Abstract
The purpose of this study was to investigate the effects of melittin on production performance, antioxidant function, immune function, heat shock protein, intestinal morphology, and cecal microbiota of heat-stressed quails. A total of 120 (30-day-old) male quails were randomly divided into 3 groups. Each group consisted of 4 replicates with 10 birds per replicate. The ambient temperature of the control group (group W) was 24°C ± 2°C. The heat stress group (group WH) and the heat stress + melittin group (group WHA2) were subjected to heat stress for 4 h from 12:00 to 16:00 every day, and the temperature was 36°C ± 2°C for 10 d. The results showed that compared with the group W, heat stress significantly decreased growth performance, serum and liver antioxidative function, immune function, intestinal villus height (VH) and villus height-to-crypt depth ratio (VH/CD), and cecal microbiota Chao and ACE index (P < 0.05). The crypt depth (CD) in the small intestine, and HSP70 and HSP90 mRNA levels in the heart, liver, spleen, and kidney were significantly increased (P < 0.05). Dietary melittin significantly increased growth performance, serum and liver antioxidative function, immune function, intestinal VH and VH/CD, and cecal microbiota Shannon index in heat-stressed quails (P < 0.05). Melittin significantly decreased small intestinal CD, and HSP70 and HSP90 mRNA levels in the viscera (P < 0.05). Furthermore, dietary melittin could have balanced the disorder of cecal microbiota caused by heat stress and increased the abundance and diversity of beneficial microbiota (e.g., Firmicutes were significantly increased). PICRUSt2 functional prediction revealed that most of the KEGG pathways with differential abundance caused by high temperature were related to metabolism, and melittin could have restored them close to normal levels. Spearman correlation analysis showed that the beneficial intestinal bacteria Anaerotruncus, Bacteroidales_S24-7_group_norank, Lachnospiraceae_unclassified, Shuttleworthia, and Ruminococcaceae_UCG-014 increased by melittin were positively correlated with average daily feed intake, the average daily gain, serum and liver superoxide dismutase, IgG, IgA, bursa of Fabricius index, and ileum VH and VH/CD. In sum, our results demonstrate for the first time that dietary melittin could improve the adverse effects of heat stress on antioxidant function, immune function, heat shock protein, intestinal morphology, and cecal microbiota in quails, consequently improving their production performance under heat stress.
Key words: quail, heat stress, melittin, antioxidant function, intestinal microbiota
INTRODUCTION
In poultry production, a wide range of factors, including the management, nutrition, environment, and disease conditions, can cause stress (Surai and Fisinin, 2016). Heat stress is one of the major environmental stressors affecting poultry production in tropical and subtropical regions. Heat stress adversely impacts the growth rate, production performance, immune response, gut function and gut microbiota, redox balance, energy bioavailability in cells, and attainment of the body's homeostasis, culminating in huge economic losses to the poultry industry (Saeed et al., 2019; Uyanga et al., 2021). Heat stress increases the body temperature, respiratory rate, and metabolic heat production, decreases feed intake, impairs animal performance, causes immune dysfunction, impairs nutrient digestibility and metabolism, alters gut integrity and microbiota composition, and increases mortality (Emami et al., 2020; Kumar et al., 2021; Brugaletta et al., 2022; Uyanga et al., 2022).
Consequently, during heat stress, there is an imminent need to devise useful strategies that would aid overcome stress effects in farm animals. Besides environmental improvements in poultry housing, dietary manipulation is a vital machinery that can contribute to alleviating the negative impacts of heat stress (Abdel-Moneim et al., 2021). Dietary manipulation involves the inclusion/supplementation of functional additives (supplements) with beneficial properties to poultry diets (Olgun et al., 2021). It is an acceptable practice that involves the inclusion of vitamins, minerals, amino acids, phytogenes, growth promoters, antioxidants, nutraceuticals, herbs, and probiotics, among others, in poultry nutrition (Abd et al., 2020; Alagawany et al., 2020).
For centuries, bee venom therapy has been used in the treatment of human acute and chronic diseases (Wehbe et al., 2019). The toxin contains various bioactive peptides and enzymes. Melittin is a kind of polypeptide that exhibits a variety of biological activities, such as anti-inflammatory, antibacterial, antifungal, antitumor, etc. (Khalil et al., 2021). Melittin was found to improve the immune function and antioxidant stress of chickens (Kim et al., 2019) and rabbits (Elkomy et al., 2021). The addition of melittin to feed could improve the laying performance, intestinal antioxidant capacity, and barrier function in 70-day-old quail (Li et al., 2023). Furthermore, it enhanced the balance of oxidation and antioxidation in mice with cognitive impairment to protect nerves (Nguyen and Lee, 2021). However, the studies on the effects of melittin on quails exposed to heat stress are very limited. According to the beneficial effects of melittin, we speculate that melittin can improve the damage of antioxidant capacity and immune function caused by heat stress and reduces undesirable bacteria of cecal microbiota. Therefore, the purpose of this study was to investigate the protective effect of melittin on quail health and to explore its molecular mechanism to provide new ideas for quail breeding to alleviate thermal stress.
MATERIALS AND METHODS
Experimental Animals, Diets, and Experimental Design
Chinese white-feather quails (male) were provided by a farm in Henan Province and fed adaptively for 10 d. The heat stress test began at 30 d of age. Quails (n = 120) with similar body weights were randomly divided into 3 groups: control (W, basic diet), heat stress (WH, basic diet), and heat stress + melittin (WHA2, melittin 0.12 g/kg) group. Each group had 4 replicates, with 10 quails per replicate. A cage was used to raise the quails (50 cm × 55 cm × 45 cm, 5 quails per cage). The ambient temperature of the group W was maintained at 24°C ± 2°C at a relative humidity of 55 ± 2%. Quails in groups WH and WHA2 were subjected to heat stress for 10 consecutive days from 12:00 to 16:00 every day for 4 h at 36°C ± 2°C and humidity of 70 ± 2%, and the remainder of the time, they were kept as the group W. In this experiment, the basic diet of quails was corn-soybean meal diet (Supplementary Table 1). The birds were fed a basic diet with ad libitum access to feed and drinking water. Melittin used in this study is a new type of antimicrobial peptide that mainly uses genetic engineering technology to construct a melittin/nisin tandem expression element gene on the chromosome of Bacillus subtilis (food grade). Finally, the product containing polypeptides (activity ≥1 MIU/g, Item No. 361001) was acquired by high-efficiency fermentation and expression technology. The study was approved by the Institutional Animal Care and Use Committee of Xinyang Agriculture and Forestry University (XAFU-2021-07056).
Production Performance
The initial and daily weights as well as feed consumption were recorded. On the day after the end of the 10-day heat stress period, the birds were weighed on an empty stomach and used for calculations of the average daily feed intake (ADFI), average daily gain (ADG), and feed-to-gain ratio (F/G).
Detection of Serum Antioxidant and Immune Indices
Upon completion of the experiment, 1 quail was chosen at random from each replicate for blood collection from the fasting right jugular vein. A centrifuge was used to separate serum at 3,500 rpm for 15 min and stored at −20°C. The antioxidant indexes for superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) and immune indexes for complement C3, interferon-γ (IFN-γ), IgG, and IgA were measured using commercial ELISA kits (Sino Best Biological Technology Co. Ltd., Shanghai, China) by following the protocol provided by the manufacturer.
Tissue Sample Collection
After collecting blood, cervical dislocation was performed to sacrifice the quails. We collected the tissues from the duodenum, jejunum, ileum, heart, liver, spleen, and kidney and immediately frozen them in liquid nitrogen, and stored at −80°C. With 4% paraformaldehyde, we fixed small intestine tissues. We weighed the thymus, spleen, and bursa of Fabricius and calculated the immune organ index. Their calculation formula is as follows: Immune organ index (mg/g) = fresh organ weight/quail weight. The intestinal microbiota composition was analyzed by aseptically collecting the contents of the quail cecum and freezing them in liquid nitrogen as soon as possible.
Histology
The intestinal tract fixed by 4% paraformaldehyde was removed and the mucous membrane was removed. Conventional paraffin sections were made by cleaning, dehydration, transparency, wax dipping, embedding, slicing (thickness 5 μm), spreading and baking, and hematoxylin and eosin (H&E) staining (Awad et al., 2009). The intestinal villus height (VH) and crypt depth (CD) were measured using a NikonNi-U microscope under 5 × multiple, and the ratio of villus height to crypt depth (VH/CD) was calculated.
Heat Shock Protein Expression
Heat shock protein (HSP) 70 and HSP90 mRNA expression was quantified in the heart, liver, spleen, and kidney tissues of quails. Total RNA was extracted using a commercial kit (Wuhan Servicebio Technology, Wuhan, China) and quantified with UV spectroscopy with a NanoDrop 2000 instrument (Thermo Fisher, Pittsburg, PA), and 1.2% agarose gel electrophoresis was used to determine RNA integrity. Reverse transcription reactions were performed with a commercial kit (Wuhan Servicebio) following the manufacturer's instructions, and cDNA was stored at −20°C. Real-time fluorescence quantitative PCR (RT-qPCR) reactions were performed using a SYBR Green qPCR Master Mix kit (Wuhan Servicebio) following the manufacturer's instructions using quail-specific PCR primers (Supplementary Table 2) synthesized by GENEWIZ, Suzhou, China. The cycling parameters were: 95°C, 30 s and 40 cycles of 95°C, 15 s; 60°C, 30 s; 72°C, 30 s. The relative expression of mRNAs was calculated using the 2−△△CT method and calculated using the software supplied with the RT-qPCR instrument (Pfaffl, 2001).
Bioinformatic Analysis of Intestinal Microbiota
Samples of cecal contents were identified using 16S rDNA detection completed by Shanghai Origin gene Bio-pharm Technology. Illumina PE250 (Illumina, San Diego, CA) sequencing was used to generate the cecal library. The QIIME software (v1.8.0) was used for raw data processing (Caporaso et al., 2010). Based on 97% sequence identity, operational taxonomic units (OTUs) were merged and classified using the UCLUST sequence alignment tool (Edgar, 2010). Species annotations were carried using the Ribosomal Database Project database, and α- and β-diversity were analyzed using QIIME. Differences in species classification of intestinal microbiota were analyzed using the LEfSe method (Segata et al., 2011) and displayed using R v.2.15.3, and functions were predicted and analyzed with PICRUSt2 (Douglas et al., 2019).
