Abstract
Chondrosarcoma, a treatment-resistant cancer with limited therapeutic options, lacks significant advancements in treatment methods. However, PR-619, a novel inhibitor of deubiquitinating enzymes, has demonstrated anti-tumor effects in various malignancies. This study aimed to investigate the impact of PR-619 on chondrosarcoma both in vitro and in vivo. Two human chondrosarcoma cell lines, SW11353 and JJ012, were utilized. Cell viability was assessed using an MTT assay, while flow cytometry enabled the detection of apoptosis and cell cycle progression. Western blotting analyses were conducted to evaluate apoptosis, cell stress, and endoplasmic reticulum (ER) stress. Furthermore, the in vivo anti-tumor effects of PR-619 were examined using a xenograft mouse model. The results revealed that PR-619 induced cytotoxicity, apoptosis, and cell cycle arrest at the G0/G1 stage by activating caspases, PARP cleavage, and p21. Moreover, PR-619 increased the accumulation of polyubiquitinated proteins and ER stress by activating IRE1, GRP78, caspase-4, CHOP, and other cellular stress responses, including JNK activation. In vivo analysis demonstrated that PR-619 effectively inhibited tumor growth with minimal toxicity in the xenograft mouse model. These findings provide evidence of the anti-tumor effects and induction of cellular and ER stress by PR-619 in human chondrosarcoma, suggesting its potential as a novel therapeutic strategy for in human chondrosarcoma.
Keywords: Deubiquitinating enzyme, chondrosarcoma, apoptosis, endoplasmic reticulum stress
Introduction
Chondrosarcoma is the second most common primary bone sarcoma and accounts for ~30% of malignant bone tumors in humans worldwide. Chondrosarcomas are resistant to most conventional treatments, making surgical resection the only cure [1,2]; the prognosis for advanced chondrosarcomas that cannot be completely resected remains grim. Chondrosarcomas are commonly chemotherapy-resistant and do not respond to any standard chemotherapy regimens [3].
This resistance to chemotherapy of chondrosarcoma has been associated with multidrug-resistance 1 gene p-glycoprotein expression, increased expression of Bcl-2 family proteins, and poor vascularity resulting in poor delivery of anti-cancer agents to chondrosarcomas [4,5]. New therapeutic targets including Hedgehog, Src, PI3k-Akt-mTOR, histone deacetylase inhibitors, and angiogenesis have been proposed, but clinical evidence is still lacking. Thus, it is thus imperative to develop novel therapeutic targets to treat such highly malignant, unresectable, or metastatic diseases [6,7].
The balance between protein synthesis and degradation must be maintained to ensure the dynamic balance of protein-dependent cellular functions [8]. Ubiquitination is a post-translational modification of a protein. During ubiquitination, the substrates can be targeted and labeled with a key regulatory molecule, ubiquitin, for effective proteasome degradation [9,10]. Ubiquitin links to the target protein through a series of enzymes to precisely regulate the function of intracellular proteins and regulate many important physiological functions in cells. Furthermore, ubiquitin regulation involves activation, coupling, and ligation, requiring ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3) [9,10].
Ubiquitination is reversible through the action of a large group of proteases called deubiquitinating enzymes (DUBs) that function to remove ubiquitin chains from target proteins [11,12]; ~100 DUBs are encoded in the human genome [7]. Proteasome-related DUBs remove ubiquitin chains before the degradation of target proteins, since they sterically inhibit the translocation of a target protein to the 20S core [13]. DUB regulation has also been a promising target for cancer treatment [14]. Deubiquitinating enzyme inhibitors (DUBIs) trigger the accumulation of polyubiquitinated proteins that can cause DNA damage, apoptosis, autophagy, and numerous other abnormal cell responses in cancer cells [15,16]. Small-molecule DUBIs have been reported to be effective against various cancers [17,18], and pharmacological research has discovered several potential clinical applications of new DUB inhibitors or antagonists. However, the cytotoxic effects of DUBIs on chondrosarcomas have not been well-studied [19]. As a pan-DUB inhibitor, PR-619 is a promising anti-cancer drug with broad specificity [20,21]. Therefore, this study aimed to analyze cell cycle, cell survival stress, and apoptosis both in vitro and in vivo, using a xenograft mouse model to investigate the anti-tumor effects of PR-619 on chondrosarcomas and the underlying mechanisms through which it suppresses human chondrosarcoma.
