Key Points
Question
In patients with nonproliferative diabetic retinopathy (NPDR) and good vision but without center-involved diabetic macular edema (CI-DME), does early aflibercept reduce disease progression and improve long-term visual acuity compared with initial observation and treatment only if disease worsens?
Findings
This study presents 4-year primary outcomes of a randomized clinical trial that included 328 patients (399 eyes), randomized to 2.0 mg aflibercept injections or sham injections. Among those receiving aflibercept, proliferative diabetic retinopathy or CI-DME developed in 33.9% vs 56.9% among those who received sham—a difference that was statistically significant. Change in visual acuity was −2.7 vs −2.4 letters, a difference that was not statistically significant.
Meaning
At 4 years, treatment of NPDR with aflibercept vs sham treatment resulted in statistically significant anatomic improvement, but no improvement in visual acuity.
Abstract
Importance
Anti–vascular endothelial growth factor (VEGF) injections in eyes with nonproliferative diabetic retinopathy (NPDR) without center-involved diabetic macular edema (CI-DME) reduce development of vision-threatening complications from diabetes over at least 2 years, but whether this treatment has a longer-term benefit on visual acuity is unknown.
Objective
To compare the primary 4-year outcomes of visual acuity and rates of vision-threatening complications in eyes with moderate to severe NPDR treated with intravitreal aflibercept compared with sham. The primary 2-year analysis of this study has been reported.
Design, Setting, and Participants
Randomized clinical trial conducted at 64 clinical sites in the US and Canada from January 2016 to March 2018, enrolling 328 adults (399 eyes) with moderate to severe NPDR (Early Treatment Diabetic Retinopathy Study [ETDRS] severity level 43-53; range, 0 [worst] to 100 [best]) without CI-DME.
Interventions
Eyes were randomly assigned to 2.0 mg aflibercept (n = 200) or sham (n = 199). Eight injections were administered at defined intervals through 2 years, continuing quarterly through 4 years unless the eye improved to mild NPDR or better. Aflibercept was given in both groups to treat development of high-risk proliferative diabetic retinopathy (PDR) or CI-DME with vision loss.
Main Outcomes and Measures
Development of PDR or CI-DME with vision loss (≥10 letters at 1 visit or ≥5 letters at 2 consecutive visits) and change in visual acuity (best corrected ETDRS letter score) from baseline to 4 years.
Results
Among participants (mean age 56 years; 42.4% female; 5% Asian, 15% Black, 32% Hispanic, 45% White), the 4-year cumulative probability of developing PDR or CI-DME with vision loss was 33.9% with aflibercept vs 56.9% with sham (adjusted hazard ratio, 0.40 [97.5% CI, 0.28 to 0.57]; P < .001). The mean (SD) change in visual acuity from baseline to 4 years was −2.7 (6.5) letters with aflibercept and −2.4 (5.8) letters with sham (adjusted mean difference, −0.5 letters [97.5% CI, −2.3 to 1.3]; P = .52). Antiplatelet Trialists’ Collaboration cardiovascular/cerebrovascular event rates were 9.9% (7 of 71) in bilateral participants, 10.9% (14 of 129) in unilateral aflibercept participants, and 7.8% (10 of 128) in unilateral sham participants.
Conclusions and Relevance
Among patients with NPDR but without CI-DME at 4 years treatment with aflibercept vs sham, initiating aflibercept treatment only if vision-threatening complications developed, resulted in statistically significant anatomic improvement but no improvement in visual acuity. Aflibercept as a preventive strategy, as used in this trial, may not be generally warranted for patients with NPDR without CI-DME.
Trial Registration
ClinicalTrials.gov Identifier: NCT02634333
This randomized clinical trial analyzes the effects of aflibercept injections vs sham for reducing disease progression and improving long-term visual acuity in patients with nonproliferative diabetic retinopathy.
Introduction
Anti–vascular endothelial growth factor (VEGF) treatment reduces but does not eliminate the incidence of vision-threatening complications of diabetes, and it is highly but not 100% effective for the complications once they occur. The DRCR Retina Network Protocol W aimed to answer the question, “does prophylactic anti-VEGF treatment in eyes at high risk of vision-threatening complications of diabetes result in better anatomic and vision outcomes at 4 years when compared with observation with anti-VEGF treatment initiated only after a vision-threatening complication has occurred?”
The 2-year primary analysis of this trial showed that aflibercept (2 mg every 16 weeks after 3 monthly loading doses) reduced diabetic retinopathy severity, lowered incidence of vision-impairing center-involved diabetic macular edema (CI-DME), and reduced risk of progression to proliferative diabetic retinopathy (PDR) compared with sham.1 The probability of developing CI-DME with vision loss or PDR within 2 years was approximately 16% (aflibercept) vs 43% (sham). These results were supported by PANORAMA, a similar study of prophylaxis that randomly assigned eyes with moderately severe to severe nonproliferative diabetic retinopathy (NPDR) to aflibercept (2 mg every 16 weeks or 2 mg every 8 weeks then as needed).2
Despite the anatomic benefits, neither this trial nor PANORAMA demonstrated an associated visual acuity benefit over 2 years with anti-VEGF therapy. Mean (SD) change in visual acuity from baseline to 2 years in this trial decreased by 0.9 (5.8) letters in aflibercept and 2.0 (6.1) letters in sham, a difference neither statistically significant (adjusted mean difference, 0.5 letters [97.5% CI, −1.0 to 1.9]; P = .47) nor clinically important.1 In PANORAMA, the visual acuity area under the curve (AUC) from baseline to 100 weeks improved by 1.5 letters (2 mg every 16 weeks) and 0.8 letters (2 mg every 8 weeks then as needed), compared with 0.6 letters with sham.
Reported herein are results for 4-year outcomes to determine whether early treatment leads to sustained anatomic benefits and longer-term superior visual acuity outcomes.
