Abstract
The diagnosis and treatment of cancer are continuously evolving in search of more efficient, safe, and personalized approaches. Therapies based on nanoparticles or physical stimuli-responsive substances have shown great potential to overcome the inherent shortcomings of conventional cancer therapies. In fact, nanoparticles may increase the half-life of chemotherapeutic agents or promote the targeting in cancer tissues while physical stimuli-responsive substances are more effective and safer with respect to traditional chemotherapeutic agents because of the possibility to be switched on only when needed. These 2 approaches can be combined by exploiting the ability of some inorganic nanomaterials to be activated by light, ultrasounds, magnetic fields, or ionizing radiations. Albeit the development of stimuli-responsive materials is still at the early stages, research in this field is rapidly growing since they have important advantages with respect to organic nanoparticles or molecular substances, like higher stability, and higher efficiency in converting the stimulus in heat or, in some cases, reactive oxygen species. On the other hand, the translation process is slowed down by issues related to safety and quality of the formulations. This literature review summarizes the current advancements in this research field, analysing the most promising materials and applications.
Keywords: photothermal therapy, photodynamic therapy, sonodynamic therapy, magnetic hyperthermia, radiotherapy, carbon, noble metals, nanoparticles
Introduction
Despite important advances in the treatments, cancer is still one of the most significant causes of death in patients, after heart and infectious diseases, with an estimated number of more than 19 million of new cases in the world in 2020 (International Agency for Research on Cancer). This is particularly relevant in low and middle incoming countries, where many people cannot afford the diagnosis and treatment of early-stage cancer because of poverty. 1 Moreover, the development of mutations and resistance in tumors poses significant additional challenges to the treatment efficacy. 2 Mutations may arise spontaneously in cancer cells or because of exposure to environmental or therapeutic factors. 3 The development of resistance to chemotherapy, radiation therapy, and other treatments is a major concern, as it may result in treatment failure and in the evolution of more aggressive and treatment-resistant forms of cancer. The ability of malignant cells to adapt and evolve in response to therapy makes it difficult to effectively treat and cure cancer. 4
Along with surgery and radiotherapy, chemotherapy is one of the foundations of cancer treatment today. 5 It is well recognized that chemotherapy and radiotherapy have serious adverse effects on the patient, mainly due to the low specificity of the treatments. On the other hand, the risk of missing tiny cancer cell clusters during bulk tumor surgical removal is considerable, and cancer cells that are left over frequently mutate their DNA to become resistant to adjuvant treatments. Because of this, the rates of tumor recurrence following traditional therapies are extremely high (eg, nearly 100% for glioblastoma, 85% for ovarian malignancies, and 30% for breast cancers). 6 This leads the scientific community to search for novel therapeutic approches.
Phototherapy, the technique of exposing tissues to light in order to alleviate illness, was already used to treat skin cancer, vitiligo and psoriasis in ancient Indian, Chinese, and Egyptian civilizations. 7 The discovery of modern phototherapy is attributed to Niels Ryberg Finsen, who employed blue light to cure cutaneous tuberculosis (lupus vulgaris) and red light to treat smallpox. For his work, he was honored with the Nobel Prize in Medicine and Physiology in 1903. 8 The first example of chemical agents having cytotoxic effects following light activation is reported in 1900 by Oscar Raab, who described the antimicrobial effect of light-activated acridine dyes. 9
Phototherapies combined with photosensitizers (PS) or photothermal agents (PTA) to increase efficiency and selectivity in tumor cell killing have been recently developed. PS and PTA are chemical agents preferentially absorbed by diseased cells and able to convert light into cytotoxic species or heat, respectively. 10 They are typically administered to the patient prior to the light exposure; then, laser light is delivered to the targeted area using a fiber-optic probe or, for superficial tumors, free-space optics. Several molecular PS have already been approved for cancer treatments. 11
The therapeutic approach that uses PS is called photodynamic therapy (PDT), while PTA are used in photothermal therapy (PTT). Both techniques have advantages and disadvantages and the choice between them depends on the specific medical condition being treated and the desired therapeutic outcome; furthermore, in some cases they can be associated.
Nanomedicine is defined as the application of nanotechnologies in medicine. It impacts several fields of medicine like diagnostics, medical imaging, nanoparticles (NPs) for therapy, vaccines, and regenerative medicine. It includes both medical devices and pharmaceutical products. The most studied and exploited medical application of nanotechnology is the design of NPs-based pharmaceutical formulations. 12
Several kinds of NPs have been nowadays developed for drug and nucleic acid delivery, for imaging, as agents for PDT and PTT, and as radiation sensitizers. Nanocarriers can be designed precisely to maximize drug accumulation in a target tissue, to achieve precise targeting of the intended cell, to enhance bioavailability, and to reduce the frequency and dosage required for currently prescribed medications. NPs can also serve as multifunctional platforms that can combine therapy, diagnosis, and imaging. 12 Materials, in the form of NPs have some advantages over molecular substances in oncology. In fact, through either passive targeting (enhanced permeability and retention effect) or active targeting (specific surface modification), these nanocomposites could significantly accumulate in the tumor location. Moreover, various drugs, enzymes, genes, and other therapeutic molecules could be specifically encapsulated or grafted to the surface of the NP via physical adsorption or chemical conjugation, giving it multifunctional properties.
Recent advances in nanomedicine have increased the development of stimuli-responsive NPs. These systems can be activated by internal stimuli (eg, pH, ionic strength, enzymes, or redox reactions), but more interestingly by external stimuli. In the latter case, the nanosystems are activated on-demand close to the target tissue, increasing efficacy and reducing side effects.
Two approaches can be adopted. One is the design of NPs to deliver stimuli-responsive molecular agents, the other is to design NPs that are stimuli-responsive themselves. 13 In this area, inorganic nanomaterials are particularly promising.
This literature review article will focus on the latter type of NPs to encourage ongoing research in this fast-growing area of oncology.
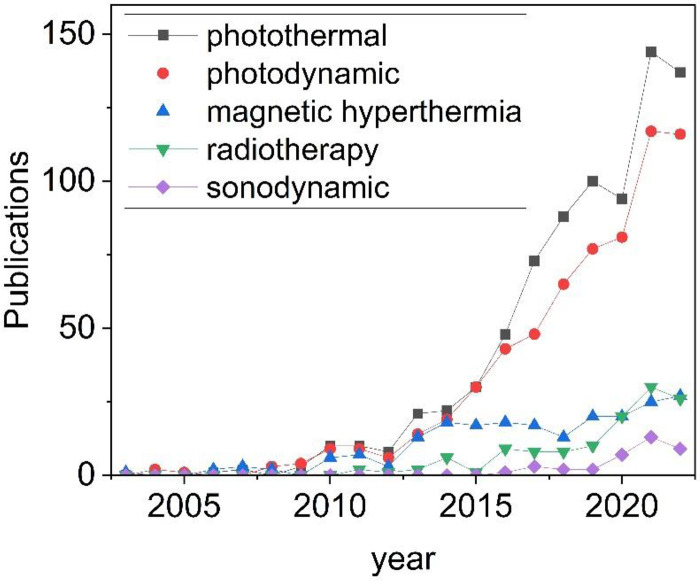
A brief description of the current oncology therapies mediated by physical stimuli in which nanomaterials are employed, in particular PDT, PTT, sonodynamic (SDT), magnetic hyperthermia, and radio therapies will be carried out along with the latest advances in these fields. Due to the no doubt wider scientific production and to the rapid increase of publications (Figure 1), a particular focus will be placed on inorganic NPs responsive to electromagnetic radiations. Finally, the clinical potential of inorganic nanomedicines and the limitations of the above described therapies will be discussed.
Figure 1.
Trend in the scientific publications containing the words “inorganic nanoparticles” and “photothermal” or “photodynamic” or “magnetic hyperthermia” or “radiotherapy” or “sonodynamic” in the past 20 years (source Web of Science).
Note that a discussion on the strategies of decoration of the surface of NPs with bioactive compounds to increase their bioavailability, stability, and reactivity, targeting and imaging capabilities is out the scope of the present review. Other exhaustive reviews on this topic can be found in literature. 14
Physical Stimuli-Mediated Cancer Therapies
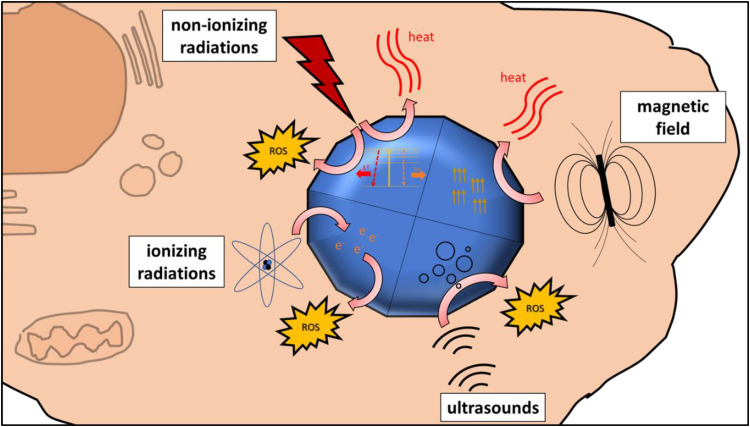
Nowadays, electromagnetic radiations are only one of the numerous kinds of physical stimuli that have been studied for medical applications. Among the others, ultrasounds, magnetic fields, or ionizing radiations found large interest. All these strategies will be briefly introduced in the present chapter underlighting advantages and disadvantages. In the choice of the type of physical stimulus 3 parameters have to be considered: the ability of the molecule/material to efficiently respond to the stimulus, the stimulus penetration depth in the tissue, and its safety.
Figure 2 shows a scheme of the type of physical stimuli that have been explored or used in NPs-mediated cancer therapies.
Figure 2.
Type of physical stimuli that can be used to induce cell death mediated by inorganic NPs. Abbreviation: NPs, nanoparticles.
Ultrasounds: Sonodynamic Therapy
Ultrasound (US) are the high-frequency sound waves commonly used for medical imaging of internal organs and tissues. The sound waves are emitted by a probe that is placed on the skin, and the echoes produced by the waves as they bounce back from the tissues are recorded and used to create images. It has a wide range of applications, including the evaluation of pregnancy, the diagnosis of abdominal and pelvic conditions, and the detection of blood flow and cardiac function. It is also commonly used in interventional procedures, such as biopsies and the tumor removal, due to its real-time imaging capabilities. 15
The term sonodynamic therapy (SDT) is frequently employed to refer to all therapeutic US approaches which are not thermally related, encompassing gene therapy, chemotherapy combinations, and the induction of apoptosis. It was first used by Yumita and Umemura in 1989. 16 These authors discover that some US-activated chemicals called sonosensitizer are cytotoxic and can therefore be used for the treatment of cancer. Where a sonochemical process is present, some researchers tend to favor this more restricted interpretation. 17
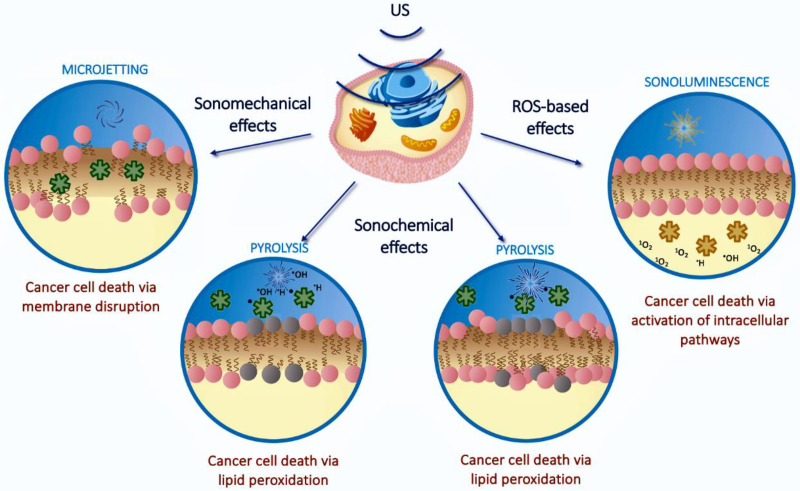
In SDT cell death can be triggered by different mechanisms that can be mediated or not by reactive oxygen species (ROS). Sonomechanical effects consist in the formation of hot areas from ultrasonic radiation release energy at a level high enough to induce bubbles to develop and oscillation, causing long-term cell damage. 18 Alternatively, sonochemical effects lead the generation of free radical species by pyrolysis of water or through excitation of the sonosensitizer. A third mechanism is based on sonoluminescence, which is the emission of light following bubble collapse that activate a sonosensitizer that, in turn, releases ROS such as singlet oxygen and hydroxyl radicals, similarly to what observed in PDT. 16 In Figure 3 a scheme of the different mechanism is reported.