Materials and methods
Cell cultures
Two human chondrosarcoma cell lines, namely JJ012 (provided by Dr. Sean P. Scully, School of Medicine, Miami University, USA) and SW1353 (from the Biological Resources Collection and Research Center, Taiwan), were used for the experiments during this study. SW1353 cells were maintained in an RPMI-1640 medium, and JJ012 cells were maintained in an L-15 medium, supplemented with 10% fetal bovine serum (Hyclone, Pittsburgh, PA, USA) and maintained at 37°C and 5% CO2 in a humidified atmosphere [19,22].
Reagents and antibodies
Different concentrations of PR-619 (MedChemExpress, Monmouth Junction, NJ, USA) were prepared as a suspension in Dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA). Western blotting analyses were performed using specific antibodies against various proteins, including phospho-histone H2A.X (Ser139, catalog No. 80312), cleaved caspase-3 (catalog No. 9664), cleaved PARP (Poly ADP-ribose Polymerase, catalog No. 5625), phospho-SAPK/JNK (stress-activated protein kinases/Jun amino-terminal kinases, Thr183/Tyr185, catalog No. 9255), CHOP (CCAAT-enhancer-binding protein homologous protein, catalog No. 2895), IRE1 (ER-resident transmembrane kinase-endoribonuclease inositol-requiring enzyme 1, catalog No. 3294), caspase-4 (catalog No. 4450), phospho-ERK (extracellular signal-regulated kinase, catalog No. 4370), AKT (protein kinase B, catalog No. 4685), phospho-AKT (catalog No. 4060), PCNA (proliferating cell nuclear antigen, catalog No. 13110), p21 (cyclin-dependent kinase inhibitor 1, catalog No. 2947) and phospho-histone H3 (catalog No. 4499), purchased from Cell Signaling Technology (Danvers, MA, USA). In addition, α-tubulin (catalog No. 112141) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; catalog No. 100118) antibodies were purchased from GeneTex (Irvine, CA, USA). Antibodies of ERK (catalog No. sc-514302), SAPK/JNK (catalog No. sc-9252), and β-actin (catalog No. sc-47778) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). All other reagents and chemicals were purchased from Sigma-Aldrich, Merck Millipore (Burlington, MA, USA), Thermo Fisher Scientific (Rockford, IL, USA), or Bio-Rad Laboratories (Hercules, CA, USA).
Measurement of cell viability
A 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine cell viability. Cell lines were cultured in a 96-well microtiter plate (5000 cells/well) and incubated at 37°C for 24 h before drug treatment. After various treatment, cells were treated with the MTT reagent at 37°C for 4 h, and the reduced crystals were dissolved in DMSO. Absorbance was then detected using the Thermo Multiskan FC microplate photometer (Thermo Fisher Scientific) at 570 nm [23].
Western blot analyses
Cells were washed with ice-cold PBS and lysed on ice using a cell lysis buffer (Cell Signaling Technology) for 15 min, followed by centrifugation at 4°C and 10,000 × g for 15 min. After collecting the clear supernatant, protein concentration was estimated using the BCA protein assay kit (Thermo Scientific). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed to analyze all samples, and the collected proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (GE Research, Niskayuna, NY, USA). The membrane was blocked for at least 1 h using 5% bovine serum albumin in Tris-buffered saline containing Tween (TBST), after which it was incubated with specific primary antibodies overnight at 4°C. The next day, the PVDF membrane was washed thrice with TBST for 10 min each, and then incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (GeneTex) for 1 h at 25°C. Finally, the antibody-bound PVDF membrane was incubated with the Immobilon ECL Ultra Western HRP Substrate (Merck Millipore) and was developed and visualized using GE ImageQuant LAS 4000. Moreover, the expression levels of target proteins were quantified using NIH Image J software (version 1.52q) with normalization to each internal control (i.e., α-tubulin, β-actin, and GAPDH) [23].
