Abstract
Background
The advantages of parenchymal-sparing resection (PSR) over anatomic resection (AR) of colorectal liver metastases (CRLM) remain controversial. Here, we aim to evaluate their safety and efficacy.
Methods
A systematic review and meta-analysis of short-term perioperative outcomes and long-term oncological outcomes for PSR and AR were performed by searching Pubmed, Embase, the Cochrane Library and Web of Science databases.
Results
Twenty-two studies were considered eligible (totally 7228 patients: AR, n = 3154 (43.6%) vs. PSR, n = 4074 (56.4%)). Overall survival (OS, HR = 1.08, 95% CI: 0.95-1.22, P = 0.245) and disease-free survival (DFS, HR = 1.09, 95% CI: 0.94-1.28, P = 0.259) were comparable between the two groups. There were no significant differences in 3-year OS, 5-year OS, 3-year DFS, 5-year DFS, 3-year liver recurrence-free survival (liver-RFS) and 5-year liver-RFS. In terms of perioperative outcome, patients undergoing AR surgery were associated with prolonged operation time (WMD = 51.48 min, 95% CI: 29.03-73.93, P < 0.001), higher amount of blood loss (WMD = 189.92 ml, 95% CI: 21.39-358.45, P = 0.027), increased intraoperative blood transfusion rate (RR = 2.24, 95% CI: 1.54-3.26, P < 0.001), prolonged hospital stay (WMD = 1.00 day, 95% CI: 0.34-1.67, P = 0.003), postoperative complications (RR = 2.28, 95% CI: 1.88-2.77, P < 0.001), and 90-day mortality (RR = 3.08, 95% CI: 1.88-5.03, P < 0.001). While PSR surgery was associated with positive resection margins (RR = 0.77, 95% CI: 0.61-0.97, P = 0.024), intrahepatic recurrence (RR = 0.90, 95% CI: 0.82-0.98, P = 0.021) and repeat hepatectomy (RR = 0.64, 95% CI: 0.55-0.76, P < 0.001).
Conclusion
Considering relatively acceptable heterogeneity, PSR had better perioperative outcomes without compromising oncological long-term outcomes. However, these findings must be carefully interpreted, requiring more supporting evidence.
Trial registration
PROSPERO registration number: CRD42023445332.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-023-03127-1.
Keywords: Parenchymal-sparing resection, Anatomic resection, Colorectal liver metastases, Outcomes, Meta-analysis
Introduction
At present, colorectal cancer (CRC) ranks the third malignancy in both incidence and mortality worldwide [1]. Up to 50% of patients develop liver metastasis, and colorectal liver metastases (CRLM) have become the leading cause of mortality in patients with CRC [2]. Liver resection has been proved to be a promising cure opportunity for CRLM, with a 5-year survival rate of more than 50%. Nearly 20% of postoperative patients would survive for more than 10 years [3]. Which is an optimal surgical resection of CRLM, either anatomic resection (AR) or parenchymal-sparing resection (PSR), has been controversial. In general, the major goal for therapy is to achieve a negative surgical margin and to preserve as much liver parenchyma as possible [4]. AR can achieve radical resection of CRLM, especially for multifocal lesions or lesions invading large intrahepatic vessels. However, AR can cause more postoperative symptoms, including postoperative liver failure [5]. PSR excises the liver tumor with the minimally sufficient resection margin to preserve as much normal liver parenchyma and the major intrahepatic vessels as possible [6, 7]. PSR is equivalent to AR in oncological outcomes and correlates with lower postoperative morbidity and shorter hospital stay [8, 9]. However, major concerns have been raised about whether PSR could increase the positive rate of surgical margin and the risk of tumor recurrence [10, 11].
There has been no consensus on whether PSR is superior to AR for CRLM. Therefore, our purpose of this study was to compare the perioperative short-term and postoperative oncological long-term outcomes of CRLM treated with AR and PSR.
Materials and methods
Literature search strategy
This meta-analysis was performed according to the PRISMA guidelines [12]. All analyses were based on previously published studies and therefore did not require ethical approval or informed consent. In order to ensure accuracy and to minimize deviation, literature retrieval, literature screening, data extraction and quality evaluation were carried out by two scientific investigators independently. A systematic literature search was conducted on medical databases PubMed, Embase, the Cochrane Library, and Web of Science to select articles that compared CRLM patients undergoing AR with PSR surgery, until January 2022. Literature retrieval was not limited by the language, type or geographical area. Specific search strategies were developed for each database using the following keywords and/or MeSH terms: “anatomic*” OR “major” OR “extended”; “nonanatomic*” OR “parenchyma* sparing” OR “wedge” OR “minor” OR “limited”; (“Colorectal Neoplasms” AND “Neoplasm Metastasis”) OR “Colorectal liver metastases*” OR “CRLM/CLM”.
Inclusion and exclusion criteria
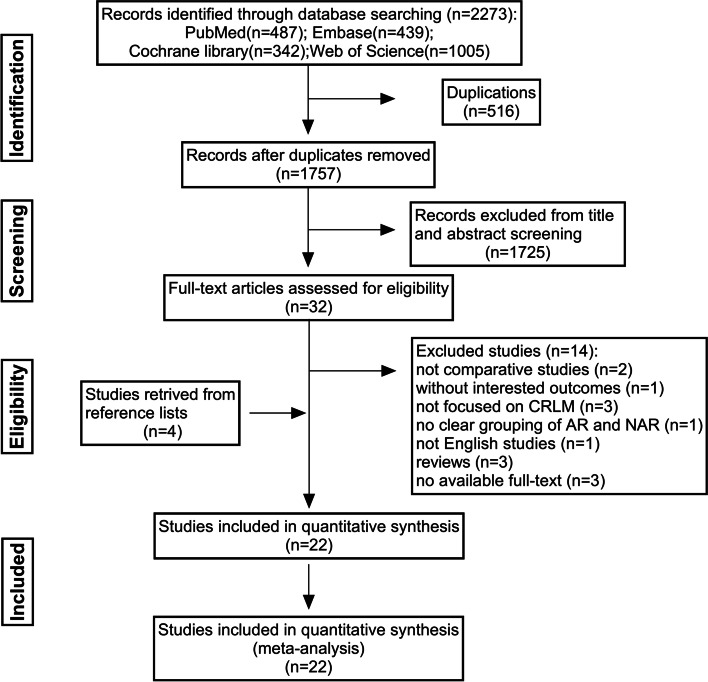
In order to ensure the reliability, candidate studies were determined according to the following inclusion and exclusion criteria. Inclusion criteria included: (1) pathologically diagnosed with CRLM and treated with surgery; (2) comparing AR with PSR, where resection approach was considered as a variable in survival analysis; (3) perioperative short-term and long-term survival outcomes; (4) human studies; (5) sample size, follow-up time, literature language: unlimited. Studies met the above inclusion criteria were included in the meta-analysis. Exclusion criteria included: (1) unfocused CRLM, AR and PSR were not clearly grouped; (2) single-arm AR or PSR studies; (3) perioperative or survival outcomes were not reported or could not be extracted; (4) non-comparative studies such as reviews, letters, case reports, and meeting abstracts; (5) full text was not available. Studies that met one of the above exclusion criteria were excluded. The detailed literature search strategy was described in Fig. 1.
