Abstract
Introduction
Recent studies have proved the diverse roles of miRs in different cancer-related processes. This study was undertaken to determine the therapeutic implications of miR-325-3p in breast cancer.
Material and methods
Expression analysis was carried out by qRT-PCR. Transfections were performed by Lipofectamine 2000 reagent. MTT assay was used for cell viability. Transwell assays were used for cell migration and invasion. Western blot analysis was used for protein expression analysis.
Results
Gene expression analysis revealed miR-325 to be significantly suppressed in breast cancer tissues and cell lines. Nonetheless, ectopic expression of miR-325 resulted in suppression of the growth and colony development potential of the SK-BR-3 and CAMA-1 cells. Transwell assays showed that miR-325 overexpression also resulted in the decline of the migration and invasion of the SK-BR-3 and CAMA-1 cells. Bioinformatic analysis showed that miR-325 targets lipocalin 15 (LNC15) in breast cancer cells. LNC15 was also overexpressed in the breast cancer tissues and cell lines. However, overexpression of miR-325 caused a significant decline in the LNC15 expression in SK-BR-3 cells. Additionally, silencing of LNC15 resulted in inhibition of the growth, migration and invasion of the SK-BR-3 cells. Rescue assay showed that overexpression of LNC15 could promote the growth, migration and invasion of the miR-325 overexpressing effects.
Conclusions
Taken together, the evidence shows that miR-325 acts as a tumor suppressor in breast cancer and may be used in the treatment of breast cancer.
Keywords: breast cancer, lipocalin, microRNA-325, invasion, proliferation
Introduction
The microRNAs (miRs) control a number of cellular processes in humans by regulating the expression of target genes [1]. Studies have pointed towards the role of miRs in various diseases which include, among others, Alzheimer’s disease, diabetes and cancer [2]. The first concrete evidence regarding the involvement of a miR in cancer came from a study which showed the involvement of miR-15 and miR-16 in lymphocytic leukemia [3]. Since then, a number of miRs have been shown to be involved in cancer-related processes such as proliferation and metastasis [4]. miR-325-3p (hereinafter referred to as miR-325) is one of the least studied miRs. However, a recent study has proved the involvement of miR-325 in the development of non-small lung carcinoma [5]. The present study was designed to investigate the role of miR-325 in breast cancer. Breast cancer causes a significant number of human deaths throughout the world. According to recent estimates, 1.3 million new breast cancer cases and 0.4 million deaths are attributed to breast cancer [6]. Studies have also shown an increase in the breast cancer incidence over the last few decades. From 2008 to 2012 around an 18% increase was observed for the breast cancer incidence as well as mortality [7]. In the US, 1 in every 8 women will develop breast cancer during her life [8]. It is believed that by 2050 there will be 3.2 million new cases of breast cancer annually [9]. All these statistics indicate that there is a pressing need to develop novel therapeutic targets and treatment regimes for breast cancer. Herein, we for the first time explored the therapeutic potential of miR-325 in breast cancer. The results showed miR-325 to be significantly suppressed in breast cancer tissues and cell lines. The overexpression of miR-325 in breast cancer cells resulted in a remarkable decline of the proliferation rate and colony development potential of breast cancer cells. Moreover, miR-325 also caused a decrease in the migration and invasion of breast cancer cells. Bioinformatic analysis revealed that miR-325 targets lipocalin 15 in breast cancer cells. Recently carried out studies have also proved the potential of lipocalins as a therapeutic target for breast cancer treatment [10]. Taken together, the evidence indicates that miR-325 may prove to be a novel therapeutic target for breast cancer treatment.
Material and methods
Breast cancer tissues, cell lines and culture conditions
Breast cancer tissue specimens and normal adjacent tissues of 15 breast cancer patients who underwent surgical resection in the Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Dadong District, Shenyang, Liaoning 110042, P.R. China from March 2017 to December 2018 were included. Patients were aged 33–75 years with an average age of 45.25 years. The breast cancer cell lines (MDA-MB-231, MDA-MB-436, SK-BR-3, and CAMA-1) and a normal breast cell line (MB 157) were procured from Type Culture Collection of Chinese Academy of Sciences, Shanghai, China. The cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 100 μg/ml streptomycin and 100 U/ml penicillin and a humidified atmosphere containing 5% CO2.