Apoptosis assay
The Muse Annexin V & Dead Cell Kit (Merck Millipore) was used for apoptosis detection, according to the manufacturer’s instructions. Flow cytometry (Muse Cell Analyzer, Merck Millipore) was used to detect and quantify the stained apoptotic cells thus obtained [24].
Cell cycle analysis via flow cytometry
Cells were seeded, cultured to 40% confluence, and treated with DMSO (i.e., the control) or PR-169 for 24 h. Cell cycle analysis was performed using a Muse Cell Cycle Assay Kit and a Muse Cell Analyzer flow cytometer (Merck Millipore) [25].
Cell proliferation assay
A commercial 5-bromo-2’-deoxyuridine (BrdU) incorporation assay (Merck Millipore) kit was used to evaluate the proliferation status of chondrosarcoma cells. After various treatments, an incorporation assay was performed according to the manufacturer’s protocol. The absorbance of each reaction was detected using the Thermo Multiskan FC microplate photometer at dual wavelengths of 450 and 540 nm [23].
In vivo xenograft experiments
A total of 5 × 105 JJ012 or SW1353 cells were suspended in 200 μL of serum-free medium and an equal amount of Matrigel (BD Biosciences, Bedford, MA, USA). The cell suspension thus derived was then injected subcutaneously into the dorsal side of 8-week-old male nude mice (National Laboratory Animal Center, Taipei, Taiwan). When the tumor grew to ~150 mm3, mice were randomly assigned to either the PR-619 treatment group or the control group. Mice in the treatment group received PR-619 10 mg/kg intraperitoneal injection twice daily for 48 days. Mice in the untreated control group received an injection of DMSO solution. Calipers were used to measure tumor volume twice a week [V = LD × (SD)2/2, where V = tumor volume, LD = longest diameter of the tumor, and SD = shortest diameter of the tumor] [23,25]. The serum testing of mice was conducted by the Animal Center at the College of Medicine, National Taiwan University. All studies involving animal experiments were approved by the National Taiwan University College of Medicine and College of Public Health Institutional Animal Care and Use Committee (IACUC) (No. 20180156) and followed ARRIVE guidelines.
Tissue array analysis
Tissue arrays (catalog number: T261b at Biomax, Rockville, MD, USA) containing normal cartilage tissue and chondrosarcoma tissues on the CREST coated glass slides from Matsunami Glass Industry (Osaka, Japan) were purchased from US Biomax-TissueArray.Com (Rockville, MD, USA). The human tissue microarray was subjected to immunohistochemical (IHC) staining with ubiquitin-specific protease 14 (USP14, catalog No. MA5-32821 from Thermo Fisher), ubiquitin-specific protease 21 (USP21, catalog No. 17856-1-AP from Proteintech, Rosemont, IL, USA) and ubiquitin C-terminal hydrolase L5 (UCHL5, catalog No. 11527-1-AP from Proteintech) antibodies, after which it was imaged using the TissueFAXS System and analyzed using the StrataQuest Analysis System (TissueGnostics, Vienna, Austria). A board-certified pathologist (Dr. Lin W.C.) assessed the immunoreactivity of the tissue.
Statistical analysis
GraphPad Prism 6 software was used for statistical analysis, and all data were expressed as mean ± standard deviation or standard error of the mean. Student’s t-test was used for statistical analyses.
Results
The expressions of deubiquitinating enzymes USP14, USP21 and UCHL5 increased in chondrosarcoma cells
Previous research has indicated that deubiquitinating enzymes (DUBs) exhibit increased expression levels in tumor cells. Therefore, the objective of this study was to examine the expression of DUBs specifically in chondrosarcomas. For this purpose, three representative DUBs were identified: ubiquitin-specific protease 14 (USP14), ubiquitin-specific protease 21 (USP21), and ubiquitin C-terminal hydrolase L5 (UCHL5). Immunohistochemistry (IHC) staining was employed on clinical samples utilizing tissue arrays. The results demonstrated a significant upregulation of USP14, USP21, and UCHL5 in chondrosarcoma tumor tissues (Figure 1A and 1B) compared to normal cartilage (Figure 1C-H). These findings suggest that DUBs may contribute to the development and progression of chondrosarcomas.