Figure 3.
Pathways of cytotoxicity in SDT. Reprinted from Advanced Drug Delivery Review, Canaparo R, Foglietta F, Barbero N, Serpe L. The promising interplay between sonodynamic therapy and nanomedicine p. 114495, Copyright (2022), with permission from Elsevier. Abbreviation: SDT, sonodynamic therapy.
There is a growing interest in using inorganic micro/nanosystems as sonosensitizers. Several inorganic nanomaterials such as titanium dioxide (TiO2), porous silicon, graphene, mesoporous silica, gold, iron oxides (Fe3O4), and fullerene, have shown appreciable SDT efficiency. 19 For example, Brazzale et al 20 reported that gold NPs can be activated by US to yield ROS and resulting in oxidative damage and consequent cancer cell death.
US therapy is noninvasive, safe, and controllable. Strength, frequency, and duration of exposure to the ultrasound waves to determine how much tumor cell killing occurs. When compared to other physical stimuli, US have clear advantages in the noninvasive treatment of nonsuperficial malignancies because of their excellent tissue penetration and low tissue attenuation. 21
However, SDT is still in its infancy and further investigation to reveal its clinical potential is needed. In particular, the lack of standardized cavitation detection and measurement is still a difficult point to address, specially to increase the comprehension into the SDT mechanism in vivo. 16
Magnetic Field: Magnetic Hyperthermia Therapy
Superparamagnetism is a property that occurs in magnetic materials in the form of NPs. Oppositely to large ferromagnetic particles, they became magnetic only in the presence of a magnetic field. The most studied superparamagnetic NPs are iron oxides (SPIONs). Several products based on them have been and are currently in clinical trials, one of them is already in the market, as discussed in Chapter 4.
The most important application of SPIONs is as T1 contrasting agents in magnetic resonance imaging. SPIONs are also studied as drug delivery agents in magnetic-guided delivery, in which accumulation in the target tissue is induced by the local application of an external magnetic field. 22 Finally, SPIONs can also be exploited to generate localized heat if stimulated by an external alternating magnetic field (magnetic hyperthermia, MHT). In these conditions, repeated alignments and relaxations of magnetic spins of particles occurs, and the associated energy is dissipated through heat.23,24
The efficacy of the particles in the different applications depends by their magnetic properties, which in turn can be modulated by particles size, composition, for example by replacing iron ions with other transition metal ions, and surface modifications. Size is a key parameter since it is directly correlated with the magnetization values, which determines the capacity of particles to respond to the applied magnetic field. However, size needs to remain low enough to avoid irreversible magnetization that would induce undesirable particles agglomeration due to particle–particle magnetic attraction. For this reason, particles size is typically between 5 and 50 nm. Size is critical also for magneto-thermal applications. For this application, heating efficiency increases with size: particles larger than 20 nm are more effective in heat induction, with an upper limit of 40 nm under clinically relevant magnetic field. 25
Iron oxide NPs are considered highly biocompatible. In fact, they can be degraded in the body releasing iron ions that are transferred to ferritin that can cage and store them or used in metabolic processes. On the other hand, iron ions might induce ROS-mediated cytotoxicity 26 or different forms of cell death. 27 Different coating materials have been investigated, such as polymers, silica, metal oxides, or carbon which can enhance biocompatibility, and allow for anchoring of molecules through covalent bonding. 28
Although this is a promising therapeutic strategy, preclinical studies often apply magnetic field strengths, frequencies or dose of NPs that are beyond what is clinically practicable. Further research is needed to improve safety and heating efficiency of NPs applied to magnetic hyperthermia, in order to achieve clinical translation. In particular, the focus should be on designing NPs that can more effectively target and heat tumors. 29
Ionizing Radiations: Radiotherapy
Radiotherapy is a conventional treatment of cancer. It is currently applied in several kinds of tumors. However, it suffers of poor selectivity and in some case poor efficacy due to radio resistance of tumor cells. In order to increase the effect of radiotherapy molecular radiosensitizers are commonly applied. 30
In the past years several NPs have been proposed as radiosensitizers like gold or heavy metal NPs, ferrites, and titanium dioxide. This application of inorganic NPs will not be reviewed here, since other exhaustive reviews can be found in literature.30,31
Nonionizing Electromagnetic Radiations: Photodynamic and Photothermal Therapies
Nonionizing electromagnetic radiations encompass ultraviolet (UV, λ = 320-400 nm), visible (λ = 400-700 nm), and near-infrared light (NIR) (λ = 700-1700 nm). NIR is the most studied range because of its higher ability to deeply penetrate in biological tissues. This is a particularly important property since it allows treating larger tumors with low invasive procedures.
NIR light is mainly divided into 2 biological windows: first (NIR-I, 700-950 nm) and second (NIR-II, 1000-1700 nm). According to the American National Standards Institute the skin maximum permissible exposure (MPE) and maximal penetration depth at 808 nm and at 1000–1100 nm light irradiation are 0.33 and 1 W/cm2, respectively. 32 This means that NIR-II lasers have an MPE power that is approximately 3 times higher than NIR-I lasers for irradiation without damaging skin. 33
Nonionizing electromagnetic radiations can induce in molecular substances or materials 2 different effects, the release of ROS or the generation of heat.
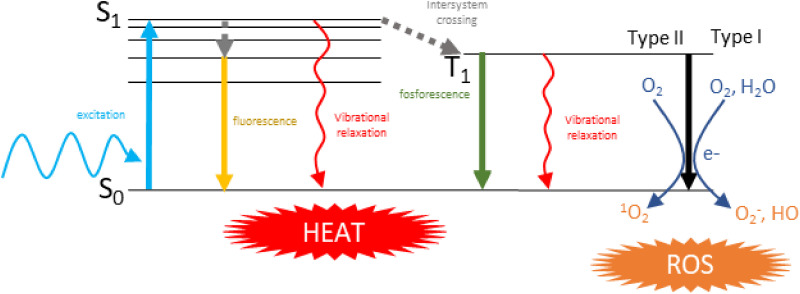
Following photoexcitation, PS and PTA in the grown singlet state (S0) are activated to an excited singlet state (S1) (Figure 4). The excited state decays back to the ground state mainly through 3 processes: emitting a photon having an energy lower than the incident one (fluorescence), by intersystem crossing to the triplet state T1 (which may further lead to phosphorescence), and by nonradiative vibrational relaxation. Heat generation arises as the result of vibrational relaxation processes (photon to phonon conversion). For a molecular substance the 3 pathways are usually competitive, resulting in limited heat generation. To improve the photothermal (PT) conversion efficiency, light emitting, and intersystem crossing must be inhibited. 34 In the triplet state some substances can transfer electrons to other molecules generating ROS (superoxide or hydroxyl radicals), or energy to oxygen generating another cytotoxic ROS, that is, singlet oxygen. The 2 mechanisms of generation of ROS are referred as types I and II, respectively. 35
Figure 4.
Mechanisms of ROS and heat production in molecular substances. Abbreviation: ROS, reactive oxygen species.
Materials are in general better PTA than molecules since have a higher absorption coefficient. They are also characterized by their thermal stability, which ensures that they can maintain their temperature under high-intensity illumination. Moreover, they have a high potential to accumulate selectively in cancer cells.
Photodynamic Therapy
PDT is a contemporary noninvasive anticancer approach with a high potential for clinical use. In PDT, local or systemic PS can be activated by light to produce cytotoxic ROS. These ROS can either cause angiotoxicity to cut off the supply of nutrients to tumor cells or induce necrosis and apoptosis in tumor cells. In addition, it has been demonstrated that acute pro-inflammatory cytokines and immunogenic cell death brought on by PDT activate the immune system and trigger the reconstruction of the tumor microenvironment. Some molecular PS has received clinical approval for the treatment of various malignancies. 36
The choice of the photosensitizer relies on a variety of variables, depending upon the structural characteristics of the PS. For effective PDT, the PS should absorb light at wavelengths that can penetrate the tissue of interest, and the excited state should have sufficient energy to produce ROS with a high quantum yield (ie, the number of ROS produced per adsorbed photon). Moreover, the PS should be stable under the light exposure used for PDT to avoid degradation and to maintain its phototoxicity. 37 Finally, the PS should preferentially accumulate in the target cells or tissue and localize in the subcellular compartments where ROS generation is desired.
Albeit PDT is a promising noninvasive cancer treatment approach with a lot of potential for clinical use it has still has a lot of drawbacks in several applications, including poorly delivered light, insufficient oxygen due to the hypoxic tumor microenvironment, and poor efficiency in metastatic tumors. 38 Some methods have been proposed to overcome the lack of oxygen like the development of NPs containing catalase, an enzyme which can react with hydrogen peroxide to produce oxygen, hemoglobin, and perfluorocarbon, which operates as an oxygen carrier. 39
Photothermal Therapy
Irreversible cell death can occur quickly when the tumor tissues are heated to a temperature over 41°C. 40 A laser is generally employed to irradiate tumor cells topically or percutaneously in order to uniformly increase the tumor temperature while minimizing destruction to healthy cells.
PTT methods may be based on 3 different mechanisms. The first one involves exposing the tumor location to extremely high temperatures (over 45°C) for a short duration of time, which causes thermal ablation and consequent cellular death. This approach typically causes hemorrhage and tumor vascular stasis, which prevent the addition of other treatments. 41 The second approach, known as mild hyperthermia, requires maintaining temperatures between 42°C and 43°C to induce cellular damage and to enhance the permeability of tumor arteries, both of which can also be exploited to increase the uptake of NPs by tumors. 42 Compared to normal tissues, tumor tissues are more acidic and hypoxic. It is assumed that these properties make them more temperature sensitive, enabling PTT to selectively destroy cancer cells and protect healthy cells surrounding the tumor. 42 To further elevate and localize the heat generation in the target tumor cells exclusively PTA can be used. In fact, PTA need less energy to reach therapeutic temperatures. 43 Several optical dyes acting as PS possess also the potential to act as PTA. The third approach consists in a mild hyperthermia to enhance drug delivery. 43
PTA features and functions are crucial toward the PTT process. Strong NIR absorption, a high extinction coefficient, outstanding PT stability, high PT conversion efficiency, and other characteristics all need to be represented inside the ideal PTA. A variety of nanomaterials can be remarkable candidates. Organic nanomaterials include semiconducting polymers like polypyrrole, polyaniline, and poly (3,4 ethylenedioxythiophene), as well as cyanine, porphyrin, phthalocyanine, and boron dipyrromethene (BODIPY). 44
Inorganic nanomaterials, including carbon nanotubes, graphene, fullerenes, and carbon dots, along with semiconductor-based nanomaterials such as sulfides, oxides, and selenides, in addition to transition metal NPs, transition metal carbides, and nitrides, are examples of NIR-absorbing materials that have been developed so far.
Sources of Nonionizing Radiation for Photothermal and Photodynamic Therapies
The most relevant sources of nonionizing radiation for tumor treatments are lasers. A laser is an optoelectronic device that exploits the mechanism of amplification by stimulated emission to emit a coherent beam of light, where the term “coherent” means that there is a fixed phase relationship between the electric field values at different locations or at different times and therefore the beam is capable of exhibiting interference effects; for the scopes of this work, it refers to a practically monochromatic beam. 45
A laser can operate in continuous wave mode (CW) or pulsed wave mode (PW); in the first case the wave is emitted continuously, while in the latter case the laser beam is alternatively switched on and off with the possibility to change the pulse duration and the duty-cycle in a range that depends on the exploited pulse generation approach. A CW treatment guarantees higher ablated regions while a PW treatment is usually preferred for precise and limited ablated areas. This is because in CW high temperatures are rapidly reached resulting in a large region ablation due water vaporization; in PW, instead, the heating is slower and the heat is absorbed by the tissue during the OFF-time, leading to smaller ablated regions.
Many types of lasers are available today, differing on the gain medium (a semiconductor alloy, a rare-earth-doped crystal or fiber, a gas mixture, or liquid dyes) and on thus the characteristics of the emitted beam. The most common are the laser diodes (LDs), the fiber lasers, and the diode-pumped solid-state lasers, which have practically replaced the early gas lasers and flash lamp pumped solid-state lasers in all the considered applications. In particular, LDs are electrically pumped lasers in which the gain is generated by an electrical current flowing through a junction made with differently doped semiconductor materials. They are the elective choice for PDT or PTT treatments because of their many advantages, such as: small footprint (leading to compact benchtop solutions), simplified driving mechanism, and high emission efficiency (the source of energy is an electrical current, which can be easily controlled and provides a high emitted to absorbed power ratio), availability of high-power versions (LDs capable of emitting several watts are off-the-shelf products), wide choice of emission wavelengths from UV to mid-infrared (the emitted wavelength depends on the combination of used semiconductors; eg, GaAs-based devices emit in the NIR around 1000 nm, while GaN devices in the blue around 440 nm). The possibility to select the emission wavelength is very important because it allows balancing between 2 constraints, namely: (i) the optimal wavelength required for the efficient excitation of the PS or to maximize the absorption for heat generation and (ii) the light penetration depth in the tissue.
