Abstract
1,3-Diamine-derived catalysts were designed, synthesized, and used in asymmetric Mannich reactions of ketones. The reactions catalyzed by one of the 1,3-diamine derivatives in the presence of acids afforded the Mannich products with high enantioselectivities under mild conditions. In most cases, bond formation occurred at the less-substituted α-position of the ketone carbonyl group. Our results indicate that the primary and the tertiary amines of the 1,3-diamine derivative cooperatively act for the catalysis.
Introduction
Amine derivatives have been used as catalysts and components of catalyst systems to accelerate chemical transformations.1,2 For example, compounds that have a primary amine group and a tertiary amine group in a geminal relationship (or 1,2-relationship), such as cinchona-derived amines, cyclohexane-1,2-diamines, and 1,2-diarylethane-1,2-diamines, have been used as catalysts with or without other molecules (such as acids) to accelerate bond-forming reactions of enolizable ketones and aldehydes as nucleophiles.1,3−5 During the catalysis by these amines, the primary amine often forms an enamine intermediate, and the tertiary amine group is protonated and functions as an acid to interact with electrophiles.3−5 When the tertiary amine group interacts with acid molecules, it may also act as a group providing steric bulk and/or shielding function.4 In addition, the primary and the tertiary amine groups appear to act cooperatively.3−5 While our understanding of the catalysis by amine derivatives has significantly advanced in recent years,1,2 the ability to design catalysts suitable for a given reaction remains challenging. To advance the development, testing of new catalyst designs and elucidating the relationships between catalyst structures and the catalytic features and mechanisms are necessary. Here, we report the design, synthesis, and use of catalyst 1a and related molecules in which the primary amine group and the tertiary amine group have a 1,3-relationship (Scheme 1). While 1,2-diamine derivatives have been extensively explored as catalysts, 1,3-diamine derivatives have not been popularly examined as catalysts. We hypothesized that the molecules bearing a primary amine group and a tertiary amine group in a 1,3-relationship in certain designs would work as catalysts. To demonstrate our hypothesis, we evaluated the use of the 1,3-diamine derivatives in Mannich reactions of ketones (Scheme 1).
Scheme 1. 1,3-Diamine-Derived Catalyst Developed in This Work and the Asymmetric Mannich Reactions of Ketones.
Results and Discussion
Design and Synthesis of the 1,3-Diamine-Derived Catalysts
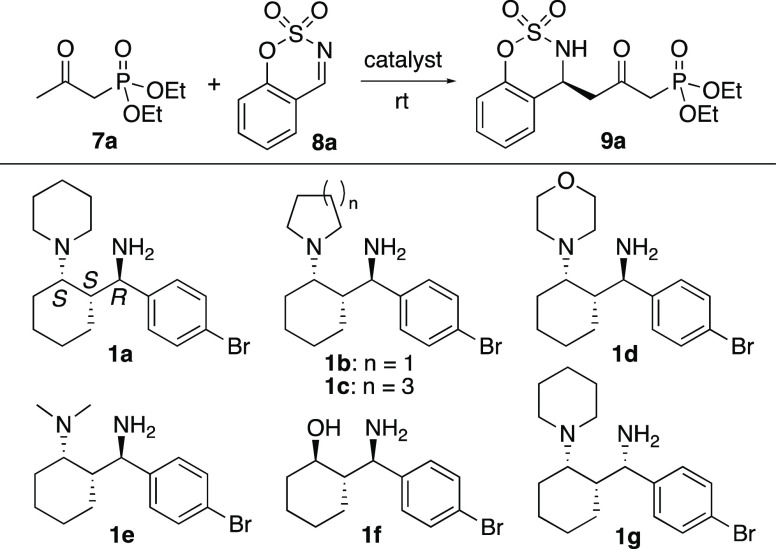
In the design of 1a, we hypothesized that the use of a primary amine group in the amino(aryl)methyl group moiety would lead to the formation of enamine intermediates during the catalysis. The tertiary amine group and the amino(aryl)methyl group moiety were attached in a trans relationship on a cyclohexane ring to position the amine groups in a 1,3-relationship. In the resulting structure, the primary amine group and the tertiary amine group would form a hydrogen bond depending on the conformation. We reasoned that the intramolecular hydrogen bonding would tune the functions of the primary and the tertiary amino groups during the catalysis. The design with the 1,3-relationship of the amine groups would provide a space that accepts various ketones to form enamines during the reaction.