Fig. 1.
PRISMA flowchart of the study selection
Data extraction
The preliminary selected studies were classified and managed using Endnote X9 software. An Excel sheet was created to collect relevant data of all included studies. The data were extracted independently by two investigators. If there were disagreements, the teams were jointly resolved to reach an agreement. Long-term oncological outcomes were the primary endpoints of this meta-analysis, including overall survival (OS), disease-free survival (DFS), and liver recurrence-free survival (Liver-RFS). Secondary endpoints were perioperative outcomes, including duration of surgery, amount of blood loss, intraoperative blood transfusion rate, hospital stay, postoperative complications, positive resection margin, 90-day mortality, intrahepatic recurrence, and repeat hepatectomy. Study characteristics (publication year, first author, number of patients), patient characteristics (age, gender), and tumor characteristics (primary site, number of metastases, size of metastases, simultaneous resection, and CEA level) were also collected.
Quality assessment
The quality of non-randomized controlled trials was evaluated by the modified Newcastle–Ottawa Scale (NOS) [13]. Two investigators independently assessed the quality of the literature. The NOS scale assesses three quality parameters (patient selection, intergroup comparability and outcome assessment) that were divided into eight specific items. There are slight differences in scoring case–control and cohort studies. The total score shall be 9 stars, the higher the score, the better the quality, while seven stars or more are considered as high quality. Low-quality studies with a NOS score less than five stars were excluded. The detailed quality evaluation of the included literature was listed in Table S1.
Statistical analysis
The meta-analysis was performed by using Stata (version 14.0, Stata Corp) software. For continuous variables, weighted mean differences (WMD) and 95% confidence interval (CI) were used for evaluation. When the mean and standard deviation (SD) were not reported, calculations were made according to the equation proposed by Hozo et al. [14]. Survival data were used as dichotomous variables at different time points (3-year, 5-year OS, DFS, liver-RFS) with relative risk (RR) and 95% CI. When no specific survival data were reported, we estimated specific survival rate using the Kaplan-Meier plots. Meanwhile, we calculated the cumulative hazard ratio (HR) and 95% CI for each study from the Kaplan–Meier plots using the Engauge digitizer software (version 11.3) [15]. The overall effect was determined using the z-test. A 2-sided p-value < 0.05 was considered statistically significant.
Statistical heterogeneity between studies would be explored by examining forest plots and by using the X2 test. When the degree of CI overlap between studies in the forest plot was relatively low, we would consider that heterogeneity existed in the included studies. A p < 0.10 in the X2 test indicated the existence of heterogeneity. Heterogeneity was quantified using the I2 statistic. The I2 values of 25-49%, 50-74%, and 75-100%, could be interpreted as low, moderate, and high heterogeneity, respectively. If I2 < 50%, fixed effects model would be applied, otherwise, random effects model would be applied.
Sensitivity analysis and publication bias
Sensitivity analysis was performed using Stata software (version 14.0, Stata Corp) to explore the robustness of the results and potential sources of heterogeneity. For each study being removed, a new meta-analysis was performed. If heterogeneity was significantly reduced, this specific study was considered to be a major source of heterogeneity and further evaluation was required. Publication bias was assessed by funnel plot and was quantified by Egger test. A p > 0.1 indicated that there was no publication bias [16].
Result
Included studies and quality assessment
The entire process of the literature search was described in Fig. 1. A total of 2273 articles were retrieved from the databases. After excluding duplicates and title-abstract, 32 articles were included in the full-text analysis. According to the selection criteria, 14 articles were further excluded, while another 4 articles were included by references. All literature quality assessment NOS scores were ≥ 7 (Table S1). Finally, 22 studies were included in this meta-analysis [8–10, 17–35]. A total of 7228 patients with CRLM who underwent liver resection from 1980 to 2019 were included, of which 4074 (56.4%) underwent PSR and 3154 (43.6%) underwent AR.
Characteristics of the included studies