Figure 1.
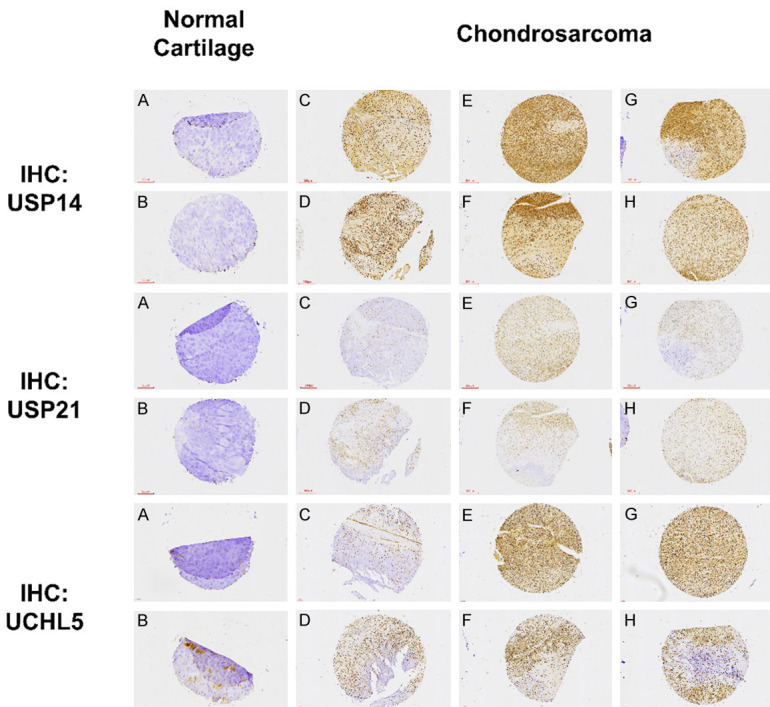
Increased expressions of USP14, USP21 and UCHL5 deubiquitinating enzymes in chondrosarcoma cells (A and B) compared to those in normal human cartilage (C-H). Tissue array slides were subjected to immunohistochemical analysis by using anti-USP14, anti-USP21 and anti-UCHL5 antibodies; all sections were digitally processed at 200 × magnification.
USP21, USP14, and UCHL5 have been found to regulate molecules involved in cell cycle regulation and cell proliferation. Inhibition of these enzymes in cancer cells has demonstrated the induction of cell death, even in the case of bone tumors [26-29]. In chondrosarcoma, DUBs are known to be upregulated, and this upregulation is thought to contribute to tumor progression [30-32]. PR-619, a broad-spectrum inhibitor of DUBs including USP21, USP14, and UCHL5, has displayed promising potential in suppressing the growth of chondrosarcomas [28]. By specifically targeting and inhibiting DUBs, PR-619 presents a potential therapeutic approach for controlling the progression of chondrosarcomas.
