Abstract
Non-alcoholic fatty liver disease (NAFLD), metabolic syndrome (MetS), and type 2 diabetes (T2DM) are metabolic disorders that belong to a highly prevalent disease cluster with a significant impact on public health worldwide. MetS is a complex condition characterized by metabolism perturbations that include glucose intolerance, insulin resistance, dyslipidaemia, associated pro-inflammatory state, and arterial hypertension. Because the components of MetS commonly co-occur, the management of these disorders cannot be considered separate issues. Thus NAFLD, recognized as a hepatic manifestation of MetS, is frequently associated with T2DM. This review analyses the underlying connections between these diseases and the risks associated with their co-occurrence. The effective management of NAFLD associated with MetS and T2DM involves an early diagnosis and optimal treatment of each condition leading to improvement in glycaemic and lipid regulation, liver steatosis, and arterial hypertension. The net effect of such treatment is the prevention of atherosclerotic cardiovascular diseases and liver fibrosis.
Keywords: non-alcoholic fatty liver disease, metabolic syndrome, type 2 diabetes mellitus, management
Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized by excessive accumulation of lipids in the liver defining the presence of steatosis in > 5% hepatocytes according to the histological findings, i.e. > 5.6% fat content measured by proton magnetic resonance spectroscopy (1H-MRS) [1]. To establish the diagnosis of NAFLD, it is necessary to exclude other possible causes of liver fat deposition, such as alcohol consumption (> 20 g daily, or > 14 drinks per week in female subjects; 30 g daily, or > 21 drinks per week in male subjects), other chronic liver diseases (viral, autoimmune, infiltrative), and the use of different medications that favour liver steatosis (amiodarone, corticosteroids, cytotoxic agents, members of highly-active antiretroviral therapy, tamoxifen, and numerous other drugs) [1–4]. Fatty liver is presented as NAFLD and/or non-alcoholic steatohepatitis (NASH) as 2 separate entities. In contrast to NAFLD, NASH may further progress to fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [1]. Thus, the fatty liver may manifest a diverse histological spectrum ranging from simple steatosis to inflammation and fibrosis, which may cause life-threatening liver and systemic complications [5, 6].
Metabolic syndrome (MetS) represents a group of interrelated risk factors that favour the emergence and development of atherosclerotic cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) [7, 8]. These factors include perturbation in glucose metabolism (impaired fasting glycaemia (IFG), impaired glucose tolerance (IGT), or T2DM), arterial hypertension, dyslipidaemia (hypertriglyceridaemia and/or decreased level of high-density lipoproteins (HDL)), and central type obesity [9, 10]. The prevalence of MetS is 10–84%, depending on geographic region, rural/urban surroundings, demographic characteristics of a population, and criteria for diagnosis of MetS [11]. Age and sex also influence the prevalence of MetS – an increase in prevalence with age and in the female sex [12, 13]. MetS has become one of the leading public health problems due to the continuous increase in the number of obese individuals [14]. The significance of MetS is more evident through higher morbidity and mortality rates of progressive atherosclerosis diseases accelerated by pro-inflammatory and pro-coagulant components of MetS compared to individuals without MetS [15–17]. Additionally, the presence of NAFLD in lean subjects becomes a diagnostic and treatment challenge and emphasizes the significance of visceral over central obesity [18].
The pathophysiological mechanism that may connect most of the above-described conditions is insulin resistance (IR) [9]. IR is a state in which the normal concentration of insulin is not sufficient to provide the expected response of peripheral tissues, such as the liver, fat, and muscle tissues, as the most important target organs for the action of insulin. Consequently, increased secretion of insulin occurs to overcome the state of hyperglycaemia. Hyperinsulinaemia is a transitory state, and if the stimulus for insulin secretion persists, pancreatic β cells cannot secrete the amount of insulin sufficient for overcoming hyperglycaemia, leading to IGT or T2DM [9, 19]. MetS and NAFLD/NASH are associated with an increased risk of CVD and T2DM [20, 21].
The presented metabolic triad are among the most frequent noncommunicable diseases met today. The health and economic burden of such a disease is extreme. Tailoring the best treatment approach is the mainstay of modern and effective management. By revealing novel agents, more effective regimens arise to manage any experienced component of the metabolic cluster. The pharmacological management of the metabolic triad represents a very active field of interactive research. Mono or combined therapy of novel agents, including insulin treatment and older agents (statins and metformin), needs to be tailored individually.
We review the recent literature data regarding the link between NAFLD, MetS, and T2DM and current treatment options for patients with this pathological condition.
NAFLD in general
The prevalence of NAFLD worldwide is estimated to be 23–25%, with the projection that the incidence will increase in the coming decades [22–25]. The burden of NAFLD differs globally, from the highest prevalence of 32% in the Middle East and 30% in South America, over 24% in North America and Europe, to the lowest prevalence of 13% in Africa [3, 26]. In addition, the prevalence of NASH was confirmed in 20% of subjects with NAFLD [25, 27]. However, there are some inconsistencies between epidemiological studies, such as NAFLD diagnostic (ultrasound-based or serum markers), the number of extensive epidemiological studies from developing countries, etc. Despite some discrepancies between the local and global prevalence of NAFLD, the disease burden is higher in subjects with elevated body mass index (BMI) and among patients with T2DM [23, 24].
The origin of NAFLD is a complex and incompletely defined process still in the realm of hypothesis. Firstly, the pathogenesis of NAFLD was explained as a two-hit hypothesis [28]. The first hit applies to hepatic lipid accumulation and IR, while the second hit refers to inflammation, mitochondrial dysfunction, and oxidative stress that promote disease progression and eventually cirrhosis [29]. The multiple-hit hypothesis for NAFLD pathogenesis is widely accepted because it involves a more comprehensive view. This theory emphasized the role of genetic and environmental factors in metabolic dysfunction and disturbing connection between organs, especially the liver, adipose tissue, pancreas, and gut [29–31].
Contemporary understanding of NAFLD as an influential metabolic disease with possible distant consequences has induced the change of the name, from NAFLD to metabolic (dysfunction)-associated fatty liver disease (MAFLD) [32–35]. Metabolic (dysfunction)-associated fatty liver disease is a clinical diagnosis and does not require histology analysis of liver biopsy specimens [36]. It includes the presence of steatosis (detected by imaging or biomarkers or liver specimen histology) and either T2DM or overweight/obesity or 2 of the following: increased waist circumference, arterial hypertension, increased serum triglycerides, decreased HDL-cholesterol lipoproteins, increased C-reactive protein level, the presence of prediabetes, or insulin resistance [34]. The definition of MAFLD emphasizes the metabolic character of the disease and points out preventive measures regarding NAFLD pathophysiology chain interruption transfer from simple steatosis to liver fibrosis and cirrhosis and HCC [33, 34].