The baseline characteristics of the included studies were summarized in Table 1, including gender, age, primary tumor site, the number of metastases, the size of metastases, synchronous resection, and the CEA level. In AR and PSR, males accounted for 58.7% (1469/2503) and 60.7% (2207/3638), respectively. The proportion of males in the PSR group was slightly higher than that in the AR group. In most studies, the mean or median age was around 65. Among 4702 primary tumor sites, 3198 (68%) were in the colon whereas 1504 (32%) in the rectum. In the AR group, 1168 (69.8%) of the 1673 tumors were located in the colon, whereas 505 (30.2%) in the rectum. In the PSR group, 2030 (67%) of the 3029 tumors were located in the colon, whereas 999 (33%) in the rectum. The mean follow-up time for the studies ranged from 0 to 235 months.
Table 1.
Characteristics of the included studies
| First Author (Year) | Enrollment period | Country | Group | No. of patients | Gender (Male%) | Age | Primary tumor (Colon/ Rectum) |
Number of metastases | Largest metastasis size(cm) | Synchronous liver metastases (%) | CEA level (ng/mL) | Type of survival outcomes | Follow-up (months) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andreou 2021 [9] | 2012–2019 | Switzerland | AR | 13 | 9(69.2%) | 61(30–79) | 9/4 | N/A | N/A | 9(100%) | N/A | OS, RFS | 55 | 8 |
| PSR | 79 | 51(64.6%) | 62(36–84) | 59/20 | N/A | N/A | 45(57%) | N/A | ||||||
| Dam 2014 [25] | 1991–2010 | The Netherlands | AR | 129 | 67(51.9%) | 64(24–82) | 79/50 | 4(1–12) | 3.0(0.0–20.0) | N/A | N/A | OS, DFS | 33(0–235) | 8 |
| PSR | 169 | 110(65.1%) | 64(28–88) | 98/71 | 1(1–3) | 3.0(0.4–13.0) | N/A | N/A | ||||||
| DeMatteo 2000 [17] | 2001–2013 | Italy | AR | 148 | 88(59%) |
< 70:110(74%); > 70:38(26%) |
110/38 | > 1:30(20%) | ≥ 5 cm:48(32%) | N/A |
< 200:101(92%); ≥ 200:9(8%) |
OS | 25(1–140) | 7 |
| PSR | 119 | 67(56%) |
< 70:83(70%); > 70:36(30%) |
96/33 | > 1:23(19%) | ≥ 5 cm:19(16%) | N/A |
< 200:84(90%); ≥ 200:9(10%) |
||||||
| Donadon 2018 [31] | 2001–2013 | Italy | AR | 110 | 67(60%) | 63.8 ± 10.2 | N/A | 3.5 ± 2.6 | 4.9 ± 2.5 | 44(40%) | N/A | OS, DFS | 33(1–83) | 8 |
| PSR | 110 | 74(67%) | 61.9 ± 11.0 | N/A | 3.7 ± 3.3 | 4.5 ± 2.5 | 43(39%) | N/A | ||||||
| Finch 2007 [21] | 1993–2003 | UK | AR | 280 | 171(61%) | 63(26–84) | N/A | 2(1–14) | 4.5(0.7–20) | 117(41.8%) | 18 ng/dl(1–37,140) | OS, DFS | 33(24–144) | 7 |
| PSR | 96 | 64(67%) | 63(24–79) | N/A | 1(1–9) | 3.3(0.4–15) | 36(38%) | 5 ng/dl(1–12,124) | ||||||
| Guzzetti 2008 [22] | 1996–2005 | Italy | AR | 102 | 58(56.8%) |
< 70:78(76.4%); > 70: 24(23.6%) |
55/21 | Single 59(61.4%); Multiple 37(38.6%) | > 5 cm:31(32.3) | N/A |
< 200:52(89.6); ≥ 200:6(10.4) |
OS, DFS | N/A | 8 |
| PSR | 106 | 63(59.5%) |
< 70: 77(72.6%); > 70: 29(27.4%) |
60/31 | Single 67(64.4%); Multiple 37(35.6%) | > 5 cm:21(20.8) | N/A |
< 200:51(86.4); ≥ 200:8(13.6) |
||||||
| Hosokawa 2017 [29] | 2000–2015 | France | AR | 242 | 141(58%) | 64.0 ± 10.0 | 158/84 | N/A | 2.04 ± 0.8 | 121(50%) | 159.7 ± 1,347.6 | OS, RFS | 41 | 8 |
| PSR | 1478 | 888(60%) | 64.1 ± 11.0 | 980/498 | N/A | 1.93 ± 0.76 | 721(49%) | 65.7 ± 303.0 | ||||||
| Joechle 2020 [34] | 2006–2016 | USA | AR | 105 | 69(65.7%) | 54(29–82) | 72/33 | 1(1–9) | 2.0(0.16–5.3) | 75(71%) | N/A | OS, RFS, liver-RFS | 43.1 | 7 |
| PSR | 105 | 71(67.6%) | 56(26–79) | 82/23 | 1(1–8) | 1.8(0.1–5.8) | 81(77%) | N/A | ||||||
| Kokudo 2001 [18] | 1980–1999 | Japan | AR | 96 | 54(56.3%) | 58.7 ± 1.0 | 71/25 |
Single:54(56.3%); ≥ 3:20(20.8%) |
5.81 ± 0.4 | 46(47.9%) | N/A | OS | N/A | 7 |
| PSR | 78 | 46(59%) | 60.3 ± 1.2 | 49/29 |
Single:42(53.8%); ≥ 3:19(24.4%) |
2.69 ± 0.16 | 56(71.8%) | N/A | ||||||
| Lalmahomed 2011 [24] | 2000–2008 | Netherlands | AR | 88 | 56(64%) | 65(30–82) | 55/33 | 2(1–7) | 4(1–15) | 35(40%) | > 200:10(12%) | OS, DFS | 35(1–111) | 8 |
| PSR | 113 | 70(62%) | 65(36–86) | 59/54 | 1(1–7) | 3(1–7) | 43(38%) | > 200:6(5%) | ||||||
| Lordan 2017 [30] | 2000–2010 | UK | AR | 238 | 130(54.6%) | 64.8(24–86) | N/A | Single:161(67.7%); Mutiple:76(31.9%) | 3.2(0.4–20) | 14 (5.9) | N/A | OS, DFS | 36(0.12–144) | 8 |
| PSR | 238 | 135(56.7%) | 65.7(31–87) | N/A | Single:153(64.3%); Mutiple:85(35.7%) | 3.1(0.5–14) | 15 (6.3) | N/A | ||||||
| Matsuki 2016 [26] | 2005–2013 | Japan | AR | 23 | 17(74%) | 62(29–84) | 15/8 | 5(1–17) | 2.0(0.5–3) | 13(57) | 4.9(0.6–230) | OS, RFS, Liver-RFS | 40(5–81) | 8 |
| PSR | 40 | 25(63%) | 64(40–81) | 26/14 | 4(1–27) | 1.8(0.5–3) | 18(45) | 4.2(0.5–117) | ||||||
| Matsumura 2016 [27] | 1999–2012 | Japan | AR | 32 | 22(68.8) | 62.5(27–80) | 22/10 | 7(4–31) | 3.0(0.8–5) | N/A | 9.35(0.6–975) | OS, RFS, Liver-RFS | N/A | 7 |
| PSR | 113 | 74(65.5) | 60(40–81) | 65/48 | 6(4–33) | 2.5(0.4–5) | N/A | 7(0.5–3097) | ||||||
| Memeo 2016 [28] | 2006–2013 | France | AR | 266 | 145(55%) | 61(29–82) | 189/77 | 4(3–15) | 3.3(0.6–20) | 127(48%) | N/A | OS, DFS | N/A | 8 |
| PSR | 266 | 146(55%) | 62.4(40–80) | 194/72 | 4(3–9) | 3.5(0.6–11) | 134(50%) | N/A | ||||||
| Mise 2016 [8] | 1993–2013 | Houston | AR | 144 | 80(56%) | 58(22–87) | 105/39 | N/A | 1.9(0.3–3.0) | 17(12%) | 2.9(0.4–250.3) | OS, RFS, Liver-RFS | 37(2—208) | 8 |
| PSR | 156 | 94(61%) | 60(30–88) | 113/43 | N/A | 1.5(0.4–3.0) | 50(32%) | 2.5(0.4–430.9) | ||||||
