Abstract
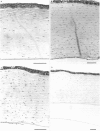
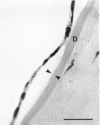
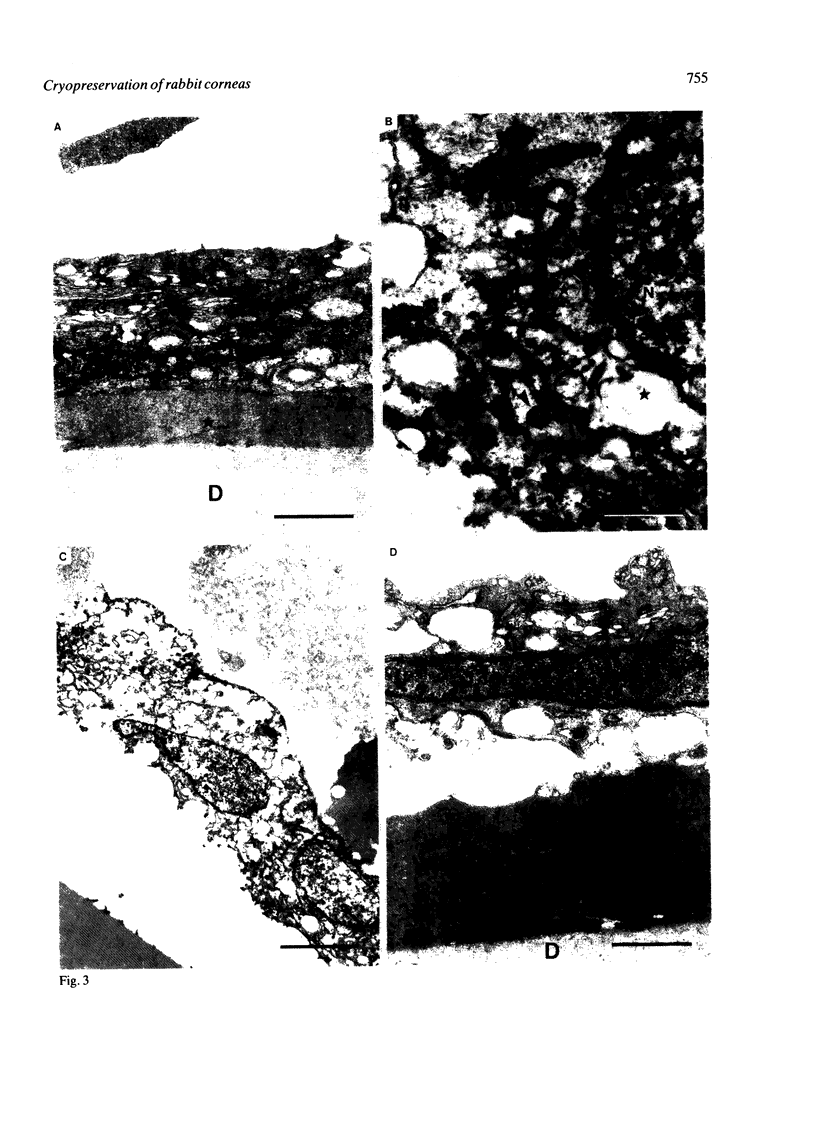
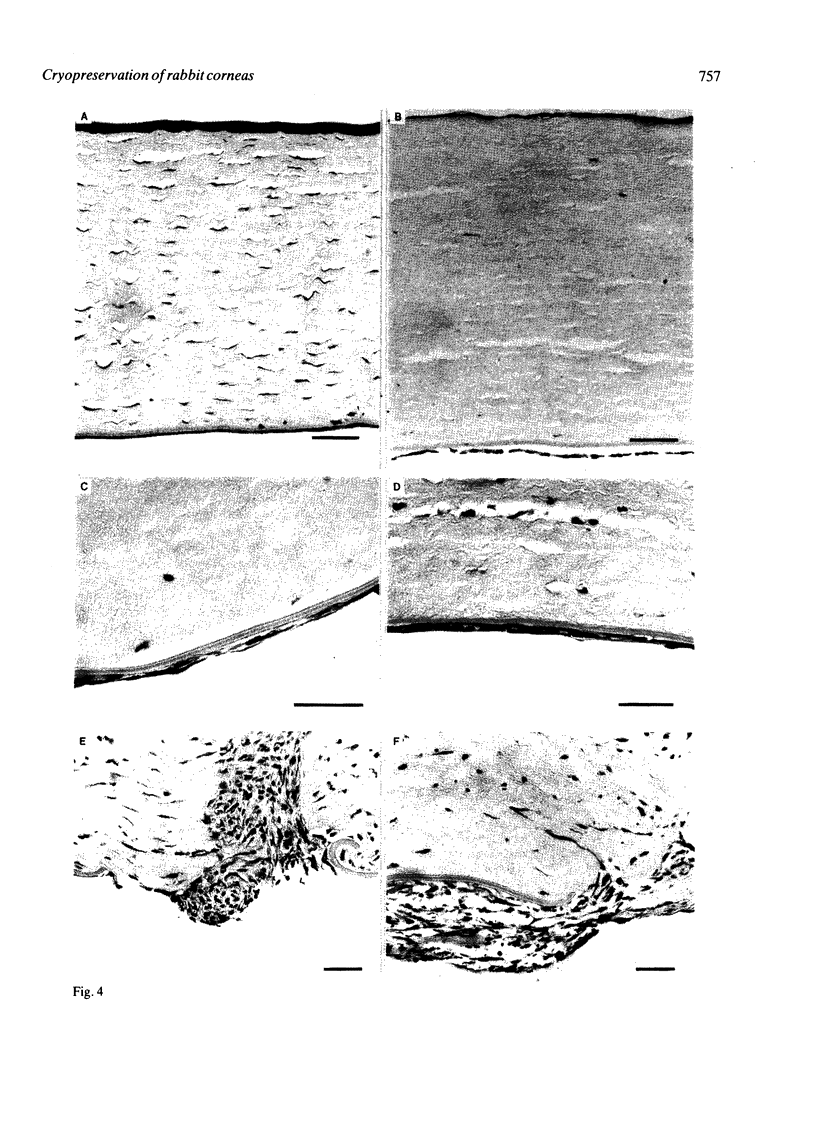
Rabbit corneas were frozen and thawed by three methods and compared by full thickness transplantation as well as specular microscopy, histology, and transmission electron microscopy. Two of the methods used a recently described technique, in which the excised cornea was immersed in a potassium-rich buffered solution containing the cryoprotectant dimethyl sulphoxide (Me2SO, 2 mol/l). This solution was designed to restrict the loss of intracellular potassium and to prevent cell swelling at low temperatures. In one group the corneas were frozen and thawed surrounded by 5 ml of medium, while in the second group corneas were drained of excess fluid and frozen in air. The third group consisted of corneas cryopreserved by Capella and colleagues' method. All the cryopreserved corneas were damaged, but those that had been frozen in air after exposure to the new medium showed better structure and function than corneas frozen by either of the other two techniques.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capella J. A., Kaufman H. E., Robbins J. E. Preservation of viable corneal tissue. Cryobiology. 1965 Nov-Dec;2(3):116–121. doi: 10.1016/s0011-2240(65)80096-7. [DOI] [PubMed] [Google Scholar]
- Clifton E. C., 3rd, Hanna C. Corneal cryopreservation and the fate of corneal cells in penetrating keratoplasty. Am J Ophthalmol. 1974 Aug;78(2):239–250. doi: 10.1016/0002-9394(74)90084-1. [DOI] [PubMed] [Google Scholar]
- Dikstein S., Maurice D. M. The metabolic basis to the fluid pump in the cornea. J Physiol. 1972 Feb;221(1):29–41. doi: 10.1113/jphysiol.1972.sp009736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhauser H. F., Van Horn D. L., Gallun A. B., Schultz R. O. Experimental rehydration of cryopreserved corneal tissue. Invest Ophthalmol. 1971 Feb;10(2):100–107. [PubMed] [Google Scholar]