PR-619 reduces cell viability and induces apoptosis of chondrosarcoma cells
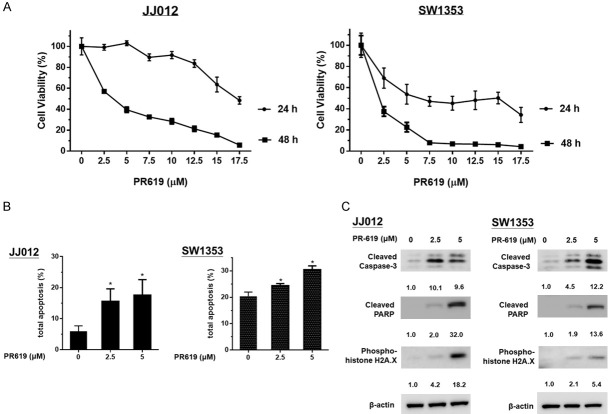
Then we examined the anti-tumor effects of PR-619 on the JJ012 and SW1353 human chondrosarcoma cell lines, with PR-619 concentrations ranging from 2.5 to 17.5 μM for 24 and 48 h. PR-619 significantly reduced the viability of chondrosarcoma cells in a dose- and time-dependent manner (see Figure 2A). Annexin V-FITC/PI labeling flow cytometry was used to analyze the apoptotic effects of PR-619-on chondrosarcoma cells; PR-619 treatment (2.5 μM and 5 μM) for 48 h significantly triggered apoptosis in chondrosarcoma cells (see Figure 2B). In addition, PR-619 treatment on both chondrosarcoma cells lines for 48 h resulted in the cleavages of caspase-3, PARP, and histone H2A.X (a DNA damage marker, see Figure 2C).
Figure 2.
PR-619 inhibited cell viability and induced apoptosis in human chondrosarcoma cells. A. JJ012 and SW1353 cells were treated with 2.5 and 5 μM of PR-619 for 24 and 48 h, respectively, after which they were harvested for MTT assays. B. JJ012 and SW1353 cells were treated with 2.5 and 5 μM PR-619 for 48 h; DMSO treatment was considered the untreated control group. Flow-assisted cell sorting with propidium iodide and annexin V-FITC staining was used to quantify apoptotic cells. C. Cell lysates were harvested after PR-619 treatment for 48 h. Following this, the expressions of cleaved caspase-3, cleaved PARP, and phosphorylated histone H2A.X were analyzed via Western blotting analysis. Results are representative of three independent experiments and data are presented as mean ± SD values; *P<0.05 was considered significant as compared with the control. Full-length blots are presented in Figure S1.
PR-619 increases the accumulation of polyubiquitinated proteins, induces cellular stress and endoplasmic reticulum stress-related apoptosis in chondrosarcoma cells
Imbalances in protein homeostasis can cause cellular stress and protein-folding stress within the endoplasmic reticulum (ER) [33], which is involved in many the progress in many cancers. ER stress is buffered by activating the unfolded protein response (UPR), a homeostatic signaling network designed to recover ER function. As a pan DUB inhibitor, PR-619 interrupts protein turnover within the ubiquitin-proteasome system and further aggravates both cellular and ER stress; this results in apoptosis if cells fail to adapt to ER stress caused by excess unfolded proteins [34]. To investigate the effects of PR-619, a drug known to accumulate polyubiquitinated proteins [35], we treated the chondrosarcoma cell lines JJ012 and SW1353 with 5 μM PR-619 for 48 hours. After treatment, we performed Western blot analysis using an anti-ubiquitin antibody. The results showed a significant increase in polyubiquitination levels in the cells exposed to PR-619 (Figure 3A). In addition, our results show that PR-619 was responsible for activating cellular stress-related molecules, such as phosphorylated SAPK/JNK, in a dose-dependent manner (Figure 3B). Additionally, PR-619 induced ER stress via the up-regulation of IRE-1, GPR78, CHOP and caspase-4, all of which are related to ER stress-induced apoptosis (Figure 3C) [36].
Figure 3.
