Abstract
BACKGROUND:
Conducting an adequately powered survival study in idiopathic pulmonary fibrosis (IPF) is challenging due to the rare nature of the disease and the need for extended follow-up. Consequently, registration trials of IPF treatments have not been designed to estimate long-term survival.
OBJECTIVE:
To predict life expectancy for patients with IPF receiving pirfenidone versus best supportive care (BSC) in a population that met the inclusion criteria of patients enrolled in the ASCEND and CAPACITY trials.
METHODS:
Kaplan-Meier survival data for pirfenidone and BSC were obtained from randomized controlled clinical studies (CAPACITY, ASCEND), an open-label extension study (RECAP), and the Inova Fairfax Hospital database. Data from the Inova registry were matched to the inclusion criteria of the CAPACITY and ASCEND trials. Life expectancy was estimated by the area under the curve of parametric survival distributions fit to the Kaplan-Meier data.
RESULTS:
Mean (95% confidence interval) life expectancy was calculated as 8.72 (7.65-10.15) years with pirfenidone and 6.24 (5.38-7.18) years with BSC. Therefore, pirfenidone improved life expectancy by 2.47 (1.26-4.17) years compared with BSC. In addition, treatment with pirfenidone recuperated 25% of the expected years of life lost due to IPF. Sensitivity analyses found that results were sensitive to the choice of parametric survival distribution, and alternative piecewise and parametric approaches.
CONCLUSIONS:
This analysis suggests that this population of patients with IPF has an improved life expectancy if treated with pirfenidone compared with BSC.
Idiopathic pulmonary fibrosis (IPF) is a rare disease of unknown etiology, characterized by progressive and irreversible fibrosis of the interstitium of the lung. IPF is fatal, resulting in the death of patients within 2-5 years from diagnosis.1-7 The 5-year survival rate of 20%-40% associated with IPF8 is similar to non-small cell lung cancer9 and worse than that of many other cancers.9,10
The prevalence of IPF in Europe and North America has been estimated to be from 3 to 9 cases per 100,000.11 Given the orphan disease status of IPF and the need for extended follow-up, executing an adequately powered survival study in IPF is challenging.12-14 Consequently, most registration trials in IPF have been conducted over a 12-month period and were not designed to estimate the long-term effect of treatment on survival.15-17
Pirfenidone is an antifibrotic drug with anti-inflammatory properties, which is approved by European and U.S. regulatory agencies for the treatment of patients with IPF. Pirfenidone was found to have a favorable benefit-risk profile, as demonstrated by evidence generated in 3 randomized placebo-controlled Phase III trials: ASCEND (Study 016)15 and CAPACITY (Studies 004 and 006).16 Pooled results from a prespecified analysis of the ASCEND and CAPACITY trials found that pirfenidone significantly reduced all-cause mortality at 12 months by 48% (hazard ratio [HR]=0.52; 95% confidence interval [CI] = 0.31-0.87; P = 0.01).15 The significant effect of pirfenidone on survival compared with placebo was further demonstrated when additional data from the CAPACITY trials up to 72 weeks were included18 and in a network meta-analysis (NMA) of treatments for IPF (see “Systematic Review and Network Meta-analysis of Idiopathic Pulmonary Fibrosis Treatments” on page S5 of this supplement). Long-term survival data for pirfenidone have been characterized through an open-label extension of the ASCEND and CAPACITY trials—RECAP (Study 012).19
Best supportive care (BSC) for IPF reflects a therapeutic approach that prioritizes symptom management, alongside the early identification and treatment of disease complications. BSC may include pulmonary rehabilitation, supplemental oxygen therapy, and/or other symptomatic treatments. In general, BSC is not thought to influence the dismal survival associated with a diagnosis of IPF.20
The objective of this analysis was to predict life expectancy in patients with IPF receiving pirfenidone or BSC in a population that met the inclusion criteria of patients enrolled in the ASCEND and CAPACITY trials.