Statistical Analysis
Statistical significance was calculated in SPSS Statistics 26 (SPSSS Inc., Chicago, IL) using 2-tailed paired t test in table. Heat shock proteins and intestinal microbiota α-diversity were analyzed and graphed using R v.3.6.3 (https://www.xiantao.love/products) with 2-tailed paired t test. STAMP 2.1.3 software was used for Welch's t test at the phylum and genus levels and for function prediction and chart construction. Spearman rank correlation analysis was performed to evaluate the relationship between changes in cecal microbiota (genus level) and growth performance and health parameters of quails using SPSS Statistics 26 (SPSSS Inc., Chicago, IL). The results were obtained as mean ± SEM, and P < 0.05 was considered a statistically significant difference.
RESULTS AND DISCUSSION
Effect of Melittin on Production Performance of Heat-Stressed Quails
At present, the poultry breeding mode is developing rapidly on the road of intensive farming. Excessive breeding density and global warming are more likely to cause poultry heat stress, endanger the health of poultry bodies, and bring huge economic losses to the poultry breeding industry. It is hot and humid in summer in southern China, where heat stress has also become one of the prime reasons restricting the development of poultry industry. Therefore, strategies to mitigate the negative effects of thermal stress must be devised. The melittin used in this study is a polypeptide product produced by modern biotechnology, which made melittin lose its original hemolytic side effect. We used quail as an experimental animal model to investigate the impacts of melittin on production performance, antioxidation, immune response, intestinal histomorphology, intestinal microbiota, and HSP gene expression.
In this study, compared with the group W, heat stress had a negative impact on all production performance traits of quails, with a significant decrease in ADG (P = 0.0028) and a significant increase in F/G (P = 0.0318) (Table 1). Previously, when quails were exposed to heat stress, their weight gain and feed efficiency decreased (El-Kholy et al., 2017; Mehaisen et al., 2017). Interestingly, heat stress did not cause a significant decrease in quail feed intake (P = 0.1540), which may be ascribed to the increase in feed intake of quails at moderate temperatures to compensate for the loss of heat stress. Birds reduce their feed intake under heat stress is intended to reduce heat production due to digestion, absorption, and nutrient utilization processes (Baumgard and Rhoads, 2013). Furthermore, high temperature can change the neuroendocrine system and inhibit the excitability of the feeding nerve center, resulting in a decrease in feed intake (Calefi et al., 2017). A significant decrease in ADG during heat stress may be due to the increase in blood corticosterone levels, which changes energy consumption and also increases protein catabolism (Siegel, 1980). In addition, at a temperature of 32°C, the activities of trypsin, chymotrypsin, and amylase decreased (Hai et al., 2001), resulting in the decrease in nutrient digestibility, and the short-term suitable temperature could not recover effectively. Therefore, the higher the ambient temperature, the lower the nutrient digestibility of animals and poultry.
Table 1.
Effect of melittin on production performance of heat-stressed quail.
| W × WH |
WH × WHA2 |
||||||
|---|---|---|---|---|---|---|---|
| Item | W | WH | WHA2 | SEM | P value | SEM | P value |
| ADFI (g) | 18.91 | 17.11 | 18.90 | 1.11 | 0.1540 | 1.43 | 0.2546 |
| ADG (g) | 4.44 | 3.68 | 4.40 | 0.16 | 0.0028 | 0.25 | 0.0285 |
| F/G | 4.26 | 4.64 | 4.29 | 0.14 | 0.0318 | 0.11 | 0.0197 |
The mean and standard error of the mean are used to express data.
P < 0.05 indicates a significant difference between the 2 groups.
Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; F/G, feed-to-gain ratio.
W, control group; WH, heat stress group; WHA2, heat stress + melittin group.
On the other hand, adding anti–heat-stress drugs or feed additives to feed can increase daily gain, daily feed intake, and feed reward, such as propolis (Mehaisen et al., 2017), resveratrol (Liu et al., 2014), antimicrobial peptides (Hu et al., 2017), chromium (El-Kholy et al., 2017), vitamins C and E (Sahin and Kucuk, 2001). We observed that melittin supplementation significantly increased the ADG (P = 0.0285) and decreased the F/G in heat-stressed quails (P = 0.0197) (Table 1). These positive effects of melittin on quail production performance may be related to the following research results.
Effect of Melittin on Antioxidant Function of Heat-Stressed Quails
Antioxidant enzyme system, as the first barrier of antioxidant defense in animals, reflects the metabolic level of reactive oxygen species and the level of tissue damage. Its enzyme system includes SOD, catalase, and GSH-Px, among others (Surai et al., 2019). High environmental temperature can lead to oxidative stress, resulting in an imbalance of the oxidant/antioxidant system in poultry (Sahin et al., 2002). Heat stress can disrupt the redox dynamic balance by increasing the production of free radicals and lead to oxidative damage of DNA, proteins, and lipids, ultimately resulting in the death of cells (Arnaud et al., 2002). In addition, increased production of free radicals results in higher levels of lipid peroxidation in plasma and tissues (Naziroglu et al., 2000). There is evidence that heat stress can reduce the activities of antioxidant enzymes in serum (Liu et al., 2014; Hosseini-Vashan et al., 2016), intestine (Yang et al., 2021), liver (Zhang et al., 2018), and bursa of Fabricius (Liu et al., 2021) and increase the concentration of MDA. Our results are consistent with the above results. Heat stress can significantly reduce the SOD and GSH-Px activities in serum and liver and significantly increase their MDA concentration (P < 0.05) (Table 2).
Table 2.
Effect of melittin on serum, hepatic, and testicular antioxidant indices in heat-stressed quails.
| W × WH |
WH × WHA2 |
||||||
|---|---|---|---|---|---|---|---|
| Item | W | WH | WHA2 | SEM | P value | SEM | P value |
| Serum | |||||||
| SOD (ng/mL) | 12.05 | 10.69 | 12.53 | 0.29 | 0.0036 | 0.72 | 0.0442 |
| GSH-Px (ng/mL) | 196.55 | 174.67 | 203.27 | 6.42 | 0.0144 | 6.68 | 0.0052 |
| MDA (nmol/mL) | 16.54 | 20.52 | 16.74 | 1.40 | 0.0297 | 1.28 | 0.0252 |
| Liver | |||||||
| SOD (ng/mL) | 14.32 | 12.68 | 14.89 | 0.41 | 0.0072 | 0.64 | 0.0135 |
| GSH-Px (ng/mL) | 242.97 | 212.05 | 247.13 | 7.21 | 0.0052 | 9.69 | 0.0111 |
| MDA (nmol/mL) | 13.79 | 18.22 | 15.42 | 0.67 | 0.0006 | 0.51 | 0.0015 |
The mean and standard error of the mean are used to express data.
P < 0.05 indicates a significant difference between the 2 groups.
Abbreviations: GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
W, control group; WH, heat stress group; WHA2, heat stress + melittin group.
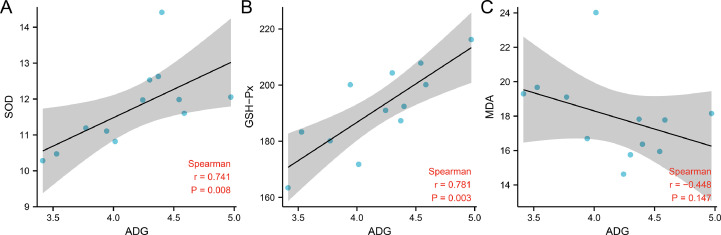
At present, studies on alleviating the effects of heat stress on poultry are largely focused on the supplementation of antioxidants, such as resveratrol (Wang et al., 2021; Yang et al., 2021), to reduce heat stress-mediated oxidative stress (Goel, 2021). Dietary melittin could significantly increase the SOD and GSH-Px activities in serum and liver in heat-stressed quails, and significantly reduce MDA content (P < 0.05) (Table 2). We also noted a significant positive correlation between the activities of SOD and GSH-Px and ADG (r = 0.741, P = 0.008 and r = 0.781, P = 0.003, respectively) (Figure 1). Melittin can increase the antioxidant properties in heat-stressed quails because of its antioxidant activity, which was found to enhance the antioxidant function of rabbit serum (Elkomy et al., 2021) as well as the antioxidant function of mouse intestine (Ahmedy et al., 2020), lung (El-Aarag et al., 2019), and kidney (Kim et al., 2021) and reduce its MDA content to prevent cell damage and reduce oxidative stress. This increase in antioxidant capacity can reduce the negative effects of thermal stress on quails. The mechanism may be that melittin can alleviate neuro-oxidative stress induced by β-amyloid protein by stimulating heme oxygenase-1 (HO-1) production and regulating the TrkB/CREB/BDNF signaling pathway through nuclear translocation of nuclear factor NF-E2-related factor (Nrf2) (Nguyen and Lee, 2021). Furthermore, it can improve the renal oxidative stress induced by lipopolysaccharide by increasing the nuclear translocation of Nrf2 and upregulating its target genes HO-1 and NAD (P) H: quinone oxidoreductase 1 (Kim et al., 2021). However, the mechanism of melittin increasing antioxidant capacity under heat stress warrants further exploration.
Figure 1.
The correlation between ADG and antioxidation index. Abbreviation: ADG, average daily gain.
Effect of Melittin on Serum Immune Indices and Immune Organ Index of Heat-Stressed Quails
The complement system is not only an important component of innate immunity in animal serum, but it also plays a pivotal role in the process of acquired immunity. It widely participates in antimicrobial defense response and immune regulation and can mediate the damaging response of immunopathology. It is cascaded mainly through 3 pathways (classical, lectin, and alternative). Complement C3 plays a key role in the process of complement activation. Both classical and alternative activation pathways can promote the chain reaction of subsequent complement components only after complement C3 is activated (Reis et al., 2019; Thorgersen et al., 2019). In this study, we noted that heat stress reduced the level of complement C3 in quails (P = 0.1692) (Table 3). However, thermal stress significantly reduced the level of complement C3 in laying hens and broilers (Zhang et al., 2012; He et al., 2019). Therefore, heat stress could inhibit immune function by reducing the content of serum complement C3. However, melittin supplementation increased the level of complement C3 (P = 0.0657) (Table 3). The cytokine IFN-γ has antiviral, anticancer, and immunomodulatory properties, and is powerful in regulating the immune system. As an essential component of the immune system, it helps the body to eliminate pathogens (Schroder et al., 2004). In this study, we found that heat stress had no significant effect on the content of serum IFN-γ (P = 0.6505), but the addition of melittin significantly increased the content of IFN-γ (P = 0.0146) (Table 3).