Methods
Methods for this trial were published previously, and the study protocol (Supplement 1) and statistical analysis plan (Supplement 2) are available online.1 This study adhered to the tenets of the Declaration of Helsinki3; sites received approval from their respective ethics board; all participants provided written informed consent, and an independent data and safety monitoring committee monitored the study every 6 months. The components of PDR and CI-DME outcomes, study procedures, sample size calculations, randomization, visit schedules, and masking are available online.1
Participants
Participants were adults with type 1 or type 2 diabetes who had at least 1 eye with severe NPDR, according to the enrolling investigator (moderate to severe NPDR [Diabetic Retinopathy Severity Scale {DRSS} levels 43-53] by central reading center grading of fundus photographs) and best-corrected visual acuity at least 79 letters (range, 0 [worst] to 100 [best]; Snellen equivalent 20/25 or better). Eyes with CI-DME, evidence of neovascularization within the 7-modified Early-Treatment Diabetic Retinopathy Study (ETDRS) fields on fluorescein angiography confirmed by a central reading center, prior panretinal photocoagulation (PRP), or history of DME or diabetic retinopathy treatment within the 12 months prior to enrollment were ineligible. Participants could have 1 (unilateral) or 2 (bilateral) study eyes enrolled, depending on the number eligible.
Randomization
Study eyes were randomized 1:1 to receive 2-mg intravitreal aflibercept injections (Regeneron) or sham injections stratified by baseline DRSS and study eye laterality, with random block sizes of 2 and 4. Treatment group assignment was masked to study participants, photographers, optical coherence tomography (OCT) technicians, reading center graders, and visual acuity testers, including refractionists.
Interventions
Prevention injections, whether aflibercept or sham, were given at baseline, 1, 2, and 4 months and then every 4 months through 2 years. In years 3 and 4, the 4-month prevention injections could be deferred if the clinical assessment of the eye indicated mild NPDR or better (DRSS level ≤35). If high-risk PDR (DRSS level ≥71) or CI-DME with vision loss (10 or more letters at 1 visit or 5 or more at 2 consecutive visits) developed, then aflibercept treatment was initiated and re-treatment for PDR (Protocol S) or CI-DME (Protocol T) was determined by DRCR Retina Network algorithms.4,5 Eyes could meet the outcome for PDR without meeting the high-risk PDR criteria for treatment.
Statistical Analyses
The primary 4-year visual acuity outcome was mean change in visual acuity (best-corrected ETDRS letter score) from baseline to 4 years. The mean treatment group difference was estimated using a linear mixed model with fixed effects to adjust for baseline visual acuity and the randomization stratification factors, a participant-level random intercept to account for correlation in bilateral participants, and Markov Chain Monte Carlo multiple imputation (100 imputations) for missing data. The imputation model assumed data are missing at random and included treatment group, study eye laterality, baseline DRSS, baseline visual acuity, and change in visual acuity from baseline to each protocol assessment visit up to and including 4 years. The primary 4-year anatomic outcome was the development of PDR or CI-DME with vision loss (whichever came first) within 4 years. The hazard ratio (HR) between treatment groups was estimated using a marginal Cox proportional hazards model with adjustment for the randomization stratification factors and a robust sandwich estimate of the covariance matrix to account for participant-level correlation. The proportional hazards assumption was verified using Martingale residuals.6 Outcome probabilities (cumulative incidence) were estimated using the Kaplan-Meier (product-limit) survival estimates.7 Eyes that did not meet the outcome were censored at the last completed visit.
The analysis of secondary outcomes mimicked the analysis of primary outcomes. The odds ratio (OR) between treatment groups was estimated for binary outcomes using logistic regression with generalized estimating equations and a participant-level exchangeable working correlation structure. A safety analysis evaluated ocular and systemic adverse events. The Workplace Productivity and Activity Impairment questionnaire was used to compare change in functional outcomes at the participant level (unilateral participants only). The primary analyses followed the as-randomized principle, analyzing all participants by randomized treatment group, regardless of treatment received, with missing data imputed with multiple imputation where applicable. Subgroup analysis was performed only for the primary outcomes in the prespecified subgroups: baseline DRSS; baseline noncentral DME; sex; and race and ethnicity (race and ethnicity were combined as a single subgroup; self-reported in National Institutes of Health–specified categories; data were collected to define the cohort and to conduct preplanned subgroup analyses). Sensitivity analyses included a per-protocol analysis of both primary outcomes (only including eyes ≥80% compliant with treatment according to protocol), as well as complete-case (no imputed values) and tipping-point (shifting of imputed values) analyses for the primary visual acuity outcome. Hypothesis tests were 2-sided with statistical significance determined at P < .025 (to account for 2.5% α spending on the 2-year analysis). At 2 and 4 years, hypothesis testing followed a hierarchal approach; a treatment group comparison of mean visual acuity change was only conducted if there was a statistically significant difference in the PDR/CI-DME composite outcome. With a planned sample size of 386 eyes, the power was estimated to be at least 89% for the primary PDR/CI-DME outcome (assuming true rates of 15% vs 30% at 2 years) and visual acuity outcome (assuming true difference was ≥3 with an SD of 8 letters). There was no formal multiplicity adjustment among secondary and safety outcomes, and thus, because of the potential for type I error, findings should be interpreted as exploratory. Descriptive statistics are based on observed data (no imputed values). Analyses were conducted with SAS SAS/STAT 15.1 (SAS Institute, Inc).
Results
This trial enrolled 399 eyes (328 participants) between January 2016 and March 2018 across 64 sites in the US and Canada. The last participant completed the 4-year visit on May 11, 2022. There were 200 eyes randomly assigned to aflibercept and 199 to sham. Baseline participant and study eye characteristics by treatment group are in Table 1, and by 4-year study visit completion are in eTable 1 in Supplement 3. Excluding deaths, 74.0% (271 of 366) of eyes completed the study and 80.2% (300 of 374) of eyes either completed the study or met the primary outcome while still enrolled (Figure 1).