Methods
A survival analysis was conducted based on methodology recommended in guidelines from the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU).21
Data Sources
Kaplan-Meier survival data for patients receiving pirfenidone 2,403 mg/day were obtained from the following 4 clinical studies, all of which were approved by the institutional review board or ethics committee at each participating center: ASCEND (Study 016), a multinational, placebo-controlled, confirmatory, Phase III trial, which included 555 patients treated with either pirfenidone 2,403 mg/day (n = 278) or placebo (n = 277)15; CAPACITY (Studies 004 and 006), 2 multinational, placebo-controlled, pivotal, Phase III trials, which included 779 patients treated with either pirfenidone 2,403 mg/day (n = 345), pirfenidone 1,197 mg/day (n = 87), or placebo (n = 347)16; and RECAP (Study 012), an open-label extension study for ASCEND and CAPACITY, which monitored safety endpoints, including survival.22
At the time of conducting the current analysis, survival data with pirfenidone were available for up to 8.78 years (median = 2.51 years). Patients were censored due to loss of follow-up or lung transplant in all 4 studies. The primary endpoints of the ASCEND and CAPACITY studies were evaluated at 52 weeks and 72 weeks, respectively.15,16 A total of 1,058 patients who completed treatment in the ASCEND or CAPACITY studies opted to enroll in the RECAP study of pirfenidone 2,403 mg/day, including 455 from ASCEND and 603 from CAPACITY.19,23
Kaplan-Meier survival data for patients receiving placebo from the CAPACITY and ASCEND studies were considered representative of BSC, and data were available for up to 52 weeks in all studies. The CAPACITY studies provided additional survival data up to 120 weeks; however, since the study design mandated that patients continued on treatment until the last patient completed 72 weeks of treatment, only 13% (45/347) of the original cohort randomized to the placebo arm in the CAPACITY studies had data at the next time point (96 weeks). Hence, placebo data post-72 weeks in the CAPACITY studies had to be treated with considerable caution, given that the data came from a small, and potentially unrepresentative, sample.
Given the short-term follow-up of patients receiving placebo in the clinical trials, IPF registries collecting long-term BSC survival data were also evaluated. An IPF registry at the Inova Fairfax Hospital, Falls Church, Virginia (herein referred to as the Inova registry), was deemed appropriate for evaluating survival with BSC after 52 weeks (the point at which data were still available for the ASCEND and CAPACITY studies).
Demographics, pulmonary function data, and diagnostic criteria for the Inova registry have been described previously.6 Patient-level data were obtained, and ethical approval to use these data for the survival analysis was received from the institutional review board at the Inova Human Research Protection Program before any protocol-defined activities. Patients in the Inova registry were enrolled between January 1997 and June 2015. Physicians at the Inova Fairfax Hospital confirmed that patients had a diagnosis of IPF according to the 2011 American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association IPF diagnosis and management guidelines.24
Patients received BSC, including pulmonary rehabilitation, supplemental oxygen, and N-acetylcysteine, where necessary. No patients received prednisolone, azathioprine, or antifibrotic therapy. Data from the Inova registry were restricted to match the inclusion criteria of the patients enrolled in the ASCEND and CAPACITY studies, with the least restrictive parameter (40-80 years of age, forced vital capacity [FVC] ≥ 50%, carbon monoxide diffusing capacity [DLco] ≥ 30%, FVC or DLco < 90%, forced expiratory volume in 1 second/FVC ratio > 0.7) selected when inclusion criteria differed. This resulted in a cohort of 286 of the original 815 Inova registry patients with relevant matching data. For this cohort, survival data were available for up to 17.50 years (median = 3.14 years). As with the ASCEND, CAPACITY, and RECAP studies, patients in the Inova registry were censored due to loss of follow-up or lung transplant.
Given the availability of data for the ASCEND and CAPACITY studies to inform short-term survival and an appropriate control group for which to inform long-term survival, a piecewise approach was used for BSC. This approach considered the Kaplan-Meier survival data from the placebo arms of the ASCEND and CAPACITY studies until 52 weeks (the point at which data were still available for both studies), followed by a parametric curve fit to Kaplan-Meier survival data after 52 weeks from the Inova registry.