Epidemiological and cohort studies indicated inheritance in NAFLD but were insufficient to enable the mapping of certain genes of interest and qualify them as treatment targets [6]. With the development of modern genotypic arrays, genome- and exome-wide association studies (GWAS and EWAS) have become feasible, thus enabling the detection of different exonic variants associated either with full-spectrum or certain categories of NAFLD (steatosis, inflammation or fibrosis) by whole-exome sequencing (WES) [37, 38]. Genetic variants associated with full-spectrum NAFLD are missense SNP in PNPLA3 (rs738409) encoding p.I148M and missense variant rs58542926 encoding p.E167K in TM6SF2 [39, 40]. Additionally, pleiotropic genetic variants, such as GCKR rs 1260326, MBOAT7, and MARC1, are associated with certain categories of NAFLD: steatosis, inflammation, and liver fibrosis/cirrhosis [41–44]. However, some genetic variants are associated with individual NAFLD stages, such as PPP1R3B, PYGO1, APOE, and GPAM with steatosis [41, 44, 45]. Additionally, LEPR and HSD17B13 variants are associated merely with NASH and NASH and cirrhosis, respectively [40, 41]. The bioinformatics analysis identifies a number of pathways, including metabolism and PPAR signalling pathways, which were included in the NAFLD pathogenesis [46].
The connection between NAFLD, MetS, and T2DM
NAFLD is not only liver disease but a multisystem disorder. It is connected with MetS, IFG, IGT, and T2DM, and they have an increased risk of CVD [47, 48]. Such a strong association promotes MAFLD, presented with overweight/obesity, T2DM, or evidence of metabolic dysregulation in subjects with liver steatosis [34]. Recently, it was shown that the blood level of betatrophin, a liver hormone that regulates glucose and lipid metabolism, tends to decline during the progress of NAFLD, which may cause glucose intolerance [49]. In addition, NAFLD is associated with numerous systemic diseases, such as chronic kidney disease (CKD) and colorectal carcinoma [50].
In clinical practice, abdominal ultrasound and liver biopsy are widely used to assess the severity of NAFLD/NASH. Ultrasound diagnosis of NAFLD is the most commonly used method because it is easily applicable with high sensitivity (84.8%) and specificity (93.6%) [51, 52]. A disadvantage of abdominal ultrasound is its limited capacity for diagnostic of degrees of hepatic steatosis, especially in patients with high BMI [53–56]. Liver biopsy is still the gold standard for assessing the severity of NAFLD, but invasiveness is a limiting factor. In addition, screening the potential of extra-hepatic manifestations is an integral part of managing NAFLD patients [50, 57]. NAFLD usually manifests itself asymptomatically or only biochemically as a slightly elevated level of serum transaminases [58]. Therefore, serum transaminases are not sensitive markers of the presence of NAFLD [58, 59]. In the case of advanced disease with NASH, liver fibrosis, and cirrhosis, clinical manifestations are more pronounced than isolated NAFLD. Occasionally, it may present as right upper abdominal pain, nausea, the urge to vomit, gastric or intestinal motility complaints, and jaundice. As chronic liver disease progresses, clinical presentations of decompensated cirrhosis and liver failure occur. The appearance of unexplained fever, weight loss, or haemorrhagic ascites may indicate the most severe complication of NAFLD, HCC, followed by an increase in serum α-fetoprotein [58, 60–63].
The frequency of liver steatosis is significantly higher in obese individuals and patients with T2DM (observed in ~45% and 70% of subjects, respectively) [64]. On the other hand, there is a significantly higher risk of development of T2DM in patients with NAFLD [65]. Therefore, NAFLD is defined as the hepatic manifestation of MetS [66]. According to meta-analyses, the prevalence of NAFLD, NASH, and severe liver fibrosis (stage F ≥ 3) in patients with T2DM is 57.8%, 65.26%, and 15.05%, respectively [3, 67]. On the other hand, the prevalence of MetS in patients with NAFLD and NASH is 41 and 47%, respectively [3, 68]. Interaction between T2DM and NAFLD/NASH is bi-directional [69]. T2DM is observed in approximately a quarter of patients with NAFLD/NASH, whereas NAFLD occurs in three-quarters of patients with T2DM [70, 71].
Unhealthy and high-calorie food, excessive consumption of saturated fats and refined carbohydrates, sweetened beverages, and fructose, and a lack of physical activity and sedentary lifestyle significantly influence the occurrence of obesity and NAFLD [72]. In a certain number of diseased individuals, it is observed that some genetic factors (such as the presence of PNPLA3I148M and TM6SF2E167K polymorphisms) may determine the severity of NAFLD and influence the course of the disease to more advanced forms [73–75]. Recently, it was found that fibroblast growth factor 21 may be a reliable marker of inflammatory processes in the liver of obese subjects [76].
Due to global epidemics of T2DM and obesity, a significant increase in prevalence is expected for MetS and CVD [9]. Most patients with T2DM also have MetS [77]. Patients with MetS have a doubled risk of CVD occurrence compared to those without MetS [77]. Finally, MetS is associated with a 5-fold higher probability of occurrence of T2DM [78].
Patients with T2DM and NAFLD/NASH have a 2-fold higher risk of development and progression of CVD [48, 79] and 2- to 3-fold higher risk of death caused by chronic liver diseases than patients with T2DM without NAFLD [80]. T2DM is an independent predictor of general mortality and mortality caused by liver disease [81]. The risk of development of T2DM in patients with NAFLD/NASH is 33–55% [82]. Patients with NAFLD/NASH have an almost 2-fold higher risk of developing T2DM in 5 years of monitoring [83]. In addition, the progression of liver disease and increased morbidity and mortality are detected in lean subjects with NAFLD [84, 85].
The quality of glycaemic regulation determines the risk of CVD. Thus, IFG, IGT, and/or increased levels of HbA1c are associated with a higher risk of coronary disease [86]. Even if in the reference range, high normal glycaemic values may predict an increased risk of coronary disease and fatal outcomes independently of traditional risk factors for CVD [87]. Treatment of T2DM decreases the proportion of fat in the liver [88].