- Ehlers N., Sperling S., Olsen T. Post-operative thickness and endothelial cell density in cultivated, cryopreserved human corneal grafts. Acta Ophthalmol (Copenh) 1982 Dec;60(6):935–944. doi: 10.1111/j.1755-3768.1982.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Elford B. C., Walter C. A. Effects of electrolyte composition and pH on the structure and function of smooth muscle cooled to -79 degrees C in unfrozen media. Cryobiology. 1972 Apr;9(2):82–100. doi: 10.1016/0011-2240(72)90015-6. [DOI] [PubMed] [Google Scholar]
- Foreman J., Pegg D. E. Cell preservation in a programmed cooling machine: the effect of variations in supercooling. Cryobiology. 1979 Aug;16(4):315–321. doi: 10.1016/0011-2240(79)90043-9. [DOI] [PubMed] [Google Scholar]
- Hayes A. R., Pegg D. E., Kingston R. E. A multirate small-volume cooling machine. Cryobiology. 1974 Aug;11(4):371–377. doi: 10.1016/0011-2240(74)90015-7. [DOI] [PubMed] [Google Scholar]
- Hunt C. J., Beadle D. J., Harris L. W. An ultrastructural study of the recovery of Chinese hamster ovary cells after freezing and thawing. Cryobiology. 1977 Apr;14(2):135–143. doi: 10.1016/0011-2240(77)90133-x. [DOI] [PubMed] [Google Scholar]
- Hunt C. J., Taylor M. J., Pegg D. E. Freeze-substitution and isothermal freeze-fixation studies to elucidate the pattern of ice formation in smooth muscle at 252 K (-21 degrees C). J Microsc. 1982 Feb;125(Pt 2):177–186. doi: 10.1111/j.1365-2818.1982.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Kaufman H. E. Corneal cryopreservation and its clinical application. Transplant Proc. 1976 Jun;8(2 Suppl 1):149–152. [PubMed] [Google Scholar]
- Kuming B. S. The assessment of endothelial viability. S Afr Med J. 1969 Aug 30;43(35):1083–1085. [PubMed] [Google Scholar]
- LOVELOCK J. E. The haemolysis of human red blood-cells by freezing and thawing. Biochim Biophys Acta. 1953 Mar;10(3):414–426. doi: 10.1016/0006-3002(53)90273-x. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E. The mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim Biophys Acta. 1953 May;11(1):28–36. doi: 10.1016/0006-3002(53)90005-5. [DOI] [PubMed] [Google Scholar]