Survival Analysis
Parametric survival distributions (exponential, Weibull, Gompertz, inverse-gamma, log-normal, and log-logistic) were fit to Kaplan-Meier survival data using STATA (StataCorp, College Station, TX) and Microsoft Excel (Microsoft Corporation, Redmond, WA). The best-fitting distribution was chosen according to the following: statistical consideration (Akaike and Bayesian information criteria); visual inspection of the fitted curve against the Kaplan-Meier data; and clinical interpretation (especially for the extrapolated posttrial portion of the curve). Life expectancy was calculated from the area under the curve of the selected parametric distributions, predicting overall survival with pirfenidone and BSC.
Sensitivity Analyses
To evaluate the impact of adopting alternative survival assumptions for pirfenidone and BSC, sensitivity analyses were performed to explore uncertainty. These included the following: selecting alternative parametric distributions for pirfenidone and BSC; applying HRs derived from an NMA of treatments for IPF (see “Systematic Review and Network Meta-analysis of Idiopathic Pulmonary Fibrosis Treatments” on page S5 of this supplement) to determine life expectancy with BSC; and adopting a parametric approach and a more conservative piecewise approach to estimate life expectancy with pirfenidone.
Results
Baseline age, gender, and pulmonary function were well balanced between the pooled placebo arms of the ASCEND and CAPACITY trials25 and the matched Inova registry population (Table 1).
TABLE 1.
Pooled Baseline Characteristics of Patients in the ASCEND and CAPACITY Clinical Studies and the Inova Registry
| Pooled ASCEND and CAPACITY Studiesa | Inova Registry | ||
|---|---|---|---|
| Pirfenidone (N = 623) | Placebo (N = 624) | BSC (N = 286) | |
| Age, years | 68.0 (45-80) | 68.0 (40-80) | 67.0 (43-80) |
| Male, % | 74.3 | 74.5 | 81.8 |
| Caucasian, % | 95.0 | 94.6 | NA |
| FVC % predicted | 71.1 (48-124) | 70.3 (48-136) | 68.0 (50-114) |
| DLco % predicted | 44.0 (27-81) | 44.1 (27-170) | 46.0 (30-102) |
| 6MWD (m) | 400.0 (112-731) | 413.5 (163-716) | NA |
| UCSD SOBQ score | 31.0 (0-100) | 31.5 (0-105) | NA |
| FEV1/FVC ratio | 0.84 (0.69-0.99) | 0.84 (0.69-0.97) | 0.84 (0.70-1.15) |
Note: Data presented are median (range) or % unless otherwise stated.
aPooled CAPACITY and ASCEND data: Reproduced with permission of the European Respiratory Society. Eur Respir J. 2016;47(1):243-53.25
6MWD = 6-minute walk distance; BSC = best supportive care; DLco = carbon monoxide diffusing capacity; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; NA = not available; UCSD SOBQ = University of California, San Diego, Shortness of Breath Questionnaire.
Weibull distributions were selected as the best-fitting distributions because these distributions provided the best goodness-of-fit to the available pirfenidone and BSC survival data, and the extrapolated posttrial portions of the curves were deemed clinically plausible by 2 IPF experts who were in agreement (Toby M. Maher and Steven D. Nathan, coauthors of this publication; Table 2 and Figure 1). Internal validation confirmed that the Weibull distributions accurately compared with trial outcomes at 52 and 72 weeks for survival (Table 3).
TABLE 2.
Statistical Goodness-of-Fit for the Parametric Distributions Evaluated Based on Data from the ASCEND, CAPACITY, and RECAP Studies, and the Inova Fairfax Hospital Database
| Distribution | Information Criteriona | |
|---|---|---|
| Akaike | Bayesian | |
| Weibull | 849 | 857 |
| Log-logistic | 849 | 857 |
| Gompertz | 856 | 865 |
| Inverse-gamma | 850 | 863 |
| Exponential | 867 | 872 |
| Log-normal | 856 | 865 |
aLower information criterion value suggests better fit to the observed data.
FIGURE 1.