Patients with T2DM have a higher chance of developing NASH from NAFLD than those without diabetes [89, 90]. In patients with T2DM, the appearance of fatty liver is registered significantly earlier than diabetes vascular complications [91]. Therefore, early detection of NAFLD/NASH in patients with T2DM is of great importance. Determination of biomarkers of oxidative stress, dyslipidaemia, and inflammation can be beneficial [88]. Also, T2DM [92] and NAFLD [93] are the most important factors of risk for the development of chronic kidney disease (CKD), and the contribution of CKD to the development of CVD is much higher when associated with these conditions [94]. This leads to the development of the so-called “triad disease” consisting of NAFLD/NASH, CKD, and T2DM, which has great significance for public health, primarily because of the increasing incidence of CVD-associated morbidity and mortality [21, 94]. Clinical manifestation of NAFLD-related CVD varies and includes endothelial dysfunction, atherogenic dyslipidaemia, hypertension, altered gastrointestinal (GIT) microbiota, systemic inflammation, and cardiomyopathy [48, 95, 96]. Considering this connection, screening for CV risk factors in patients with NAFLD is strongly recommended [1, 97, 98]. Also, IR and T2DM are significant factors in liver fibrosis occurrence and development in patients with NAFLD [99, 100]. Four entities describe the link between T2DM and liver diseases: incidental and simultaneous existence of entities (which occurs very frequently), diabetic hepatopathy (a liver disease caused by diabetes), hepatogenous diabetes (diabetes caused by chronic liver disease), and liver disease that occurs coincidentally in patients with DM [101]. NAFLD/NASH, NASH-induced liver cirrhosis, and HCC belong to the subgroup of chronic liver diseases worsened by diabetes [62, 102]. Liver diseases that coincide with diabetes com prise haemochromatosis, autoimmune liver disease, and biliary duct diseases [103–105].
The pathophysiological pathway in which T2DM leads to NAFLD/NASH could be partially explained by chronic inflammation associated with IR, increased uptake of free fatty acids (FFA) by the liver, lipotoxicity, and development necroinflammation [106]. Accumulation of products of lipid metabolism (ceramides, diacylglycerol) leads to hepatic IR, increased gluconeogenesis, oxidative stress, and depletion of pancreatic β-cells [107]. On the other hand, NAFLD in patients with T2DM is associated with IR, poor metabolic control [108], and a higher incidence of micro- and macrovascular complications of diabetes [109, 110]. In contrast, improvement of NAFLD decreases the risk of occurrence of T2DM by approximately 70% [111].
Due to the frequent and strong association between T2DM and NAFLD/NASH, it is necessary to screen all patients with T2DM for the presence of NAFLD and vice versa [101, 112, 113]. For diagnostics of perturbations of glucose metabolism in patients with chronic liver diseases, the most reliable is the oral glucose tolerance test (OGTT) [114] because most of these patients have normal values of morning glycaemia [112]. HbA1c can serve as an indicator of the quality of retrograde glycaemic regulation in compensated chronic hepatic liver disease, as opposed to decompensated conditions, where it is advised to determine the level of fructosamine as an alternative marker. To assess the quality of daily glycaemic regulation and efficiency of diabetes therapy, it is helpful to measure glycaemia frequently during the day or optimally to perform continuous monitoring of glycaemia, if available [101, 112, 113].
Management of NAFLD/NASH, MetS, and T2DM
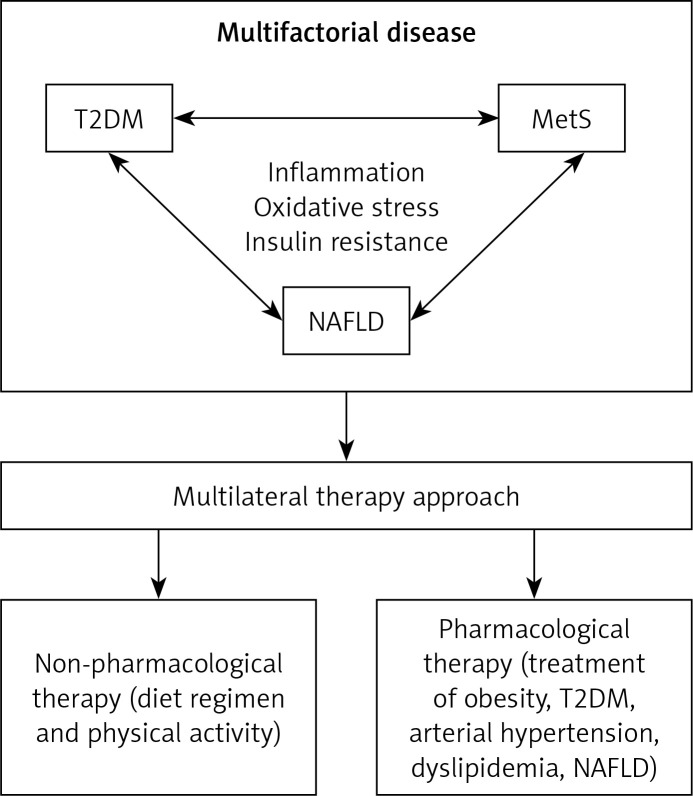
Treatment of NAFLD and MetS, which frequently co-exist in the same patient, requires a multifactorial approach consisting of non-pharmacological measures (such as diet regimen and physical activity) and pharmacological measures (treatment of obesity, arterial hypertension, T2DM, dyslipidaemia) to achieve improvement in the biochemical and histological presentation of NAFLD/NASH and reduction of cardiovascular risk [20, 115] (Figure 1). The primary goal of MetS treatment is to decrease the risk of CVD [9], and therefore the management is directed towards lowering elevated levels of atherogenic lipids and treating arterial hypertension and T2DM [116].
Figure 1.
Multilateral therapy approach for the treatment of multifactorial disease
MetS – metabolic syndrome, NAFLD – non-alcoholic fatty liver disease, T2DM – type 2 diabetes mellitus.
If non-pharmacological measures, such as body weight reduction, and change in physical activity do not yield favourable results, it is necessary to introduce pharmacological therapy [9, 117]. Also, no unified diet is recommended for NAFLD management [118]. There is still no single drug that can be used alone to treat MetS [9]. Currently used medications for individual components of MetS, such as arterial hypertension, T2DM, and atherogenic dyslipidaemia, positively reduce inflammation [117, 119].