Catalyst 1a and related derivatives 1b–f were synthesized from compound 2, which was generated by the (S)-β-proline-catalyzed anti-selective Mannich reaction of cyclohexanone with an aryl imine derivative in the presence of K2CO36 (Scheme 2 and Table 1). For the synthesis of 1a, the route via 3–6 shown in Scheme 2 was employed. Depending on workup conditions after the deprotection of the Boc group, 1a was obtained as either the CF3COOH salt form or the free form (Supporting Information).
Scheme 2. Synthesis of 1a.
Table 1. Evaluations of Catalysts 1 in the Mannich Reaction of 7a with 8a To Afford 9aa.
| entry | catalyst system (equiv) | time (h) | yield (%)b | erc |
|---|---|---|---|---|
| 1 | 1a (0.3) | 96 | <5 | 82:18 |
| 2 | 1a·CF3COOH (0.3) | 72 | 99 | 87:13 |
| 3 | 1a·CF3COOH (0.3), CF3COOH (0.3) | 48 | 85 | 94:6 |
| 4 | 1a·CF3COOH (0.3), (PhCH2O)2POOH (0.3) | 48 | 99 | 96:4 |
| 5 | 1a (0.3), (PhCH2O)2POOH (0.3) | 72 | 99 | 92:8 |
| 6 | 1a (0.3), CF3COOH (0.3), (PhCH2O)2POOH (0.3) | 48 | 99 | 96:4 |
| 7 | 1b·CF3COOH (0.3), (PhCH2O)2POOH (0.3) | 72 | 77 | 87:13 |
| 8 | 1c·CF3COOH (0.3), (PhCH2O)2POOH (0.3) | 48 | >99 | 63:37 |
| 9 | 1d·CF3COOH (0.3), (PhCH2O)2POOH (0.3) | 120 | 48 | 93:7 |
| 10 | 1e·CF3COOH (0.3), (PhCH2O)2POOH (0.3) | 48 | 89 | 94:6 |
| 11 | 1f (0.3) | 168 | 11 | – |
| 12 | 1f (0.3), (PhCH2O)2POOH (0.3) | 120 | 0 | – |
| 13 | 1g/CF3COOH (1:0.8) (0.3), (PhCH2O)2POOH (0.3) | 96 | 80 | 16:84 |
Conditions: 7a (0.75 mmol, 3.0 equiv), 8a (0.25 mmol, 1.0 equiv), and catalyst system (as indicated, equiv relative to 8a) in toluene (1.65 mL) at rt (25 °C).
Determined by 1H NMR analysis before purification.
Determined by HPLC analysis after purification.
Evaluations of the 1,3-Diamine-Derived Catalysts in Enantioselective γ-Position-Selective Mannich Reactions of β-Ketophosphonates
First, we evaluated the use of 1 in the presence or absence of acids in catalysis of the Mannich reaction of β-ketophosphonate 7a with imine 8a to afford γ-position-selective reaction product 9a (Table 1). β-Ketophosphonates, including those bearing chiral centers, have been used in the Horner–Wadsworth–Emmons version of Wittig reactions to form enone derivatives and in other reactions.7−9 Previously, only three substrate examples of the enantioselective γ-position-selective Mannich reaction of a β-ketophosphonate were reported, in which a cinchona-derived amine with an acid was used as a catalyst.9 While the catalyst system was useful for the corresponding reactions of β-ketoesters,9 it was less efficient for the reactions of β-ketophosphonates,9 and further development was required. Among the catalysts 1 and conditions tested, the reactions in the presence of 1a·CF3COOH with dibenzyl phosphate or of 1a with CF3COOH and dibenzyl phosphate at room temperature (25 °C) afforded 9a in high yields with high enantioselectivities (Table 1, entries 4 and 6). 1,3-Amino alcohol 1f was not an efficient catalyst (Table 1, entries 11 and 12). Thus, the 1,3-diamine moiety of 1 was necessary for the catalysis. While the reaction of 7a and 8a affording 9a catalyzed by a cinchona-derived amine with an acid was previously reported,9 the reaction catalyzed by 1,3-diamine derivative 1a with the acids under the optimized conditions was faster to afford 9a and provided 9a with higher enantioselectivity than the previously reported reaction catalyzed by the cinchona-derived amine with the acid.