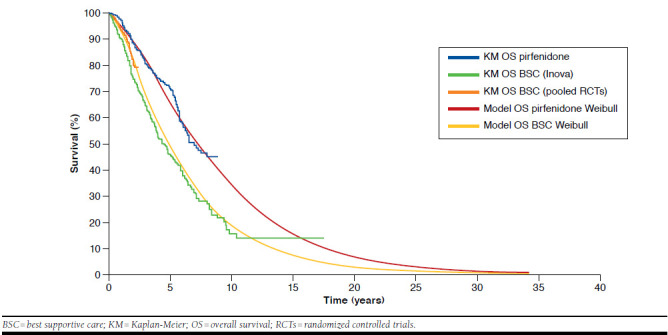
Kaplan-Meier Survival Data and Selected Parametric Survival Distributions
TABLE 3.
Internal Validation: Comparison of Weibull Distribution-Predicted Outcomes with Clinical Trial Outcomes (Pirfenidone) and Registry Data (BSC)
| Overall Survival | Pirfenidone | BSC | |||
|---|---|---|---|---|---|
| Weibull Distribution Prediction | Observed Data | Weibull Distribution Prediction | Observed Data (Clinical Trial) | Observed Data (Inova Registry) | |
| 52 weeks | 96 | 97 | 93 | 93 | 90 |
| 72 weeks | 93 | 94 | 87 | 91 | 84 |
Note: All values expressed in percentages.
BSC = best supportive care.
Using the Weibull model, mean life expectancy (95% CI) was calculated as 8.72 (7.65-10.15) years with pirfenidone and 6.24 (5.38-7.18) years with BSC. This suggests that for this population, pirfenidone results in a mean improvement in life expectancy of 2.47 (1.26-4.17) years compared with BSC (Table 4). Based on the parametric survival distributions, median survival was 7.25 years with pirfenidone and 4.67 years with BSC, which was a gain of 2.58 years (Table 4).
TABLE 4.
Comparison of Survival in Patients Receiving Pirfenidone and BSC Using the Weibull Distribution
| Survival (Years) | |||
|---|---|---|---|
| Pirfenidone | BSC | Difference | |
| Median | 7.25 | 4.67 | 2.58 |
| Mean (95% CI) | 8.72 (7.65-10.15) | 6.24 (5.38-7.18) | 2.47 (1.26-4.17) |
BSC = best supportive care; CI = confidence interval.
Mean life expectancy for a U.K. age- and gender-matched general population was calculated as 15.93 years using life tables from the Office of National Statistics.26 Therefore, patients with IPF receiving BSC lose 9.69 years of life compared with this general population. The current analysis demonstrated that treatment with pirfenidone could recuperate 25% (2.47 years/9.69 years × 100) of the expected years of life lost due to IPF compared with the general population (Figure 2).
FIGURE 2.
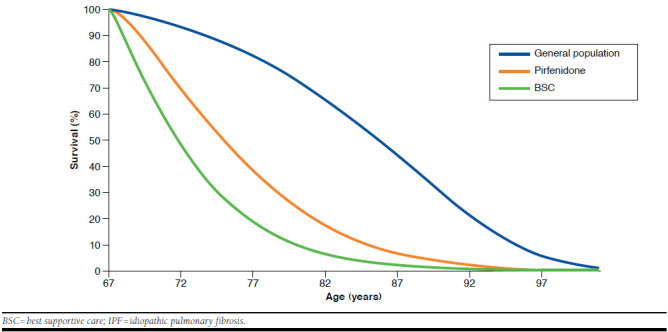
Survival with Pirfenidone and BSC in Patients with IPF Versus Survival in a Matched Population from the United Kingdom
Sensitivity analyses conducted to explore uncertainties using alternative parametric distributions (including the log-logistic, Gompertz, inverse-gamma, exponential, and lognormal distributions) all demonstrated a survival advantage for pirfenidone versus placebo, with the improvement in life expectancy with pirfenidone ranging between 1.95 and 5.34 years (Table 5).
TABLE 5.
