Abstract
BACKGROUND:
The introduction of anti-vascular endothelial growth factor (anti-VEGF) drugs to ophthalmology has revolutionized the treatment of neovascular age-related macular degeneration (nAMD). Despite this significant progress, gaps and challenges persist in the diagnosis of nAMD, initiation of treatment, and management of frequent intravitreal injections. Thus, nAMD remains a leading cause of blindness in the United States.
OBJECTIVE:
To present current knowledge, evidence, and expert perspectives on anti-VEGF therapies in nAMD to support managed care professionals and providers in decision making and collaborative strategies to overcome barriers to optimize anti-VEGF treatment outcomes among nAMD patients.
SUMMARY:
Three anti-VEGF therapies currently form the mainstay of treatment for nAMD, including 2 therapies approved by the FDA for treatment of nAMD (aflibercept and ranibizumab) and 1 therapy approved by the FDA for oncology indications and used off-label for treatment of nAMD (bevacizumab). In clinical trials, each of the 3 agents maintained visual acuity (VA) in approximately 90% or more of nAMD patients over 2 years. However, in long-term and real-world settings, significant gaps and challenges in diagnosis, treatment, and management pose barriers to achieving optimal outcomes for patients with nAMD. Many considerations, including individual patient characteristics, on-label versus off-label treatment, repackaging, and financial considerations, add to the complexity of nAMD decision making and management. Many factors may contribute to additional challenges leading to suboptimal long-term outcomes among nAMD patients, such as delays in diagnosis and/or treatment approval and initiation, individual patient response to different anti-VEGF therapies, lapses in physician regimentation of anti-VEGF injection and monitoring, and inadequate patient adherence to treatment and monitoring. These latter factors highlight the considerable logistical, emotional, and financial burdens of long-term, frequent intravitreal injections and the vital importance of personalized approaches to anti-VEGF treatment decision making and management for patients with nAMD. To address these challenges and reduce the number of yearly injections, studies have examined alternative dosing regimens, including extended fixed intervals, as needed, and treat-and-extend strategies in specific nAMD patient populations. New clinical evidence and insights into expert clinical practice discussed in this article can support managed care professionals in the key role they play in addressing challenges in nAMD treatment and management and optimizing patient outcomes through appropriate management of anti-VEGF treatment.
Age-related macular degeneration (AMD) is the most common cause of vision loss and blindness among people aged 60 years and older in industrialized nations.1-3 Characterized initially by a blurred area in the center of vision, the vision loss that occurs with inadequately treated AMD has profoundly negative effects on patients’ independence, productivity, and quality of life. The condition is associated with increased risks of cognitive dysfunction, depression, and falls and related injuries, along with requirements for extensive and costly caregiving services.4-8 Loss of central vision in AMD affects patients’ abilities to drive, read, write, recognize faces, and participate in social activities.
The condition is defined by 2 main types: (1) non-neovascular, also called dry or nonexudative, AMD and (2) neovascular, also called wet or exudative, AMD (nAMD). The clinical manifestations of early disease are macular pigmentary changes and the formation of extracellular lipid deposits, called drusen, under the retinal pigment epithelium (RPE). In the AMD classification scheme developed by the Age-Related Eye Disease Study (AREDS) research group, large drusen indicate intermediate AMD, which poses a high risk of progression to nAMD.9 Advanced stages are characterized by geographic atrophy of retinal tissue (advanced non-neovascular AMD) and/or choroidal neovascularization (CNV; advanced nAMD). The latter condition, which is the clinical manifestation of nAMD, results from the upregulation of pro-inflammatory and angiogenic cytokines, including vascular endothelial growth factor (VEGF).10 VEGF is an important signaling protein involved in angiogenesis. In nAMD, blood vessels grow from the choroid into the subretinal or sub-RPE space (Figure 1).10 If inadequately treated, nAMD leads to vision loss or blindness caused by leakage, hemorrhage, RPE detachments, and scar formation.
FIGURE 1.
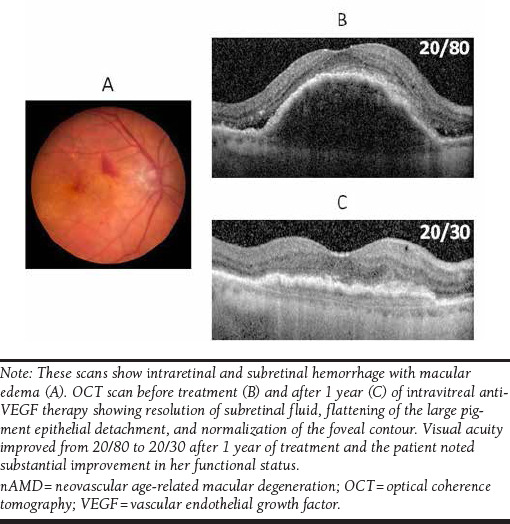
Color Fundus Photograph of a Patient with nAMD Who Noted Progressive Visual Acuity Loss in Her Right Eye Over 3 Weeks
Beyond a dilated ophthalmic examination, the main technology for detecting nAMD is optical coherence tomography (OCT), and the diagnosis may be confirmed by fluorescein angiography (FA), which assesses the extent of CNV lesions, leakage, and fluid presence. Combined approaches and advanced technologies, such as spectral-domain OCT and OCT-angiography, can improve diagnostic accuracy and monitoring and inform clinical decision making.11-13 Whereas these modalities are essential for nAMD diagnosis and monitoring, they contribute to the burden and costs of office visits. Moreover, given the brief time over which CNV lesions can grow and cause vision loss, even monthly office-based assessments may not be sufficient for identifying treatment needs in some patients.13 Several home-based methods of detecting nAMD are noteworthy, including Amsler charts, near-vision charts, preferential hyperacuity perimetry, shape-discrimination hyperacuity tests, and macular mapping tests. The benefits, disadvantages, and cost-effectiveness of these home-based methods, including the U.S. Food and Drug Administration (FDA)-approved ForeseeHome device, have been reviewed in the literature.12-15
In the United States, approximately 14 million people have AMD; the prevalence of advanced disease, including nAMD, was estimated to be 1.75 million in 2004.16,17 Based on projected U.S. aging demographics, an estimated 3 million people will have advanced AMD by 2020.16 Whereas only 10%-20% of AMD patients have the neovascular type, it is responsible for severe vision loss or blindness in approximately 90% of cases.3
Although early signs of vision loss and advanced cases can occur between ages 40 and 50, most patients with AMD are 50 years or older. The prevalence of nAMD increases sharply with age, from an estimated 0.7% in people aged 65-74 years to 8.5% in people aged 85 years and older.18 Higher rates of AMD have been reported for Caucasian populations than Hispanic or African American populations.3 In addition, some studies have identified associations between AMD and cardiovascular disease, including hypertension and high cholesterol.10
Excellent educational resources on AMD for patients are available from the American Academy of Ophthalmology (AAO; https://www.aao.org/eye-health) and the American Society of Retina Specialists (http://www.asrs.org/patients).
Current Management of AMD
Although there is no cure, timely treatment can help achieve the nAMD treatment goals of drying affected eyes by inhibiting new blood vessels that leak blood and fluid and improving or maintaining visual acuity (VA) over long periods. However, in real-world settings, significant gaps and challenges pose barriers to achieving these goals. This article addresses these barriers and reviews key study evidence and expert clinical perspectives to support managed care professionals in decision making to optimize anti-VEGF treatment outcomes among nAMD patients.