Laser light has the further advantage of being easily routed to the tumor site using small size optical fibers. This especially is useful in the case of internal organs because it lowers the invasive impact, thanks to the percutaneous delivery of the laser light. Moreover, the same delivery fiber can also be used for sensing to assess the effectiveness of the laser action.46–48
Drawbacks and Limitations of the Application of NPs in PTT and PDT
Several critical points ampere the clinical application of inorganic NPs-mediated PTT and PDT. First of all, the extent of PT and photodynamic (PD) conversion are directly correlated with the intensities of NIR light that will gradually decrease as it crosses the body tissues. 49 Consequently, the efficacy might be poor in deep tissues. To solve this problem, a typical strategy is the use of optical fibers-assisted PTT for interstitial illumination or other ultra-deep PTT strategies, 50 as discussed in the “Sources of nonionizing radiation for photothermal and photodynamic therapies” section. Other strategies that as being explored to bypass this issue are the combination of PTT and PTD with other kind of therapies like chemotherapy, immunotherapy, or genetherapy 51 to obtain a synergic effect.49,52
Photoresponsive Nanomaterials
A wide variety of inorganic compounds can adsorb light converting the energy in heat and/or reactive species. The most studied are transition metals, transition metals oxide, transition metals chalcogenide, and elemental carbon allotropes. Some transition metal semiconductors like TiO2, Fe2O3, and ZnO are powerful photoactive agents, able to generate large amount of ROS. 53 Albeit they can be modified to shift the adsorption maximum toward low-energy radiation, most of these compounds adsorb in the UV or visible region, making them poorly suitable for medical purposes. Moreover, in some cases they are known to dissolve in biological environment releasing toxic ions. 54
In this chapter the most promising categories of inorganic nanomaterials are considered focusing on those that can be activated by NIR electromagnetic radiations, and their properties and mechanism of action discussed. In Table 1 examples of inorganic materials that have been proposed for these applications are reported.
Table 1.
Example of Inorganic-Based Nanomaterials for NIR-Triggered PD, PT, SD, Magnetic Hyperthermia or Combined Therapies.
| Inorganic material | Surface modification | Targeting agent | Drug | Application | Tumour type | Model | References# |
|---|---|---|---|---|---|---|---|
| Carbon nanoparticles | - | - | - | PTT/photoacustic imaging | Prostate cancer | In vitro / in vivo | 55 |
| Carbon nanoparticles | - | - | - | PTT | Lung cancer | In vitro | 56 |
| GO-C60 | Methoxypolyethylene glycol | - | - | PDT/PTT | HeLa cell | In vitro | 57 |
| Single-walled carbon nanohorns | Indocyanine green | - | - | PTT/PDT | Breast cancer | In vitro | 58 |
| SWCNTs/Au nanorods | PEGylated phospholipids | - | - | PTT/imaging | Breast cancer | In vivo | 59 |
| SWCNTs/MWCTs | Tripeptide lipid/sucrose laurate - | - | siRNA | PTT/gene therapy | Cervical cancer | In vitro / in vivo | 60 |
| GO | Gelatine/bovine serum albumine | - | Doxorubicin | PTT/ chemotherapy |
Breast cancer | In vitro | 61 |
| Graphene | PEG | PTT | Breast cancer | In vivo | 62 | ||
| CNT/AgNPs | PEG | - | - | PTT | Melanoma | In vitro / in vivo | 63 |
| r-GO/Au nanocages | Spinach extract hydrogel | - | - | PTT/PDT/chemotherapy | HeLa cells | In vitro | 64 |
| Au nanorods | PEG | Lactoferrin | - | PTT | Liver cancer | In vitro / in vivo | 65 |
| Au nanocages | Liposome | - | - | PTT/ chemotherapy |
Breast cancer | In vitro /in vivo | 66 |
| Au nanorods | PEG | - | - | PTT | In vitro / in vivo | 67 | |
| Au nanorods | PEG | - | - | PTT | Squamous cell carcinoma | In vitro | 68 |
| Au nanorods | Polyamidoamine dendrimer | RGD | - | PTT | Melanoma | In vitro / in vivo | 69 |
| Au nanocages | - | Macrophage membrane and surface anti-PDL1 antibody | Galunisertib | PTT/immunotherapy | Colorectal cancer | In vitro / in vivo | 52 |
| Au stars | PVP | PTT | Prostate carcinoma | In vitro / in vivo | 70 | ||
| Au nanocages | Monoclonal antibodies | PTT | Breast cancer | In vitro | 71 | ||
| Au spheres | Targeting peptides | PTT | HSC-3 and HaCaT cells | In vitro | 72 | ||
| Au-TiO2 nanoparticle | Doxorubicin | PDT | MCF-7, breast cancer cell line | In vitro | 73 | ||
| Au nanochains@SiO2 | Photoacoustic imaging/PTT | Breast cancer | In vivo and in vitro | 74 | |||
| AgNPs | PEG /bovine serum albumin/ indocyanine green | - | - | PTT | Melanoma | In vitro / in vivo | 75 |
| Ag@Fe3O4@C NPs | PEG | FA | Doxorubicin | PTT/chemotherapy | HeLa cells | In vitro and in vivo | 76 |
| Indium NPs | PEG | Photoacoustic imaging/PTT | Breast cancer | In vitro and in vivo | 77 | ||
| Porous Pt NPs | Poly(diallyldimethylammonium chloride) | - | - | PTT | Glioblastoma | In vitro | 78 |
| Pt NPs | - | TPP peptides | PTT | HepG2 cells/ | In vitro / in vivo | 79 | |
| Pt NPs | - | - | - | PTT | Colon cancer | In vitro | 80 |
| Pt NPs | PVP | - | - | PTT | Bone metastasis | In vitro / in vivo | 81 |
| Au spheres | folate-PEG | SDT | KB and HCT-116 cells | In vitro | 20 | ||
| gold nanoparticle–protoporphyrin IX | SDT | Colon carcinoma tumors in BALB/c mice | In vivo | 82 | |||
| AuNP@SiO2 | PEG-perfluorohexane | High intensity focused ultrasound surgical therapy-ultrasound (US) imaging | rabbit VX2 xenograft tumor | Ex vivo / in vivo | 83 | ||
| Iron oxide | 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000 | Magnetic hyperthermia | Mouse xenograft tumour model for glioblastoma multiforme | In vivo | 25 | ||
| Iron oxide | Polyethylenimine | Magnetic hyperthermia | Human neuroblastoma SH-SY5Y cells | In vitro | 84 | ||
| Iron oxide | Chitosan-based nanomicelles | Anti-αvβ3 integrin antibody | Doxorubicin | Magnetic hyperthermia / magnetic particle imaging | MDA-MB-231 and 4T1 cells | In vitro | 85 |
| Iron oxide | Polyethylene glycol | Magnetic hyperthermia / magnetic particle imaging | Mice - tumor xenografts: U87MG and MDA-MB-231-Luc cell | In vivo | 86 |
Abbreviations: CNT, carbon nanotube; GO, graphene oxide; PEG, polyethylene glycol; PTT, photothermal therapy; PVP, polyvinylpyrrolidone; r-GO, reduced GO; SDT, sonodynamic therapy; siRNA, small interfering RNA; SWCNTs, single-walled carbon nanotubes; TPP, triphenyl phosphonium; PDT, photodynamic therapy.; RGD, arginylglycylaspartic acid; FA, folic acid.
Carbon-Based Nanomaterials
Since the discovery of fullerene (C60) in 1985, 87 carbon structures in the nanometric scale have been created in a variety of morphologies and microstructures, including carbon NPs, carbon dots, fullerenes, graphene, carbon nanotubes, hybrid carbon-based nanostructures. Carbon-based nanomaterials have showed excellent potential for the aim of medication delivery. The main reason of their popularity in medical research is the rich chemistry of carbon, that allows them to be functionalized by drugs or targeting molecules. 87 When designed with dimensions between 5 and 50 nm, they are easily internalized by cells and therefore can be used as nanoscale drug delivery systems. However, carbon nanostructures are not studied only as nanocarriers. In fact, depending upon their structure, they may exhibit PD/PT properties, or fluorescence when activated by NIR light. 88
Fullerenes
Fullerenes are a family of carbon allotropes consisting in closed-cage carbon molecules. They can be considered both NPs and molecules. The most common is also known as Buckminster fullerene or C60. Fullerenes can be modified to introduce different functional groups that can bind drugs, genes, or targeting agents. Due to their small size, fullerenes are rapidly internalized by cells, making them suitable as delivery agents. 89
One interesting property of fullerenes is their antioxidant activity, which is due to their capacity to scavenge free radicals. In the tumor microenvironment, the increased levels of ROS can lead to cellular damage and promote tumor growth. Thus, fullerenes can help to reduce oxidative stress in the tumor microenvironment, potentially slowing down tumor growth and sensitizing the tumor to other therapies, such as chemotherapy or radiotherapy. Additionally, they could prevent tumor growth, angiogenesis, and proto-oncogene activation, enhancing anticancer efficacy. 89 On the other hand, once activated by light, they can act as PS. 90 Similar to molecular substances, fullerene can be excited by light from ground state to 1C60. This short-lived species is readily converted to long-lived 3C60 via intersystem crossing, but in presence of molecular oxygen, the fullerene can transfer its energy to it, generating the very reactive ROS singlet oxygen (1O2). 1C60 and 3C60 are also excellent one-electron acceptor being easily reduced to the fullerene radical anion C60•− by electron transfer. Again, in the presence of oxygen, the fullerene radical anion can transfer one electron, producing a superoxide anion radical O2•- and hydroxyl radical •OH. 91
Unfortunately, fullerene exhibits a maximum of adsorption in the visible region, making them of limited application. Some methods have been proposed to extend the adsorption in the NIR range, such as the association with graphene oxide (GO). 57
Carbon Nanotubes
Carbon nanotubes (CNTs) are carbon allotropes with hollow, elongated cylindrical nanostructures. CNTs were first discovered by Sumio Iijima in 1991, and since then, they have been widely studied in the biomedical field thanks to their appealing structures and distinctive properties. 92
CNTs have been proposed as nanocarriers to carry chemotherapeutics, antibodies, proteins, peptide-based medicines, and therapeutic genes for the treatment of cancer with the appropriate functionalization. 93 In addition, functionalization can improve CNTs stability in water and biocompatibility, or affect how cells interact with them, which can significantly lessen harmful effects. Two common methods to modify CNTs are covalent and noncovalent functionalization with a range of chemical groups or targeting agents. When compared to noncovalent functionalization, covalent modification can generate more stable functionalized platforms for drug administration. 94
CNTs are categorized as single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs). SWCNTs are more versatile agents, since depending upon their structure (defects and chirality), they can be metallic (m-SWCNT) or semimetallic (s-SWCNT). NIR light is converted by m-SWCNT rapidly into heat via fast nonradiative decay while in s-SWCNTs it can be used also for the generation of ROS making them possible agents for PDT. 95
The efficiency of conversion of light into heat largely depends upon the structure of CNTs. In fact, the properties of CNTs determine its electronic band structure, which can affect how the nanotube absorbs and dissipates energy. For example, CNTs with smaller diameters tend to have larger band gaps, which can enhance their optical absorption properties of NIR light and increase their efficiency in converting light into heat. Similarly, CNTs with a higher degree of chirality (ie, the way in which the graphene lattice is rolled up to form the nanotube) can have unique band structures that also enhance their absorption and conversion properties. 96
The structure of CNTs also influences its phonon dispersion relations, which describe how heat is carried through the nanotube. For example, CNTs with a smaller diameter tend to have a higher density of phonon modes, which can lead to more efficient thermal transport and dissipation. Additionally, defects or impurities in the CNTs structure can create localized states that can trap energy and reduce the efficiency of light-to-heat conversion. 97
A huge number of studies on CNTs medical applications have been published. However, CNTs exhibit some limitations that hamper the translation to clinics, among them the cost of production and some concern on their toxicity related to the elongate shape. 98
Graphene and Graphene Oxide
Graphene is a carbon allotrope consisting in one atom-thick planar sheet of sp2-hybridized carbon atoms. The fundamental physical characteristics of defect-free graphene, such as its superior mechanical strength, high thermal conductivity, and high charge carrier mobility, make it an ideal candidate for a variety of applications, including energy, electronics, molecular sensing, and catalysis. In addition, graphene and graphene derivatives have been largely studied in the biomedical field. They include single-layer graphene, bilayer graphene, multilayer graphene, GO, and reduced GO (r-GO).