| Okumura 2019 [10] | 2004–2017 | France | AR | 82 | 51(62.2%) | 64(43–85) | 55/27 | 2(1–8) | 2.8(0.5–13) | 50(61%) | N/A | OS, RFS, Liver-RFS | 33.9(6–120) | 8 |
| PSR | 82 | 50(61%) | 65(33–83) | 48/34 | 2(1–7) | 2.5(0.5–15) | 45(54.9%) | N/A | ||||||
| Pandanaboyana 2018 [32] | 1993–2011 | UK | AR | 582 | N/A |
< 65:282(48.5%); > 65:300(51.5%) |
N/A | 2 | 4.5(3–7) | 294(50.5%) | N/A | OS, DFS | 32.2(17.5–56.9) | 8 |
| PSR | 409 | N/A |
< 65:175(42.8%); > 65:234(57.2%) |
N/A | 2 | 2.7(2–4) | 228(55.7%) | N/A | ||||||
| Sarpel 2009 [23] | 1987–2007 | USA | AR | 94 | 54(57%) | 60.8 ± 10.4 | 60/10 | 1.7 ± 1.2 | 6.6 ± 4.7 | 8(8%) | N/A | OS, DFS | 34 | 8 |
| PSR | 89 | 51(57%) | 62.3 ± 11.6 | 59/12 | 1.4 ± 1.0 | 3.5 ± 2.3 | 8(9%) | N/A | ||||||
| She 2020 [35] | 1990–2017 | China | AR | 70 | 38(54.3%) | 61.0(29–85) | N/A | 1(1–7) | 2.45(1.0–11.0) | 38(55.1%) | 8.8(0.7–802) | OS, DFS | 39.8(2.9–183.9) | 8 |
| PSR | 70 | 47(67.1%) | 61.0(31–85) | N/A | 1(1–multiple) | 2.5(0.9–11.0) | 27(38.6%) | 8.85(1–526) | ||||||
| Spelt 2018 [33] | 2006–2014 | Sweden | AR | 60 | 39(65%) | 65(61–69) | N/A | 3(2–5) | 2.65(1.7–4.0) | 38(64.4%) | 5(3–18) | OS | 35 | 7 |
| PSR | 59 | 35(59.3%) | 69(63–76) | N/A | 2(2–4) | 2.2(1.5–3.0) | 29(51.8%) | 5(3–17) | ||||||
| Stewart 2004 [19] | 1988–2001 | UK | AR | 69 | N/A | 62(23–79) | N/A |
1–3:65(97%); > 3:2/67(3%) |
< 5 cm:14(23.3%); > 5 cm:46(76.7%) |
N/A | 625U/l(14–308,040) | OS | N/A | 7 |
| PSR | 27 | N/A | 64(28–82) | N/A |
1–3:22(91.7%); > 3:2(8.3%) |
< 5 cm:14(77.7%); > 5 cm:4(22.3%) |
N/A | 105U/l(1–2662) | ||||||
| Zorzi 2006 [20] | 1991–2004 | Italy | AR | 181 | 113(62.4%) |
< 65:120(66.3%); > 65:61(33.7%) |
113/46 | Single:99(54.7%); Multiple:82(45.3%) | 3(0.3–18) | 73(40.3%) |
< 200:160(88.4%); > 200:7(3.9%) |
OS | 25 | 7 |
| PSR | 72 | 46(64%) |
< 65:44(61%); > 65:28(39%) |
42/17 | Single:45(62.5%); Multiple:27(37.5%) | 2.1(0.5–6) | 25(34.7%) |
< 200:63(87.5%); > 200:1(1.4%) |
Abbreviations: AR Anatomic resection, DFS Disease-free survival, N/A Not available, OS Overall survival, PSR Parenchymal-sparing resection, RFS Recurrence‐free survival
Overall survival (OS)
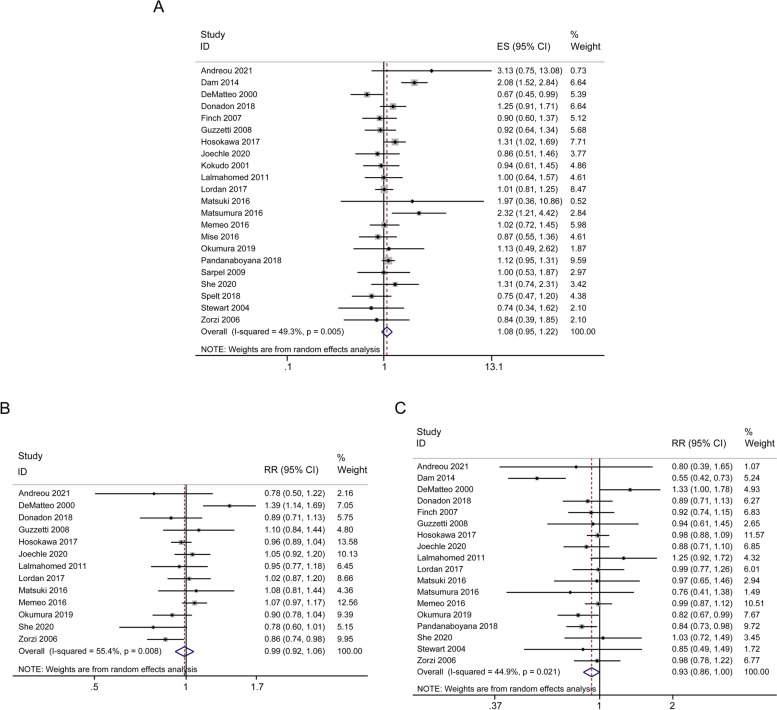
The primary long-term outcome of OS was summarized in Table 2 and Fig. 2. HR-values extracted from 22 studies were incorporated into the assessment of OS. However, no clear evidence of any benefit of PSR on survival was found (HR = 1.08; 95% CI, 0.95-1.22; p = 0.245; I2 = 49.3%), as shown in Fig. 2A. The 3-year OS was comparable between AR and PSR groups (RR = 0.99; 95% CI, 0.92-1.06; p = 0.728; I2 = 55.4%) (Fig. 2B). The 5-year OS was slightly higher in PSR group than that in AR group (RR = 0.93; 95% CI, 0.86-1.00; p = 0.054; I2 = 44.9%) (Fig. 2C). The studies were moderately heterogeneous and used a random effect model.
Table 2.
Results of meta-analysis comparing AR and PSR for CRLM
| Patients | Study heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes of interest | Studies | AR | PSR | WMD/RR/HR (95% CI) | P value | I2(%) | P value | Effect model |
| Long-term | ||||||||
| Overall survial (OS) | 22 | 3154 | 4074 | 1.08(0.95–1.22) | 0.245 | 49.3 | 0.005 | Random |
| 3‐year OS | 13 | 1668 | 2878 | 0.99(0.92–1.06) | 0.728 | 55.4 | 0.008 | Random |
| 5‐year OS | 18 | 2760 | 3692 | 0.93(0.86–1.00) | 0.054 | 44.9 | 0.021 | Random |
| Disease‐free survival (DFS) | 14 | 2260 | 3368 | 1.09(0.94–1.28) | 0.259 | 75.1 | < 0.001 | Random |
| 3‐year DFS | 10 | 1237 | 2581 | 0.98(0.89–1.07) | 0.66 | 0 | 0.897 | Fixed |
| 5‐year DFS | 14 | 2260 | 3368 | 0.88(0.73–1.07) | 0.212 | 70.1 | < 0.001 | Random |
| 3‐year Liver-RFS | 5 | 386 | 496 | 1.02(0.9–1.15) | 0.789 | 0 | 0.79 | Fixed |
| 5‐year Liver-RFS | 5 | 386 | 496 | 1.00(0.88–1.14) | 0.981 | 0 | 0.592 | Fixed |
| Short‐term | ||||||||
| Duration of operation (min) | 13 | 1234 | 1364 | 51.48(29.03–73.93) | < 0.001 | 98.6 | < 0.001 | Random |
| Estimated blood loss (mL) | 10 | 886 | 992 | 189.92(21.39–358.45) | 0.027 | 98.6 | < 0.001 | Random |
| Intraoperative blood transfusion | 12 | 1799 | 3004 | 2.24(1.54–3.26) | < 0.001 | 74 | < 0.001 | Random |
| Length of hospital stay (day) | 15 | 1813 | 3011 | 1.00(0.34–1.67) | 0.003 | 66.6 | < 0.001 | Random |
| Positive margin (mm) | 12 | 1244 | 1080 | 0.77(0.61–0.97) | 0.024 | 33.9 | 0.119 | Fixed |
| Postoperative complications | 12 | 1880 | 3116 | 2.28(1.88–2.77) | < 0.001 | 0 | 0.639 | Fixed |
| 90‐day mortality | 7 | 1711 | 2826 | 3.08(1.88–5.03) | < 0.001 | 0 | 0.796 | Fixed |
| Intrahepatic recurrence | 15 | 2299 | 2036 | 0.90(0.82–0.98) | 0.021 | 26.2 | 0.166 | Fixed |
| Repeat hepatectomy | 14 | 2362 | 3398 | 0.64(0.55–0.76) | < 0.001 | 12.4 | 0.317 | Fixed |
Abbreviations: AR Anatomic resection, CI Confidence interval, CRLM Colorectal liver metastases, DFS Disease-free survival, HR Hazard ratio, RR Risk ratio, OS Overall survival, PSR Parenchymal-sparing resection, RFS Recurrence‐free survival, WMD Weighted mean difference
Fig. 2.