Currently available treatment options include photodynamic therapy, laser surgery, and anti-VEGF therapies. In photodynamic therapy, verteporfin is injected into the bloodstream and activated via laser to close off and stunt the growth of new blood vessels, thereby slowing the rate of vision loss. Although less common, laser surgery may also be used to destroy abnormal blood vessels in nAMD. The management of nAMD has been transformed by VEGF inhibitor therapies, the first of which, pegaptanib, was approved by the FDA in 2004. Three additional, more effective, anti-VEGF agents that block all VEGF isoforms, administered by intravitreal injections, currently form the mainstay of guideline-directed treatment: ranibizumab, aflibercept, and bevacizumab.3 As described in Table 1, the 3 agents bind VEGF, thereby inhibiting the interaction of VEGF to Flt1 and KDR receptors on the surface of endothelial cells, preventing endothelial cell proliferation, new blood vessel formation, and vascular leakage. The FDA approved ranibizumab (0.5 mg) in 2006, with recommended intravitreal injections once a month (approximately 28 days). Although not as effective, patients may be treated with 3 monthly doses followed by less frequent dosing or with 1 dose every 3 months after 4 monthly doses with regular assessment.19 Aflibercept was approved in 2011 with a recommended dose of 2.0 mg administered by intravitreal injection every 4 weeks (monthly) for the first 3 months, followed by a 2.0 mg dose once every 8 weeks (2 months).20 Some patients may require monthly dosing after the first 3 months. Bevacizumab, which is FDA-approved for several systemic cancers, is used off-label for nAMD in 1.25 mg doses, commonly initiated at 4-week intervals.21 The agents differ in structure and molecular weight, which may account for greater ocular penetration and VEGF binding affinity of aflibercept and ranibizumab compared with bevacizumab and differences in clinical efficacy between the pharmaceuticals.22
TABLE 1.
Overview of Anti-VEGF Therapies Used in Clinical Practice for nAMD
| Agent/FDA Approval | Description | Recommended Dosagea,b | Key Clinical Trials |
|---|---|---|---|
| Ranibizumab (Lucentis) 2006 |
|
|
|
| Aflibercept (Eylea) 2011 |
|
|
|
| Bevacizumab (Avastin) 2004c |
|
|
|
aAll 3 agents administered as intravitreal injection.
bDosage for ranibizumab and aflibercept based on FDA prescribing information.
cNot FDA-approved for the treatment of patients with nAMD.
FDA = U.S. Food and Drug Administration; kd = kilodalton; mAb = monoclonal antibody; nAMD = neovascular age-related macular degeneration; VEGF = vascular endothelial growth factor.
Changes in vision are assessed by measuring VA, which is the ability of the eye to distinguish details and shapes at a set distance, on specialized charts such as the Snellen chart and the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. The ETDRS chart has been established as the gold standard for objective VA measurement in clinical trials and consists of 14 rows of 5 letters each, for a total of 70 letters.23 At 4 meters, the ETDRS chart measures VA from 20/10 to 20/200, with an ETDRS score of 100 corresponding to perfect VA of 20/10.23 In clinical trials over periods up to 2 years, each of the 3 agents maintained VA in at least 90% of nAMD patients, as evaluated by the endpoint of losing fewer than 15 letters (out of a total of 70 letters) on the ETDRS chart, a threshold that has been recognized by the FDA as representative of stabilization of vision, and which reflects a doubling of visual angle (e.g., 20/20 to 20/40).21,24-27 Approximately 30%-40% of patients improved VA, gaining 15 or more letters.
Efficacy and Safety of Anti-VEGF Therapies: Results of Key Clinical Trials
As summarized in Table 2, clinical trials on ranibizumab, aflibercept, and bevacizumab demonstrated their efficacy in maintaining or improving vision in patients with nAMD over 1 year.21,25,27 The MARINA trial on ranibizumab enrolled 716 patients with nAMD whose mean age was 77 years and mean VA was 53.5 letters (approximate Snellen equivalent = 20/80). Patients were randomly assigned to sham injections or ranibizumab, either 0.3 mg or 0.5 mg, monthly.27 At 1 year, mean VA scores increased by 6.5 and 7.2 letters in the 2 ranibizumab groups, respectively, and decreased by 10.4 letters in the sham group (P < 0.001 for both comparisons). Compared with the sham group, the ranibizumab groups had significantly greater proportions of patients who maintained or improved their VA scores (P < 0.001 for both groups). Ranibizumab treatment was also associated with better anatomic outcomes, including arrested CNV growth and leakage.27
TABLE 2.
Summary of Year 1 Results from Key Phase 3 Clinical Trials on Anti-VEGF Therapies
| Clinical Trial | Treatment Groups | Mean Δ in Letters | Loss of < 15 Letters (%) | P Value | Gain of ≥ 15 Letters (%) | P Value |
|---|---|---|---|---|---|---|
| ANCHOR (2006)24 | Verteporfin (n = 143) | – | 64.3 | < 0.001 | 5.6 | < 0.001 |
| Ranibizumab 0.3 mg (n = 140) | – | 94.3 | 35.7 | |||
| Ranibizumab 0.5 mg (n = 140) | – | 96.4 | 40.3 | |||
| MARINA (2006)27 | Sham (n = 238) | -10.4 | 62.2 | < 0.001 | 5.0 | < 0.001 |
| Ranibizumab 0.3 mg (n = 238) | 6.5 (P < 0.001) | 94.5 | 24.8 | |||
| Ranibizumab 0.5 mg (n = 240) | 7.2 (P < 0.001) | 94.6 | 33.8 | |||
| CATT (2011)21 | Ranibizumab 0.5 mg monthly (n = 284) | 8.5 ± 0.8 | 94.4 | 0.29 | 34.2 | 0.09 |
| Bevacizumab 1.25 mg monthly (n = 265) | 8.0 ± 1.0 | 94.0 | 31.3 | |||
| Ranibizumab 0.5 mg PRN (n = 285) | 6.8 ± 0.8 | 95.4 | 24.9 | |||
| Bevacizumab 1.25 mg PRN (n = 271) | 5.9 ± 1.0 | 91.5 | 28.0 | |||
| VIEW 1 (V1) VIEW 2 (V2) (2012)a,25 | Ranibizumab 0.5 mg every 4 weeks (n = 304/291) | 8.1 ± 15.3 (VI) 9.4 ± 13.5 (V2) |
93.8 (VI) 94.8 (V2) |
– | 30.9 (VI) 34.0 (V2) |
– |
| Aflibercept 0.5 mg every 4 weeks (n = 301/296) | 6.9 ± 13.4 (VI) 9.7 ± 14.1 (V2) |
95.0 (VI) 95.3 (V2) |
24.9 (VI) 34.8 (V2) |
|||
| Aflibercept 2.0 mg every 4 weeks (n = 304/309) | 10.9 ± 13.8 (VI) 7.6 ± 12.6 (V2) |
95.1 (VI) 94.5 (V2) |
37.5 (VI) 29.4 (V2) |
|||
| Aflibercept 2.0 mg every 8 weeks (n = 301/306) | 7.9 ± 15.0 (VI) 8.9 ± 14.4 (V2) |
94.4 (VI) 95.4 (V2) |
30.6 (VI) 31.4 (V2) |
aVIEW 1 was conducted in the United States and Canada. VIEW 2 was conducted in Europe, the Asia-Pacific region, Japan, and Latin America.
PRN = pro re nata; VEGF = vascular endothelial growth factor.
