± 0.5 | 3.88 | 97% | 38.8 |
| PVA (1%) | ||||||||
| 05 | PTX 72 | 1:10 | 244 ± 0.19 | 0.3 ± 0.02 | −3 ± 0.2 | 0.78 | 78% | 7.8 |
| 06 | PTX 73 | 2:10 | 349 ± 12.08 | 0.5 ± 0.03 | −4 ± 0.6 | 1.92 | 96% | 19.2 |
| 07 | PTX 74 | 3:10 | 327 ± 23.2 | 0.7 ± 0.03 | −2.20 ± 0.3 | 2.52 | 84% | 25.2 |
| 08 | PTX 75 | 4:10 | 365 ± 10.91 | 0.6 ± 0.04 | −5.11 ± 0.4 | 3.80 | 95% | 38 |
| PVA (1.5%) | ||||||||
| 09 | PTX 76 | 1:10 | 408 ± 26.13 | 0.2 ± 0.02 | −3.25 ± 0.2 | 0.94 | 94% | 9.4 |
| 10 | PTX 77 | 2:10 | 496 ± 7.09 | 0.3 ± 0.01 | −3.71 ± 0.1 | 0.19 | 95% | 1.9 |
| 11 | PTX 78 | 3:10 | 1042 ± 11.1 | 1.0 ± 0.04 | −7.75 ± 0.6 | 2.91 | 97% | 29.1 |
| 12 | PTX 79 | 4:10 | 1040 ± 0.19 | 1.0 ± 0.05 | −4.31 ± 0.5 | 3.84 | 96% | 38.4 |
| PVA (2%) | ||||||||
| 13 | PTX 80 | 1:10 | 315 ± 18.45 | 0.3 ± 0.01 | −2.23 ± 0.3 | 0.94 | 94% | 9.4 |
| 14 | PTX 81 | 2:10 | 402 ± 23.66 | 0.6 ± 0.02 | −1.53 ± 0.2 | 1.84 | 92% | 18.4 |
| 15 | PTX 82 | 3:10 | 529 ± 34.24 | 0.9 ± 0.01 | −1.15 ± 0.1 | 2.64 | 88% | 26.4 |
| 16 | PTX 83 | 4:10 | 601 ± 43.21 | 0.5 ± 0.03 | −1.1 ± 0.1 | 3.36 | 84% | 33.6 |
D: Paclitaxel, P: PLGA.
Fig. 4.
Effect of varying concentrations of PVA on particle size.
Fig. 5.
Effect of varying concentrations of PVA on the zeta potential.
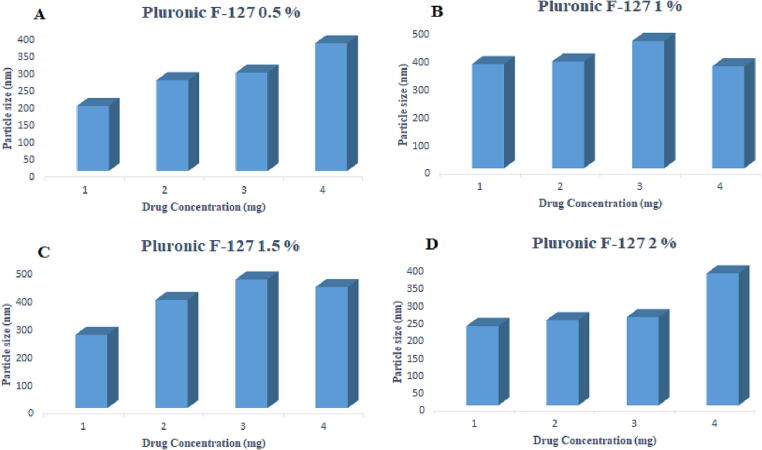
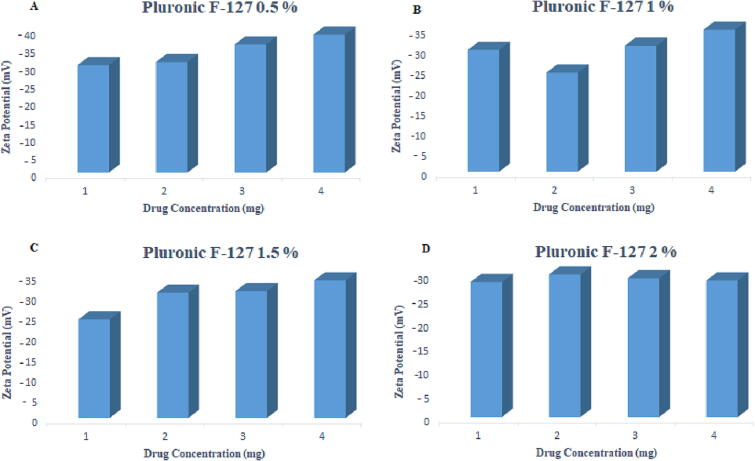
The particle size was within the range of 190 ± 12.42 to 373 ± 14.47 nm for 0.5 %, 371 ± 22.61 to 363 ± 44.0 nm for 1 %, 261 ± 11.8 to 431 ± 23.9 nm for 1.5 % and 226 ± 34.37 to 376 ± 20.41 nm for 2 % pluronic F-127. The zeta potential values ranged from −28.28 ± 1.2 to −38.5 ± 1.9 mV as the drug content was increased from 1 mg to 4 mg, respectively, as given in Table 5 and Fig. 6, Fig. 7.