Graphene is a lipophilic material, a property that facilitates the membrane barrier penetration. This property has been exploited to produce antimicrobial formulations, 99 but it makes graphene poorly suitable for cancer therapy. GO is a chemically modified form of graphene. The presence of hydroxyl and carboxyl groups increases its compatibility with water. This material has several advantages, like adsorption in a wide range of wavelength, the possibility to bind drug via π-π stacking or covalent bonding thus acting as drug delivery agent. GO has good PT conversion efficiency, making it ideal agent for PTT. Moreover, it demonstrated photoluminescence spanning the visible to the NIR, enabling cellular imaging.
Like fullerene and CNTs, graphene and its derivatives gain interest in thermal therapies. The broad-spectrum NIR absorption of graphene derivatives enable efficient absorption of a wide range of NIR wavelengths, enhancing the effectiveness of the treatment. 100 GO is generally used to design hybrid nanomaterials. Modification can be performed to improve colloidal stability, or to design combined therapeutic agents that promise enhanced anticancer activity and reduced side effects. The association with molecular photosensitizer makes possible a combined PD/PTT approach, where the cytotoxic effect of heat is associated to the release of ROS. For example, Ding et al recently synthesized a multifunctional platform by conjugating r-GO, indocyanine green (a NIR-PS), and the targeting agent folic acid. 101 The association of GO with magnetic NPs, such as SPIONs enable the design of PD/MHT agents, while the association with chemotherapeutic agents allow the heat-triggered release of the drug (PTT/chemotherapy). 102 For example, Chang et al 64 synthesized a multifunctional r-GO hydrogel as drug carriers for synergistic chemo/PTT/PDT application.
Carbon Nanoparticles and Carbon Quantum Dots
Graphene and CNTs nanostructures are characterized by high crystallinity. Oppositely, spherical carbon NPs are composed mainly by an amorphous carbonaceous matrix, that might contain variable number of crystalline patches. The main categories of nanostructures are carbon quantum dots (CQDs), mesoporous and hydrothermal carbon NPs.
CQDs are small carbon NPs (<10 nm in size) discovered accidentally by Xu et al, 103 in 2004 during the purification of SWCNTs.
The main feature of CQDs is the fluorescence, making them suitable for imaging. In this application, CQDs are alternative to semiconductor quantum dots (QDs), having the advantage of not containing heavy metal elements, that raise significant concerns regarding their potential toxicity in biological systems. 104 CQDs may also be PS, thus enabling their use as PDT agents. A great advantage is the high hydrophilicity and colloidal stability of this carbon nanomaterial. 105 There are numerous methods of producing CQDs. The top-down methods include chemical (strong oxidizing acids) or laser ablation and electrochemical carbonization, 105 while bottom-up methods include microwave irradiation and hydrothermal/solvothermal treatment starting from organic precursors.
Like for graphene, CQDs have been mixed with other substances to create hybrid multifunctional materials. For example, the team of Wu et al, in 2018, obtained a hybrid material by electrostatic interaction of the negatively charged CQD with porphyrin tetraplatin complex. The complex showed a higher efficacy than the 2 separated precursors and shows high biocompatibility, good hydrophilicity, good colloidal stability, and can be detected by fluorescence. Additionally, it exhibits strong antineoplastic activity, which suggests that it has a great potential for clinical use in patients with cancer.105,106 In 2014, the team of Zheng et al, 107 produce a complex based on QDs with the purpose of obtaining a bioimaging agent. The team produced a CQD-Oxa complex synthesized by a condensation reaction between the amine groups of oxaliplatin. Exploiting the reducing environment in tumor cells, it acts as a drug carrier and controlled drug release system, while fluorescence makes possible to monitor the course and distribution of drug molecules. 107
Mesoporous carbon NPs exhibit pores (mesopores) and are produced by carbonization of an organic precursor in the presence of a template. This kind of material has an established role in other fields like catalysis, gas separation and storage, and energy harvesting, 108 while biological applications are relatively new. Mesoporous carbon NPs may be used to store and release molecular drugs in the target tissue. In this application they appear more promising that other mesoporous materials like silica, since in this case the release may be induced by NIR-triggered PT effect.57,109
Carbon NPs made by hydrothermal carbonization (HCNPs) has generated widespread interest in recent years in several applications. 109 The synthesis consists in the thermal treatment of water mixed with organic substances such as saccharides (glucose, sucrose, or starch) or simpler compounds such as furfural at temperatures in the 150 °C to 350°C range under autogenous pressure. This synthesis has advantages with respect to other kind of methods like, for example, pyrolysis at high temperatures. 110 In fact, HCNPs can be prepared with a defined size by an environmentally friendly one-pot synthesis. Moreover, the NPs are hydrophilic and form stable colloidal suspensions in water. 111
HCNPs are composed of elemental carbon, mainly amorphous, and are decorated by acidic carboxylic groups at the surface. Exhibiting a high negative surface charge, HCNPss form highly stable suspensions at all pH values. These functionalities also make them suitable as carriers of drugs, such as cationic peptides that might be bonded to the NP surface by electrostatic interactions. 112
Other important advantages of HCNPs are their biocompatibility. In fact, they exhibit low toxicity against the cells of the immune system and act as antioxidants 113 and are hemo compatible. 114 In addition, previous data suggest that HCNPs are slowly degraded by neutrophils. 56 Finally, depending upon the bulk structure, some of these materials exhibit physical properties like fluorescence, PT and PD capabilities that suggest a possible use as both therapeutic and diagnostic agents themselves. 111
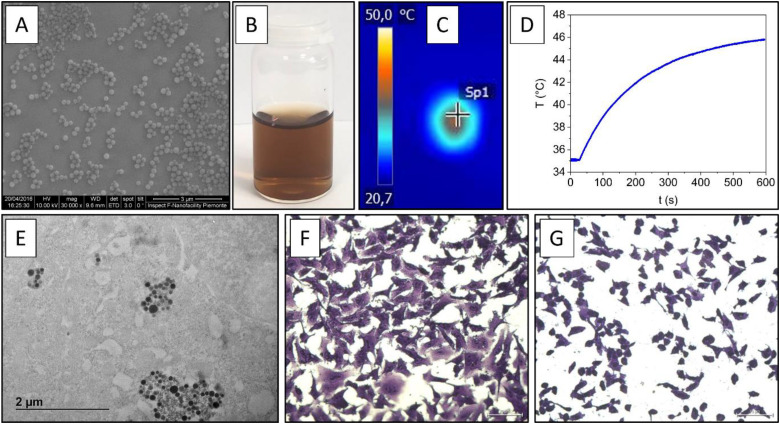
Previous studies by some of us found that, when irradiated with an NIR laser beam (945 nm, 1.3 W/cm2), HCNPs efficiently generate heat 112 (Figure 5). The same particles have been shown to be efficiently internalized by human A549 cells without exhibiting any cytotoxicity. On the other hand, HCNPs, when irradiated and thus activated by NIR light elicited rapid cell death, characterized by the elevation of heat shock proteins and the induction of DNA damage, resulting as promising platforms for PTT of lung cancer. 56
Figure 5.
(A) Representative SEM images of HCNP; (B) colloidal suspension of HCNP in water; (C) and (D) photothermal activity of HCNP: (C) thermal camera image of colloidal suspension of HCNP in water after irradiation by NIR laser and (D) temperature change curves during NIR laser irradiation of HCNP; (E) internalization of HCNP: representative TEM image showing HCNP internalized by the human A549 lung adenocarcinoma cell line treated for 24 h with 80 µg/mL of HCNP; (F) and (G) effect of NIR-activated HCNP on A549 cells (irradiance 3 W/cm2, 15 min): (F) cells irradiated by NIR laser and (G) cells exposed to HCNPs (80 μg/mL) and irradiated by NIR laser (HCNPs + NIR). Abbreviations: HCNP, hydrothermal carbonization; NIR, near-infrared; SEM, scanning electron microscope; TEM, transmission electron microscopy.
HCNP are also promising as theranostic platforms: Miao et al demonstrated in 2016 that glucose-derived HCNP not only have good capability of PT destruction of cancer cells but also exhibit excellent photoacoustic signal enhancement for both in vitro and in vivo photoacoustic imaging. 55
Noble Metal Nanomaterials
In the past decades there has been an exponential increase of research into noble metal nanostructures aims at several fields, including catalysis, electronics, photonics, biosensing, and biomedicine, mainly because of their tunable optical properties.115, 116 Noble metal NPs, such as gold (AuNP), silver (AgNP) and platinum (PtNP) are so-called plasmonic NPs, presenting unique optical features that occurs because the conduction electrons on these NPs surface undergo a collective oscillation when excited by light at specific wavelengths. This process is known as localized surface plasmon resonance.117,118 Plasmonic NPs absorb light strongly, and convert photon energy into heat quickly and efficiently, thereby making them excellent thermal emitters. 119 Noble metal nanostructures have high photostability, consequently do not suffer from photobleaching. For these reasons, plasmonic PTT is being explored as promising strategy to treat different cancer types. 120 The excitation wavelength can be tuned from the visible to the NIR regions of the electromagnetic spectrum by changing NP chemical composition, shape, size, and aggregation state.121–123
AuNPs with specific shapes such as stars, 70 rods, 124 shells, 125 cages, 71 cubes, 126 and spheres 72 have received great attention for PTT due to their high optical adsorption coefficients both, NIR-I and NIR-II regions. 127 Kim et al 128 summarized the PT conversion efficiencies of AuNPs of various shapes and PTT or PDT application. In particular, gold nanorods (AuNRs) and nanoshells, have been widely studied for PTT of cancer, both for PT ablation of solid tumors and mild hyperthermia treatments, because of their strong PT conversion efficiency of NIR light and simple tuning of their optical properties.67,68,129
A further interesting feature of AuNPs is the simple functionalization of their surface with tumor targeting molecules/macromolecules, to minimize the side effect and to increase the dosage of particles at sites where PTT is needed.130,131 For example, the arginine-glycine-aspartic (RGD) acid peptide conjugate to dendrimer-modified AuNR showed highly selective targeting and destructive effects on the cancer cells and solid tumors under NIR laser irradiation. 69 AuNPs can also be functionalized with stealthy polymers such as polyethylene glycol (PEG), which maximizes NPs blood circulation times and apparently promotes accumulation at tumor sites.65, 132
Functionalization with anticancer drugs allows the combination of plasmonic PTT with chemotherapy.49, 52 In particular, hyperthermia has been shown to increase the drug release kinetic into the tumor along with an enhancement of the drug effect.133, 134
AuNPs also present the possibility to be used for diagnostic purpose, thus in combination with their PT properties they can be exploited as cancer theranostic agents.135–137
AuNPs, per se, present a good biocompatibility.138–141 However, the long retention time of gold particles within the human body due to their chemical stability, could result in adverse effects.142–144
Surface modifications may improve AuNPs bioavailability/biocompatibility.145–147 However, in some cases they have been shown to be source of adverse effects. For example, the cationic surfactant cetyltrimethylammonium bromide (CTAB), essential as shape-directing agent for the synthesis of the most promising AuNRs, which also forms their coating, presents a high cytotoxicity. 148 Although the toxicity is associated with excess CTAB in solution,149,150 simple purification is not possible since removing excess of surfactant leads to the aggregation of the AuNRs and to the consequent loss of their PT properties. 150 This has limited the potential use of CTAB-coated AuNRs in biomedical applications.
Other plasmonic NPs have been proposed, like PtNPs and AgNPs. However, they have been less studied for PTT application probably due to more challenging synthetic strategy and poor chemical stability, respectively. Despite that, several interesting studies have been published on this type of NPs.63, 75, 78–81
Clinical Potential of Photoresponsive Inorganic Nanomaterials and Obstacles to Clinical Translation
NPs have a great potential for more efficient and specific anticancer therapies, testified by the large number of studies focused on the design of nanomedicines. 151 However, today, most of the approved nanoformulations are lipid or protein-based, while a minor part is based on inorganic NPs. 152
To the best of our knowledge, only 2 physical stimuli-responsive inorganic nanoparticles-based drugs have been approved: a formulation based on amino silane-coated iron oxide NPs (NanothermTD), that has been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for glioblastoma and prostate cancer treatment by local administration exploiting the magnetic hyperthermia, 153 and HensifyTD, a product containing coated crystalline hafnium oxide radiosensitizer. 154 This formulation is administered locally by injection in the tumor mass, and it is approved for the treatment of soft tissue sarcoma in combination with radiotherapy. Beside NanothermTD and HensifyTD described above Ferumoxytol, a compound composed by carboxymethyl-dextran-coated superparamagnetic iron oxide NP, has been approved by the FDA in 2009 for the treatment of iron deficiency anemia in adults with renal disease, 155 while formulations based on FerumoxsilTD (siloxane-coated iron oxide NPs) are approved as contrasting agent for magnetic resonance of the gastrointestinal tract.