The ANCHOR trial enrolled 423 patients (mean age = 77 years; mean baseline VA = 45.5-47.1 letters) with predominantly classic, subfoveal CNV not previously treated with verteporfin photodynamic therapy (PDT) or antiangiogenic drugs. Patients were randomized 1:1:1 to monthly verteporfin PDT, 0.3 mg ranibizumab, or 0.5 mg ranibizumab arms. At 2 years, there was significant VA benefit in the ranibizumab arms compared to PDT (P < 0.0001). Compared with only 6.3% who received PDT, 34%-41% of patients who were administered ranibizumab gained ≥ 15 letters. In the ranibizumab group, 89.9%-90.0% of patients lost < 15 letters compared with 65.7% in the PDT group. Patients in the ranibizumab arms showed a mean improvement in VA from baseline by 8.1 and 10.7 letters, respectively, compared with a mean decline of 9.8 letters in the PDT arm. Overall, ranibizumab provided greater clinical benefit than verteporfin PDT.24
The CATT trial compared outcomes of ranibizumab (0.5 mg) and bevacizumab (1.25 mg), both administered monthly or as needed (pro re nata [PRN]), in 1,208 patients with nAMD (mean age = 79 years; mean VA = 60.5 letters; approximate Snellen equivalent = 20/63).21 From baseline to 1 year, mean VA scores increased in all 4 groups by a range of 5.9 letters in the PRN bevacizumab group to 8.5 letters in the monthly ranibizumab group. Based on noninferiority criteria, magnitudes of improvement were deemed statistically equivalent for ranibizumab and bevacizumab when given monthly or when given as needed. Ranibizumab was more effective than bevacizumab in treating the exudative component of disease, as evidenced by a greater proportion of patients with no intraretinal or subretinal fluid on optical coherence tomography (OCT) at 4 weeks (27.5% vs 17.3%; P < 0.001). Subsequent clinical trials have supported the CATT results indicating the noninferiority of bevacizumab compared with ranibizumab based on VA changes.28,29
The VIEW 1 and VIEW 2 trials were similarly designed studies that compared outcomes of ranibizumab and aflibercept.25 Patients with nAMD (n = 2,419; mean age = 76 years; mean VA = 53.8; approximate Snellen equivalent = 20/80) were randomly assigned to ranibizumab (0.5 mg) every 4 weeks (Rq4) or to 1 of 3 aflibercept doses: 0.5 mg every 4 weeks (0.5q4), 2 mg every 4 weeks (2q4), or 2 mg every 8 weeks after 3 monthly loading doses (2q8). In both studies, compared with monthly ranibizumab, all 3 aflibercept groups were statistically noninferior and clinically equivalent for the primary endpoint of maintained baseline VA scores. At 1 year, the proportion of patients who lost less than 15 letters ranged from 93.8% for ranibizumab (VIEW 1) to 95.4% for aflibercept dosed every 8 weeks (VIEW 2). In an integrated analysis of the 2 studies, the proportions of patients without intraretinal edema and sub-retinal fluid were 62.0% in the ranibizumab group and 60.3%, 72.4%, and 67.7% for the 0.5q4, 2q4, and 2q8 aflibercept groups, respectively.25 The evidence from VIEW 1 and VIEW 2 demonstrates the potential to extend anti-VEGF treatment to once every 8 weeks and to achieve similar outcomes compared with monthly dosing. Post hoc analysis of VIEW 1 and VIEW 2 including patients with early persistent retinal fluid (N = 1,815) showed that mean best corrected visual acuity (BCVA) gain from baseline to Week 52 was greater in eyes in the 2q4 group compared with those in the Rq4 (P < 0.01) or 2q8 (P < 0.05) groups. Although there was no significant difference in the proportion of eyes that gained ≥ 15 letters among the 3 groups, a lower percentage of eyes lost ≥ 5 letters in the 2q4 group (6.5%, 95% confidence interval [CI] = 1.8-11.1) compared with that of Rq4 (16.6%, 95% CI = 10.9-22.3) and 2q8 (16.2%, 95% CI = 9.4-23.1). Thus, aflibercept 2q4 may offer greater benefit to the subgroup of patients with early persistent retinal fluid compared to Rq4 and aflibercept 2q8.30
Injections of all 3 anti-VEGF agents potentially pose risks of serious ocular adverse events, including endophthalmitis, retinal detachment, and subretinal and vitreous hemorrhage. However, as reported in the key clinical trials, rates of these events were approximately 1% or lower.21,25,27 Risks of treatment-related serious systemic events, including thromboembolic events, are also low in nAMD patients who receive intravitreal anti-VEGF injections.21,25,27,31
Concerns about the safety of bevacizumab are associated with its requirement for repackaging before intravitreal administration. Vials of bevacizumab are often divided into single-dose, prefilled syringes for intravitreal use by compounding pharmacies. Serious outbreaks of endophthalmitis have been reported among nAMD patients who received bevacizumab injections that were repackaged by the same pharmacies.32,33 Moreover, analyses have demonstrated that product aliquoting, handling, and distribution can reduce the protein concentration and potency of bevacizumab for intravitreal use.34 However, additional oversight has been instituted and compounding pharmacies must comply with United States Pharmacopeia Chapter 797,35 which sets standards for the compounding, transportation, and storage of compounded sterile products. In addition, sources of bevacizumab should be verified.36 Repackaged bevacizumab has been shown to be stable for 3-6 months.37,38 The AAO guidelines recommend that “the informed consent process should include a discussion of the risks and benefits of treatment and treatment alternatives. The off-label status of bevacizumab for neovascular AMD should be included in the discussion.”3
Neovascular AMD Management Gaps, Challenges, and Opportunities
As demonstrated in extensions to the key clinical trials summarized above, rates of maintained or improved VA remained high among study patients who were treated with ranibizumab, aflibercept, or bevacizumab over 96 weeks or 2 years.24,26,39 Similarly low risks of treatment-related adverse ocular or systemic events were also reported in the extension trials. However, long-term observational studies and clinical experiences have revealed that a substantial proportion of nAMD patients who begin treatment with anti-VEGF agents experience significant vision loss over periods of 3 to 8 years.40-42 The SEVEN-UP study evaluated VA and anatomic measures in 65 patients who participated in key clinical trials on ranibizumab.40 For example, after a mean of 7.3 years following entry into the MARINA or ANCHOR trials, 23% of study eyes had a Snellen VA score of 20/40 or better; however, 37% of study eyes were legally blind, defined as VA of 20/200 or worse. Better visual outcomes were identified among patients in the highest quartile for the number of anti-VEGF injections received (≥ 11 injections at the time of the SEVEN-UP evaluation).40,41
Suboptimal long-term outcomes among nAMD patients who receive anti-VEGF treatment may be attributable to many complex factors. These include inexorable CNV progression due to increased activation of VEGF or other angiogenic mediators, effects of other AMD- and age-related ocular conditions, lack of patient adherence to treatment, lack of patient monitoring, and lapses in physician regimentation of anti-VEGF injections and monitoring. Many nAMD patients present with advanced disease and experience long delays between CNV formation and anti-VEGF treatment initiation, which predicts poor outcomes.12,13 Moreover, nAMD management is complicated by age- and disease-related comorbidities that can compromise quality of life and treatment outcomes.7
Among all stakeholders, the costs associated with nAMD management raise challenges. For a newly diagnosed patient, the estimated costs of monthly anti-VEGF treatment can range from $65,000 to more than $250,000 over 20 years.43 A central issue involves disparity in cost between off-label bevacizumab and the 2 FDA-approved agents. Using data from comparative clinical trials including CATT, studies have concluded that bevacizumab is more cost-effective than ranibizumab.43,44 However, comparative and cost-effectiveness evidence is either inconsistent or lacking to address the influences of many patient-specific factors on anti-VEGF treatment outcomes. These include inter-individual differences in age and general health; starting VA scores and anatomic characteristics, including the location and extent of CNV lesions, and presence of retinal fluid leakage, hemorrhage, and fibrotic scarring; risks of AMD in the second eye; willingness and ability to adhere to treatment and monitoring regimens; initial treatment responses; and threats to quality of life associated with vision loss. These factors highlight the vital importance of individualized approaches to anti-VEGF treatment decision making and management for patients with nAMD. With study evidence and insights into expert clinical practices that directly address these challenges, managed care professionals can play key roles in optimizing outcomes through appropriate management and monitoring of anti-VEGF treatment.
