Abstract
In the pre-vaccination era, the prevalence of chronic hepatitis B virus (HBV) infection in the World Health Organization (WHO) Eastern Mediterranean Region (EMR) ranged from two to seven percent in a total population of over 580 million people. Mortality estimates place cirrhosis among the top ten causes of years of life lost in the EMR. The region has made notable achievements, improving coverage from only 6% in 1992, when WHO recommended hepatitis B vaccination of all infants, to 83% in 2014. Member states adopted a hepatitis B control target in 2009 to reduce chronic hepatitis B virus infection prevalence to less than one percent among children aged <5 years by 2015. This report reviews progress toward achievement, challenges faced, and the next steps forward of hepatitis B control among children in the EMR.
Keywords: Hepatitis B virus, HBV, Vaccination, birth dose, North Africa, Middle East, EMRO
1. Background
Two billion persons have been infected with hepatitis B virus (HBV) worldwide, and >350 million have chronic infection [1]. Globally, in 2010, over 700,000 deaths were attributed to HBV infection: >340,000 from liver cancer, >312,000 from cirrhosis and >132,000 from acute infection [2]. Low and middle-income countries bear the majority of the burden of hepatitis B-related liver cancer deaths [3].
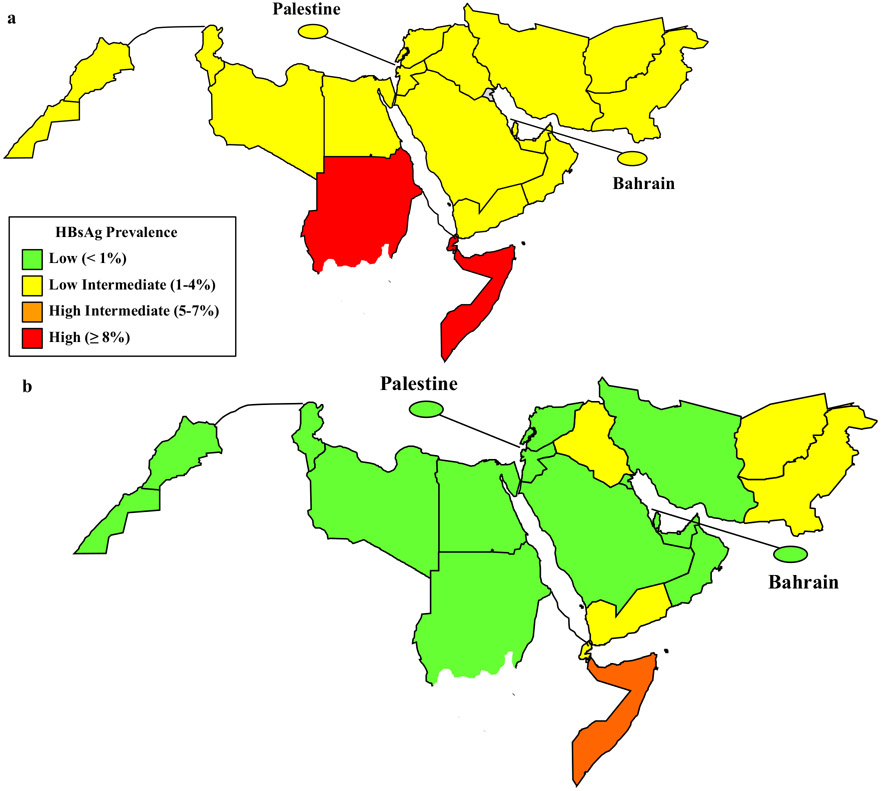
The World Health Organization (WHO) estimates that more than four million people are infected yearly with HBV in the Eastern Mediterranean Region (EMR) [4]. EMR countries have a prevalence of chronic HBV infection ranging from low intermediate (2–4%) in most countries to high intermediate (5–7%) in Somalia and Sudan (Fig. 1a) [5,6].
Fig. 1.
Estimated prevalence of chronic hepatitis B virus infection among children in the WHO Eastern Mediterranean Region (pre-vaccine prevalence of chronic hepatitis B was previously estimated [5]. Post-vaccine prevalence estimates come from nationally representative serosurveys [Al Awaidy et al., Egypt unpublished, Sudan unpublished] or were estimated by inputting WHO-UNICEF national immunization coverage estimates for 2014 into a mathematical model [5,15].) before vaccination (a) and in 2014 (b).
The EMR is composed of 21 member states [a total of 22 countries or areas, including Palestine, which reports status toward vaccination control goals to the WHO Regional Office for the Eastern Mediterranean (EMRO)] that stretch from Morocco in the west to Pakistan in the east, and from Syria in the north to Somalia in the south. In 2011, Sudan split into two independent countries, Sudan and South Sudan. Sudan remained in the EMR and South Sudan joined the WHO African Region.