No guidance recommends treatment with one particular medication for NAFLD/NASH with or without T2DM. Newer antihyperglycaemic agents, such as dipeptidyl peptidase-4 inhibitors (DPP4i) (sitagliptin, saxagliptin, vildagliptin, alogliptin, linagliptin) and glucagon-like peptide-1 receptor agonists (GLP1-RA) (exenatide, lixisenatide, liraglutide, dulaglutide, and semaglutide), or sodium-glucose transport protein 2 inhibitors (SGLT2i) (cana-/empa-/dapagliflozin), may be helpful in the treatment of NAFLD/NASH, decreasing the overall content of fat in the liver, and probably inflammation and fibrosis [120]. Additionally, they decrease the occurrence of CKD, which manifests either independently or in association with NAFLD/NASH, T2DM, or CVD [89]. GLP1-RAs, the structural homologues to the natural incretin glucagon-like peptide-1 (GLP-1), stimulate glucose-dependent pancreatic insulin secretion, reduce glucagon secretion, and slow down gastric emptying [121, 122]. Similarly, gliptins or DPP4i promote an increase in natural incretin levels such as GLP-1 and gastric inhibitory peptide (GIP) by blocking the dipeptidyl peptidase-4 (DPP4) enzyme involved in the degradation of natural incretins. The elevated incretin levels act on a simultaneous increase in glucose-dependent insulin secretion and decrease glucagon secretion [123, 124].
Other medications used for the treatment of T2DM, metformin and pioglitazone, lead to improved biochemical, ultrasonographic, and/or histological presentation in patients with NAFLD/NASH [125–133]. Thiazolidinedione, such as pioglitazone, activates the PPARγ receptors and decreases insulin resistance in various tissues, predominantly in skeletal muscles and the liver [134, 135]. Conflicting results exist regarding the use of sitagliptin [136, 137]. In patients with T2DM and NAFLD/NASH, combined therapy consisting of statin and liraglutide with or without SGLT2i is very potent and helpful, especially in reducing hepatic and cardiovascular morbidity and mortality in such patients [89, 138–140]. SGLT-2i reduces proximal tubule glucose reabsorption by binding to the SGLT-2 receptors, thus eliminating glucose urinary. Some studies emphasize the favourable effects of SGLT2i regarding major adverse cardiovascular events and congestive heart failure deterioration [141–143]. Renin-angiotensin blockers and antihypertensive medications are helpful additional therapy for patients with NAFLD/NASH [144]. Treatment with insulin degludec/aspart (IDegAsp) co-formulation improves hypoglycaemia, insulin requirement, and body mass of T2DM patients [145]. Besides novel therapies, statins and metformin have been shown as well-proven treatments for metabolic triad. Statins, as the inhibitors of the hydroxymethylglutaryl-CoA (HMG-CoA) reductase enzyme, inhibit a key step in the sterol biosynthetic pathway [146], while metformin acts on complex I inhibition, leading to AMPK activation, and alters cellular redox balance [147].
Growing evidence supports the effectiveness without adverse effects of various lipid-lowering nutraceuticals [148]. Appropriate therapy with silymarin, vitamin D and E, polyunsaturated fatty acids of the omega-3 series, coenzyme Q10, curcumin, and berberine with concomitant lifestyle changes can beneficially affect subjects with NAFLD [149]. Because CVD is the leading cause of fatal outcome in patients with NAFLD, it is important to use adequate therapy to prevent disease progression. Although a few guidelines for managing NAFLD are recommended by various scientific societies related to hepatology, some important issues remain unclear. The most important doubts are related to the definition of NAFLD, some directions for clinical practice, the need to monitor high-risk patients, and affordable non-invasive tests, including the new biomarkers for NAFLD diagnosis, etc. [150, 151]. One doubt is concerned with systematic screening for NAFLD in diabetic or obese subjects to prevent NASH and advanced fibrosis development, but it is not cost-effective [70, 152, 153]. Furthermore, currently using hepatic biomarkers for NAFLD screening is insufficient, considering false-negative results [153, 154]. In addition, the reason for differences between various guidelines is caused more by population characteristics, including genetics and lifestyle, screening strategies, and primary health care, than the inability to find unique attitudes [150, 151]. Despite some inconsistencies, we strongly recommended using existing Clinical Practice Guidelines, with preference being given to the use of local or institutional guidelines. The Position Paper endorsed by the International Lipid Expert Panel (ILEP) upgrades currently used guidelines on managing lipids in patients with atherosclerotic CVD [155]. Numerous experts from several European countries summarized data and presented draft practice recommendations and strategies for optimal lipid management considering the cost-effectiveness. The ILEP Position Paper explains in more detail practical solutions focusing on immediate combined lipid-medicated therapy in patients with high cardiovascular risk [155]. In addition, lipid-lowering therapy is suggested and the term “extremely high risk” has been proposed for a group of patients with acute coronary syndrome [155].
Conclusions
The timed treatment of NAFLD and MetS contributes to the prevention of T2DM. Non-pharmacological measures carried out in the management of NAFLD and MetS present the non-pharmacological mainstream in T2DM management at the same time. The optimal pharmacological treatment of T2DM is provided by novel antihyperglycaemic drug classes (i.e. SGLT2i, DPPi, GLP1-RA), sometimes combined with insulin sensitizers (metformin and pioglitazone), preventing the occurrence or progression of NAFLD. If all three entities already co-existed in the same patient, the concomitant management and nutraceuticals significantly decreased cardiovascular morbidity and mortality. Experience from everyday clinical practice shows that the treatment of the primarily presented entity of the “metabolic triad” significantly determines the presentation and treatment of another entity.
A more thorough investigation involving different specialities is needed to answer the complex natural history of NAFLD, enabling its more effective management. Connections between NAFLD, MetS, and T2DM lead to a complex multifactorial disease. The proper understanding of these connections and an early diagnosis and monitoring of existing conditions contribute to establishing effective targeted treatment.
Acknowledgments
Bojan Mitrovic and Zoran Gluvic share the first authorship.
The authors would like to thank Dr. M. Macvanin for her valuable comments during the preparation of the manuscript. This work is part of a collaboration between the Department of Radiobiology and Molecular Genetics, “VINČA” Institute of Nuclear Sciences - National Institute of the Republic of Serbia, University of Belgrade, Belgrade, Serbia, Clinic for Internal Medicine, Department of Endocrinology and Diabetes, Zemun Clinical Hospital, School of Medicine, University of Belgrade, Belgrade, Serbia, Department of Internal Medicine and Medical Specialties, University of Palermo, Italy and the Department of Hypertension, Medical University of Lodz, Poland.