Table 3.
Effect of melittin on serum immune indices and immune organ index in heat-stressed quails.
| W × WH |
WH × WHA2 |
||||||
|---|---|---|---|---|---|---|---|
| Item | W | WH | WHA2 | SEM | P value | SEM | P value |
| Serum immune indices | |||||||
| Complement C3 (μg/mL) | 1095.75 | 1030.03 | 1247.72 | 42.06 | 0.1692 | 96.88 | 0.0657 |
| IFN-γ (pg/mL) | 113.88 | 114.92 | 131.04 | 2.18 | 0.6505 | 4.75 | 0.0146 |
| IgG (mg/mL) | 487.56 | 372.01 | 467.37 | 38.54 | 0.0241 | 28.64 | 0.0158 |
| IgA (μg/mL) | 432.85 | 346.06 | 443.00 | 30.24 | 0.0284 | 33.66 | 0.0281 |
| Immune organ index1 | |||||||
| Thymus | 3.16 | 2.43 | 2.76 | 0.34 | 0.0765 | 0.31 | 0.3170 |
| Bursa of Fabricius | 1.73 | 1.14 | 1.70 | 0.11 | 0.0019 | 0.08 | 0.0005 |
| Spleen | 0.84 | 0.69 | 0.82 | 0.08 | 0.1185 | 0.08 | 0.1844 |
The mean and standard error of the mean are used to express data.
P < 0.05 indicates a significant difference between the 2 groups.
The unit of immune organ index is mg/g body weight.
W, control group; WH, heat stress group; WHA2, heat stress + melittin group.
The determination of serum immunoglobulin is the most commonly employed method to evaluate the humoral immune function. Poultry exposure to high ambient temperature can reduce humoral immune response (Lara and Rostagno, 2013). IgG is the most abundant immunoglobulin in animals and participates in immune responses such as antibacterial, antiviral, antitoxin, etc. (Lundqvist et al., 2006). IgA is an important part of the mucosal defense system of the body, and it is the first line of defense against the invasion of pathogenic microorganisms (Lundqvist et al., 2006). We found that heat stress significantly decreased the IgG and IgA levels in serum of quails (P = 0.0241, 0.0284, respectively) (Table 3). Our findings are consistent with a previous study that found that heat stress significantly reduced the IgG and IgA contents in broilers (Zaglool et al., 2019). Melittin significantly increased the IgG and IgA contents in serum of quails (P = 0.0158, 0.0281, respectively) (Table 3), and the level of antibody affected by heat stress was alleviated.
The bursa of Fabricius, thymus, and spleen are the main immune organs for the development, differentiation, and antibody production of immune cells. Their relative weight is typically used as an indicator of the immune function of birds, and a high immune organ index indicates that the immune organs are well developed (Lara and Rostagno, 2013). Heat stress could significantly decrease the immune organ index of Wenchang chicks, accompanied by a series of changes, such as a decrease in thymus volume, thymus lobule, and lymphoid follicle, and medulla/cortex ratio, among others (Tang and Chen, 2016). Heat stress could lead to lower relative weight of the spleen and the bursa of Fabricius (Zhu et al., 2014) and decrease of cortex and medulla of lymphocytes in the bursa of Fabricius (Lara and Rostagno, 2013). We observed that thermal stress significantly decreased the bursa of Fabricius index (P = 0.019), but we found no significant difference in the decrease of thymus and spleen indices (P = 0.0765, 0.1185, respectively) (Table 3). After adding melittin, the bursa of Fabricius index was significantly increased (P = 0.0005), but the thymus and the spleen indices were not significantly increased (P = 0.3170, 0.1844, respectively) (Table 3). Therefore, melittin could improve the immune function of heat-stressed quails and enhance the ability of the body to resist pathogenic infection.
Effect of Melittin on the Intestinal Morphology in Heat-Stressed Quails
Growth performance and intestinal health are closely related to the morphology of the small intestine (Ao and Kim, 2020). The increase in VH is related to the increase in the villus absorption surface area, which can promote the absorption of nutrients (Dai et al., 2021). The shallower the depth of CD in the intestine, the better the maturity of intestinal mucosal epithelial cells, which could increases the secretion of digestive enzymes and nutrient absorption and improves the growth performance of broilers (Stamilla et al., 2020). The VH/CD ratio is not only a significant indicator of intestinal absorptive capacity but also an important parameter to describe the intestinal health of birds (Jayaraman et al., 2013). With the increase in the ratio, the mucosa was repaired, and the digestion and absorption were enhanced, while the decrease in the ratio would lead to an opposite effect (Zhang et al., 2020). The intestinal morphological changes we observed can explain the effect of thermal stress on production performance and the effect of melittin. In the WH group, VH and VH/CD in duodenum, jejunum, and ileum decreased significantly, while CD increased significantly (P < 0.05) (Table 4 and Supplementary Figure 1). Similar results have been obtained in previous studies on heat-stressed quails (Mehaisen et al., 2017). Several possible factors could lead to changes in the intestinal morphology due to increased temperature, including the result of reduced feed intake in heat-stressed quails, as previously reported in chickens (Shamoto and Yamauchi, 2000). Furthermore, heat stress can cause intestinal tissue ischemia, increase intestinal toxins, and lead to intestinal epithelial exfoliation and damage (Lambert, 2009; Liu et al., 2009), which in turn leads to the shortening of VH and deepening of CD. Therefore, exposure to thermal stress may affect intestinal digestion and absorption function (Yu et al., 2010), and result in changes that may impair quail production performance, as we have observed. After melittin supplementation (WHA2 group), VH, VH/CD in duodenum, jejunum, and ileum increased significantly, while CD in duodenum and ileum decreased significantly (P < 0.05) (Table 4 and Supplementary Figure 1). Therefore, melittin supplementation had a positive effect on intestinal morphology and mucosal development in heat-stressed quails.
Table 4.
Effect of melittin on histology of the intestinal tract in heat-stressed quails.
| W × WH |
WH × WHA2 |
||||||
|---|---|---|---|---|---|---|---|
| Item | W | WH | WHA2 | SEM | P value | SEM | P value |
| Duodenum | |||||||
| VH (μm) | 669.23 | 569.44 | 639.02 | 33.30 | 0.0067 | 30.09 | 0.0305 |
| CD (μm) | 60.37 | 66.80 | 56.79 | 1.79 | 0.0016 | 2.48 | 0.0005 |
| VH/CD | 11.00 | 9.08 | 11.77 | 0.53 | 0.0015 | 0.80 | 0.0028 |
| Jejunum | |||||||
| VH (μm) | 392.87 | 265.26 | 324.61 | 13.88 | 1.42 × 10−6 | 23.70 | 0.0202 |
| CD (μm) | 39.21 | 44.70 | 43.97 | 1.20 | 0.0001 | 1.52 | 0.6375 |
| VH/CD | 10.09 | 6.02 | 7.48 | 0.26 | 5.34 × 10−10 | 0.47 | 0.0056 |
| Ileum | |||||||
| VH (μm) | 405.94 | 305.44 | 383.10 | 25.67 | 0.0007 | 30.00 | 0.0168 |
| CD (μm) | 41.62 | 51.00 | 42.95 | 2.29 | 0.0005 | 2.30 | 0.0020 |
| VH/CD | 10.03 | 6.18 | 9.07 | 0.41 | 9.07 × 10−6 | 0.48 | 1.07 × 10−5 |
The mean and standard error of the mean are used to express data.
P < 0.05 indicates a significant difference between the 2 groups.
Abbreviations: CD, crypt depth; VH, villus height; VH/CD, ratio of villus height to crypt depth.
W, control group; WH, heat stress group; WHA2, heat stress + melittin group.
Effect of Melittin on the Expression Levels of HSP70 and HSP90 in the Viscera of Heat-Stressed Quails
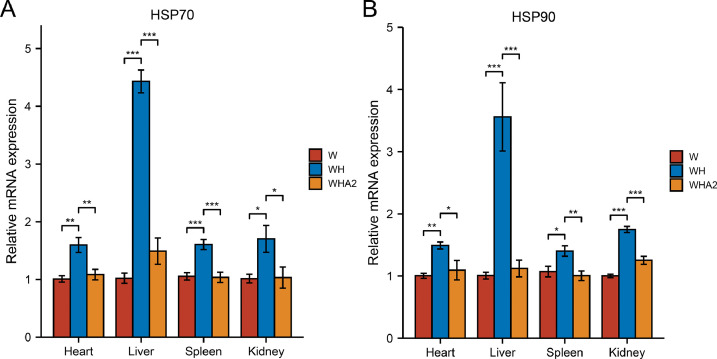
HSP is a specific protein produced when an organism encounters an adverse environmental state that causes a stress response in the body, such as HSP70 and HSP90 (Pirkkala et al., 2001). Physiologically and pathologically, these proteins play a key role in the maintenance of protein homeostasis (Doyle et al., 2019). Currently, they are considered general tissue damaged markers. In quails exposed to heat stress, HSP70 and HSP90 mRNA levels were significantly increased in the heart, liver, spleen, and kidney (P < 0.05) (Figure 2). There was a significant reduction in HSP70 and HSP90 mRNA expression in quail viscera after treatment with melittin (P < 0.05) (Figure 2). It has been shown that melittin has a relieving effect on oxidative stress.
Figure 2.
Effect of melittin on the expression levels of HSP70 and HSP90 in viscera of heat-stressed quails. Data are represented as the mean ± SEM. W, control group; WH, heat stress group; WHA2, heat stress + melittin group. ns, P > 0.05; *, P < 0.05; **, P <0.01; ***, P <0.001.