Forest plots of the effect of AR versus PSR on overall survival (OS). Cumulative hazard ratio (HR) of overall survival (OS) (A), risk ratio (RR) of 3-year OS (B), and 5-year OS (C). HR and RR are presented with 95% CI
Disease-free survival (DFS)
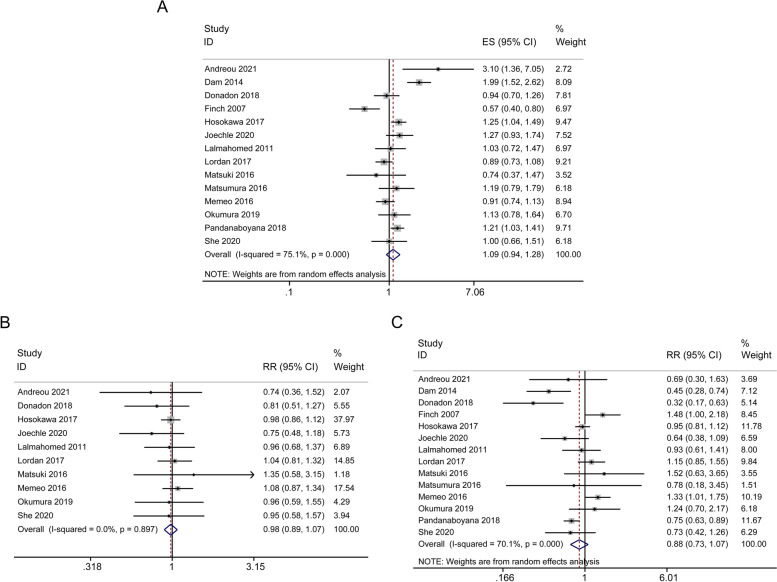
The primary long-term outcome of DFS was summarized in Table 2 and Fig. 3. HR-values extracted from 14 studies were incorporated into the assessment of DFS, moderate heterogeneity was observed among these studies, the random effect model was applied. The combined effect was HR = 1.09; 95% CI, 0.94-1.28; p = 0.259. The related forest plots were shown in Fig. 3A. 3-year DFS was reported in 10 studies, low heterogeneity was observed among these studies (I2 = 0.0%, p = 0.897). The fixed effect model was applied, the combined effect was RR = 0.98; 95% CI, 0.89-1.07; p = 0.660 (Fig. 3B). The combined effect of 5-year DFS was RR = 0.88; 95% CI, 0.73-1.07; p = 0.212 (Fig. 3C). There was no significant difference in DFS between the AR group and the PSR group.
Fig. 3.
Forest plots of the effect of AR versus PSR on disease-free survival (DFS). Cumulative hazard ratio (HR) of DFS (A), risk ratio (RR) of 3-year DFS (B), and 5-year DFS (C)
Liver recurrence-free survival (Liver-RFS)
Results from 5 included literatures showed that 3-year liver-RFS (RR = 1.02; 95% CI, 0.90-1.15; p = 0.789; I2 = 0.0%) and 5-year liver-RFS (RR = 1.00; 95% CI, 0.88-1.14; p = 0.981; I2 = 0.0%) in both AR and PSR procedures were comparable, as shown in Fig. S1A-B.
Short-term outcomes
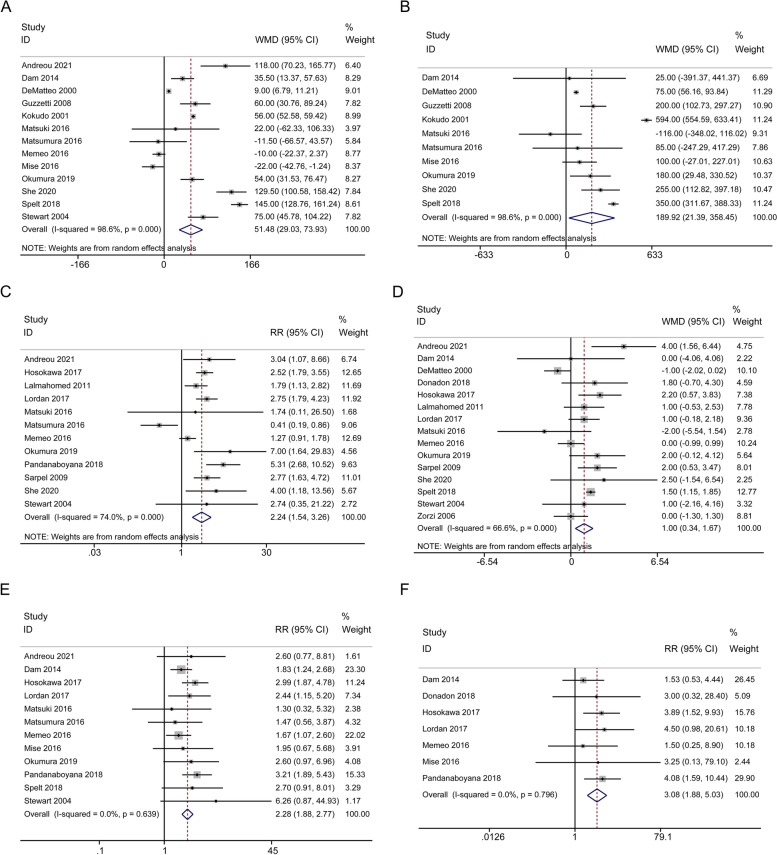
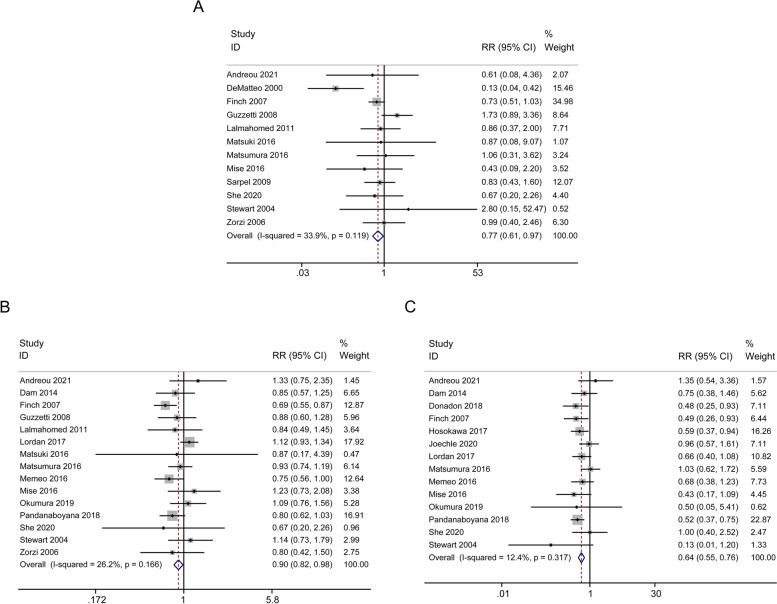
Short-term outcomes included duration of operation, blood loss, intraoperative blood transfusion rate, length of hospital stay, postoperative complications, 90-day mortality, positive resection margin, intrahepatic recurrence, and repeat hepatectomy. As summarized in Table 2, Fig. 4 and Fig. 5, compared with PSR group, AR group was associated with longer operative time (13 studies, WMD = 51.48 min; 95% CI, 29.03-73.93; p < 0.001; I2 = 98.6%, Fig. 4A), higher amount of blood loss (10 studies, WMD = 189.92 ml; 95% CI, 21.39-358.45; p = 0.027; I2 = 98.6%, Fig. 4B), increased intraoperative blood transfusion rate (12 studies, RR = 2.24; 95% CI, 1.54-3.26; p < 0.001; I2 = 74.0%, Fig. 4C), prolonged hospital stay (15 studies, WMD = 1.00d; 95% CI, 0.34-1.67; p = 0.003; I2 = 66.6%, Fig. 4D), increased postoperative complications (12 studies, RR = 2.28; 95% CI, 1.88-2.77; p < 0.001; I2 = 0.0%, Fig. 4E) and increased 90-day mortality (7 studies, RR = 3.08; 95% CI, 1.88-5.03; p < 0.001; I2 = 0.0%, Fig. 4F). 12 studies showed that PSR group was associated with a higher rate of positive resection margin (RR = 0.77; 95% CI, 0.61-0.97; p = 0.024; I2 = 33.9%, Fig. 5A). 15 studies indicated that intrahepatic recurrence was more obvious in PSR group (RR = 0.90; 95% CI, 0.82-0.98; p = 0.021; I2 = 26.2%, Fig. 5B). 14 studies suggested a higher repeat hepatectomy rate in PSR group (RR = 0.64; 95% CI, 0.55-0.76; p < 0.001; I2 = 12.4%, Fig. 5C). In terms of short-term outcomes, there were certain differences between the PSR and AR groups.