- Lee W. R., Mueller F. O., Trevor-Roper P. D. In vivo survival of the endothelium of freeze-stored corneal homografts in the rabbit. Br J Ophthalmol. 1967 May;51(5):321–330. doi: 10.1136/bjo.51.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER F. O. TECHNIQUES FOR FULL-THICKNESS KERATOPLASTY IN RABBITS USING FRESH AND FROZEN CORNEAL TISSUE. Br J Ophthalmol. 1964 Jul;48:377–393. doi: 10.1136/bjo.48.7.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden P. W., Easty D. L. Assessment and interpretation of corneal endothelial cell morphology and function following cryopreservation. Br J Ophthalmol. 1982 Feb;66(2):136–140. doi: 10.1136/bjo.66.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P., Rall W. F., Rigopoulos N. Relative contributions of the fraction of unfrozen water and of salt concentration to the survival of slowly frozen human erythrocytes. Biophys J. 1981 Dec;36(3):653–675. doi: 10.1016/S0006-3495(81)84757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology. 1977 Jun;14(3):251–272. doi: 10.1016/0011-2240(77)90175-4. [DOI] [PubMed] [Google Scholar]
- Mueller F. O., O'Neill P., Trevor-Roper P. D. Full-thickness corneal grafts in Addis Ababa, Ethiopia. Br J Ophthalmol. 1967 Apr;51(4):227–245. doi: 10.1136/bjo.51.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller F. O. Short-term experiments on grafting fresh and frozen corneal tissue in dogs. Br J Ophthalmol. 1968 Oct;52(10):752–762. doi: 10.1136/bjo.52.10.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer L., Laing R. A., Leibowitz H. M., Oak S. S. Coalescence of endothelial cells in the traumatized cornea. I. Experimental observations in cryopreserved tissue. Arch Ophthalmol. 1983 Nov;101(11):1787–1790. doi: 10.1001/archopht.1983.01040020789026. [DOI] [PubMed] [Google Scholar]
- Pegg D. E., Diaper M. P., Skaer H. L., Hunt C. J. The effect of cooling rate and warming rate on the packing effect in human erythrocytes frozen and thawed in the presence of 2 M glycerol. Cryobiology. 1984 Oct;21(5):491–502. doi: 10.1016/0011-2240(84)90047-6. [DOI] [PubMed] [Google Scholar]