Table 5.
Formulation of paclitaxel with PLGA, 0.05% SLS and Pluronic F-127.
| NO | Code | D:P (mg) | Size (nm) | PDI | Z.P (mv) | Amount Encapsulate (mg/mL) | (%) E.E | (%) Drug Loading |
|---|---|---|---|---|---|---|---|---|
| Pluronic F-127 (0.5%) | ||||||||
| 01 | PTX 84 | 1:10 | 190 ± 12.42 | 0.13 ± 0.02 | −30.2 ± 1.1 | 0.95 | 95 | 9.5 |
| 02 | PTX 85 | 2:10 | 265 ± 3.38 | 0.5 ± 0.03 | −30.9 ± 0.8 | 1.1 | 55 | 11 |
| 03 | PTX 86 | 3:10 | 287 ± 13.51 | 0.2 ± 0.01 | −35.8 ± 1.2 | 2.43 | 81 | 24.3 |
| 04 | PTX 87 | 4:10 | 373 ± 14.47 | 0.9 ± 0.08 | −38.5 ± 1.9 | 2.52 | 63 | 25.2 |
| Pluronic F-127 (1 %) | ||||||||
| 05 | PTX 88 | 1:10 | 371 ± 22.61 | 0.7 ± 0.02 | −29.77 ± 0.2 | 0.66 | 66 | 6.6 |
| 06 | PTX 89 | 2:10 | 380 ± 38.97 | 0.6 ± 0.04 | −24.2 ± 0.18 | 1.22 | 61 | 12.2 |
| 07 | PTX 90 | 3:10 | 453 ± 48.10 | 0.6 ± 0.01 | −30.8 ± 0.1 | 1.26 | 42 | 12.6 |
| 08 | PTX 91 | 4:10 | 363 ± 44.0 | 0.4 ± 0.04 | −34.7 ± 0.2 | 1.52 | 38 | 15.2 |
| Pluronic F-127 (1.5 %) | ||||||||
| 09 | PTX 92 | 1:10 | 261 ± 11.8 | 0.4 ± 0.03 | −24.05 ± 0.9 | 0.47 | 47 | 4.7 |
| 10 | PTX 93 | 2:10 | 385 ± 33.4 | 1.0 ± 0.04 | −30.59 ± 0.11 | 1.96 | 98 | 19.6 |
| 11 | PTX 94 | 3:10 | 458 ± 18.5 | 0.5 ± 0.04 | −31 ± 0.25 | 1.35 | 45 | 13.5 |
| 12 | PTX 95 | 4:10 | 431 ± 23.9 | 0.19 ± 0.01 | –33.62 ± 1.7 | 2.0 | 50 | 20 |
| Pluronic F-127 (2 %) | ||||||||
| 13 | PTX 96 | 1:10 | 226 ± 34.37 | 0.5 ± 0.02 | −28.28 ± 1.2 | 0.44 | 44 | 4.4 |
| 14 | PTX 97 | 2:10 | 243 ± 10.04 | 0.1 ± 0.02 | −29.89 ± 0.9 | 0.38 | 19 | 3.8 |
| 15 | PTX 98 | 3:10 | 251 ± 23.02 | 0.1 ± 0.01 | −29.07 ± 0.21 | 0.24 | 08 | 2.4 |
| 16 | PTX 99 | 4:10 | 376 ± 20.41 | 0.1 ± 0.04 | −28.61 ± 0.11 | 0.28 | 07 | 2.8 |
D: Paclitaxel, P: PLGA.
Fig. 6.
Effect of varying concentrations of Pluronic F-127 on particle size.
Fig. 7.
Effect of varying concentrations of Pluronic F-127 on the zeta potential.