The upregulation of HSP70 induced by heat stress reduced the level of intracellular ROS by regulating glutathione metabolism to protect cells from oxidative stress (Baek et al., 2000; Kregel, 2002). In addition, HSP90 regulates many signaling pathways related to apoptosis and protein transcription to enhance stress tolerance (Padmini and Usha, 2011). By forming multichaperone complexes, ATP-dependent HSP70 and HSP90 chaperones collaborate to fold client proteins into active conformations, stabilize proteins, and turnover proteins (Morán et al., 2019). HSP90 acts downstream of HSP70, and its recruitment and regulation are related to the function of HSP70 and several other cochaperones. After oxidative damage is induced, HSP90 attempts to buffer the effect, followed by HSP70-dependent protective responses (Morán et al., 2019). We observed that the expression patterns of HSP70 and HSP90 mRNA were similar in the viscera of heat-stressed quails.
Microbiota Diversity
The stability of the intestinal microbiota is extremely important to the health and function of intestinal tract (Zhang et al., 2017; Dai et al., 2021). Dysregulation of intestinal microbiota can reduce overall performance and may result in a variety of infectious diseases (Mishra and Jha, 2019). Furthermore, heat stress alters the structure of intestinal microbial communities, which may be a key factor in host animal health (Zhang et al., 2017; Awad et al., 2020; Chen et al., 2021). The distribution of intestinal microbiota also affects the stress response of hosts (Karl et al., 2018). Therefore, it is a feasible strategy to alleviate thermal stress by regulating intestinal microbiota (Wang et al., 2018a; Humam et al., 2019). Our previous studies have demonstrated that melittin can increase significantly the Bacteroidales abundance at the genus level of intestinal microbiota in quails to improve intestinal health (Li et al., 2023). However, there is a dearth of information pertaining to the effects of melittin on the cecal microbiota of quails exposed to heat stress.
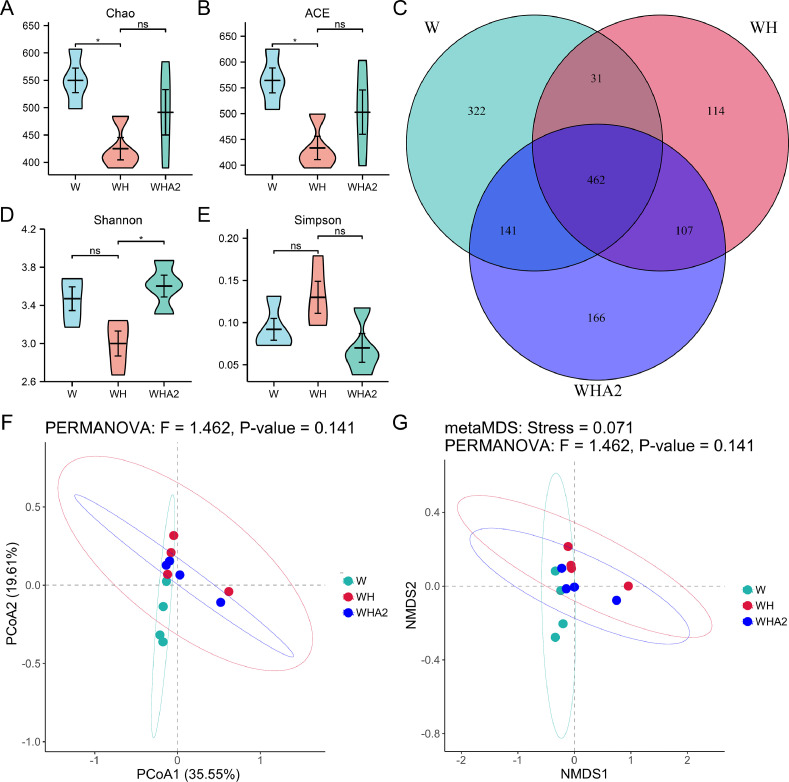
The abundance (Chao and ACE indices) and diversity (Shannon and Simpson indices) of intestinal microbiota were evaluated by α-diversity value. In this study, thermal stress decreased significantly the Chao and ACE indices of cecum in quails (P < 0.05) (Figure 3A and B), which was consistent with the results of Venn analysis. Thermal stress decreased the number of OUTs in the microbial community in cecum of quails (Figure 3C), and heat stress significantly decreased the Chao index in the ileum of broilers (Wang et al., 2018a). However, heat stress had no significant effect on Shannon and Simpson indices of quail cecum (P > 0.05) (Figure 3D and E). The results were consistent in the ileum of thermal-stressed broilers and the small intestine of Shaoxing ducks (Wang et al., 2018a; Tian et al., 2020; Jin et al., 2022), indicating that high temperature had no significant impact on the α-diversity of intestinal microbiota in those birds. Melittin could alleviate the negative effects of heat stress on Chao and ACE indices of quail cecum, which increased by 12.64 and 12.91%, respectively (Figure 3A and B). Venn map showed that the number of OUTs in the group WHA2 increased. At the same time, melittin significantly increased the Shannon index (P < 0.05) (Figure 3D), and the Simpson index decreased by 46.03% (P > 0.05) (Figure 3E), indicating that melittin increased the abundance and diversity of heat-stressed quail cecal microbiota. The results of principal co-ordinates analysis and Bray-Curtis-based NMDS analysis revealed no significant differences among the 3 groups (Figure 3F and G), indicating that heat stress and melittin did not significantly alter the microbial community in the cecal content of quails.
Figure 3.
Effects of melittin on the cecal microbiota diversity in heat-stressed quails. (A) Chao index, (B) ACE index, (C) Venn diagram, (D) Shannon index, (E) Simpson index (the larger the index value, the lower the community diversity), (F) β-diversity analysis of microbial communities using principal co-ordinates analysis (PCoA) based on OTUs, (G) NMDS based on Bray-Curtis distance. Abbreviation: ACE, abundance-based coverage estimator. W, control group; WH, heat stress group; WHA2, heat stress + melittin group. ns, P > 0.05; *, P < 0.05.
Analysis of the Difference in the Abundance of Intestinal Microbiota
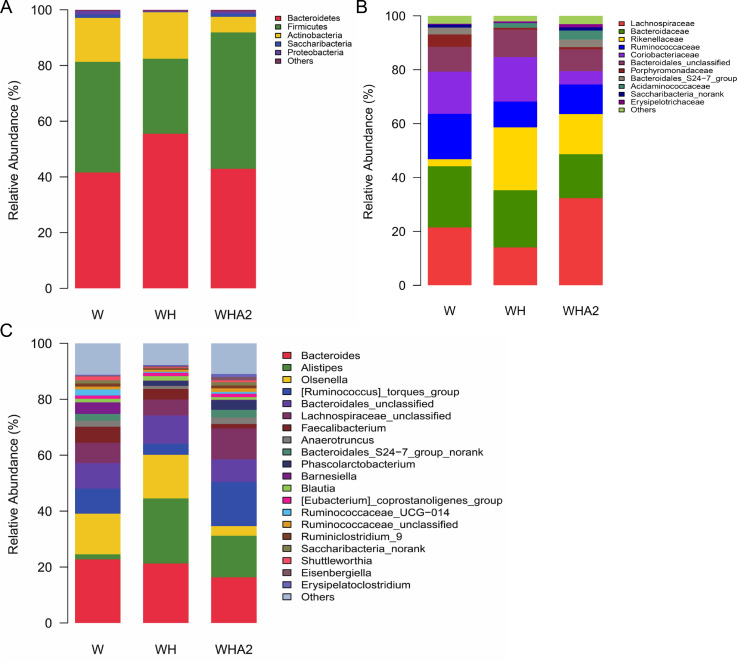
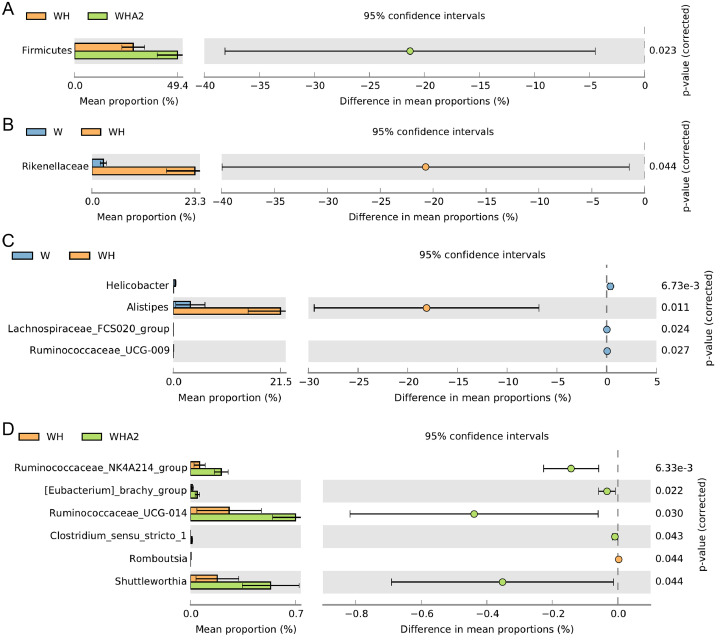
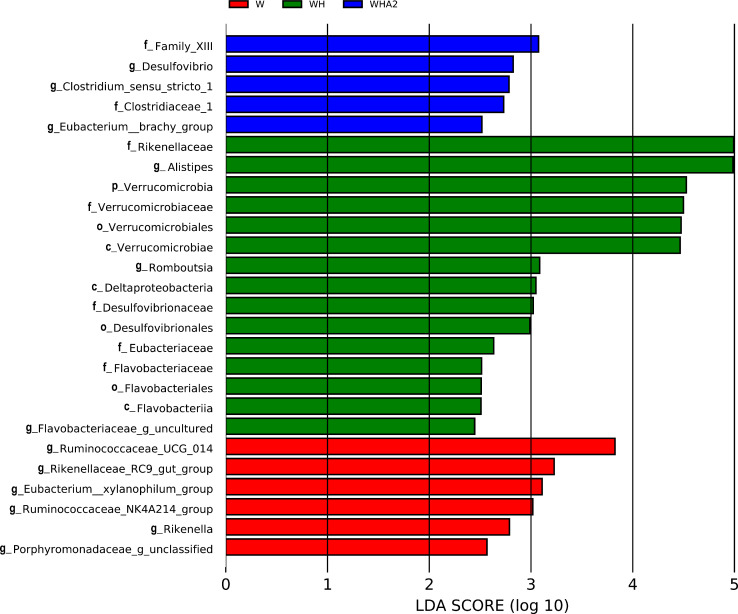
According to the results of 16s rRNA gene sequencing and classification analysis, the main phylum level of cecal microbiota in quails were Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Saccharibacteria (Figure 4A), which were consistent with other studies (Wang et al., 2018a,b, 2020; Awad et al., 2020; Tian et al., 2020). However, heat stress reduced the abundance of Firmicutes, Proteobacteria, and Saccharibacteria (P = 0.3073, 0.0661, 0.3774, respectively) and increased the abundance of Actinobacteria and Bacteroidetes (P = 0.6987, 0.5641, respectively) (Figure 4A, Supplementary Table 3), which was consistent with the results of another study (Zhu et al., 2019). After the addition of melittin, the abundance of Actinobacteria and Bacteroidetes decreased (P = 0.4085, 0.2071, respectively), while the abundance of Firmicutes increased significantly (P = 0.023) (Figures 4A and 5A, Supplementary Table 3). At the family level, heat stress decreased the abundance of Lachnospiraceae (P = 0.1529) and Ruminococcaceae (P = 0.7431) in the cecal microbial community of quails and significantly increased the abundance of Rikenellaceae (P = 0.044), while melittin increased the abundance of Lachnospiraceae and Ruminococcaceae (P = 0.1179, 0.5465, respectively) and decreased the abundance of Rikenellaceae (P = 0.235) (Figures 4B and 5B, Supplementary Table 3). At the genus level, heat stress significantly increased the Alistipes abundance of microbial community in the cecum of quails (P = 0.011) and significantly reduced the abundance of Helicobacter, Lachnospiraceae_FCS020_group, Ruminococcaceae_UCG-009, and Ruminococcaceae_NK4A214_group (P = 0.0038, 0.0242, 0.0268, 0.0327, respectively) (Figures 4C and 5C). Melittin significantly increased the abundance of Ruminococcaceae_NK4A214_group, [Eubacterium]_brachy_group, Ruminococcaceae_UCG-014, Clostridium_sensu_stricto_1, and Shuttleworthia (P = 0.0065, 0.0135, 0.0271, 0.0146, 0.0410, respectively) and reduced the abundance of Romboutsia (P = 0.044) (Figures 4C and 5D, Supplementary Table 4). It is necessary to use the Lefse method to clarify the biomarkers of cecal microbiota between groups. The results showed that Alistipes, Romboutsia, and Flavobacteriaceae_g_uncultured were the markers of cecal microbiota at the genus level in group WH (Figure 6). Desulfovibrio, Clostridium_sensu_stricto_1, and Eubacterium_brachy_group are markers of cecal microbiota at the genus level in group WHA2 (Figure 6).
