Abstract
BACKGROUND:
As lung ultrasound (LUS) has emerged as a diagnostic tool in patients with COVID-19, we sought to investigate the association between LUS findings and the composite in-hospital outcome of ARDS incidence, ICU admission, and all-cause mortality.
METHODS:
In this prospective, multi-center, observational study, adults with laboratory-confirmed SARS-CoV-2 infection were enrolled from non-ICU in-patient units. Subjects underwent an LUS evaluating a total of 8 zones. Images were analyzed off-line, blinded to clinical variables and outcomes. A LUS score was developed to integrate LUS findings: ≥ 3 B-lines corresponded to a score of 1, confluent B-lines to a score of 2, and subpleural or lobar consolidation to a score of 3. The total LUS score ranged from 0–24 per subject.
RESULTS:
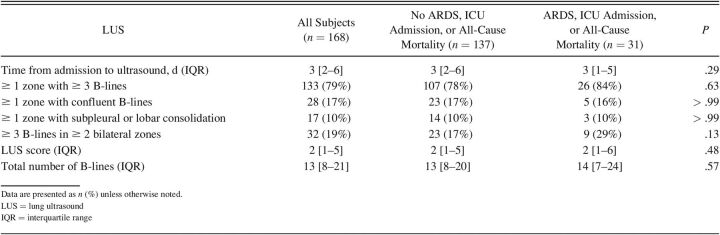
Among 215 enrolled subjects, 168 with LUS data and no current signs of ARDS or ICU admission (mean age 59 y, 56% male) were included. One hundred thirty-six (81%) subjects had pathologic LUS findings in ≥ 1 zone (≥ 3 B-lines, confluent B-lines, or consolidations). Markers of disease severity at baseline were higher in subjects with the composite outcome (n = 31, 18%), including higher median C-reactive protein (90 mg/L vs 55, P < .001) and procalcitonin levels (0.35 μg/L vs 0.13, P = .033) and higher supplemental oxygen requirements (median 4 L/min vs 2, P = .001). However, LUS findings and score did not differ significantly between subjects with the composite outcome and those without, and were not associated with outcomes in unadjusted and adjusted logistic regression analyses.
CONCLUSIONS:
Pathologic findings on LUS were common a median of 3 d after admission in this cohort of non-ICU hospitalized subjects with COVID-19 and did not differ among subjects who experienced the composite outcome of incident ARDS, ICU admission, and all-cause mortality compared to subjects who did not. These findings should be confirmed in future investigations. The study is registered at Clinicaltrials.gov (NCT04377035).
Keywords: COVID-19, lung ultrasound, risk stratification, in-hospital outcomes
Introduction
The SARS-CoV-2 pandemic has resulted in numerous deaths, increased hospitalization burden, and ICU admissions worldwide. A serious complication and important cause of death and ICU admission among patients with COVID-19 is the development of ARDS.1 A recent meta-analysis of 50,466 subjects reported an incidence of ARDS of 14.8% in hospitalized subjects with SARS-CoV-2 infection.2
Prior studies have provided evidence for a promising role of lung ultrasound (LUS) in the diagnosis, management, and prognostic assessment of subjects with ARDS3-8 LUS has emerged as a useful tool in the bedside assessment of patients, and its noninvasive, portable, and rapid use makes it an attractive tool in evaluating critically ill patients. Several studies have already identified the potential utility of LUS in the initial assessment of patients with COVID-19 and described the typical findings on LUS seen with this infection including bilateral B-lines, confluent B-lines, consolidations, and pleural line abnormalities.9-13 LUS findings have also been shown to correlate well with findings on computed tomography (CT) of the chest in subjects with COVID-19.12,14-17 An international expert consensus statement on the clinical utility of point-of-care ultrasound in patients with COVID-19 recommends the use of LUS to diagnose COVID-19 pneumonia with appropriate integration of clinical information and to help guide clinical decisions on respiratory support in patients with respiratory failure in addition to standard respiratory monitoring.18
Although there is evidence supporting the use of LUS in the early assessment and diagnosis of patients with COVID-19, there is limited knowledge regarding the use of LUS for risk stratification in these patients. LUS could potentially allow clinicians to perform fast and noninvasive risk assessments in patients with COVID-19, enabling individualized tailoring of therapy and patient management.19,20 Therefore, this prospective, multi-center, observational study aimed to investigate the association between LUS findings and the composite in-hospital outcome of incident ARDS, ICU admission, and all-cause mortality in non-ICU hospitalized subjects with COVID-19.
QUICK LOOK.
Current Knowledge
Lung ultrasound (LUS) has emerged as a useful tool in the early assessment and diagnosis of patients with COVID-19. The typical findings on LUS seen with this infection include bilateral B-lines, confluent B-lines, consolidations, and pleural line abnormalities. However, there is limited knowledge regarding the use of LUS for risk stratification in this population.
What This Paper Contributes to Our Knowledge
In this prospective, multi-center, observational study, pathologic findings on LUS were common in non-ICU hospitalized subjects with COVID-19. Moreover, there was a significant positive correlation between LUS findings and C-reactive protein, ferritin, and level of oxygen therapy. However, LUS scores were not associated with in-hospital incident ARDS, ICU admission, or all-cause mortality.