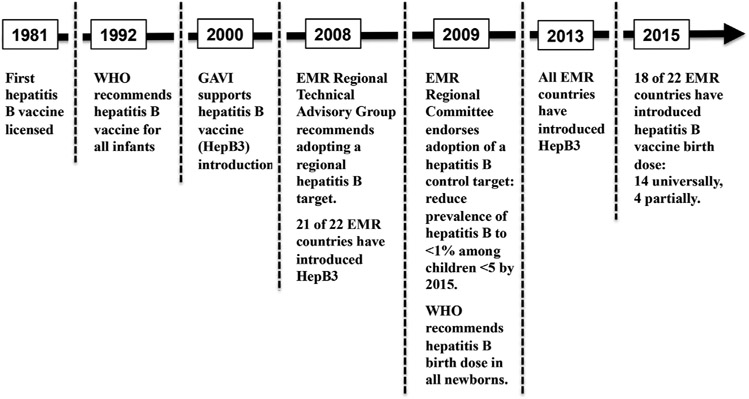
To raise awareness of the significant HBV burden in the EMR and the urgent need for action to prevent HBV transmission, the member states of the EMR adopted in 2009 a target for hepatitis B control through childhood vaccination to be achieved by 2015. A timeline of hepatitis B vaccination milestones in the EMR is given in Fig. 2. This report reviews progress toward achievement, challenges faced, and the next steps forward of the regional hepatitis B control target.
Fig. 2.
Timeline of hepatitis B vaccination milestones in the WHO Eastern Mediterranean Region.
2. Methods
EMR member states and Palestine annually report vaccination coverage using the WHO/UNICEF Joint Reporting Form (JRF) [7]. Methodology used by the WHO and UNICEF working group to derive coverage estimates from JRF data, vaccine coverage surveys and consultation with national authorities has been previously published [8]. Vaccination coverage data reported in this paper are WHO/UNICEF estimates of national immunization coverage, unless specified otherwise.
A mathematical model, developed by Goldstein et al., was used to estimate the impact of hepatitis B vaccination in the EMR on HBV prevalence and HBV-related deaths [5]. The natural history of HBV infection is age-related. Therefore, infection and progression to chronic HBV was calculated in one of three age ranges: perinatal (at birth); early childhood (after birth to <5 years); and late (≥5 years). The annual infection rate prior to vaccine introduction was estimated by the model using HBV prevalence reported in studies conducted prior to the introduction of hepatitis B vaccination in EMR countries. The number of perinatal infections was calculated from the prevalence of hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) and the probability of transmission. The number of early childhood infections was calculated based on the prevalence of HBV infection in five year olds after subtracting perinatal infections. The number of infections in the late period was determined from the prevalence of HBV infection among individuals ≥30 years of age, excluding infections acquired in perinatal and early periods. HBV-related deaths were estimated from age-specific HBV-related cirrhosis and hepatocellular cancer mortality curves and adjusted for background mortality. In the absence of sufficient data for Palestine, HBV pre-vaccination prevalence, HBV prevalence at age five, and age-specific all-cause mortality, rates in Palestine were assumed to be similar to those rates in Jordan. Since the Goldstein et al. model was published before the division of Sudan into two countries, baseline prevalence inputs for the model for Sudan were adjusted to exclude data from areas now in South Sudan, because of the highly differential HBV prevalence of these two countries [6].
3. Regional hepatitis B control target and strategies
In 2009, EMR member states endorsed Resolution EM/RC56/R.5 that established a regional target to reduce the prevalence of chronic HBV infection to <1% among children below five years of age by 2015. The main strategies for achieving and verifying achievement of the hepatitis B control target include: (1) reaching high hepatitis B vaccine birth dose coverage within 24 h of birth; (2) reaching high coverage of three-doses of hepatitis B vaccine; (3) completion of serological surveys of HBsAg prevalence, representative of the target population, to document the impact of hepatitis B immunization programs and achievement of the regional target; and (4) verification of achieving the regional target by an independent body of experts.
3.1. Reaching high hepatitis B birth dose coverage within 24 h of birth
Ninety-percent of persons infected with HBV perinatally will develop chronic infection compared with six percent of those infected ≥5 years of age [5]. Hepatitis B vaccination within 24 h of birth is highly effective, preventing perinatal transmission in 70–95% of infants whose mothers are chronically infected [9]. It has been estimated that 90% birth dose coverage in a population could result in an 84% reduction in HBV-related deaths [5]. In 2009, WHO recommended delivery of hepatitis B birth dose as soon as possible after birth (<24 h) in all countries, regardless of disease burden [1].
