Abstract
Peer-driven interventions can be effective in reducing HIV injection risk behaviors among people who inject drugs (PWID). We employed a causal mediation framework to examine the mediating role of recall of intervention knowledge in the relationship between a peer-driven intervention and subsequent self-reported HIV injection-related risk behavior among PWID in the HIV Prevention Trials Network (HPTN) 037 study. For each intervention network, the index participant received training at baseline to become a peer educator, while non-index participants and all participants in the control networks received only HIV testing and counseling; recall of intervention knowledge was measured at the 6-month visit for each participant, and each participant was followed to ascertain HIV injection-related risk behaviors at the 12-month visit. We used inverse probability weighting to fit marginal structural models to estimate the total effect (TE) and controlled direct effect (CDE) of the intervention on the outcome.The proportion eliminated (PE) by intervening to remove mediation by the recall of intervention knowledge was computed. There were 385 participants (47% in intervention networks) included in the analysis. The TE and CDE risk ratios for the intervention were 0.47 (95% confidence interval (CI): 0.28, 0.78) and 0.73 (95% CI: 0.26, 2.06) and the PE was 49%. Compared to participants in the control networks, the peer-driven intervention reduced the risk of HIV injection-related risk behavior by 53%. The mediating role of recall of intervention knowledge accounted for less than 50% of the total effect of the intervention, suggesting that other potential causal pathways between the intervention and the outcome, such as motivation and skill, self-efficacy, social norms and behavior modeling, should be considered in future studies.
Keywords: People who inject drugs, Causal mediation analysis, HIV risk networks, Peer-driven intervention, HIV injection-related risk behavior
Introduction
Despite remarkable scientific progress over the last 40 years in the development of new antiretroviral therapies and implementation of treatment-as-prevention programs,1 marginalized populations around the world continue to face significant barriers accessing HIV treatment and prevention services.2,3 Approximately two million new cases of HIV are reported annually, and 1 in 5 people who inject drugs (PWID) are living with HIV worldwide.4 Since 2015, several clusters and outbreaks of HIV have been reported among PWID in counties that span the rural-urban spectrum across the US. Long-term declining trends in HIV incidence among PWID have stalled, and in fact, HIV diagnoses increased by 16.7% nationally from 2016 to 2018.5
Social networks play an important role in HIV transmission6–9 because sharing of injection equipment is one of the major predictors of HIV infection among PWID.10 Because of limited access and uptake of pre-exposure prophylaxis (PrEP) among PWID in the US,11–13 behavioral interventions are designed to curb HIV transmission through reductions of injection and sexual risk behavior remain an essential component of HIV prevention.14,15 Indeed, prior research suggests that injection risk networks can be leveraged for HIV prevention using peer-driven interventions that train PWID to educate members of their existing personal HIV risk networks. Risk networks consist of groups of individuals possibly engaged in sexual or injection risk practices with each other. Peer education interventions are based on the rationale that peers are trusted members of this hard-to-reach population and likely have a strong influence on the individual behavior of their network members.16 These peer-driven interventions have been shown to reduce injection-related HIV risk behaviors when compared with HIV testing and counseling alone.17–20 In the HIV Prevention Trials Network (HPTN) 037 study, index participants and their networks were randomized to a peer education intervention or a control condition. For intervention knowledge we focused on responses to five intervention-specific phrases that were intended to improve participant recall of intervention information (i.e., index participant) was trained to be a peer educator, while other network members in the intervention and control networks were not, and experienced possible effects of the interv18%ention only through their interaction with the trained index participant or being a member of a network with a trained index participant. The peer educators in the intervention networks were trained on how to promote safer drug injection skills, taught communication strategies to conduct peer outreach, promote norms about HIV risk reduction, and encouraged to model safer behavior among peers. Everyone in the study received HIV testing and counseling. Because adequate knowledge about HIV transmission can be a prerequisite for HIV risk behavior change,21 the success of these interventions may depend on the diffusion of intervention knowledge in a personal risk network – group of individuals engaged in risky sexual or injection behaviors with each other.22–24 However, it is possible participants changed their injection-related HIV risk behavior in response not only to exposure to the intervention material, but by interacting with trained index participants who also provided them with information, encouraged and modeled behavior change, or promoted new social norms. Therefore, recall of intervention knowledge alone may not necessarily lead to behavior change as other factors such as motivation and skill,21 self-efficacy,25 social norms26–28 and behavior modeling may play a role as well.
Although there is strong evidence that peer education interventions are associated with increased HIV knowledge,29 in a recent study using data from an HIV prevention intervention among PWID in Ukraine, Smyth et al.25 found that neither self-efficacy to practice safer behavior nor communication with network members causally mediated the effects of the intervention on HIV incidence. There is limited research evaluating the potential mediating role of intervention knowledge recall and other mediators in the pathway linking a peer-driven intervention to changes in injection-related HIV risk behaviors among PWID. Understanding causal pathways between a peer-driven intervention and changes in HIV risk behaviors is important in informing policy decisions and HIV prevention study designs allowing us to test theories to inform HIV prevention programs.30,31 In this study, we analyzed data from HPTN 037 study to examine whether recall of intervention knowledge mediates the causal effects of a peer-driven intervention on changes in self-reported injection-related HIV risk behavior.
Methods
Study design, recruitment, and participants:
This analysis was conducted using data from HPTN 037—a phase 3 randomized controlled trial to evaluate the efficacy of a network-oriented peer education intervention —in reducing HIV transmission and related risk behaviors among PWID and their network members. The study was implemented in Philadelphia, USA, and Chiang Mai, Thailand. However, this analysis is restricted only to participants in the Philadelphia site, where meaningful overall peer-driven intervention effects on injection-related risk behaviors were observed. In Chiang Mai, where a government’s war on drugs altered interactions among PWID, data may not have been reliable, and no intervention effects were observed.26
Two types of participants were enrolled in the trial: index participants and members of their HIV risk networks. Index participants who were HIV negative were actively recruited by outreach workers from areas with a high prevalence of HIV and injection drug use. Subsequently, index participants recruited individuals with whom they had injected drugs or had sex within the prior three months. To be eligible, index participants had to be ≥18 years of age, report injecting drugs at least 12 times in the last three months, test negative for HIV, not have received medications for opioid use disorder in the past three months, and be able to recruit at least one member from their injection risk network into the study. The index participants and their network members were randomized to the intervention or control condition. Network members were recruited by the index participants and were not trained to be peer educators. To be eligible, network members had to be ≥18 years of age, be recruited by an eligible index participant regardless of HIV status, and report injecting drugs or had sex together with the index participant that recruited them within the prior three months. Index participants in one network could not serve as a network member in another network and overlap between networks was possible, but was not captured in the study.32 Recruitment of participants into the HPTN 037 study began in December 2002 and follow-up ended in August 2006.