Methods
This was a prospective, multi-center, observational cohort study of hospitalized subjects with laboratory-confirmed SARS-CoV-2. In total, 215 consecutive patients were enrolled from dedicated COVID-19 non-ICU in-patient units in 8 hospitals in eastern Denmark during the inclusion period (March 30, 2020–June 3, 2020). Subjects were not recruited from ICUs. The inclusion criteria were laboratory-confirmed SARS-CoV-2 infection, age ≥ 18 y, and ability to provide written informed consent as previously reported and described in 2 studies published on the same cohort.21,22 For the purpose of this analysis, we excluded patients who had ARDS or were admitted to the ICU prior to the LUS examination and those who did not have a LUS performed or had missing or uninterpretable images for all 8 LUS zones. ARDS prior to study inclusion was defined according to the Berlin criteria,23 incorporating chest x-ray findings and not LUS findings (Supplemental material, Table 1, see related supplemental material at http://www.rcjournal.com). For example, some of the enrolled subjects had been admitted to the ICU prior to the time of study enrollment and then transferred to the non-ICU in-patient units. These subjects were excluded from the current analysis. The study was conducted in accordance with the second Declaration of Helsinki and approved by the regional ethics committee in the greater Copenhagen area. All subjects provided written informed consent. The study is registered at Clinicaltrials.gov (NCT04377035).
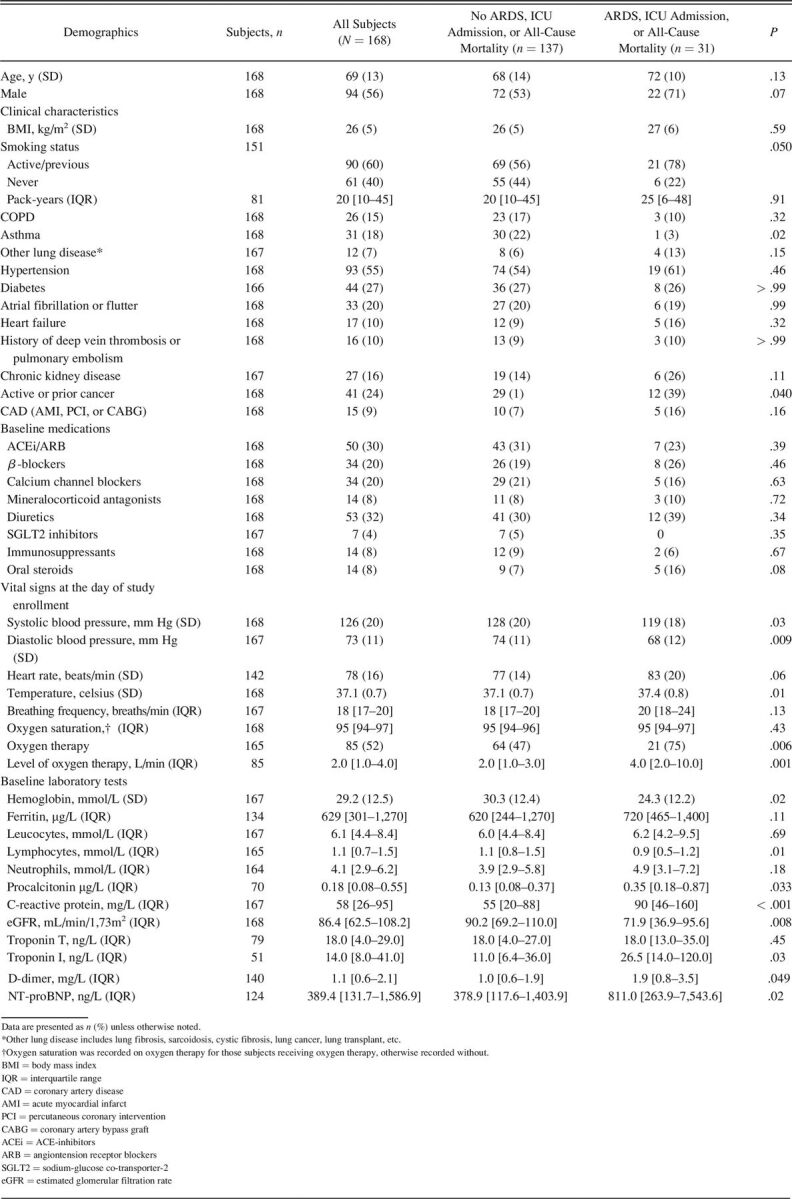
Table 1.
Baseline Characteristics Stratified According to the In-Hospital Composite Outcome of Incident ARDS, ICU Admission, and All-Cause Mortality (n = 168)
All subjects answered an extensive questionnaire regarding their medical history and health-related behavior. Vital signs from the day of study enrollment (ie, the day of LUS and echocardiography) and closest to the time of ultrasound were obtained from the subjects’ electronic health records. Information on medical history, comorbidities, and medication was retrieved from the subjects’ electronic health records and the questionnaire as previously described.21,22 Laboratory tests were performed during the hospitalization and were recorded from the day of study enrollment if available, otherwise recorded from the day with available laboratory data closest to the study enrollment date.