This work was supported by the Ministry of Education, Science, and Technological Development of the Republic of Serbia (Contract #451-03-9/2021-14/ 200017).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388-402. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine J, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55: 2005-23. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73-84. [DOI] [PubMed] [Google Scholar]
- 4.Banach M, Burchardt P, Chlebus K, et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch Med Sci 2021; 17: 1447-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cengiz M, Candır BA, Yılmaz G, Akyol G, Ozenirler S. Is increased red cell distribution width an indicating marker of nonalcoholic steatohepatitis and fibrotic stage? World J Gastroenterol 2013; 19: 7412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016; 65: 1038-48. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005; 112: 3066-72. [DOI] [PubMed] [Google Scholar]
- 8.Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. Metabolic syndrome and non-cardiac vascular diseases: an update from human studies. Curr Pharm Des 2014; 20: 4944-52. [DOI] [PubMed] [Google Scholar]
- 9.Gluvic Z, Zaric B, Resanovic I, et al. Link between metabolic syndrome and insulin resistance. Curr Vasc Pharmacol 2017; 15: 30-9. [DOI] [PubMed] [Google Scholar]
- 10.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640-5. [DOI] [PubMed] [Google Scholar]
- 11.Kolovou GD, Anagnostopoulou KK, Salpea KD, Mikhailidis DP. The prevalence of metabolic syndrome in various populations. Am J Med Sci 2007; 333: 362-71. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002; 287: 356-9. [DOI] [PubMed] [Google Scholar]
- 13.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinology and metabolism clinics of North America 2004; 33: 351-75. [DOI] [PubMed] [Google Scholar]
- 14.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med 2007; 356: 213-5. [DOI] [PubMed] [Google Scholar]
- 15.Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med 2004; 164: 1066-76. [DOI] [PubMed] [Google Scholar]
- 16.Girman CJ, Rhodes T, Mercuri M, et al. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am J Cardiol 2004; 93: 136-41. [DOI] [PubMed] [Google Scholar]
- 17.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002; 288: 2709-16. [DOI] [PubMed] [Google Scholar]
- 18.Chrysavgis L, Ztriva E, Protopapas A, Tziomalos K, Cholongitas E. Nonalcoholic fatty liver disease in lean subjects: prognosis, outcomes and management. World J Gastroenterol 2020; 26: 6514-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med 2006; 119: S10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsiki N, Perez-Martinez P, Anagnostis P, Mikhailidis DP, Karagiannis A. Is nonalcoholic fatty liver disease indeed the hepatic manifestation of metabolic syndrome? Curr Vasc Pharmacol 2018; 16: 219-27. [DOI] [PubMed] [Google Scholar]
- 21.Przybyszewski EM, Targher G, Roden M, Corey KE. Nonalcoholic fatty liver disease and cardiovascular disease. Clin Liver Dis 2021; 17: 19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019; 69: 2672-82. [DOI] [PubMed] [Google Scholar]
- 24.Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5: 739-52. [DOI] [PubMed] [Google Scholar]
- 25.Lazarus JV, Mark HE, Anstee QM, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat Rev Gastroenterol Hepatol 2022; 19: 60-78. [DOI] [PubMed] [Google Scholar]
- 26.Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol 2020; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018; 69: 896-904. [DOI] [PubMed] [Google Scholar]
- 28.Ghrénassia E, Avouac J, Khanna D, et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case-control study. J Rheumatol 2014; 41: 99-105. [DOI] [PubMed] [Google Scholar]
- 29.Siddique A, Nelson JE, Aouizerat B, Yeh MM, Kowdley KV. Iron deficiency in patients with nonalcoholic Fatty liver disease is associated with obesity, female gender, and low serum hepcidin. Clinic Gastroenterol Hepatol 2014; 12: 1170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood 2006; 107: 1747-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J Gastroenterol 2018; 24: 2974-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valenti L, Pelusi S. Redefining fatty liver disease classification in 2020. 2020; 40: 1016-7. [DOI] [PubMed] [Google Scholar]
- 33.Eslam M, Sanyal AJ, George J. Toward more accurate nomenclature for fatty liver diseases. Gastroenterology 2019; 157: 590-3. [DOI] [PubMed] [Google Scholar]
- 34.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73: 202-9. [DOI] [PubMed] [Google Scholar]
- 35.Méndez-Sánchez N, Bugianesi E, Gish RG, et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol 2022; 7: 388-90. [DOI] [PubMed] [Google Scholar]
- 36.Du X, DeForest N, Majithia AR. Human genetics to identify therapeutic targets for NAFLD: challenges and opportunities. Front Endocrinol 2021; 12: 777075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirschhorn JN. Genomewide association studies: illuminating biologic pathways. N Engl J Med 2009; 360: 1699-701. [DOI] [PubMed] [Google Scholar]
- 38.Rabbani B, Tekin M, Mahdieh N. The promise of whole-exome sequencing in medical genetics. J Hum Genetic 2014; 59: 5-15. [DOI] [PubMed] [Google Scholar]
- 39.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol 2018; 68: 268-79. [DOI] [PubMed] [Google Scholar]
- 40.Parisinos CA, Wilman HR, Thomas EL, et al. Genome-wide and Mendelian randomisation studies of liver MRI yield insights into the pathogenesis of steatohepatitis. J Hepatol 2020; 73: 241-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anstee QM, Darlay R, Cockell S, et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J Hepatol 2020; 73: 505-15. [DOI] [PubMed] [Google Scholar]
- 42.Buch S, Stickel F, Trépo E. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet 2015; 47: 1443-8. [DOI] [PubMed] [Google Scholar]
- 43.Chambers JC, Zhang W, Sehmi J, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet 2011; 43: 1131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jamialahmadi O, Mancina RM, Ciociola E, et al. Exome-wide association study on alanine aminotransferase identifies sequence variants in the GPAM and APOE associated with fatty liver disease. Gastroenterology 2021; 160: 1634-46.e1637. [DOI] [PubMed] [Google Scholar]
- 45.Speliotes EK, Yerges-Armstrong LM, Wu J, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genetics 2011; 7: e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Lin B, Chen Z, et al. Identification of key pathways and genes in nonalcoholic fatty liver disease using bioinformatics analysis. Arch Med Sci 2020; 16: 374-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsiki N, Athyros VG, Karagiannis A, Wierzbicki AS, Mikhailidis DP. Should we expand the concept of coronary heart disease equivalents? Curr Opin Cardiol 2014; 29: 389-95. [DOI] [PubMed] [Google Scholar]