cDNA synthesis and qRT-PCR
The TRIzol reagent (Invitrogen/Life Technologies, Carlsbad, CA, USA) was used for the extraction of RNA from the tissues and cell lines. Subsequently, the total RNA was reverse-transcribed by a RevertAid cDNA synthesis kit (Fermentas). Thereafter quantitative RT-PCR was performed using SYBR Green master mix (Applied Biosystems; Foster City, Calif, USA) on an ABI 7900HT system. The reaction mixture consisted of 20 μl containing 1.5 mM MgCl2, 2.5 units of Taq DNA Polymerase, 200 μM dNTP, 0.2 μM of each primer and 0.5 μg of DNA. The cycling conditions were as follows: 95°C for 20 s, followed by 40 cycles of 95°C for 15 s, and 58°C for 1 min. All reactions were carried out in triplicate and the gene expression was determined by the 2–ΔΔCt method.
Cell transfection
The miR-325 mimics and miR-NC were ordered from RiboBio (Guangzhou, China). Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) was then used to perform the transfections in accordance with the manufacturer’s guidelines. As the SK-BR-3 and CAMA-1 cells reached 80% confluence, the appropriate concentration of miR-325 mimics or miR-NC was transfected into these cells.
Cell proliferation assay
The SK-BR-3 and CAMA-1 cells were seeded in a 96-well tissue-culture plates with approximately 2500 cells/well. The viability of the cells was evaluated at different time intervals by Vybrant MTT Cell Proliferation Assay (Invitrogen, USA) as per the manufacturer’s guidelines. Finally, the optical density was measured at 570 nm using a spectrophotometer.
Cell migration and invasion assay
Transwell chambers with Matrigel were employed to monitor invasion of the SK-BR-3 and CAMA-1. In brief, the cells were transfected with appropriate constructs and 48 h after transfection, the cells were harvested and suspended in fresh media. While 200 μl of the cell suspension containing approximately 5 × 104 cells was placed in the upper compartment, 500 μl of fresh media was placed in the lower compartment. After 24 h, cells present in the upper compartment were removed by swabbing while cells that invaded to the lower surface were fixed and then subsequently stained with 0.05% crystal violet. Finally, ten random fields were selected to determine the invasion under the light microscope. A similar procedure was used for cell migration except that Matrigel was not used.
Immunohistochemical analysis
4-μm sections cut from the paraffin blocks were mounted on silane-coated glass slides. This was followed by deparaffinization and soaking for around 25 min in H2O2/methanol (0.3%) at room temperature. The sections were subsequently heated in hot water and Immunosaver (Nishin EM, Co., Ltd., Tokyo, Japan) at 98°C for 50 min. Non-specific binding sites were blocked by incubating with Protein Block serum-free for 30 min. A mouse monoclonal anti-LNC1 antibody was applied at a dilution of 1 : 100 for 24 h at 4°C. The primary antibody was visualized using the Histofine Simple Stain PO (M) kit (Nichirei, Tokyo, Japan), as per the manufacturer’s guidelines. Chromogen 3,3′-diaminobenzidine tetrahydrochloride containing 0.005% H2O2 in 50 mM ammonium acetate-citrate acid buffer (pH 6.0) was applied as 0.02% solution. The sections were lightly counterstained with Mayer’s hematoxylin and mounted.
Western blotting
The breast cancer tissues and cell lines were lysed and the protein concentration in each sample was measured by the Bradford assy. Equal concentrations of the proteins from each sample were loaded on 10% SDS polyacrylamide gels (SDS-PAGE) and then followed by shifting to polyvinylidene fluoride membranes. Blocking of the membrane was then performed by fat-free milk (5%) in TBST. This was followed by incubation with a primary antibody for 24 h at 4°C. Subsequently, a secondary antibody was added at 25°C for about 2 h. The bands of interest were finally observed by chemiluminescence.
Statistical analysis
The experiments were carried out in three biological replicates. The data are presented as the mean of the replicates ± SD. The statistical analysis was carried out by Student’s t test or one-way ANOVA followed by Tukey’s test. P < 0.05 was considered as statistically significant.