Promoting Early AMD Detection, Diagnosis, and Treatment
Through clinical trials and studies on the natural history of AMD, researchers have identified the importance of early detection, diagnosis, and treatment.12,13,45 Subanalyses of anti-VEGF clinical trials have demonstrated that the key predictors of better long-term outcomes are higher VA scores and smaller CNV lesion areas at treatment initiation.46-48 In addition, better outcomes are associated with a shorter duration between initial symptoms and anti-VEGF treatment initiation.12,13,49 Even minor delays in anti-VEGF treatment can have profoundly negative effects within days or weeks. These include severe vision loss or blindness (due to new CNV lesions growing and causing leakage), hemorrhage, and other complications.45 Based on an analysis of CNV lesion sizes among patients who participated in key anti-VEGF clinical trials, researchers have estimated that the earliest enrolled patients had nAMD for nearly 8 months before entering the studies.50 Early detection of CNV in an affected eye is also important because the condition subsequently develops in the second eye in a substantial proportion of patients. In a meta-analysis of 4,362 patients with nAMD, the second eye developed AMD in 12.2% of patients by 1 year and in 26.8% of patients by 4 years.51
A number of studies have reported underdiagnosis of AMD and long delays between initial symptoms and treatment initiation for nAMD.12,13,49,52 A recent U.S.-based study investigated the accuracy of AMD diagnosis among 644 older adults (1,288 eyes) who received a dilated comprehensive eye examination from a primary care ophthalmologist or optometrist.53 Participants were eligible if they had no indication of an AMD diagnosis in their medical records. In follow-up exams using color fundus photography, expert graders identified AMD in 25% of the eyes. Among these undiagnosed cases, 30% had large drusen. As determined through the extensive AREDS research program, the use of antioxidant vitamins and minerals (i.e., daily high doses of vitamins C and E, beta-carotene, zinc, and copper) can reduce the risk of progression from intermediate to advanced AMD by approximately 25% over a 5-year period.3,9,54 As reasoned by the authors of the study indicating a high rate of AMD underdiagnosis, if the patients with large drusen had been diagnosed correctly, they might have benefited from AREDS-based nutritional supplements.53 The AAO guidelines recommend AREDS-based nutritional supplements for patients with intermediate or advanced AMD to reduce progression.3 When AREDS-based supplementation is used, care must to taken in the dosage and forms of supplementation to avoid complications. For example, copper should be supplemented as cupric oxide to avoid zinc-induced copper deficiency anemia, and lutein and zeaxanthin may be substituted for beta-carotene.3
A key factor that contributes to delays in AMD detection, diagnosis, and treatment initiation is a lack of awareness and knowledge about the disease among the public and nonspecialist health care professionals.55,56 In primary care settings, the AREDS authors recommended more advanced training in identifying AMD, along with the use of high-quality retinal imaging modalities. In a study associated with the National Health and Nutrition Examination Survey, 84% of people with AMD were unaware of their condition.56
Accounting for Treatment Burden and Other Patient-Centered Factors
Patients generally understand the severe implications of inadequate AMD treatment and many initially express fears of having injections in the eye, anticipating pain or discomfort.8 In addition, due to age-related challenges, many patients have difficulty arranging and traveling to appointments for treatment and monitoring. In a survey of 75 patients with nAMD (mean age = 79 years), the reported mean time per visit was 11.7 hours, accounting for appointment preparation, travel, waiting time, treatment time, and post-appointment recovery.57 Most patients (72%) reported that they were driven to their appointments by a caregiver, requiring a significant amount of caregiver time away from work or personal activities.
Treatment and follow-up monitoring for patients with nAMD can also pose considerable burdens on physicians and their clinical staff. In a multicenter survey conducted in the United States, 57 retina specialists reported time requirements for care provided to patients with nAMD.57 The mean duration for a patient visit was 90 minutes, and AMD patient care accounted for 20% of office staff time per week. Most physicians (58%) reported that billing and filing for reimbursement placed a major burden on staff resources. Moreover, 67% of the physicians indicated that it would be very desirable to reduce the number of office visits for the treatment and monitoring of patients with nAMD.57
Optimizing Anti-VEGF Therapeutic Strategies
In response to the burdens and high costs of anti-VEGF treatment dosed by the schedules in registration clinical trials, retina specialists have developed alternative regimens in which patients receive injections at extended fixed intervals, PRN based on disease activity, and/or by the treat-and-extend strategy.58-60 The goals of these approaches are to minimize evidence of exudative disease activity such as intraretinal fluid, subretinal fluid, and hemorrhage as efficiently and safely as possible and maintain or improve VA while also reducing the number of yearly injections. Comparisons across the studies are somewhat limited by differences in patient characteristics, baseline VA and disease activity, treatment methods, re-treatment thresholds, and study duration. Nonetheless, consistent patterns in the evidence are noteworthy and useful for guiding decisions about anti-VEGF dosing for effective outcomes and reduced burden and costs.
Extended Fixed-Interval Treatment
Several early studies on alternative anti-VEGF dosing strategies investigated the effects of quarterly fixed-interval injections.61,62 The PIER trial included patients (n = 184) who received 3 monthly injections of sham or ranibizumab (0.3 mg or 0.5 mg) followed by quarterly injections.61 At 1 year, mean VA scores decreased by 16.3, 1.6, and 0.2 letters in the 3 groups, respectively (P ≤ 0.0001 for comparisons of sham and both ranibizumab groups). These losses occurred after mean gains of 2.9 and 4.3 letters in the 2 ranibizumab groups. These results indicate that quarterly fixed-interval dosing is not sufficient to maintain the initially gained BCVA from the monthly treatment.
Pro Re Nata
In the PRN strategy, patients receive a series of monthly loading injections of anti-VEGF therapy and then have regular office visits for assessment of VA and anatomic measures based on OCT, FA, or other imaging modalities. The CATT trial included 1,208 patients (mean age = 78.4-80.1 years; baseline VA = 60.1-61.5 letters) who were treated with ranibizumab or bevacizumab on monthly or PRN dosing schedules.21,26 Based on re-treatment criteria, only patients with active disease received subsequent anti-VEGF injections. Patients in the PRN groups were evaluated every month and received treatment if their affected eye had retinal fluid on OCT, new or persistent hemorrhage, decreased VA, or dye leakage on FA. Mean VA scores increased in all 4 groups at 1 year (Table 2). However, compared with monthly ranibizumab and bevacizumab, the 1-year improvement in VA was 1.7 letters and 2.1 letters less in the PRN groups, respectively. Over 2 years, VA scores increased more in the monthly versus PRN groups, with a mean difference of 2.4 letters (P = 0.046). The proportion of eyes without retinal fluid was higher for monthly versus PRN treatment, with a mean difference of 19% (P < 0.0001). Over 2 years, patients in the ranibizumab and bevacizumab PRN groups received 12.6 and 14.1 injections (P = 0.01), respectively, of a maximum 26 injections.21,26 Thus, whereas the treatment burden was reduced relative to monthly injections, the VA and anatomical outcomes were worse. Moreover, as stated above, CATT PRN subjects still required the substantial burden of monthly visits for evaluation. Hence, a conscientious PRN approach may require monthly visits.