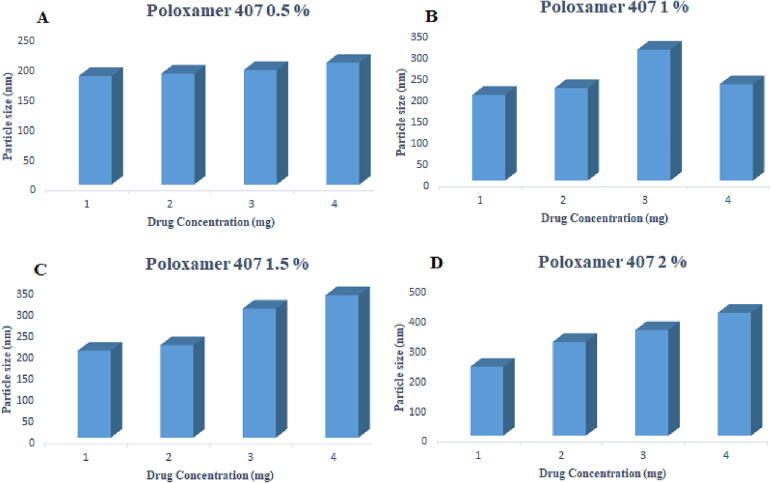
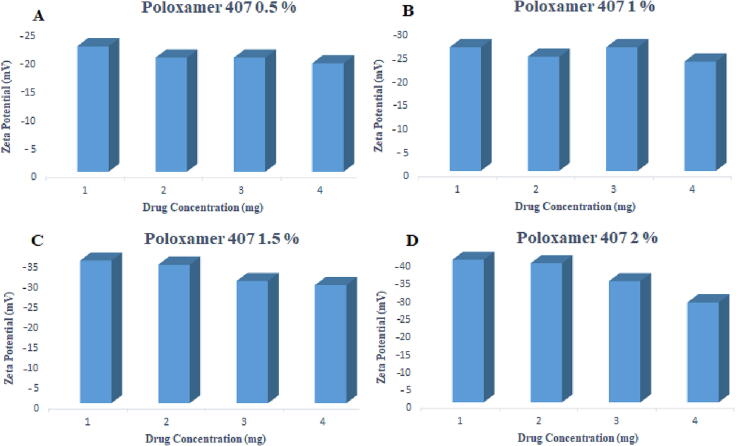
The particle size was within the range of 180 ± 1.22 to 202 ± 36.17 nm for 0.5 %, 199 ± 21.80 to 224 ± 26.98 nm for 1 %, 202.3 ± 14.5 to 224 ± 26.98 nm for 1.5 % and 229 ± 13.24 to 408 ± 11.27 nm for 2 % poloxamer 407. Paclitaxel loaded polymeric nanoformulations prepared by using poloxamer 407 (0.5, 1, 1.5, 2 %) and SLS (0.05 %) as stabilizers show a negative charge, and the zeta potential values decrease as the concentration of the drug is increased from 1 mg to 4 mg, as shown in Table 7 and Fig. 8, Fig. 9. There was an increase in zeta potential ranging from −19.1 ± 1.5 to −40.4 ± 1.6 mV with an increase in the concentration of poloxamer 407 from 0.5 to 2 %.
Table 7.
Validation Parameters of the UV–Visible Spectrophotometric Method of Paclitaxel Analysis.
| Parameters | Results |
|---|---|
| Linearity | |
| Calibration Range | 1 – 30 µg/ml |
| Wavelength (λ max) | 237 nm |
| Regression Equation | Y = 0.0288x – 0.1511 |
| Correlation Co Efficient (R2) | 0.993 |
| Accuracy (% Recovery) | Mean ± S.D; % RSD |
| Sample Without Excipients | 98.46 ± 0.13; 0.13 |
| Sample With Excipients | 99.76 ± 0.27; 0.27 |
| Stability (Amount Recovered) | Mean ± S.D; % RSD |
| Day 01 (n = 3) | 9.94 ± 0.04. 0.40 |
| Day 02 (n = 3) | 9.88 ± 0.03. 0.30 |
| Day 03 (n = 3) | 9.94 ± 0.02. 0.20 |
Fig. 8.
Effect of varying concentrations of poloxamer 407 on particle size.
Fig. 9.
Effect of varying concentrations of poloxamer 407 on the zeta potential.
3.4. Development and validation of U.V Visible spectrophotometric method
The method was validated according to ICH guidelines. The drug response was found to be linear (R2 = 0.993) in the given range. The regression equation and correlation coefficient are given in Table 9. Paclitaxel was found to be stable under these storage conditions. The results indicate that there is no significant effect of excipients on percent recovery. The average amount recovered, standard deviation (±SD), and coefficient of variation (%RSD) are given in Table 7.
Table 9.