Fig. 4.
Forest plots of potential effects of AR versus PSR on short-term outcomes. Duration of operation (A), estimated blood loss (B), intraoperative blood transfusion (C), length of hospital stay (D), postoperative complications (E), and 90-day mortality (F)
Fig. 5.
Forest plots of potential effects of AR versus PSR on short-term outcomes. Positive margin (A), intrahepatic recurrence (B), and repeat hepatectomy (C)
Sensitivity analysis and publication bias
Sensitivity analysis of long-term and short-term outcomes obtained robust results. No significant changes in effect values were observed after sequentially removing one study compared to the overall analysis. Funnel plots were used to display publication bias. A symmetrical distribution of funnel plots could be observed. The publication bias test (Egger) confirmed that there was no publication bias in all included studies (Fig. S2-3).
Discussion
Hepatectomy is a well-established treatment option for CRLM, in an attempt to achieve complete tumor resection while preserving sufficient residual healthy liver parenchyma to limit the risk of postoperative liver dysfunction/failure [36]. AR is recommended for therapeutic resection when liver metastases are relatively large or multiple, or when tumors invade the portal veins. However, extensive liver resection may be associated with post-hepatectomy liver failure [37]. PSR has been increasingly recognized as an appropriate and effective treatment in recent years, however, whether the non-anatomical nature of PSR leads to recurrence and worse long-term outcomes remains debated.
This meta-analysis has been focused on the differences in perioperative and long-term outcomes between AR and PSR for CRLM therapy. We retrospectively analyzed 7228 patients with CRLM from 22 independent studies. Our results indicated that compared with AR, PSR had better perioperative prognosis, shorter operative time, less intraoperative bleeding, a lower blood transfusion rate, fewer postoperative complications, and reduced 90-day mortality. Despite this benefit, there was a slightly higher incidence of positive margin, an increased risk of postoperative intrahepatic recurrence, and an increased rate of repeat resections for PSR. Long-term outcomes, including OS, DFS and liver-RFS, were comparable between AR and PSR without significant differences. We performed an updated meta-analysis and added new outcomes, such as positive resection margin, intrahepatic recurrence, and repeat hepatectomy rate, which is an innovation of this meta-analysis.
AR was likely to remove undetected micro-metastases and to obtain adequate tumor-free margins. The risk of postoperative recurrence of PSR was generally limited, while AR may not have a preventive effect on intrahepatic or extrahepatic recurrence [18, 31]. Recent studies have demonstrated that PSR is preferred for the treatment of resectable CRLM when permitted by the tumor size and location, without increasing the risk of remnant liver recurrence, associated with lower postoperative morbidity and shorter hospital stay, and with an equal oncological outcome [9, 38–40]. Burlaka et al. confirmed that parenchyma-sparing surgery should be a priority pathway for complex treatment of patients with deeply located lesions of the right liver lobe and bilobar liver metastases [41].
At present, the biggest question about PSR is whether it increases intrahepatic recurrence, which is an important predictor of survival outcome of CRLM patients after hepatectomy. Given that the goal of PSR is to minimize resection of the normal liver without sacrificing oncologic outcome, an increased risk of intrahepatic recurrence and a higher rate of repeat resections may not be surprising. In patients with small solitary CRLM, parenchymal-sparing hepatectomy (PSH) has no negative effect on OS, RFS, Liver-RFS, and does not increase the recurrence of liver remnants. However, in the case of liver recurrence, salvage repeat hepatectomy after PSR improves 5-year survival rate in patients with recurrence [8, 10, 42–44]. The greatest advantage of PSR lies in the increased treatment options after recurrence, especially the increased chance of reoperation, resulting in prolonged survival. Therefore, if PSR is performed first, with sufficient remnants of healthy liver tissues, repeat hepatectomy can be associated with improved OS [8, 43, 45]. For patients with more than 6 lesions, the survival time of patients with PSR was significantly longer than that of patients with major hepatectomy [46]. In a short-term and long-term study of 1720 patients with liver tumors < 30 mm in the right lobe who underwent PSH or right-lobe hepatectomy, 5-year RFS and OS were comparable between the two groups. However, repeated hepatectomy was performed more frequently in PSH. And the 5-year OS in PSH group was significantly higher than right-lobe hepatectomy group [29]. These data suggest that PSR has better oncologic benefit for repeat hepatectomy in the setting of recurrence. Our meta-analysis results showed that patients treated with PSR had certain risk of intrahepatic recurrence, however, the two procedures yielded comparable results in terms of 3-year and 5-year liver-RFS. The 5 year-OS was increased in PSR group by parenchyma-sparing repeat hepatectomy. Therefore, this meta-analysis helps to strengthen the application of PSR in CRLM. In the surgical decision-making of CRLM, it is necessary to ensure the radical resection of all lesions, retain as much liver parenchyma as possible, with little impact on liver function and low postoperative complications, and can increase the possibility of second resection after recurrence, thereby improving survival.
However, this meta-analysis has several limitations. Although we have conducted an extensive review of the literature available, all the included studies were non-randomized, single-center studies, which introduced selection bias and the risk of non-sufficient clinical evidence. Second, the choice of resection methods and patients’ baseline parameters may influence the analytic results. Our study collected 7228 CRLM patients from 22 studies, as an update of the current discussion of AR and PSR surgical outcomes.