Peer-driven education intervention:
All participants received counseling during HIV testing, and an interviewer-administered behavioral risk assessment once every 6 months for up to 30 months. Only index participants were randomized to the two study conditions: either an intervention condition (with training on how to be a peer educator from study staff) or a control condition (with no training). A network was considered exposed to the intervention if their index member was randomized to the intervention group. The network-level exposure was a package intervention consisting of six 2-hour peer-educator sessions during the first four weeks and two booster sessions at the 6- and 12-month visits, respectively. In this analysis, we considered this a single multifaceted peer-driven intervention and did not disentangle the effects of different components. The training focused on educating index subjects in the intervention arm on: 1) how to promote safer sex and drug injection skills among network members, and 2) communication strategies to conduct peer outreach and promote norms about HIV risk reduction with network members. Index participants randomized to the training intervention engaged in role-playing activities to practice peer education and risk reduction skills and were encouraged to model safer injection behaviors when they were with their peers. A major component of the training focused on developing communication skills on how to talk with network members about HIV injection-related risk reduction. It was anticipated that peers’ modeling and discussing methods of reducing HIV injection risk behavior within their social networks would lead to changes in injection-related risk behaviors for all non-index participants in their network. Index participants randomized into the control condition did not participate in any intervention training sessions.
Study outcome:
One of the secondary outcomes in the original study was self-reported injection-related HIV risk behavior. At each study visit, for all study participants, an interviewer administered a behavioral survey that included self-reports of HIV injection-related risk behaviors in the prior month. In this analysis, we focused our assessment on five descriptive injection risk behaviors: needle sharing, sharing cooker, sharing cotton, sharing rinse water, and front/back loading. These are drug preparation and injection practices conceptualized as unique behaviors that may have been affected by the peer-driven intervention designed to reduce the potential risk of HIV acquisition/transmission among PWID.19 The intervention targeted reductions across these HIV injection-related risk behaviors and following a prior analysis of this study, a comprehensive clinical outcome was defined as report of any injection-related risk behavior at the 12-month visit. The outcome was assessed among participants who reported injection drug use in the last 3 months at baseline. We modeled the probability of any unsafe injection practices versus none at the 12-month visit. The analyses were adjusted for the baseline report of HIV risk behaviors.
Potential mediators:
The intervention training sessions included a number of specific phrases and terms designed to assess the diffusion of intervention messages. To measure recall of terminology associated with the intervention training, participants were shown a list of terms at the 6-month visit and asked, “Which of these exact words or phrases have you heard before?” In this analysis, we examined three types of potential mediators: intervention terms, positive control terms, and negative control terms. Intervention terms were specific to the training program to which only index participants randomized to the intervention arm were provided as part of their training with some repeatedly, and others only once or twice. For intervention knowledge, we focused on responses to five intervention-specific phrases intended to improve participant recall of intervention information: “SPEAKK”, “Injection risk ladder”, “Ribbon game”, “Sex risk ladder”, and “Project FAST”. For example, “SPEAKK” was an intervention term used by index members to recall communication skills specific to the training program and “Injection risk ladder” was a term for the “levels of risk” associated with injection drug use. If a participant recognized any of the phrases at the 6-month visit, recall of intervention knowledge was coded as Yes (1) or else No (0). All participants were exposed to positive control terms at all visits including “harm reduction”. No participants were exposed to negative control terms. Negative control terms were technical terms, unrelated to HIV prevention, used by study staff but to which study participants should not have been exposed, because they were not used in the intervention training. Positive control terms were terms to which all study participants were exposed during HIV counseling and testing, but not specific to the intervention. Negative control terms included “EXPLORE”, “Matrix method”, and “SCHARP”. There was only one positive control term, “harm reduction”. A binary variable was created based on recall of any of the negative control terms. Based on theory of diffusion of innovation,33 we hypothesized that for recall of intervention knowledge to serve as a useful measure of social diffusion of the intervention material in intervention networks, some of the peer-driven intervention effects were expected to be mediated by the recall of intervention terms. This is because HIV knowledge is a prerequisite for behavioral change.21 Similarly, recall of the positive control or negative control terms were not expected to have any significant association with the outcome of interest.
Baseline demographic and contextual factors:
Covariates were selected to include factors that may confound the relationship between peer-driven intervention or recall of intervention knowledge and HIV injection-related risk behavior. The following baseline social and demographic factors were used in our analysis: index status, age, marital status, educational level, employment status, drinking habits, non-injection drug use, risky sexual behavior (i.e., multiple sexual partners, unprotected sex with a non-primary partner, transactional sex), and recent incarceration, homelessness, and treatment for substance use (a description of covariates is provided in Table 1A of the Appendix).
Analytic sample and study timeline:
Of the 696 individuals who enrolled in the HPTN 037 study at the Philadelphia site, our study sample included 385 (55%) individuals who reported having injected drugs within three months of their baseline visit and completed the 6- and 12-month visits with their intervention knowledge and injection-related HIV risk behavior assessed at the 6- and 12-month visits, respectively (Figure 1). Peer-driven education intervention was determined at baseline, recall of intervention knowledge was measured at the 6-month visit, and an assessment of injection-related HIV risk behavior was completed at the 12-month visit.
Figure 1.

Flowchart of study participants in the HIV Prevention Trials Network 037 Study, Philadelphia, Pennsylvania, 2002-2006 showing selection of the analytic sample
Overview of causal mediation framework:
Mediation analysis can be used to advance our understanding of the dynamics of a peer-driven intervention on behavioral outcomes. Recent methodological developments in causal inference have provided definitions of causal effects that can be estimated under certain identifying conditions (i.e., consistency, positivity, exchangeability) from models with nonlinearities and interactions.34–36 Although natural direct effect (NDE) and natural indirect effect (NIE) allow for the decomposition of the total effect (TE) of an exposure into a direct and indirect component and the calculation of the proportion mediated (PM),37 estimation of NDE and NIE requires stronger assumptions for identification.38,39 Because these natural effects do not correspond to any intervention that can be implemented in practice, in this study, we estimate the controlled direct effect (CDE) that requires weaker assumptions (i.e., no uncontrolled exposure-outcome confounding and no uncontrolled mediator-outcome confounding) as compared to those for natural effects. CDE correspond to the intervention effect that remains after intervening to set the mediator at a specific level (Table A2).
Statistical analysis:
We conducted descriptive analyses of baseline substance use and sociodemographic characteristics of participants: overall, by intervention arm and by our mediator of interest (i.e., recall of intervention knowledge). Injection-related HIV risk behaviors were assumed to be correlated among participants from the same network. Unadjusted and adjusted associations between the peer-driven intervention and potential mediators and the outcome of interest were evaluated using log-Binomial regression using generalized estimating equations (GEE) to account for correlation within networks. We also assessed the association between the intervention and possible mediators. In some instances, multivariable log-Binomial models did not converge and GEE Poisson regression models with a log link were used, which provided consistent but less efficient estimates of the risk ratio (RR).40,41
The approach for causal mediation analysis was based on the potential outcomes framework whereby the TE and CDE can be estimated separately and used to calculate the proportion eliminated (PE) by intervening on the mediator.37,42–44 The average TE was defined as the average effect of the peer-driven intervention on injection-related HIV risk behavior among participants in intervention networks contrasted with the average effect among participants in control networks. CDE was defined as the average effect of recall of intervention knowledge on injection-related HIV risk behaviors if the pathway between the exposure (peer-driven education intervention) and the mediator (recall of intervention knowledge) was removed (hypothetically) so that the mediator was no longer dependent on the exposure. The PE quantified the potential for reducing the risk of HIV injection-related risk behavior by intervening to remove recall of intervention knowledge.