Scope and Mechanisms of Enantioselective γ-Position-Selective Mannich Reactions of β-Ketophosphonates Catalyzed by the Catalyst System Containing 1,3-Diamine Derivative 1a
Next, using 1a·CF3COOH with dibenzyl phosphate as the catalyst system, the scope of the γ-position-selective Mannich reactions of β-ketophosphonates was evaluated (Table 2). Various δ-amino β-ketophosphonates 9 were obtained mostly with high enantioselectivities. No formation of the α-position reaction products was detected. Although the rate of the reaction was affected by the substituents on the imine, products from the reactions with hindered imines (such as imines bearing o-substituents relative to the imine group) and with imines bearing electron-donating substituents were also obtained (formation of 9f–h). A tetrasubstituted carbon center was also constructed when a ketimine derivative was used in the catalyzed reaction (formation of 9j).
Table 2. Mannich Reactions of β-Ketophosphates 7 with Imines 8 To Afford 9 Catalyzed by the Catalyst System Containing 1aa.

Conditions: 7 (0.75 mmol), 8 (0.25 mmol), 1a·CF3COOH (0.075 mmol), and dibenzyl phosphate (0.075 mmol) in toluene (1.65 mL) at rt (25 °C). Isolated yield and enantiomer ratio (er) after purification are shown. 1H NMR yield before purification is shown in parenthesis.
A 1.0 mmol-scale reaction.
Determined after derivatization (see the Supporting Information).
To understand the mechanisms of the catalyzed reactions, the reactions were analyzed at various time points and the stabilities of product 9a and its regioisomer 10a under the catalytic conditions were evaluated. When the reaction of 7a and 8a to form 9a in the presence of the catalyst system 1a·CF3COOH and dibenzyl phosphate was analyzed at various time points, no formation of α-position reaction product 10a was detected at any time point analyzed, and the enantiopurity of 9a was essentially unchanged (er 95:5–96:4) from 10 h (47% conversion) to 54 h (>99% conversion) (Scheme 3a). It should be noted that the reaction of 7a and 8a in the presence of pyrrolidine–CH3COOH (1:1) or in the presence of benzylamine–CH3COOH (1:1) afforded both 10a and 9a during the consumption of imine 8a (Scheme 3a).
Scheme 3. (a) Time Course Analysis of the Catalyzed Reactions, (b) Evaluation of the Stability of 10a under the Catalytic Conditions, and (c) Evaluation of the Stability of 9a under the Catalytic Conditions.

When 10a was treated under the catalytic conditions with 1a·CF3COOH and dibenzyl phosphate that were used for the formation of 9, no decomposition of 10a and no formation of 9a, were detected (Scheme 3b). Treatment of (±)-9a or (S)-9a with 1a·CF3COOH and dibenzyl phosphate also did not alter 9a, and the enantiopurity of 9a was unchanged in either case (Scheme 3c). These results indicated that product 9a was formed kinetically under the conditions in the presence of 1a·CF3COOH and dibenzyl phosphate. These results also indicated that the use of the catalyst system with 1a resulted in the bond formation at only the γ-position of the β-ketophosphonate. Because the bond formation at the α-position of the β-ketophosphonate occurred in the presence of pyrrolidine-acid or benzylamine-acid, the functions of 1a were distinct from those of pyrrolidine and benzylamine.
Extended Scope and Mechanisms of the Mannich Reactions Catalyzed by the Catalyst System Containing 1,3-Diamine Derivative 1a
To further understand the features of the catalysis by 1a, catalyst 1a was used in reactions of various ketones with imine 8a under the conditions used for the formation of 9 (Table 3). Mannich products 11 were obtained with high enantioselectivities, although the reaction rate varied depending on the ketone. Except for the reaction to form 11c, in which the regioisomer was also formed, the bond formation enantioselectively occurred at the less-substituted α-position of the ketone carbonyl group (or at the methyl group) in the reactions to form 9 or 11 in the presence of 1a and the acids. These results indicate that catalyst 1a provides a space to accept ketones bearing bulky moieties (such as diethyl phosphonate and diethylamide) and functions to selectively form a bond at the less-substituted methyl group of the ketone when more than one enolizable position is present.
Table 3. Mannich Reactions of Ketones in the Presence of the Catalyst System Containing 1aa.
See the Supporting Information. In parenthesis, 1H NMR yield before purification is shown.
Determined after derivatization.