Results of paclitaxel nanoformulations with different concentrations of mannitol after freeze drying.
| Code | Formulations |
Blank |
2 % Mannitol |
5 % Mannitol |
10 % Mannitol |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS(nm) | PDI | ZP(mv) | PS(nm) | PDI | ZP(mv) | PS(nm) | PDI | ZP(mv) | PS(nm) | PDI | ZP(mv) | PS(nm) | PDI | ZP(mv) | |
| PTX 84 | 190 ± 12.42 | 0.13 ± 0.02 | −30 ± 1.1 | 237 ± 1.15 | 0.19 ± 0.01 | −24.3 ± 2.2 | 244 ± 13.2 | 0.2 ± 0.08 | −31.1 ± 1.5 | 185 ± 2.8 | 0.1 ± 0.2 | −31.2 ± 1.2 | 298 ± 9.1 | 0.35 ± 1.8 | −24.4 ± 1.1 |
| PTX 86 | 287 ± 13.51 | 0.2 ± 0.01 | −35.8 ± 1.2 | 329 ± 15.5 | 0.3 ± 0.1 | −34.3 ± 1.5 | 350 ± 1.9 | 0.19 ± 0.03 | −30 ± 2.5 | 293 ± 17.2 | 0.18 ± 1.2 | −36.4 ± 2.9 | 377 ± 6.9 | 0.4 ± 1.2 | −29.7 ± 1.1 |
| PTX 108 | 202 ± 14.5 | 0.17 ± 0.03 | −35.2 ± 0.1 | 293 ± 12.3 | 0.2 ± 0.15 | −29.0 ± 2.3 | 280 ± 2.4 | 0.23 ± 0.02 | –33.4 ± 1.4 | 190 ± 14.1 | 0.2 ± 0.11 | −35.1 ± 1.3 | 356 ± 17.3 | 0.2 ± 1.2 | −34.3 ± 1.2 |
| PTX 112 | 229 ± 13.24 | 0.2 ± 0.01 | −40.4 ± 1.6 | 312 ± 6.09 | 0.4 ± 0.11 | −38.4 ± 1.7 | 305 ± 3.31 | 0.24 ± 0.02 | −36.9 ± 1 | 211 ± 3.1 | 0.2 ± 0.9 | −38.9 ± 2.9 | 308 ± 24.3 | 0.5 ± 1.3 | −29.1 ± 2.4 |
The method precision was evaluated on the basis of repeatability and intermediate precision using a solution of paclitaxel in the concentration range of 10, 20 and 30 µg/mL, as shown in Table 8.
Table 8.
Intra Day and Inter Day studies of UV–Visible Spectrophotometric Method of Paclitaxel Analysis.
| Sampling time | Concentration | Concentration Recovered µg/mL | %RSD |
|---|---|---|---|
| 0 h | 10 µg/mL | 9.94 ± 0.04 | 0.40 |
| 6 h | 9.91 ± 0.02 | 0.20 | |
| 12 h | 9.87 ± 0.04 | 0.30 | |
| 18 h | 9.84 ± 0.03 | 0.41 | |
| 24 h | 9.88 ± 0.03 | 0.31 | |
| 48 h | 9.79 ± 0.02 | 0.20 | |
| 72 h | 9.80 ± 0.02 | 0.20 | |
| 0 h | 20 µg/mL | 19.88 ± 0.03 | 0.30 |
| 6 h | 19.87 ± 0.03 | 0.30 | |
| 12 h | 19.84 ± 0.04 | 0.40 | |
| 18 h | 19.81 ± 0.03 | 0.30 | |
| 24 h | 19.80 ± 0.02 | 0.20 | |
| 48 h | 19.78 ± 0.02 | 0.20 | |
| 72 h | 19.76 ± 0.02 | 0.20 | |
| 0 h | 30 µg/mL | 29.89 ± 0.04 | 0.40 |
| 6 h | 29.87 ± 0.04 | 0.40 | |
| 12 h | 29.83 ± 0.02 | 0.20 | |
| 18 h | 29.81 ± 0.02 | 0.20 | |
| 24 h | 29.81 ± 0.02 | 0.20 | |
| 48 h | 29.77 ± 0.04 | 0.40 | |
| 72 h | 29.76 ± 0.03 | 0.30 |
From freshly prepared paclitaxel nanoformulations, a 2 mL sample was taken and centrifuged at 15000 rpm at 25 °C for 30 min. The supernatant was discarded, and the precipitate was dissolved in ACN and analyzed by a UV–visible spectrophotometer at 237 nm. The same procedure was applied for all prepared nanoformulations. The paclitaxel solution (100 µg/mL) was analyzed in the range of 200 to 400 nm using ACN as a blank (Fig. 10).
Fig. 10.
UV Absorbance of Pclitaxel Solution in ACN (1 µg/m-30 µg/ml).