The TE and CDE were estimated separately using inverse-probability-weighted (IPW) to fit marginal structural models (MSM). To estimate the TE, we used a log-Binomial regression of the outcome, injection-related HIV risk behavior, with the peer-driven intervention as exposure using GEE to account for the correlation of outcomes within networks. Because only a subset of the original study population was used in the analysis and randomization at the network level was no longer guaranteed, the inverse-probability-of-treatment weights (IPTW) were estimated for each study participant. This was defined as the ratio of the estimated marginal probability of the individual’s observed intervention to the estimated probability of each individual’s observed intervention, conditional on their baseline covariates including injection-related risk behaviors. The IPTW essentially creates a pseudo-population in which baseline covariates were no longer associated with the peer-driven education intervention, thus eliminating measured confounding in the analysis.45 To estimate the CDE, a corresponding MSM used a product of the IPTW for the exposure and an additional inverse-probability-of-mediator weight (IPMW). The IPMW was estimated for each study participant as the ratio of the estimated marginal probability of the individual’s observed mediator to the estimated probability of each individual’s observed mediator, conditional on their baseline covariates including injection-related risk behaviors. The IPMW also creates a pseudo-population in which the recall of intervention knowledge was no longer dependent on peer-driven education intervention, thus eliminating any mediation by measured recall of intervention knowledge.45 The distribution of the exposure, mediator and combined weights suggested no evidence of model misspecification or violations of positivity of the exposure and mediator models (Table A3 and Fig. A1). We estimated causal risk ratios (RR) with their 95% confidence intervals (CIs) using a robust estimator of the variance.
The proportion of the TE eliminated by intervening on the mediator, i.e., the proportion eliminated, was computed on the excess relative risk scale by43 () and reported if the RRs of CDE and TE were in the same direction and the CDE did not exceed the TE.37,42 We explored exposure–mediator interaction to allow adequate model flexibility and to more fully capture the dynamics of mediation, and the interaction term was included in the final model because its magnitude was large and omission would have changed the estimate of CDE and PE in a meaningful way.42 We also examined the effect modification of the total effect by network size. Finally, we performed a sensitivity analysis for unmeasured confounding using a negative control and positive control terms.
All statistical tests performed were two-sided and conducted at the .05 significance level. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, US).
Results
This analysis included 385 participants with 182 (47%) in the intervention networks (Table 1). There were 141 index participants with 68 (37%) in the intervention networks. The network size in the full cohort ranged from 2 to 7 with an average of 3.7 participants; mean age was 42 years; 70% were male; 60% white; 22% were married or living with a partner; and 79% were unemployed. Due in part to randomization, sociodemographic characteristics and relevant substance use behaviors were comparable between the two arms of the study. However, at baseline, a higher proportion of participants in the intervention networks reported injection risk behaviors than participants in the control networks. Non-injection drug use was common, with the primary drugs of choice being crack cocaine and benzodiazepines in the intervention and control networks, respectively. Heavy alcohol use was also more common among the intervention group than the control networks. The baseline characteristics of participants included in the analysis were similar to those of the original cohort (Table A4). The distribution of network size by intervention group among study participants is shown in Table A5. Among study participants, 18% were enrolled in dyads (networks with two participants) while 40% belonged to a network with two other network members. In each intervention group and overall, the most common network size observed was three.
Table 1.
Baseline characteristics of participants in the HPTN 037 study, by intervention group, Philadelphia, Pennsylvania, 2002-2006 (n = 385).
| Characteristics | Overall n (%) | Intervention arm n (%) | Control arm n (%) |
|---|---|---|---|
| Number enrolled | 385 | 182 (47.3) | 203 (52.7) |
|
| |||
| Individual participant’s role | |||
| Index participant | 141 (36.6) | 68 (37.4) | 73 (36.0) |
| Network member | 244 (63.4) | 114 (62.6) | 130 (64.0) |
|
| |||
| Network size | 3.7 (1.4) | 3.9 (1.6) | 3.5 (1.2) |
|
| |||
| Age, years | 42.0 (9.0) | 42.8 (8.6) | 41.2 (9.3) |
|
| |||
| Age | |||
| 18–30 | 50 (13.0) | 16 (8.8) | 34 (16.8) |
| 31–40 | 111 (28.8) | 58 (31.9) | 53 (26.1) |
| 41–50 | 158 (41.0) | 78 (42.9) | 80 (39.4) |
| 50+ | 66 (17.1) | 30 (16.5) | 36 (17.7) |
|
| |||
| Gender | |||
| Male | 271 (70.4) | 130 (71.4) | 141 (69.5) |
| Female | 114 (29.6) | 52 (28.6) | 62 (30.5) |
|
| |||
| Race/ethnicity | |||
| White | 230 (59.7) | 117 (74.3) | 113 (55.7) |
| Nonwhite | 155 (40.3) | 65 (35.7) | 90 (44.3) |
|
| |||
| Marital status | |||
| Separated/divorced/widowed | 217 (56.4) | 98 (53.9) | 119 (58.6) |
| Single | 82 (21.3) | 39 (21.4) | 43 (21.2) |
| Married/Living with partner | 86 (22.3) | 45 (24.7) | 41 (20.2) |
|
| |||
| Education | |||
| Up to secondary school | 120 (31.2) | 48 (26.4) | 72 (35.5) |