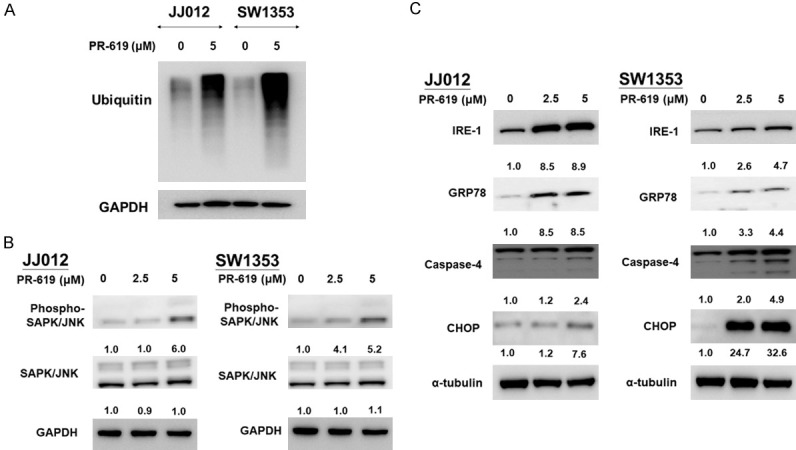
PR-619 induced cellular stress and ER stress-related apoptosis in chondrosarcoma cells. JJ012 and SW1353 cells were treated with 2.5 and 5 μM PR-619, respectively, or DMSO for the nontreated control, for 48 h. A, B. Cell lysates were harvested and analyzed using Western blot analysis with specific antibodies against ubiquitin, phspho-SAPK/JNK and SAPK/JNK. C. Expressions of IRE-1, GRP78, caspase-4, and CHOP in total cell lysates analyzed using western blotting analyses; all results are representative of at least three independent experiments. Full-length blots are presented in Figure S2.
PR-619 inhibits cell proliferation and cell cycle progress via ERK and AKT inactivation
The impact of PR-619 on cell proliferation was examined using the bromodeoxyuridine (BrdU) incorporation assay. Treatment with 5 μM of PR-619 for 48 h significantly reduced cell proliferation in human chondrosarcoma cells (Figure 4A). The effects of PR-619 on cycle progression in chondrosarcoma cells were analyzed through flow cytometric analyses; PR-619 induced cell cycle arrest at the G0/G1 stage in chondrosarcoma cells (see Figure 4B). The ERK-MAPK and PI3K/AKT/mTOR pathways are involved in cell differentiation, proliferation, and cell cycle, having been demonstrated to promote tumorigenesis. ERK or AKT inhibitors are effective in treating various human cancers [37,38], and the dual inhibition of the ERK and AKT signaling pathways has synergistically inhibited chondrosarcoma growth [33]. Western blot analyses showed that PR-619 inactivated phospho-ERK and phospho-AKT without any effect on the expression of pan ERK and pan AKT (Figure 4C). In addition, PR-619 increased the expression levels of p21 while suppressing the expression of phosphor-histone H3 and the PCNA cell proliferation marker in cell cycle regulatory proteins (Figure 4C).
Figure 4.
PR-619 inhibited cell proliferation and induced cell cycle arrest at G0/G1 in chondrosarcoma cells. JJ012 and SW1353 cells were treated with 5 μM PR-619 and DMSO (for the untreated control) for 48 h. A. Cell proliferation was analyzed using the BrdU assay. B. Flow cytometric analyses were used for cell cycle progression and quantitative data are presented as the means ± SD from at least three independent experiments; *P<0.05 was considered significant as compared with the control. C. JJ012 and SW1353 cells were treated with 2.5 and 5 μM PR-619, respectively, with DMSO for the nontreated control, for 48 h. Cell lysates were harvested for western blot analyses with specific antibodies for phospho-AKT, AKT, phospho-ERK, ERK, p21, phospho-histone H3, and PCNA; all results are representative of at least three independent experiments. Full-length blots are presented in Figure S3.
PR-619 suppresses tumor growth of in vivo human chondrosarcoma xenograft
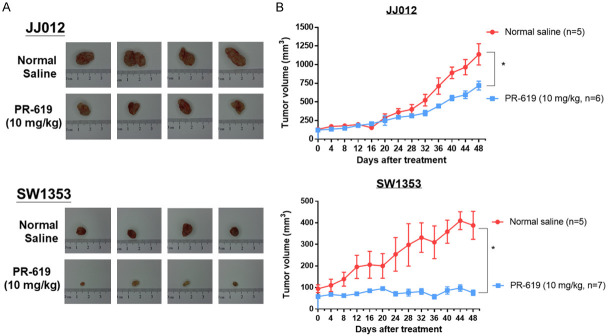
Furthermore, this study evaluated the anti-tumor effects of in vivo PR-619 treatment using a xenograft mouse model. JJ012 and SW1353 cells were mixed with Matrigel and injected subcutaneously into the flanks of nude mice. PR-619 showed significant anti-tumor effects on xenograft tumors from both cell lines (see Figure 5A and 5B). To monitor the toxicity of PR-619 in mice, body weight was measured every 8 days. At the end of the experiment, blood was collected, and serum was separated to measure aspartate aminotransferase (abbreviated as AST or GOT) and creatinine levels. These measurements served as indicators of potential liver and kidney toxicity, respectively. There were no significant changes in body weight, serum GOT levels, or serum creatinine levels between the normal saline group and the PR-619 group throughout the treatment period. This suggests that PR-619 did not cause any apparent toxicity to the mice (see Figure S4).