For compound 1a to function as the catalyst, acids were required. In 1H NMR analyses, chemical shifts of 1a were shifted downfield upon the addition of the acids (Supporting Information). The changes of the chemical shifts were observed not only for the protons on the carbons that are attached to the nitrogen of the tertiary amine group but also for other protons including protons on the carbon bearing the primary amine group. In contrast, the addition of acetic acid to benzylamine did not alter the chemical shifts. Thus, the changes in the chemical shifts of 1a upon the addition of the acid indicate that 1a directly interacts with the acids and that the tertiary amine and the primary amine groups of 1a cooperatively function.
Utility of 1a and the 1a-Catalyzed Reactions
To demonstrate the utility of 1a and the catalyzed reactions, Mannich reaction product 9a was transformed to 12 and 13 (Scheme 4). Compounds 12 and 13 were obtained in highly enantiomerically enriched forms.
Scheme 4. Transformations of 9a.
Conclusions
We designed and synthesized 1,3-diamine-derived catalyst 1a and related diamine derivatives. The use of 1a with acids as a catalyst system resulted in highly regio- and enantioselective Mannich reactions of various ketones, including enantioselective γ-position-selective Mannich reactions of β-ketophosphonates, under mild conditions. Analyses indicated that the primary and the tertiary amine groups of 1a, which are in a 1,3-relationship, function cooperatively during the catalysis. The results and the information described here will be useful for the development of new catalysts and for the understanding of the relationships between catalyst structures and the catalytic features and mechanisms.
Acknowledgments
We thank Dr. Michael Chandro Roy, Research Support Division, Okinawa Institute of Science and Technology Graduate University for mass analyses. This study was supported by the Okinawa Institute of Science and Technology Graduate University.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c01051.
Experimental procedures; synthesis of catalysts and related molecules; additional evaluations of catalysts; NMR analyses of 1a with acids; plausible transition state; NMR spectra; and HPLC chromatograms (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Doyle A. G.; Jacobsen E. N. Small-Molecule H-Bond Donors in Asymmetric Synthesis. Chem. Rev. 2007, 107, 5713–5743. 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; b Mukherjee S.; Yang J. W.; Hoffmann S.; List B. Asymmetric Enamine Catalysis. Chem. Rev. 2007, 107, 5471–5569. 10.1021/cr0684016. [DOI] [PubMed] [Google Scholar]; c Melchiorre P. Cinchona-based Primary Amine Catalysis in the Asymmetric Functionalization of Carbonyl Compounds. Angew. Chem., Int. Ed. 2012, 51, 9748–9770. 10.1002/anie.201109036. [DOI] [PubMed] [Google Scholar]; d Zhang L.; Fu N.; Luo S. Pushing the Limits of Aminocatalysis: Enantioselective Transformations of α-Branched β-Ketocarbonyls and Vinyl Ketones by Chiral Primary Amines. Acc. Chem. Res. 2015, 48, 986–997. 10.1021/acs.accounts.5b00028. [DOI] [PubMed] [Google Scholar]; e Lee H.-J.; Maruoka K. Design of Bifunctional Amino Tf-Amide Organocatalysts and Application in Various Asymmetric Transformations. Chem. Rec. 2022, 22, e202200004 10.1002/tcr.202200004. [DOI] [PubMed] [Google Scholar]; f Tanaka F. Amines as Catalysts: Dynamic Features and Kinetic Control of Catalytic Asymmetric Chemical Transformations to form C-C Bonds and Complex Molecules. Chem. Rec. 2023, 23, e202200207 10.1002/tcr.202200207. [DOI] [PubMed] [Google Scholar]