Fourteen (64%) of twenty-two EMR countries have introduced a universal birth dose of hepatitis B vaccine (Table 1). By 2014, Jordan, Somalia, Sudan and Yemen had not yet introduced birth dose; Afghanistan, Egypt and Pakistan had introduced birth dose sub-nationally; and Bahrain had introduced birth dose selectively (Table 1). Afghanistan has introduced birth dose in health facilities only. Egypt began a phased approach to birth dose introduction, starting with health facilities in pilot districts (phase 1) and then all districts (phase 2) of Alexandria, Gharbia and Sohag governorates. Expansion of birth dose to health facilities in Ismailia, Port Saeed and Suez governorates (phase 3) and then Fayoum and Aswan governorates (phase 4) is planned in 2015–2016. Pakistan introduced birth dose vaccine in secondary and tertiary hospitals in one province and plans phased introduction in other provinces. Before 2015, Bahrain’s national policy was to screen all pregnant women for HBsAg, and then selectively administer birth dose plus hepatitis B immune-globulin (HBIG) within 24 h to neonates of HBsAg-positive and HBsAg-unknown mothers. However, Bahrain instituted universal hepatitis B birth dose vaccination of all newborns, regardless of maternal status, in 2015.
Table 1.
Universal hepatitis B vaccine birth dose by country—WHO Eastern Mediterranean Region, 2010–2014.
| Category | Country | Live birthsa | Introduction of birth dose (year) |
Hepatitis B birth dose vaccination coverageb % |
Institutional deliveriesc % |
Births attended by skilled health personnelc % |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | ||||||
| No universal birth dose | Afghanistan | 1034 | Subnational (2014) | 0 | 0 | 0 | 0 | 4 | 33 | 38.6 |
| Bahrain | 20 | Partiald (n/a) | n/a | n/a | n/a | n/a | n/a | 98 | 99.5 | |
| Egypt | 1902 | Subnational (2014) | 0 | 0 | 0 | 0 | 0 | 87 | 91.5 | |
| Jordan | 194 | No | 0 | 0 | 0 | 0 | 0 | 99 | 99.6 | |
| Pakistan | 4597 | Subnational (2014) | 0 | 0 | 0 | 0 | 0 | 48 | 52.1 | |
| Somalia | 469 | No | 0 | 0 | 0 | 0 | 0 | 9 | 33 | |
| Sudan | 1287 | No | 0 | 0 | 0 | 0 | 0 | 28 | 23.1 | |
| Yemen | 767 | No | 0 | 0 | 0 | 0 | 0 | 30 | 44.7 | |
| Universal birth dose | Djibouti | 24 | National (2011) | 0 | 0 | 87 | 86 | 94 | 87 | 87.4 |
| Iran | 1449 | National (<1998) | 99 | 99 | 96 | 99 | 97 | 95 | 96.4 | |
| Iraq | 1068 | National (2004) | 27 | 32 | 37 | 43 | 43 | 77 | 90.9 | |
| Kuwait | 70 | National (<1998) | 99 | 98 | 97 | 98 | 96 | 98 | 100 | |
| Lebanon | 65 | National (2006) | 94 | 94 | 94 | 94 | 94 | 98 | 98 | |
| Libya | 127 | National (<1998) | 99 | 99 | 99 | 99 | 99 | n/a | 99.8 | |
| Morocco | 753 | National (1999) | 20 | 4 | 9 | 14 | 14 | 73 | 73.6 | |
| Oman | 75 | National (<1998) | 99 | 99 | 99 | 99 | 99 | 99 | 98.6 | |
| Palestine | 124 | National (n/a) | 96 | 96 | 97 | 97 | 97 | 99 | 99.6 | |
| Qatar | 24 | National (1989) | 99 | 96 | 95 | 96 | 92 | 99 | 100 | |
| Saudi Arabia | 557 | National (<1995) | 98 | 98 | 99 | 99 | 98 | 91 | 97 | |
| Syria | 533 | National (1993) | 97 | 89 | 81 | 81 | 78 | 78 | 96.2 | |
| Tunisia | 189 | National (2006) | 82 | 80 | 73 | 82 | 80 | 99 | 98.6 | |
| United Arab Emirates | 130 | National (2010) | 98 | 97 | 96 | 96 | 91 | 100 | 100 | |
n/a, data not available.
In thousands; data from WHO for year 2014, accessed at http://apps.who.int/ghodata (Palestine data are from 2014 JRF).
Coverage data come from the WHO-UNICEF estimates of national immunization coverage (updated July 2015).
Data from UNICEF (updated June 2015), accessed at www.data.unicef.org.
HBsAg is screened at prenatal visit and birth dose given <24 h to neonates of positive mothers or mothers with unknown HBsAg status. Birth dose is not universally given.