Findings from other representative trials that included PRN arms are summarized in Table 3. These include the PRONTO trial, which demonstrated that under rigorous assessment and re-treatment criteria, the PRN strategy can elicit substantial improvements in VA scores and anatomic measures while sharply reducing the number of yearly injections compared with monthly dosing.63,64 In this trial, 40 patients received 3 monthly loading injections of ranibizumab before switching to PRN dosing. The extensive re-treatment criteria were a loss of 5 letters with retinal fluid on OCT, an increase in central retinal thickness (CRT) of at least 100 μm, new-onset classic CNV, new macular hemorrhage, or persistent retinal fluid on OCT at least 1 month after the previous injection. At 2 years, mean VA scores increased by 11.2 letters, and mean CRT decreased by 212 μm (P < 0.001 for both results); 43% of patients gained 15 letters or more. These improvements occurred with a mean of 9.9 injections over the 2-year PRONTO study.64
TABLE 3.
Methods and Findings of Representative PRN and Treat-and-Extend Trials of Anti-VEGF Therapies
| Trial | Groups/Baseline Mean VA/Protocol | Re-Treatment or Extension Criteria | Key Findings (Means) |
|---|---|---|---|
| PRN | |||
| PRONTO (2009) 64,66 |
|
Re-treatment: ≥ 5-letter loss with fluid, ↑ CRT of ≥ 100 µm, new-onset CNV, new hemorrhage, or persistent fluid |
|
| SAILOR (2009) 66 |
|
|
|
| HARBORa(2013)65 |
|
Re-treatment: ≥ 5-letter loss or any evidence of disease activity on SD-OCT |
|
| VIEW 96-week (2014)39 | Baseline to 52 weeks:
|
Re-treatment: New or persistent fluid, ↑ CRT of ≥ 100 µm, ≥ 5-letter loss with fluid, new-onset CNV, new or persistent leak, new hemorrhage, or lapse of 12 weeks since the previous injection |
|
| Treat and extend | |||
| LUCAS 201668 |
|
Re-treatment: Fluid on OCT, new or persistent hemorrhage, dye leakage, or increased lesion size on FA |
|
| TREX-AMD 201771 |
|
Re-treatment: Intraretinal or subretinal fluid on SD-OCT |
|
| Treat and extend | |||
| ATLAS 201772 |
|
Extension based on absence of the following: macular fluid on OCT, vision loss of ≥ 5 letters, new macular hemorrhage, and increased lesion size or leakage on FA |
|
aData not shown for HARBOR groups that received monthly and PRN ranibizumab 2.0 mg.
CFT = central foveal thickness; CNV = choroidal neovascularization; CRT = central retinal thickness; FA = fluorescein angiography; FP = fundus photography; OCT = optical coherence tomography; PRN = pro re nata; SD = standard deviation; VA = visual acuity; VEGF = vascular endothelial growth factor.
The HARBOR trial further investigated the potential for PRN dosing to promote effective treatment outcomes and reduce injection burden (n = 1,098 patients; mean age = 79 years; mean baseline VA = 53.5-54.5 letters).65 Among 550 patients who received ranibizumab 0.5 mg monthly or PRN, 1-year improvements in mean VA scores were 10.1 letters and 8.2 letters, respectively. Despite these large improvements, compared with monthly dosing, the PRN regimen did not meet the prespecified noninferiority margin of 4 letters. The 2 groups received a mean of 11.3 and 7.7 injections over 1 year. The HARBOR and PRONTO trials had rigorous re-treatment criteria and required monthly office visits for assessing VA and disease activity in the PRN groups. Contrasting PRN assessment protocols and outcomes were reported in the SAILOR trial, which included patients who received ranibizumab 0.5 mg (n = 1,209).66 These patients had monthly office visits for 3 months and quarterly visits thereafter. The re-treatment criteria were a loss of 5 letters and/or an increase in CFT of at least 100 μm with retinal fluid. From baseline to 3 months, mean VA increased by 7.0 letters in this group of patients; however, from 3 months to 1 year, they lost 4.7 letters. Over 1 year, the mean number of injections was 4.9. The authors concluded that the reduced VA benefits may have been due to the relatively long (quarterly) interval between office visits for assessment and PRN treatment.66
The 96-week VIEW extension trial compared outcomes of among 2,419 patients who, at Week 52, were switched from monthly ranibizumab (0.5 mg) or aflibercept (0.5 mg every 4 weeks, 2 mg every 4 weeks, or 2 mg every 8 weeks) to PRN dosing with a capped 12-week maximum treatment interval.39 The re-treatment criteria were new or persistent retinal fluid, an increase in CRT of 100 μm or more, a loss of 5 or more letters with retinal fluid presence, new-onset CNV, new or persistent leak, new hemorrhage, or a lapse of 12 weeks since the previous injection. From baseline to 96 weeks, mean VA increased by 7.9, 6.6, 7.6, and 7.6 letters in the 4 groups, respectively; the mean numbers of injections were 16.5, 16.2, 16.0, and 11.2. In all groups, the proportions of patients who gained 15 or more letters were similar during the fixed-interval (29.8%-33.4%) and PRN phases (28.1%-33.4%). However, across the 2 phases, the proportion of patients with no intraretinal or subretinal fluid on OCT decreased by 16.5%, 18.0%, 15.7%, and 17.6% in the 4 groups, respectively.39
In the studies that have compared monthly and PRN dosing, statistically similar proportions of patients experienced ocular or systemic serious adverse events, the rates of which were comparable to those reported in the key clinical trials reviewed above.39,67
Treat and Extend
In a 2007 commentary that addressed the challenges and potential high costs of frequent assessment for disease activity in the PRN approach, Spaide (2007) described a treat-and-extend regimen, which is now used by many ophthalmologists and retina specialists.59 In this approach, patients receive a series of loading anti-VEGF injections, usually at 4-week intervals, with VA and anatomic assessment. When criteria indicating no disease activity are met, patients receive an injection and the treatment interval is extended, usually by 2 weeks at a time, until a maximum interval of 12 to 16 weeks is reached. If CNV lesions are reactivated, the treatment interval is similarly reduced. Findings from key clinical trials that have included treat-and-extend arms are summarized in Table 3.
The LUCAS trial compared outcomes of patients who received ranibizumab 0.5 mg (n = 218) or bevacizumab 1.25 mg (n = 213) according to a treat-and-extend regimen similar to the one described above.68,69 Treatment intervals were extended by 2 weeks at a time when OCT and fundus examination indicated no signs of active neovascular disease. The maximum treatment interval was 12 weeks. Re-treatment criteria were retinal fluid on OCT, new or persistent hemorrhage, dye leakage, or increased lesion size on FA. At 2 years, there were no significant differences between the 2 groups, respectively, for mean changes in VA scores (+6.6 and +7.4 letters) or CRT (-122 and -113 μm).68 Significantly more injections were given to patients treated with bevacizumab (18.2 injections) compared with ranibizumab (16.0 injections; P ≤ 0.001). The proportion of patients who received treatment every 12 weeks was greater in the ranibizumab (17%) compared with the bevacizumab (10%) group.