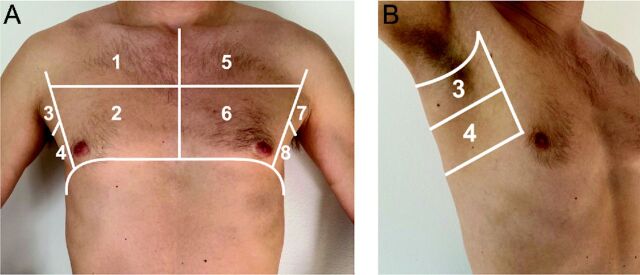
LUS examinations were performed at the bedside using the portable Vivid iq 4D ultrasound system (GE Healthcare, Chicago, Illinois) by 4 trained investigators (MHL, KGS, JNL, NDJ) following a standardized 8-zone imaging protocol (4 zones on each hemithorax, 6 s per clip)24 with subjects in a semi-recumbent position. Figure 1 illustrates the location of the evaluated LUS zones. After initial identification of the most representative images/intercostal space within one zone, the sonographer held the probe still and recorded a 6-s clip in that location. The recorded LUS clip corresponded to the most representative images of the particular zone according to the sonographer and was not necessarily the most abnormal or least abnormal pattern. The LUS examinations were performed with regular echocardiographic equipment with the cardiac preset using a phased array (1.7–3.3 MHz) transducer18,25 in sagittal orientation at an imaging depth of 18–20 cm.
Fig. 1.
Overview of evaluated 8 LUS zones with A: anterior and lateral zones and B: lateral zones. LUS = Lung ultrasound.
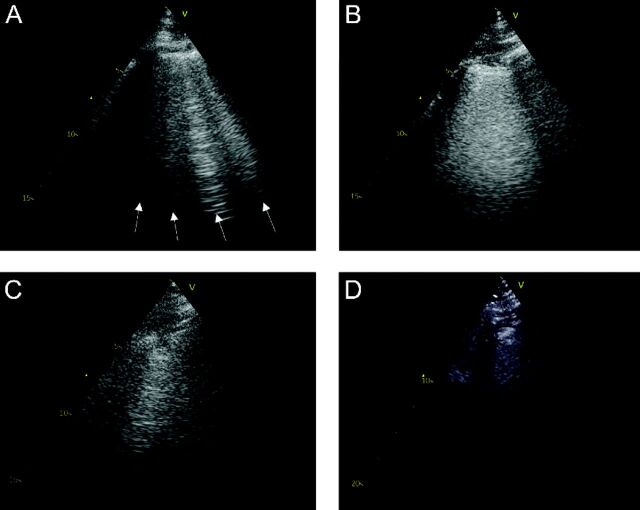
The LUS video clips were subsequently analyzed off-line by a trained investigator (CE) using commercially available software (EchoPAC version 203, GE Healthcare) in consultation with an LUS expert (EP). The following findings were recorded from each zone: (1) the maximum number of B-lines visualized in one intercostal space in a freeze-frame (Figure 2A), (2) presence of confluent B-lines (a single B-line comprising ≥ 50% of the intercostal space without separation into individual B-lines during the clip) (Figure 2B), (3) presence of a subpleural consolidation (Figure 2C), and (4) presence of a lobar consolidation (Figure 2D).
Fig. 2.
Examples of LUS images with A: 4 B-lines, B: a confluent B-line, C: a subpleural consolidation, and D: a lobar consolidation. LUS = Lung ultrasound.
In order to incorporate the above findings into a single parameter, a LUS score was constructed (Table 2, Supplemental Material, see related supplemental material at http://rc.rcjournal.com):
Table 2.
Baseline LUS Findings Stratified According to the In-Hospital Composite Outcome of Incident ARDS, ICU Admission, and All-Cause Mortality (n = 168)
(1) LUS score: One point was assigned to each zone if there was ≥ 3 B-lines, 2 points if a confluent B-line was present, and 3 points for a subpleural or lobar consolidation. If a zone had 2 findings, eg, both ≥ 3 B-lines and subpleural consolidation, the score would be determined based on the highest attainable score for the individual LUS findings; in the example provided, 3 points would be assigned based on the presence of a subpleural consolidation (total score range: 0–24 points). The LUS score was similar to the previously investigated aeration score.13,20,26-28
In addition, 2 B-line quantification methods previously described in subjects with heart failure29 were investigated:
1) Total number of B-lines: We also calculated the total number of B-lines per patient as the sum of the maximum number of B-lines in each of the 8 zones.29 To include observations with confluent B-lines in the parameter total number of B-lines, confluent B-lines were counted as the highest number of B-lines assigned to a single zone in this data set (7 B-lines).
2) ≥ 3 B-lines in ≥ 2 zones on each hemithorax: Based on a method commonly used in patients with acute heart failure for the detection of pulmonary edema, we considered ≥ 3 B-lines in ≥ 2 bilateral zones as diagnostic for bilateral pneumonitis (yes/no).30
Subjects also underwent an echocardiogram at the time of the LUS. The echocardiogram was performed by the same investigators who performed the LUS. The echocardiographic findings have been published previously.21,22
The primary end point was the composite in-hospital outcome of incident ARDS (as defined by the Berlin criteria,23 Supplemental material, Table 1, see related supplemental material at http://rc.rcjournal.com), ICU admission, and in-hospital all-cause mortality. Adjudication of events was based on review of the subjects’ electronic medical records. Subjects were followed until June 17, 2020, by which all subjects were either discharged or had died.
