Antiphospholipid syndrome(APS) is a systemic autoimmune disorder characterized by vascular thrombosis and/or pregnancy morbidity in the presence of persistently elevated antiphospholipid antibodies(aPL) (i.e., lupus anticoagulant[LA], anticardiolipin[aCL] and anti-beta-2 glycoprotein-I[anti-β2GPI] antibodies)[1]. Cognitive dysfunction occurs in aPL-positive patients[2, 3], but a posterior cortical atrophy(PCA)-like syndrome has not yet been described. Primarily caused by Alzheimer’s disease, PCA is a rare, clinico-radiological syndrome characterized by occipito-temporo-parietal degeneration and progressive decline in basic visual, visuoperceptual, visuospatial, and other higher sensory functions[4]. We aimed to describe three patients with APS who presented with clinico-radiologic features of PCA(PCA-APS) and report neuroimaging findings that could help differentiate PCA-APS from true neurodegenerative-PCA.
1. METHODS
Retrospective review of medical records from the Neurodegenerative Research Group (NRG) collected between September 2009 and December 2022 (n=717) was performed for patients with a clinical diagnosis of PCA, including clinico-radiologic features suggestive of PCA[4](n=74) and a diagnosis of APS[1](n=3). All three PCA-APS identified had undergone neurological and neuropsychological evaluation, genetic/metabolic testing, and neuroimaging with MRI and 18F-fluorodeoxyglucose(FDG)-PET. One patient had additional 11C-Pittsburgh compound B (amyloid-β) and 18F-flortaucipir(tau)-PET scans. All had signed written informed consent forms. This study was approved by the Mayo Clinic IRB.
2. RESULTS
2.1. Clinical presentations
Three PCA-APS patients (one woman,two men) had varying age at onset (29-69 years). Detailed information on sign/symptoms and workup are shown in Supplementary Table 1. At onset, patient-1 and patient-2 had progressive visuospatial, visuoperceptual, and other higher cortical visual function deficits consistent with clinical PCA criteria[4]. These included space and object perception deficits, simultanagnosia, environmental agnosia, ideomotor apraxia, dressing apraxia, alexia, and Gerstmann syndrome (acalculia, left-right confusion, finger agnosia, agraphia). Patient-3 manifested atypical visual complaints, including transient vision spells with tunnel and kaleidoscope vision, disorientation, and altered awareness of surroundings. Neuropsychological testing detected mild visuospatial/perceptual deficits. Twelve years later, marked visuospatial/perceptual impairment and dressing apraxia were observed. In all patients, neurological examinations were unremarkable at presentation but later showed variable hyperreflexia, increased muscle tone, Babinski and/or Hoffmann signs. Neuropsychological evaluations at presentation and follow-up showed exclusive or greater impairment of visuospatial/perceptual and constructional abilities. Genetic and metabolic workup and cerebrospinal fluid analyses were negative or non-diagnostic.
Subsequent circumstances prompted testing for a hypercoagulable state and aPLs:patient-1 had acute stroke; patient-2 showed thrombocytopenia with musculoskeletal pains, psoriatic plaques, and renal insufficiency; and patient-3 had prolonged aPTT, transient right-sided weakness during vision/dizzy spells, livedo reticularis, and renal insufficiency. Laboratory criteria for APS were met with elevated titers of aCL (n=3), LA (n=3) and anti-β2GPI (n=2) on two rounds of testing. Clinical criteria for APS were met as patient-1 had unequivocal imaging findings of acute stroke, while a brain and a renal biopsy showed evidence of vascular thrombosis and injury in patient-2 and patient-3, respectively. Patients received anticoagulants or antiplatelets, as appropriate.
2.2. Neuroimaging findings
T1-weighted MRI showed predominant atrophy of bilateral parieto-occipital lobes with patchy sensorimotor and visual cortex involvement (not shown). T2 and FLAIR showed hyperintensities in periventricular, deep, and parasagittal superficial subcortical white matter (Figure 1a). Head and neck MR angiograms showed no abnormalities (not shown). FDG-PET scans showed a distinct pattern of hypometabolism forming parallel lines running in the anteroposterior direction corresponding to watershed areas where the anterior, middle, and/or posterior cerebral arteries converged (Figure 1b). This pattern resembled railroad tracks when seen from the superior and anterior views, hence the moniker “railroad track” sign (Figure 1c). Amyloid-PET and tau-PET scans from patient-1 showed no increased retention.