In the TREX-AMD trial, patients received ranibizumab 0.5 mg monthly (n = 20) or in a treat-and-extend regimen after 3 monthly loading doses (n = 40). Upon inactive disease, patients in the latter group received an injection, and the treatment interval was extended by 2 weeks at a time for a maximum interval of 12 weeks.70,71 Recurrence of disease activity was designated by intraretinal or subretinal fluid on OCT. At 2 years, the monthly and treat-and-extend groups did not differ significantly in improvements of VA (+10.5 and +8.7 letters) or CRT (-117 and -118 μm). Significantly more injections were given to patients treated monthly (25.5) compared with the alternate regimen (18.6; P < 0.001).
The ATLAS trial assessed VA and anatomic outcomes in 40 patients who received aflibercept 2.0 mg in a treat-and-extend regimen that involved an initial injection and repeated evaluations every 4 weeks until no disease activity was observed.72 Treatment intervals were extended by 2 weeks at a time, for a maximum of 16 weeks, when the following conditions were absent: macular fluid on OCT, vision loss of 5 letters or more, new macular hemorrhage, and increased lesion size or leakage on FA. At 2 years, mean letter gain was 2.4 and the number of injections was 6.5. Mean CFT decreased by 139 μm at the end of 2 years and treatment intervals were 8 weeks or longer and 12 weeks or longer for 75% and 38% of patients, respectively.
The clinical trials on the treat-and-extend regimen have reported low rates of serious ocular and systemic adverse events. In the LUCAS trial, rates of endophthalmitis at 2 years were 0% and 0.5% in the ranibizumab and bevacizumab groups, respectively.68 No cases of this event were reported in the TREX-AMD trial, and 1 of the 40 patients in the ATLAS trial had culture-positive endophthalmitis.71
The recently published results from the large TREND trial showed that the treat-and-extend regimen of 0.5 mg ranibizumab was noninferior to monthly ranibizumab dosing with a least squares mean BCVA change from baseline of 6.2 and 8.1 letters, respectively. These BCVA changes occurred within 6 months of treatment and were stable in both arms. Fewer injections were required in the treat-and-extend group (8.7) compared with the monthly dosing group (11.1). There was no significant difference in types and rates of adverse events between the 2 groups.73
Managed Care Implications and Strategies to Improve AMD Treatment and Outcomes
Clinical trials have demonstrated the efficacy of anti-VEGF therapies in the treatment of nAMD. Each of the 3 commonly used agents, aflibercept, ranibizumab, and bevacizumab, maintained VA in ≥ 90% of nAMD patients in clinical trials up to 2 years. VA improved in a subset of patients, approximately 30%-40%, as measured by a gain in 15 or more letters on the ETDRS chart. Clinical trials have also demonstrated potential benefits for different dosing regimens for certain patient subpopulations, such as every-4-week dosing with aflibercept over every-8-week dosing with aflibercept or every-4-week dosing with ranibizumab for patients with early persistent retinal fluid, while other patients may have similar outcomes with every-8-week dosing. However, several complex factors, such as inadequate adherence to treatment and monitoring, can negatively affect long-term outcomes. Managed care professionals can help improve long-term outcomes for patients with nAMD by working with physicians to identify appropriate anti-VEGF treatment selection and dosing regimens based on patient characteristics, balancing outcomes and burden of therapy, including cost of therapy and burden on the patient and caregiver.
Through educational programs and resources, managed care organizations can support efforts to increase public awareness about AMD, which may encourage at-risk individuals to note potential symptoms, have regular dilated fundus examinations, and modify lifestyle behaviors to reduce risks.12,13
Educational programs should promote awareness about common AMD risk factors, which include increasing age, family history, cardiovascular risk factors, and cigarette smoking.3,10,18 For patients who have developed nAMD, managed care professionals can educate and support patients for consistent adherence to therapy and monitoring to optimize patient outcomes.
Current Debates in AMD Treatment and Management
While the anti-VEGF therapies have revolutionized management, we have a long way to go to achieve optimal outcomes for all nAMD patients. To address these challenges, authors of this manuscript, consisting of 3 retina specialists, Dr. Lloyd Clark, Dr. Jared Nielsen, and Dr. Charles Wykoff, and an expert in health care management, Dr. Joel Brill, share their perspectives and viewpoints on key questions relating to contemporary considerations in a candid and open discussion.
How can modifiable risk factors for AMD development and progression be recognized and addressed earlier?
Dr. Charles Wykoff: While over half of one’s risk of developing AMD may be genetically determined, there are specific lifestyle changes that can slow the progression of this common disease: smoking cessation, optimal cardiovascular risk factor control, and AREDS supplementation in appropriate patient populations. Identical twin studies have reported that smoking in one twin leads to nAMD diagnosis about 1 decade earlier than in a nonsmoking twin. Once someone has nAMD, smoking is associated with a more aggressive disease course. In the appropriate patient population, consumption of the 6 specific supplements within the AREDS formation can also help mitigate risk. It is important to understand that AREDS supplements have only been proven valuable in modifying the AMD disease course among patients with a diagnosis of intermediate dry AMD or advanced AMD in 1 eye. Toward this end, appropriate ocular screening exams for patients at risk of AMD, which can be asymptomatic in its intermediate dry stage, is important and should be encouraged. Furthermore, primary eye care teams should be educated regarding which patients should be prescribed AREDS supplements and the possible side effects and contraindications to supplement use.
When and how should patients be treated?
Dr. Charles Wykoff: Once diagnosed with nAMD, earlier treatment leads to better absolute visual outcomes with a reduced treatment burden. To achieve earlier treatment initiation, patients and caregivers need to be educated about the signs and symptoms of AMD, and appropriate screening eye exams should be encouraged. Once nAMD is diagnosed, efficient transfer of care to a retina specialist skilled in managing nAMD is important.
Dr. Jared Nielsen: Individuals with nAMD may live with this disease for 20 years or longer. Permanent vision loss, quality of life, and financial considerations are at stake in managing nAMD. The burden of management can be high and, unfortunately, many patients do not receive the intensity of treatment required to achieve optimal outcomes. Efforts to reduce treatment burden using advanced imaging and a customized treatment approach, along with patient education and support, can help minimize disease burden and improve patient outcomes.
Dr. Lloyd Clark: Yes, initial studies pointed toward monthly therapy as the best choice for patients with nAMD. However, early on, it was recognized that this dosing strategy, employed indefinitely, would be difficult to maintain. Therefore, investigators and clinicians sought to evaluate alternative dosing strategies to reduce treatment burden. Initially, changes to treatment intervals were made independent of disease activity using either fixed quarterly intervals or PRN schedules. Regardless of anti-VEGF agent chosen, these strategies were unsatisfactory in maintaining vision gains achieved with early monthly dosing.
The breakthrough occurred when nAMD disease activity was accounted for in extending treatment intervals. Treat-and-extend dosing is based on the concept of disease-free intervals between treatments to maintain VA gains. As we have learned from a number of natural history and interventional studies, the disease severity and response to anti-VEGF therapy of patients with nAMD is highly variable. Thus, if outcomes approaching monthly therapy are to be achieved with treat-and-extend dosing, a personalized approach to therapy must be employed that minimizes or eliminates recurrent CNV activity.
This concept will drive future therapies in nAMD. Newer agents targeting different cytokines used either alone or in conjunction with anti-VEGF agents may reduce treatment burden, and their effective use will likely require a treat-and-extend approach. Longer-acting molecules, extended drug-delivery devices, and gene therapy platforms that offer long-acting control of CNV will also require careful monitoring of breakthrough disease. Currently, and in the near future, maintenance of disease-free intervals between treatments is key to optimal management of nAMD.