Results
miR-325 is aberrantly downregulated in breast cancer
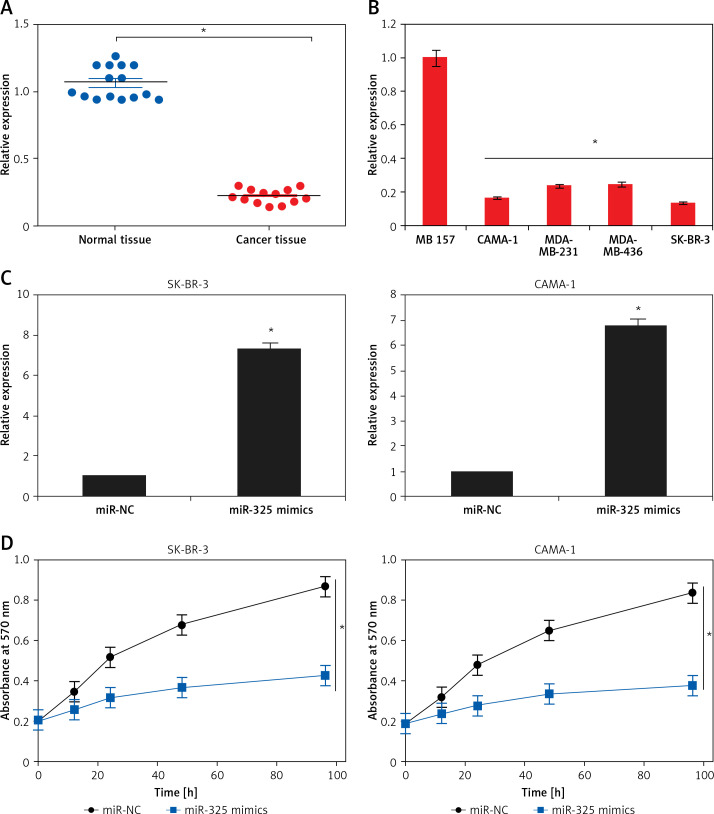
The gene expression analysis of miR-325 carried out by qRT-PCR revealed that in all the breast cancer tissues, miR-325 was significantly downregulated. The downregulation was found to be up to 7.5-fold relative to the normal tissues (Figure 1 A). Moreover, the expression of miR-325 was also downregulated in all the breast cancer cell lines by up to 6.5-fold relative to the normal tissues (Figure 1 B).
Figure 1.
A – Expression of miR-325 in normal and breast cancer tissues. B – Expression of miR-325 in normal MB 157 and different breast cancer cell lines. C – Expression of miR-325 in miR-NC and miR-325 mimic transfected SK-BR-3 and CAMA-1 cells. D – Cell viability of miR-NC and miR-325 mimic transfected SK-BR-3 and CAMA-1 cells. The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05)
miR-325 inhibits proliferation of breast cancer cells
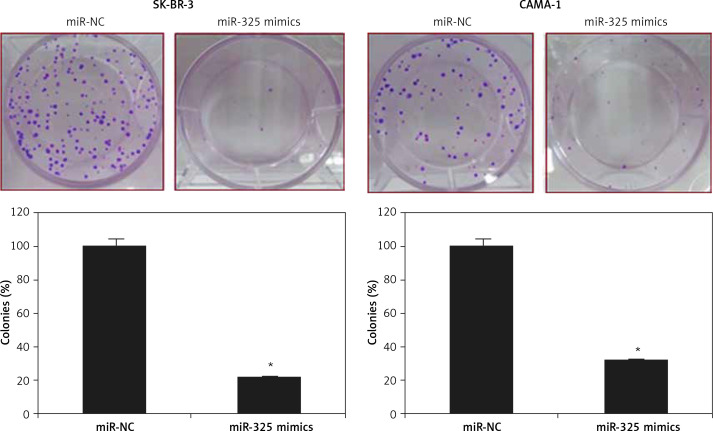
The SK-BR-3 and CAMA-1 breast cancer cell lines were transfected with miR-325 mimics and miR-NC and the overexpression of miR-325 was confirmed by qRT-PCR. We observed that transfection of miR-325 mimics caused up to 7.3-fold upregulation of miR-325 in SK-BR-3 and CAMA-1 cells (Figure 1 C). The miR-325 overexpressing breast cancer cells were then subjected to MTT assay and the viability was measured at different time intervals. We observed that miR-325 overexpression in the SK-BR-3 and CAMA-1 cells caused a time-dependent decrease in the viability as compared to the miR-NC transfected cells (Figure 1 D). It was also observed that miR-325 overexpression resulted in inhibition of colony formation of SK-BR-3 and CAMA-1 cells (Figure 1 E).