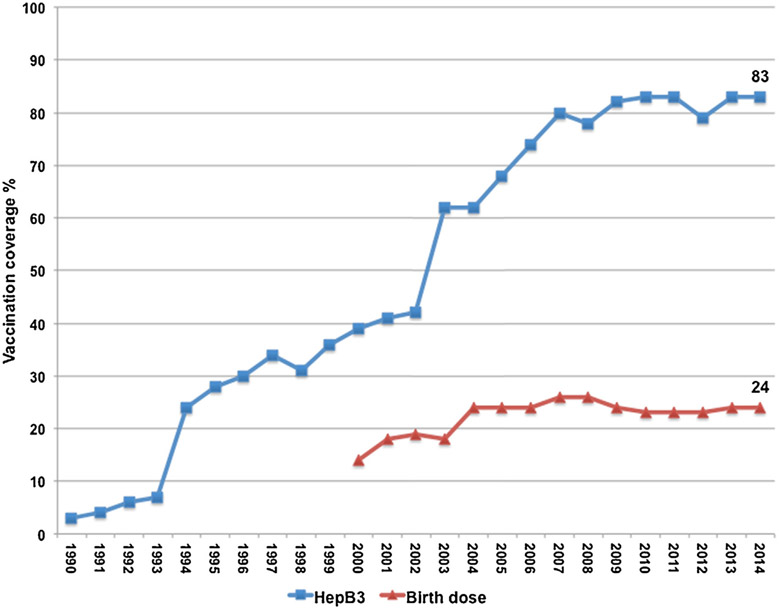
Overall, hepatitis B birth dose vaccination coverage in the region was 24% in 2014 compared with 14% in 2000 (Fig. 3). In 2014, among 14 EMR countries with a universal birth dose program, 71% of newborns received a birth dose within 24 h; Iraq, Morocco and Syria had <80% birth dose coverage (Table 1).
Fig. 3.
Hepatitis B vaccination coverage in the WHO Eastern Mediterranean Region, 1990–2014. Data from final WHO-UNICEF Estimates of National Immunization Coverage (July 2015). HepB3 – third dose of hepatitis B vaccine. Birth dose – hepatitis B vaccine given within 24 h of birth.
Children delivered in health facilities and by skilled birth attendants are more likely to receive hepatitis B birth dose vaccine [10]. In 2014, 62% of all births in the EMR were delivered in health institutions and 67% were attended by skilled health personnel. However, in the eight countries without universal birth dose, only 49% of births were delivered in health institutions, compared with 86% in countries with universal birth dose. Similarly, in countries without universal birth dose, only 54% of deliveries were attended by skilled health personnel, compared with 92% in countries with universal birth dose. Countries with the lowest rates of institutional births (Iraq, Morocco and Syria) also had the lowest rates of birth dose coverage among those countries with a nationwide universal birth dose policy (Table 1).
3.2. Reaching high three-dose hepatitis B vaccine coverage in infancy
In 1992, the WHO recommended that three doses of hepatitis B vaccination be included in the routine childhood immunization programs of all countries, as three doses protect up to 95% of healthy infants from chronic HBV infection [9]. Prior to this recommendation, six (27%) of 22 EMR countries had already introduced three doses of hepatitis B vaccine (HepB3), including Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates (UAE). By 2000, 17 (77%) had introduced the series, and by 2013 all EMR countries had introduced HepB3. Overall, HepB3 coverage in the region rose from only three percent in 1990 to 39% in 2000 and to 83% in 2014 (Fig. 3). However, six (27%) countries [Afghanistan, Djibouti, Iraq, Pakistan, Somalia and Syria], representing 50% of the EMR birth cohort, had HepB3 coverage <80% in 2014.
3.3. Measuring impact with seroprevalence surveys
The verification of achievement of the hepatitis B control target will primarily be based on HBsAg prevalence <1% among children aged <5 years, as determined by at least one nationally representative serological survey. The sample size should be adequate to show <1% prevalence with 95% confidence and the accuracy of the estimate should be within ±0.5%. Seroprevalence data from children ≥5 years of age may be used for verification if hepatitis B vaccine had been introduced for a period greater than the ages of the children included the survey. Serosurvey methodology must be consistent with WHO survey guidelines [11].
3.4. Verifying achievement of the control target
Verification of achieving the EMR hepatitis B control target will require establishment of guidelines and processes modeled on processes for verification of hepatitis B control in the WHO Western Pacific Region (WPR), verification of measles elimination, and certification of polio eradication [12-14]. Similar to the polio certification and measles verification processes in the EMR and other regions, each EMR country will create a National Verification Committee (NVC). The NVC will be an independent panel of persons with expertise in key areas such as clinical medicine, epidemiology, immunizations, public health or virology that will develop and monitor the verification activities in their country. The NVC will make the formal request for regional verification and will submit their country’s documentation to the regional level. The Regional Director of the EMR will appoint an independent panel of experts to establish the Regional Verification Commission (RVC). Once established, the RVC will advise NVCs on the processes for collecting, analyzing and reporting data required to verify hepatitis B control. Finally, the RVC will receive, validate and accept or reject the final reports from the NVCs.
4. Status and impact of hepatitis B control
Eleven EMR countries have either completed nationally representative hepatitis B serosurveys (Oman and Sudan), are in the process of analyzing serosurveys (Egypt and Tunisia), or are in planning and development stages (Bahrain, Iran, Lebanon, Morocco, Palestine, Qatar and Saudi Arabia). Oman and Sudan have likely met the regional control target by showing that HBsAg prevalence is <1% in their target population of children. Oman conducted a nationally representative, school-based hepatitis B seroprevalence survey in 2005 comparing children who were eligible for routine hepatitis B vaccine after its introduction in August of 1990 with children who were born before hepatitis B introduction [15]. Of 1890 children born after vaccine introduction, 10 (0.5%) were HBsAg-positive compared to 16 HBsAg-positives (2.3%) among 175 born before vaccine introduction. Oman has maintained >98% birth dose and ≥97% HepB3 vaccination coverage since completion of its seroprevalence study and is likely ready for national and regional verification of achieving the regional hepatitis B control target.
