Abstract
Background:
Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of ulcerative colitis (UC).
Objective:
To assess colectomy incidence rates (IRs) and baseline characteristics for the presence of identified colectomy risk factors among patients in the tofacitinib OCTAVE UC clinical program.
Design:
This post hoc analysis evaluated patients in the 8-week OCTAVE Induction 1 and 2, 52-week OCTAVE Sustain, and OCTAVE Open (open-label, long-term extension) studies.
Methods:
IRs [95% confidence interval (CI)] for colectomy were analyzed. Baseline risk factors based on clinical guidelines: aged <40 years at diagnosis, extensive colitis, severe endoscopic disease [Mayo endoscopic subscore (MES) = 3], hospitalization for UC within 12 months, C-reactive protein (CRP) >3 mg/L, and serum albumin <3.5 g/dL. Baseline risk factors were evaluated in patients who underwent colectomy by study and summarized descriptively.
Results:
Over a maximum of 7.8 years of tofacitinib exposure, 14 patients underwent colectomy: 3/1139 (0.3%) in OCTAVE Induction 1 and 2 [tofacitinib 10 mg twice daily (BID): n = 2; placebo: n = 1], 3/593 (0.5%) in OCTAVE Sustain (placebo: n = 3), and 8/944 (0.8%) in OCTAVE Open (tofacitinib 10 mg BID: n = 8). Colectomy IR per 100 patient-years for all patients who received ⩾1 tofacitinib dose was 0.34 (95% CI: 0.16–0.63). All patients who underwent colectomy had ⩾1 risk factor and prior tumor necrosis factor inhibitor (TNFi) failure, among which the most common risk factors were a MES of 3 (n = 13), CRP >3 mg/L (n = 11), and aged <40 years at diagnosis (n = 9).
Conclusions:
Among patients with moderate to severe UC receiving tofacitinib, colectomies were infrequent; all patients undergoing colectomy had prior TNFi failure, and most had multiple additional risk factors. This provides important information to discuss with patients and inform management decisions.
Registration:
NCT01465763; NCT01458951; NCT01458574; and NCT01470612.
Keywords: colectomy, tofacitinib, ulcerative colitis
Graphical abstract.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory condition that is characterized by inflammation of the colorectal mucosa. 1 The aim of current treatment strategies for UC is to achieve and maintain steroid-free remission and prevent morbidity, including hospitalization and surgery, as well as disease complications such as cancer.1–3 Although medical interventions are key in the management of UC, it is estimated that up to 15% of patients develop refractory disease or become intolerant to long-term maintenance therapy and may require proctocolectomy.3–5
Real-world studies have demonstrated that colectomy rates among patients with UC have varied over time and by geographic location.6,7 Overall, up to one in five patients with UC will require surgery within 10 years of diagnosis. 8 A database review of patients with UC in Canada reported a decrease in the incidence rate (IR) of colectomy from 3.6 per 100 patient-years (PY) between 1998 and 2004 to 3.0 per 100 PY between 2005 and 2011, following the introduction of biologic therapy. 6 A systematic review of major abdominal surgery among patients with inflammatory bowel disease found that, among contemporary cohorts of patients with UC (diagnosis after 2000), the cumulative colectomy risk at 1, 5, and 10 years was 2.8% [95% confidence interval (CI): 2.0–3.9], 7.0% (95% CI: 5.7–8.6), and 9.6% (95% CI: 6.3–14.2), respectively. 9
The following risk factors have been identified in the American College of Gastroenterology Clinical Guidelines as being associated with poor prognosis (as measured by the likelihood of colectomy): aged <40 years at diagnosis, extensive colitis, severe endoscopic disease [defined as a Mayo endoscopic subscore (MES) of 3], hospitalization for UC within 12 months, C-reactive protein (CRP) level >3 mg/L, and serum albumin level <3.5 g/dL. 3
Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of UC. The efficacy and safety of tofacitinib 10 mg twice daily (BID) as induction and maintenance therapy in patients with moderately to severely active UC have been demonstrated in one phase II and two phase III trials of 8 weeks’ duration,10,11 a phase III trial of 52 weeks’ duration, 11 and an open-label, long-term extension (OLE) study. 12
Colectomy data from the global tofacitinib OCTAVE UC clinical program have not been reported previously. Herein, we report colectomy IRs and the results of a post hoc analysis to assess baseline characteristics for the presence of identified risk factors for colectomy among all patients in the global tofacitinib OCTAVE UC clinical program.
Methods
Patients and study design
This post hoc analysis included data from all patients who participated in the phase III induction studies (OCTAVE Induction 1 and 2: NCT01465763 and NCT01458951), the phase III maintenance study (OCTAVE Sustain: NCT01458574), and the OLE study (OCTAVE Open: NCT01470612).