| Completed secondary school | 180 (46.7) | 94 (51.6) | 86 (42.4) |
| Beyond secondary school | 85 (22.1) | 40 (22.0) | 45 (22.2) |
|
| |||
| Employment | |||
| Employed | 79 (20.5) | 37 (20.3) | 42 (20.7) |
| Unemployed | 306 (79.5) | 145 (79.7) | 161 (79.3) |
|
| |||
| Non-injection drug use | |||
| Crack (smoke) | 203 (52.7) | 109 (59.9) | 94 (46.31) |
| Cocaine (snort or sniff) | 70 (18.2) | 30 (16.5) | 40 (19.7) |
| Opiates (smoked) | 149 (38.7) | 71 (39.0) | 78 (38.4) |
| Benzodiazepines | 207 (53.8) | 88 (48.4) | 119 (58.6) |
|
| |||
| Alcohol use | |||
| Did not drink | 143 (37.1) | 67 (36.8) | 76 (37.4) |
| Never got drunk | 107 (27.8) | 41 (22.5) | 66 (32.5) |
| Got drunk | 135 (35.1) | 74 (40.7) | 61 (30.1) |
|
| |||
| Drug treatment program in past 6 months | 124 (32.2) | 48 (26.4) | 76 (37.4) |
|
| |||
| Housing | |||
| Spent night on the street | 82 (21.3) | 34 (18.7) | 48 (23.7) |
| Spent time in jail | 54 (14.0) | 28 (15.4) | 26 (12.8) |
|
| |||
| Sexual risk in the last month | |||
| Low | 203 (52.7) | 93 (51.1) | 110 (54.2) |
| High | 182 (47.3) | 89 (48.9) | 93 (45.8) |
|
| |||
| Types of Injection drugs used in last month | 375 (97.4) | 178 (97.8) | 197 (97.0) |
| Heroin | 349 (90.6) | 163 (89.6) | 186 (91.6) |
| Heroin with cocaine | 134 (34.8) | 72 (39.6) | 62 (30.5) |
| Heroin with amphetamine | 3 (0.8) | 2 (1.1) | 1 (0.5) |
| Cocaine | 122 (31.7) | 61 (33.5) | 61 (30.1) |
| Amphetamine | 7 (1.8) | 4(2.2) | 3 (1.5) |
|
| |||
| Injection-related risk behavior in last month | |||
| Shared rinse water | 184 (47.8) | 73 (40.1) | 111 (54.7) |
| Shared cooker | 235 (61.0) | 98 (53.8) | 137 (67.5) |
| Shared cotton | 172 (44.7) | 69 (37.9) | 103 (50.7) |
| Used front or back loaded syringe | 84 (21.8) | 41 (22.5) | 43 (21.2) |
| Passed a needle to someone else | 203 (52.7) | 85 (46.7) | 118 (58.1) |
Table A6 presents baseline characteristics by the mediator level of recall of intervention knowledge which was not randomized. Of a total of 134 (35%) participants who reported recall of at least one intervention-specific term at the 6-month visit, 75 (56%) were index participants in the intervention networks. Of the 251 study participants who did not recall any of the intervention terms, 185 (74%) were non-index network members. The rate of recall of intervention knowledge was higher among white study participants than nonwhite participants. Rates of recall of intervention knowledge were also lower among participants who reported benzodiazepine use than those who did not report benzodiazepine use. The prevalence of recall of intervention knowledge did not differ in terms of sexual risk behavior, alcohol use, non-injection drug use and injection-related risk behaviors measured at baseline. Table 2 shows prevalence of recognition of intervention terms compared to positive control and negative control terms at the 6-month visit. Participants in the intervention networks recognized intervention terms at a higher frequency than those in the control networks (p < .05). Prevalence of recognition of positive control and composite negative control terms were similar for both study arms (p > .20) .
Table 2.
Prevalence of recall of intervention terms, and positive and negative control terms at the 6-month visit among PWID in HPTN 037 study, Philadelphia, Pennsylvania, 2002-2006 (n = 385).a
| Terms used in assessment of intervention knowledge | Overall, n (%) | Intervention arm, n (%) | Control arm, n (%) |
|---|---|---|---|
| Recall of intervention knowledge | 134 (34.8) | 85 (46.7) | 49 (24.1) |
| Intervention-specific terms | |||
| SPEAKK | 62 (16.1) | 49 (26.9) | 13 (6.4) |
| Injection risk Ladder | 76 (19.7) | 56 (30.8) | 20 (9.9) |
| Project FAST | 54 (14.0) | 34 (18.7) | 20 (9.9) |
| Ribbon game | 50 (13.0) | 44 (24.2) | 6 (3.0) |
| Sex risk ladder | 86 (22.3) | 64 (35.2) | 22 (10.8) |
|
| |||
| Positive control term | |||
| Harm reduction | 106 (27.5) | 55 (30.2) | 51 (25.1) |
|
| |||
| Any negative control terms | 79 (20.5) | 42 (23.1) | 37 (18.2) |
| Specific negative control terms | |||
| EXPLORE | 55 (14.3) | 28 (15.4) | 27 (13.3) |
| Matrix method | 37 (9.6) | 27 (14.8) | 10 (4.9) |
| SCHARP | 29 (7.5) | 19 (10.4) | 10 (4.9) |
Only index participants in the intervention arm received training with exposure to intervention-specific test terms; all participants were exposed to positive control terms; and no participants were exposed to negative control terms.
At baseline, only 25% of study participants reported none of the five injection-related HIV risk behaviors used to define the main outcome, while 10% reported all five risk behaviors. At the 12-month visit, 79% of participants reported none and only 1% reported all five HIV risk behaviors (Figure 2 and Table A7). A total of 105 (27%) reported safer injection practices at the 12-month visit compared to baseline injection-related HIV risk behaviors. This proportion includes participants with safer injection practices at the 12-month visit as well as those who remained free of injection risk behavior. Both the peer-driven intervention and recall of intervention knowledge were associated with safer injection-related risk behavior, with RR of 0.47 (95% confidence interval (CI): 0.28, 0.78) and 0.62 (95% CI: 0.37, 1.03), respectively (Table 3). Compared to participants in the control networks, the peer-driven intervention reduced the risk of HIV injection-related risk behavior by 53%. Recall of positive control or negative control terms was not associated with injection-related risk behavior (p > .20).
Figure 2.
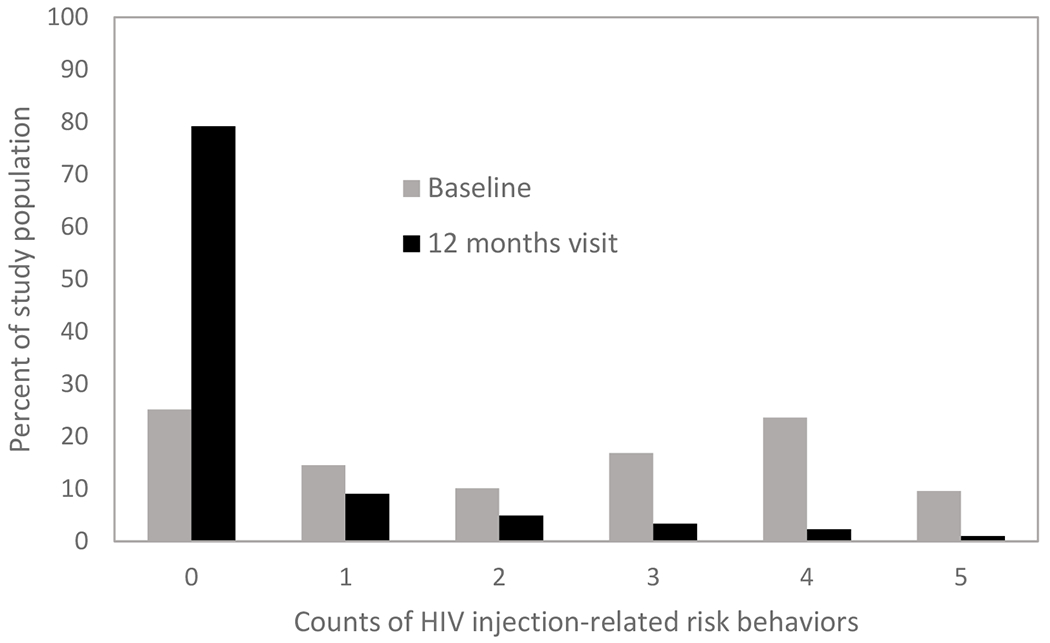
Distribution of counts of injection-related risk behaviors at baseline and 12-month visit among people who inject drugs in HPTN 037
Table 3.