Figure 4.
Taxonomic composition and distribution map of major phylum (A), family (B), and genus (C) in the cecal contents of quails. W, control group; WH, heat stress group; WHA2, heat stress + melittin group.
Figure 5.
Analysis on the difference of major phylum (A), family (B), and genus (C and D) of in cecal microbiota of quails. W, control group; WH, heat stress group; WHA2, heat stress + melittin group.
Figure 6.
Linear discriminant analysis (LDA) of cecal microbiota in quail. LDA score is represented by the length of the histogram. Abscissa, species LDA score. LDA > 2 represents statistically significant biomarkers. W, control group; WH, heat stress group; WHA2, heat stress + melittin group.
The Firmicutes secrete extracellular polysaccharide-degrading enzymes so that animals can better absorb nutrients in food (Murphy et al., 2010; Lamendella et al., 2011). Heat stress decreased the abundance of Firmicutes in quail intestinal microbiota and decreased the hydrolysis of carbohydrate in quail intestine, that is, the digestibility of carbohydrate and crude protein in diet decreased, the energy intake of quail body was insufficient. Melittin can effectively improve this negative effect by significantly increasing Firmicutes. Actinobacteria includes many pathogens, which destroys intestinal mucosal barrier function (Xue et al., 2018) and have a negative correlation with body weight (Farkas et al., 2022). We found that melittin decreased the Actinobacteria abundance in heat-stressed quails and significantly increased their ADG.
It is important for Rikenellaceae to degrade structural carbohydrates (Tao et al., 2019), and most can ferment unabsorbed polysaccharides in the host gut to produce short-chain fatty acids such as acetate, propionate, and butyrate (Su et al., 2014). At the genus level, Alistipes belonging to Rikenellaceae can produce acetate and propionate (Parker et al., 2020), which can inhibit the release of proinflammatory cytokines by macrophages and are potential anti-inflammatory mediators (Zafar and Saier, 2021). Cyclic heat stress significantly increased Alistipes, possibly to alleviate the effect of heat stress on the intestinal immune function of quails. As producers of short-chain fatty acids, Lachnospiraceae_FCS020_group and Ruminococcaceae_UCG-009 can regulate immune and inflammatory responses (Shang et al., 2016; Chen et al., 2017). However, heat stress significantly reduced their abundance and, therefore, reduced the amount of short-chain fatty acids (Wang et al., 2022). Nevertheless, they accounted for a relatively small proportion of cecal microbiota, and the extent of their impact on the body requires further investigation. Ruminococcaceae_NK4A214_group, [Eubacterium]_brachy_group, Ruminococcaceae_UCG-014, Clostridium_sensu_stricto_1, and Shuttleworthia, which were significantly increased by melittin, belong to Firmicutes, which can produce butyric acid, a short-chain fatty acid that can induce the reconstruction of epithelial tight junction, enhance the barrier integrity of gastrointestinal epithelial cells, and inhibit intestinal mucosal permeability (Reimer et al., 2014). Moreover, Ruminococcaceae is used as the best indicator for low residual feed intake in digestion of caecum and is also abundant in chickens with low F/G (Stanley et al., 2016; Siegerstetter et al., 2017). Ruminococcaceae produce ruminococin C1 (RumC1). RumC1 has a bactericidal effect on multiple drug-resistant bacteria and pathogenic Clostridium by inhibiting nucleic acid synthesis (Chiumento et al., 2019). Melittin also significantly reduced the abundance of the harmful bacteria genus (Romboutsia). The correlation between anxiety-like behavior and Romboutsia indicates that it plays a role in the formation of anxiety-like phenotype (Grant et al., 2021). In the mouse anxiety model induced by chronic unpredictable mild stress, Romboutsia also increased (Sun et al., 2019), further suggesting the relationship between this genus and anxiety. In summary, melittin improved the imbalance of intestinal microbiota induced by heat stress and alleviated the anxiety behavior of quails caused by heat stress.
Functional Prediction of Cecal Microbiota
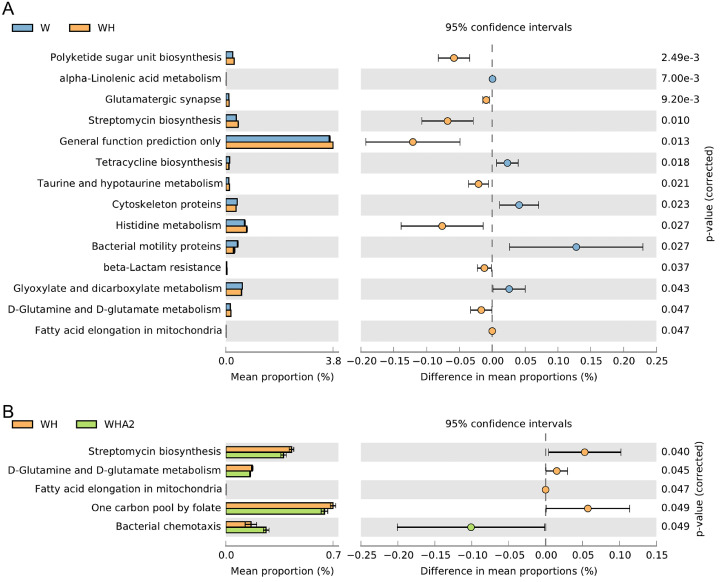
We utilized a predictive exploratory tool to describe the molecular functions of microbial communities using PICRUSt. Based on the KEGG L3 database, we found that heat stress significantly (P < 0.05) increased polyketide sugar unit biosynthesis, glutamatergic synapse, streptomycin biosynthesis, taurine and hypotaurine metabolism, histidine metabolism, beta-lactam resistance, D-glutamine and D-glutamate metabolism, and fatty acid elongation in mitochondria in quails, all of which belonged to the metabolism in KEGG L1. Therefore, we speculate that when quails were exposed to heat stress, to cope with high ambient temperature, blood was transferred to peripheral tissues to better dissipate heat, resulting in a decrease in intestinal blood and oxygen supply, which may lead to intestinal oxidative stress, inflammation and barrier dysfunction, and digestive and absorption dysfunction (Lambert, 2009; Tabler et al., 2020). Therefore, in order to meet the nutritional requirements of daily activities, and the body needs to be compensated by another metabolism. Heat stress significantly downregulated alpha-linolenic acid metabolism, tetracycline biosynthesis, cytoskeleton proteins, bacterial motility proteins, glyoxylate, and dicarboxylate metabolism (P < 0.05) (Figure 7A). Cytoskeleton plays an important role in maintaining the order of cell morphological structure, and internal structure as well as in cell movement, cell differentiation, material transport, energy conversion, and information transmission (Benoit et al., 2021). Heat stress significantly decreased cytoskeleton proteins in quails, which affected the integrity of intestinal mucosal tight junction complex and increased intestinal mucosal permeability. After melittin intervention, the above gene functions gradually returned to the normal level (Figure 7B and Supplementary Table 5), suggesting that the regulation of gene function related to intestinal microbiota metabolism may be one of the ways for melittin to alleviate intestinal injury caused by heat stress.
Figure 7.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) L3 orthologs in quails were used to compare the functional properties of cecal metagenomic sequences. A 2-sided Welch's t test was used to determine the differences between the predicted functions. W, control group; WH, heat stress group; WHA2, heat stress + melittin group.