Full study design details have been previously published.10–12 Briefly, patients in OCTAVE Induction 1 and 2 were randomized to receive tofacitinib 10 or 15 mg BID, or placebo, with the final efficacy assessment at week 8. The tofacitinib dose of 15 mg BID in OCTAVE Induction 1 and 2 was subsequently discontinued following a protocol amendment. Patients who completed OCTAVE Induction 1 and 2 with a clinical response could enter OCTAVE Sustain and were re-randomized to receive tofacitinib 5 or 10 mg BID or placebo, with the final efficacy assessment at week 52. Patients in remission at week 52 of OCTAVE Sustain received tofacitinib 5 mg BID in OCTAVE Open. Patients who were nonresponders after completing OCTAVE Induction 1 or 2, who had completed OCTAVE Sustain and were not in remission, or who demonstrated treatment failure in OCTAVE Sustain, were eligible for OCTAVE Open and received tofacitinib 10 mg BID.
All studies were registered with ClinicalTrials.gov and were conducted in compliance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice Guidelines and were approved by the Institutional Review Boards and/or Independent Ethics Committees at each investigational center participating in the studies or at a Central Institutional Review Board. All patients provided written informed consent.
Assessments and statistical analysis
IRs [unique patients with events per 100 PY of exposure; events included those outside the 28-day risk period (defined as the period up to 28 days beyond the last dose of the study drug)] and 95% CIs for colectomy among patients enrolled in the tofacitinib OCTAVE UC clinical program were analyzed in three cohorts: the Induction Cohort included patients from OCTAVE Induction 1 and 2 who received tofacitinib 10 mg BID or placebo; the Maintenance Cohort included patients from OCTAVE Sustain who received tofacitinib 5 or 10 mg BID or placebo; and the Overall Cohort included patients who received ⩾1 dose of tofacitinib in the phase III and OLE studies. As doses could be switched across studies, tofacitinib doses in the Overall Cohort were categorized based on the average daily tofacitinib dose and defined as follows: predominant dose (PD) tofacitinib 5 mg BID, average total daily dose of tofacitinib <15 mg; and PD tofacitinib 10 mg BID, average total daily dose of tofacitinib ⩾15 mg.
Baseline demographics and clinical characteristics were evaluated by study and summarized descriptively.
Risk factors for colectomy that were evaluated at baseline of OCTAVE Induction 1 and 2 included those identified in the American College of Gastroenterology Clinical Guidelines 3 : aged <40 years at diagnosis, extensive colitis, severe endoscopic disease (defined as a MES of 3), hospitalization for UC within 12 months prior to baseline of the induction studies, CRP level >3 mg/L, and serum albumin level <3.5 g/dL. The following additional factors were analyzed to provide better clinical characterization of patients who underwent colectomy: baseline oral corticosteroid use, prior Clostridium difficile (C. difficile) infection, and prior cytomegalovirus (CMV) infection.13,14
Results
In total, 14 patients underwent colectomy across studies in the tofacitinib OCTAVE UC clinical program over a maximum of 7.8 years of tofacitinib exposure: 3 of 1139 patients (0.3%) (tofacitinib 10 mg BID: n = 2; placebo: n = 1) in OCTAVE Induction 1 and 2; 3 of 593 patients (0.5%) (placebo: n = 3) in OCTAVE Sustain; and 8 of 944 patients (0.8%) (tofacitinib 10 mg BID: n = 8) in OCTAVE Open.
IRs for colectomy
IRs and 95% CIs for colectomy, stratified by cohort, are presented in Figure 1. Numerical differences in IRs were observed for placebo versus tofacitinib 5 and 10 mg BID in the Induction and Maintenance Cohorts; however, the colectomy rates were low across the treatment groups and 95% CIs for IRs overlapped. In the Induction Cohort, IRs (95% CIs) were 2.47 (0.06–13.74) in the placebo group and 1.26 (0.15–4.55) in the tofacitinib 10 mg BID group. The difference in IR (95% CI) for the tofacitinib 10 mg BID versus placebo group was −1.21 (−6.35 to 3.93) and was not statistically significantly different from 0. In the Maintenance Cohort, IRs (95% CIs) were 2.90 (0.60–8.47) in the placebo group, 0.00 (0.00–2.48) in the tofacitinib 5 mg BID group, 0.00 (0.00–2.35) in the tofacitinib 10 mg BID group, and 0.00 (0.00–1.21) for all patients who received tofacitinib. The difference in IR (95% CI) for the tofacitinib all versus placebo group was −2.90 (−6.18 to 0.38) and was not statistically significantly different from 0. In the Overall Cohort, the IRs (95% CI) were 0.47 (0.23–0.87) in the PD tofacitinib 10 mg BID group, 0.00 (0.00–0.46) in the PD tofacitinib 5 mg BID group, and 0.34 (0.16–0.63) in the tofacitinib all group.
Figure 1.
IRs for colectomy in the Induction, Maintenance, and Overall Cohorts from the tofacitinib OCTAVE UC clinical program.
The Induction Cohort comprised patients who received placebo or tofacitinib 10 mg BID in OCTAVE Induction 1 and 2 (data from patients who received tofacitinib 15 mg BID were excluded from the analysis). The Maintenance Cohort comprised patients who received placebo, tofacitinib 5 mg BID, or tofacitinib 10 mg BID in OCTAVE Sustain. The Overall Cohort comprised patients who received ⩾1 dose of tofacitinib 5 or 10 mg BID in OCTAVE Induction 1 and 2, OCTAVE Sustain, or OCTAVE Open. All events, including those that were outside the 28-day risk period, are included in the figure.