Unadjusted and adjusted risk ratios of association between the intervention and potential mediators and self-reported injection-related HIV risk behaviors among PWID in HPTN 037 study, Philadelphia, Pennsylvania, 2002-2006 (n = 385)a
| Terms used in assessment of intervention knowledge | RR (95% CI)b | aRR (95% CI)c |
|---|---|---|
| Peer-driven intervention | 0.47 (0.28, 0.78) | 0.47 (0.28, 0.79) |
| Recall of intervention knowledgec | 0.59 (0.37, 0.94) | 0.62 (0.37, 1.03) |
| Intervention-specific terms | ||
| SPEAKK | 0.41 (0.20, .84) | 0.33 (0.16, 0.72) |
| Injection risk Ladder | 0.56 (0.32, 0.98) | 0.58 (0.31, 1.10) |
| Project FAST | 1.02 (0.60, 1.74) | 1.11 (0.64, 1.90) |
| Ribbon game | 0.44 (0.21, 0.91) | 0.42 (0.18, 1.01) |
| Sex risk ladder | 0.52 (0.29, 0.92) | 0.54 (0.28, 1.05) |
| Positive control term | ||
| Harm reduction | 1.00 (0.68, 1.47) | 0.93 (0.63, 1.39) |
| Any negative control termsd | 0.92 (0.58, 1.47) | 0.97 (0.59, 1.58) |
| Specific negative control term | ||
| EXPLORE | 1.07 (0.64, 1.79) | 1.18 (0.69, 2.03) |
| Matrix method | 0.66 (0.32, 1.34) | 0.80 (0.39, 1.63) |
| SCHARP | 1.01 (0.45, 2.22) | 0.99 (0.43, 2.34) |
Only index participants in the intervention arm received training with exposure to intervention-specific test terms; all participants were exposed to positive control terms; and no participants were exposed to negative control terms.
Multilevel Log-binomial regression model;
Multilevel Poisson regression model adjusted for baseline network-randomized exposure and other baseline covariates.
Recall of any intervention terms,
Recall of any negative control terms.
We found that peer-driven intervention was also associated with the recall of intervention knowledge but not with positive control or negative control terms. The likelihood of recall of intervention knowledge was 1.9 times higher among participants in the intervention networks compared to those in the control networks (RR=1.86; 95% CI: 1.39, 2.48) (Table 4).
Table 4.
Estimated effects of a peer education intervention on potential mediators of the causal effect of the intervention on self-reported HIV injection-related risk behaviors among PWID in HPTN 037 study, Philadelphia, Pennsylvania, 2002-2006 (n = 385).a
| Potential mediators | RR (95% CI)b | aRR (95% CI)c |
|---|---|---|
| Recall of intervention knowledge | 1.93 (1.46, 2.56) | 1.86 (1.39, 2.48) |
| Intervention-specific terms | ||
| SPEAKK | 4.23 (2.43, 7.35) | 3.26 (1.81, 5.85) |
| Injection risk Ladder | 3.12 (1.90, 5.14) | 2.95 (1.76, 4.95) |
| Project FAST | 1.90 (1.80, 3.06) | 1.83 (1.13, 2.98) |
| Ribbon game | 8.62 (3.82, 19.46) | 6.68 (2.94, 15.17) |
| Sex risk ladder | 3.20 (2.12, 4.83) | 3.06 (2.00, 4.70) |
| Positive control term | ||
| Harm reduction | 1.20 (0.87, 1.66) | 1.17 (0.84, 1.63) |
| Any negative control terms | 1.27 (0.88, 1.83) | 1.16 (0.82, 1.65) |
| Specific negative control terms | ||
| EXPLORE | 1.16 (0.72, 1.88) | 1.02 (0.64, 1.64) |
| Matrix method | 2.54 (1.43, 4.54) | 2.81 (1.55, 5.12) |
| SCHARP | 2.10 (1.00, 4.43) | 1.92 (0.95,3.91) |
Only index participants in the intervention arm received training with exposure to intervention-specific test terms; all participants were exposed to positive control terms; and no participants were exposed to negative control terms.
Multilevel Log-binomial regression model;
Multilevel Poisson regression model adjusted for baseline network-randomized exposure and other baseline covariates.
For mediation analysis including the exposure-mediator interaction in the model, we observed that the total effect of the intervention on injection-related HIV risk behaviors on the risk ratio scale was 0.47 (95% CI: 0.28, 0.78; p < .01) (Table 5) and with recall of intervention knowledge as the mediator of interest, the CDE on the risk ratio scale was 0.48 (95% CI: 0.27, 0.84; p = .01) (Table 8A). Compared to only voluntary HIV counseling and testing, the estimated proportion of the total effect of the peer-driven intervention on injection-related HIV risk behavior that could be reduced by “eliminating” the mediating role of recall of intervention knowledge was 49% on the excess relative risk scale. Using intervention-specific terms as potential mediators, estimates of CDEs reveal evidence of inconsistent mediation leading to smaller TE of the intervention on the outcome in some cases. By intervening to “eliminate” the positive control term (harm reduction), only 5% of the total effect of peer-driven education intervention on injection-related HIV risk behavior would be reduced. Intervening to “eliminate” negative control terms would not change the total effect of the intervention on the outcome.
Table 5.
Risk ratios (RR) for total effect (TE) and controlled direct effect (CDE) of the effect of a network-randomized peer education intervention on self-reported HIV injection-related risk behaviors with mediators recall intervention terms, positive and negative control terms among PWID in HPTN 037 study, Philadelphia, Pennsylvania, 2002-2006 (models with exposure-mediator interaction) (n = 385).a
| Terms tested in multilevel IPW marginal structural models for causal mediation | (95% CI) | ||
|---|---|---|---|
| Peer-driven intervention | 0.47 (0.28, 0.78) | ||
| Potential mediators | (95% CI) | Proportion eliminated | |
|
| |||
| Recall of intervention knowledge | 0.73 (0.26, 2.06) | 0.49 | |
| Intervention-specific terms | |||
| SPEAKK | 0.28 (0.04, 2.13) | NA | |
| Injection risk Ladder | 0.19 (0.04, 0.78) | NA | |
| Project FAST | 0.24 (0.07, 0.81) | NA | |
| Ribbon game | 0.31 (0.02, 4.62) | NA | |
| Sex risk ladder | 0.66 (0.16, 2.68) | 0.37 | |
|
| |||
| Positive control term | |||
| Harm reduction | 0.47 (0.18, 1.24) | 0.01 | |
|
| |||
| Any negative control term | 0.41 (0.13, 1.32) | NA | |
| Specific negative control terms | |||
| EXPLORE | 0.45 (0.13, 1.61) | NA | |
| Matrix method | 0.21 (0.03, 1.28) | NA | |
| SCHARP | 0.25 (0.05, 1.36) | NA | |
Only index participants in the intervention arm received training with exposure to intervention-specific test terms; all participants were exposed to positive control terms; and no participants were exposed to negative control terms.