Sudan completed a nationally representative, community-based seroprevalence study among children <5 years of age in 2012 [16] [Unpublished data]. In the survey, 3600 children were tested by a rapid point of care test; 16 (0.4%) were positive for HBsAg. The average HepB3 coverage was 80% during 2006–2012 (the period between HepB3 introduction and completion of the serosurvey). Since the serosurvey, Sudan has maintained ≥92% HepB3 coverage. Though Sudan has not yet introduced birth dose, and its HepB3 coverage before the serosurvey was only at a moderate level, a sufficiently low HBsAg seroprevalence and sustained high HepB3 coverage in the last few years would make Sudan a good candidate to begin national and regional verification of achievement of the hepatitis B target.
Using the model by Goldstein et al., it is estimated that, in addition to Oman and Sudan, 13 countries would meet the regional control target of <1% HBsAg seroprevalence (Table 2) [5]. Fig. 1a and b demonstrates the estimated impact of hepatitis B vaccination in the region on the prevalence of chronic HBV infection before and after vaccination, respectively. Pre-vaccine prevalence was previously estimated [5]. Post-vaccine prevalence estimates come from serosurveys [15,16] [Sudan, unpublished], or were estimated by inputting WHO-UNICEF national immunization coverage estimates for 2014 into a mathematical model [5]. Fig. 1b illustrates that 15 (68%) of 22 EMR countries achieved <1% chronic HBV infection prevalence by the end of 2014.
Table 2.
Hepatitis B vaccine type, schedule, third-dose coverage and verification status by country—WHO Eastern Mediterranean Region, 2010–2014.
| Country | Hepatitis B vaccineb | Hepatitis B vaccine schedule |
HepB3 vaccination coveragea % | HBsAg prevalence estimatec % |
Verification status | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | |||||
| Afghanistan | Pentavalent | 6, 10, 14 wks | 66 | 68 | 67 | 70 | 75 | 1.6 | Requires increased coverage |
| Bahrain | Hexavalent (2 mo), pentavalent (4, 6, 18 mo) | 2, 4, 6, 18 mo | 99 | 99 | 99 | 99 | 99 | 0.5 | Serosurvey planned |
| Djibouti | Pentavalent | 6, 10, 14 wks | 88 | 87 | 81 | 82 | 78 | 1.9 | Requires increased coverage |
| Egypt | Pentavalent | 2, 4, 6 mo | 97 | 96 | 93 | 97 | 94 | 1.0 (<1.0)d | Serosurvey completed |
| Iran | Pentavalent | 2, 4, 6 mo | 99 | 99 | 98 | 99 | 99 | 0.1 | Serosurvey planned |
| Iraq | Pentavalent | 2, 6 mo | 72 | 77 | 61 | 66 | 62 | 1.5 | Requires increased coverage |
| Jordan | Monovalent | 3, 4, 5 mo | 98 | 98 | 98 | 98 | 98 | 0.5 | Requires serosurvey |
| Kuwait | Pentavalent | 2, 4, 6, 18 mo | 99 | 99 | 98 | 99 | 96 | 0.2 | Requires serosurvey |
| Lebanon | Pentavalent | 2, 4, 6, 18 mo | 81 | 81 | 81 | 81 | 81 | 0.4 | Serosurvey in progress |
| Libya | Hexavalent | 2, 4, 6 mo | 98 | 98 | 98 | 96 | 94 | 0.1 | Requires serosurvey |
| Morocco | Pentavalent | 2, 3, 4 mo | 98 | 98 | 99 | 99 | 99 | 0.8 | Requires serosurvey |
| Oman | Pentavalent | 2, 4, 6 mo | 98 | 99 | 97 | 97 | 98 | 0.5 (0.2)d | Serosurvey completed |
| Pakistan | Pentavalent | 6, 10, 14 wks | 82 | 74 | 72 | 73 | 73 | 1.7 | Requires increased coverage |
| Palestine | Pentavalent | 2, 4, 6 mo | 98 | 99 | 99 | 99 | 99 | 0.2 | Serosurvey planned |
| Qatar | Hexavalent (2 mo), pentavalent (4, 6 mo) | 2, 4, 6 mo | 97 | 93 | 92 | 99 | 99 | 0.1 | Serosurvey in progress |
| Saudi Arabia | Hexavalent | 2, 4, 6 mo | 98 | 98 | 98 | 98 | 98 | 0.1 | Serosurvey planned |
| Somalia | Pentavalent | 6, 10, 14 wks | 0 | 0 | 0 | 42 | 42 | 6.2 | Requires increased coverage |
| Sudan | Pentavalent | 6, 10, 14 wks | 75 | 93 | 92 | 93 | 94 | 1.3 (0.4)d | Serosurvey completed |
| Syria | Pentavalent | 2, 4, 6 mo | 84 | 66 | 43 | 71 | 71 | 0.6 | Requires increased coverage |
| Tunisia | Pentavalent | 2, 3, 6 mo | 98 | 98 | 97 | 98 | 98 | 0.2 | Serosurvey in progress |
| United Arab Emirates | Hexavalent (2 mo), pentavalent (4, 6 mo) | 2, 4, 6 mo | 94 | 94 | 94 | 94 | 94 | 0.2 | Requires serosurvey |
| Yemen | Pentavalent | 6, 10, 14 wks | 87 | 81 | 82 | 88 | 88 | 1.1 | Requires increased coverage |
wks, weeks; mo, months.