BID, twice daily; CI, confidence interval; IR, incidence rate; N, number of patients evaluable for colectomy; n, number of patients with the event; PD, predominant dose; PY, patient-years; UC, ulcerative colitis.
Risk factors for colectomy
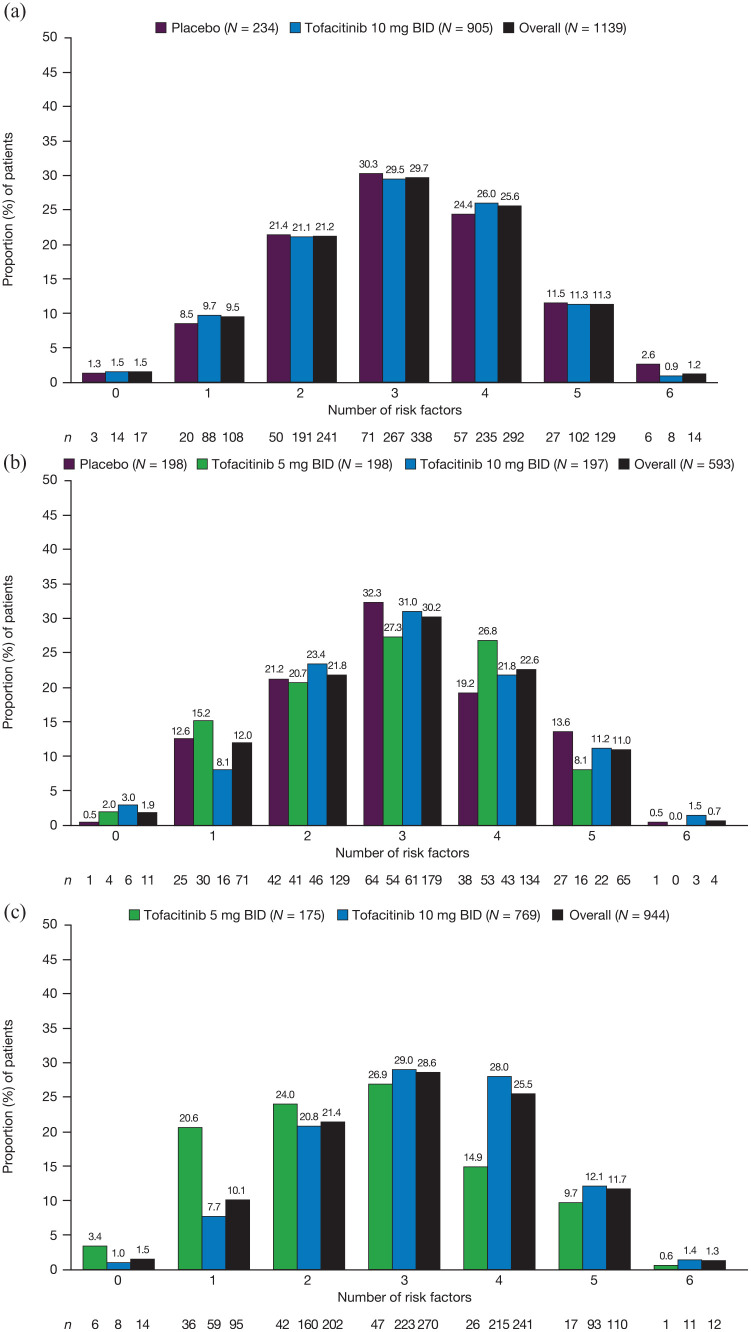
The proportion of patients in the tofacitinib OCTAVE UC clinical program with risk factors for colectomy, stratified by treatment group and number of risk factors, is shown in Figure 2. Within each respective phase of the program, across treatment groups, >98% (OCTAVE Induction 1 and 2: 1122 of 1139 patients; OCTAVE Sustain: 582 of 593 patients; OCTAVE Open: 930 of 944 patients) of patients had ⩾1 risk factor for colectomy, and >70% (OCTAVE Induction 1 and 2: 871 of 1139 patients; OCTAVE Sustain: 442 of 593 patients; OCTAVE Open: 713 of 944 patients) of patients had 2–4 risk factors for colectomy.
Figure 2.
Proportion of patients in each treatment group in (a) OCTAVE Induction 1 and 2, (b) OCTAVE Sustain, and (c) OCTAVE Open, stratified by number of risk factors.
BID, twice daily; N, number of patients in the treatment group; n, number of patients in the treatment group by specified number of colectomy risk factors.
Risk factors for each patient who underwent colectomy, by study, are shown in Table 1. All patients who underwent colectomy had ⩾1 risk factor for colectomy, with most patients (n = 11) having ⩾3 risk factors. The three most common risk factors among patients who underwent colectomy were a MES of 3 (n = 13), CRP >3 mg/L (n = 11), and being aged <40 years at diagnosis (n = 9) (Table 2). The three patients in OCTAVE Sustain who underwent colectomy had four, three, and two risk factors for colectomy. In OCTAVE Open, seven of eight patients who underwent colectomy had ⩾4 risk factors. In all studies, only 1 of 14 patients who underwent colectomy had a serum albumin level <3.5 g/dL. No patients who underwent colectomy in any of the studies had experienced prior C. difficile or CMV infections.