As shown in Table 5, there was an increase in encapsulation efficiency as the pluronic F-127 concentration was increased from 0.5 to 1.5 % while keeping the PLGA and SLS concentrations constant; however, at a2% concentration, there was a decline in encapsulation efficiency, which is in accordance with the previous data available. There was no significant effect on encapsulation efficiency by increasing the concentration of drug from 1 to 4 mg. Those nanoformulations with encapsulation efficiencies greater than 80% were selected for further studies.
As shown in Table 6, there was an increase in encapsulation efficiency as the concentration of poloxamer 407 was increased from 0.5 to 2% while maintaining the PLGA and SLS concentrations. There is an increase in encapsulation efficiency as the initial concentration of the drug is increased. Those nanoformulations with encapsulation efficiencies greater than 80% were selected for further studies.
Table 6.
Formulation of paclitaxel with PLGA, 0.05% SLS and poloxamer 407.
| NO | Code | D:P (mg) | Size (nm) | PDI | Z.P (mv) | Amount Encapsulate (mg/mL) | (%) E.E | (%) Drug Loading |
|---|---|---|---|---|---|---|---|---|
| Poloxamer 407 (0.5 %) | ||||||||
| 01 | PTX 100 | 1:10 | 180 ± 1.22 | 0.11 ± 0.01 | –22.1 ± 1.5 | 0.64 | 64 | 6.4 |
| 02 | PTX 101 | 2:10 | 184.6 ± 1.03 | 0.13 ± 0.01 | −20.1 ± 1.1 | 0.90 | 45 | 9.0 |
| 03 | PTX 102 | 3:10 | 190 ± 3.48 | 0.13 ± 0.03 | −20.7 ± 1.8 | 1.83 | 61 | 18.3 |
| 04 | PTX 103 | 4:10 | 202 ± 36.17 | 0.3 ± 0.01 | −19.1 ± 1.5 | 2.12 | 53 | 21.2 |
| Poloxamer 407 (1 %) | ||||||||
| 05 | PTX 104 | 1:10 | 199 ± 21.80 | 0.4 ± 0.01 | −26.85 ± 0.03 | 0.77 | 77 | 7.7 |
| 06 | PTX 105 | 2:10 | 215 ± 18.72 | 0.6 ± 0.01 | −24.1 ± 0.15 | 1.30 | 65 | 0.13 |
| 07 | PTX 106 | 3:10 | 304 ± 12.99 | 0.6 ± 0.04 | −26.8 ± 0.23 | 1.95 | 65 | 19.5 |
| 08 | PTX 107 | 4:10 | 224 ± 26.98 | 0.8 ± 0.02 | –23.08 ± 0.1 | 2.52 | 63 | 25.2 |
| Poloxamer 407 (1.5 %) | ||||||||
| 09 | PTX 108 | 1:10 | 202.3 ± 14.5 | 0.17 ± 0.03 | −35.2 ± 0.12 | 0.89 | 89 | 8.9 |
| 10 | PTX 109 | 2:10 | 215 ± 28.3 | 0.2 ± 0.03 | −34.5 ± 0.03 | 1.42 | 71 | 14.2 |
| 11 | PTX 110 | 3:10 | 300 ± 17.1 | 0.19 ± 0.02 | −30.25 ± 0.25 | 1.95 | 65 | 19.5 |
| 12 | PTX 111 | 4:10 | 331 ± 22.5 | 0.3 ± 0.01 | −29.75 ± 0.11 | 2.28 | 57 | 22.8 |
| Poloxamer 407 (2 %) | ||||||||
| 13 | PTX 112 | 1:10 | 229 ± 13.24 | 0.2 ± 0.01 | −40.4 ± 1.6 | 0.84 | 84 | 8.4 |
| 14 | PTX 113 | 2:10 | 312 ± 12.41 | 0.3 ± 0.02 | −39.08 ± 0.6 | 1.38 | 69 | 13.8 |
| 15 | PTX 114 | 3:10 | 351 ± 10.49 | 0.3 ± 0.03 | −34.21 ± 1.7 | 2.04 | 68 | 20.4 |
| 16 | PTX 115 | 4:10 | 408 ± 11.27 | 0.7 ± 0.02 | −28.11 ± 0.7 | 1.88 | 47 | 18.8 |
D: Paclitaxel, P: PLGA.
3.5. Nanoparticle characteristics before and after freeze drying
Freeze drying of the selected formulations was performed using mannitol (2%, 5% and 10%) as a cryoprotectant. Selected formulations were also freeze dried without cryoprotectant. Samples were frozen (-20 °C) for 30 mins and placed in a freeze-dried chamber. The drying process was performed for 24 hr at −45 °C. Freeze-dried nanoparticles were reconstituted by adding double distilled water (2 mL). The difference in the particle size, PDI and surface charge after freeze drying was measured to check the process validity as well as the stability of the nanoformulation. The changes observed in particle size, PDI and zeta potential were non-significant, as shown in Table 9.