Among the other kind of inorganic NPs, AuNPs are the most studied. However, despite the large amount of literature and patents,156,157 just 2 formulations containing such class of NPs have been evaluated in clinical trials for PTT application. 158 Specifically, 2 formulations containing silica-gold nanoshells were tested for the PT ablation of different types of cancers, and of atherosclerotic plaques. The formulation developed for cancer therapy (AuroShellTD) was administered intravenously as infusion followed by interstitial laser illuminations. Three clinical trials provided very promising results (NCT00848042, NCT01679470, and NCT02680535) and a new 1 is in place (NCT04240639).
The success of photoresponsive inorganic nanomaterials-based therapies depends upon several factors. The first is the penetration capacity of the physical stimulus (considering its safety), and the availability of cheap and user-friendly instrumentation to precisely deliver the physical stimulus. As discussed above, NIR light have a lower penetration capacity than ultrasounds or magnetic fields, thus requiring optical fibers to deliver the light in the case of deep tumors.
Another important factor is the ability of the NPs to accumulate into the tumor mass (if systemically administered) and their safety profile. Both these aspects are modulated by the NPs physicochemical properties including surface modifications. 154
Among the different properties, particle size plays a key role. It is known to modulate both biodistribution and efficacy. In order to have a long circulation time for accumulating in tumors, NPs with diameters between 5 and 200 nm are necessary. In fact, while particles larger than 200 nm typically become held by Kupffer cells in reticuloendothelial systems (RES), those smaller than 5 nm are quickly removed from the circulation through extravasation or renal clearance. 151 Size also controls the uptake by nonphagocytic cells. It is generally accepted that optimal uptake is for particles having a diameter around 50 nm. Finally, size is a key parameter for systemically administered nanomedicines reaching tumors by passive targeting. In this case, a large size (>70 nm) is needed, to avoid particles extravasation in healthy tissue and accumulation in tumor. 151
Although NPs shape is known to play a significant influence in biodistribution, cellular uptake and subcellular localization, its precise effect is still under debate. Rods were reported to have the maximum cellular uptake when NPs were larger than 100 nm, followed by spheres and cubes. However, in experiments using NPs that were smaller than 100 nm, spheres showed higher cellular internalization over rods. 151
The behavior of materials is known to be dictated by the early events occurring when they came in contact with biofluids. 159 This also applies to NPs. Several exhaustive reviews describing the molecular basis and the effects of the interaction of biomolecules with NPs have been published159–161 and therefore we refer to them for details. One of the main processes is the formation of a layer of proteins adsorbed the surface of particles called protein corona 162 which in turn modulates the fate of NPs in the body. In fact, proteins in the corona may contain ligands for several receptors, like opsonins, plasma proteins that are recognized by immune cell 163 or apolipoprotein E, a protein secreted by several cells that can drive particle uptake by hepatocytes. 163 This applies not only in blood, but also in other biofluids, like lung surfactant, or gastrointestinal fluids, that contains proteins that can potentially modulate NPs toxicity and barrier crossing. For examples, surfactant proteins that are abundant in the alveolar fluid have been shown to rapidly associate to NPs and mediate their uptake by alveolar macrophages 164 thus affecting cell-mediated clearance. Concerning the oral route, we previously reported that NPs acquire during the transit in the gastrointestinal tract a protein corona that contains proteases potentially able to modulate the NP crossing and effect on the gastrointestinal barrier since involved in the regulation of the intestinal permeability. 165
To impede the formation of the protein corona and to stabilize the NPs avoiding dissolution, biodegradation or agglomeration, surface modifications are normally applied. PEGylation is the most popular one. PEG is able to enhance the pharmacokinetic properties of NPs by forming a hydrophilic coating that is resistant to protein adsorption thus reducing opsonin proteins adsorption and immune system clearance. On the other hand, PEGylation itself has been shown in some cases to interfere with the activity of active groups on the surface of NPs, such as therapeutic moieties or targeting ligands, which reduces the effectiveness of NPs in biomedical applications. Moreover, allergic reactions due to anti-PEG antibody are known.166, 167
Different kinds of targeting strategies have been proposed to promote NPs accumulation in the target tissue. Extracellular and intracellular targeting represent a big promise to improve drug selectivity and safety. On the other hand, surface decoration introduces complexity to the formulation, potentially affecting cost and quality of the nanoformulation.
The control of the stimuli-responsive NPs physicochemical properties (size, shape, and surface properties) is particularly challenging. This is because the same features modulate the PD, PT, or magnetic properties of the material. Furthermore, the scale-up of the synthetic methods is often difficult to be achieved. This affects the reproducibility of the manufacturing process leading to batch-to-batch variability. 168
Conclusions and Perspectives
Stimuli-responsive inorganic NPs represent a new and promising alternative to conventional molecular agents or organic NPs. Their high stability and light to heat conversion capacity make them interesting, in particular in the field of PTT. On the other hand, their development is still in its early stages, with few formulations already in the market. Extensive investigations aimed at the definition of accurate structure-activity relationships, and at the development of reproducible and scalable synthetic methods are needed to accelerate future clinical translation.
Acknowledgment
The authors are grateful to Prof. Roberto Canaparo, Department of Drug Science and Technology, University of Torino, for his critical input in the paragraph “Ultrasound-based therapies”; thanks to dr. Giulia Antonello, Department of Chemistry, University of Torino, Aurora Bellone, Department of Electronics and Telecommunications, Politecnico di Torino and dr. Jessica Ponti, Joint Research Centre Physical Research Infrastructures of the European Commission, for their contribution to Figure 5. Figure 5E was generated through access to the Nanobiotechnology laboratory under the Framework for access to the Joint Research Centre physical Research Infrastructures of the European Commission (project CETECAN—Research Infrastructure Access Agreement N° 35194/2).
Abbreviations
- BODIPY
boron dipyrromethene
- CNTs
carbon nanotubes
- CQDs
carbon quantum dots
- CTAB
cetyltrimethylammonium bromide
- CW
continuous wave mode
- FDA
Food and Drug Administration
- GO
graphene oxide
- HCNP
hydrothermal carbonization
- LDs
laser diodes
- MHT
magnetic hyperthermia
- MPE
maximum permissible exposure
- NIR
near-infrared
- NPs
nanoparticles
- PD
photodynamic
- PDT
photodynamic therapy
- PEG
polyethylene glycol
- PS
photosensitizers
- PT
photothermal
- PTA
photothermal agents
- PTT
photothermal therapy
- PVP
polyvinylpyrrolidone
- PW
pulsed wave
- PtNP
platinum NP
- QDs
quantum dots
- r-GO
reduced GO
- ROS
reactive oxygen species
- SDT
sonodynamic therapy
- SPIONs
superparamagnetic NPs are iron oxides
- SWCNTs
single-walled carbon nanotubes RGD, arginine-glycine-aspartic acid; MWCNTs, multi-walled carbon nanotubes; FA, folic acid
- TPP
triphenyl phosphonium
- US
ultrasound
- UV
ultraviolet.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: SG is recipients of PhD grant from Ministero dell’Università` e della Ricerca (MUR), Italy.
ORCID iD: Ivana Fenoglio https://orcid.org/0000-0002-6946-3105
References
- 1.Vicini A, Landrigan P, Straif k, The Rising Global Cancer Pandemic J. Moral Theology. 2022;2:1-221. doi: 10.55476/001c.38724 [DOI]
- 2.Shewach DS, Kuchta RD. Introduction to cancer chemotherapeutics. Chem Rev. 2009;109:2859‐2861. doi: 10.1021/CR900208X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Q, Wang K, Zhang X, et al. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small. 2020;16(22):2001704. doi: 10.1002/smll.202001704 [DOI] [PubMed] [Google Scholar]
- 4.Goenka A, Tiek D, Song X, et al. The many facets of therapy resistance and tumor recurrence in glioblastoma. Cells. 2021;10:484. doi: 10.3390/cells10030484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duman F, Forgan RS. Applications of nanoscale metal–organic frameworks as imaging agents in biology and medicine. J Mater Chem B. 2021;9:3423‐3449. doi: 10.1039/D1TB00358E [DOI] [PubMed] [Google Scholar]
- 6.Han HS, Choi KY. Advances in nanomaterial-mediated photothermal cancer therapies: toward clinical applications. Biomedicines. 2021;9:305. doi: 10.3390/biomedicines9030305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-kader M. Abdel-kader MH. In: Kostron H and Hasan T, eds. Photodynamic Medicine: From Bench to Clinic, The Royal Society of Chemistry, 2016;1-21.
- 8.El-Hussein A, Manoto SL, Ombinda-Lemboumba S, Alrowaili ZA, Mthunzi-Kufa P. A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anticancer Agents Med Chem. 2020;21:149‐161. doi: 10.2174/1871520620666200403144945 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Xu Y, Guo X, et al. Enhanced antimicrobial activity through the combination of antimicrobial photodynamic therapy and low-frequency ultrasonic irradiation. Adv Drug Delivery Rev 2022;183:114168. doi: 10.1016/j.addr.2022.114168 [DOI] [PubMed] [Google Scholar]
- 10.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23:217‐228. doi: 10.1007/S10103-007-0470-X [DOI] [PubMed] [Google Scholar]
- 11.Quazi MZ, Park J, Park N. Phototherapy: a conventional approach to overcome the barriers in oncology therapeutics. Technol Cancer Res Treat. 2023;22:1-6. doi: 10.1177/15330338231170939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Z, Gao L, Chen K, et al. Nanoparticles: a new approach to upgrade cancer diagnosis and treatment. Nanoscale Res Lett. 2021;16:88-105. doi: 10.1186/S11671-021-03489-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham S, Choi Y, Pharmaceutics JC. Stimuli-responsive nanomaterials for application in antitumor therapy and drug delivery. Pharmaceutics. 2020;12:630. doi: 10.3390/pharmaceutics12070630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph T, Mahapatra DK, Esmaeili A, et al. Nanoparticles: taking a unique position in medicine. Nanomaterials. 2023;13:574. doi: 10.3390/nano13030574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma G, Kesharwani R, Veeresh P, et al. Advances in diagnostic techniques for therapeutic intervention. Biomed Eng Appl Healthcare. 2019;105‐121. doi: 10.1007/978-981-13-3705-5_5/COVER [DOI] [Google Scholar]
- 16.Canaparo R, Foglietta F, Barbero N, Serpe L. The promising interplay between sonodynamic therapy and nanomedicine. Adv Drug Delivery Rev 2022;189:114495. doi: 10.1016/j.addr.2022.114495 [DOI] [PubMed] [Google Scholar]