Table 1.
Risk factors among patients who underwent colectomy in the tofacitinib OCTAVE UC clinical program.
| Treatment group at time of colectomy | Number of risk factors present a | Risk factors b | ||||||
|---|---|---|---|---|---|---|---|---|
| Age at UC diagnosis, years | Disease extent | Baseline MES | Hospitalization for UC within 12 months c | Baseline CRP, mg/L | Baseline serum albumin, g/dL | |||
| OCTAVE Induction 1 and 2 | ||||||||
| 1 | Tofacitinib 10 mg BID | 3 | 26 | Left-sided colitis | 3 | No | 53.53 | 4.0 |
| 2 | Tofacitinib 10 mg BID | 1 | 41 | Left-sided colitis | 3 | No | 1.90 | 4.3 |
| 3 | Placebo | 3 | 14 | Extensive colitis/pancolitis | 2 | No | 4.36 | 3.6 |
| OCTAVE Sustain | ||||||||
| 1 | Placebo | 4 | 52 | Extensive colitis/pancolitis | 3 | Yes | 4.65 | 4.0 |
| 2 | Placebo | 3 | 67 | Left-sided colitis | 3 | Yes | 19.59 | 3.9 |
| 3 | Placebo | 2 | 23 | Left-sided colitis | 3 | No | 1.08 | 4.5 |
| OCTAVE Open | ||||||||
| 1 | Tofacitinib 10 mg BID | 4 | 35 | Extensive colitis/pancolitis | 3 | No | 13.42 | 4.1 |
| 2 | Tofacitinib 10 mg BID | 4 | 22 | Extensive colitis/pancolitis | 3 | No | 4.02 | 4.2 |
| 3 | Tofacitinib 10 mg BID | 2 | 44 | Proctosigmoiditis | 3 | No | 4.50 | 3.7 |
| 4 | Tofacitinib 10 mg BID | 4 | 20 | Extensive colitis/pancolitis | 3 | No | 36.80 | 4.2 |
| 5 | Tofacitinib 10 mg BID | 4 | 31 | Extensive colitis/pancolitis | 3 | Yes | 1.71 | 4.3 |
| 6 | Tofacitinib 10 mg BID | 5 | 45 | Extensive colitis/pancolitis | 3 | Yes | 40.53 | 3.2 |
| 7 | Tofacitinib 10 mg BID | 4 | 31 | Left-sided colitis | 3 | Yes | 4.34 | 4.1 |
| 8 | Tofacitinib 10 mg BID | 5 | 29 | Extensive colitis/pancolitis | 3 | Yes | 7.50 | 4.0 |
Risk factors for colectomy included: aged <40 years at diagnosis, extensive colitis, severe endoscopic disease (MES of 3), hospitalization for UC within 12 months (prior to induction study baseline), CRP level >3 mg/L, and serum albumin level <3.5 g/dL.
Data were taken from baseline of OCTAVE Induction 1 and 2.
Hospitalization for UC was considered a risk factor if it occurred within 12 months prior to enrollment into the induction studies.
BID, twice daily; CRP, C-reactive protein; MES, Mayo endoscopic subscore; UC, ulcerative colitis.
Table 2.
Number of patients who underwent colectomy in the tofacitinib OCTAVE UC clinical program with each risk factor.
| Treatment group at time of colectomy | Risk factors | |||||
|---|---|---|---|---|---|---|
| Aged <40 years at UC diagnosis | Extensive colitis | Severe endoscopic disease | Hospitalization for UC within 12 months | CRP >3 mg/L |
Serum albumin <3.5 g/dL |
|
| Tofacitinib 10 mg BID, n/N | 7/10 | 6/10 | 10/10 | 4/10 | 8/10 | 1/10 |
| Placebo, n/N | 2/4 | 2/4 | 3/4 | 2/4 | 3/4 | 0/4 |
| Total, n/N | 9/14 | 8/14 | 13/14 | 6/14 | 11/14 | 1/14 |
Data were taken from baseline of OCTAVE Induction 1 and 2. Severe endoscopic disease was defined as a MES of 3. Hospitalization for UC was required to be within 12 months prior to enrollment into OCTAVE Induction 1 and 2.
BID, twice daily; CRP, C-reactive protein; MES, Mayo endoscopic subscore; N, number of patients in the treatment group with colectomy; n, number of patients with the specified risk factor; UC, ulcerative colitis.
Demographics and clinical characteristics of patients who underwent colectomy
Baseline demographics and clinical characteristics of patients who underwent colectomy are presented, by study, in Table 3. Of the 14 patients who underwent colectomy, the majority were female, and most had a disease duration of ⩾6 years, a MES of 3, and a total Mayo score ⩾6 at the last visit prior to their colectomy events. All patients had prior tumor necrosis factor inhibitor (TNFi) failure; regarding disease extent, eight patients had extensive colitis or pancolitis (Tables 1 and 3).