Table 1. Baseline Participant and Study Eye Characteristics.
| Baseline characteristicsa | No. (%) | |
|---|---|---|
| Aflibercept (n = 200) | Sham (n = 199) | |
| Study eyes laterality | ||
| Unilateral | 129 (64.5) | 128 (64.3) |
| Bilateral | 71 (35.5) | 71 (35.7) |
| Female sex | 83 (41.5) | 86 (43.2) |
| Male sex | 117 (58.5) | 113 (56.8) |
| Age, y | ||
| Mean (SD) | 56.6 (10.1) | 55.0 (10.6) |
| Median (IQR) | 57 (51-63) | 56 (48-62) |
| Race and ethnicity | ||
| American Indian or Alaska Native | 1 (<1) | 0 |
| Asian | 10 (5.0) | 9 (4.5) |
| Black or African American | 29 (14.5) | 32 (16.1) |
| Hispanic or Latino | 62 (31.0) | 67 (33.7) |
| More than 1 race | 3 (1.5) | 1 (<1) |
| Native Hawaiian or Other Pacific Islander | 1 (<1) | 1 (<1) |
| Unknown or not reported | 2 (1.0) | 3 (1.5) |
| White | 92 (46.0) | 86 (43.2) |
| Diabetes | ||
| Type 1 | 12 (6.0) | 23 (11.6) |
| Type 2 | 188 (94.0) | 176 (88.4) |
| Duration, y | ||
| Mean (SD) | 16.4 (8.9) | 17.3 (9.7) |
| Median (IQR) | 17 (10-22) | 16 (11-22) |
| Insulin used | 139 (69.5) | 139 (69.8) |
| Hemoglobin A1c, %b | ||
| Mean (SD) | 8.8 (2.2) | 8.7 (2.0) |
| Median (IQR) | 8.6 (7.1-10.0) | 8.3 (7.3-9.7) |
| Mean arterial blood pressure, mm Hg | ||
| Mean (SD) | 99.7 (11.2) | 98.9 (11.2) |
| Median (IQR) | 100 (92-107) | 98 (91-106) |
| Body mass indexc | ||
| Mean (SD) | 32.9 (6.9) | 32.2 (6.6) |
| Median (IQR) | 31 (28-37) | 31 (27-36) |
| Prior myocardial infarction | 16 (8.0) | 19 (9.5) |
| Prior stroke | 13 (6.5) | 11 (5.5) |
| Preexisting kidney disease | 6 (3.0) | 6 (3.0) |
| Study eye characteristics | ||
| Visual acuity (best-corrected ETDRS)d | ||
| Mean (SD), letter score | 87.4 (4.5) | 87.9 (4.6) |
| Median (IQR), letter score | 88 (84-90) | 88 (85-91) |
| Median, Snellen equivalent | 20/20 | 20/20 |
| 20/20 or better (≥84 letters) | 156 (78.0) | 161 (80.9) |
| OCT machinee | ||
| Heidelberg Spectralis | 111 (55.5) | 112 (56.3) |
| Zeiss Cirrus | 89 (44.5) | 87 (43.7) |
| OCT central subfield thickness, μmf | ||
| Mean (SD) | 281 (26) | 280 (24) |
| Median (IQR) | 283 (264-300) | 283 (265-299) |
| OCT retinal volume, mm3g,h | ||
| Mean (SD) | 7.4 (0.7) | 7.4 (0.7) |
| Median (IQR) | 7.4 (7.0-7.9) | 7.4 (6.9-7.8) |
| Diabetic retinopathy severity score | ||
| Moderate NPDR (level 43) | 33 (16.5) | 35 (17.6) |
| Moderately severe NPDR (level 47A) | 65 (32.5) | 61 (30.7) |
| Moderately severe NPDR (level 47B-D) | 54 (27.0) | 55 (27.6) |
| Severe NPDR (level 53) | 48 (24.0) | 48 (24.1) |
| Intraocular pressure, mm Hgi | ||
| Mean (SD) | 15.3 (3.1) | 15.1 (3.1) |
| Median (IQR) | 15 (13-17) | 15 (13-17) |
| Lens status | ||
| Phakic (natural lens) | 166 (83.0) | 167 (83.9) |
| Posterior chamber intraocular lens (surgically replaced lens) | 34 (17.0) | 32 (16.1) |
| Noncentral DMEj,k | 75 (39.9) | 83 (42.6) |
| Prior treatment for DMEl | 19 (9.5) | 22 (11.1) |
| Prior anti-VEGF for DME | 8 (4.0) | 5 (2.5) |
| Prior focal/grid laser for DME | 12 (6.0) | 19 (9.5) |
Abbreviations: DME, diabetic macular edema; ETDRS, Early Treatment Diabetic Retinopathy Study; NPDR, nonproliferative diabetic retinopathy; OCT, optical coherence tomography; VEGF, vascular endothelial growth factor.
All baseline characteristics reflect data collected at the randomization visit except for diabetic retinopathy severity, which was collected on fundus photos at the screening visit.
Values for hemoglobin A1c were missing for 11 aflibercept and 9 sham participants.
Calculated as weight in kilograms divided by height in meters squared.
Visual acuity letter score measured after protocol-defined refraction using an electronic ETDRS test (range, 0 [worst] to 100 [best] letters [Snellen equivalent of <20/800 to 20/10]).
Technology uses light waves to obtain cross-sectional images of the retina.
Heidelberg Spectralis machine equivalent (normative mean ≈ 262 μm for females; 278 μm for males).8
Values for OCT retinal volume were missing for 12 aflibercept and 4 sham eyes.
Intraocular pressure is the fluid pressure inside the eye measured with a tonometry test (normative mean ≈ 15 mm Hg).10
Indicates at least 2 noncentral macular subfields with OCT thickness above threshold (mean [SD] 2) or at least 1 noncentral macular subfield with OCT thickness at least 15 μm above threshold (mean [SD] 2).
Values for noncentral DME status were missing for 12 aflibercept and 4 sham eyes.
Indicates no treatment for DME or retinopathy in the 12 months preceding baseline.
Figure 1. Study Selection, Randomization, and Flow of Participants’ Eyes.
Protocol-specified visits occurred at baseline (randomization) and at target periods of 1 month (± 2-wk range), 2 months (± 1-wk range), 4 months (± 8-wk range); then every 4 months (± 12-wk range for annual visits; ± 8-wk range otherwise) through 4 years (range, −12 weeks to +24 weeks).
aOf the 328 participants who were randomized, 71 bilateral participants (30 females and 41 males) had 1 eye randomized to receive afibercept and 1 eye randomized to receive sham.
bThe 137 patients’ eyes that completed the 4-year visit indicates 68.5% of the 200 randomized (74.9% excluding 17 patients who died).
cThe 134 patients’ eyes that completed the 4-year visit indicates 67.3% of the 199 randomized (73.2% excluding 16 patients who died).
dDeaths exclude 1 participant (1 study eye) in the aflibercept group who completed a visit in the 4-year visit window but died before completing the designated 4-year study visit.
eThe primary analysis of the key outcomes included all randomized participants using all available follow-up time for the anatomical outcome (time to development of proliferative diabetic retinopathy or center-involved diabetic macula edema) and multiple imputation for missing data of the visual acuity outcome (change in visual acuity from baseline to 4 years).
fThe per-protocol analysis included eyes that were at least 80% adherent with protocol injections (aflibercept or sham) prior to the event or censoring time (for the primary anatomical outcome) and prior to the 4-year visit (for the primary visual acuity outcome). The compete-case analysis for the primary visual acuity outcome included all eyes that completed the 4-year visit.