Due to the small size and large surface area of nanoparticles, they tend to agglomerate. The most suitable method for the long-term stability of nanoparticles and to overcome these stability issues is freeze drying (Heurtault, Saulnier et al. 2003). It is a widely used process for improving the stability of pharmaceutical products, including vaccines, proteins, peptides and colloidal drug delivery systems. To protect a product from the stress of freezing and dehydration and to ensure maximum stability during storage, a suitable cryoprotectant is selected, and its concentration is optimized (Abdelwahed, Degobert et al. 2006). Freeze drying of the selected formulations was performed using mannitol (2%, 5% and 10%) as a cryoprotectant. No significant changes were observed in particle size, PDI and zeta potential with 5% mannitol.
3.6. Long-term stability
The stability of paclitaxel-loaded polymeric nanoparticles was evaluated by comparison of the drug encapsulation efficiency, particle size, PDI and zeta potential of initial formulations with those that were stored for 6 months at 25 ˚C and 4 ˚C in closed glass vials. No significant changes were observed in these parameters when stored at 4 ˚C. However, there were significant changes in the physicochemical properties when stored at 25 ˚C as shown in Table 10. These changes may be attributed to agglomeration of particles upon long-term storage, while at low temperature, particle agglomeration is prevented due to low kinetic energy, which prevents particle collision. These results suggest thatto achieve maximum therapeutic efficacy and to prevent physical and chemical instability, nanoformulations should be stored at low temperature, i.e., at 4 °C.
Table 10.
Stability Studies of Paclitaxel Nanoformulations.
| Time | Code | Stored at 4 °C |
Stored at 25 °C |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PS | PDI | ZP | %EE | PS | PDI | ZP | %EE | ||
| Day 1 | PTX 84 | 190 | 0.13 | −30 | 95% | 190 | 0.13 | −30 | 95% |
| PTX 86 | 287 | 0.2 | −35.8 | 81% | 287 | 0.2 | −35.8 | 81% | |
| PTX 108 | 202 | 0.17 | −35.2 | 89% | 202 | 0.17 | −35.2 | 89% | |
| PTX 112 | 229 | 0.2 | −40.4 | 84% | 229 | 0.2 | −40.4 | 84% | |
| 4th Week | PTX 84 | 190 | 0.13 | −30 | 95% | 198 | 0.13 | −31 | 95% |
| PTX 86 | 289 | 0.2 | −34 | 81% | 291 | 0.21 | −36 | 81% | |
| PTX 108 | 203 | 0.1 | −35.2 | 89% | 210 | 0.5 | −35.9 | 90% | |
| PTX 112 | 229 | 0.2 | −38.4 | 84% | 238 | 0.2 | −39 | 84% | |
| 16th Week | PTX 84 | 194 | 0.14 | −29 | 95% | 199 | 0.3 | −25 | 90% |
| PTX 86 | 289 | 0.21 | −35 | 80% | 299 | 0.3 | −30 | 80% | |
| PTX 108 | 209 | 0.3 | −35 | 84% | 225 | 0.4 | −36 | 91% | |
| PTX 112 | 231 | 0.2 | −39 | 84% | 220 | 0.21 | −40 | 82% | |
| 20th Week | PTX 84 | 195 | 0.2 | −31 | 96% | 219 | 0.4 | −28 | 91% |
| PTX 86 | 290 | 0.3 | –33 | 83% | 307 | 0.3 | −27 | 81% | |
| PTX 108 | 210 | 0.4 | −34 | 89% | 234 | 0.6 | –32 | 86% | |
| PTX 112 | 233 | 0.2 | −36 | 80% | 244 | 0.4 | –33 | 84% | |
| 24th Week | PTX 84 | 198 | 0.2 | −28 | 97% | 234 | 0.3 | −29 | 96% |
| PTX 86 | 290 | 0.4 | –33 | 83% | 322 | 0.5 | −27 | 81% | |
| PTX 108 | 211 | 0.4 | –33 | 88% | 239 | 0.5 | –32 | 82% | |
| PTX 112 | 234 | 0.4 | −36 | 81% | 253 | 0.4 | −38 | 85% | |
4. Conclusion
More than 150 paclitaxel PLGA nanoformulations were prepared and then optimized according to the requirements. In these formulations, four (04) were selected as required for breast cancer targeting. Nanoformulations PTX 84, 86, 108 and 112 were selected on the basis of their physicochemical characteristics, freeze-drying stability and long-term stability. All the formulations with PVA, poloxamer and Pluronic showed better results in terms of particle size, PDI, zeta potential, % EE and drug loading, but the best results were obtained at 1:10 and 3:10 drug-to-polymer ratios. At these particular ratios, the nanoparticles produced have a size of 189–287 nm, zeta potential ≥ ±30 mv, PDI 0.1–0.2, and % EE > 80%. The other ratios failed due to one or more reasons, such as stability, particle aggregation and sedimentation during washing. Higher concentrations of pluronic F-127 and poloxamer 407 were used to prevent the detachment of the polypropylene glycol part from the surface of the nanoparticles, which ultimately led to stable formulations. SLS was utilized to determine the stability of the nanoparticles in water, particularly during the washing period. The increased surface charge is also a contributing factor towards the stability and long circulation half-life of nanoformulations. The findings of this particular research will ultimately be a basis for the development of new paclitaxel formulations for the treatment of cancer.