Table 3.
Baseline demographics and clinical characteristics of patients who underwent colectomy in the tofacitinib OCTAVE UC clinical program.
| Treatment group at time of colectomy | Induction/ maintenance treatment group a |
Subpopulation | Sex; age, b years | Disease duration, b years | Smoking status | Baseline oral corticosteroid use b | Prior TNFi failure c | Duration of treatment prior to colectomy, b days | Duration of tofacitinib treatment prior to colectomy, c days | Total Mayo score at last visit prior to colectomy | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OCTAVE Induction 1 and 2 | |||||||||||
| 1 | Tofacitinib 10 mg BID | – | Male; 35 | ⩾6 | Ex-smoker | No | Yes | 21 | 21 | 11 | |
| 2 | Tofacitinib 10 mg BID | – | Male; 45 | <6 | Never smoked | No | Yes | 38 | 38 | 11 | |
| 3 | Placebo | – | Female; 19 | <6 | Never smoked | No | Yes | 58 | 0 | 9 | |
| OCTAVE Sustain | |||||||||||
| 1 | Placebo | Tofacitinib 10 mg BID | – | Female; 71 | ⩾6 | Ex-smoker | Yes | Yes | 126 | 63 | 6 |
| 2 | Placebo | Tofacitinib 10 mg BID | – | Female; 71 | <6 | Ex-smoker | Yes | Yes | 143 | 70 | 10 |
| 3 | Placebo | Tofacitinib 10 mg BID | – | Female; 30 | ⩾6 | Never smoked | Yes | Yes | 63 | 61 | 4 |
| OCTAVE Open | |||||||||||
| 1 | Tofacitinib 10 mg BID | Placebo | Maintenance treatment failure d | Female; 45 | ⩾6 | Never smoked | Yes | Yes | 62 | 131 | 12 |
| 2 | Tofacitinib 10 mg BID | Tofacitinib 10 mg BID | Maintenance treatment failure d | Female; 31 | ⩾6 | Never smoked | Yes | Yes | 132 | 282 | 6 |
| 3 | Tofacitinib 10 mg BID | Tofacitinib 10 mg BID | Maintenance treatment failure d | Male; 49 | <6 | Ex-smoker | Yes | Yes | 44 | 265 | 11 |
| 4 | Tofacitinib 10 mg BID | Placebo | Induction nonresponder e | Female; 33 | ⩾6 | Never smoked | No | Yes | 92 | 92 | 4 |
| 5 | Tofacitinib 10 mg BID | Tofacitinib 10 mg BID | Induction nonresponder e | Male; 32 | <6 | Current smoker | Yes | Yes | 56 | 114 | 8 |
| 6 | Tofacitinib 10 mg BID | Tofacitinib 10 mg BID | Maintenance treatment failure d | Male; 52 | ⩾6 | Ex-smoker | No | Yes | 375 | 555 | 11 |
| 7 | Tofacitinib 10 mg BID | Placebo | Induction nonresponder e | Female; 43 | ⩾6 | Never smoked | Yes | Yes | 47 | 47 | 11 |
| 8 | Tofacitinib 10 mg BID | Tofacitinib 10 mg BID | Induction nonresponder e | Female; 34 | <6 | Never smoked | Yes | Yes | 174 | 237 | 6 |
Induction treatment group allocations are presented for induction nonresponders. Maintenance treatment group allocations are presented for maintenance treatment failures.
Data were taken from baseline of the respective studies in which the colectomy occurred.
Data were taken from baseline of OCTAVE Induction 1 and 2.
Maintenance treatment failures comprised induction responders who experienced treatment failure (defined as a ⩾3-point increase from OCTAVE Sustain baseline total Mayo score plus a ⩾1-point increase in rectal bleeding subscore and endoscopic subscore, and an absolute endoscopic subscore of ⩾2 points after ⩾8 weeks of treatment) in OCTAVE Sustain and received tofacitinib 10 mg BID in OCTAVE Open.
Induction nonresponders comprised patients who did not achieve a clinical response (defined as a decrease from induction study baseline total Mayo score of ⩾3 points and ⩾30%, plus a decrease in rectal bleeding subscore of ⩾1 point or an absolute rectal bleeding subscore of 0 or 1) after 8 weeks of induction treatment with tofacitinib 10 mg BID or placebo and continued to receive tofacitinib 10 mg BID in OCTAVE Open.
–, not applicable; BID, twice daily; TNFi, tumor necrosis factor inhibitor; UC, ulcerative colitis.