miR-325 suppresses migration and invasion of breast cancer cells
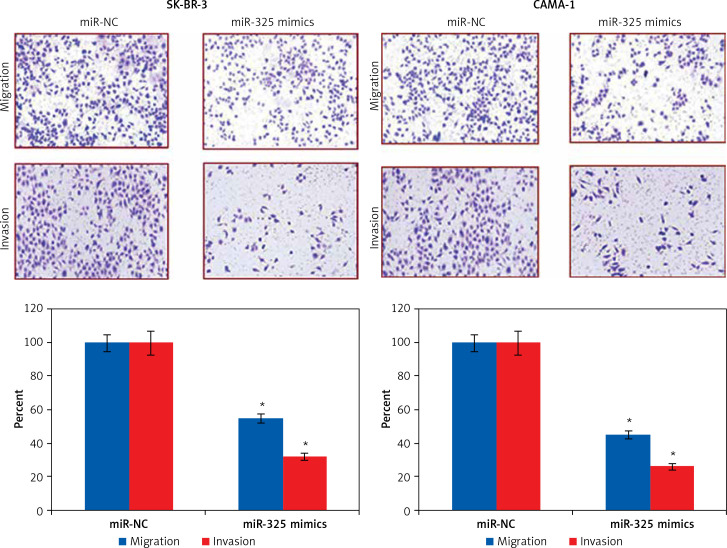
The transwell assays showed that miR-325 overexpression suppressed the migration of both the SK-BR-3 and CAMA-1 cells. The migration was inhibited by 47% and 58% in miR-325 mimic transfected SK-BR-3 and CAMA-1 cells as compared to around 100% in the miR-NC transfected SK-BR-3 and CAMA-1 cells respectively (Figure 2). The transwell assays also showed that the invasion of the breast cancer cells was also suppressed. The invasion of the SK-BR-3 and CAMA-1 cells was suppressed by 67% and 74% relative to the controls respectively (Figure 3).
Figure 2.
Colony formation of miR-NC and miR-325 mimic transfected SK-BR-3 and CAMA-1 cells
The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05).
Figure 3.
Transwell assay showing migration and invasion of miR-NC and miR-325 mimic transfected SK-BR-3 and CAMA-1 cells
The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05).
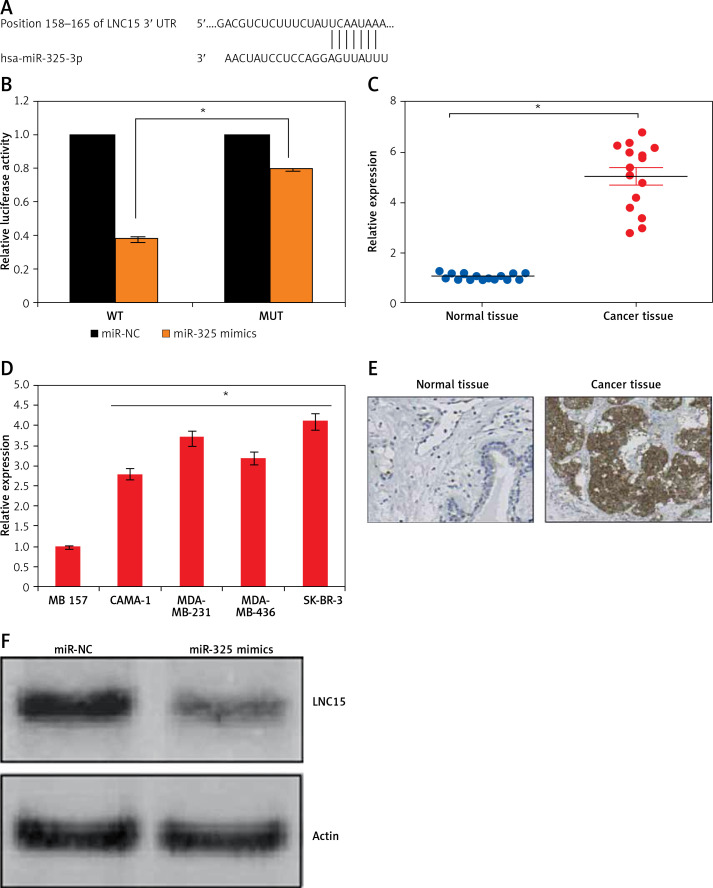
miR-325 targets lipocalin 15 in breast cancer cells
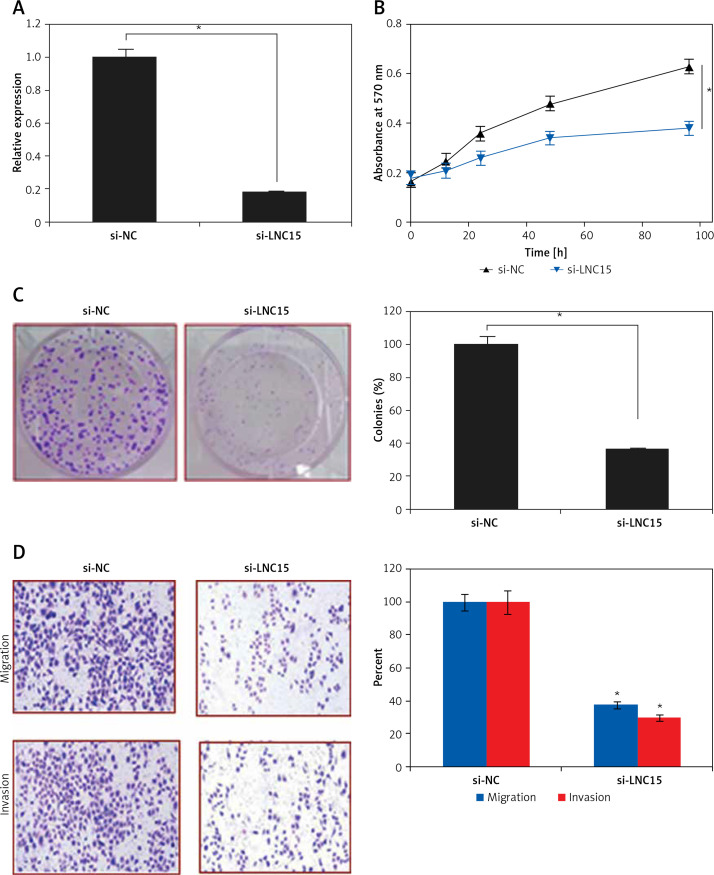
Bioinformatic approaches such as TargetScan were employed to identify the targets of miR-325. TargetScan revealed several targets of miR-325 and most of them have been studied in different cancer types. However, we observed that LNC15 has not been studied as the target of miR-325 (Figure 4 A). For this reason, LNC15 was selected as the target of miR-325 for investigation in the present study. Next, the dual luciferase assay also confirmed the interaction between miR-325 and LNC15 (Figure 4 B). We also examined the expression of LNC15 in all the breast cancer tissues and the cell lines and