Figure 1. Imaging findings in PCA-APS.
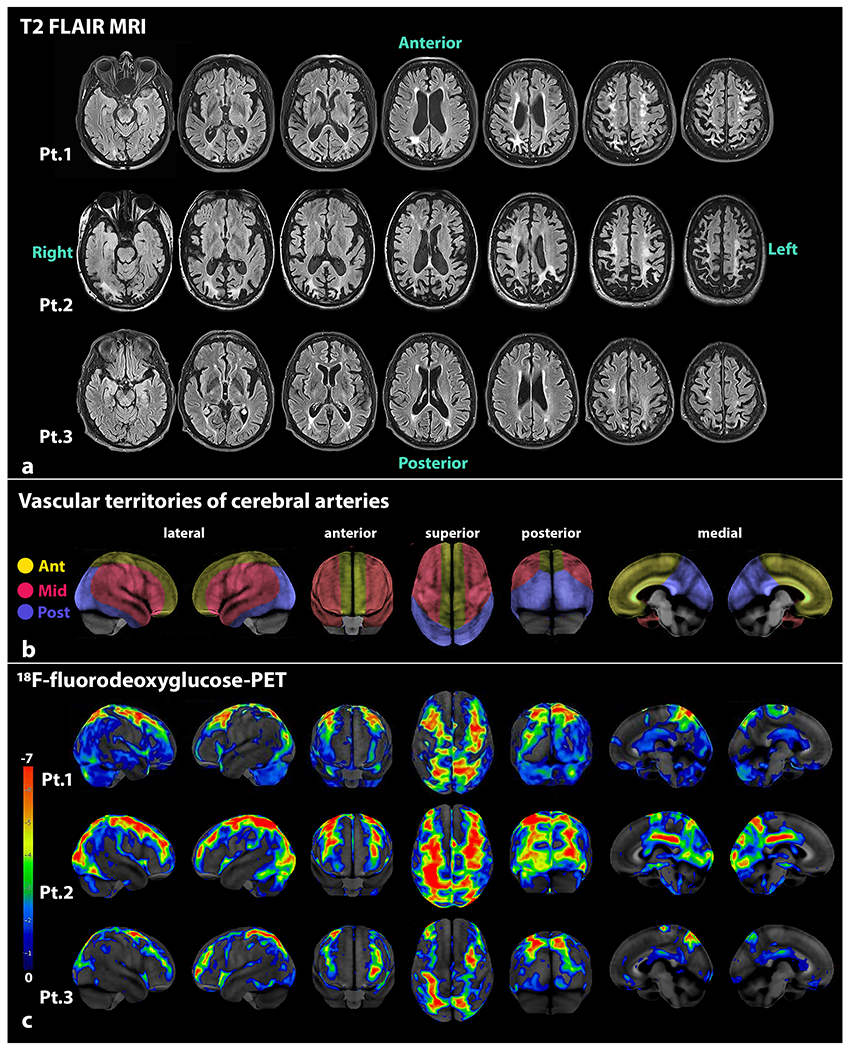
Serial T2 FLAIR MRI supratentorial sequences are shown from the three PCA-APS patients, where asymmetric bilateral atrophy involving the posterior parietal and occipital regions is seen in the top three rows (a). The frontal areas show relatively less atrophy. Multiple white matter lesions of variable sizes are seen in periventricular, deep, and subcortical white matter in watershed areas. The vascular territories supplied by the three main cerebral arteries (depicted in yellow for the anterior cerebral arteries, red for middle cerebral arteries, and blue for posterior cerebral arteries) are shown in the middle row (b). The pattern of glucose hypometabolism on 18F-fluorodeoxyglucose PET scans from the three PCA-APS patients are shown in the lower three rows (c). The PET images of each patient were processed with CortexID Suite. The “railroad track” sign is best appreciated in the superior views of the brain, where marked hypometabolism is observed in watershed regions where the anterior, middle, and posterior cerebral arteries converge. The posterior views of the brain also show the considerable hypometabolism in the posterior parietal and occipital lobes, with some patchy involvement of the visual cortices. Abbreviations: Ant = anterior cerebral artery; APS = antiphospholipid syndrome; FLAIR MRI = fluid attenuated inversion recovery magnetic resonance imaging; Mid = middle cerebral artery; PCA = posterior cortical atrophy; Post = posterior cerebral artery; Pt = patient
3. DISCUSSION
We report three patients with clinicoradiologic features suggestive of neurodegenerative-type PCA, who posed a diagnostic dilemma as some MRI and FDG-PET abnormalities did not follow the typical recognized patterns of focal neurodegenerative diseases, but instead showed white matter T2 hyperintensities and hypometabolism in watershed areas. Ultimately, all patients were diagnosed with APS.