It can be revealed from our research that by controlling different processing parameters and using suitable stabilizers, nanoparticles of the desired particle size, surface charge, encapsulation efficiency and drug release profile can be controlled. By attaching ligands on the surface of nanoparticles, targeted therapy can be achieved, which is the main goal of the whole study.
Author contributions
Mirina Sakhi carried out the experimental work and manuscript writing. Abad Khan, Zafar Iqbal and Ismail Khan designed the project and wrote the manuscript. Saeed Ahmad Khan, Sumaira Irum Khan and Muzna Ali Khattak helped in data analysis. Mohammad N. Uddin and Mohsin Kazi helped in manuscript editing and evaluation. All authors read and approved the final manuscript for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R301), King Saud University, Riyadh, Saudi Arabia. The research support and facilitation provided by the Higher Education Commission (HEC), of Pakistan and Department of Pharmacy, University of Peshawar is also acknowledged.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mirina Sakhi, Email: mirina.sakhi@yahoo.com.
Abad Khan, Email: drabadkhan@uoswabi.edu.pk.
Ismail Khan, Email: ismailkhan@uoswabi.edu.pk.
Saeed Ahmad Khan, Email: saeedkhan@kust.edu.pk.
Sumaira Irum Khan, Email: sumaira.pharmacy@must.edu.pk.
Muzna Ali Khattak, Email: muznaqazi@yahoo.com.
Mohammad N. Uddin, Email: uddin_mn@mercer.edu.
Mohsin Kazi, Email: mkazi@ksu.edu.sa.
Fazli Nasir, Email: fazlinasir@uop.edu.pk.
References
- Abdelwahed, W., G. Degobert, S. Stainmesse and H. Fessi., 2006. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv. drug deliv. rev.58(15): 1688-1713. https://doi.org/10.1016/jaddr.2006. 09.017. [DOI] [PubMed]
- Ali A.M.A., El-Enin H.A.A. In-vitro/in-vivo evaluation of Paclitaxel Freeze-Dried micellar nanoparticles intended for buccal delivery. J. Drug Deliv. Sci. Tech. 2021;62 doi: 10.1016/j.jddst.2021.102424. [DOI] [Google Scholar]
- Aslam, M., M. A. Kalyar and Z. A. Raza., 2018. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Poly. Eng. Sci.58(12): 2119-2132.https://doi.org/10.1002/pen. 24855.