What are the considerations for on-label versus off-label treatment of AMD?
Dr. Charles Wykoff: While we have 3 distinct pharmaceuticals that all block the activity of VEGF, some patients appear to respond optimally to one agent more than another. In some patients with nAMD, it appears that the FDA-approved products may be more effective at achieving optimal anatomic and/or visual outcomes. It is important to recognize that the bevacizumab used in the CATT trial discussed in this manuscript and the large DRCR.net Protocol T trial comparing the 3 anti-VEGF agents in patients with diabetic macular edema employed bevacizumab delivered in glass, whereas the bevacizumab that most retina physicians use regularly is delivered in plastic syringes, which may affect safety and efficacy. Ideally patients and physicians would have access to all of the available pharmaceuticals so that therapy can be individualized as needed. It is valuable for insurance plans to communicate to physicians any restrictions or requirements for use of pharmaceutical therapies to treat AMD.
Dr. Jared Nielsen: Yes, we are fortunate to have 2 FDA-approved and 1 off-label treatment for nAMD. With disparities in cost, there may be a tendency to use the least expensive option. However, in nAMD decision making, it is important to consider other factors. Repackaged anti-VEGF therapy is not identical to the drug used in comparative effectiveness trials. In addition, results from clinical trials, which evaluate the efficacy and safety of agents within a select patient population over a relatively short period of time, may not be generalizable to all patients who suffer from nAMD. Biopharmacologic response can differ between patients, with some demonstrating resistance to one anti-VEGF agent but responding to another. Preserving treatment options and removing barriers to appropriate treatment are essential to keeping patients seeing and living well with AMD.
Dr. Joel V. Brill: Health care costs continue to increase. The Centers for Medicare & Medicaid Services projects that U.S. health care spending will reach nearly $5.5 trillion by 2025 and will account for 19.9% of the gross domestic product by 2025, up from 17.8% in 2015.74 To mitigate costs, treatment of nAMD with off-label anti-VEGF therapy has been incorporated into some health plan formularies. Although concerns have been raised about repackaging of off-label anti-VEGF therapy for use in nAMD, additional oversight has been implemented over the repackaging process since the passage of the Drug Quality and Security Act in 2013. Physicians and payers have a duty and obligation to work together to provide medically necessary, value-based therapies that are safe and effective. I agree with my colleagues that preserving options and removing barriers to appropriate treatment are essential to keeping patients seeing and living well with nAMD. Medicare has multiple avenues, including the Center for Medicare and Medicaid Innovation and the Physician-Focused Payment Model Technical Advisory Committee, to innovate and propose reimbursement policies that equalize physician reimbursement for the administration of nAMD agents.
Conclusions
A key theme that emerges from studies on anti-VEGF agents and therapeutic strategies is that optimal outcomes depend on individualized approaches to treatment decision making and management. For example, in the PIER trial, after 3 monthly injections of ranibizumab, mean VA scores decreased in patients who switched to quarterly injections; however, 40% of the cohort maintained the vision gains that they achieved during the first 3 months.61 As noted in Table 3, some clinical trials have reported high standard deviations and wide ranges for the number of injections received by patients in PRN and treat-and-extend groups. Through clinical experience, nAMD experts have learned that, to dry affected eyes and arrest CNV growth, some patients may need injections even more frequently than recommended in FDA labels for ranibizumab or aflibercept. However, the VIEW studies demonstrated the potential to extend anti-VEGF treatment with aflibercept to once every 8 weeks in some patients and achieve similar outcomes compared with monthly dosing.
The efficacy of anti-VEGF therapy for nAMD has been consistently demonstrated in clinical trials; however, real-world gaps and challenges can pose significant barriers to achieving treatment goals. In collaboration with ophthalmologists and retina specialists, managed care professionals can play key roles in overcoming barriers associated with underdiagnosis of nAMD, delays between CNV growth and anti-VEGF treatment initiation, lack of awareness about AMD among the public, and the logistic, emotional, and financial burdens of frequent intravitreal injections.
References
- 1.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-85. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933-43. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Ophthmalogy. Age-related macular degeneration preferred practice patterns . Available at: https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp-2015. Accessed January 10, 2018.
- 4.Mathew RS, Delbaere K, Lord SR, Beaumont P, Vaegan, Madigan MC. Depressive symptoms and quality of life in people with age-related macular degeneration. Ophthalmic Physiological Opt. 2011;31(4):375-80. [DOI] [PubMed] [Google Scholar]
- 5.Rovner BW, Casten RJ, Leiby BE, Tasman WS. Activity loss is associated with cognitive decline in age-related macular degeneration. Alzheimers Dementia. 2009;5(1):12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown GC, Brown MM, Sharma S, et al. The burden of age-related macular degeneration: a value-based medicine analysis. Trans Am Ophthalmol Soc. 2005;103:173-84. [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Quality Life Outcomes. 2006;4:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23(2):127-40. [DOI] [PubMed] [Google Scholar]
- 9.Age-Related Eye Disease Study Research Group. A randomized, placebocontrolled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8 . Arch Ophthalmol. 2001;119(10):1417-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller JW. Age-related macular degeneration revisited--piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am J Ophthalmol. 2013;155(1):1-35.e13. [DOI] [PubMed] [Google Scholar]
- 11.Castillo MM, Mowatt G, Elders A, et al. Optical coherence tomography for the monitoring of neovascular age-related macular degeneration: a systematic review. Ophthalmology. 2015;122(2):399-406. [DOI] [PubMed] [Google Scholar]
- 12.Keane PA, de Salvo G, Sim DA, Goverdhan S, Agrawal R, Tufail A. Strategies for improving early detection and diagnosis of neovascular age-related macular degeneration. Clin Ophthalmol. 2015;9:353-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz R, Loewenstein A. Early detection of age related macular degeneration: current status. Int J Retina Vitreous. 2015;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121(2):535-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wittenborn JS, Clemons T, Regillo C, Rayess N, Liffmann Kruger D, Rein D. Economic evaluation of a home-based age-related macular degeneration monitoring system. JAMA Ophthalmol. 2017;135(5):452-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-72. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the U.S. population. Arch Ophthalmol. 2011;129(1):75-80. [DOI] [PubMed] [Google Scholar]
- 18.Lambert NG, ElShelmani H, Singh MK, et al. Risk factors and biomarkers of age-related macular degeneration. Prog Retin Rye Res. 2016;54:64-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucentis (ranibizumab injection) for intravitreal injection. Genentech. January 2017 . Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125156s111lbl.pdf. Accessed January 10, 2018.
- 20.Eyelea (aflibercept) injection for intravitreal injection. Regeneron Pharmaceuticals. November 2011 . Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125387lbl.pdf. Accessed January 10, 2018.