In OCTAVE Induction 1 and 2, the two patients who underwent colectomy and were receiving treatment with tofacitinib 10 mg BID had received treatment for 21 and 38 days, and both had a total Mayo score of 11 at the last visit prior to their colectomy events. All three patients who underwent colectomy in OCTAVE Sustain were receiving placebo after achieving clinical response with tofacitinib 10 mg BID during OCTAVE Induction 1 and 2; these patients were all female, aged 71, 71, and 30 years, had received treatment for 126, 143, and 63 days, and had total Mayo scores of 6, 10, and 4, respectively, at the last visit prior to their colectomy events. Of the eight patients who had colectomies during OCTAVE Open (all assigned to the tofacitinib 10 mg BID group), four were maintenance treatment failures (i.e. induction responders who experienced treatment failure in OCTAVE Sustain and received tofacitinib 10 mg BID in the OLE study), of which three received tofacitinib 10 mg BID during OCTAVE Sustain. The remaining patients were induction nonresponders (i.e. patients who did not achieve a clinical response after 8 weeks of induction treatment with tofacitinib 10 mg BID or placebo and continued to receive tofacitinib 10 mg BID in OCTAVE Open).
Discussion
In this post hoc analysis, we analyzed data from all patients in the tofacitinib OCTAVE UC clinical program. Across the program, colectomies were infrequent, with an overall IR of 0.34 (95% CI: 0.16–0.63) per 100 PY among all patients who received ⩾1 dose of tofacitinib. All colectomies occurred in patients with recognized risk factors for poor prognosis, as well as with prior TNFi failure, even in a patient population who were not required to have been exposed to TNFi therapy prior to entry in these studies.
Some numerical differences in IRs for colectomy were observed between the treatment groups in each of the three cohorts. However, it should be noted that a small number of colectomy events was observed, and 95% CIs for IRs overlapped. Furthermore, for the Overall Cohort, due to the design of the tofacitinib OCTAVE UC clinical program, comparisons of the findings for the PD tofacitinib 5 versus 10 mg BID groups are limited by the differences in the populations. For example, the PD tofacitinib 10 mg BID group mainly comprised patients who were not in remission (either induction nonresponders or maintenance treatment failures) and were non-randomly assigned to receive tofacitinib 10 mg BID in OCTAVE Open, while patients in remission received tofacitinib 5 mg BID. In the Induction and Maintenance Cohorts, proportions and IRs for colectomies were numerically higher for patients receiving placebo versus tofacitinib 5 and 10 mg BID; however, CIs for the differences in IRs, when tofacitinib treatment was compared to placebo, indicated that these differences may be due to normal variation. No colectomies occurred in patients receiving active treatment with tofacitinib in the Maintenance Cohort. All three patients who underwent colectomy in OCTAVE Sustain had received induction therapy with tofacitinib 10 mg BID in OCTAVE Induction 1 and 2 and were subsequently re-randomized to receive placebo in OCTAVE Sustain, which supports the importance of patients remaining on maintenance therapy; however, this finding is limited by the fact that colectomies occurred in three patients in OCTAVE Sustain.
In OCTAVE Open, none of the colectomies occurred in patients who received tofacitinib 5 mg BID, with all eight colectomies reported in patients in the tofacitinib 10 mg BID group. This may, in part, be because in contrast with patients in the tofacitinib 5 mg BID group, patients who received tofacitinib 10 mg BID in OCTAVE Open were not in remission at study entry and demonstrated varying degrees of active UC, ranging from moderate to severe. 12 All eight patients with colectomies in OCTAVE Open were either induction nonresponders (four patients) or maintenance treatment failures (four patients); therefore, these patients represent a more treatment-refractory population. No colectomies were reported among patients who entered OCTAVE Open in remission, and, although the patients who required lower tofacitinib doses to maintain remission may represent a population that is easier to treat, this supports the importance of patients remaining in remission as a long-term treatment goal.
Colectomy and UC disease-related surgery rates for other UC therapies have been reported in the GEMINI and UNIFI clinical programs. The GEMINI clinical program evaluated the efficacy and safety of vedolizumab 300 mg in adults with inflammatory bowel disease. In GEMINI 1, a phase III, randomized, placebo-controlled trial in patients with moderately to severely active UC, colectomy occurred in 15 of 620 (2.4%) and 3 of 149 (2.0%) patients in the vedolizumab (maximum exposure: 52 weeks) and placebo groups, respectively. In the GEMINI long-term safety study, which included patients who had previously participated in the vedolizumab clinical studies and patients naïve to vedolizumab treatment, colectomy occurred in 55 of 894 patients (6.2%) with UC, after a maximum exposure of 196 weeks. 15 The UNIFI phase III, randomized, placebo-controlled trial evaluated the efficacy and safety of ustekinumab 130 mg or 6 mg per kg in patients with UC. In that trial, UC-related surgery was reported in 2 of 348 (0.6%) and 3 of 175 (1.7%) patients in the ustekinumab and placebo groups, respectively, through week 44 of the maintenance study. 16 Proportions of patients who underwent colectomy reported here are comparable to the UNIFI clinical program [OCTAVE Induction 1 and 2: 3 of 1139 patients (0.3%); OCTAVE Sustain: 3 of 593 patients (0.5%); OCTAVE Open: 8 of 944 patients (0.8%)]. However, given the differences between trial designs and patient populations in the GEMINI, UNIFI, and OCTAVE programs, any comparisons between clinical trials should be interpreted cautiously.