Cognitive dysfunction exists in primary-APS[2, 3], secondary-APS[5], and otherwise normal aPL-positive elderly[6]. A subcortical pattern with impaired attention, concentration, visuomotor speed, and executive functioning is mostly reported, implicating damage to fronto-subcortical pathways[2]. Contrarily, our PCA-APS patients manifested apraxia, agnosia, and higher visuospatial/visuoconstruction dysfunction, implying damage to cortical association areas and consistent with the severe posterior parieto-occipital cortex atrophy. While visuomotor/spatial problems had been previously described[2, 5, 7], to our knowledge, a PCA-like syndrome has not been reported. LA and aCL are associated with ischemic stroke onset before age 50, especially in female patients[2]. They are also independently linked to cognitive impairment[2, 3, 6, 7]. A silent cerebral damage possibly ensues allowing cognitive dysfunction to occur independent of stroke history[6]. Only our female triple-positive patient developed a stroke and the two male patients developed cognitive problems before age 50.
In APS, MRI findings include single/multiple infarcts, hemorrhages, cortical atrophy, and white matter lesions (WMLs)[3]. WMLs can represent thrombotic, inflammatory, or demyelinating processes[3]. They are common in deep subcortical and periventricular regions and supposedly cause the attentional and executive impairments, resembling multi-infarct dementia[8]. We additionally found WMLs in parasagittal subcortical areas in conjunction with the watershed hypometabolism. A previous study had found no associations between FDG-PET hypometabolism and aPLs[9]. We hypothesize a possible mechanism whereby repetitive, partial yet prolonged hypoxic-ischemic insults affect the watershed regions, particularly the triple watershed area located at the junction of the three major cerebral arteries corresponding to the parieto-occipital lobes. Eventually, chronic hypoperfusion leads to glucose hypometabolism and degeneration/atrophy of the parieto-occipital regions. This consequently triggers the PCA-like symptoms. Thrombo-occlusions of small or medium-sized vessels, especially of the middle and posterior cerebral arteries, are common in aPL-associated hypercoagulable state[10]. Indeed, patient-2 had a brain biopsy revealing remote cortical microinfarcts involving multiple leptomeningeal vessels. Nevertheless, increased vascular tone and endothelial proliferation with intimal hyperplasia due to direct endothelial injury are other possible causes[8]. Watershed hypometabolism in PCA patients should prompt investigation for a hypercoagulable state and aPLs.
A strength of this study is the specificity of the “railroad track” sign for PCA-APS, which was not detected in the hundreds of other neurodegenerative disease patients and healthy controls from our database. While limited by the small numbers, our PCA-APS patients had been identified from a large cohort of PCA patients diagnosed and followed by leading experts.
4. CONCLUSION
APS can present as PCA before the onset of stroke or other non-criteria manifestations of APS. Distinct MRI hyperintensities and FDG-PET hypometabolism in watershed areas can help differentiate PCA-APS from true PCA, thus favoring early diagnosis and treatment.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank our patients and their families for their participation.