Baseline clinical and demographic characteristics and LUS findings were stratified according to the composite in-hospital outcome of incident ARDS, ICU admission, and all-cause mortality. Continuous variables were compared using the Student t test or Wilcoxon rank-sum test for normally and non-normally distributed variables, respectively. Categorical variables were compared using Fischer exact or chi-square test as appropriate. We assessed the correlation between LUS findings (LUS score and total number of B-lines) and markers of disease severity (C-reactive protein, ferritin, procalcitonin, lymphocyte count, D-dimer, and level of oxygen therapy) or markers of cardiac stress (NT-proBNP and troponin I/T) using Spearman correlation coefficient. Intra-reader agreement analysis for the total number of B-lines (total of 8 zones) was performed in 15 randomly selected subjects using Bland-Altman analysis. Unadjusted and adjusted logistic regression models were used to investigate the association between LUS findings at baseline and the composite in-hospital outcome of incident ARDS, ICU admission, and all-cause mortality. Model 1 was adjusted for age and sex, as these variables have been shown to be associated with mortality in subjects with COVID-19.31 Models were checked for interactions between LUS findings and time to LUS and level of oxygen requirement. To account for missing zones on LUS, 3 additional logistic regression analyses were conducted as sensitivity analyses: (1) a model with the LUS score or B-line number adjusted for the number of missing zones (n = 168), (2) an unadjusted model with the LUS score or B-line number based on only 6 zones32 (excluding the basal zones on each hemithorax [zones 4 and 8]) (n = 168), and (3) an unadjusted model with the LUS score or B-line number based on only 6 zones (excluding the basal zones on each hemithorax [zones 4 and 8]) and excluding observations with missing zones (n = 144). Statistical analyses were performed using Stata SE version 14.1 (StataCorp, College Station, Texas). A 2-sided P value of <. 05 was considered statistically significant.
Results
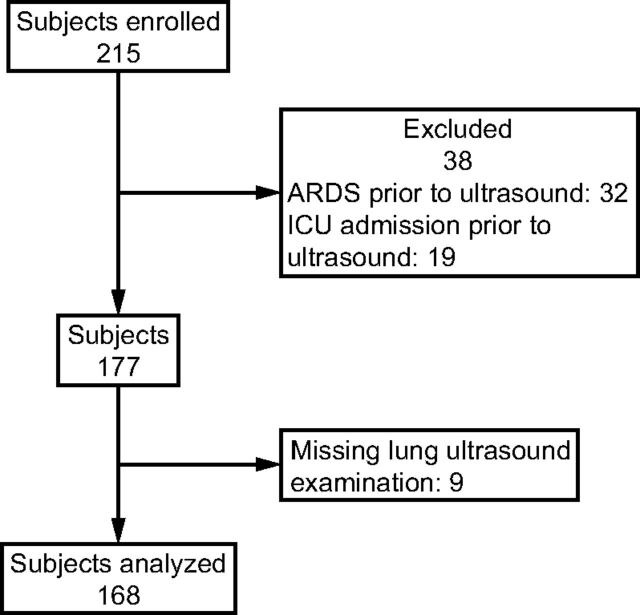
Of the 215 enrolled subjects, 38 subjects had ARDS or were admitted to the ICU prior to the LUS examination (ARDS: n = 32, ICU admission: n = 19) and 9 subjects did not have a LUS performed or had uninterpretable LUS images for all 8 zones (Fig. 3). Therefore, 168 subjects were included in this analysis. Mean age was 69 ± 13 y, and 56% of subjects were male. Median time from admission to LUS examination was 3 d (interquartile range [IQR] 2–6).
Fig. 3.
Flow chart.
Baseline demographic and clinical characteristics are summarized in Table 1. There were no significant differences with regard to demographic characteristics, body mass index (BMI), and medications at baseline between subjects who experienced the composite outcome and those who did not. Interestingly, subjects who did not experience the composite outcome were more likely to have a diagnosis of asthma at baseline. Subjects who experienced the composite outcome were more often treated with supplemental oxygen and with higher levels of oxygen at baseline (median 4 L/min vs 2, P = .001). Moreover, subjects with the composite outcome had higher markers of disease severity at baseline with higher median C-reactive protein levels (90 mg/L vs 55, P < .001) and median procalcitonin levels (0.35 µg/L vs 0.13, P = .033). Subjects with the composite outcome also had worse renal function with lower median estimated glomerular filtration rate levels (71.9 mL/min/1.73m2 vs 90.2, P = .008) and higher median troponin I (26.5 ng/L vs 11.0, P = .027), NT-proBNP (811.0 ng/L vs 378.9, P = .020), and D-dimer (1.9 mg/L vs 1.0, P = .049) levels.
Of the 168 subjects included in the analysis, 47 subjects (28%) had ≥ 1 missing zone on LUS due to uninterpretable images. The median number of missing zones was 1 (IQR 1–2). One hundred and thirty-three (79%) subjects had ≥ 3 B-lines, 28 (17%) had confluent B-lines, and 17 (10%) had subpleural or lobar consolidations in ≥ 1 zones on LUS. In total, 136 subjects (81%) had any pathologic finding (eg, ≥ 3 B-lines, confluent B-lines, or subpleural or lobar consolidations) in at least 1 zone on LUS. The median number of zones with pathologic findings was 2 (IQR 1–4), and 32 subjects (19%) had ≥ 3 B-lines in ≥ 2 zones on each hemithorax corresponding with bilateral pneumonitis (Table 2). With regard to the intra-reader agreement for the total number B-lines (total of 8 zones), the mean B-line difference in 15 subjects from the re-analysis to the original analysis was −1.6 (95% limits of agreement −6.3 to 3.1). There were no differences in LUS findings in subjects with and without heart failure or other lung disease at baseline (eg, lung fibrosis, sarcoidosis) (data not shown).