A variety of predictors of colectomy have been proposed, including demographic, genetic, serologic, and endoscopic factors. Disease extent has been identified as one of the strongest predictors of colectomy among patients with UC. 13 A retrospective review of patients with severe UC found that male patients, those diagnosed between the ages of 34 and 64 years, and those with pancolitis, had an increased likelihood of early colectomy. 17 Furthermore, a previous systematic review that evaluated the risk factors for colectomy in patients with chronic refractory UC found that prior TNFi exposure was associated with a higher risk of colectomy. 18 In the present analysis, all patients who underwent colectomy had prior TNFi failure, compared with 37.7–55.3% across the treatment groups of each study included in this analysis.12,19 These results suggest that patients with risk factors for colectomy and those with prior TNFi failure may represent a more treatment-refractory population, and this population is at higher risk for colectomy than a population with no prior risk factors for colectomy, or one that is TNFi-naïve. This finding is of particular relevance in environments where product labeling for tofacitinib requires previous failure of TNFi therapy. 20 Most patients also had severe endoscopic disease (MES of 3) on program entry and moderately or severely active disease, based on the total Mayo score, prior to colectomy; 10 however, the extent of disease varied, with a small majority having extensive colitis/pancolitis. Out of all the patients who underwent colectomy, only one patient in OCTAVE Open had a serum albumin level <3.5 g/dL, suggesting that the risk factors for colectomy in an outpatient population differ from those in an inpatient population. Further studies are required to determine factors predictive of colectomy among patients with chronic refractory UC.
All patients who underwent colectomy in the tofacitinib OCTAVE UC program had ⩾1 risk factor, the most common being severe endoscopic disease at the start of treatment, and most patients had ⩾3 risk factors. The identification of risk factors for colectomy plays an important role in influencing treatment choices among patients with UC and can, ultimately, impact patient morbidity and mortality. 3 Further studies are required to determine whether specific risk factors other than those analyzed in this study, or a greater number of risk factors, increase the risk for colectomy. Moreover, the degree to which each specific risk factor contributes toward risk of colectomy is not yet understood and future studies may elucidate this.
While the tofacitinib OCTAVE UC clinical program followed patients for a maximum of 7.8 years of tofacitinib exposure, this study was limited by the fact that it was a post hoc analysis with a small number of colectomy events. This study was further limited because specific reasons for colectomy were not collected during the studies included in this analysis. However, most colectomy events were presumably related to active disease, suggested by the fact that most patients had a total Mayo score ⩾6 at the last visit prior to colectomy. Furthermore, the number of events in OCTAVE Open may have been underestimated, due to the use of case report form identification.
In conclusion, in this analysis of patients with moderate to severe UC in the tofacitinib OCTAVE UC clinical program, colectomies were infrequent; as expected, all events occurred in patients with prior TNFi failure, and most patients had multiple additional risk factors that were previously identified in clinical guidelines. This provides important information to discuss with patients and inform management decisions. Recognition of factors associated with risk of poor prognosis or colectomy is significant when evaluating the risk and benefits of different surgery or treatment options and identifying the appropriate, individualized treatment options for a UC patient.
Acknowledgments
The authors would like to thank the patients, investigators, and study teams who were involved in the tofacitinib UC program. Medical writing support and editorial support, under the direction of the authors, were provided by Chimwemwe Chibambo, MBChB, and Liz Bailey, respectively, at CMC Connect, a division of IPG Health Medical Communications, and were funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med 2022; 175: 1298–304).
Footnotes
ORCID iD: Paulo G. Kotze  https://orcid.org/0000-0002-9632-6691
https://orcid.org/0000-0002-9632-6691
Contributor Information
David T. Rubin, University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA
Leonardo Salese, Pfizer Inc, Collegeville, PA, USA Former employee of Pfizre.
Mitchell Cohen, Pfizer Inc, 500 Arcola Road, Collegeville, PA 19426, USA.
Paulo G. Kotze, IBD Outpatient Clinics, Colorectal Surgery Unit, Pontifícia Universidade Católica do Paraná (PUCPR), Curitiba, Brazil
John C. Woolcott, Pfizer Inc, Collegeville, PA, USA
Chinyu Su, Pfizer Inc, Collegeville, PA, USA.
Rajiv Mundayat, Pfizer Inc, New York, NY, USA.
Jerome Paulissen, Pfizer Inc, New York, NY, USA.
Joana Torres, Gastroenterology Division, Hospital Beatriz Ângelo, Loures, Portugal; Division of Gastroenterology, Hospital da Luz, Lisbon, Portugal; Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal.
Millie D. Long, Center for Gastrointestinal Biology and Disease, University of North Carolina, Chapel Hill, NC, USA
Declarations
Ethics approval and consent to participate: All studies were registered with ClinicalTrials.gov, were conducted in compliance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice Guidelines and were approved by the Institutional Review Boards and/or Independent Ethics Committees at each investigational center participating in the studies, or at a Central Institutional Review Board. All patients provided written informed consent.
Consent for publication: Not applicable.
Author contributions: David T. Rubin: Conceptualization; Writing – original draft; Writing – review & editing.
Leonardo Salese: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Mitchell Cohen: Conceptualization; Writing – original draft; Writing – review & editing.
Paulo G. Kotze: Conceptualization; Writing – original draft; Writing – review & editing.