- 48.Kasper P, Martin A, Lang S, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol 2021; 110: 921-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonmez A, Dogru T, Ercin CN, Genc H, Celebi G. Betatrophin levels are related to the early histological findings in nonalcoholic fatty liver disease. Metabolites 2021; 11: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015; 62: S47-64. [DOI] [PubMed] [Google Scholar]
- 51.Paige JS, Bernstein GS, Heba E, et al. A pilot comparative study of quantitative ultrasound, conventional ultrasound, and MRI for predicting histology-determined steatosis grade in adult nonalcoholic fatty liver disease. AJR Am J Roentgenol 2017; 208: W168-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozturk A, Grajo JR, Gee MS, et al. Quantitative hepatic fat quantification in non-alcoholic fatty liver disease using ultrasound-based techniques: a review of literature and their diagnostic performance. Ultrasound Med Biol 2018; 44: 2461-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bohte AE, Koot BG, van der Baan-Slootweg OH, et al. US cannot be used to predict the presence or severity of hepatic steatosis in severely obese adolescents. Radiology 2012; 262: 327-34. [DOI] [PubMed] [Google Scholar]
- 54.Hamaguchi M, Kojima T, Itoh Y, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007; 102: 2708-15. [DOI] [PubMed] [Google Scholar]
- 55.Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 2009; 51: 1061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol 2007; 189: W320-3. [DOI] [PubMed] [Google Scholar]
- 57.Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol 2014; 20: 13306-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macut D, Božić-Antić I, Bjekić-Macut J, Tziomalos K. Management of endocrine disease: polycystic ovary syndrome and nonalcoholic fatty liver disease. Eur J Endocrinol 2017; 177: R145-58. [DOI] [PubMed] [Google Scholar]
- 59.Loria P, Adinolfi L, Bellentani S, et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis 2010; 42: 272-82. [DOI] [PubMed] [Google Scholar]
- 60.Mitrovic B, Gluvic Z, Macut D, et al. Effects of metformin-single therapy on the level of inflammatory markers in serum of non-obese T2DM patients with NAFLD. Endocr Metab Immune Disord Drug Targets 2022; 22: 117-24. [DOI] [PubMed] [Google Scholar]
- 61.Sebastiani G. Non-invasive assessment of liver fibrosis in chronic liver diseases: implementation in clinical practice and decisional algorithms. World J Gastroenterol 2009; 15: 2190-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 2020; 158: 1822-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Younossi ZM, Noureddin M, Bernstein D, et al. Role of noninvasive tests in clinical gastroenterology practices to identify patients with nonalcoholic steatohepatitis at high risk of adverse outcomes: expert panel recommendations. Am J Gastroenterol 2021; 116: 254-62. [DOI] [PubMed] [Google Scholar]
- 64.Vernon G, Baranova A, Younossi Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274-85. [DOI] [PubMed] [Google Scholar]
- 65.Bae J, Rhee E, Lee W, et al. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: a 4-year retrospective longitudinal study. Diabetes Care 2011; 34: 727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katsiki N, Imprialos K, Vlachopoulos C. Editorial: arterial stiffness, central haemodynamics and non-alcoholic fatty liver disease: links with cardiovascular risk and effects of drug treatment. Curr Vasc Pharmacol 2018; 16: 401-4. [DOI] [PubMed] [Google Scholar]
- 67.Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation 2019; 103: 22-7. [DOI] [PubMed] [Google Scholar]
- 68.Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014; 2: 901-10. [DOI] [PubMed] [Google Scholar]
- 69.Grgurevic I, Podrug K, Mikolasevic I, Kukla M. Natural history of nonalcoholic fatty liver disease: implications for clinical practice and an individualized approach. Can J Gastroenterol Hepatol 2020; 2020: 9181368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut 2016; 65: 1359-68. [DOI] [PubMed] [Google Scholar]
- 71.Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol 2010; 105: 1567-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerber L, Otgonsuren M, Mishra A, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther 2012; 36: 772-81. [DOI] [PubMed] [Google Scholar]
- 73.Valenti L, Al-Serri A, Daly AK, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2010; 51: 1209-17. [DOI] [PubMed] [Google Scholar]
- 74.Liu YL, Reeves HL, Burt AD, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun 2014; 5: 4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iqbal U, Perumpail BJ, Akhtar D, Kim D. The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease. Medicines 2019; 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cantero I, Abete I, Bullón-Vela V, et al. Fibroblast growth factor 21 levels and liver inflammatory biomarkers in obese subjects after weight loss. Arch Med Sci 2022; 18: 36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 2008; 28: 629-36. [DOI] [PubMed] [Google Scholar]
- 78.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol 2005; 4: 198-203. [DOI] [PubMed] [Google Scholar]
- 79.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007; 30: 2119-21. [DOI] [PubMed] [Google Scholar]
- 80.Zoppini G, Fedeli U, Gennaro N, Saugo M, Targher G, Bonora E. Mortality from chronic liver diseases in diabetes. Am J Gastroenterol 2014; 109: 1020-5. [DOI] [PubMed] [Google Scholar]
- 81.Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut 2010; 59: 1410-5. [DOI] [PubMed] [Google Scholar]
- 82.Bae JC, Rhee EJ, Lee WY, et al. Combined effect of nonalcoholic fatty liver disease and impaired fasting glucose on the development of type 2 diabetes: a 4-year retrospective longitudinal study. Diabetes Care 2011; 34: 727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan WK, Tan AT, Vethakkan SR, Tah PC, Vijayananthan A, Goh KL. Non-alcoholic fatty liver disease in diabetics--prevalence and predictive factors in a multiracial hospital clinic population in Malaysia. J Gastroenterol Hepatol 2013; 28: 1375-83. [DOI] [PubMed] [Google Scholar]