- Ruusuvaara P. Effects of corneal preservation, donor age, cadaver time and postoperative period on the graft endothelium. A specular microscopic study. Acta Ophthalmol (Copenh) 1979 Oct;57(5):868–881. doi: 10.1111/j.1755-3768.1979.tb01854.x. [DOI] [PubMed] [Google Scholar]
- Ruusuvaara P. The fate of preserved and transplanted human corneal entothelium. Acta Ophthalmol (Copenh) 1980 Jun;58(3):440–453. doi: 10.1111/j.1755-3768.1980.tb05745.x. [DOI] [PubMed] [Google Scholar]
- Sherrard E. S. Full thickness keratoplasty in the rabbit: an unsatisfactory index of donor integrity. Exp Eye Res. 1974 Feb;18(2):135–142. doi: 10.1016/0014-4835(74)90099-2. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Hunt C. J. A new preservation solution for storage of corneas at low temperatures. Curr Eye Res. 1985 Sep;4(9):963–973. doi: 10.3109/02713689509000003. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Hunt C. J. Dual staining of corneal endothelium with trypan blue and alizarin red S: importance of pH for the dye-lake reaction. Br J Ophthalmol. 1981 Dec;65(12):815–819. doi: 10.1136/bjo.65.12.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Pegg D. E. The effect of ice formation on the function of smooth muscle tissue stored at -21 or -60 degrees C. Cryobiology. 1983 Feb;20(1):36–40. doi: 10.1016/0011-2240(83)90057-3. [DOI] [PubMed] [Google Scholar]
- Taylor M. J. The role of pH and buffer capacity in the recovery of function of smooth muscle cooled to -13 degrees C in unfrozen media. Cryobiology. 1982 Dec;19(6):585–601. doi: 10.1016/0011-2240(82)90188-2. [DOI] [PubMed] [Google Scholar]
- Van Horn D. L., Edelhauser H. F., DeBruin J. Functional and ultrastructural changes in cryopreserved corneas. Arch Ophthalmol. 1973 Oct;90(4):312–318. doi: 10.1001/archopht.1973.01000050314016. [DOI] [PubMed] [Google Scholar]
- Van Horn D. L., Schultz R. O. Endothelial survival in cryopreserved human corneas: a scanning electron microscopic study. Invest Ophthalmol. 1974 Jan;13(1):7–16. [PubMed] [Google Scholar]
- Van Horn D. L., Sendele D. D., Seideman S., Buco P. J. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci. 1977 Jul;16(7):597–613. [PubMed] [Google Scholar]