John C. Woolcott: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Chinyu Su: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Rajiv Mundayat: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Jerome Paulissen: Conceptualization; Formal analysis; Writing – original draft; Writing – review & editing.
Joana Torres: Conceptualization; Writing – original draft; Writing – review & editing.
Millie D. Long: Conceptualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Pfizer. Authors who are employees of Pfizer Inc contributed to the study design, analysis, and data interpretation. Medical writing support was funded by Pfizer. Jerome Paulissen is an employee of Syneos Health, which was a paid contractor to Pfizer in connection with the development of this article and related statistical analysis.
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DTR has received consulting fees from AbbVie, AltruBio, Arena Pharmaceuticals, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corp/Syneos, Connect Biopharma, Eli Lilly, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, InDex Pharmaceuticals, Iterative Scopes, Janssen, Pfizer Inc, Prometheus Biosciences, Reistone, Takeda, and Techlab Inc, and has received a research grant from Takeda. LS is a stockholder of Pfizer Inc and was an employee of Pfizer Inc at the time of this analysis. MC, JCW, CS, and RM are employees and stockholders of Pfizer Inc. PGK has received consulting and speaker fees from AbbVie, Janssen, Pfizer Inc, and Takeda, and has received research grants from Pfizer Inc and Takeda. JP is an employee of Syneos Health, which was a paid contractor to Pfizer in connection with the development of this article and related statistical analysis. JT has received advisory board fees from AbbVie, Arena Pharmaceuticals, Bristol Myers Squibb, Galapagos, Janssen, and Pfizer Inc, research grants from AbbVie and Janssen, and speaker fees from AbbVie, Galapagos, Janssen, and Pfizer Inc. MDL has received consulting fees from AbbVie, Bristol Myers Squibb, Calibr, Eli Lilly, Genentech, Janssen, Pfizer Inc, Prometheus Biosciences, Roche, Salix, Takeda, Target PharmaSolutions, Theravance, and Valeant, and has received research grants from Pfizer Inc and Takeda.
Availability of data and materials: Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017; 389: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meier J, Sturm A. Current treatment of ulcerative colitis. World J Gastroenterol 2011; 17: 3204–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019; 114: 384–413. [DOI] [PubMed] [Google Scholar]
- 4. Spinelli A, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: surgical treatment. J Crohns Colitis 2022; 16: 179–189. [DOI] [PubMed] [Google Scholar]
- 5. Ross H, Steele SR, Varma M, et al. Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum 2014; 57: 5–22. [DOI] [PubMed] [Google Scholar]
- 6. Abou Khalil M, Boutros M, Nedjar H, et al. Incidence rates and predictors of colectomy for ulcerative colitis in the era of biologics: results from a provincial database. J Gastrointest Surg 2018; 22: 124–132. [DOI] [PubMed] [Google Scholar]
- 7. Parragi L, Fournier N, Zeitz J, et al. Colectomy rates in ulcerative colitis are low and decreasing: 10-year follow-up data from the Swiss IBD Cohort Study. J Crohns Colitis 2018; 12: 811–818. [DOI] [PubMed] [Google Scholar]
- 8. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013; 145: 996–1006. [DOI] [PubMed] [Google Scholar]
- 9. Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn’s disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol 2021; 19: 2031–2045.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012; 367: 616–624. [DOI] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Lawendy N, Danese S, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther 2022; 55: 464–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dias CC, Rodrigues PP, da Costa-Pereira A, et al. Clinical predictors of colectomy in patients with ulcerative colitis: systematic review and meta-analysis of cohort studies. J Crohns Colitis 2015; 9: 156–163. [DOI] [PubMed] [Google Scholar]
- 14. Khoudari G, Singh A, Alkhadra Y, et al. Predictors of colectomy in patients admitted with inflammatory bowel disease: a population-based study [Abstract]. Am J Gastroenterol 2019; 114(S1): 804.31021833 [Google Scholar]
- 15. Shen B, Blake A, Lasch K, et al. Vedolizumab use in patients with inflammatory bowel diseases undergoing surgery: clinical trials and post-marketing experience. Gastroenterol Rep (Oxf) 2019; 7: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019; 381: 1201–1214. [DOI] [PubMed] [Google Scholar]
- 17. Al-Darmaki A, Hubbard J, Seow CH, et al. Clinical predictors of the risk of early colectomy in ulcerative colitis: a population-based study. Inflamm Bowel Dis 2017; 23: 1272–1277. [DOI] [PubMed] [Google Scholar]
- 18. Macaluso FS, Cavallaro F, Felice C, et al. Risk factors and timing for colectomy in chronically active refractory ulcerative colitis: a systematic review. Dig Liver Dis 2019; 51: 613–620. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, D'Haens GR, Sands BE, et al. Tofacitinib for the treatment of ulcerative colitis: an integrated summary of up to 7.8 years of safety data from the global clinical programme. J Crohns Colitis 2023; 17: 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. US Food and Drug Administration. XELJANZ® (tofacitinib) highlights of prescribing information, https://labeling.pfizer.com/showlabeling.aspx?id=959 (2022).