Coverage data come from the WHO-UNICEF estimates of national immunization coverage (updated July 2015).
Pentavalent vaccine contains diphtheria, tetanus, pertussis, Haemophilus influenzae and hepatitis B antigens (DTP-HiB-HepB), except in Jordan, where pentavalent vaccine contains DTP-HiB-IPV; IPV, inactivated polio virus. Hexavalent vaccine contains DTP-HiB-HepB-IPV.
Prevalence from HBsAg serosurveys in Oman and Sudan; preliminary analysis from Egypt’s serosurvey indicates <1% prevalence; other countries are estimated using mathematical model [Goldstein et al.].
HBsAg prevalence % from mathematical model (actual HBsAg prevalence % from serosurvey).
Based on modeled data, the region as a whole has achieved 1.21% prevalence of chronic HBV infection in young children, based on country-specific prevalence estimates and the region’s 2014 birth cohort of 15.5 million. Among those born between 2005 and 2014, it is estimated that nearly five million chronic HBV infections have been prevented and almost 700,000 future hepatitis B-related deaths have been averted due to the impact of hepatitis B vaccination programs in the region.
5. Goals, challenges and the way forward
5.1. Introduce universal birth dose and achieve high birth dose coverage
Low rates of health institution deliveries create challenges to introduce birth dose and to increase birth dose coverage [17]. Home births are more likely to occur in remote areas without access to an adequate cold chain. Without a cold chain, these areas do not have a continuous supply of vaccine. While outreach activities can be conducted to provide vaccine doses after birth, the urgency in the timing of birth dose precludes that solution. Birth dose vaccine needs to be available every day as close as possible to the place of birth.
Though hepatitis B vaccine is not licensed for use outside the cold chain (OCC), WHO considers it to be a candidate for controlled temperature chain use (CTC) because of its thermal stability and vulnerability to freeze damage during refrigerated storage and transport [10]. In addition, field studies in China, Indonesia and Vietnam have shown high potency and equivalent seroconversion when hepatitis B vaccine was stored outside the cold chain compared to hepatitis B vaccine maintained in the cold chain [18-20]. Indonesia has taken on a national policy of providing vaccine OCC to help increase coverage among home births.
Birth attendants without formal health training delivered nearly all of the home births in EMR countries with low rates of health facility deliveries. Unskilled birth attendants infrequently have training in vaccine administration and safety. If accessibility to hepatitis B birth dose were improved by acceptance of its storage and use OCC by EMR countries, accessibility to trained health workers to handle and deliver vaccine injections would still remain a barrier. Many studies have shown this barrier can be overcome with use of a simple, compact, pre-filled auto-disabled device (CPADs, e.g., Uniject™) that allow persons with minimal training to effectively deliver vaccine in rural settings [10,21]. Uniject has been used safely and effectively for the administration of hepatitis B vaccine at birth, both within and outside the cold chain [18,22,23], and was reported to have high acceptability among parents [22]. In Indonesia, where 53% of births occur at home in rural areas, the combination of both strategies, the administration of birth dose OCC with Uniject was shown to improve birth dose (BD) coverage by 66% [22]. Analyses in Indonesia and Cambodia have shown that administration of BD with Uniject can be cost-effective compared to single-dose and ten-dose HepB vaccine vials [24].
While low rates of institutional births and birth attendance by skilled personnel pose challenges, they should not impede national introduction of birth dose in health facilities. Using the model by Goldstein et al., it is estimated that if Afghanistan, Pakistan, Somalia, Sudan and Yemen introduced birth dose in all health facilities and achieved coverage equal to the percent of their health facility births, an additional 24,000 chronic HBV infections and 3400 related deaths would be prevented every year.
Iraq, Syria and Morocco have a universal birth dose policy but have low birth dose coverage despite >70% births occurring in health facilities (Table 1). In these cases, WHO recommends developing or improving integration of birth dose vaccination into newborn and maternal care [10]. Specific recommendations include ensuring that there is a clear birth dose policy in each health facility, that birth dose vaccine is available, that there are standing orders in the delivery room or prenatal ward, and that the personnel responsible for administering vaccine are clearly defined [10].