PE was not reported if CDE and TE were not in the same direction or if CDE exceed TE.
When the exposure-mediator interaction term was excluded from the model, the estimates of CDE and PE changed in a meaningful way (Table A8). The total effect of the peer-driven intervention on self-reported injection-related HIV risk behaviors may be modified by network size (Table A9). The total effect of the intervention on injection-related HIV risk behaviors on the risk ratio scale was 0.36 (95%CI: 0.17, 0.74; p=.01) among participants within larger networks compared to 0.66 (95% CI: 0.34, 1.26; p=.20) among participants within smaller networks (dyads and triads).
Discussion
In this study, we found that a peer-driven education intervention was effective in reducing the risk of injection-related HIV risk behaviors among PWID in Philadelphia, and this causal effect was mediated, in part, by the recall of intervention knowledge. The mediating role of recall of intervention knowledge was relatively small when the exposure-mediator interaction was ignored while a large direct effect of the peer-driven education intervention on injection-related HIV risk behaviors was observed, suggesting there may be other pathways through which peer-driven education intervention affects the injection-related HIV risk behavior, other than recall of intervention knowledge. However, when the exposure–mediator interaction was included to allow adequate model flexibility and to more fully capture the dynamics of mediation, we found that the estimated proportion of the total effect of the peer-driven intervention on injection-related HIV risk behavior that could be reduced by “eliminating” the mediating role of recall of intervention knowledge was 49% on the excess relative risk scale. These findings support results from the original analysis, which showed that this peer-driven education intervention had a significant effect in reducing HIV risk behaviors.32 The study design was based on a number of social diffusion theories46 related to communication of the intervention knowledge, promotion of social norms and modeling of safer HIV behaviors which can be tested using mediation analysis if appropriate data is collected on these other potential mediators.30
Peer-driven education intervention had significant effects on recall of intervention knowledge measured at the 6-month visit, and the recall of intervention knowledge also had significant effects on injection-related HIV risk behavior measured at the 12-month visit. The study design consisted of recruiting index participants who were trained to promote HIV prevention by sharing the skills and knowledge acquired from intervention training with members of their personal risk networks. Network members did not participate in the intervention training as peer educators themselves but may have received HIV prevention information from their index participants and participated in baseline and follow-up assessments. The intervention training sessions included information on communication strategies and how to promote social norms and act as a role model within a personal social risk network. It is plausible peer-driven intervention effects are mediated by the recall of intervention knowledge.21
Prior studies have shown that peer-driven interventions may have substantial resonance beyond the individual who received the intervention. Buchanan et al described estimators for individual, disseminated and overall intervention effects and showed peer-driven intervention may have substantial impact within the network beyond direct effects. Without assessing such disseminated (spillover) effects, the effect of an intervention could be underestimated.47 This study extends our understanding of the causal pathways between a peer-driven intervention and changes in HIV injection-related risk behaviors. The findings may inform the design of future HIV prevention studies.
Our analysis relies on several assumptions. First, for an unbiased estimation of mediation effects, the intervention must precede the mediator, and both must precede the outcome. This condition is met because in HPTN 037 the peer-driven education intervention preceded the 6-month measurement of recall of intervention knowledge, and both preceded the assessment of injection-related HIV risk behavior at the 12-month visit. Second, for valid inference, mediation analysis assumes the absence of unmeasured confounding in the exposure-outcome relationship as well as the mediator-outcome relationship.42,48 The HPTN 037 met the exposure-outcome assumption primarily via randomization. However, randomization may not have held in the subset of the study population used and does not protect against confounding in the mediator-outcome relationship because the level of recall of intervention knowledge was not randomized. Although we adjusted for index status, baseline injection-related risk behavior, age, marital status, educational level, employment status, drinking habits, non-injection drug use, risky sexual behavior, incarceration, homelessness, and treatment for substance use disorder, we cannot exclude unmeasured confounding. The absence of a relationship between negative control and positive control terms or exposures and our outcome of interest when analyzed using the same model as for the main exposure-outcome possibly suggests the absence of significant unmeasured confounding.49 Finally, we assumed the absence of selection bias and measurement error.
Limitations:
Our study has some limitations. First, for the peer-driven intervention to succeed, the index participants must be motivated, willing, and able to recruit network members, and deliver accurate information to members of their personal social network. If peer educators alter the contents of the intervention or fail to model safer behavior, it could reduce the effectiveness of the intervention.50 The reliability of recall of intervention knowledge is unknown. An unreliable measurement of the mediator can bias an estimate of the effect of the mediator on the outcome toward the null.51,52 Recall of intervention knowledge or buzzwords may not be the best way to assess what was learned in the intervention because it does not translate into large reductions in injection-related HIV risk behavior.Among the intervention index participants in Philadelphia 85% attended at least one peer education training session and 72% attended at least four sessions. Second, as with many ratio measures, the PE may be unstable and highly variable, making it difficult to do inference and draw conclusions beyond assessing the importance of mediation.42,51 Instability become problematic when the TE and NDE operate in opposite directions (“inconsistent mediation”), leading to smaller TE of the intervention on the outcome.42 Third, by design an index participant in one network in HPTN 037 could not serve as a network member in another network and although not captured in the observed data, overlap among study networks was possible. This overlap may result in contamination whereby individuals in the control networks are inadvertently exposed to the intervention training material. For example, individuals in intervention networks could converse with and encourage those in control networks to change their injection behaviors. As a result, contamination could impact evaluation of the intervention effect. In a separate published study, Simmons et al.53 used recall of intervention terms to assess contamination in HPTN 037, an approach that was supported by our findings using a mediation framework. Fourth, because our definition of injection-related risk behavior did not include study participants who reported a decrease in the number of injection-related risk behaviors at the 12-month visit and those who remained free of injection risk behavior during the study, the intervention benefits may have been underesstimated. We acknowledge that a person going from five injection-related risk behaviors to none may have shown greater benefit than someone going from two to one. Unfortunately, our sample size is not sufficiently large to examine all these different patterns of behavior. Fifth, selection bias may have been introduced into the analysis by conditioning inclusion in the analysis on completion of the 6- and 12-month visits with an assessment of recall of intervention knowledge and injection-related HIV risk behavior assessed at the 6- and 12-month visits, respectively. Future studies examining time-varying mediation may consider using inverse probability of censoring weights to address potential selection bias.