- 17.Xing X, Zhao S, Xu T, et al. Advances and perspectives in organic sonosensitizers for sonodynamic therapy. Coord Chem Rev. 2021;445:214087. doi: 10.1016/J.CCR.2021.214087 [DOI] [Google Scholar]
- 18.Nowak K, Schwartz M, Breza V. Sonodynamic therapy: rapid progress and new opportunities for non-invasive tumor cell killing with sound. Cancer Lett. 2022;532:215592. doi: 10.1016/j.canlet.2022.215592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan P, Liu L, Wang P. Sonodynamic therapy (SDT) for cancer treatment: advanced sensitizers by ultrasound activation to injury tumor. ACS Appl Bio Mater. 2020;3:3456‐3475. doi: 10.1021/acsabm.0c00156 [DOI] [PubMed] [Google Scholar]
- 20.Brazzale C, Canaparo R, Racca L. Enhanced selective sonosensitizing efficacy of ultrasound-based anticancer treatment by targeted gold nanoparticles. Future Med. 2016;12(23):3053‐3070. doi: 10.2217/nnm-2016-0293 [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Wang M, Dai Z. The molecular design of and challenges relating to sensitizers for cancer sonodynamic therapy. Mater Chem Front. 2020;4:2223‐2234. doi: 10.1039/D0QM00232A [DOI] [Google Scholar]
- 22.Fernández-Pacheco R, Valdivia JG, Ibarra MR. Magnetic nanoparticles for local drug delivery using magnetic implants. Methods Mol Biol. 2009;544:559‐569. doi: 10.1007/978-1-59745-483-4_35 [DOI] [PubMed] [Google Scholar]
- 23.Fernández-Pacheco R, Marquina C, Gabriel Valdivia J, et al. Magnetic nanoparticles for local drug delivery using magnetic implants. J Magn Magn Mater. 2007;311:318‐322. doi: 10.1016/J.JMMM.2006.11.192 [DOI] [Google Scholar]
- 24.Lee N, Yoo D, Ling D, Cho MH, Hyeon T, Cheon J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem Rev. 2015;115(19):10637‐10689. doi: 10.1021/ACS.CHEMREV.5B00112 [DOI] [PubMed] [Google Scholar]
- 25.Tong S, Quinto C, Zhang L, Mohindra P, Bao G. Size-dependent heating of magnetic iron oxide nanoparticles. ACS Nano. 2017;11:6808‐6816. doi: 10.1021/acsnano.7b01762 [DOI] [PubMed] [Google Scholar]
- 26.Gaharwar U, Meena R, Rajamani P. Iron oxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in lymphocytes. J Appl Toxicol 2017;37(10):1232‐1244. doi: 10.1002/jat.3485 [DOI] [PubMed] [Google Scholar]
- 27.Lomphithak T, Fadeel B. Die hard: cell death mechanisms and their implications in nanotoxicology. Toxicol Sci 2023;192:141‐154. doi: 10.1093/TOXSCI/KFAD008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vercellino S, Kokalari I, Cantoral M. Biological interactions of ferromagnetic iron oxide–carbon nanohybrids with alveolar epithelial cells. Biomater Sci. 2022;10(13):3514‐3526. doi: 10.1039/D2BM00220E [DOI] [PubMed] [Google Scholar]
- 29.Chang D, Lim M, Goos JACM, et al. Biologically targeted magnetic hyperthermia: potential and limitations. Front Pharmacol. 2018;9(AUG):386237. doi: 10.3389/FPHAR.2018.00831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong L, Zhang Y, Liu C, Zhang M, Han S. Application of radiosensitizers in cancer radiotherapy. Int J Nanomedicine. 2021;16:1083‐1102. doi: 10.2147/IJN.S290438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubey P, Sertorio M, Cancers VT. Therapeutic advancements in metal and metal oxide nanoparticle-based radiosensitization for head and neck cancer therapy. Cancers (Basel) 2022;14:514. doi: 10.3390/cancers14030514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An D, Fu J, Zhang B, et al. NIR-II responsive inorganic 2D nanomaterials for cancer photothermal therapy: recent advances and future challenges. Adv Funct Mater 2021;31(32):1-45. doi: 10.1002/adfm.202101625 [DOI] [Google Scholar]
- 33.Yang J, Xie R, Feng L, et al. Hyperthermia and controllable free radical coenhanced synergistic therapy in hypoxia enabled by near-infrared-II light irradiation. ACS Nano. 2019;13(11):13144‐13160. doi: 10.1021/ACSNANO.9B05985 [DOI] [PubMed] [Google Scholar]
- 34.Zhao L, Liu Y, Chang R. Supramolecular photothermal nanomaterials as an emerging paradigm toward precision cancer therapy. Adv Funct Mater 2019;29:1806877. doi: 10.1002/adfm.201806877 [DOI] [Google Scholar]
- 35.Mohanty S, Paul S. Nanotechnology-based ROS-triggered therapeutic strategies in multiple cancer. In: Chakraborti S, ed, Handbook of Oxidative Stress in Cancer: Therapeutic Aspects. 2022:2753-2777 doi: 10.1007/978-981-16-5422-0_119 [DOI]
- 36.Robertson C, Evans D, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol, B 2009;96:1‐8. doi: 10.1016/j.jphotobiol.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 37.Aziz B, Aziz I, Khurshid A, Raoufi E. An overview of potential natural photosensitizers in cancer photodynamic therapy. Biomedicines. 2023;11:224. doi: 10.3390/biomedicines11010224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng X, Shao Z, Science YZA. Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Adv Sci. 2021;8:1-16. doi: 10.1002/advs.202002504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Kwon N, Guo T, Liu Z, Yoon J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angewandte Chemie Int Edn. 2018;57(36):11522‐11531. doi: 10.1002/ANIE.201805138 [DOI] [PubMed] [Google Scholar]
- 40.Bao Z, Liu X, Liu Y, Liu H. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J Pharm Sci 2016;11:349‐364. doi: 10.1016/j.ajps.2015.11.123 [DOI] [Google Scholar]
- 41.Xu C. Second near-infrared photothermal materials for combinational nanotheranostics. Chem Soc Rev. 2021;50:1111‐1137. doi: 10.1016/j.ajps.2015.11.123 [DOI] [PubMed] [Google Scholar]
- 42.Emami B. Physiological mechanisms in hyperthermia: a review. Int J Rad Oncol Biol Phys. 1984;10:289‐295. doi: 10.1016/0360-3016(84)90015-4 [DOI] [PubMed] [Google Scholar]
- 43.Verma J, Lal S, Van Noorden CJ, Verma J, van Noorden CJ. Nanoparticles for hyperthermic therapy: synthesis strategies and applications in glioblastoma. Int J Nanomedicine. 2014;9:2863‐2877. doi: 10.2147/IJN.S57501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L, Zhang X, Wang X, Guan X, Zhang W, Ma J. Recent advances in selective photothermal therapy of tumor. J Nanobiotechnology. 2021;19:335-350:335–350. doi: 10.1186/S12951-021-01080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitz C, Ewing J, Hecht J. In: Introduction to laser technology. 2012;1-320.
- 46.Schena E, Saccomandi P. Laser ablation for cancer: past, present and future. J Funct Biomater. 2017;8:19. doi: 10.3390/jfb8020019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beccaria A, Bellone A, Mirigaldi A. Temperature monitoring of tumor hyperthermal treatments with optical fibers: comparison of distributed and quasi-distributed techniques. Optical Fiber Technology. 2020;60:102340. doi: 10.1016/j.yofte.2020.102340 [DOI] [Google Scholar]
- 48.Gassino R, Liu Y, Konstantaki M. A fiber optic probe for tumor laser ablation with integrated temperature measurement capability. J Lightwave Technol 2016;35:3447‐3454. doi: 10.1109/JLT.2016.2618618 [DOI] [Google Scholar]
- 49.Deinavizadeh M, Kiasat A, Hooshmand N, et al. Smart NIR-light and pH responsive doxorubicin-loaded GNRs@SBA-15-SH nanocomposite for chemo-photothermal therapy of cancer. Nanophotonics. 2021;10(12):3303‐3319. doi: 10.1515/nanoph-2021-0207 [DOI] [Google Scholar]
- 50.Chu Y, Xu XQ, Wang Y. Ultradeep photothermal therapy strategies. J Phys Chem Lett 2022;13(41):9564‐9572. doi: 10.1021/ACS.JPCLETT.2C02642 [DOI] [PubMed] [Google Scholar]
- 51.Mu X, Li J, Yan S, et al. SiRNA delivery with stem cell membrane-coated magnetic nanoparticles for imaging-guided photothermal therapy and gene therapy. ACS Biomater Sci Eng. 2018;4(11):3895‐3905. doi: 10.1021/acsbiomaterials.8b00858 [DOI] [PubMed] [Google Scholar]
- 52.Wang S, Song Y, Cao K, et al. Photothermal therapy mediated by gold nanocages composed of anti-PDL1 and galunisertib for improved synergistic immunotherapy in colorectal cancer. Acta Biomater. 2021;134:621‐632. doi: 10.1016/J.ACTBIO.2021.07.051 [DOI] [PubMed] [Google Scholar]
- 53.Fenoglio I, Ponti J, Alloa E, Ghiazza M, Nanoscale IC. Singlet oxygen plays a key role in the toxicity and DNA damage caused by nanometric TiO2 in human keratinocytes. Nanoscale. 2013;5(14):6567‐6576. doi: 10.1039/C3NR01191G [DOI] [PubMed] [Google Scholar]
- 54.Ivask A, Juganson K, Bondarenko O, et al. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: a comparative review. Nanotoxicology. 2014;8(Suppl. 1):57‐71. doi: 10.3109/17435390.2013.855831 [DOI] [PubMed] [Google Scholar]
- 55.Miao ZH, Wang H, Yang H, Li Z, Zhen L, Xu CY. Glucose-derived carbonaceous nanospheres for photoacoustic imaging and photothermal therapy. ACS Appl Mater Interfaces. 2016;8(25):15904‐15910. doi: 10.1021/ACSAMI.6B03652 [DOI] [PubMed] [Google Scholar]
- 56.Kokalari I, Keshavan S, Rahman M, et al. Efficacy, biocompatibility and degradability of carbon nanoparticles for photothermal therapy of lung cancer. Future Med. 2021;16:689‐707. doi: 10.2217/nnm-2021-0009 [DOI] [PubMed] [Google Scholar]
- 57.Li Q, Hong L, Li H, Liu C. Graphene oxide-fullerene C60 (GO-C60) hybrid for photodynamic and photothermal therapy triggered by near-infrared light. Biosens Bioelectron. 2017;89:477‐482. doi: 10.1016/J.BIOS.2016.03.072 [DOI] [PubMed] [Google Scholar]
- 58.Gao C, Dong P, Lin Z, et al. Near-infrared light responsive imaging-guided photothermal and photodynamic synergistic therapy nanoplatform based on carbon nanohorns for efficient cancer. Chemistry. 2018;24(49):12827‐12837. doi: 10.1002/chem.201802611 [DOI] [PubMed] [Google Scholar]
- 59.Robinson JT, Welsher K, Tabakman SM, et al. High performance in vivo near-IR (>1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res. 2010;3(11):779‐793. doi: 10.1007/s12274-010-0045-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Y, Zhao T, Cao Y, et al. Temperature-sensitive lipid-coated carbon nanotubes for synergistic photothermal therapy and gene therapy. ACS Nano. 2021;15:6517‐6529. doi: 10.1021/acsnano.0c08790 [DOI] [PubMed] [Google Scholar]
- 61.Wu B, Li M, Wang L, et al. Size-transformable nanohybrids with pH/redox/enzymatic sensitivity for anticancer therapy. J Mater Chem B. 2021;9:4319‐4328. doi: 10.1039/D1TB00396H [DOI] [PubMed] [Google Scholar]
- 62.Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318‐3323. doi: 10.1021/nl100996u [DOI] [PubMed] [Google Scholar]
- 63.Behnam MA, Emami F, Sobhani Z, et al. Novel combination of silver nanoparticles and carbon nanotubes for plasmonic photo thermal therapy in melanoma cancer model. Adv Pharm Bull. 2018;8:49. doi: 10.15171/APB.2018.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang G, Li S, Huang F, Zhang X. Multifunctional reduced graphene oxide hydrogel as drug carrier for localized and synergic photothermal/photodynamics/chemo therapy. J Mater Sci Technol 2016;32:735‐762. doi: 10.1016/j.jmst.2016.06.014 [DOI] [Google Scholar]
- 65.Yang H, He H, Tong Z, Xia H, Mao Z, Gao C. The impact of size and surface ligand of gold nanorods on liver cancer accumulation and photothermal therapy in the second near-infrared window. J Colloid Interface Sci. 2020;565:186‐196. doi: 10.1016/J.JCIS.2020.01.026 [DOI] [PubMed] [Google Scholar]
- 66.He H, Liu L, Zhang S, et al. Smart gold nanocages for mild heat-triggered drug release and breaking chemoresistance. J Controlled Release. 2020;323:387‐397. doi: 10.1016/j.jconrel.2020.04.029 [DOI] [PubMed] [Google Scholar]