Sensitivity Analyses of Survival in Patients Receiving Pirfenidone and BSC Using Alternative Distributions
| Sensitivity Analysis | Mean Survival (95% CI), Years | ||
|---|---|---|---|
| Pirfenidone | BSC | Difference | |
| Parametric Distributions for Pirfenidone and BSC | |||
| Log-logistic | 11.24 (9.87-12.82) | 7.72 (6.51-9.01) | 3.52 (1.86-5.57) |
| Gompertz | 8.35 (7.44-10.31) | 6.39 (5.54-7.96) | 1.95 (1.27-3.24) |
| Inverse-gamma | 9.53 (7.98-12.37) | 6.40 (5.44-7.58) | 3.12 (1.56-6.05) |
| Exponential | 11.95 (11.82-12.06) | 6.61 (6.54-6.68) | 5.34 (5.20-5.49) |
| Log-normal | 12.74 (10.93-14.40) | 8.76 (7.29-10.24) | 3.98 (1.79-6.14) |
| Alternative Methods for Calculating BSC Survival | |||
| Applying NMA HRa to the pirfenidone survival distribution | 8.72 (7.75-10.26) | 5.42 (3.16-8.15) | 3.29 (0.79-5.77) |
| Parametric approach; Weibull distributions calculated using only Inova registry data | 8.72 (7.66-10.19) | 5.88 (5.05-6.96) | 2.83 (1.54-4.46) |
| Alternative Methods for Calculating Pirfenidone Survival | |||
| Conservative piecewise approach; pirfenidone has the same effect on survival as BSC after 5 years | 7.79 (6.95-8.74) | 6.24 (5.39-7.33) | 1.54 (1.06-1.95) |
aSee “Systematic Review and Network Meta-analysis of Idiopathic Pulmonary Fibrosis Treatment” on page S5 of this supplement.
BSC = best supportive care; CI = confidence interval; HR = hazard ratio; NMA = network meta-analysis.
An NMA conducted by Fleetwood et al. estimated that, compared with placebo, pirfenidone reduced all-cause mortality by 48% over 12 months (HR = 0.52; 95% CI = 0.28-0.92; see “Systematic Review and Network Meta-analysis of Idiopathic Pulmonary Fibrosis Treatments” on page S5 of this supplement). A sensitivity analysis was used to calculate the BSC survival distribution by applying the HR from the NMA to the pirfenidone survival distribution. This resulted in a decrease in life expectancy with BSC versus the base-case analysis, resulting in an improvement of 3.29 years with pirfenidone compared with BSC (Table 5).
Using a Weibull parametric survival distribution fit to Kaplan-Meier survival data from the Inova registry to estimate survival with BSC resulted in a decrease in life expectancy with BSC compared with the base-case analysis. This resulted in a 2.83-year improvement in life expectancy with pirfenidone compared with BSC (Table 5).
Finally, a conservative sensitivity analysis employing a piecewise approach was conducted, which assumed that after 5 years, pirfenidone and BSC have the same effect on survival. This model demonstrated that pirfenidone would continue to maintain improvements in survival compared with BSC; however, the magnitude of the improvement in life expectancy was reduced to 1.54 years (Table 5).
Discussion
IPF is associated with significant disease burden and has a 5-year survival rate of only 20%-40%,8 and there exists a pressing clinical need for effective treatments that improve prognosis. Despite the high mortality associated with IPF, survival studies are clinically, financially, and ethically unrealistic or unjustifiable.13,14,27 Furthermore, most clinical studies in IPF are of limited duration. The possibility of a “trial effect,” whereby trial enrollment confers a survival benefit,28,29 and the fact that patients recruited to studies tend to have milder physiologic impairment than that seen in real-life cohorts should also be considered.21
In our survival analysis, mean survival was estimated to be 8.72 years with pirfenidone and 6.24 years with BSC; hence, pirfenidone improved life expectancy by an average of 2.47 years compared with BSC. The 95% CI for the magnitude of improvement suggested that treatment with pirfenidone could result in a minimum improvement of at least 1 year and a maximum improvement of more than 4 years compared with BSC. Sensitivity analyses found that pirfenidone was associated with a consistent improvement in life expectancy compared with BSC. Together with data from the Phase III clinical trials showing a mortality benefit of pirfenidone versus placebo after 52 weeks of treatment,15 our results show that pirfenidone could provide a tangible benefit to patients with IPF by improving their life expectancy.