5.2. Recognize and respond to the impact of conflict on immunization
Nearly half of EMR countries have been challenged with violent conflict or civil strife in recent years. War and conflict cause vaccine shortages, cold chain failures, and logistical challenges to reach children for vaccination [25,26]. Lack of security in conflict areas has been independently associated with poor childhood immunization rates [27]. While the impact of conflict on immunization is multifactorial, and a full analysis of this situation in the EMR is outside the scope of this paper, a few illustrations highlight this point. Since 2011, conflict in Syria has resulted in hundreds of thousands of deaths, and millions of people have been internally displaced or have fled Syria [28]. From 2010, one year prior to the Syrian conflict, to 2014, birth dose and HepB3 coverage dropped by 19% (97–78%) and by 13% (84–71%), respectively.
With a recent re-emergence of war and over a decade of conflict, Iraq has experienced a decline in hepatitis B birth dose coverage from 93% in 2005 to 43% in 2014 and a HepB3 coverage during the same period of ~60%, leaving 3.3 million infants and children unvaccinated. Afghanistan and Pakistan are persistently security-compromised regions, and they achieved only minimal gains in HepB3 coverage over the last decade, from 63% to 75% (4.7 million unvaccinated) and from 70% to 73% (11 million unvaccinated), respectively. Though HepB3 was only introduced in recent years, Somalia is unlikely to improve its current 42% HepB3 coverage significantly until the government regains greater control of the country from insurgents and public health infrastructure is rebuilt. The effect of internal conflict in Libya and Yemen on hepatitis B vaccination coverage has not yet been assessed, though these countries remain at high risk.
The first steps to mitigating the negative impact of conflict on childhood immunization include understanding and quantifying the effects of underlying factors in the local context. For example, findings from an assessment of vaccination capacity in post-war Iraq were used to revise vaccine distribution methods to overcome a damaged cold chain system [29]. A greater understanding of the effects and associated factors could help partner organizations such as the WHO and UNICEF to respond more quickly and effectively. As noted in the previous section, hepatitis B vaccine birth dose could be used OCC and administered by lay health workers using CPADs in regions where the cold chain has been disrupted and/or where trained health care workers do not have safe access. Further, it is important to bring together policy makers, local authorities, community representatives and frontline skilled and lay health workers to determine how to best maintain immunization services in insecure conditions [27].
6. Conclusions
The significant prevalence of chronic HBV infection in countries of the WHO Eastern Mediterranean Region and the burden of resultant cirrhosis, liver cancer and death have made HBV control a public health priority. The region has responded by introducing infant hepatitis B vaccination into all national programs and by establishing a regional control target to reduce the prevalence of chronic HBV infection to less than one percent in children aged <5 years. But many challenges remain. Five of eight countries that have not introduced universal birth dose have low rates of institutional deliveries and birth attendance by skilled health workers. The use of hepatitis B vaccine OCC in these countries, with or without CPADs, could help overcome these barriers. Childhood immunization programs, including hepatitis B, have had persistently low or decreasing coverage in many EMR countries due to war and conflict. Underlying factors need to be understood and quantified to develop mitigation and prevention strategies.
Each EMR country that achieves <1% chronic HBV infection seroprevalence among young children will mark the region’s commitment to achieving hepatitis B control, will highlight the success of overcoming its challenges, and will serve as a milestone toward the desired impact of eventual elimination of HBV-related cirrhosis, liver cancer and premature death.
Funding:
The work was supported by the U.S. Centers for Disease Control and Prevention.
Footnotes
Conflict of interest: All authors declare that there are no conflicts of interest.
References
- [1].World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec 2009;84(October(40)):405–19. [PubMed] [Google Scholar]
- [2].Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(December(9859)):2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45(October(4)):529–38. [DOI] [PubMed] [Google Scholar]
- [4].World Health Organization. Technical Paper. The growing threats of hepatitis B and C in the Eastern Mediterranean Region: a call for action; 2009. Available at http://www.emro.who.int/surveillance-forecasting-response/strategy/regional-committee-resolutions.html [accessed 01.07.15]. [Google Scholar]
- [5].Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005;34(December(6)):1329–39. [DOI] [PubMed] [Google Scholar]
- [6].Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386(October (10003)):1546–55. [DOI] [PubMed] [Google Scholar]
- [7].World Health Organization. WHO/UNICEF joint reporting form on immunization: WHO vaccine preventable diseases monitoring system; 2015. Available at http://apps.who.int/immunizatiommonitoring/globalsummary [accessed 26.07.15].