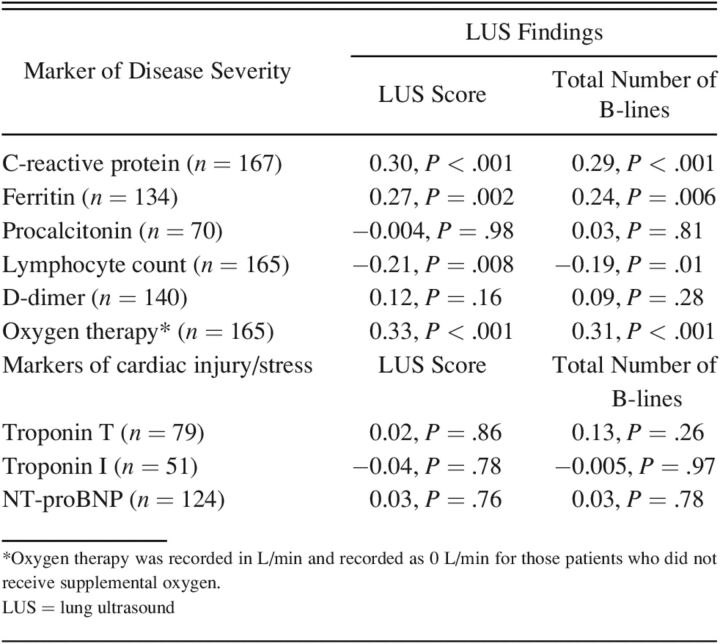
The LUS findings were associated with several markers of disease severity (Table 3). Among subjects with available laboratory data, there was a significant positive correlation between the LUS findings (LUS score and total number of B-lines) and CRP, ferritin, and level of oxygen therapy. Moreover, there was a significant inverse relationship between LUS findings (LUS score and total number of B-lines) and lymphocyte levels. However, LUS findings were not associated with D-dimer or procalcitonin levels. Moreover, LUS findings were not associated with markers of cardiac injury or stress as measured with troponin I/T and NT-proBNP levels (Table 3).
Table 3.
Correlation Between LUS Findings and Laboratory Markers of Disease Severity Using Spearman Correlation Coefficients
Among the 168 included subjects, 24 subjects (14%) developed ARDS, 9 (5%) were admitted to the ICU, and 17 (10%) died in hospital during follow-up (Supplemental Material, Table 3, see related supplemental material at http://rc.rcjournal.com). In total, 31 subjects (18%) experienced the composite outcome of incident ARDS, ICU admission, or all-cause mortality during the hospital stay after the LUS examination. The median number of d from study enrollment to event or discharge for the entire cohort was 3 d (IQR 1–7). For those admitted to the ICU after enrollment (n = 9), the median number of d from admission to discharge or death was 21 d (IQR 17–26).
The number of subjects with ≥ 3 B-lines, confluent B-lines, and subpleural or lobar consolidations in ≥ 1 zones on LUS did not differ significantly between subjects with an event versus those without, neither did the number of zones with pathologic findings on LUS (P = .40). The proportion of subjects with bilateral pneumonitis was numerically higher in subjects with the composite outcome but did not reach statistical significance (29% vs 17, P = .13). None of the other LUS quantification methods differed significantly between the 2 groups.
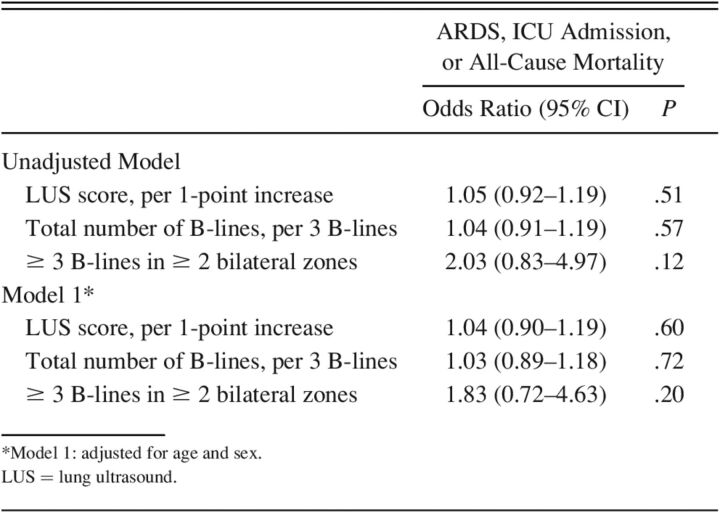
There were no significant associations between LUS findings at baseline and the in-hospital composite outcome in unadjusted or adjusted logistic regression analyses (Table 4). There were no significant interactions between LUS findings and time to LUS or supplemental oxygen requirement. For each of the 3 sensitivity analyses described in the Methods section, the direction and significance of the association between LUS findings and the composite in-hospital outcome remained similar (Supplemental Material, Table 4, see related supplemental material at http://rc.rcjournal.com). Furthermore, in a sensitivity analysis of subjects with ≤ 2 missing zones (n = 159), the associations between LUS findings and outcome remained similar (data not shown).
Table 4.
The Association Between LUS Findings and the In-Hospital Composite Outcome of Incident ARDS, ICU Admission, and All-Cause Mortality Using Logistic Regression (n = 168)
Discussion
In this prospective, multi-center, observational study of non-ICU hospitalized subjects with laboratory-confirmed SARS-CoV-2, pathologic findings on LUS were common after a median of 3 d after admission. LUS findings were associated with several markers of disease severity, including C-reactive protein, oxygen therapy, and lymphocyte count. However, in this cohort there were no significant differences in the LUS findings between subjects who experienced the composite outcome of incident ARDS, ICU admission, or all-cause mortality during the hospital stay and those who did not. Despite similar LUS findings, subjects who experienced the composite outcome had higher markers of disease severity including higher temperature, C-reactive protein, and procalcitonin levels and were treated with higher levels of oxygen. To our knowledge, this was one of the largest prospective, multi-center studies investigating LUS findings in subjects with COVID-19 and their association with in-hospital outcomes of incident ARDS, ICU admission, or all-cause mortality.