- 67.Ali MRK, Rahman MA, Wu Y, et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc Natl Acad Sci U S A. 2017;114(15):E3110‐E3118. doi: 10.1073/pnas.1619302114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacKey MA, Ali MRK, Austin LA, Near RD, El-Sayed MA. The most effective gold nanorod size for plasmonic photothermal therapy: theory and in vitro experiments. J Phys Chem B 2014;118:1319‐1326. doi: 10.1021/jp409298f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Huang P, Zhang X, et al. RGD-conjugated dendrimer-modified gold nanorods for in vivo tumor targeting and photothermal therapy. Mol Pharm. 2010;7:94‐104. doi: 10.1021/MP9001415 [DOI] [PubMed] [Google Scholar]
- 70.Espinosa A, Silva AKA, Sánchez-Iglesias A, et al. Cancer cell internalization of gold nanostars impacts their photothermal efficiency in vitro and in vivo: toward a plasmonic thermal fingerprint in tumoral environment. Adv Healthc Mater. 2016;5:1040‐1048. doi: 10.1002/adhm.201501035 [DOI] [PubMed] [Google Scholar]
- 71.Chen J, Wang D, Xi J, et al. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007;7:1318‐1322. doi: 10.1021/NL070345G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panikkanvalappil S, Hooshmand N, El-Sayed MA. Intracellular assembly of nuclear-targeted gold nanosphere enables selective plasmonic photothermal therapy of cancer by shifting their absorption wavelength. Bioconjug Chem. 2017;28:2452-2460. doi: 10.1021/acs.bioconjchem.7b00427 [DOI] [PubMed] [Google Scholar]
- 73.Akram MW, Raziq F, Fakhar-e-Alam M, et al. Tailoring of Au-TiO2 nanoparticles conjugated with doxorubicin for their synergistic response and photodynamic therapy applications. J Photochem Photobiol A Chem. 2019;384:112040. doi: 10.1016/J.JPHOTOCHEM.2019.112040 [DOI] [Google Scholar]
- 74.Zhou C, Zhang L, Sun T, et al. Activatable NIR-II plasmonic nanotheranostics for efficient photoacoustic imaging and photothermal cancer therapy. Adv Mater 2021;33:2006532. doi: 10.1002/adma.202006532 [DOI] [PubMed] [Google Scholar]
- 75.Park T, Lee S, Amatya R, et al. ICG-Loaded PEGylated BSA-silver nanoparticles for effective photothermal cancer therapy. Int J Nanomedicine. 2020;15:5459. doi: 10.2147/IJN.S255874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang M, Liang Y, Zhang Z, et al. Ag@Fe3O4@C nanoparticles for multi-modal imaging-guided chemo-photothermal synergistic targeting for cancer therapy. Anal Chim Acta. 2019;1086:122‐132. doi: 10.1016/J.ACA.2019.08.035 [DOI] [PubMed] [Google Scholar]
- 77.Chen Y, Wu H, Zhou H, et al. PEGylated indium nanoparticles: a metallic contrast agent for multiwavelength photoacoustic imaging and second near-infrared photothermal therapy. ACS Appl Mater Interfaces. 2021;13(39):46343‐46352. doi: 10.1021/ACSAMI.1C13578 [DOI] [PubMed] [Google Scholar]
- 78.Zhu XM, Wan HY, Jia H, et al. Porous pt nanoparticles with high near-infrared photothermal conversion efficiencies for photothermal therapy. Adv Healthc Mater. 2016;5(24):3165‐3172. doi: 10.1002/ADHM.201601058 [DOI] [PubMed] [Google Scholar]
- 79.Ma Z, Zhang Y, Zhang J, et al. Ultrasmall peptide-coated platinum nanoparticles for precise NIR-II photothermal therapy by mitochondrial targeting. ACS Appl Mater Interfaces. 2020;12(35):39434‐39443. doi: 10.1021/ACSAMI.0C11469 [DOI] [PubMed] [Google Scholar]
- 80.Depciuch J, Stec M, Klebowski B, et al. Size effect of platinum nanoparticles in simulated anticancer photothermal therapy. Photodiagnosis Photodyn Ther. 2020;29:101594. doi: 10.1016/J.PDPDT.2019.101594 [DOI] [PubMed] [Google Scholar]
- 81.Wang C, Cai X, Zhang J, et al. Trifolium-like platinum nanoparticle-mediated photothermal therapy inhibits tumor growth and osteolysis in a bone metastasis model. Small. 2015;11(17):2080‐2086. doi: 10.1002/SMLL.201403315 [DOI] [PubMed] [Google Scholar]
- 82.Sazgarnia A, Shanei Ahmad, Meibodi Naser Tayyebi, Eshghi Hossein, Nassirli Hooriyeh, 2011. A novel nanosonosensitizer for sonodynamic therapy: in vivo study on a colon tumor model. J Ultrasound Med. 2011;30(10):1321‐1329. doi: 10.7863/jum.2011.30.10.1321 [DOI] [PubMed] [Google Scholar]
- 83.Wang X, Chen H, Zheng Y, et al. Au-nanoparticle coated mesoporous silica nanocapsule-based multifunctional platform for ultrasound mediated imaging, cytoclasis and tumor ablation. Biomaterials. 2013;34:2057‐2068. doi: 10.1016/J.BIOMATERIALS.2012.11.044 [DOI] [PubMed] [Google Scholar]
- 84.Sanz B, Calatayud M, Torres T, Biomaterials MF. Magnetic hyperthermia enhances cell toxicity with respect to exogenous heating. Biomaterials. 2017;114:62‐70. doi: 10.1016/j.biomaterials.2016.11.008 [DOI] [PubMed] [Google Scholar]
- 85.Manigandan A, Handi V, Sundaramoorthy NS, et al. Responsive nanomicellar theranostic cages for metastatic breast cancer. Bioconjung Chem. 2017;29:275‐286. doi: 10.1021/acs.bioconjchem.7b00577 [DOI] [PubMed] [Google Scholar]
- 86.Wei Tay Z, Chandrasekharan P, Chiu-Lam A, et al. Magnetic particle imaging-guided heating in vivo using gradient fields for arbitrary localization of magnetic hyperthermia therapy. ACS Nano. 2018;12:3699‐3713. doi: 10.1021/acsnano.8b00893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Werengowska-Ciećwierz K, Wiśniewski M, Terzyk AP, Furmaniak S. The chemistry of bioconjugation in nanoparticles-based drug delivery system. Adv Condensed Matter Phys. 2015;2015:198175. doi: 10.1155/2015/198175 [DOI] [Google Scholar]
- 88.Kamimura M. Recent progress of near-infrared fluorescence in vivo bioimaging in the second and third biological window. Anal Sci 2021;37:691‐697. doi: 10.2116/analsci.20SCR11 [DOI] [PubMed] [Google Scholar]
- 89.Bakry R, Vallant RM, Najam-ul-Haq M, et al. Medicinal applications of fullerenes. Int J Nanomedicine. 2007;2:639‐649. [PMC free article] [PubMed] [Google Scholar]
- 90.Hamblin MR. Fullerenes as photosensitizers in photodynamic therapy: pros and cons. Photochem Photobiol Sci. 2018;17(11):1515‐1533. doi: 10.1039/C8PP00195B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamakoshi Y, Umezawa N, Ryu A, et al. Active oxygen species generated from photoexcited fullerene (C 60) as potential medicines: O2-. versus 1O2. J Am Chem Soc. 2003;125(42):12803‐12809. doi: 10.1021/JA0355574 [DOI] [PubMed] [Google Scholar]
- 92.Gupta N, Gupta SM, Sharma SK. Carbon nanotubes: synthesis, properties and engineering applications. Carbon Letters. 2019;29:419‐447. doi: 10.1007/S42823-019-00068-2 [DOI] [Google Scholar]
- 93.Tang L, Zhang A, Zhang Z, et al. Multifunctional inorganic nanomaterials for cancer photoimmunotherapy. Cancer Comun. 2022;42:141‐163. doi: 10.1002/cac2.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H, Song S, Song G. Non-covalently functionalized carbon nanostructures for synthesizing carbon-based hybrid nanomaterials. J Nanosci Nanotechnol. 2014;14:1425‐1440. doi: 10.1166/jnn.2014.9048 [DOI] [PubMed] [Google Scholar]
- 95.Murakami T, Nakatsuji H, Inada M, et al. Photodynamic and photothermal effects of semiconducting and metallic-enriched single-walled carbon nanotubes. J Am Chem Soc. 2012;134(43):17862‐17865. doi: 10.1021/ja3079972 [DOI] [PubMed] [Google Scholar]
- 96.Alrahili M. Light to heat conversion efficiency of single-walled carbon nanotubes. J Taibah Univ Sci 2022;16:923‐932. doi: 10.1080/16583655.2022.2125700 [DOI] [Google Scholar]
- 97.Ruoff R, Lorents DC. Mechanical and thermal properties of carbon nanotubes. Carbon N Y 1995;33:925‐930. doi: 10.1016/0008-6223(95)00021-5 [DOI] [Google Scholar]
- 98.Murjani BO, Kadu PS, Bansod M, Vaidya SS, Yadav MD. Carbon nanotubes in biomedical applications: current status, promises, and challenges. Carbon Lett. 2022;32:1207‐1226. doi: 10.1007/S42823-022-00364-4 [DOI] [Google Scholar]
- 99.Sanchez VC, Jachak A, Hurt RH, Kane AB. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem Res Toxicol. 2012;25:15‐34. doi: 10.1021/tx200339h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim H, Chung K, Lee S. Near-infrared light-responsive nanomaterials for cancer theranostics. WIRES Nanomed Nanobiotechnol. 2016;8:23‐45. doi: 10.1002/wnan.1347 [DOI] [PubMed] [Google Scholar]
- 101.Ding M, Zhang W, Zhang X, Shang H. A multifunctional FA/rGO/MSN-IR820 nanotherapeutic platform for photodynamic/photothermal therapy of tumor. Mater Lett. 2023;331:133508. doi: 10.1016/j.matlet.2022.133508 [DOI] [Google Scholar]
- 102.Liu J, Cui L, Losic D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013;9(12):9243‐9257. doi: 10.1016/j.actbio.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 103.Ghosh D, Sarkar K, Devi P, Kim K. Current and future perspectives of carbon and graphene quantum dots: from synthesis to strategy for building optoelectronic and energy devices. Renew Sustain Energy Rev 2021;135:110391. doi: 10.1016/j.rser.2020.110391 [DOI] [Google Scholar]
- 104.Yaghini E, Seifalian AM, MacRobert AJ. Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine. 2009;4:353‐363. doi: 10.2217/NNM.09.9 [DOI] [PubMed] [Google Scholar]
- 105.Kościk I, Jankowski D, Jagusiak A. Carbon nanomaterials for theranostic use. J Carbon Res. 2021;8:3. doi: 10.3390/c8010003 [DOI] [Google Scholar]
- 106.Wu F, Yue L, Su H, Wang K, Yang L, Zhu X. Carbon dots @ platinum porphyrin composite as theranostic nanoagent for efficient photodynamic cancer therapy. Nanoscale Res Lett. 2018;13:357–367. doi: 10.1186/S11671-018-2761-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng M, Liu S, Li J, et al. Integrating oxaliplatin with highly luminescent carbon dots: an unprecedented theranostic agent for personalized medicine. Adv Mater. 2014;26(21):3554‐3560. doi: 10.1002/adma.201306192 [DOI] [PubMed] [Google Scholar]
- 108.Liang C, Li Z, Dai S. Mesoporous carbon materials: synthesis and modification. Angewandte Chemie Int Edn. 2008;47(20):3696‐3717. doi: 10.1002/ANIE.200702046 [DOI] [PubMed] [Google Scholar]
- 109.Zhou M, Zhao Q, Wu Y, et al. Mesoporous carbon nanoparticles as multi-functional carriers for cancer therapy compared with mesoporous silica nanoparticles. AAPS PharmSciTech. 2020;21:42–54. doi: 10.1208/S12249-019-1604-8 [DOI] [PubMed] [Google Scholar]
- 110.Titirici M, Antonietti M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem Soc Rev. 2010;39:103‐116. doi: 10.1039/B819318P [DOI] [PubMed] [Google Scholar]
- 111.Sevilla M, Carbon AF. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon N Y 2009;47:2281‐2289. doi: 10.1016/j.carbon.2009.04.026 [DOI] [Google Scholar]
- 112.Kokalari I, Gassino R, Giovannozzi A. Pro-and anti-oxidant properties of near-infrared (NIR) light responsive carbon nanoparticles. Free Radical Biol Med 2019;134:165‐176. doi: 10.1016/j.freeradbiomed.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 113.Barzan G, Kokalari I, Gariglio G, et al. Molecular aspects of the interaction with gram-negative and gram-positive bacteria of hydrothermal carbon nanoparticles associated with Bac8c2,5Leu. ACS Omega. 2022;7(19):16402‐16413. doi: 10.1021/acsomega.2c00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Federici G, Soddu S. Variants of uncertain significance in the era of high-throughput genome sequencing: a lesson from breast and ovary cancers. J Exp Clin Cancer Res. 2020;39:45-57. doi: 10.1186/S13046-020-01554-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dreaden EC, Alkilany AM, Huang X, et al. The golden age: gold nanoparticles for biomedicine. Che. Soc. Rev. 2012;41: 2740-2779. doi: 10.1039/C1CS15237H [DOI] [PMC free article] [PubMed]
- 116.Gramotnev D, Bozhevolnyi SI. Plasmonics beyond the diffraction limit. Nat Photonics 2010;4:83‐91. doi:10.1038/nphoton.2009.282 [Google Scholar]
- 117.Bigall NC, Härtling T, Klose M, Simon P, Eng LM, Eychmüller A. Monodisperse platinum nanospheres with adjustable diameters from 10 to 100 nm: synthesis and distinct optical properties. Nano Lett. 2008;8(12):4588‐4592. doi: 10.1021/NL802901T [DOI] [PubMed] [Google Scholar]