Visual Acuity
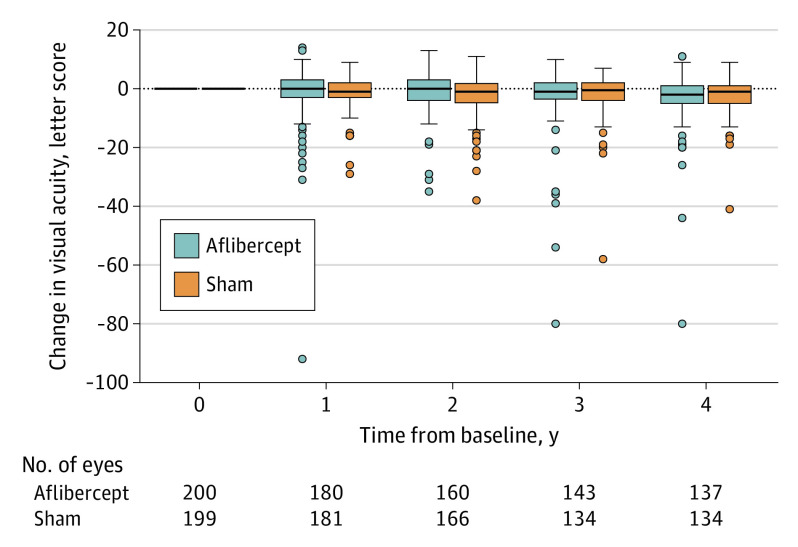
Visual acuity decreased from baseline to 4 years by a mean (SD) of 2.7 (6.5) letters for eyes in the aflibercept group vs 2.4 (5.8) letters for eyes in the sham group (adjusted mean difference, −0.5 [97.5% CI, −2.3 to 1.3]; P = .52), with mean (SD) AUC in visual acuity change over 4 years of −1.0 (4.2) letters for eyes in the aflibercept group vs −1.3 (3.6) letters for eyes in the sham group (adjusted mean difference, −0.2 [97.5% CI, −1.2 to 0.8]; P = .65) (Figure 2, Table 2). At 4 years, 13.1% (18 of 137) of eyes in the aflibercept group vs 12.7% (17 of 134) in the sham group were 10 or more letters worse than baseline (adjusted OR, 1.11 [97.5% CI, 0.55 to 2.22]; P = .74). Visual acuity was 20/40 or better in 95.6% (131 of 137) of eyes in the aflibercept group and 96.3% (129 of 134) of eyes in the sham group at 4 years, and visual acuity was 20/20 or better in 65.0% (89 of 137) of eyes in the aflibercept group and 68.7% (92 of 134) of eyes in the sham group (Table 2). Treatment effect on visual acuity was not meaningfully different between any preplanned subgroups analyzed (baseline DRSS, baseline noncentral DME, race and ethnicity, or sex; eFigure 1 and eTable 2 in Supplement 3).
Figure 2. Distribution of Visual Acuity Change From Baseline Through 4 Years.
The horizontal line in the box plots indicates the median, the top of the box indicates the 75th percentile, the bottom of the box indicates the 25th percentile, the whiskers indicate the nearest quartile to the most extreme data point within 1.5 times the IQR, and circles indicate values beyond these limits.
Table 2. Visual Acuity Outcomes at 4 Years.
| Visual acuity (best-corrected Early Treatment Diabetic Retinopathy Study) outcomes | No. (%)a | Aflibercept vs sham | |||
|---|---|---|---|---|---|
| Aflibercept (n = 137) | Sham (n = 134) | Primary (n = 399)b | Complete case (n = 271)c | Per-protocol (n = 196)d | |
| At baseline, letter score, mean (SD) | 87.4 (4.5) | 87.8 (4.7) | |||
| At 4 y, letter score, mean (SD) | 84.1 (11.1) | 85.3 (7.8) | |||
| Change from baseline to 4 y (primary outcome), letters, mean (SD)e | −2.7 (6.5) | −2.4 (5.8) | Mean difference, −0.5 (97.5% CI, −2.3 to 1.3)f | Mean difference, 0.0 (97.5% CI, −1.4 to 1.4)f | Mean difference, 0.2 (97.5% CI, −1.5 to 2.0)f |
| P value | .52 | >.99 | .78 | ||
| Average change over 4 y (area under the curve), letters, mean (SD) | −1.0 (4.2) | −1.3 (3.6) | Mean difference, −0.2 (97.5% CI, −1.2 to 0.8)f | ||
| P value | .65 | ||||
| Worsened ≥10 letters from baseline to 4 y | 18 (13.1) | 17 (12.7) | Odds ratio, 1.11 (97.5% CI, 0.55 to 2.22)g | ||
| P value | .74 | ||||
| Worsened ≥15 letters from baseline to 4 y | 8 (5.8) | 6 (4.5) | |||
| Worsened ≥5 letters from baseline to 4 y and the previous study visit | 25 (18.2) | 23 (17.2) | |||
| Improved ≥5 letters from baseline to 4 y and the previous study visit | 7 (5.1) | 5 (3.7) | |||
| Visual acuity of 20/20 or better (≥84 letters) at 4 y | 89 (65.0) | 92 (68.7) | |||
| Visual acuity of 20/40 or better (≥69 letters) at 4 y | 131 (95.6) | 129 (96.3) | |||
| Visual acuity of 20/200 or worse (≤38 letters) at 4 y | 2 (1.5) | 0 | |||
Numeric values are reported as No. (%) unless otherwise indicated.
The primary analysis followed the as-randomized principle, analyzing all participants by randomized treatment group (n = 200 aflibercept; n = 199 sham) regardless of treatment received, with multiple imputation (100 imputations) for missing data in the analysis of visual acuity change, area under the curve, and loss of 10 or more letters. The imputation model included treatment group, study eye laterality, baseline diabetic retinopathy severity score, baseline visual acuity, and change in visual acuity from baseline to each protocol assessment visit up to and including 4 years. A tipping point analysis identified shift parameters of 4 letters for aflibercept to be superior and 3 letters for sham to be superior.