the qRT-PCR revealed LNC15 to be aberrantly upregulated in both the breast cancer tissues and cell lines (Figures 4 C, D). Additionally, immunohistochemical analysis showed higher expression of LNC15 in breast cancer tissue (Figure 4 E). However, miR-325 overexpression in the MDA-MBA-325 cells caused post-transcriptional suppression of LNC15 (Figure 4 F). We also sought to ascertain whether silencing of LNC15 caused similar effects on the SK-BR-3 cells as that of miR-325. We found that LNC15 silencing caused a remarkable decrease in the proliferation of the SK-BR-3 cells (Figures 5 A, B). The colony formation of the SK-BR-3 cells was also inhibited by 58% and 68% relative to the control upon silencing of LNC15 (Figure 5 C). The transwell assays showed that the migration and invasion of SK-BR-3 cells was inhibited by 68 and 73% respectively.
Figure 4.
A – TargetScan analysis showing LCN15 as the target of miR-325. B – Dual luciferase assay. C – Expression of LNC15 in breast cancer and adjacent normal tissues. Expression of LNC15 in normal and breast cancer cell lines (D) and immunohistochemical analysis (E) showing expression of LNC15 in normal and cancer tissue. F – Western blots showing expression of LNC15 in miR-NC and miR-325 mimic transfected SK-BR-3 cells
The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05).
Figure 5.
A – Expression of miR-325 in si-NC and si-LCN15 transfected SK-BR-3 cells. B – Cell viability of si-NC and si-LCN15 transfected SK-BR-3 cells. C – Colony formation assay of si-NC and si-LCN15 transfected SK-BR-3 cells. D – cell migration and invasion of si-NC and si-LCN15 transfected SK-BR-3 cells
The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05).