- 84.Golabi P, Paik J, Fukui N, Locklear CT, de Avilla L, Younossi ZM. Patients with lean nonalcoholic fatty liver disease are metabolically abnormal and have a higher risk for mortality. Clin Diabetes 2019; 37: 65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patoulias DI, Kalogirou MS. Lean non-alcoholic fatty liver disease: do not forget diabetes. Arch Med Sci Atheroscler Dis 2019; 4: e248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk). BMJ 2001; 322: 15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anand SS, Dagenais GR, Mohan V, et al. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: the EpiDREAM cohort study. Eur J Prev Cardiol 2012; 19: 755-64. [DOI] [PubMed] [Google Scholar]
- 88.Klisic A, Isakovic A, Kocic G, et al. Relationship between oxidative stress, inflammation and dyslipidemia with fatty liver index in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2018; 126: 371-8. [DOI] [PubMed] [Google Scholar]
- 89.Athyros VG, Polyzos SA, Kountouras J, et al. Non-alcoholic fatty liver disease treatment in patients with type 2 diabetes mellitus; new kids on the block. Curr Vasc Pharmacol 2020; 18: 172-81. [DOI] [PubMed] [Google Scholar]
- 90.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007; 30: 1212-8. [DOI] [PubMed] [Google Scholar]
- 91.Giorda C, Forlani G, Manti R, et al. Occurrence over time and regression of nonalcoholic fatty liver disease in type 2 diabetes. Diabetes Metab Res Rev 2017; 33: doi: 10.1002/dmrr.2878. [DOI] [PubMed] [Google Scholar]
- 92.Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol 2017; 13: 297-310. [DOI] [PubMed] [Google Scholar]
- 93.Targher G, Chonchol M, Pichiri I, Zoppini G. Risk of cardiovascular disease and chronic kidney disease in diabetic patients with non-alcoholic fatty liver disease: just a coincidence? J Endocrinol Invest 2011; 34: 544-51. [DOI] [PubMed] [Google Scholar]
- 94.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296-305. [DOI] [PubMed] [Google Scholar]
- 95.Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J Am Coll Cardiol 2019; 73: 948-63. [DOI] [PubMed] [Google Scholar]
- 96.Lechner K, McKenzie AL, Kränkel N, et al. High-risk atherosclerosis and metabolic phenotype: the roles of ectopic adiposity, atherogenic dyslipidemia, and inflammation. Metab Syndr Relat Disord 2020; 18: 176-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bonapace S, Valbusa F, Bertolini L, et al. Nonalcoholic fatty liver disease is associated with aortic valve sclerosis in patients with type 2 diabetes mellitus. PLoS One 2014; 9: e88371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Przybyszewski EM, Targher G, Roden M, Corey KE. Nonalcoholic fatty liver disease and cardiovascular disease. Clin Liver Dis 2021; 17: 19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valenti L, Bugianesi E, Pajvani U, Targher G. Nonalcoholic fatty liver disease: cause or consequence of type 2 diabetes? Liver Int 2016; 36: 1563-79. [DOI] [PubMed] [Google Scholar]
- 100.Ercin CN, Dogru T, Genc H, et al. Insulin resistance but not visceral adiposity index is associated with liver fibrosis in nondiabetic subjects with nonalcoholic fatty liver disease. Metab Syndr Relat Disord 2015; 13: 319-25. [DOI] [PubMed] [Google Scholar]
- 101.Hamed AE, Elwan N, Naguib M, et al. Diabetes association with liver diseases: an overview for clinicians. Endocr Metab Immune Disord Drug Targets 2019; 19: 274-80. [DOI] [PubMed] [Google Scholar]
- 102.Francque SM, Marchesini G, Kautz A, et al. Non-alcoholic fatty liver disease: a patient guideline. JHEP Rep 2021; 3: 100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barton JC, Acton RT. Diabetes in HFE hemochromatosis. J Diabetes Res 2017; 2017: 9826930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X, Xu H, Zhan M, Niu J. The potential effects of diabetes mellitus on liver fibrosis in patients with primary biliary cholangitis. Med Sci Monit 2019; 25: 6174-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Sousa G, Prinz N, Becker M, et al. Diabetes mellitus and autoimmune hepatitis: demographical and clinical description of a relatively rare phenotype. Horm Metab Res 2018; 50: 568-74. [DOI] [PubMed] [Google Scholar]
- 106.Valenti L, Bugianesi E, Pajvani U, Targher G. Nonalcoholic fatty liver disease: cause or consequence of type 2 diabetes? Liver Int 2016; 36: 1563-79. [DOI] [PubMed] [Google Scholar]
- 107.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010; 363: 1341-50. [DOI] [PubMed] [Google Scholar]
- 108.Lomonaco R, Bril F, Portillo-Sanchez P, et al. Metabolic impact of nonalcoholic steatohepatitis in obese patients with type 2 diabetes. Diabetes Care 2016; 39: 632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jia G, Di F, Wang Q, et al. Non-alcoholic fatty liver disease is a risk factor for the development of diabetic nephropathy in patients with type 2 diabetes mellitus. PLoS One 2015; 10: e0142808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mantovani A, Pernigo M, Bergamini C, et al. Nonalcoholic fatty liver disease is independently associated with early left ventricular diastolic dysfunction in patients with type 2 diabetes. PLoS One 2015; 10: e0135329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes. Diabetes Care 2015; 38: 1673-9. [DOI] [PubMed] [Google Scholar]
- 112.Hamed AE, Elsahar M, Elwan NM, et al. Managing diabetes and liver disease association: practice guidelines from the Egyptian Association for the Study of Liver and Gastrointestinal Disease (EASLGD). Arab J Gastroenterol 2019; 20: 61-3. [DOI] [PubMed] [Google Scholar]
- 113.Hamed AE, Elsahar M, Elwan NM, et al. Managing diabetes and liver disease association. Arab J Gastroenterol 2018; 19: 166-79. [DOI] [PubMed] [Google Scholar]
- 114.Hudacko RM, Sciancalepore JP, Fyfe BS. Diabetic microangiopathy in the liver: an autopsy study of incidence and association with other diabetic complications. Am J Clin Pathol 2009; 132: 494-9. [DOI] [PubMed] [Google Scholar]
- 115.Gluvic Z, Tomasevic R, Bojovic K, Obradovic M, Isenovic ER. Non-alcoholic fatty liver disease: a multidisciplinary clinical practice approach – the institutional adaptation to existing Clinical Practice Guidelines. Emerg Critic Care Med 2022; 2: 12-22. [Google Scholar]
- 116. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev 2005; 13: 322-7. [PubMed] [Google Scholar]
- 117.Rizzo M, Rizvi AA, Rini GB, Berneis K. The therapeutic modulation of atherogenic dyslipidemia and inflammatory markers in the metabolic syndrome: what is the clinical relevance? Acta Diabetol 2009; 46: 1-11. [DOI] [PubMed] [Google Scholar]
- 118.Katsiki N, Stoian AP, Rizzo M. Dietary patterns in non-alcoholic fatty liver disease (NAFLD): stay on the straight and narrow path! Clin Investig Arterioscler 2022; 34 Suppl 1: S24-S31. [DOI] [PubMed] [Google Scholar]