- Bai J., Li Y., Yang S., Du J., Wang S., Zheng J., Wang Y., Yang Q., Chen X., Jing X. A simple and effective route for the preparation of poly (vinylalcohol)(PVA) nanofibers containing gold nanoparticles by electrospinning method. Sol. Sta. Comm. 2007;141(5):292–295. doi: 10.1016/j.ssc.2006.10.024. [DOI] [Google Scholar]
- BAYINDIR Z.S., BADILLI U. Preparation of polymeric nanoparticles using different stabilizing agents. J. Fac. Pharm. Ankara Uni. 2009;38(4):257–268. doi: 10.1501/Eczfak_0000000543. [DOI] [Google Scholar]
- das Neves, J., J. Michiels, K. K. Ariën, G. Vanham, M. Amiji, M. F. Bahia and B. Sarmento., 2012. Polymeric nanoparticles affect the intracellular delivery, antiretroviral activity and cytotoxicity of the microbicide drug candidate dapivirine. Pharm. res.29: 1468-1484. https://doi.org/10.1007/s11095-011-0622-3 [DOI] [PubMed]
- Dumortier, G., J. L. Grossiord, F. Agnely and J. C. Chaumeil., 2006. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. res.23: 2709-2728. https://doi.org/10.1007/s1109 5-006-9104-4. [DOI] [PubMed]
- Fereja T.H., Seifu M.F., Mola T.Y. UV-visible spectrophotometric method development and quantification of ciprofloxaciline in tablets dosage form. J. Pharm. Pharmacol. 2015;2:1–8. [Google Scholar]
- Heurtault B., Saulnier P., Pech B., Proust J.-E., Benoit J.-P. Physico-chemical stability of colloidal lipid particles. Biomat. 2003;24(23):4283–4300. doi: 10.1016/S0142-9612(03)00331-4. [DOI] [PubMed] [Google Scholar]
- Huang C.-Y., Chen C.-M., Lee Y.-D. Synthesis of high loading and encapsulation efficient paclitaxel-loaded poly (n-butyl cyanoacrylate) nanoparticles via miniemulsion. Int. J. Pharm. 2007;338(1–2):267–275. doi: 10.1016/j.ijpharm.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Kalepu S., Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm. Sin. B. 2015;5(5):442–453. doi: 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanage, S. G., P. B. Mohite and S. Jadhav.,2013. Development and validation of UV-visible spectrophotometric method for simultaneous determination of eperisone and paracetamol in solid dosage form. Adv. pharm. bulletin 3(2):447. https://doi.org/10.5681%2Fapb.2013.073. [DOI] [PMC free article] [PubMed]
- Mainardes, R. M. and R. C. Evangelista., 2005. PLGA nanoparticles containing praziquantel: effect of formulation variables on size distribution. Int. j. pharm.290(1-2):137-144. https://doi.org/10.1016/j.ijpharm. 2004.11.027. [DOI] [PubMed]
- Pongpeerapat A., Wanawongthai C., Tozuka Y., Moribe K., Yamamoto K. Formation mechanism of colloidal nanoparticles obtained from probucol/PVP/SDS ternary ground mixture. Int. J. Pharm. 2008;352(1–2):309–316. doi: 10.1016/j.ijpharm.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Redhead H., Davis S., Illum L. Drug delivery in poly (lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J. Controll. Release. 2001;70(3):353–363. doi: 10.1016/S0168-3659(00)00367-9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Hachair A., Hofmann A. Hexavalent chromium quantification in solution: Comparing direct UV–visible spectrometry with 1, 5-diphenylcarbazide colorimetry. Comptes Rendus Chimie. 2018;21(9):890–896. doi: 10.1016/j.crci.2018.05.002. [DOI] [Google Scholar]
- Schmitt, P. D., N. S. Trasi, L. S. Taylor and G. J. Simpson.,2015. Finding the needle in the haystack: characterization of trace crystallinity in a commercial formulation of paclitaxel protein-bound particles by Raman spectroscopy enabled by second harmonic generation microscopy. Mol. Pharmaceutics 12(7): 2378-2383. https:// doi.org/ 10. 1021/acs.molpharmaceut.5b00065. [DOI] [PubMed]
- Shkodra-Pula B., Grune C., Traeger A., Vollrath A., Schubert S., Fischer D., Schubert U. “Effect of surfactant on the size and stability of PLGA nanoparticles encapsulating a protein kinase C inhibitor. Int. J. Pharm. 2019;566:756–764. doi: 10.1016/j.ijpharm.2019.05.072. [DOI] [PubMed] [Google Scholar]
- Stolnik, S., B. Daudali, A. Arien, J. Whetstone, C. Heald, M. Garnett, S. Davis and L. Illum., 2001. The effect of surface coverage and conformation of poly (ethylene oxide)(PEO) chains of poloxamer 407 on the biological fate of model colloidal drug carriers. BBA. Biomembranes 1514(2): 261-279. https://doi.org/10.1016/S0005-2736(01)00376-5 [DOI] [PubMed]
- Tuomela A., Hirvonen J., Peltonen L. Stabilizing agents for drug nanocrystals: effect on bioavailability. Pharmaceutics. 2016;8(2):16. doi: 10.3390/pharmaceutics8020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Hao J., Yuan S., Li Y., Juan W., Sha X., Fang X. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int. J. Pharma. 2009;376(1–2):176–185. doi: 10.1016/j.ijpharm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- Win K.Y., Feng S.-S. In vitro and in vivo studies on vitamin E TPGS-emulsified poly (D, L-lactic-co-glycolic acid) nanoparticles for paclitaxel formulation. Biomaterials. 2006;27(10):2285–2291. doi: 10.1016/j.biomaterials.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Zhao L., Feng S.-S. Enhanced oral bioavailability of paclitaxel formulated in vitamin E-TPGS emulsified nanoparticles of biodegradable polymers: in vitro and in vivo studies. J. Pharm. Sci. 2010;99(8):3552–3560. doi: 10.1002/jps.22113. [DOI] [PubMed] [Google Scholar]