Funding/Support
This study was funded by the National Institutes of Health grant R01-AG50603 (PI: J.L.W.). We thank AVID Radiopharmaceuticals, Inc., for their support in supplying the AV-1451 precursor, chemistry production advice and oversight, and FDA regulatory cross-filing permission and documentation needed for the flortaucipir-PET imaging.
Role of Funder/Sponsor
The National Institutes of Health and AVID Radiopharmaceuticals, Inc. had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest Disclosures
Drs. Carlos, Crum, Machulda and Ms. Pham report no disclosures. Drs. Graff-Radford, Whitwell and Josephs are funded by the NIH. Dr. Lowe serves as a consultant for Bayer Schering Pharma, Philips Molecular Imaging, Piramal Imaging and GE Healthcare, Siemens Molecular Imaging and AVID Radiopharmaceuticals related to PET imaging.
Footnotes
Conflicts of interest: We have no conflicts of interest pertinent to this manuscript
REFERENCES:
- [1].Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, PG DEG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA, International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS), Journal of thrombosis and haemostasis : JTH 4(2) (2006) 295–306. [DOI] [PubMed] [Google Scholar]
- [2].Jacobson MW, Rapport LJ, Keenan PA, Coleman RD, Tietjen GE, Neuropsychological deficits associated with antiphospholipid antibodies, Journal of clinical and experimental neuropsychology 21(2) (1999) 251–64. [DOI] [PubMed] [Google Scholar]
- [3].Tektonidou MG, Varsou N, Kotoulas G, Antoniou A, Moutsopoulos HM, Cognitive deficits in patients with antiphospholipid syndrome: association with clinical, laboratory, and brain magnetic resonance imaging findings, Archives of internal medicine 166(20) (2006) 2278–84. [DOI] [PubMed] [Google Scholar]
- [4].Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, Dickerson BC, Vandenberghe R, Ahmed S, Bak TH, Boeve BF, Butler C, Cappa SF, Ceccaldi M, de Souza LC, Dubois B, Felician O, Galasko D, Graff-Radford J, Graff-Radford NR, Hof PR, Krolak-Salmon P, Lehmann M, Magnin E, Mendez MF, Nestor PJ, Onyike CU, Pelak VS, Pijnenburg Y, Primativo S, Rossor MN, Ryan NS, Scheltens P, Shakespeare TJ, Suárez González A, Tang-Wai DF, Yong KXX, Carrillo M, Fox NC, Consensus classification of posterior cortical atrophy, Alzheimer’s & dementia : the journal of the Alzheimer’s Association 13(8) (2017) 870–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Conti F, Alessandri C, Perricone C, Scrivo R, Rezai S, Ceccarelli F, Spinelli FR, Ortona E, Marianetti M, Mina C, Valesini G, Neurocognitive dysfunction in systemic lupus erythematosus: association with antiphospholipid antibodies, disease activity and chronic damage, PloS one 7(3) (2012) e33824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schmidt R, Auer-Grumbach P, Fazekas F, Offenbacher H, Kapeller P, Anticardiolipin antibodies in normal subjects. Neuropsychological correlates and MRI findings, Stroke 26(5) (1995) 749–54. [DOI] [PubMed] [Google Scholar]
- [7].Maeshima E, Yamada Y, Yukawa S, Nomoto H, Higher cortical dysfunction, antiphospholipid antibodies and neuroradiological examinations in systemic lupus erythematosus, Intern Med 31(10) (1992) 1169–74. [DOI] [PubMed] [Google Scholar]
- [8].Fleetwood T, Cantello R, Comi C, Antiphospholipid Syndrome and the Neurologist: From Pathogenesis to Therapy, Frontiers in neurology 9 (2018) 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kao CH, Lan JL, ChangLai SP, Liao KK, Yen RF, Chieng PU, The role of FDG-PET, HMPAO-SPET and MRI in the detection of brain involvement in patients with systemic lupus erythematosus, Eur J Nucl Med 26(2) (1999) 129–34. [DOI] [PubMed] [Google Scholar]
- [10].Jacobson MW, Abraham E, Tietjen GE, A review of neurological sequelae and cognitive deficits associated with antiphospholipid antibodies, J Stroke Cerebrovasc Dis 6(2) (1996) 61–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