Figure 5.
PR-619 suppressed tumor growth in an in vivo human chondrosarcoma xenograft. Nude mice inoculated with JJ012 and SW1353 xenografts were treated twice daily for 48 days with DMSO/saline (untreated control group, n=5) and PR-619 (10 mg/kg, i.p. n=5). A. Tumor images representing excised tumors from each group. B. Tumor volume for each group during three weeks of treatment; data are presented as means ± standard error of mean values; *P<0.05 was considered significant as compared with the control (normal saline group).
Discussion
The ubiquitination of proteins plays a key role in cellular signal transduction pathways and mediates protein stability. Ubiquitin-proteasome system (UPS) dysregulation has been reported to be associated with various cancers [39]; cancer cells take advantage of the UPS by alternating the degradation of specific proteins, thereby promoting cancer proliferation and reducing apoptosis [17,39]. UPS processes are thus potential anti-cancer targets. DUBs are proteases that reverse protein ubiquitination and are crucial for maintaining cellular protein homeostasis; ~100 DUBs are encoded within the human genome and have been identified as promising targets for cancer therapy [18,19]. PR-619 has previously been reported to exert a broad DUB-inhibitory profile that results in the accumulation of polyubiquitinated proteins and 26S proteasome complexes [21,35,40]. PR-619 effectively treats some cancers [18,35,41]. This study is the first to indicate that chondrosarcomas are a good candidate for DUB inhibitor treatment.
When cells in our study encountered stress, protein synthesis or degradation was initiated to cope with the stress [35]. Meanwhile, the dysregulated protein synthesis, modification, and degradation triggered ER stress and unfolded protein response (UPR). If ER stress is intense and irreversible, or the adaptive response fails, apoptosis would occur. UPR was reported to be associated with the progress of chondrosarcoma [22,42]. Targeting the pathway to disturb protein homeostasis and potentiate ER stress is a promising strategy for chondrosarcoma treatment. In this study, we noted that PR-619 aggravated ER stress with activation of IRE-1 and GRP78 and triggered ER stress-related downstream apoptotic effectors, such as caspase-4 and CHOP. The present study confirmed our assumption that DUBs were a promising therapeutic target for the treatment of chondrosarcoma.
The ERK-MAPK and the PI3K/AKT/mTOR pathways play essential roles in cell differentiation, proliferation, and cell cycle progression. ERK and AKT have been attributed to many malignancies. Both ERK and AKT inhibitors are effective in the treatment of a variety of human cancers [6,43,44]. This study found that PR-619 treatment resulted in decreased ERK and AKT phosphorylation. PR-619 also induced cell cycle arrest at the G0/G1 stage, thereby increasing p21 expression and decreasing the levels of phospho-histones H3 and PCNA. The results suggest that PR-619 significantly inhibits molecules that promote cell growth in the MAPK/ERK and AKT pathways. However, the precise roles of AKT and ERK in the anti-tumor effect of DUBs inhibitors are yet to be explored.
Conclusions
In conclusion, this study showed that PR-619 induced cytotoxicity, cell cycle arrest, ER stress, and ER stress-related apoptosis in chondrosarcoma cells both in vitro and in vivo. DUB inhibition is potentially a promising target for chondrosarcoma therapy. Further studies for preclinical or clinical trials of new DUB inhibitors with high specificity may confirm the clinical efficacy of DUB inhibitors on chondrosarcoma treatments. Our findings thus provide important evidence for future clinical trials involving DUB inhibitors for treating human chondrosarcomas.