Conclusion
Causal mediation provides a framework for investigating the causal pathways between an intervention and an outcome of interest. We evaluated what would happen to the effect of a peer-driven education intervention on injection-related HIV risk behavior if we were to hypothetically remove the role of recall of intervention knowledge. We found that a large portion of the causal effect of a peer-driven education intervention on injection-related HIV risk behavior that was mediated through the recall of intervention knowledge. Because the mediating role of the recall of intervention knowledge was less than 50%, other important pathways through which peer-driven intervention affects injection-related HIV risk behaviors such as changes in social norms and ability to model behaviors should be considered as potential mediators. Many behavioral interventions tend to focus on knowledge because increased knowledge is believed to be a prerequisite for behavior change and it is easy to measure. It may be more prudent to focus on skills, self-efficacy, and promoting social factors such as norms. Changes in norms may be an important causal pathway between a peer-driven intervention and behavior change, especially in social behaviors involved in HIV transmission dynamics.
Supplementary Material
Acknowledgements:
We thank Dr. Carl Latkin for providing access to the HPTN 037 data.
Funding:
Drs. Ashley Buchanan, Natallia Katenka and TingFang Lee were supported by the National Institute on Drug Abuse (NIDA) Avenir Award Number 1DP2DA046856-01 of the National Institutes of Health. Dr. Hilary Aroke is supported by a NIDA Diversity Supplement grant (DP2 DA046856). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- CI
Confidence interval
- TE
Total effect
- CDE
Controlled direct effect
- PE
Proportion eliminated
- IPTW
Inverse-probability-of-treatment weight
- IPMW
Inverse-probability-of-mediator weight
- GEEs
Generalized estimating equations
- HPTN
HIV Prevention Trials Network
- HIV
Human Immunodeficiency Virus
- PWID
People who inject drugs
- RR
Risk ratio
Footnotes
Conflicts of interest/Competing interests: None.
Ethics approval: Approved by the University of Rhode Island Institutional Review Board
Consent to participate: Not applicable.
Consent for publication: Not applicable.
Code availability: Not applicable.
Availability of data and material:
Data was obtained from through a third-party and authors cannot make these data publicly available due to data use agreement.
References
- 1.Rodger AJ, Cambiano V, Bruun T, et al. Sexual Activity without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171–181. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald V, Verster A and Baggaley R. A Call for Differentiated Approaches to Delivering HIV Services to Key Populations. Journal of the International AIDS Society. 2017;20:21658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Risher K, Mayer K and Beyrer C. The HIV Treatment Cascade in Men Who Have Sex with Men, People Who Inject Drugs and Sex Workers. Current Opinion in HIV and AIDS. 2015;10(6):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathers BM, Degenhardt L, Phillips B, et al. Global Epidemiology of Injecting Drug Use and HIV among People Who Inject Drugs: A Systematic Review. The Lancet. 2008;372(9651):1733–1745. [DOI] [PubMed] [Google Scholar]
- 5.Gant Z, Johnson SD, Li J, et al. Diagnoses of HIV Infection in the United States and Dependent Areas (Updated), 2018. 2020.
- 6.Rhodes T, Singer M, Bourgois P, et al. The Social Structural Production of HIV Risk among Injecting Drug Users. Social Science and Medicine. 2005;61(5):1026–1044. [DOI] [PubMed] [Google Scholar]
- 7.Koram N, Liu H, Li J, et al. Role of Social Network Dimensions in the Transition to Injection Drug Use: Actions Speak Louder Than Words. AIDS and Behavior. 2011;15(7):1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakon CM, Ennett ST and Norton EC. Mechanisms through Which Drug, Sex Partner, and Friendship Network Characteristics Relate to Risky Needle Use among High Risk Youth and Young Adults. Social Science and Medicine. 2006;63(9):2489–2499. [DOI] [PubMed] [Google Scholar]
- 9.Tsang MA, Schneider JA, Sypsa V, et al. Network Characteristics of People Who Inject Drugs within a New HIV Epidemic Following Austerity in Athens, Greece. Journal of Acquired Immune Deficiency Syndromes. 2015;69(4):499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggaley RF, Boily M-C, White RG, et al. Risk of HIV-1 Transmission for Parenteral Exposure and Blood Transfusion: A Systematic Review and Meta-Analysis. AIDS. 2006;20(6):805–812. [DOI] [PubMed] [Google Scholar]
- 11.Hacker E, Cohn J, Golden MR, et al. HIV Pre-Exposure Prophylaxis (Prep) Uptake, Initiation, and Persistence in the Detroit Public Health STD Clinic. In Open Forum Infectious Diseases, 2017. (Vol. 4, No. suppl_1, pp. S437–S437). US: Oxford University Press. [Google Scholar]
- 12.Hess KL, Johnson SD, Hu X, et al. Diagnoses of HIV Infection in the United States and Dependent Areas, 2017. HIV Surveillance Report 2 (28): 1–25. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Google Scholar]
- 13.Smith DK, Van Handel M, Wolitski RJ, et al. Vital Signs: Estimated Percentages and Numbers of Adults with Indications for Preexposure Prophylaxis to Prevent HIV Acquisition--United States, 2015. MMWR. Morbidity and Mortality Weekly Report. 2015;64(46):1291–1295. [DOI] [PubMed] [Google Scholar]
- 14.Mathers BM, Degenhardt L, Phillips B, et al. Global Epidemiology of Injecting Drug Use and HIV among People Who Inject Drugs: A Systematic Review. The Lancet. 2008;372(9651):1733–1745. [DOI] [PubMed] [Google Scholar]
- 15.Degenhardt L, Mathers B, Vickerman P, et al. Prevention of HIV Infection for People Who Inject Drugs: Why Individual, Structural, and Combination Approaches Are Needed. The Lancet. 2010;376(9737):285–301. [DOI] [PubMed] [Google Scholar]
- 16.Kerrigan D and Weiss E. Peer Education and HIV/AIDS: Past Experience, Future Directions: Report of a Consultation. Population Council, Horizons Project; 2000. [Google Scholar]
- 17.Latkin CA. Outreach in Natural Settings: The Use of Peer Leaders for HIV Prevention among Injecting Drug Users’ Networks. Public Health Report. 1998;113 Suppl 1(Suppl 1):151–159. [PMC free article] [PubMed] [Google Scholar]
- 18.Weeks MR, Li J, Dickson-Gomez J, et al. Outcomes of a Peer HIV Prevention Program with Injection Drug and Crack Users: The Risk Avoidance Partnership. Substance Use &Misuse. 2009;44(2):253–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latkin CA, Donnell D, Metzger D, et al. The Efficacy of a Network Intervention to Reduce HIV Risk Behaviors among Drug Users and Risk Partners in Chiang Mai, Thailand and Philadelphia, USA. Social Science & Medicine. 2009;68(4):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobin KE, Kuramoto SJ, Davey-Rothwell MA, et al. The Step into Action Study: A Peer-Based, Personal Risk Network-Focused HIV Prevention Intervention with Injection Drug Users in Baltimore, Maryland. Addiction. 2011;106(2):366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher JD and Fisher WA. Changing AIDS-Risk Behavior. Psychological Bulletin. 1992;111(3):455. [DOI] [PubMed] [Google Scholar]