The choice of the Weibull distribution was important in quantifying the magnitude of survival benefit with pirfenidone. NICE DSU guidelines21 were adhered to in the selection of the base-case survival distribution. Although the log-logistic distribution was found to have a similar goodness-of-fit to the Weibull distribution, it was not selected in the base case due to its long tail, which resulted in clinically unrealistic and optimistic survival expectations for patients receiving pirfenidone. Other distributions were found to have a significantly worse goodness-of-fit compared with the Weibull distribution and were therefore not chosen in the base case.
Median survival with BSC was calculated, using the Weibull distribution, as 4.67 years, which is at the upper end of the published estimates of 2-5 years,1-8 suggesting that the prediction of survival with pirfenidone is comparable with a clinically plausible prediction of survival with BSC. In addition, the median survival data associated with pirfenidone and BSC (7.25 years and 4.67 years, respectively) used in our analyses were similar to data used in analyses that used both the National Jewish Health Interstitial Lung Disease database (7.91 years and 4.70 years, respectively)30 and the Clinical Practice Research Datalink database (6.87 years and 3.41 years, respectively).31 However, the mean survival with BSC calculated from the Weibull distribution in our analysis (6.24 years) was considered to be high.
The length of survival time in IPF is affected by several factors, including the presence of comorbidities,32,33 the time since diagnosis,34 and the severity of disease at baseline.4 While the Inova registry data used to model long-term survival with BSC were taken from clinical practice and should be representative of real-world patients, the entry criteria for the ASCEND and CAPACITY studies were applied to the registry data to generate a population similar to that of the combined placebo arms of these trials. Therefore, patients with more severe disease at baseline would not have been included (only patients with FVC ≥ 50% and/or DLco ≥ 30% were included).
Limitations
The current analysis has limitations. The Inova registry was used to estimate survival with BSC past 52 weeks, which may under- or overestimate the survival expectations of patients receiving BSC. As an observational study, the Inova registry is subject to biases in terms of the selected population, interventions used as a BSC strategy, and the potential for comorbidities, all of which may affect survival outcomes.
In this study, biases with regard to the elected population were mitigated by only considering patients from the Inova registry who would meet the inclusion criteria from the ASCEND and CAPACITY studies. As a consequence, baseline characteristics were similar between the Inova registry and the placebo arms of the ASCEND and CAPACITY studies. Characteristics at 52 weeks could not be compared because these data were not available from the Inova registry. However, there is no clinical reason to suggest that disease characteristics would have been different between the 2 populations at 52 weeks, given that they had similar characteristics at baseline and neither population received any treatment that has been shown to slow disease progression.
A further potential limitation is that the definition of BSC in IPF has changed over time. One of the most important changes to BSC has been the rapid decline in the use of triple therapy with prednisolone, azathioprine, and N-acetylcysteine, following evidence that this combination may be harmful in patients with IPF.35 It should be noted that no patients in the Inova registry received prednisolone or azathioprine, and therefore biases related to the BSC strategy used are likely to be small.
Finally, survival outcomes in the Inova registry and the placebo arms of the ASCEND and CAPACITY studies were not identical at 52 or 72 weeks, which raises the question of whether the registry was appropriate for use in a piecewise methodology. It could be hypothesized that this difference is due to patients from the Inova registry having more life-threatening comorbidities compared with patients in the ASCEND and CAPACITY studies. Nevertheless, the Inova registry was found to be more representative of both baseline characteristics and survival outcomes compared with other registries investigated. Importantly, the estimated median survival using the registry was deemed clinically plausible and within the expectations of published estimates of survival with BSC.1-8
The possibility of a “trial effect” should be considered when estimating survival expectations of patients receiving pirfenidone, since trial enrollment may confer a survival benefit. For example, clinical trial populations are potentially healthier, with fewer comorbidities compared with populations observed in clinical practice.21,28,29 Nevertheless, survival outcomes in the placebo arms of the ASCEND and CAPACITY studies were similar to those of the real-life, observational Inova registry, suggesting that any potential “trial effect” did not greatly influence results.