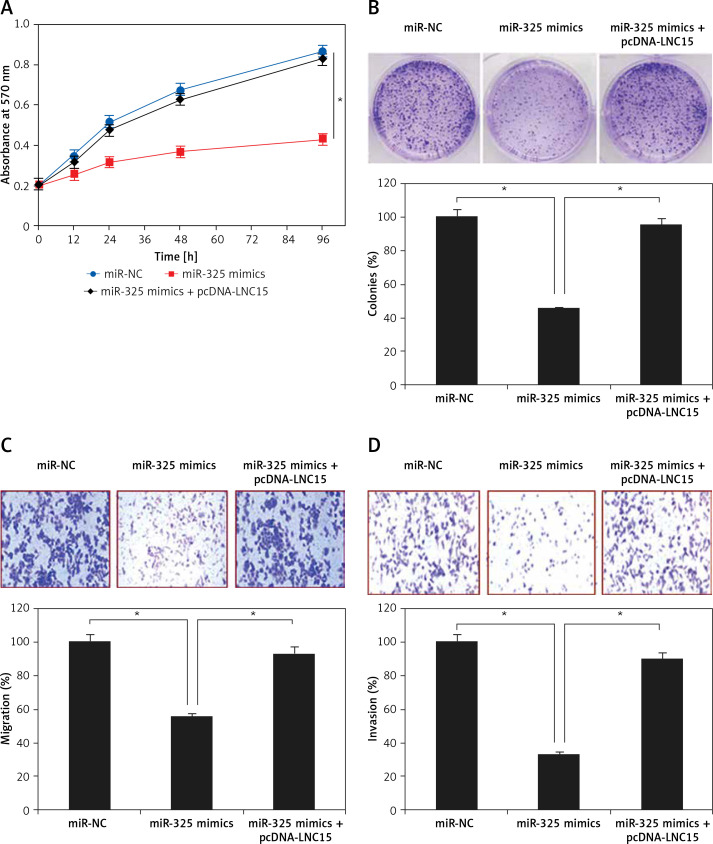
Lipocalin 15 avoids the tumor suppressive effects of miR-325
The effects of LNC15 overexpression on the proliferation and colony formation of the SK-BR-3 breast cancer cells overexpressing miR-325 were also investigated. We observed that overexpression of LNC15 in the SK-BR-3 breast cancer cells overexpressing miR-325 promoted their proliferation and colony formation, migration and invasion, thereby avoiding the tumor suppressive effects of miR-325 (Figures 6 A–D).
Figure 6.
Effect of LCN15 overexpression on proliferation (A), colony formation (B), migration (C) and invasion (D) of miR-325 mimic transfected SK-BR-3 cells
The experiments were performed in triplicate and expressed as mean ± SD (*p < 0.05).
Discussion
Breast cancer causes tremendous mortality and its treatment is mainly hampered by the unavailability of therapeutic targets, lack of efficient chemotherapy, emergence of drug resistance and frequent relapses [11]. Herein the therapeutic implications of miR-325 have been explored in breast cancer. The gene expression analysis revealed that miR-325 was significantly suppressed in breast cancer tissues and cell lines. The expression of miR-325 has also been shown to be downregulated in lung cancer and hepatocellular carcinoma [6]. To decipher the role of miR-325 in breast cancer, it was overexpressed in CAMA-1 and SK-BR-3 cells. miR-325 overexpression caused a remarkable decrease in breast cancer cell proliferation and colony formation suggestive of the tumor suppressive role of miR-325 in breast cancer. Previous studies have also reported the tumor suppressive roles of several miRs. For example, miR-466 has been reported to inhibit the proliferation of prostate cancer [12]. miR-124 has been shown to suppress the growth of colorectal cancer cells [13]. Similarly, miR-22 has been found to suppress the growth of gastric cancer cells [14]. Next the effects of miR-325 overexpression were also examined on the migration and invasion of breast cancer cells by transwell assays. The results revealed that miR-325 overexpression causes a decrease in both migration and invasion of breast cancer cells. These observations are in agreement with previous studies wherein miRs have been shown to suppress the migration and invasion of cancer cells; for example, miR-101 was shown to decrease the migration and invasion of glioblastoma cells [15]. In another study, miR-340 was found to inhibit migration and invasion of breast cancer cells [16]. Studies have shown that miRs exert their effects by regulating the expression of the target genes [17]. Herein we found that miR-325 targets lipocalin 15 (LCN15). LCN15 was found to be significantly upregulated in the breast cancer tissues and silencing of LNC15 also suppresses the proliferation, migration and invasion of the breast cancer cells. Nonetheless, overexpression of LNC15 in miR-325 mimic transfected cells could reverse the effects of LNC15. These observations are in agreement with previous studies wherein lipocalins such as lipocalin 2 were shown to promote metastasis of breast cancer [18]. In yet another study, lipocalin 2 was shown to promote invasion of cervical cancer cells [19]. Overexpression of lipocalins has also been shown to suppress migration and invasion of prostate cancer [20].