The complete case analysis included 137 aflibercept eyes and 134 sham eyes that completed the 4-year visit.
The per-protocol analysis included 106 aflibercept eyes and 90 sham eyes that completed the 4-year visit, received at least 80% of injections (aflibercept or sham) according to protocol through the first 2 years and through 4 years, and had no other treatment for proliferative diabetic retinopathy or diabetic macular edema before the 4-year visit.
Outliers in visual acuity change from baseline to 4 years were truncated to within the overall mean (SD) 3 (−1.6 [3] × 6.7) of visual acuity change from baseline to 2 years. Values of outliers for the aflibercept group were −80, −44, and −26, and there was 1 outlier for the sham group (−41).
Mean differences between treatment groups were estimated using linear mixed models with a random intercept for the correlation between eyes of participants having 2 study eyes and fixed effects for treatment group, study eye laterality, baseline diabetic retinopathy severity score, and baseline visual acuity.
This adjusted odds ratio was estimated using generalized estimating equations with an exchangeable correlation structure for the correlation between eyes of participants having 2 study eyes and regression terms for treatment group, study eye laterality, baseline diabetic retinopathy severity score, and baseline visual acuity.
PDR and CI-DME
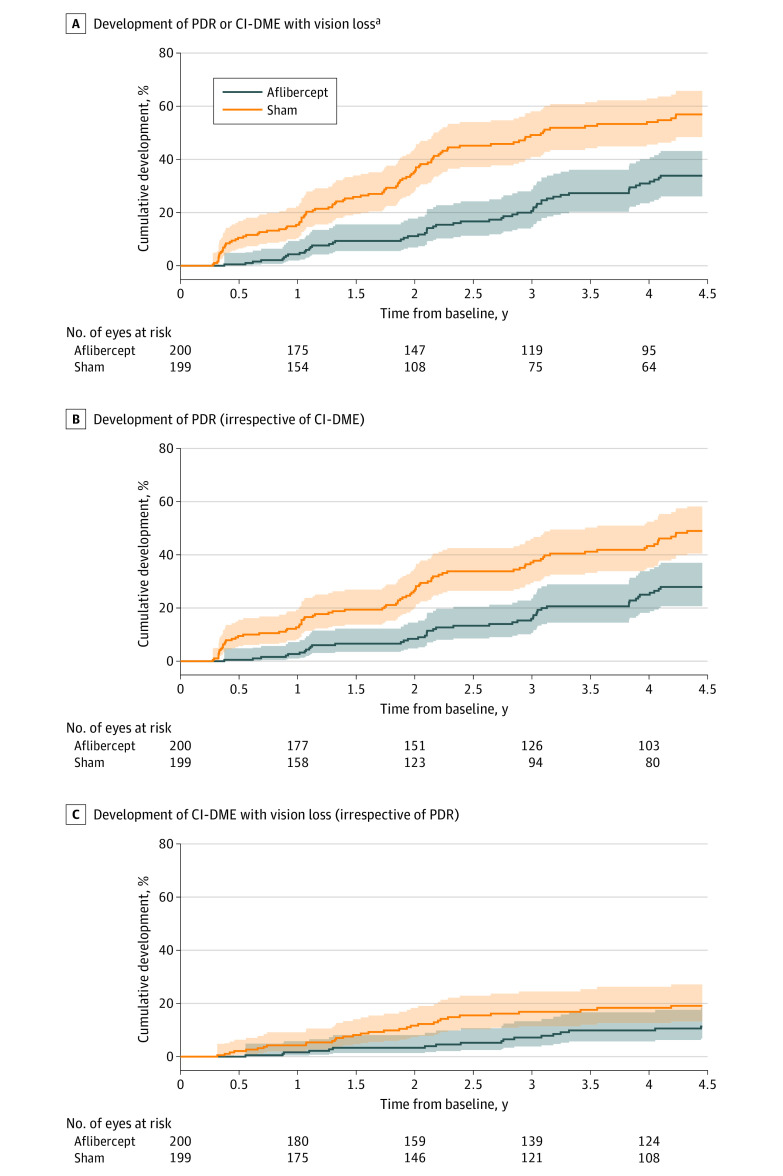
The 4-year cumulative incidence for PDR or CI-DME with vision loss was 33.9% in eyes in the aflibercept group vs 56.9% in eyes in the sham group (adjusted HR, 0.40 [97.5% CI, 0.28 to 0.57]; P < .001; Table 3 and Figure 3A). The probability of developing PDR within 4 years was 27.9% for eyes in the aflibercept group vs 49.0% for eyes in the sham group (adjusted HR, 0.42 [97.5% CI, 0.29 to 0.61]; P = <.001; Table 3 and Figure 3B), and the probability of developing CI-DME with vision loss was 11.3% for eyes in the aflibercept group vs 19.1% for eyes in the sham group (adjusted HR, 0.51 [97.5% CI, 0.27 to 0.97]; P = .02; Table 3 and Figure 3C). Difference in 4-year cumulative incidence (calculated from Kaplan-Meier estimates and standard errors) was 23.1% (97.5% CI, 10.8% to 35.3%) for any PDR or CI-DME outcome; 21.1% (97.5% CI, 9.0% to 33.1%) for any PDR outcome; and 7.8% (97.5% CI, −1.1% to 16.7%) for any CI-DME outcome. The most common event was neovascularization of the disc or elsewhere for PDR, with event probabilities of 26.9% for eyes in the aflibercept group and 44.0% for eyes in the sham group through 4 years. The 4-year cumulative incidence for all PDR and CI-DME outcome events are in Table 3, and the frequencies of the first qualifying events that determined the composite PDR and CI-DME outcomes are in eTable 3 in Supplement 3. The probability of developing high-risk PDR was 5.6% with aflibercept and 12.1% with sham. There was faster progression in eyes with higher baseline DRSS (P < .001), but the treatment effects within baseline DRSS subgroups were similar (eFigure 2 in Supplement 3). Treatment by subgroup interactions were not statistically significant for baseline DRSS, baseline noncentral DME, race and ethnicity, or sex (eFigure 3 and eTable 4 in Supplement 3).