- 21.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. NEJM. 2011;364(20):1897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart MW, Rosenfeld PJ, Penha FM, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina. 2012;32(3):434-57. [DOI] [PubMed] [Google Scholar]
- 23.Early Treatment Diabetic Retinopathy Study. Early Treatment Diabetic Retinopathy Study Design and baseline patient characteristics. ETDRS report number 7 . Ophthalmology. 1991;98(5):741-56. [DOI] [PubMed] [Google Scholar]
- 24.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57-65.e55. [DOI] [PubMed] [Google Scholar]
- 25.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-48. [DOI] [PubMed] [Google Scholar]
- 26.Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. NEJM. 2006;355(14):1419-31. [DOI] [PubMed] [Google Scholar]
- 28.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258-67. [DOI] [PubMed] [Google Scholar]
- 29.Kodjikian L, Souied EH, Mimoun G, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology. 2013;120(11):2300-09. [DOI] [PubMed] [Google Scholar]
- 30.Jaffe GJ, Kaiser PK, Thompson D, et al. Differential response to anti-VEGF regimens in age-related macular degeneration patients with early persistent retinal fluid. Ophthalmology. 2016;123(9):1856-64. [DOI] [PubMed] [Google Scholar]
- 31.Modi YS, Tanchon C, Ehlers JP. Comparative safety and tolerability of anti-VEGF therapy in age-related macular degeneration. Drug Safety. 2015;38(3):279-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg RA, Flynn HW Jr, Isom RF, Miller D, Gonzalez S. An outbreak of streptococcus endophthalmitis after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012;153(2):204-208.e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez S, Rosenfeld PJ, Stewart MW, Brown J, Murphy SP. Avastin doesn’t blind people, people blind people. Am J Ophthalmol. 2012;153(2):196-203.e191. [DOI] [PubMed] [Google Scholar]
- 34.Yannuzzi NA, Klufas MA, Quach L, et al. Evaluation of compounded bevacizumab prepared for intravitreal injection. JAMA Ophthalmol. 2015;133(1):32-39. [DOI] [PubMed] [Google Scholar]
- 35.Pharmaceutical Compounding-Sterile Preparations. United States Pharmacopeia: 2008-2009 USP Pharmacists’ Pharmacopeia . 2nd ed. 5th supplement. Rockville, MD: United States Pharmacopeia Convention. April 21-24, 2010. Washington, DC. [Google Scholar]
- 36.American Academy of Ophthalmology. Clinical statement. Verifying the source of compounded bevacizumab for intravitreal injections - 2014 . Available at: https://www.aao.org/clinical-statement/verifying-source-of-compounded-bevacizumab-intravi-2. Accessed January 10, 2018.
- 37.Paul M, Vieillard V, Roumi E, et al. Long-term stability of bevacizumab repackaged in 1mL polypropylene syringes for intravitreal administration. Ann Pharm Fr. 2012;70(3):139-54. [DOI] [PubMed] [Google Scholar]
- 38.Khalili H, Sharma G, Froome A, Khaw PT, Brocchini S. Storage stability of bevacizumab in polycarbonate and polypropylene syringes. Eye. 2015;29(6):820-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193-201. [DOI] [PubMed] [Google Scholar]
- 40.Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292-99. [DOI] [PubMed] [Google Scholar]
- 41.Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6):1175-83. [DOI] [PubMed] [Google Scholar]
- 42.Maguire MG, Martin DF, Ying GS, et al. Five-year outcomes with antivascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(8):1751-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein JD, Newman-Casey PA, Mrinalini T, Lee PP, Hutton DW. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology. 2014;121(4):936-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno TA, Kim SJ. Ranibizumab (Lucentis) versus bevacizumab (Avastin) for the treatment of age-related macular degeneration: an economic disparity of eye health. Semin Ophthalmol. 2016;31(4):378-84. [DOI] [PubMed] [Google Scholar]
- 45.Ho AC, Albini TA, Brown DM, Boyer DS, Regillo CD, Heier JS. The potential importance of detection of neovascular age-related macular degeneration when visual acuity is relatively good. JAMA Ophthalmology. 2017;135(3):268-73. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144(6):850-57. [DOI] [PubMed] [Google Scholar]
- 47.Ying GS, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114(2):246-52. [DOI] [PubMed] [Google Scholar]
- 49.Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV. Time to first treatment: the significance of early treatment of exudative age-related macular degeneration. Retina. 2012;32(7):1260-64. [DOI] [PubMed] [Google Scholar]
- 50.Liu TY, Shah AR, Del Priore LV. Progression of lesion size in untreated eyes with exudative age-related macular degeneration: a meta-analysis using Lineweaver-Burk plots. JAMA Ophthalmol. 2013;131(3):335-40. [DOI] [PubMed] [Google Scholar]
- 51.Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115(1):116-26. [DOI] [PubMed] [Google Scholar]
- 52.Canan H, Sizmaz S, Altan-Yaycioglu R, Sariturk C, Yilmaz G. Visual outcome of intravitreal ranibizumab for exudative age-related macular degeneration: timing and prognosis. Clin Interv Aging. 2014;9:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neely DC, Bray KJ, Huisingh CE, Clark ME, McGwin G Jr, Owsley C. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017;135(6):570-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chew EY, SanGiovanni JP, Ferris FL, et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013;131(7):843-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boxell EM, Amoaku WM, Bradley C. Healthcare experiences of patients with age-related macular degeneration: have things improved? Cross-sectional survey responses of Macular Society members in 2013 compared with 1999. BMJ Open. 2017;7(2):e012790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibson DM. Diabetic retinopathy and age-related macular degeneration in the U.S. Am J Prev Med. 2012;43(1):48-54. [DOI] [PubMed] [Google Scholar]
- 57.Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725-31.e721. [DOI] [PubMed] [Google Scholar]
- 58.Agarwal A, Rhoades WR, Hanout M, et al. Management of neovascular age-related macular degeneration: current state-of-the-art care for optimizing visual outcomes and therapies in development. Clin Ophthalmol. 2015;9:1001-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spaide R. Ranibizumab according to need: a treatment for age-related macular degeneration. Am J Ophthalmol. 2007;143(4):679-80. [DOI] [PubMed] [Google Scholar]
- 60.Stewart MW. Individualized treatment of neovascular age-related macular degeneration: what are patients gaining? Or losing? J Clin Med. 2015;4(5):1079-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145(2):239-48. [DOI] [PubMed] [Google Scholar]
- 62.Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011;118(5):831-39. [DOI] [PubMed] [Google Scholar]
- 63.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(4):566-83. [DOI] [PubMed] [Google Scholar]
- 64.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43-58.e41. [DOI] [PubMed] [Google Scholar]
- 65.Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046-56. [DOI] [PubMed] [Google Scholar]
- 66.Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1731-39. [DOI] [PubMed] [Google Scholar]
- 67.Schmucker CM, Rucker G, Sommer H, et al. Treatment as required versus regular monthly treatment in the management of neovascular age-related macular degeneration: a systematic review and meta-analysis. PloS One. 2015;10(9):e0137866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berg K, Hadzalic E, Gjertsen I, et al. Ranibizumab or bevacizumab for neovascular age-related macular degeneration according to the Lucentis compared to Avastin study treat-and-extend protocol: two-year results. Ophthalmology. 2016;123(1):51-59. [DOI] [PubMed] [Google Scholar]
- 69.Berg K, Pedersen TR, Sandvik L, Bragadottir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122(1):146-52. [DOI] [PubMed] [Google Scholar]
- 70.Wykoff CC, Croft DE, Brown DM, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology. 2015;122(12):2514-22. [DOI] [PubMed] [Google Scholar]
- 71.Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmology Retina. 2017;1(4):314-321. [DOI] [PubMed] [Google Scholar]
- 72.DeCroos FC, Reed D, Adam MK, et al. Treat-and-extend therapy using aflibercept for neovascular age-related macular degeneration: a prospective clinical trial. Am J Ophthalmol. 2017;180:142-50. [DOI] [PubMed] [Google Scholar]
- 73.Silva R, Berta A, Larsen M, et al. . Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57-65. [DOI] [PubMed] [Google Scholar]
- 74.Keehan SP, Stone DA, Poisal JA, et al. National health expenditure projections, 2016-25: price increases, aging push sector to 20 percent of economy. Health Aff (Millwood). 2017;36(3):553-63. [DOI] [PubMed] [Google Scholar]