In conclusion, miR-325 acts as a tumor suppressor in breast cancer. It also inhibits migration and invasion of breast cancer cells by targeting LNC15. Taken together, the findings of the present study point towards a novel therapeutic target in breast cancer.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer – a brief overview. Adv Biol Regul 2015; 57: 1-9. [DOI] [PubMed] [Google Scholar]
- 2.Chan B, Manley J, Lee J, Singh SR. The emerging roles of microRNAs in cancer metabolism. Cancer Letters 2015; 356: 301-8. [DOI] [PubMed] [Google Scholar]
- 3.Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 2015; 15: 321-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017; 16: 203-22. [DOI] [PubMed] [Google Scholar]
- 5.Yao S, Zhao T, Jin H. Expression of MicroRNA-325-3p and its potential functions by targeting HMGB1 in non-small cell lung cancer. Biomed Pharmacother 2015; 70: 72-9. [DOI] [PubMed] [Google Scholar]
- 6.Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast cancer: epidemiology and etiology. Cell Biochem Biophys 2015; 72: 333-8. [DOI] [PubMed] [Google Scholar]
- 7.McPherson K, Steel C, Dixon JM. Breast cancer – epidemiology, risk factors, and genetics. BMJ 2000; 321: 624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koczkodaj P, Sulkowska U, Gotlib J, Mańczuk M. Breast cancer mortality trends in Europe among women in perimenopausal and postmenopausal age (45+). Arch Med Sci DOI: 10.5114/aoms.2019.85198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast cancer: epidemiology and etiology. Cell Biochem Biophys 2015; 72: 333-8. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Bielenberg DR, Rodig SJ, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci 2009; 106: 3913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast cancer: epidemiology and etiology. Cell Biochem Biophys 2015; 72: 333-8. [DOI] [PubMed] [Google Scholar]
- 12.Colden M, Dar AA, Saini S, et al. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis 2017; 8: e2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taniguchi K, Sugito N, Kumazaki M, et al. MicroRNA-124 inhibits cancer cell growth through PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Letters 2015; 363: 17-27. [DOI] [PubMed] [Google Scholar]
- 14.Zuo QF, Cao LY, Yu T, et al. MicroRNA-22 inhibits tumor growth and metastasis in gastric cancer by directly targeting MMP14 and Snail. Cell Death Dis 2015; 6: e2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu N, Zhang L, Wang Z, et al. MicroRNA-101 inhibits proliferation, migration and invasion of human glioblastoma by targeting SOX9. Oncotarget 2017; 8: 19244-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadi-Yeganeh S, Paryan M, Arefian E, et al. MicroRNA-340 inhibits the migration, invasion, and metastasis of breast cancer cells by targeting Wnt pathway. Tumor Biol 2016; 37: 8993-9000. [DOI] [PubMed] [Google Scholar]
- 17.Acunzo M, Croce CM. MicroRNA in cancer and cachexia – a mini-review. J Infect Dis 2015; 212 (Suppl 1): S74-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauvois B, Susin S. Revisiting neutrophil gelatinase-associated lipocalin (NGAL) in cancer: saint or sinner? Cancers 2018; 10: E336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung IH, Wu TI, Liao CJ, et al. Overexpression of lipocalin 2 in human cervical cancer enhances tumor invasion. Oncotarget 2016; 7: 11113-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding G, Fang J, Tong S, et al. Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. Prostate 2015; 75: 957-68. [DOI] [PubMed] [Google Scholar]