LUS is a useful tool in critically ill patients and can be used to detect and quantify pulmonary congestion29 and rule out pneumothorax33 among other conditions. The noninvasive, rapid performance makes LUS highly applicable in the acute setting for diagnosis and early triaging in patients with COVID-19 pneumonia. Similar to prior investigations, we found that pathologic findings on 8-zone LUS were common in this cohort of non-ICU hospitalized subjects with COVID-19 after a median of 3 d after admission. In our cohort, 81% of subjects had a pathologic finding in at least 1 zone. In comparison, Bonadia et al34 and Casella et al35 found that 93% and 96% of subjects had a pathologic finding on LUS, and Brahier et al36 found that 91% had an abnormal LUS in the emergency department (ED).
In order to incorporate the different LUS findings in a single parameter, we investigated a LUS score that followed a similar approach to the aeration score ranging from 0–3 per zone,13,20,26-28 although in our study it was based on an 8 rather than a 12-zone protocol. As lung involvement in subjects with COVID-19 is most often detected in the posterior areas based on CT findings, imaging of posterior zones will likely improve sensitivity of the LUS examination.37,38 However, as this was not yet known at the time the present study was designed, our LUS protocol did not include posterior zones, which may have resulted in a lower rate of detection of LUS findings in some subjects. Despite the evaluation of only anterior and lateral zones in our study, there was a high prevalence of abnormal LUS findings in this cohort, and the LUS score and total number of B-lines correlated significantly with laboratory markers of disease severity.
Although COVID-19 pneumonia has some distinctive features on LUS, there is currently no finding on LUS that is pathognomonic of COVID-19 pneumonia.13,39 B-lines on LUS can be seen in various disease processes including pneumonia, pulmonary congestion, and ARDS.39 LUS findings related to COVID pneumonia, non-COVID viral pneumonia/pneumonitis, and ARDS may, therefore, overlap. Moreover, patients with severe COVID-19 pneumonia often meet the Berlin criteria for ARDS, even though COVID-19 pneumonia is a distinct disease.40 In order to systematically examine the differences between ARDS, COVID pneumonia, and non-COVID viral pneumonia/pneumonitis, future prospective studies are needed in which LUS images are interpreted off-line by readers blinded to the ultimate diagnoses.
Although we did not find a statistically significant association between LUS findings and the composite outcome of incident ARDS, ICU admission, or all-cause mortality in our cohort, prior single-center studies in subjects with COVID-19 have demonstrated significant associations between LUS findings and adverse outcomes.20,28,34,36,41-43 An important difference to note is that the LUS examinations in our study were performed in hospitalized subjects with a median time from admission to LUS of 3 d. In 3 smaller, prospective studies, the LUS examinations were performed in subjects presenting to the ED.34,36,41 Five studies20,28,35,42 investigated the prognostic value of LUS in hospitalized subjects with COVID-19. However, unlike our study, the LUS examinations were performed on the d of admission or within 24 or 48 h of admission. In 2 retrospective studies, LUS aeration score based on 12-zone LUS examinations was associated with adverse outcome and critical illness in 120 and 211 hospitalized subjects, respectively.20,28 Additionally, a LUS score based on 8-zone LUS could distinguish between critical-type patients versus severe-type patients in 128 critically ill patients with COVID-19.43 In a prospective study in 52 subjects, Perrone et al42 found that the LUS score on a 14-zone LUS was associated with clinical worsening (defined as a combination of high-flow oxygen support, ICU admission, and 30-d mortality). Moreover, in a prospective study of 190 hospitalized subjects, total LUS score within 48 h of admission was associated with an increased risk of death and transfer to the ICU in univariable but not multivariable analyses.35 In a subset of subjects with an additional LUS performed after 72 h of admission, the total LUS score was associated with an increased risk of death and transfer to the ICU in both univariable and multivariable analyses.35
The difference in the timing of the LUS, the study population (in-patient/out-patient, number of included subjects), study design (retrospective/prospective), and the LUS imaging protocols may explain the differences in LUS findings and association with outcome in our study compared to the prior investigations. Some of the subjects in our study might have already been improving as the LUS was performed a median of 3 d (IQR 2–6) after admission. Furthermore, the wide range of d from admission to LUS may have led to subjects being in different stages of the disease at the time of the LUS. As a team of investigators visited the 8 different hospitals during the study period and enrolled hospitalized patients with COVID-19, it was not feasible to strictly perform the LUS within 24 h of admission. Moreover, although the prior studies used a LUS score of 0–3 to quantify the LUS findings in each zone, the LUS imaging protocols and the total number of zones evaluated varied across the different studies (8–14 zones). The differing LUS imaging protocols make it difficult to compare findings across studies and warrant a standardized LUS imaging and quantification approach for future studies in order to better compare the results. The international expert consensus statement on the use of LUS in patients with COVID-19 also recommend standardization of zones and scanning techniques.18 However, similar to the results of our study, the LUS score was not associated with outcomes, the occurrence of death on d 28, in a multi-center, retrospective study in 57 subjects with COVID-19 admitted to the ICU for acute respiratory failure.44 Thus, there are conflicting results regarding the association between LUS findings and outcomes in patients with COVID-19, and standardization of LUS imaging protocols could facilitate comparison across the studies. Interestingly, comorbidities such as obesity, hypertension, diabetes, and COPD were not associated with the in-hospital outcomes in our study. This could be due to a low prevalence of these comorbidities in this cohort as compared to other cohorts, eg, lower BMI. Alternatively, the low event rate may not have allowed to assess these associations.