Correlation Analysis
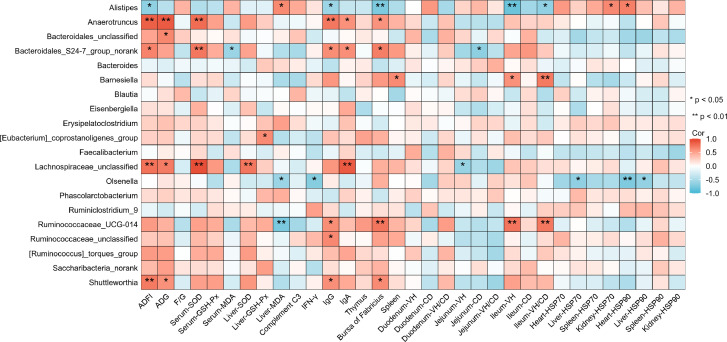
The benefits of Alistipes were mentioned earlier, but the correlation analysis revealed that the abundance values of Alistipes were negatively correlated with ADFI, IgG, bursa of Fabricius index, ileum VH, and VH/CD, which was positively correlated with liver MDA, kidney HSP70, and heart HSP90 (P < 0.05) (Figure 8). Therefore, its increase may damage the immune function, increase oxidative stress, and intestinal digestion and absorption of the body. Other studies have demonstrated that Alistipes has a positive correlation with anxiety and depression because it can increase lipopolysaccharide in the intestinal tract, and it can reduce the amount of phenylalanine and tryptophan by increasing lipopolysaccharide in the intestine and inhibiting bacteria responsible for synthesizing phenylalanine and tryptophan, leading to depression and anxiety (Qin et al., 2007; Kofler et al., 2019). Interestingly, there is also evidence that the presence of Alistipes is associated with the promotion of healthy phenotypes, such as protective effects in colitis, autism spectrum disorders, and various liver fibrosis and cardiovascular fibrosis diseases (Parker et al., 2020). Furthermore, dietary melittin did not significantly reduce the cecal Alistipes abundance in heat-stressed quails (Supplementary Table 4). According to the conclusions of related studies, the genus may play a leading role in the regulation of the disease, or may only play an auxiliary or coinducing role. Further animal studies are required to decipher the mechanisms of complex multimodal diseases and targeted studies on subtype phenotypes.
Figure 8.
Correlation analysis between health parameters and cecal microbial composition in the genus levels in quails. With SPSS Statistics 26.0, Spearman's correlation analysis was conducted, and the results were visualized with R (https://www.xiantao.love/products). The red and blue circles represent positive correlation and negative correlation, respectively. Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; F/G, feed-to-gain ratio; GSH-Px, glutathione peroxidase; HSP, heat shock protein; MDA, malondialdehyde; SOD, superoxide dismutase. Thymus, bursa of Fabricius, and spleen refer to their organ index. * P < 0.05, ** P < 0.01.
Heat stress decreased the abundance of Anaerotruncus, Bacteroidales_S24-7_group_norank, Lachnospiraceae_unclassified, and Shuttleworthia of butyric acid-producing bacteria and were positively correlated with ADFI (P = 0.0005, 0.0174, 0.0045, and 0.0082, respectively), ADG (P = 0.0002, 0.0925, 0.0102, and 0.0415, respectively), serum SOD (P = 0.0026, 0.0068, 1.86 × 105, and 0.0849, respectively), liver SOD (P = 0.0709, 0.4038, 0.0065, and 0.0513, respectively), IgG (P = 0.0074, 0.0342, 0.0513, and 0.03581, respectively), IgA (P = 0.0415, 0.0369, 0.0002, and 0.1309, respectively), bursa of Fabricius index (P = 0.0446, 0.0117, 0.1591, and 0.0283, respectively). The abundance values of Ruminococcaceae_UCG-014 were negatively correlated with liver MDA, which was positively correlated with IgG, bursa of Fabricius index, and ileum VH and VH/CD (P < 0.05) (Figure 8). The addition of melittin increased these butyric acid-producing bacteria. The abundance values of Barnesiella were positively correlated with spleen index, and ileum VH and VH/CD (P < 0.05) (Figure 8). The abundance values of Olsenella were negatively correlated with MDA, HSP70, and HSP90 of liver, IFN-γ, and heart HSP90 (P < 0.05) (Figure 8). Interestingly, heat stress and melittin continuously decreased the abundance of Barnesiella and Olsenella (Supplementary Table 4). The former is a beneficial intestinal bacteria that protects the host from enterococcus pathogens and participates in immune regulation (Presley et al., 2010; Ubeda et al., 2013). Olsenella regulates and enhances the immunotherapy response by producing the metabolite inosine (Mager et al., 2020). Therefore, we speculate that both heat stress and melittin have inhibitory effects on Barnesiella and Olsenella, and these 2 bacteria are highly sensitive to melittin, and their effects on intestinal health need to be further studied. In summary, the gut microbiota of quails was disturbed when they were subjected to heat stress, and melittin could have regulated the structure of intestinal microbiota and increased the abundance of beneficial bacteria, which was helpful in improving intestinal morphology, mucosal barrier function, and growth performance.
CONCLUSIONS
Our experiments showed that dietary melittin could enhance the growth performance, antioxidant function, immune function, and intestinal morphology and reduce the expression of HSP70 and HSP90 in the viscera of quails under heat stress. We observed that melittin could increase the diversity of cecal microbiota and the abundance of beneficial bacteria in heat-stressed quails (e.g., Rikenellaceae). Melittin restored the metabolic decline of heat stress on functional prediction (e.g., cytoskeleton proteins). At the same time, correlation analysis proved that melittin could improve the health index of quails by balancing intestinal microbiota. These results provide a theoretical basis for the application of melittin in the poultry industry.
ACKNOWLEDGMENTS
This work was supported by the Project of Science and Technology of Henan Province (grant no. 222102110192), Innovative Research Team of Poultry Germplasm Resources Application and Healthy Breeding of Dabie Mountain Area in Xinyang Agriculture and Forestry University (grant no. XNKJTD-013), and Young Backbone Teacher Training Program of Xinyang Agriculture and Forestry University (grant no. GGJS-2021003).
DISCLOSURES
The authors declare that there are no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102713.
Appendix. Supplementary materials
REFERENCES
- Abd E.M., Abdelnour S.A., Taha A.E., Khafaga A.F., Arif M., Ayasan T., Swelum A.A., Abukhalil M.H., Alkahtani S., Aleya L., Abdel-Daim M.M. Herbs as thermoregulatory agents in poultry: an overview. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.134399. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.E., Shehata A.M., Khidr R.E., Paswan V.K., Ibrahim N.S., El-Ghoul A.A., Aldhumri S.A., Gabr S.A., Mesalam N.M., Elbaz A.M., Elsayed M.A., Wakwak M.M., Ebeid T.A. Nutritional manipulation to combat heat stress in poultry - a comprehensive review. J. Therm. Biol. 2021;98 doi: 10.1016/j.jtherbio.2021.102915. [DOI] [PubMed] [Google Scholar]
- Ahmedy O.A., Ibrahim S.M., Salem H.H., Kandil E.A. Antiulcerogenic effect of melittin via mitigating TLR4/TRAF6 mediated NF-kappaB and p38MAPK pathways in acetic acid-induced ulcerative colitis in mice. Chem. Biol. Interact. 2020;331 doi: 10.1016/j.cbi.2020.109276. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Abd E.M., Saeed M., Naveed M., Arain M.A., Arif M., Tiwari R., Khandia R., Khurana S.K., Karthik K., Yatoo M.I., Munjal A., Bhatt P., Sharun K., Iqbal H., Sun C., Dhama K. Nutritional applications and beneficial health applications of green tea and l-theanine in some animal species: a review. J. Anim. Physiol. Anim. Nutr. (Berl.) 2020;104:245–256. doi: 10.1111/jpn.13219. [DOI] [PubMed] [Google Scholar]
- Ao X., Kim I.H. Effects of grape seed extract on performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poult. Sci. 2020;99:2078–2086. doi: 10.1016/j.psj.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud C., Joyeux M., Garrel C., Godin-Ribuot D., Demenge P., Ribuot C. Free-radical production triggered by hyperthermia contributes to heat stress-induced cardioprotection in isolated rat hearts. Br. J. Pharmacol. 2002;135:1776–1782. doi: 10.1038/sj.bjp.0704619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Abdel-Raheem S., Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Awad E.A., Najaa M., Zulaikha Z.A., Zulkifli I., Soleimani A.F. Effects of heat stress on growth performance, selected physiological and immunological parameters, caecal microflora, and meat quality in two broiler strains. Asian-Australas. J. Anim. Sci. 2020;33:778–787. doi: 10.5713/ajas.19.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek S.H., Kim J.Y., Choi J.H., Park E.M., Han M.Y., Kim C.H., Ahn Y.S., Park Y.M. Reduced glutathione oxidation ratio and 8 ohdG accumulation by mild ischemic pretreatment. Brain Res. 2000;856:28–36. doi: 10.1016/s0006-8993(99)02376-8. [DOI] [PubMed] [Google Scholar]
- Baumgard L.H., Rhoads R.J. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013;1:311–337. doi: 10.1146/annurev-animal-031412-103644. [DOI] [PubMed] [Google Scholar]
- Benoit B., Baillet A., Poüs C. Cytoskeleton and associated proteins: pleiotropic JNK substrates and regulators. Int. J. Mol. Sci. 2021;22:8375. doi: 10.3390/ijms22168375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugaletta G., Teyssier J.R., Rochell S.J., Dridi S., Sirri F. A review of heat stress in chickens. Part I: insights into physiology and gut health. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.934381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calefi A.S., Quinteiro-Filho W.M., Ferreira A.J.P., Palermo-Neto J. Neuroimmunomodulation and heat stress in poultry. Worlds Poult. Sci. J. 2017;73:493–504. [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Wilson J.E., Koenigsknecht M.J., Chou W.C., Montgomery S.A., Truax A.D., Brickey W.J., Packey C.D., Maharshak N., Matsushima G.K., Plevy S.E., Young V.B., Sartor R.B., Ting J.P. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat. Immunol. 2017;18:541–551. doi: 10.1038/ni.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Zhang H., Zhao N., Yang X., Du E., Huang S., Guo W., Zhang W., Wei J. Effect of chlorogenic acid on intestinal inflammation, antioxidant status, and microbial community of young hens challenged with acute heat stress. Anim. Sci. J. 2021;92:e13619. doi: 10.1111/asj.13619. [DOI] [PubMed] [Google Scholar]