Validation of the survival analysis would ideally have involved the use of other external data sources; however, IPF is a rare disease and there is a paucity of robust survival datasets. For patients treated with pirfenidone, survival data were only available from the studies that were used in the survival analysis, and therefore no other data were available for external validation.
It should also be noted that the NMA conducted by Fleetwood et al., which provided the HR used to calculate the BSC survival distribution in our sensitivity analyses, also used the same survival data as were used in our survival analysis. Similarly, of the registries evaluating patients with IPF receiving BSC, survival data from the most appropriate source were used in the survival analysis, and therefore no other data were available for external validation. As such, the survival analysis was validated by comparison of the Weibull distributions with the 2 primary data sources.
Given the challenges of conducting an adequately powered survival study in IPF, determining the most appropriate methods to predict survival for licensed IPF treatments is an important topic. Future research may consider collecting long-term survival data for pirfenidone in a real-world clinical setting to aid comparison with the long-term datasets currently available for BSC. In addition, information pertaining to the long-term survival expectations of nintedanib (the only other licensed treatment for IPF) would be useful for clinicians and decision makers, since the only information on the effects of nintedanib on mortality comes from the 2 pivotal Phase III INPULSIS studies, which found no significant effect of nintedanib on all-cause mortality after 12 months of treatment (HR = 0.70; 95% CI = 0.43-1.12; P = 0.14).17
Conclusions
Using the inclusion criteria of the patients enrolled in the ASCEND and CAPACITY studies, our survival analysis suggested that in this population of patients with IPF, treatment with pirfenidone improves life expectancy compared with BSC. The estimated magnitude of benefit with pirfenidone was substantial, suggesting that pirfenidone treatment improves life expectancy by 2.47 years compared with BSC. This could represent a 25% recuperation of the years of life lost due to IPF compared with the life expectancy in a corresponding general population.26
Acknowledgments
Medical writing support was provided by Katharine Howe on behalf of Complete Medical Communications, funded by F. Hoffmann-La Roche.
References
- 1.Strand MJ, Sprunger D, Cosgrove GP, et al.. Pulmonary function and survival in idiopathic vs secondary usual interstitial pneumonia. Chest. 2014;146(3):775-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Chen SY, Yeh WS, et al.. Idiopathic pulmonary fibrosis in U.S. Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566-72. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Lee JH, Ryu YJ, Chang JH.. Clinical predictors of survival in idiopathic pulmonary fibrosis. Tuberc Respir Dis (Seoul). 2012;73(3):162-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mura M, Porretta MA, Bargagli E, et al.. Predicting survival in newly diagnosed idiopathic pulmonary fibrosis: a 3-year prospective study. Eur Respir J. 2012;40(1):101-09. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Ryu JH, Elicker BM, et al.. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(12):1390-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan SD, Shlobin OA, Weir N, et al.. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011;140(1):221-29. [DOI] [PubMed] [Google Scholar]
- 7.Fernández Pérez ER, Daniels CE, Schroeder DR, et al.. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137(1):129-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DS, Collard HR, King TE Jr.. Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3(4):285-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society. Cancer facts & figures 2016. Available at: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed February 10, 2017.