Our study findings should be considered in the context of their limitations. First, we did not include LUS-specific exclusion criteria for this study; eg, subjects were included irrespective of whether they had pulmonary fibrosis, lung cancer, or were on dialysis. Second, our imaging protocol did not include scanning of posterior zones that are commonly affected in patients with COVID-19 pneumonia. In addition, a subset of subjects had uninterpretable images, most commonly in the lateral zones just above the diaphragm (zones 4 and 8). Since some of the subjects were very ill and optimal positioning of patients was sometimes challenging, it was difficult to acquire some of the LUS images, in particular zones 4 and 8. Third, we only observed a limited number of events, which only allowed us to adjust for 2 important variables in the logistic regression analyses. Moreover, our study may not have enrolled an adequate number of subjects or observed an adequate number of events to detect a statistically significant association between LUS findings and the composite outcome. The proposed number of enrolled subjects (n = 1,000) was estimated prior to the peak of the pandemic in Denmark. The number of hospitalized COVID-19 patients did not reach as many as initially anticipated. Therefore, we were not able to enroll as many subjects as initially proposed. The association between LUS findings and in-hospital events should, therefore, be investigated further in larger, prospective studies with the use of standardized imaging protocols. This would also allow for end points to be evaluated individually and not as part of a composite outcome.
Conclusions
Among non-ICU hospitalized subjects with COVID-19, pathologic findings on LUS were common a median of 3 d after admission and were associated with laboratory markers of disease severity. However, there were no apparent differences in LUS findings between subjects who developed ARDS, were admitted to the ICU, or died during hospitalization and those without an event. These findings should be investigated further in larger, prospective studies.
Supplementary Material
Footnotes
Dr Biering-Sørensen discloses relationships with Sanofi Pasteur, GE Healthcare, Amgen, and Novartis. Dr Platz discloses relationships with the National Institutes of Health and Novartis. Dr Ulrik discloses relationships with AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Teva Pharmaceutical, Orion, Novartis, Actelion, Mundipharma, Sanofi Genzyme, ALK- Abelló, and Chiesi. The remaining authors have disclosed no conflicts of interest.
Drs Espersen and Platz contributed equally to this study.
Supplementary material related to this paper is available at http://www.rcjournal.com.
References
- 1.Qiu P, Zhou Y, Wang F, Wang H, Zhang M, Pan X, et al. Clinical characteristics, laboratory outcome characteristics, comorbidities, and complications of related COVID-19 deceased: a systematic review and meta-analysis. Aging Clin Exp Res 2020;32(9):1869-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single-arm meta-analysis. J Med Virol 2020;92(6):612-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisani L, Vercesi V, van Tongeren PSI, Lagrand WK, Leopold SJ, Huson MAM, et al. ; Lung Ultrasound Consortium. The diagnostic accuracy for ARDS of global versus regional lung ultrasound scores - a post hoc analysis of an observational study in invasively ventilated ICU patients. Intensive Care Med Exp 2019;7(Suppl 1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corradi F, Brusasco C, Pelosi P. Chest ultrasound in acute respiratory distress syndrome. Curr Opin Crit Care 2014;20(1):98-103. [DOI] [PubMed] [Google Scholar]
- 5.Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound 2008;6(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004;100(1):9-15. [DOI] [PubMed] [Google Scholar]
- 7.Stefanidis K, Dimopoulos S, Tripodaki ES, Vitzilaios K, Politis P, Piperopoulos P, et al. Lung sonography and recruitment in patients with early acute respiratory distress syndrome: a pilot study. Crit Care 2011;15(4):R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Z, Jiang L, Xi X, Jiang Q, Zhu B, Wang M, et al. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm Med 2015;15(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing C, Li Q, Du H, Kang W, Lian J, Yuan L. Lung ultrasound findings in patients with COVID-19 pneumonia. Crit Care 2020;24(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahu AK, Mathew R, Bhoi S, Sinha TP, Nayer J, Aggarwal P. Lung sonographic findings in COVID-19 patients. Am J of Emerg Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng Q-Y, Wang X-T, Zhang L-N; Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel coronavirus pneumonia during the 2019–2020 epidemic. Intensive Care Med 2020;46(5):849-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trauer MM, Matthies A, Mani N, McDermott C, Jarman R. The utility of lung ultrasound in COVID-19: a systematic scoping review. Ultrasound 2020;28(4):208-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpicelli G, Lamorte A, Villén T. What's new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med 2020;46(7):1445-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benchoufi M, Bokobza J, Anthony CA, Dion E, Baranne M-L, Levan F, et al. Lung injury in patients with or suspected COVID-19: a comparison between lung ultrasound and chest CT scanner severity assessments, an observational study. medRxiv: [Google Scholar]
- 15.Poggiali E, Dacrema A, Bastoni D, Tinelli V, Demichele E, Ramos PM, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology 2020;295(3):E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyu G, Zhang Y, Tan G. Transthoracic ultrasound evaluation of pulmonary changes in COVID-19 patients during treatment using modified protocols. Adv Ultrasound Diagn Ther 2020;4(2):79-83. [Google Scholar]