In conclusion, our meta-analysis suggests that PSR has comparable safety and efficacy to AR, with favorable perioperative outcomes without compromising oncological outcomes. However, high-quality multicenter randomized controlled trials are needed in the future to validate the robustness of our findings.
Supplementary Information
Additional file 1: Fig. S1. Forest plots of the effect of AR versus PSR on 3-year liver recurrence-free survival (liver-RFS) (A) and 5-year liver-RFS (B). Fig. S2. Funnel plots of cumulative OS (A), 3-year OS (B), 5-year OS (C), cumulative DFS (D), 3-year DFS (E), 5-year DFS (F), 3-year liver-RFS (G), and 5-year liver-RFS (H). Fig. S3. Funnel plots of short-term outcomes. Duration of operation (A), estimated blood loss (B), intraoperative blood transfusion (C), length of hospital stay (D), postoperative complications (E), 90‐day mortality (F), positive margin (G), intrahepatic recurrence (H), and repeat hepatectomy (I).
Additional file 2: Table S1. Quality of studies evaluated by modified Newcastle-Ottawa scale.
Additional file 3. Supplemental file. The full electronic search strategy for each database.
Acknowledgements
We would like to thank all of the participants.
Authors’ contributions
Study conception, design and data analysis were performed by K.W. and Y.L. Material preparation and data collection were performed by M.D.H. and H.M.L.; X.Q.L. and D.J.Y. prepared Fig. 4 and Fig. 5. L.D. led the conceptualization and provided valuable supervision and oversight of the project. K.W. wrote the manuscript, all authors discussed and revised the previous versions of the manuscript. All authors reviewed and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (Grant # 82170525).
Availability of data and materials
The data analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval was not necessary, as this study was a “Systematic Review and Meta-analysis”. There are no individual person’s data and presentations of case reports involved in this article.
Consent for publication
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with ICMJE criteria.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–254. doi: 10.3322/caac.21772. [DOI] [PubMed] [Google Scholar]
- 2.Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin. 2022;72(4):372–401. doi: 10.3322/caac.21728. [DOI] [PubMed] [Google Scholar]
- 3.Ivey GD, Johnston FM, Azad NS, Christenson ES, Lafaro KJ, Shubert CR. Current surgical management strategies for colorectal cancer liver metastases. Cancers (Basel) 2022;14(4):1063. doi: 10.3390/cancers14041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RP, Kokudo N, Folprecht G, Mise Y, Unno M, Malik HZ, et al. Colorectal liver metastases: a critical review of State of the Art. Liver Cancer. 2016;6(1):66–71. doi: 10.1159/000449348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cady B, Jenkins RL, Steele GD, Jr, Lewis WD, Stone MD, McDermott WV, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227(4):566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moris D, Dimitroulis D, Vernadakis S, Papalampros A, Spartalis E, Petrou A, et al. Parenchymal-sparing hepatectomy as the new doctrine in the treatment of liver-metastatic colorectal disease: beyond oncological outcomes. Anticancer Res. 2017;37(1):9–14. doi: 10.21873/anticanres.11283. [DOI] [PubMed] [Google Scholar]
- 7.Keck J, Gaedcke J, Ghadimi M, Lorf T. Surgical therapy in patients with colorectal liver metastases. Digestion. 2022;103(4):245–252. doi: 10.1159/000524022. [DOI] [PubMed] [Google Scholar]
- 8.Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg. 2016;263(1):146–152. doi: 10.1097/SLA.0000000000001194. [DOI] [PubMed] [Google Scholar]
- 9.Andreou A, Gloor S, Inglin J, Di Pietro MC, Banz V, Lachenmayer A, et al. Parenchymal-sparing hepatectomy for colorectal liver metastases reduces postoperative morbidity while maintaining equivalent oncologic outcomes compared to non-parenchymal-sparing resection. Surg Oncol. 2021;38:101631. doi: 10.1016/j.suronc.2021.101631. [DOI] [PubMed] [Google Scholar]
- 10.Okumura S, Tabchouri N, Leung U, Tinguely P, Louvet C, Beaussier M, et al. Laparoscopic parenchymal-sparing hepatectomy for multiple colorectal liver metastases improves outcomes and salvageability: a propensity score-matched analysis. Ann Surg Oncol. 2019;26(13):4576–4586. doi: 10.1245/s10434-019-07902-x. [DOI] [PubMed] [Google Scholar]
- 11.Andreou A, Knitter S, Schmelzle M, Kradolfer D, Maurer MH, Auer TA, et al. Recurrence at surgical margin following hepatectomy for colorectal liver metastases is not associated with R1 resection and does not impact survival. Surgery. 2021;169(5):1061–1068. doi: 10.1016/j.surg.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMatteo RP, Palese C, Jarnagin WR, Sun RL, Blumgart LH, Fong Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4(2):178–184. doi: 10.1016/s1091-255x(00)80054-2. [DOI] [PubMed] [Google Scholar]
- 18.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg. 2001;181(2):153–159. doi: 10.1016/s0002-9610(00)00560-2. [DOI] [PubMed] [Google Scholar]
- 19.Stewart GD, O'Súilleabháin CB, Madhavan KK, Wigmore SJ, Parks RW, Garden OJ. The extent of resection influences outcome following hepatectomy for colorectal liver metastases. Eur J Surg Oncol. 2004;30(4):370–376. doi: 10.1016/j.ejso.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Zorzi D, Mullen JT, Abdalla EK, Pawlik TM, Andres A, Muratore A, et al. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg. 2006;10(1):86–94. doi: 10.1016/j.gassur.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Finch RJ, Malik HZ, Hamady ZZ, Al-Mukhtar A, Adair R, Prasad KR, et al. Effect of type of resection on outcome of hepatic resection for colorectal metastases. Br J Surg. 2007;94(10):1242–1248. doi: 10.1002/bjs.5640. [DOI] [PubMed] [Google Scholar]
- 22.Guzzetti E, Pulitanò C, Catena M, Arru M, Ratti F, Finazzi R, et al. Impact of type of liver resection on the outcome of colorectal liver metastases: a case-matched analysis. J Surg Oncol. 2008;97(6):503–507. doi: 10.1002/jso.20979. [DOI] [PubMed] [Google Scholar]
- 23.Sarpel U, Bonavia AS, Grucela A, Roayaie S, Schwartz ME, Labow DM. Does anatomic versus nonanatomic resection affect recurrence and survival in patients undergoing surgery for colorectal liver metastasis? Ann Surg Oncol. 2009;16(2):379–384. doi: 10.1245/s10434-008-0218-2. [DOI] [PubMed] [Google Scholar]
- 24.Lalmahomed ZS, Ayez N, van der Pool AE, Verheij J, JN IJ, Verhoef C. Anatomical versus nonanatomical resection of colorectal liver metastases: is there a difference in surgical and oncological outcome? World J Surg. 2011; 35 (3): 656–661. [DOI] [PMC free article] [PubMed]