- 22.Des Jarlais DC, Friedman SR, Friedmann P, et al. HIV/AIDS-Related Behavior Change among Injecting Drug Users in Different National Settings. AIDS. 1995;9(6):611–617. [DOI] [PubMed] [Google Scholar]
- 23.Sikkema KJ, Heckman TG, Kelly JA, et al. HIV Risk Behaviors among Women Living in Low-Income, Inner-City Housing Developments. American Journal of Public Health. 1996;86(8_Pt_1):1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latkin CA, Mandell W, Vlahov D, et al. The Long‐Term Outcome of a Personal Network‐Oriented HIV Prevention Intervention for Injection Drug Users: The Safe Study. American Journal of Community Psychology. 1996;24(3):341–364. [DOI] [PubMed] [Google Scholar]
- 25.Smyth HL, Pitpitan EV, MacKinnon DP, et al. Assessing Potential Outcomes Mediation in HIV Interventions. AIDS and Behavior. 2021:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latkin C, Donnell D, Liu TY, et al. The Dynamic Relationship between Social Norms and Behaviors: The Results of an HIV Prevention Network Intervention for Injection Drug Users. Addiction. 2013;108(5):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latkin C, Donnell D, Celentano DD, et al. Relationships between Social Norms, Social Network Characteristics, and HIV Risk Behaviors in Thailand and the United States. Health Psychology. 2009;28(3):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latkin C, Kuramoto S, Davey-Rothwell M, et al. Social Norms, Social Networks, and HIV Risk Behavior among Injection Drug Users. AIDS and Behavior 2010;14(5):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medley A, Kennedy C, O’Reilly K, et al. Effectiveness of Peer Education Interventions for HIV Prevention in Developing Countries: A Systematic Review and Meta-Analysis. AIDS Education and Prevention. 2009;21(3):181–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen HT. Theory-Driven Evaluations. Sage Publications; 1990. [Google Scholar]
- 31.MacKinnon DP. Analysis of Mediating Variables in Prevention and Intervention Research. NIDA Research Monograph. 1994;139:127–127. [PubMed] [Google Scholar]
- 32.Latkin CA, Donnell D, Metzger D, et al. The Efficacy of a Network Intervention to Reduce HIV Risk Behaviors among Drug Users and Risk Partners in Chiang Mai, Thailand and Philadelphia, USA. Social Science & Medicine. 2009;68(4):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers EM. Diffusion of Innovations. Simon and Schuster; 2010. [Google Scholar]
- 34.Robins JM and Greenland S. Identifiability and Exchangeability for Direct and Indirect Effects. Epidemiology. 1992:143–155. [DOI] [PubMed] [Google Scholar]
- 35.VanderWeele TJ and Vansteelandt S. Odds Ratios for Mediation Analysis for a Dichotomous Outcome. American Journal of Epidemiology. 2010;172(12):1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valeri L and VanderWeele TJ. Mediation Analysis Allowing for Exposure–Mediator Interactions and Causal Interpretation: Theoretical Assumptions and Implementation with Sas and Spss Macros. Psychological Methods. 2013;18(2):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanderWeele TJ. Policy-Relevant Proportions for Direct Effects. Epidemiology (Cambridge, Mass). 2013;24(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrews RM and Didelez V. Insights into the Cross-World Independence Assumption of Causal Mediation Analysis. Epidemiology. 2021;32(2):209–219. [DOI] [PubMed] [Google Scholar]
- 39.Naimi AI, Kaufman JS and MacLehose RF. Mediation Misgivings: Ambiguous Clinical and Public Health Interpretations of Natural Direct and Indirect Effects. International Journal of Epidemiology. 2014;43(5):1656–1661. [DOI] [PubMed] [Google Scholar]
- 40.Spiegelman D and Hertzmark E. Easy SAS Calculations for Risk or Prevalence Ratios and Differences. American Journal of Epidemiology. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 41.Zou G A Modified Poisson Regression Approach to Prospective Studies with Binary Data. American Journal of Epidemiology. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 42.VanderWeele T Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford University Press; 2015. [Google Scholar]
- 43.Suzuki E, Evans D, Chaix B, et al. On the “Proportion Eliminated” for Risk Differences Versus Excess Relative Risks. Epidemiology (Cambridge, Mass). 2014;25(2):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Y, Liew Z, Wang A, et al. Mediating Roles of Preterm Birth and Restricted Fetal Growth in the Relationship between Maternal Education and Infant Mortality: A Danish Population-Based Cohort Study. PLoS Medicine. 2019;16(6):e1002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robins JM, Hernan MA and Brumback B. Marginal Structural Models and Causal Inference in Epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 46.Winett RA, Anderson ES, Desiderato LL, et al. Enhancing Social Diffusion Theory as a Basis for Prevention Intervention: A Conceptual and Strategic Framework. Applied and Preventive Psychology. 1995;4(4):233–245. [Google Scholar]
- 47.Buchanan AL, Vermund SH, Friedman SR, et al. Assessing Individual and Disseminated Effects in Network-Randomized Studies. American Journal of Epidemiology. 2018;187(11):2449–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richiardi L, Bellocco R and Zugna D. Mediation Analysis in Epidemiology: Methods, Interpretation and Bias. International Journal of Epidemiology. 2013;42(5):1511–1519. [DOI] [PubMed] [Google Scholar]
- 49.Lipsitch M, Tchetgen ET and Cohen T. Negative Controls: A Tool for Detecting Confounding and Bias in Observational Studies. Epidemiology (Cambridge, Mass). 2010;21(3):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh D, Krishnan A, Gibson B, et al. Social Network Strategies to Address HIV Prevention and Treatment Continuum of Care among at-Risk and HIV-Infected Substance Users: A Systematic Scoping Review. AIDS and Behavior. 2017;21(4):1183–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacKinnon DP. Introduction to Statistical Mediation Analysis. Routledge; 2008. [Google Scholar]
- 52.Hoyle RH and Kenny DA. Sample Size, Reliability, and Tests of Statistical Mediation. Statistical Strategies for Small Sample Research. 1999;1:195–222. [Google Scholar]
- 53.Simmons N, Donnell D, Ou SS, et al. Assessment of Contamination and Misclassification Biases in a Randomized Controlled Trial of a Social Network Peer Education Intervention to Reduce HIV Risk Behaviors among Drug Users and Risk Partners in Philadelphia, Pa and Chiang Mai, Thailand. AIDS and Behavior. 2015;19(10):1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data was obtained from through a third-party and authors cannot make these data publicly available due to data use agreement.