Table 3. Development of PDR and CI-DME Outcomes Through 4 Years.
| PDR and CI-DME outcomesa | No. (4-y %) | Aflibercept vs sham, hazard ratio (97.5% CI)b | |||
|---|---|---|---|---|---|
| Aflibercept (n = 200) | Sham (n = 199) | Primary (n = 399)c | P value | Per-protocol (n = 327)d | |
| Development of PDR or CI-DME with vision loss (whichever came first, primary outcome)e | |||||
| Any PDR or CI-DME outcome | 54 (33.9) | 97 (56.9) | 0.40 (0.28 to 0.57) | <.001 | 0.37 (0.25 to 0.55) |
| Development of PDRe,f | |||||
| Any PDR outcome | 44 (27.9) | 82 (49.0) | 0.42 (0.29 to 0.61) | <.001 | |
| Neovascularization of the disc or elsewhere | 42 (26.9) | 74 (44.0) | |||
| Vitreous hemorrhage due to PDR | 12 (7.4) | 26 (16.2) | |||
| Preretinal hemorrhage due to PDRg | 6 (3.9) | 19 (11.6) | |||
| Traction retinal detachment due to PDR | 0 | 2 (1.3) | |||
| Neovascularization of the irish | 2 (1.4) | 1 (<1) | |||
| Neovascularization of the angle | 0 | 1 (<1) | |||
| Neovascular glaucoma | 0 | 2 (1.5) | |||
| Anti-VEGF for PDRi | 16 (10.2) | 42 (25.5) | |||
| Panretinal photocoagulation | 2 (1.2) | 6 (3.8) | |||
| Vitrectomy | 1 (<1) | 1 (<1) | |||
| Development of CI-DME with vision losse,j | |||||
| Any CI-DME outcome | 18 (11.3) | 32 (19.1) | 0.51 (0.27 to 0.97) | .02 | |
| CI-DME with ≥10% increase in center subfield thickness and ≥10 letter decrease in visual acuity at a single visitk | 12 (7.5) | 20 (12.0) | |||
| CI-DME with ≥10% increase in center subfield thickness and ≥5 letter decrease in visual acuity at 2 consecutive visitsk,l | 0 | 5 (3.0) | |||
| Anti-VEGF for CI-DMEm | 16 (9.9) | 29 (17.1) | |||
| Focal/grid laser | 3 (2.0) | 11 (7.0) | |||
| Corticosteroid | 2 (1.2) | 0 | |||
| Development of secondary outcomese | |||||
| Any PDR or CI-DME with vision loss criteria based on objective components defined in composite outcomen | 48 (30.3) | 88 (51.7) | |||
| CI-DME with ≥10% and ≥25 um increase in center subfield thickness | 18 (11.5) | 20 (12.6) | |||
| High-risk PDR (level ≥71) | 9 (5.6) | 20 (12.1) | |||
| Anti-VEGF for PDR or CI-DME | 30 (18.7) | 68 (40.7) | |||
Types of assessment used: reading center only (for neovascularization of the disc or elsewhere, CI-DME, and change in CST); reading center or clinical examination (for traction retinal detachment, vitreous hemorrhage, and preretinal hemorrhage); and clinical examination only (for neovascularization of the angle, neovascular glaucoma, neovascularization of the iris, change in visual acuity, and any treatment for PDR or CI-DME).
Hazard ratios were estimated using a marginal Cox regression model that adjusted for study eye laterality and baseline diabetic retinopathy severity score. The correlation between eyes of participants having 2 eyes in the study were modeled with a robust sandwich estimate of the covariance matrix.
The as-randomized principle was used to analyze all participants by randomized treatment group, regardless of treatment received. See Results for difference in 4-year cumulative incidence.
Included 163 eyes in aflibercept and 164 eyes in the sham group (that received ≥80% of injections according to protocol before meeting the outcome through the first 2 y and through 4 y when applicable; P <.001 for the hazard ratio).
Cumulative 4-year percentages were estimated using Kaplan-Meier estimates at the end of the 4-year visit window (1629 days after baseline). Eyes were considered at risk for an outcome until the outcome developed. Eyes that did not meet the outcome were censored at the time of the last completed visit. Censoring times for eyes censored at the 4-year visit were set to the end of the 4-year visit window for all analyses to prevent small numbers at risk from artificially inflating the outcome percentages at the end of the window.
Outcomes developed if PDR developed at any time during the study (irrespective of if/when CI-DME developed).
Indicates preretinal hemorrhage greater than one-half of the disc area.
Indicates neovascularization of the iris for at least 2 cumulative clock hours.
Not given without meeting another PDR outcome first.
Outcomes developed if CI-DME with vision loss developed at any time during the study (irrespective of if/when PDR developed).
Vision loss presumed to be from diabetic macular edema.
Consecutive visits at least 21 days apart.
Anti-VEGF for CI-DME was given to 6 aflibercept and 5 sham eyes without meeting another diabetic macular edema outcome first.
Included outcomes with reading center assessments on optical coherence tomography (CI-DME with visual acuity loss) or on fundus photos or fluorescein angiography (neovascularization of the disc or elsewhere, traction retinal detachment, vitreous hemorrhage, preretinal hemorrhage); excluded those based on clinical examinations.
Figure 3. Cumulative Percentages for Time From Baseline to Development of PDR or CI-DME With Vision Loss, PDR, and CI-DME With Vision Loss.
Cumulative percentages were estimated using Kaplan-Meier (product-limit) method. Shading indicates 97.5% CIs. Median time from baseline to event or censoring time: for panel A, 1370 (IQR, 698 to 1465) days in the aflibercept group and 783 (IQR, 378 to 1428) days in the sham group; for panel B, 1411 (IQR, 740 to 1465) days in the aflibercept group and 1040 (IQR, 413 to 1457) days in the sham group; and for panel C, 1421 (IQR, 859 to 1474) days in the aflibercept group and 1408 (IQR, 707 to 1463) days in the sham group.
aIndicates proliferative diabetic retinopathy (PDR) or center-involved diabetic macular edema (CI-DME), whichever develops first.