- 17.Tung-Chen Y, Martí de Gracia M, Díez-Tascón A, Alonso-González R, Agudo-Fernández S, Parra-Gordo ML, et al. Correlation between chest computed tomography and lung ultrasonography in patients with coronavirus disease 2019 (COVID-19). Ultrasound Med Biol 2020;46(11):2918-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain A, Via G, Melniker L, Goffi A, Tavazzi G, Neri L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care 2020;24(1):702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dargent A, Chatelain E, Kreitmann L, Quenot J-P, Cour M, Argaud L; COVID-LUS Study Group. Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. Plos One 2020;15(7):e0236312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichter Y, Topilsky Y, Taieb P, Banai A, Hochstadt A, Merdler I, et al. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med 2020;46(10):1873-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skaarup KG, Lassen MCH, Lind JN, Alhakak AS, Sengeløv M, Nielsen AB, et al. Myocardial impairment and acute respiratory distress syndrome in hospitalized patients with COVID-19. JACC Cardiovasc Imaging 2020;13(11):2474-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lassen MCH, Skaarup KG, Lind JN, Alhakak AS, Sengeløv M, Nielsen AB, et al. Echocardiographic abnormalities and predictors of mortality in hospitalized COVID-19 patients: the ECHOVID-19 study. ESC Heart Fail 2020;7(6):4189-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307(23):2526-2533. [DOI] [PubMed] [Google Scholar]
- 24.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. ; International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38(4):577-591. [DOI] [PubMed] [Google Scholar]
- 25.Bobbia X, Chabannon M, Chevallier T, de La Coussaye JE, Lefrant JY, Pujol S, et al. Assessment of five different probes for lung ultrasound in critically ill patients: a pilot study. Am J Emerg Med 2018;36(7):1265-1269. [DOI] [PubMed] [Google Scholar]
- 26.Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby J-J. Bedside ultrasound assessment of positive end-expiratory pressure–induced lung recruitment. Am J Respir Crit Care Med 2011;183(3):341-347. [DOI] [PubMed] [Google Scholar]
- 27.Bouhemad B, Dransart-Rayé O, Mojoli F, Mongodi S. Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann Transl Med 2018;6(21):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boero E, Rovida S, Schreiber A, Berchialla P, Charrier L, Cravino MM, et al. The COVID-19 worsening score (COWS)—a predictive bedside tool for critical illness. Echocardiography 2021;38(2):207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, et al. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short- and long-term outcomes. JACC Heart Fail 2019;7(10):849-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pivetta E, Goffi A, Nazerian P, Castagno D, Tozzetti C, Tizzani P, et al. ; Study Group on Lung Ultrasound from the Molinette and Careggi Hospitals. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial. Eur J Heart Fail 2019;21(6):754-766. [DOI] [PubMed] [Google Scholar]
- 31.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19–related death using OpenSAFELY. Nature 2020;584(7821):430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pivetta E, Goffi A, Lupia E, Tizzani M, Porrino G, Ferreri E, et al. ; SIMEU Group for Lung Ultrasound in the Emergency Department in Piedmont. Lung ultrasound–implemented diagnosis of acute decompensated heart failure in the ED: a SIMEU multi-center study. Chest 2015;148(1):202-210. [DOI] [PubMed] [Google Scholar]
- 33.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995;108(5):1345-1348. [DOI] [PubMed] [Google Scholar]
- 34.Bonadia N, Carnicelli A, Piano A, Buonsenso D, Gilardi E, Kadhim C, et al. Lung ultrasound findings are associated with mortality and need for intensive care admission in COVID-19 patients evaluated in the emergency department. Ultrasound Med Biol 2020;46(11):2927-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casella F, Barchiesi M, Leidi F, Russo G, Casazza G, Valerio G, et al. Lung ultrasonography: a prognostic tool in non-ICU hospitalized patients with COVID-19 pneumonia. Eur J Intern Med 2021;85:34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brahier T, Meuwly JY, Pantet O, Brochu Vez MJ, Gerhard Donnet H, Hartley MA, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study. Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manivel V, Lesnewski A, Shamim S, Carbonatto G, Govindan T. CLUE: COVID-19 lung ultrasound in emergency department. Emerg Med Australas 2020;32(4):694-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sultan LR, Sehgal CM. A review of early experience in lung ultrasound in the diagnosis and management of COVID-19. Ultrasound Med Biol 2020;46(9):2530-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allinovi M, Parise A, Giacalone M, Amerio A, Delsante M, Odone A, et al. Lung ultrasound may support diagnosis and monitoring of COVID-19 pneumonia. Ultrasound Med Biol 2020;46(11):2908-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care 2020;24(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosso G, Allegorico E, Pagano A, Porta G, Serra C, Minerva V, et al. Lung ultrasound as diagnostic tool for SARS-CoV-2 infection. Intern Emerg Med 2021;16(2):471-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perrone T, Soldati G, Padovini L, Fiengo A, Lettieri G, Sabatini U, et al. A new lung ultrasound protocol able to predict worsening in patients affected by severe acute respiratory syndrome coronavirus 2 pneumonia. J Ultrasound Med 2021;40(8):1627-1635. [DOI] [PubMed] [Google Scholar]
- 43.Deng Q, Zhang Y, Wang H, Chen L, Yang Z, Peng Z, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol 2020;27(10):1363-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duclos G, Bazalguette F, Allaouchiche B, Mohammedi N, Lopez A, Gazon M, et al. Can thoracic ultrasound on admission predict the outcome of critically ill patients with SARS-CoV-2? A French multi-centric ancillary retrospective study. Adv Ther 2021;38(5):2599-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.