- Chiumento S., Roblin C., Kieffer-Jaquinod S., Tachon S., Leprètre C., Basset C., Aditiyarini D., Olleik H., Nicoletti C., Bornet O., Iranzo O., Maresca M., Hardré R., Fons M., Giardina T., Devillard E., Guerlesquin F., Couté Y., Atta M., Perrier J., Lafond M., Duarte V. Ruminococcin C, a promising antibiotic produced by a human gut symbiont. Sci. Adv. 2019;5:w9969. doi: 10.1126/sciadv.aaw9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Shang L., Wang F., Zeng X., Yu H., Liu L., Zhou J., Qiao S. Effects of antimicrobial peptide Microcin C7 on growth performance, immune and intestinal barrier functions, and cecal microbiota of broilers. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.813629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas G., Maffei V., Zaneveld J., Yurgel S., Brown J., Taylor C., Huttenhower C., Langille M. PICRUSt2: an improved and extensible approach for metagenome inference. BioRxiv. 2019 doi: 10.1101/672295. [DOI] [Google Scholar]
- Doyle S.M., Hoskins J.R., Kravats A.N., Heffner A.L., Garikapati S., Wickner S. Intermolecular Interactions between Hsp90 and Hsp70. J. Mol. Biol. 2019;431:2729–2746. doi: 10.1016/j.jmb.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- El-Aarag B., Magdy M., AlAjmi M.F., Khalifa S.A.M., El-Seedi H.R. Melittin exerts beneficial effects on paraquat-induced lung injuries in mice by modifying oxidative stress and apoptosis. Molecules. 2019;24:1498. doi: 10.3390/molecules24081498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kholy M.S., El-Hindawy M.M., Alagawany M., Abd E.M., El-Sayed S. Dietary supplementation of chromium can alleviate negative impacts of heat stress on performance, carcass yield, and some blood hematology and chemistry indices of growing Japanese quail. Biol. Trace Elem. Res. 2017;179:148–157. doi: 10.1007/s12011-017-0936-z. [DOI] [PubMed] [Google Scholar]
- Elkomy A., El-Hanoun A., Abdella M., El-Sabrout K. Improving the reproductive, immunity and health status of rabbit does using honey bee venom. J. Anim. Physiol. Anim. Nutr. 2021;105:975–983. doi: 10.1111/jpn.13552. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Jung U., Voy B., Dridi S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants (Basel) 2020;10:35. doi: 10.3390/antiox10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas V., Csitári G., Menyhárt L., Such N., Pál L., Husvéth F., Rawash M.A., Mezőlaki Á., Dublecz K. Microbiota composition of mucosa and interactions between the microbes of the different gut segments could be a factor to modulate the growth rate of broiler chickens. Animals (Basel) 2022;12:1296. doi: 10.3390/ani12101296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A. Heat stress management in poultry. J. Anim. Physiol. Anim. Nutr. (Berl.) 2021;105:1136–1145. doi: 10.1111/jpn.13496. [DOI] [PubMed] [Google Scholar]
- Grant C.V., Loman B.R., Bailey M.T., Pyter L.M. Manipulations of the gut microbiome alter chemotherapy-induced inflammation and behavioral side effects in female mice. Brain Behav. Immun. 2021;95:401–412. doi: 10.1016/j.bbi.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai L., Rong D., Zhang Z.Y. The effect of thermal environment on the digestion of broilers. J. Anim. Physiol. Anim. Nutr. 2001;83:57–64. [Google Scholar]
- He S., Yu Q., He Y., Hu R., Xia S., He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Vashan S.J., Golian A., Yaghobfar A. Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Int. J. Biometeorol. 2016;60:1183–1192. doi: 10.1007/s00484-015-1112-9. [DOI] [PubMed] [Google Scholar]
- Hu F., Gao X., She R., Chen J., Mao J., Xiao P., Shi R. Effects of antimicrobial peptides on growth performance and small intestinal function in broilers under chronic heat stress. Poult. Sci. 2017;96:798–806. doi: 10.3382/ps/pew379. [DOI] [PubMed] [Google Scholar]
- Humam A.M., Loh T.C., Foo H.L., Samsudin A.A., Mustapha N.M., Zulkifli I., Izuddin W.I. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals (Basel) 2019;9:644. doi: 10.3390/ani9090644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- Jin Y.Y., Guo Y., Zheng C.T., Liu W.C. Effect of heat stress on ileal microbial community of indigenous yellow-feather broilers based on 16S rRNA gene sequencing. Vet. Med. Sci. 2022;8:642–653. doi: 10.1002/vms3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl J.P., Hatch A.M., Arcidiacono S.M., Pearce S.C., Pantoja-Feliciano I.G., Doherty L.A., Soares J.W. Effects of psychological, environmental and physical stressors on the gut microbiota. Front. Microbiol. 2018;9:2013. doi: 10.3389/fmicb.2018.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A., Elesawy B.H., Ali T.M., Ahmed O.M. Bee venom: from venom to drug. Molecules. 2021;26:4941. doi: 10.3390/molecules26164941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Han S.M., Choi Y.S., Kang H.K., Lee K.W. Effects of dietary bee venom on serum characteristic, antioxidant activity and liver fatty acid composition in broiler chickens. Korean J. Poult. Sci. 2019;46:39–46. [Google Scholar]
- Kim J., Leem J., Hong H. Melittin ameliorates endotoxin-induced acute kidney injury by inhibiting inflammation, oxidative stress, and cell death in mice. Oxid. Med. Cell Longev. 2021;2021 doi: 10.1155/2021/8843051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler M., Schiefecker A.J., Gaasch M., Sperner-Unterweger B., Fuchs D., Beer R., Ferger B., Rass V., Hackl W., Rhomberg P., Pfausler B., Thomé C., Schmutzhard E., Helbok R. A reduced concentration of brain interstitial amino acids is associated with depression in subarachnoid hemorrhage patients. Sci. Rep. 2019;9:2811. doi: 10.1038/s41598-019-39569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel K.C. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. (1985) 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Kumar M., Ratwan P., Dahiya S.P., Nehra A.K. Climate change and heat stress: impact on production, reproduction and growth performance of poultry and its mitigation using genetic strategies. J. Therm. Biol. 2021;97 doi: 10.1016/j.jtherbio.2021.102867. [DOI] [PubMed] [Google Scholar]
- Lambert G.P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 2009;87(Suppl. 14):E101–E108. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- Lamendella R., Domingo J.W., Ghosh S., Martinson J., Oerther D.B. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 2011;11:103. doi: 10.1186/1471-2180-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals (Basel) 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu R., Wang X., Wu H., Yi X., Huang L., Qin Q. Effects of melittin on laying performance and intestinal barrier function of quails. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.L., He J.H., Xie H.B., Yang Y.S., Li J.C., Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Liu W.C., Ou B.H., Liang Z.L., Zhang R., Zhao Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Yin J., Du M., Yan P., Xu J., Zhu X., Yu J. Heat-stress-induced damage to porcine small intestinal epithelium associated with downregulation of epithelial growth factor signaling. J. Anim. Sci. 2009;87:1941–1949. doi: 10.2527/jas.2008-1624. [DOI] [PubMed] [Google Scholar]
- Lundqvist M.L., Middleton D.L., Radford C., Warr G.W., Magor K.E. Immunoglobulins of the non-galliform birds: antibody expression and repertoire in the duck. Dev. Comp. Immunol. 2006;30:93–100. doi: 10.1016/j.dci.2005.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager L.F., Burkhard R., Pett N., Cooke N., Brown K., Ramay H., Paik S., Stagg J., Groves R.A., Gallo M., Lewis I.A., Geuking M.B., McCoy K.D. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369:1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- Mehaisen G., Ibrahim R.M., Desoky A.A., Safaa H.M., El-Sayed O.A., Abass A.O. The importance of propolis in alleviating the negative physiological effects of heat stress in quail chicks. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán L.T., Mayer M.P., Rüdiger S. The Hsp70-Hsp90 chaperone cascade in protein folding. Trends Cell Biol. 2019;29:164–177. doi: 10.1016/j.tcb.2018.10.004. [DOI] [PubMed] [Google Scholar]
- Murphy E.F., Cotter P.D., Healy S., Marques T.M., O'Sullivan O., Fouhy F., Clarke S.F., O'Toole P.W., Quigley E.M., Stanton C., Ross P.R., O'Doherty R.M., Shanahan F. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635–1642. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- Naziroglu M., Sahin K., Simsek H., Aydilek N., Ertas O.N. The effects of food withdrawal and darkening on lipid peroxidation of laying hens in high ambient temperatures. Dtsch. tierarztl. wochenschr. 2000;107:199–202. [PubMed] [Google Scholar]
- Nguyen C.D., Lee G. Neuroprotective activity of melittin – the main component of bee venom-against oxidative stress induced by Aβ(25-35) in in vitro and in vivo models. Antioxidants (Basel) 2021;10:1654. doi: 10.3390/antiox10111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgun O., Abdulqader A.F., Karabacak A. The importance of nutrition in preventing heat stress at poultry. World's Poult. Sci. J. 2021;77:661–678. [Google Scholar]
- Padmini E., Usha R.M. Heat-shock protein 90 alpha (HSP90α) modulates signaling pathways towards tolerance of oxidative stress and enhanced survival of hepatocytes of Mugil cephalus. Cell Stress Chaperones. 2011;16:411–425. doi: 10.1007/s12192-011-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B.J., Wearsch P.A., Veloo A.C.M., Rodriguez-Palacios A. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020;11:906. doi: 10.3389/fimmu.2020.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkkala L., Nykänen P., Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- Presley L.L., Wei B., Braun J., Borneman J. Bacteria associated with immunoregulatory cells in mice. Appl. Environ. Microbiol. 2010;76:936–941. doi: 10.1128/AEM.01561-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Wu X., Block M.L., Liu Y., Breese G.R., Hong J.S., Knapp D.J., Crews F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer R.A., Maathuis A.J., Venema K., Lyon M.R., Gahler R.J., Wood S. Effect of the novel polysaccharide PolyGlycopleX® on short-chain fatty acid production in a computer-controlled in vitro model of the human large intestine. Nutrients. 2014;6:1115–1127. doi: 10.3390/nu6031115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis E.S., Mastellos D.C., Hajishengallis G., Lambris J.D. New insights into the immune functions of complement. Nat. Rev. Immunol. 2019;19:503–516. doi: 10.1038/s41577-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Abbas G., Alagawany M., Kamboh A.A., Abd E.M., Khafaga A.F., Chao S. Heat stress management in poultry farms: a comprehensive overview. J. Therm. Biol. 2019;84:414–425. doi: 10.1016/j.jtherbio.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Sahin K., Kucuk O. Effects of vitamin C and vitamin E on performance, digestion of nutrients and carcass characteristics of Japanese quails reared under chronic heat stress (34 degrees C) J. Anim. Physiol. Anim. Nutr. (Berl.) 2001;85:335–341. doi: 10.1046/j.1439-0396.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- Sahin K., Sahin N., Onderci M., Gursu F., Cikim G. Optimal dietary concentration of chromium for alleviating the effect of heat stress on growth, carcass qualities, and some serum metabolites of broiler chickens. Biol. Trace Elem. Res. 2002;89:53–64. doi: 10.1385/BTER:89:1:53. [DOI] [PubMed] [Google Scholar]
- Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoto K., Yamauchi K. Recovery responses of chick intestinal villus morphology to different refeeding procedures. Poult. Sci. 2000;79:718–723. doi: 10.1093/ps/79.5.718. [DOI] [PubMed] [Google Scholar]
- Shang Q., Shan X., Cai C., Hao J., Li G., Yu G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016;7:3224–3232. doi: 10.1039/c6fo00309e. [DOI] [PubMed] [Google Scholar]
- Siegel H.S. Physiological stress in birds. Bioscience. 1980;30:529–534. [Google Scholar]
- Siegerstetter S.C., Schmitz-Esser S., Magowan E., Wetzels S.U., Zebeli Q., Lawlor P.G., O'Connell N.E., Metzler-Zebeli B.U. Intestinal microbiota profiles associated with low and high residual feed intake in chickens across two geographical locations. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamilla A., Messina A., Sallemi S., Condorelli L., Antoci F., Puleio R., Loria G.R., Cascone G., Lanza M. Effects of microencapsulated blends of organics acids (OA) and essential oils (EO) as a feed additive for broiler chicken. A focus on growth performance, gut morphology and microbiology. Animals (Basel) 2020;10:442. doi: 10.3390/ani10030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Geier M.S., Moore R.J. Bacteria within the gastrointestinal tract microbiota correlated with improved growth and feed conversion: challenges presented for the identification of performance enhancing probiotic bacteria. Front. Microbiol. 2016;7:187. doi: 10.3389/fmicb.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X.L., Tian Q., Zhang J., Yuan X.Z., Shi X.S., Guo R.B., Qiu Y.L. Acetobacteroides hydrogenigenes gen. nov., sp. nov., an anaerobic hydrogen-producing bacterium in the family Rikenellaceae isolated from a reed swamp. Int. J. Syst. Evol. Microbiol. 2014;64(Pt. 9):2986–2991. doi: 10.1099/ijs.0.063917-0. [DOI] [PubMed] [Google Scholar]
- Sun L., Zhang H., Cao Y., Wang C., Zhao C., Wang H., Cui G., Wang M., Pan Y., Shi Y., Nie Y. Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice. Int. J. Med. Sci. 2019;16:1260–1270. doi: 10.7150/ijms.37322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F., Fisinin V.I. Vitagenes in poultry production: Part 1. Technological and environmental stresses. World's Poult. Sci. J. 2016;72:721–734. [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants (Basel) 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler T.W., Greene E.S., Orlowski S.K., Hiltz J.Z., Anthony N.B., Dridi S. Intestinal barrier integrity in heat-stressed modern broilers and their ancestor wild jungle fowl. Front. Vet. Sci. 2020;7:249. doi: 10.3389/fvets.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Chen Z. The protective effect of γ-aminobutyric acid on the development of immune function in chickens under heat stress. J. Anim. Physiol. Anim. Nutr. (Berl.) 2016;100:768–777. doi: 10.1111/jpn.12385. [DOI] [PubMed] [Google Scholar]
- Tao S., Bai Y., Zhou X., Zhao J., Yang H., Zhang S., Wang J. In vitro fermentation characteristics for different ratios of soluble to insoluble dietary fiber by fresh fecal microbiota from growing pigs. ACS Omega. 2019;4:15158–15167. doi: 10.1021/acsomega.9b01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgersen E.B., Barratt-Due A., Haugaa H., Harboe M., Pischke S.E., Nilsson P.H., Mollnes T.E. The role of complement in liver injury, regeneration, and transplantation. Hepatology. 2019;70:725–736. doi: 10.1002/hep.30508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Li G., Chen L., Bu X., Shen J., Tao Z., Zeng T., Du X., Lu L. High-temperature exposure alters the community structure and functional features of the intestinal microbiota in Shaoxing ducks (Anas platyrhynchos) Poult. Sci. 2020;99:2662–2674. doi: 10.1016/j.psj.2019.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda C., Bucci V., Caballero S., Djukovic A., Toussaint N.C., Equinda M., Lipuma L., Ling L., Gobourne A., No D., Taur Y., Jenq R.R., van den Brink M.R., Xavier J.B., Pamer E.G. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013;81:965–973. doi: 10.1128/IAI.01197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyanga V.A., Oke E.O., Amevor F.K., Zhao J., Wang X., Jiao H., Onagbesan O.M., Lin H. Functional roles of taurine, L-theanine, L-citrulline, and betaine during heat stress in poultry. J. Anim. Sci. Biotechnol. 2022;13:23. doi: 10.1186/s40104-022-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyanga V.A., Wang M., Tong T., Zhao J., Wang X., Jiao H., Onagbesan O.M., Lin H. L-Citrulline influences the body temperature, heat shock response and nitric oxide regeneration of broilers under thermoneutral and heat stress condition. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.671691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.J., Feng J.H., Zhang M.H., Li X.M., Ma D.D., Chang S.S. Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poult. Sci. 2018;97:2153–2158. doi: 10.3382/ps/pey032. [DOI] [PubMed] [Google Scholar]
- Wang M., Lin X., Jiao H., Uyanga V., Zhao J., Wang X., Li H., Zhou Y., Sun S., Lin H. Mild heat stress changes the microbiota diversity in the respiratory tract and the cecum of layer-type pullets. Poult. Sci. 2020;99:7015–7026. doi: 10.1016/j.psj.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Shao D., Wu S., Song Z., Shi S. Heat stress-induced intestinal barrier damage and dimethylglycine alleviates via improving the metabolism function of microbiota gut brain axis. Ecotoxicol. Environ. Saf. 2022;244 doi: 10.1016/j.ecoenv.2022.114053. [DOI] [PubMed] [Google Scholar]
- Wang W.C., Yan F.F., Hu J.Y., Amen O.A., Cheng H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018;96:1654–1666. doi: 10.1093/jas/sky092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhao F., Li Z., Jin X., Chen X., Geng Z., Hu H., Zhang C. Effects of resveratrol on growth performance, intestinal development, and antioxidant status of broilers under heat stress. Animals (Basel) 2021;11:1427. doi: 10.3390/ani11051427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehbe R., Frangieh J., Rima M., El O.D., Sabatier J.M., Fajloun Z. Bee venom: overview of main compounds and bioactivities for therapeutic interests. Molecules. 2019;24:2997. doi: 10.3390/molecules24162997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Ji X., Liang H., Liu Y., Wang B., Sun L., Li W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018;9:1214–1223. doi: 10.1039/c7fo01677h. [DOI] [PubMed] [Google Scholar]
- Yang C., Luo P., Chen S.J., Deng Z.C., Fu X.L., Xu D.N., Tian Y.B., Huang Y.M., Liu W.J. Resveratrol sustains intestinal barrier integrity, improves antioxidant capacity, and alleviates inflammation in the jejunum of ducks exposed to acute heat stress. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Yin P., Liu F., Cheng G., Guo K., Lu A., Zhu X., Luan W., Xu J. Effect of heat stress on the porcine small intestine: a morphological and gene expression study. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010;156:119–128. doi: 10.1016/j.cbpa.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Zafar H., Saier M.H. Gut Bacteroides species in health and disease. Gut Microbes. 2021;13:1–20. doi: 10.1080/19490976.2020.1848158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaglool A.W., Roushdy E.M., El-Tarabany M.S. Impact of strain and duration of thermal stress on carcass yield, metabolic hormones, immunological indices and the expression of HSP90 and Myogenin genes in broilers. Res. Vet. Sci. 2019;122:193–199. doi: 10.1016/j.rvsc.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Zhang C., Chen K., Zhao X., Geng Z. Protective effects of resveratrol against high ambient temperature-induced spleen dysplasia in broilers through modulating splenic redox status and apoptosis. J. Sci. Food Agric. 2018;98:5409–5417. doi: 10.1002/jsfa.9084. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhao Q., Ci X., Chen S., Xie Z., Li H., Zhang H., Chen F., Xie Q. Evaluation of the efficacy of chlorogenic acid in reducing small intestine injury, oxidative stress, and inflammation in chickens challenged with Clostridium perfringens type A. Poult. Sci. 2020;99:6606–6618. doi: 10.1016/j.psj.2020.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhao X.H., Yang L., Chen X.Y., Jiang R.S., Jin S.H., Geng Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]
- Zhang M., Zou X.T., Li H., Dong X.Y., Zhao W. Effect of dietary γ-aminobutyric acid on laying performance, egg quality, immune activity and endocrine hormone in heat-stressed Roman hens. Anim. Sci. J. 2012;83:141–147. doi: 10.1111/j.1740-0929.2011.00939.x. [DOI] [PubMed] [Google Scholar]
- Zhu W., Jiang W., Wu L.Y. Dietary L-arginine supplement alleviates hepatic heat stress and improves feed conversion ratio of Pekin ducks exposed to high environmental temperature. J. Anim. Physiol. Anim. Nutr. (Berl.) 2014;98:1124–1131. doi: 10.1111/jpn.12195. [DOI] [PubMed] [Google Scholar]
- Zhu L., Liao R., Wu N., Zhu G., Yang C. Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl. Microbiol. Biotechnol. 2019;103:461–472. doi: 10.1007/s00253-018-9465-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.