Four-year diabetic retinopathy severity was mild NPDR or better (DRSS level ≤35) for 50.4% (64 of 127) of eyes in the aflibercept group and 29.3% (36 of 123) for eyes in the sham group. From baseline to 4 years, more eyes in the aflibercept group improved by 2 or more steps in DRSS (54.3% [69 of 127] vs 28.5% [35 of 123] in the sham group; adjusted OR, 2.97 [97.5% CI, 1.70 to 5.19]; P < .001) and fewer in the aflibercept group worsened by 2 or more steps (11.0% [14 of 127] vs 22.8% [28 of 123] in the sham group; adjusted OR, 0.51 [97.5% CI, 0.28 to 0.91]; P = .009) (eTable 5 in Supplement 3).
Central subfield thickness, obtained using OCT, decreased from baseline to 4 years by a mean (SD) of 4 (33) μm for eyes in the aflibercept group vs 6 (27) μm for eyes in the sham group (adjusted difference, 2 (97.5% CI, −7 to 11; P = .62]; eFigure 4 in Supplement 3). At 4 years, 9.8% (13 of 133) of eyes in the aflibercept group vs 8.2% (11 of 134) of eyes in the sham group had CI-DME (with or without vision loss) according to sex and OCT machine thresholds.8 Additional OCT outcomes are in eFigure 5 and eTable 6 in Supplement 3.
Treatment for Diabetic Retinopathy and Diabetic Macular Edema
Among 4-year completers, eyes assigned to the aflibercept group received a mean (SD) of 13.0 (3.7) injections (8.7 [1.4] during years 1-2, and 4.3 [2.8] during years 3-4) with 79.6% (109 of 137) receiving at least 1 injection in year 4. Among eyes randomly assigned to receive sham, 40.3% (54 of 134) of eyes that completed the 4-year visit initiated aflibercept treatment, receiving a mean (SD) of 8.7 (5.1) aflibercept injections (3.1 [3.5] during years 1-2 and 5.6 [4.4] during years 3-4) (eTable 7 in Supplement). The probability of initiating anti-VEGF treatment for PDR or CI-DME within 4 years (adjusted for censoring) was 18.7% with aflibercept and 40.7% with sham (Table 3). Of all aflibercept injections administered to the aflibercept group, 90.1% (2011 of 2231) were for prevention only. Additional treatment information, including PRP and focal/grid laser administration, are in Table 3 and eTable 7 in Supplement 3. The mean (SD) number of study visits from baseline to 4 years among 4-year completers was 16.4 (4.5) for unilateral aflibercept participants and 19.4 (7.3) for unilateral sham participants.
Safety
Overall frequency of endophthalmitis per aflibercept injection was 0.1% (3 of 2794) in study eyes, and 0 of 1346 in nonstudy eyes receiving study aflibercept. Antiplatelet Trialists’ Collaboration cardiovascular/cerebrovascular adverse event rates were 9.9% (7 of 71) in bilateral participants,11 10.9% (14 of 129) in unilateral aflibercept participants, and 7.8% (10 of 128) in unilateral sham participants (unilateral aflibercept vs unilateral sham P = .53). Ocular and systemic adverse events of interest and complete adverse event lists are in eTables 8, 9, 10, and 11 in Supplement 3. Follow-up hemoglobin A1c appeared similar between treatment groups (eTable 12 in Supplement 3).
Workplace Productivity and Activity Impairment
Functional work-related outcomes due to vision problems over the past week including absenteeism (percentage of work time missed), presenteeism (percentage of impairment while working), and work productivity loss scores (a combination of absenteeism and presenteeism) are in eTable 13 in Supplement 3. The 4-year activity impairment score (percentage of activity impairment due to vision problems over the past week) in the unilateral participants of both treatment groups was a median of 0% (IQR, 0% to 30%). There were no meaningful differences between unilateral participants in change activity impairment or in work productivity loss from baseline to 2 or 4 years (eTable 13 in Supplement 3).
Discussion
Although early treatment with aflibercept reduced the risk of PDR or CI-DME with vision loss development in eyes with moderate to severe NPDR, it did not improve visual acuity over 4 years compared with aflibercept treatment only after the onset of high-risk PDR or CI-DME. Indeed, no subgroups were identified based on baseline DRSS, noncentral DME, race and ethnicity, or sex, for which there was an apparent benefit of early aflibercept treatment on visual acuity.
The fact that no additional visual acuity benefit was identified with early aflibercept treatment reflects the efficacy of aflibercept once high-risk PDR or CI-DME with vision loss develops. A minority of eyes (13% of each randomization group) lost 2 or more lines of vision from baseline to 4 years, even though the 4-year cumulative incidence of high-risk PDR was 5.6% in the aflibercept group and 12.1% in the sham group, and 4-year cumulative incidence of CI-DME with vision loss was 11.3% in the aflibercept group and 19.1% in the sham group. Eyes in both groups returned for study visits at least every 4 months so that vision-threatening complications could be promptly identified and treated. Although, on average, the aflibercept group had 3 fewer visits than the sham group, this study did not identify any favorable effect in terms of other variables, including visual acuity area under the curve, activity impairment, or work productivity loss that might reflect the quality of life due to vision problems.
An unanswered question is how long eyes that receive early anti-VEGF will need to continue receiving injections to maintain anatomic benefits. In this study, aflibercept treatment was administered routinely for 2 years and then only given as needed if an eye had moderate NPDR or more severe disease. Using this strategy, approximately 80% of eyes in the aflibercept group that completed the 4-year visit received at least 1 injection in the fourth year, and approximately 20% needed additional treatment within 4 years. The treatment and visit burden beyond 4 years is unknown, but a preventive strategy that requires continued injections over multiple years will likely increase the overall treatment burden. Moreover, preventive treatment did not eliminate the risk of developing PDR or CI-DME with vision loss.
In the aflibercept group, anatomic disease progression was experienced by approximately 16% of eyes within 2 years and 34% within 4 years, raising the question of whether the prophylactic treatment regimen used in this protocol undertreated patients. However, more frequent treatments would increase the treatment burden and risks. A prophylactic regimen of intravitreal injections given more frequently than every 4 months was not considered acceptable in this cohort with good baseline vision by the DRCR Retina Network investigators when the study was designed.