- a Erkkilä A.; Majander I.; Pihko P. M. Iminium Catalysis. Chem. Rev. 2007, 107, 5416–5470. 10.1021/cr068388p. [DOI] [PubMed] [Google Scholar]; b Nielsen M.; Worgull D.; Zweifel T.; Gschwend B.; Bertelsen S.; Jørgensen L. A. Mechanisms in Aminocatalysis. Chem. Commun. 2011, 47, 632–649. 10.1039/C0CC02417A. [DOI] [PubMed] [Google Scholar]; c Jensen K. L.; Dickmeiss G.; Jiang H.; Albrecht Ł.; Jørgensen K. A. The Diarylprolinol Silyl Ether System: A General Organocatalyst. Acc. Chem. Res. 2012, 45, 248–264. 10.1021/ar200149w. [DOI] [PubMed] [Google Scholar]; d Li J.-L.; Liu T.-Y.; Chen Y.-C. Aminocatalytic Asymmetric Diels-Alder Reactions via HOMO Activation. Acc. Chem. Res. 2012, 45, 1491–1500. 10.1021/ar3000822. [DOI] [PubMed] [Google Scholar]; e Chauhan P.; Mahajan S.; Enders D. Achieving Molecular Complexity via Stereoselective Multiple Domino Reactions Promoted by a Secondary Amine Organocatalyst. Acc. Chem. Res. 2017, 50, 2809–2821. 10.1021/acs.accounts.7b00406. [DOI] [PubMed] [Google Scholar]
- a Lam Y.; Houk K. N. Origins of Stereoselectivity in Intramolecular Aldol Reactions Catalyzed by Cinchona Amines. J. Am. Chem. Soc. 2015, 137, 2116–2127. 10.1021/ja513096x. [DOI] [PubMed] [Google Scholar]; b Yu P.; He C. Q.; Simon A.; Li W.; Mose R.; Thøgersen M. K.; Jørgensen K. A.; Houk K. N. Organocatalytic [6+4] Cycloadditions via Zwitterionic Intermediates: Chemo-, Regio-, and Stereoselectivities. J. Am. Chem. Soc. 2018, 140, 13726–13735. 10.1021/jacs.8b07575. [DOI] [PubMed] [Google Scholar]
- a Chen X.; Thøgersen M. K.; Yang L.; Lauridsen R. F.; Xue X.-S.; Jørgensen K. A.; Houk K. N. [8+2] vs [4+2] Cycloadditions of Cyclohexadienamines to Tropone and Heptafulvenes – Mechanisms and Selectivities. J. Am. Chem. Soc. 2021, 143, 934–944. 10.1021/jacs.0c10966. [DOI] [PubMed] [Google Scholar]; b Cui H.-L.; Chouthaiwale P. V.; Yin F.; Tanaka F. Reaction-based Mechanistic Investigations of Asymmetric Hetero-Diels-Alder Reactions of Enones with Isatins Catalyzed by Amine-based Three-Component Catalyst Systems. Asian J. Org. Chem. 2016, 5, 153–161. 10.1002/ajoc.201500412. [DOI] [Google Scholar]; c He X.-L.; Xiao Y.-C.; Du W.; Chen Y.-C. Enantioselective Formal [3+3] Cycloadditions of Ketones and Cyclic 1-Azadienes by Cascade Enamine-Enaminde Catalysis. Chem. – Eur. J. 2015, 21, 3443–3448. 10.1002/chem.201404550. [DOI] [PubMed] [Google Scholar]
- a Luo S.; Xu H.; Li J.; Zhang L.; Cheng J.-P. A Simple Primary-Tertiary Diamine-Brønsted Acid Catalyst for Asymmetric Direct Aldol Reactions of Linear Aliphatic Ketones. J. Am. Chem. Soc. 2007, 129, 3074–3075. 10.1021/ja069372j. [DOI] [PubMed] [Google Scholar]; b Shi M.; Zhang Q.; Gao J.; Mi X.; Luo S. Catalytic Asymmetric α-Alkylsulfenylation with a Disulfide Reagent. Angew. Chem., Int. Ed. 2022, 61, e202209044 10.1002/anie.202209044. [DOI] [PubMed] [Google Scholar]; c Fu N.; Zhang L.; Li J.; Luo S.; Cheng J.-P. Chiral Primary Amine Catalyzed Enantioselective Protonation via an Enamine Intermediate. Angew. Chem., Int. Ed. 2011, 50, 11451–11455. 10.1002/anie.201105477. [DOI] [PubMed] [Google Scholar]; d Moran A.; Hamilton A.; Bo C.; Melchiorre P. A Mechanistic Rationale for the 9-Amino(9-deoxy)epi Cinchona Alkaloids Catalyzed Asymmetric Reactions via Iminium Ion Activation of Enones. J. Am. Chem. Soc. 2013, 135, 9091–9098. 10.1021/ja404784t. [DOI] [PubMed] [Google Scholar]; e Scharinger F.; Pàlvölgyi Á. M.; Weisz M.; Weil M.; Stanetty C.; Schnürch M.; Bica-Schröder K. Sterically Demanding Flexible Phosphoric Acids for Constructing Efficient and Multi-Purpose Asymmetric Organocatalysts. Angew. Chem., Int. Ed. 2022, 61, e202202189 10.1002/anie.202202189. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Huang M.; Zhang L.; Pan T.; Luo S. Deracemization through Photochemical E/Z Isomerization of Enamines. Science 2022, 375, 869–874. 10.1126/science.abl4922. [DOI] [PubMed] [Google Scholar]; g Rezayee N. M.; Enemærke V. J.; Linde S. T.; Lamhauge J. N.; Reyes-Rodríguez G. J.; Jørgensen K. A.; Lu C.; Houk K. N. An Asymmetric SN2 Dynamic Kinetic Resolution. J. Am. Chem. Soc. 2021, 143, 7509–7520. 10.1021/jacs.1c02193. [DOI] [PubMed] [Google Scholar]
- Garg Y.; Tanaka F. Enantioselective Direct anti-Selective Mannich-Type Reactions Catalyzed by 3-Pyrrolidinecarboxylic Acid in the Presence of Potassium Carbonate: Addition of Potassium Carbonate Improves Enantioselectivities. Org. Lett. 2020, 22, 4542–4546. 10.1021/acs.orglett.0c01561. [DOI] [PubMed] [Google Scholar]
- a Nicolau K. C.; Pan S.; Shelke Y.; Das D.; Ye Q.; Lu Y.; Sau S.; Bao R.; Rigol S. A Reverse Approach to the Total Synthesis of Halichondrin B. J. Am. Chem. Soc. 2021, 143, 9267–9276. 10.1021/jacs.1c05270. [DOI] [PubMed] [Google Scholar]; b Lowell A. N.; DeMars M. D. II; Slocum S. T.; Yu F.; Anand K.; Chemler J. A.; Korakavi N.; Priessnitz J. K.; Park S. R.; Koch A. A.; Schultz P. J.; Sherman D. H. Chemoenzymatic Total Synthesis and Structural Diversification of Tylactone-based Macrolide Antibiotics through Late-Stage Polyketide Assembly, Tailoring, and C-H Functionalization. J. Am. Chem. Soc. 2017, 139, 7913–7920. 10.1021/jacs.7b02875. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhu W.; Jiménez M.; Jung W.-H.; Camarco D. P.; Balachandran R.; Vogt A.; Day B. W.; Curran D. P. Streamlined Syntheses of (−)-Dictyostatin, 16-Desmethyl-25,26-dihydrodictyostatin, and 6-epi-16-Desmethyl-25,26-dihydrodictyostatin. J. Am. Chem. Soc. 2010, 132, 9175–9187. 10.1021/ja103537u. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Evans D. A.; Adams D. J. Total Synthesis of (+)-Galbulimima Alkaloid 13 and (+)-Himagaline. J. Am. Chem. Soc. 2007, 129, 1048–1049. 10.1021/ja0684996. [DOI] [PubMed] [Google Scholar]; e Gaul C.; Njardarson J. T.; Danishefsky S. J. The Total Synthesis of (+)-Migrastatin. J. Am. Chem. Soc. 2003, 125, 6042–6043. 10.1021/ja0349103. [DOI] [PubMed] [Google Scholar]; f Trost B. M.; Zhang L.; Lam T. M. Synthesis of the Aminocyclitol Core of Jogyamycin via an Enantioselective Pd-Catalyzed Trimethylenemethane (TMM) Cycloaddition. Org. Lett. 2018, 20, 3938–3942. 10.1021/acs.orglett.8b01518. [DOI] [PubMed] [Google Scholar]; g Umezawa T.; Seino T.; Matsuda F. Novel One-pot Three-component Coupling Reaction with Trimethylsilylmethyl-phosphonate, Acyl Fluoride, and Aldehyde through the Horner-Wadsworth-Emmons Reaction. Org. Lett. 2012, 14, 4206–4209. 10.1021/ol301879a. [DOI] [PubMed] [Google Scholar]
- a Davis F. A.; Wu Y.; Xu H.; Zhang J. Asymmetric Synthesis of cis-5-Substituted Pyrrolidine 2-Phosphonates Using Metal Carbenoid NH Insertion and δ-Amino β-Ketophosphonates. Org. Lett. 2004, 6, 4523–4525. 10.1021/ol048157+. [DOI] [PubMed] [Google Scholar]; b Davis F. A.; Xu H.; Zhang J. Asymmetric Synthesis of Ring Functionalized trans-2,5-Disubstituted Piperidines from N-Sulfinyl δ-Amino β-Keto Phosphonates. J. Org. Chem. 2007, 72, 2046–2052. 10.1021/jo062365t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethi V.; Tanaka F. Organocatalytic Enantioselective-Position-Selective Mannich Reactions of β-Ketocarbonyl Derivatives. Org. Lett. 2022, 24, 6711–6715. 10.1021/acs.orglett.2c02433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.