- [8].Burton A, Monasch R, Lautenbach B, Gacic-Dobo M, Neill M, Karimov R, et al. WHO and UNICEF estimates of national infant immunization coverage: methodology and processes. Bull World Health Organ 2009;87(July (7)):535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(December(RR-16)):1–31. [PubMed] [Google Scholar]
- [10].World Health Organization Department of Immunization, Vaccines Biologicals. Practices to improve coverage of the hepatitis B birth dose vaccine. Geneva: World Health Organization; 2012. Report No.: WHO/IVB/11.08. [Google Scholar]
- [11].World Health Organization Department of lmmunization, Vaccines Biologicals. Documenting the impact of hepatitis B immunization: best practices for conducting a serosurvey. Geneva: World Health Organization; 2011. Report No.: WHO/IVB/11.08. [Google Scholar]
- [12].Hennessey K, Mendoza-Aldana J, Bayutas B, Lorenzo-Mariano KM, Diorditsa S. Hepatitis B control in the World Health Organization’s Western Pacific Region: targets, strategies, status. Vaccine 2013;31(December(Suppl. 9)):J85–92. [DOI] [PubMed] [Google Scholar]
- [13].Teleb N, Lebo E,Ahmed H, Hossam AR, El Sayed T, Dabbagh A, et al. Progress toward measles elimination – Eastern Mediterranean Region, 2008–2012. MMWR Morb Mortal Wkly Rep 2014;63(June (23)):511–5. [PMC free article] [PubMed] [Google Scholar]
- [14].Smith J, Leke R, Adams A, Tangermann RH. Certification of polio eradication: process and lessons learned. Bull World Health Organ 2004;82(January(1)):24–30. [PMC free article] [PubMed] [Google Scholar]
- [15].Al Awaidy ST, Bawikar SP, Al Busaidy SS, Al Mahrouqi S, Al Baqlani S, Al Obaidani I, et al. Progress toward elimination of hepatitis B virus transmission in Oman: impact of hepatitis B vaccination. Am J Trop Med Hyg 2013;89(October (4)):811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Widaa NM [Thesis] Sero-epidemiology of hepatitis B among children <10 years of age in Khartoum State 2004–2005. University of Khartoum; 2006. Kartoum: n.p. Print. [Google Scholar]
- [17].Mao B, Patel MK, Hennessey K, Duncan RJ, Wannemuehler K, Soeung SC. Prevalence of chronic hepatitis B virus infection after implementation of a hepatitis B vaccination program among children in three provinces in Cambodia. Vaccine 2013;31(September (40)):4459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang L, Li J, Chen H, Li F, Armstrong GL, Nelson C, et al. B vaccination of newborn infants in rural China: evaluation of a village-based, out-of-cold-chain delivery strategy. Bull World Health Organ 2007;85(September(9)):688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Otto BF, Suarnawa IM, Stewart T, Nelson C, Ruff TA, Widjaya A, et al. At-birth immunisation against hepatitis B using a novel pre-filled immunization device stored outside the cold chain. Vaccine 1999;18(October(5–6)):498–502. [DOI] [PubMed] [Google Scholar]
- [20].Hipgrave DB, Tran TN, Huong VM, Dat DT, Nga NT, Long HT, et al. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Trop Med Hyg 2006;74(February (2)):255–60. [PubMed] [Google Scholar]
- [21].Glenton C, Khanna R, Morgan C, Nilsen ES. The effects, safety and acceptability of compact, pre-filled, auto disable injection devices when delivered by lay health workers. Trop Med Int Health 2013;18(August (8)):1002–16. [DOI] [PubMed] [Google Scholar]
- [22].Sutanto A, Suarnawa IM, Nelson CM, Stewart T, Soewarso TI. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ 1999;77(2):119–26. [PMC free article] [PubMed] [Google Scholar]
- [23].Joshi N, Kumar A, Raghu MB, Bhave S, Arulprakash R, Bhusari P, et al. Immunogenicity and safety of hepatitis B vaccine (Shanvac-B) using a novel pre-filled single use injection device Uniject in Indian subjects. Indian J Med Sci 2004;58(November (11)):472–7. [PubMed] [Google Scholar]
- [24].Levin CE, Nelson CM, Widjaya A, Moniaga V, Anwar C. The costs of home delivery of a birth dose of hepatitis B vaccine in a prefilled syringe in Indonesia. Bull World Health Organ 2005;83(June (6)):456–61. [PMC free article] [PubMed] [Google Scholar]
- [25].Obradovic Z, Balta S, Obradovic A, Mesic S. The impact of war on vaccine preventable diseases. Mater Sociomed 2014;26(December (6)):382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cetorelli V. The impact of the Iraq War on neonatal polio immunization coverage: a quasi-experimental study. J Epidemiol Community Health 2015;69(March (3)):226–31. [DOI] [PubMed] [Google Scholar]
- [27].Mashal T, Nakamura K, Kizuki M, Seino K, Takano T. Impact of conflict on infant immunisation coverage in Afghanistan: a countrywide study 2000–2003. lnt J Health Geogr 2007;6(June):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].United Nations Office for the Coordination of Humanitarian Affairs. Syria crisis: regional overview. Available at http://www.unocha.org/syrian-arab-republic/syria-country-profile/about-crisis [accessed 05.07.15].
- [29].Centers for Disease Control and Prevention (CDC). Vaccination services in postwar Iraq May 2003. MMWR Morb Mortal Wkly Rep 2003;52(August (31)):734–5. [PubMed] [Google Scholar]