- 25.van Dam RM, Lodewick TM, van den Broek MA, de Jong MC, Greve JW, Jansen RL, et al. Outcomes of extended versus limited indications for patients undergoing a liver resection for colorectal cancer liver metastases. HPB (Oxford) 2014;16(6):550–559. doi: 10.1111/hpb.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuki R, Mise Y, Saiura A, Inoue Y, Ishizawa T, Takahashi Y. Parenchymal-sparing hepatectomy for deep-placed colorectal liver metastases. Surgery. 2016;160(5):1256–1263. doi: 10.1016/j.surg.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura M, Mise Y, Saiura A, Inoue Y, Ishizawa T, Ichida H, et al. Parenchymal-sparing hepatectomy does not increase intrahepatic recurrence in patients with advanced colorectal liver metastases. Ann Surg Oncol. 2016;23(11):3718–3726. doi: 10.1245/s10434-016-5278-0. [DOI] [PubMed] [Google Scholar]
- 28.Memeo R, de Blasi V, Adam R, Goéré D, Azoulay D, Ayav A, et al. Parenchymal-sparing hepatectomies (PSH) for bilobar colorectal liver metastases are associated with a lower morbidity and similar oncological results: a propensity score matching analysis. HPB (Oxford) 2016;18(9):781–790. doi: 10.1016/j.hpb.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosokawa I, Allard MA, Mirza DF, Kaiser G, Barroso E, Lapointe R, et al. Outcomes of parenchyma-preserving hepatectomy and right hepatectomy for solitary small colorectal liver metastasis: a LiverMetSurvey study. Surgery. 2017;162(2):223–232. doi: 10.1016/j.surg.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Lordan JT, Roberts JK, Hodson J, Isaac J, Muiesan P, Mirza DF, et al. Case-controlled study comparing peri-operative and cancer-related outcomes after major hepatectomy and parenchymal sparing hepatectomy for metastatic colorectal cancer. HPB (Oxford) 2017;19(8):688–694. doi: 10.1016/j.hpb.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Donadon M, Cescon M, Cucchetti A, Cimino M, Costa G, Pesi B, et al. Parenchymal-sparing surgery for the surgical treatment of multiple colorectal liver metastases is a safer approach than major hepatectomy not impairing patients' prognosis: a bi-institutional propensity score-matched analysis. Dig Surg. 2018;35(4):342–349. doi: 10.1159/000479336. [DOI] [PubMed] [Google Scholar]
- 32.Pandanaboyana S, Bell R, White A, Pathak S, Hidalgo E, Lodge P, et al. Impact of parenchymal preserving surgery on survival and recurrence after liver resection for colorectal liver metastasis. ANZ J Surg. 2018;88(1–2):66–70. doi: 10.1111/ans.13588. [DOI] [PubMed] [Google Scholar]
- 33.Spelt L, Ansari D, Swanling M, Holka P, Andersson R. Parenchyma-sparing hepatectomy (PSH) versus non-PSH for bilobar liver metastases of colorectal cancer. Ann Gastroenterol. 2018;31(1):115–120. doi: 10.20524/aog.2017.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joechle K, Vreeland TJ, Vega EA, Okuno M, Newhook TE, Panettieri E, et al. Anatomic resection is not required for colorectal liver metastases with RAS mutation. J Gastrointest Surg. 2020;24(5):1033–1039. doi: 10.1007/s11605-019-04299-6. [DOI] [PubMed] [Google Scholar]
- 35.She WH, Cheung TT, Ma KW, Tsang SHY, Dai WC, Chan ACY, et al. Anatomical versus nonanatomical resection for colorectal liver metastasis. World J Surg. 2020;44(8):2743–2751. doi: 10.1007/s00268-020-05506-1. [DOI] [PubMed] [Google Scholar]
- 36.Jo HS, Kim DS, Jung SW, Yu YD, Choi SB, Kim WB, et al. Clinical significance of post-hepatectomy hepatic failure in patients with liver metastases from colorectal cancer. Ann Hepatobiliary Pancreat Surg. 2018;22(2):93–100. doi: 10.14701/ahbps.2018.22.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149(5):713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Sui CJ, Cao L, Li B, Yang JM, Wang SJ, Su X, et al. Anatomical versus nonanatomical resection of colorectal liver metastases: a meta-analysis. Int J Colorectal Dis. 2012;27(7):939–946. doi: 10.1007/s00384-011-1403-5. [DOI] [PubMed] [Google Scholar]
- 39.Tang H, Li B, Zhang H, Dong J, Lu W. Comparison of anatomical and nonanatomical hepatectomy for colorectal liver metastasis: a meta-analysis of 5207 patients. Sci Rep. 2016;6:32304. doi: 10.1038/srep32304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng G, Li H, Jia GQ, Fang D, Tang YY, Xie J, et al. Parenchymal-sparing versus extended hepatectomy for colorectal liver metastases: a systematic review and meta-analysis. Cancer Med. 2019;8(14):6165–6175. doi: 10.1002/cam4.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burlaka AA, Makhmudov DE, Lisnyi II, Paliichuk AV, Zvirych VV, Lukashenko AV. Parenchyma-sparing strategy and oncological prognosis in patients with colorectal cancer liver metastases. World J Surg Oncol. 2022;20(1):122. doi: 10.1186/s12957-022-02579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viganò L, Capussotti L, Lapointe R, Barroso E, Hubert C, Giuliante F, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014;21(4):1276–1286. doi: 10.1245/s10434-013-3421-8. [DOI] [PubMed] [Google Scholar]
- 43.Ahmad A, Chen SL, Bilchik AJ. Role of repeated hepatectomy in the multimodal treatment of hepatic colorectal metastases. Arch Surg. 2007;142(6):526–531. doi: 10.1001/archsurg.142.6.526. [DOI] [PubMed] [Google Scholar]
- 44.Andreou A, Brouquet A, Abdalla EK, Aloia TA, Curley SA, Vauthey JN. Repeat hepatectomy for recurrent colorectal liver metastases is associated with a high survival rate. HPB (Oxford) 2011;13(11):774–782. doi: 10.1111/j.1477-2574.2011.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Battula N, Tsapralis D, Mayer D, Isaac J, Muiesan P, Sutcliffe RP, et al. Repeat liver resection for recurrent colorectal metastases: a single-centre, 13-year experience. HPB (Oxford) 2014;16(2):157–163. doi: 10.1111/hpb.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka K, Shimada H, Matsumoto C, et al. Impact of the degree of liver resection on survival for patients with multiple liver metastases from colorectal cancer[J] World J Surg. 2008;32(9):2057–2069. doi: 10.1007/s00268-008-9610-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Forest plots of the effect of AR versus PSR on 3-year liver recurrence-free survival (liver-RFS) (A) and 5-year liver-RFS (B). Fig. S2. Funnel plots of cumulative OS (A), 3-year OS (B), 5-year OS (C), cumulative DFS (D), 3-year DFS (E), 5-year DFS (F), 3-year liver-RFS (G), and 5-year liver-RFS (H). Fig. S3. Funnel plots of short-term outcomes. Duration of operation (A), estimated blood loss (B), intraoperative blood transfusion (C), length of hospital stay (D), postoperative complications (E), 90‐day mortality (F), positive margin (G), intrahepatic recurrence (H), and repeat hepatectomy (I).
Additional file 2: Table S1. Quality of studies evaluated by modified Newcastle-Ottawa scale.
Additional file 3. Supplemental file. The full electronic search strategy for each database.
Data Availability Statement
The data analyzed during the current study are available from the corresponding author on reasonable request.