- 118.Sepúlveda B, Angelomé PC, Lechuga LM, Liz-Marzán LM. LSPR-based nanobiosensors. nanotoday. 2009;4:244‐251. doi: 10.1016/j.nantod.2009.04.001 [DOI] [Google Scholar]
- 119.Huang X, El-Sayed MA. Plasmonic photo-thermal therapy (PPTT). Alexandria J Med. 2011;47:1‐9. doi: 10.1016/J.AJME.2011.01.001 [DOI] [Google Scholar]
- 120.Ali M, Wu Y, El-Sayed MA. Gold-nanoparticle-assisted plasmonic photothermal therapy advances toward clinical application. J Phys Chem C. 2019;123(25):15375‐15393. doi: 10.1021/acs.jpcc.9b01961 [DOI] [Google Scholar]
- 121.Barbero F, Mayall C, Drobne D. Formation and evolution of the nanoparticle environmental corona: the case of Au and humic acid. Sci Total Environ 2021;768:144792. doi: 10.1016/j.scitotenv.2020.144792 [DOI] [PubMed] [Google Scholar]
- 122.Bastús N, Comenge J, Langmuir VP. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200nm: size focusing versus Ostwald ripening. Langmuir. 2011;27(17):11098‐11105. doi: 10.1021/la201938u [DOI] [PubMed] [Google Scholar]
- 123.Grzelczak M, Pérez-Juste J, Mulvaney P, Liz-Marzán LM. Shape control in gold nanoparticle synthesis. Chem Soc Rev. 2008;37:1783‐1791. doi: 10.1039/B711490G [DOI] [PubMed] [Google Scholar]
- 124.Liao S, Yue W, Cai S, et al. Improvement of gold nanorods in photothermal therapy: recent progress and perspective. Front Pharmacol. 2021;12:664123. doi: 10.3389/FPHAR.2021.664123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rastinehad AR, Anastos H, Wajswol E, et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci U S A. 2019;116(37):18590‐18596. doi: 10.1073/PNAS.1906929116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu X, Ming T, Wang X, Wang P, Wang J, Chen J. High-photoluminescence-yield gold nanocubes: for cell imaging and photothermal therapy. ACS Nano. 2010;4:113‐120. doi: 10.1021/nn901064m [DOI] [PubMed] [Google Scholar]
- 127.Vijayaraghavan P, Liu CH, Vankayala R, Chiang CS, Hwang KC. Designing multi-branched gold nanoechinus for NIR light activated dual modal photodynamic and photothermal therapy in the second biological window. Adv Mater 2014;26(39):6689‐6695. doi: 10.1002/ADMA.201400703 [DOI] [PubMed] [Google Scholar]
- 128.Kim H, Lee D. Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers (Basel) 2018;10:961. doi:10.3390/polym10090961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou R, Zhang M, Xi J, et al. Gold nanorods-based photothermal therapy: interactions between biostructure, nanomaterial, and near-infrared irradiation. Nanoscale Res Lett 2022;17:1‐17. doi: 10.1186/S11671-022-03706-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu L, Lin B, Yang H, et al. Enzyme-responsive multifunctional peptide coating of gold nanorods improves tumor targeting and photothermal therapy efficacy. Acta Biomater. 2019;86:363‐372. doi: 10.1016/J.ACTBIO.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 131.Xu C, Zhang T, Lu G, et al. Disulfiram-gold-nanorod integrate for effective tumor targeting and photothermal-chemical synergistic therapy. Biomater Sci. 2020;8(12):3310‐3319. doi: 10.1039/D0BM00062K [DOI] [PubMed] [Google Scholar]
- 132.Paciotti GF, Myer L, Weinreich D, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv: J Deliv Targeting Therapeutic Agents. 2004;11:169‐183. doi: 10.1080/10717540490433895 [DOI] [PubMed] [Google Scholar]
- 133.Kong G, Braun R, Dewhirst M. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60(16):4440‐4445. PMID: 10969790. [PubMed] [Google Scholar]
- 134.Milani V, Lorenz M, Weinkauf M, et al. Combination of hyperthermia and bortezomib results in additive killing in mantle cell lymphoma cells. Taylor & Francis. 2009;25:262‐272. doi: 10.1080/02656730902835664 [DOI] [PubMed] [Google Scholar]
- 135.Barabadi H, Vahidi H, Damavandi Kamali K, et al. Emerging theranostic gold nanomaterials to combat lung cancer: a systematic review. J Clust Sci. 2020;31:323‐330. doi: 10.1007/S10876-019-01650-4 [DOI] [Google Scholar]
- 136.Mostafavi E, Zarepour A, Barabadi H, Zarrabi A, Truong LB, Medina-Cruz D. Antineoplastic activity of biogenic silver and gold nanoparticles to combat leukemia: beginning a new era in cancer theragnostic. Biotechnol Rep. 2022;34:e00714. doi: 10.1016/J.BTRE.2022.E00714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barabadi H, Vahidi H, Damavandi Kamali K, Rashedi M, Hosseini O, Saravanan M. Emerging theranostic gold nanomaterials to combat colorectal cancer: a systematic review. J Clust Sci. 2020;31:651‐658. doi: 10.1007/S10876-019-01681-X [DOI] [Google Scholar]
- 138.Ferrari E, Barbero F, Busquets-Fité M. Growth-promoting gold nanoparticles decrease stress responses in Arabidopsis seedlings. Nanomaterials. 2021;11(12):3161. doi: 10.3390/nano11123161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kadhim RJ, Karsh EH, Taqi ZJ, Jabir MS. Biocompatibility of gold nanoparticles: in-vitro and in-vivo study. Mater Today Proc. 2021;42:3041‐3045. doi: 10.1016/J.MATPR.2020.12.826 [DOI] [Google Scholar]
- 140.Michelini S, Barbero F, Prinelli A, et al. Gold nanoparticles (AuNPs) impair LPS-driven immune responses by promoting a tolerogenic-like dendritic cell phenotype with altered endosomal structures. Nanoscale. 2021;13(16):7648‐7666. doi: 10.1039/D0NR09153G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stern JM, Kibanov Solomonov VV, Sazykina E, Schwartz JA, Gad SC, Goodrich GP. Initial evaluation of the safety of nanoshell-directed photothermal therapy in the treatment of prostate disease. Int J Toxicol. 2016;35:38‐46. doi: 10.1177/1091581815600170 [DOI] [PubMed] [Google Scholar]
- 142.Li X, Hu Z, Ma J, et al. The systematic evaluation of size-dependent toxicity and multi-time biodistribution of gold nanoparticles. Colloids Surf B Biointerfaces. 2018;167:260‐266. doi: 10.1016/J.COLSURFB.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 143.Singh P, Mijakovic I. Advances in gold nanoparticle technology as a tool for diagnostics and treatment of cancer. Expert Rev Mol Diagn. 2021;21:627‐630. doi: 10.1080/14737159.2021.1933447 [DOI] [PubMed] [Google Scholar]
- 144.Yang W, Liang H, Ma S, Wang D, Huang J. Gold nanoparticle based photothermal therapy: development and application for effective cancer treatment. Sustainable Mater Technol. 2019;22:e00109. doi: 10.1016/J.SUSMAT.2019.E00109 [DOI] [Google Scholar]
- 145.Grabinski C, Schaeublin N, Wijaya A, et al. Effect of gold nanorod surface chemistry on cellular response. ACS Nano. 2011;5:2870‐2879. doi: 10.1021/nn103476x [DOI] [PubMed] [Google Scholar]
- 146.Locatelli E, Monaco I, Comes Franchini M. Surface modifications of gold nanorods for applications in nanomedicine. RSC Adv. 2015;5(28):21681‐21699. doi: 10.1039/C4RA16473C [DOI] [Google Scholar]
- 147.Yu C, Varghese L, Irudayaraj J. Surface modification of cetyltrimethylammonium bromide-capped gold nanorods to make molecular probes. Langmuir. 2007;23(17):9114‐9119. doi: 10.1021/LA701111e [DOI] [PubMed] [Google Scholar]
- 148.Lohse SE, Murphy CJ. The quest for shape control: a history of gold nanorod synthesis. Chem Mater 2013;25:1250‐1261. doi: 10.1021/CM303708p [DOI] [Google Scholar]
- 149.Alkilany AM, Nagaria PK, Hexel CR, Shaw TJ, Murphy CJ, Wyatt MD. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small. 2009;5:701‐708. doi: 10.1002/smll.200801546 [DOI] [PubMed] [Google Scholar]
- 150.Barbero F, Moriones OH, Bastús NG, Puntes V. Dynamic equilibrium in the cetyltrimethylammonium bromide–au nanoparticle bilayer, and the consequent impact on the formation of the nanoparticle protein Corona. Bioconjug Chem. 2019;30(11):2917‐2930. doi: 10.1021/acs.bioconjchem.9b00624 [DOI] [PubMed] [Google Scholar]
- 151.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discovery 2020;20:101‐124. doi: 10.1038/s41573-020-0090-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Germain M, Caputo F, Metcalfe S. Delivering the power of nanomedicine to patients today. J Controlled Release. 2020;326:164‐171. doi: 10.1016/j.jconrel.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rodríguez F, Caruana P, la Fuente ND. Nano-based approved pharmaceuticals for cancer treatment: present and future challenges. Biomolecules. 2022;12:784. doi: 10.3390/biom12060784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shan X, Gong X, Li J, et al. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12:3028‐3048. doi: 10.1016/j.apsb.2022.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rosner MH, Auerbach M. Ferumoxytol for the treatment of iron deficiency. Expert Rev Hematol. 2011;4:399‐406. doi: 10.1586/EHM.11.31 [DOI] [PubMed] [Google Scholar]
- 156.Chen H, Sun D. Core-Satellite Nanocomposites For MRI And Photothermal Therapy. Patent number: US20150065858A1.
- 157.El-Sayed M, Ali MRK. 2021. Methods for inhibiting cancer cell migration with gold nanomaterials and photothermal therapy. Patent number: 11045548.
- 158.Zhang R, Kiessling F, Lammers T, Pallares RM. Clinical translation of gold nanoparticles. Drug Deliv Transl Res. 2023;13:378‐385. doi: 10.1007/S13346-022-01232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fenoglio I, Fubini B, Ghibaudi E. Multiple aspects of the interaction of biomacromolecules with inorganic surfaces. Adv Drug Delivery Rev. 2011;63(13):1186‐1209. doi: 10.1016/j.addr.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 160.Walczyk D, Bombelli FB, Monopoli MP, Lynch I, Dawson KA. What the cell “sees” in bionanoscience. J Am Chem Soc 2010;132(16):5761‐5768. doi: 10.1021/ja910675v [DOI] [PubMed] [Google Scholar]
- 161.Nienhaus K, Nienhaus GU. Mechanistic understanding of protein Corona formation around nanoparticles: old puzzles and new insights. Small. 2023;19(28):e2301663. doi: 10.1002/smll.202301663 [DOI] [PubMed] [Google Scholar]
- 162.Lynch I, Dawson K. Protein-nanoparticle interactions. nanotoday. In: Balogh, LP (Ed.). (2020). Nano-Enabled Medical Applications (1st ed.). Jenny Stanford Publishing 2008;3(1-2):40‐47. doi: 10.1201/9780429399039 [DOI] [Google Scholar]
- 163.Haroon HB, Hunter AC, Farhangrazi ZS, Moghimi SM. A brief history of long circulating nanoparticles. Adv Drug Delivery Rev 2022;188:114396. doi: 10.1016/j.addr.2022.114396 [DOI] [PubMed] [Google Scholar]
- 164.Francia V, Schiffelers RM, Cullis PR, Witzigmann D. The biomolecular corona of lipid nanoparticles for gene therapy. Bioconjug Chem. 2020;31:2046‐2059. doi: 10.1021/acs.bioconjchem.0c00366 [DOI] [PubMed] [Google Scholar]
- 165.Antonello G, Marucco A, Gazzano E, et al. Changes of physico-chemical properties of nano-biomaterials by digestion fluids affect the physiological properties of epithelial intestinal cells and barrier models. Part Fibre Toxicol. 2022;19:49–77. doi: 10.1186/S12989-022-00491-W [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Luxi N, Giovanazzi A, Arcolaci A, et al. Allergic reactions to COVID-19 vaccines: risk factors, frequency, mechanisms and management. BioDrugs. 2022;36:443‐458. doi: 10.1007/S40259-022-00536-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Padín-González E, Lancaster P, Bottini M, et al. Understanding the role and impact of poly (ethylene glycol) (PEG) on nanoparticle formulation: implications for COVID-19 vaccines. Front Bioeng Biotechnol. 2022;10:882363. doi: 10.3389/FBIOE.2022.882363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Caputo F, Clogston J, Calzolai L, Rösslein M, Prina-Mello A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J Control Release. 2019;299:31‐43. doi: 10.1016/j.jconrel.2019.02.030. [DOI] [PubMed] [Google Scholar]