- 10.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson J, Fogarty A, Hubbard R, McKeever T.. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795-806. [DOI] [PubMed] [Google Scholar]
- 12.Bradford WZ, Cohen AH, Leff JA.. Selection of clinically meaningful primary endpoints in phase 3 clinical trials in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2013;187(11):1269-70. [DOI] [PubMed] [Google Scholar]
- 13.du Bois RM, Nathan SD, Richeldi L, Schwarz MI, Noble PW.. Idiopathic pulmonary fibrosis: lung function is a clinically meaningful endpoint for phase III trials. Am J Respir Crit Care Med. 2012;186(8):712-15. [DOI] [PubMed] [Google Scholar]
- 14.King TE Jr, Albera C, Bradford WZ, et al.. All-cause mortality rate in patients with idiopathic pulmonary fibrosis. Implications for the design and execution of clinical trials. Am J Respir Crit Care Med. 2014;189(7):825-31. [DOI] [PubMed] [Google Scholar]
- 15.King TE Jr, Bradford WZ, Castro-Bernardini S, et al.. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083-92. [DOI] [PubMed] [Google Scholar]
- 16.Noble PW, Albera C, Bradford WZ, et al.. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760-69. [DOI] [PubMed] [Google Scholar]
- 17.Richeldi L, du Bois RM, Raghu G, et al.. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-82. [DOI] [PubMed] [Google Scholar]
- 18.Nathan S, Albera C, Bradford WZ, et al.. Effect of pirfenidone on treatment-emergent (TE) all-cause mortality (ACM) in patients with idiopathic pulmonary fibrosis (IPF): Pooled data analysis from ASCEND and CAPACITY. Eur Respir J. 2015;46(suppl 59). [Google Scholar]
- 19.Costabel U, Albera C, Lancaster L, Hormel P, Hulter H, Noble PW.. Final analysis of RECAP, an open-label extension study of pirfenidone in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2016;48. [Google Scholar]
- 20.Bradley B, Branley HM, Egan JJ, et al.. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl 5):v1-58. [DOI] [PubMed] [Google Scholar]
- 21.Latimer NR. Survival analysis for economic evaluations alongside clinical trials--extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making. 2013;33(6):743-54. [DOI] [PubMed] [Google Scholar]
- 22.ClinicalTrials.gov. Open-label study of the long term safety of pirfenidone in patients with idiopathic pulmonary fibrosis (IPF) (PIPF-012). 2016. Available at: https://clinicaltrials.gov/ct2/show/NCT00662038. Accessed February 10, 2017.
- 23.Costabel U, Albera C, Bradford WZ, et al.. Analysis of lung function and survival in RECAP: an open-label extension study of pirfenidone in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(3):198-205. [PubMed] [Google Scholar]
- 24.Raghu G, Collard HR, Egan JJ, et al.. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble PW, Albera C, Bradford WZ, et al.. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47(1):243-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Office for National Statistics. Patient characteristics from pooled trials applied to ONS data to simulate expected time of death for general population. 2014. [Google Scholar]
- 27.Wells AU, Behr J, Costabel U, Cottin V, Poletti V, Richeldi L.. Hot off the breath: mortality as a primary end-point in IPF treatment trials: the best is the enemy of the good. Thorax. 2012;67(11):938-40. [DOI] [PubMed] [Google Scholar]
- 28.Chow CJ, Habermann EB, Abraham A, et al.. Does enrollment in cancer trials improve survival? J Am Coll Surg. 2013;216(4):774-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unger JM, Barlow WE, Martin DP, et al.. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106(3):dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher M, Maher TM, Dolan P, Hill C, Marshall J.. Disease progression modeling in idiopathic pulmonary fibrosis: a prediction of time to disease progression and life expectancy with pirfenidone. Am J Respir Crit Care Med. 2015;191:A4413. [Google Scholar]
- 31.Roskell N, Saunders O, Lee D, Fisher M.. Long-term survival analysis: pirfenidone compared to standard care for the treatment of patients with idiopathic pulmonary fibrosis. Eur Respir J. 2014;44(Suppl 58):1905. [Google Scholar]
- 32.Kreuter M, Ehlers-Tenenbaum S, Palmowski K, et al.. Impact of comorbidities on mortality in patients with idiopathic pulmonary fibrosis. PLoS One. 2016;11(3):e0151425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyldgaard C, Hilberg O, Bendstrup E.. How does comorbidity influence survival in idiopathic pulmonary fibrosis? Respir Med. 2014;108(4):647-53. [DOI] [PubMed] [Google Scholar]
- 34.Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ.. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184(7):842-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghu G, Anstrom KJ, King TE Jr, Lasky JA, Martinez FJ.. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968-77. [DOI] [PMC free article] [PubMed] [Google Scholar]