- 119.Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis 2016; 5: 2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rizzo M, Montalto G, Al-Rasadi K. Treatment options for managing atherogenic dyslipidemia and fatty liver disease. Expert Opin Pharmacother 2014; 15: 1065-8. [DOI] [PubMed] [Google Scholar]
- 121.Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clin Diabetes 2018; 36: 14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Flint A, Andersen G, Hockings P, et al. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther 2021; 54: 1150-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deacon CF. Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of type 2 diabetes. Front Endocrinol 2019; 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Subrahmanyan NA, Koshy RM, Jacob K, Pappachan JM. Efficacy and cardiovascular safety of DPP-4 inhibitors. Curr Drug Safe 2021; 16: 154-64. [DOI] [PubMed] [Google Scholar]
- 125.Katsiki N, Christou GA, Kiortsis DN. Liraglutide and cardiometabolic effects: more than just another antiobesity drug? Curr Vasc Pharmacol 2016; 14: 76-9. [DOI] [PubMed] [Google Scholar]
- 126.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016; 387: 679-90. [DOI] [PubMed] [Google Scholar]
- 127.Olaywi M, Bhatia T, Anand S, Singhal S. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int 2013; 12: 584-8. [DOI] [PubMed] [Google Scholar]
- 128.Gluud LL, Knop FK, Vilsbøll T. Effects of lixisenatide on elevated liver transaminases: systematic review with individual patient data meta-analysis of randomised controlled trials on patients with type 2 diabetes. BMJ Open 2014; 4: e005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Macauley M, Hollingsworth KG, Smith FE, et al. Effect of vildagliptin on hepatic steatosis. J Clin Endocrinol Metab 2015; 100: 1578-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mashitani T, Noguchi R, Okura Y, et al. Efficacy of alogliptin in preventing non-alcoholic fatty liver disease progression in patients with type 2 diabetes. Biomed Rep 2016; 4: 183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Michurina SV, Ishenko IJ, Klimontov VV, et al. Linagliptin alleviates fatty liver disease in diabetic db/db mice. World J Diabetes 2016; 7: 534-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Katsiki N, Athyros VG, Mikhailidis DP. Cardiovascular effects of sodium-glucose cotransporter 2 inhibitors: multiple actions. Curr Med Res Opin 2016; 32: 1513-4. [DOI] [PubMed] [Google Scholar]
- 133.Katsiki N, Mikhailidis DP, Theodorakis MJ. Sodium-glucose cotransporter 2 inhibitors (SGLT2i): their role in cardiometabolic risk management. Curr Pharm Des 2017; 23: 1522-32. [DOI] [PubMed] [Google Scholar]
- 134.Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004; 351: 1106-18. [DOI] [PubMed] [Google Scholar]
- 135.Skat-Rørdam J, Højland Ipsen D, Lykkesfeldt J, Tveden-Nyborg P. A role of peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. Basic Clin Pharmacol Toxicol 2019; 124: 528-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Joy TR, McKenzie CA, Tirona RG, et al. Sitagliptin in patients with non-alcoholic steatohepatitis: a randomized, placebo-controlled trial. World J Gastroenterol 2017; 23: 141-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg 2012; 75: 240-4. [PubMed] [Google Scholar]
- 138.Athyros VG, Katsiki N, Karagiannis A. Editorial: can glucagon like peptide 1 (GLP1) agonists or sodium-glucose co-transporter 2 (SGLT2) inhibitors ameliorate non-alcoholic steatohepatitis in people with or without diabetes? Curr Vasc Pharmacol 2016; 14: 494-7. [DOI] [PubMed] [Google Scholar]
- 139.Katsiki N, Athyros VG, Mikhailidis DP. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: effects of statins and antidiabetic drugs. J Diabetes Compications 2017; 31: 521-2. [DOI] [PubMed] [Google Scholar]
- 140.Magan-Fernandez A, Rizzo M. Statins in liver disease: not only prevention of cardiovascular events. Expert Rev Gastroenterol Hepatol 2018; 12: 743-4. [DOI] [PubMed] [Google Scholar]
- 141.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393: 31-9. [DOI] [PubMed] [Google Scholar]
- 142.Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation 2019; 139: 2516-27. [DOI] [PubMed] [Google Scholar]
- 143.Wiviott SD, Raz I, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. Reply. N Engl J Med 2019; 380: 1881-2. [DOI] [PubMed] [Google Scholar]
- 144.Milic S, Mikolasevic I, Krznaric-Zrnic I, et al. Nonalcoholic steatohepatitis: emerging targeted therapies to optimize treatment options. Drug Des Devel Ther 2015; 9: 4835-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Özçelik S, Çelik M, Vural A, Aydın B, Özçelik M, Gozu H. Outcomes of transition from premixed and intensive insulin therapies to insulin aspart/degludec co-formulation in type 2 diabetes mellitus: a real-world experience. Arch Med Sci 2020; 17: 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sirtori CR. The pharmacology of statins. Pharmacol Res 2014; 88: 3-11. [DOI] [PubMed] [Google Scholar]
- 147.LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev 2021; 42: 77-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cicero AFG, Fogacci F, Stoian AP, et al. Nutraceuticals in the management of dyslipidemia: which, when, and for whom? could nutraceuticals help low-risk individuals with non-optimal lipid levels? Curr Athero Rep 2021; 23: 57-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cicero AFG, Colletti A, Bellentani S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): the available clinical evidence. Nutrients 2018; 10: 1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Monelli F, Venturelli F, Bonilauri L, et al. Systematic review of existing guidelines for NAFLD assessment. Hepatoma Res 2021; 7: 25-37. [Google Scholar]
- 151.Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroenterol 2018; 24: 3361-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Koehler EM, Plompen EP, Schouten JN, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology 2016; 63: 138-47. [DOI] [PubMed] [Google Scholar]
- 153.Corey KE, Klebanoff MJ, Tramontano AC, Chung RT, Hur C. Screening for nonalcoholic steatohepatitis in individuals with type 2 diabetes: a cost-effectiveness analysis. Dig Dis Sci 2016; 61: 2108-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chalasani N, Younossi Z. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328-57. [DOI] [PubMed] [Google Scholar]
- 155.Banach M, Penson PE, Vrablik M, et al. Optimal use of lipid-lowering therapy after acute coronary syndromes: a position paper endorsed by the International Lipid Expert Panel (ILEP). Pharmacol Res 2021; 166: 105499. [DOI] [PubMed] [Google Scholar]