The results did show a favorable effect of early treatment on 2-step or more improvement in DRSS from baseline to 4 years (P < .001). Still, given no established meaningful visual acuity advantage, this trial does not support earlier treatment for severe NPDR, at least up to 4 years. Furthermore, while DRSS improvement may seem desirable, this alone may not confer clinically relevant benefit from prophylaxis as there is evidence that underlying nonperfusion is not substantially affected by anti-VEGF treatment and progression of the disease can still occur.12
Limitations
This study has several limitations. First, participant retention was supported by closely monitoring participants who missed their follow-up visit window, conducting third-party searches for participants lost to follow-up, streamlining visits to prioritize key testing procedures during the COVID-19 pandemic, having patients who moved follow up at another DRCR site, and increasing participant compensation for completion of lengthier annual visits. Despite these measures, participant retention was approximately 75% at 4 years (excluding deaths). While less than ideal, this retention rate is in line with previous studies enrolling participants with more advanced diabetic retinopathy and highlights the challenge faced with loss to follow-up in clinical care in the longer term.4
Second, measures of visual function other than best-corrected central visual acuity were not obtained (eg, visual fields), as DRCR Retina Network Protocol S revealed that peripheral visual field loss likely occurs over time in some eyes of patients with diabetes, even in the setting of anti-VEGF treatment. The primary anatomical outcome was a composite outcome that consisted of any PDR development, including cases that might not meet high-risk criteria and hence may not be visually threatening or always need treatment. Thus, the combined end point of PDR and vision-impairing CI-DME development may not necessarily be associated with vision decline, and a more stringent composite outcome definition could have been used. Nonetheless, the study's conclusion of no visual acuity benefit with early aflibercept would not have changed.
Third, a study duration of 4 years is a restriction when evaluating prophylaxis in a lifelong disease like diabetes. Longer studies would provide valuable longer-term outcomes but also face more retention challenges.
Fourth, it is possible that an anti-VEGF agent other than aflibercept or a different re-treatment algorithm could produce different results.
Conclusions
Among patients with NPDR but without CI-DME at 4 years treatment with aflibercept vs sham, initiating aflibercept treatment only if vision-threatening complications developed, resulted in statistically significant anatomic improvement but no improvement in visual acuity. Aflibercept as a preventive strategy, as used in this trial, may not be generally warranted for patients with NPDR without CI-DME.
Trial Protocol
Statistical Analysis Plan
eFigure 1. Subgroup Treatment Effects for Visual Acuity Change
eFigure 2. Time to PDR or CI-DME Development by Baseline DR Severity
eFigure 3. Subgroup Treatment Effects for Time to PDR or CI-DME
eFigure 4. CST Change From Baseline Through 4 Years
eFigure 5. OCT Retinal Volume Change From Baseline Through 4 Years
eTable 1. Baseline Characteristics Overall and by 4-Year Completion
eTable 2. Subgroup Analysis for Visual Acuity Change From Randomization to 4 Years
eTable 3. First PDR and CI-DME With Vision Loss Outcomes Met
eTable 4. Subgroup Analysis for the Development of PDR and CI-DME With Vision Loss
eTable 5. Diabetic Retinopathy Severity Outcomes at 4 Years
eTable 6. OCT Outcomes at 4 Years
eTable 7. Cumulative Treatments for PDR and CI-DME With Vision Loss by Year
eTable 8. Ocular Adverse Events of Interest in Study Eyes
eTable 9. Systemic Adverse Events of Interest
eTable 10. All Ocular Adverse Events
eTable 11. All Systemic Adverse Events
eTable 12. Follow-up Hemoglobin A1c
eTable 13. Workplace Productivity and Activity Impairment for Unilateral Study Participants
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Maturi RK, Glassman AR, Josic K, et al. ; DRCR Retina Network . Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the Protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139(7):701-712. doi: 10.1001/jamaophthalmol.2021.0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;139(9):946-955. doi: 10.1001/jamaophthalmol.2021.2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 4.Gross JG, Glassman AR, Liu D, et al. ; Diabetic Retinopathy Clinical Research Network . Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-1148. doi: 10.1001/jamaophthalmol.2018.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells JA, Glassman AR, Ayala AR, et al. ; Diabetic Retinopathy Clinical Research Network . Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359. doi: 10.1016/j.ophtha.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80(3):557-572. doi: 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 8.Chalam KV, Bressler SB, Edwards AR, et al. ; Diabetic Retinopathy Clinical Research Network . Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(13):8154-8161. doi: 10.1167/iovs.12-10290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bressler SB, Edwards AR, Chalam KV, et al. ; Diabetic Retinopathy Clinical Research Network Writing Committee . Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol. 2014;132(9):1113-1122. doi: 10.1001/jamaophthalmol.2014.1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YX, Xu L, Wei WB, Jonas JB. Intraocular pressure and its normal range adjusted for ocular and systemic parameters: the Beijing Eye Study 2011. PLoS One. 2018;13(5):e0196926. doi: 10.1371/journal.pone.0196926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antiplatelet Trialists’ Collaboration . Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994;308(6921):81-106. doi: 10.1136/bmj.308.6921.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couturier A, Rey PA, Erginay A, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti–vascular endothelial growth factor. Ophthalmology. 2019;126(12):1685-1694. doi: 10.1016/j.ophtha.2019.06.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eFigure 1. Subgroup Treatment Effects for Visual Acuity Change
eFigure 2. Time to PDR or CI-DME Development by Baseline DR Severity
eFigure 3. Subgroup Treatment Effects for Time to PDR or CI-DME
eFigure 4. CST Change From Baseline Through 4 Years
eFigure 5. OCT Retinal Volume Change From Baseline Through 4 Years
eTable 1. Baseline Characteristics Overall and by 4-Year Completion
eTable 2. Subgroup Analysis for Visual Acuity Change From Randomization to 4 Years
eTable 3. First PDR and CI-DME With Vision Loss Outcomes Met
eTable 4. Subgroup Analysis for the Development of PDR and CI-DME With Vision Loss
eTable 5. Diabetic Retinopathy Severity Outcomes at 4 Years
eTable 6. OCT Outcomes at 4 Years
eTable 7. Cumulative Treatments for PDR and CI-DME With Vision Loss by Year
eTable 8. Ocular Adverse Events of Interest in Study Eyes
eTable 9. Systemic Adverse Events of Interest
eTable 10. All Ocular Adverse Events
eTable 11. All Systemic Adverse Events
eTable 12. Follow-up Hemoglobin A1c
eTable 13. Workplace Productivity and Activity Impairment for Unilateral Study Participants
Nonauthor Collaborators
Data Sharing Statement