Acknowledgements
This research was funded by the Taiwan National Science and Technology Council (grant numbers: MOST 110-2314-B-006-020 and MOST 108-2314-B-006-048-MY), and National Taiwan University Hospital (111-X0048, 111S3037, MS316). We would like to acknowledge the staff of National Cheng Kung University Hospital, Taipei City Hospital, Taiwan Health Foundation, Taiwan Digestive Transplantation Foundation, and the National Taiwan University Hospital - Far Eastern Hospital Joint Research Program for their administrative and experimental assistances. We also appreciate the staff of the Second, Third and Sixth Core Lab, Department of Medical Research, National Taiwan University Hospital for technical support during this research.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Chow WA. Chondrosarcoma: biology, genetics, and epigenetics. F1000Res. 2018;7:F1000 Faculty Rev-1826. [Google Scholar]
- 2.Weinschenk RC, Wang WL, Lewis VO. Chondrosarcoma. J Am Acad Orthop Surg. 2021;29:553–562. doi: 10.5435/JAAOS-D-20-01188. [DOI] [PubMed] [Google Scholar]
- 3.Monga V, Mani H, Hirbe A, Milhem M. Non-conventional treatments for conventional chondrosarcoma. Cancers (Basel) 2020;12:1962. doi: 10.3390/cancers12071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyman JJ, Hornstein AM, Meitner PA, Mak S, Verdier P, Block JA, Pan J, Terek RM. Multidrug resistance-1 and p-glycoprotein in human chondrosarcoma cell lines: expression correlates with decreased intracellular doxorubicin and in vitro chemoresistance. J Orthop Res. 1999;17:935–940. doi: 10.1002/jor.1100170619. [DOI] [PubMed] [Google Scholar]
- 5.van Oosterwijk JG, Herpers B, Meijer D, Briaire-de Bruijn IH, Cleton-Jansen AM, Gelderblom H, van de Water B, Bovee JV. Restoration of chemosensitivity for doxorubicin and cisplatin in chondrosarcoma in vitro: BCL-2 family members cause chemoresistance. Ann Oncol. 2012;23:1617–1626. doi: 10.1093/annonc/mdr512. [DOI] [PubMed] [Google Scholar]
- 6.Chen JC, Huang C, Lee IN, Wu YP, Tang CH. Amphiregulin enhances cell migration and resistance to doxorubicin in chondrosarcoma cells through the MAPK pathway. Mol Carcinog. 2018;57:1816–1824. doi: 10.1002/mc.22899. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald IJ, Lin CY, Kuo SJ, Su CM, Tang CH. An update on current and future treatment options for chondrosarcoma. Expert Rev Anticancer Ther. 2019;19:773–786. doi: 10.1080/14737140.2019.1659731. [DOI] [PubMed] [Google Scholar]
- 8.Cockram PE, Kist M, Prakash S, Chen SH, Wertz IE, Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28:591–605. doi: 10.1038/s41418-020-00708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansour MA. Ubiquitination: friend and foe in cancer. Int J Biochem Cell Biol. 2018;101:80–93. doi: 10.1016/j.biocel.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Islam MT, Zhou X, Chen F, Khan MA, Fu J, Chen H. Targeting the signalling pathways regulated by deubiquitinases for prostate cancer therapeutics. Cell Biochem Funct. 2019;37:304–319. doi: 10.1002/cbf.3401. [DOI] [PubMed] [Google Scholar]
- 11.Sun T, Liu Z, Yang Q. The role of ubiquitination and deubiquitination in cancer metabolism. Mol Cancer. 2020;19:146. doi: 10.1186/s12943-020-01262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mennerich D, Kubaichuk K, Kietzmann T. DUBs, hypoxia, and cancer. Trends Cancer. 2019;5:632–653. doi: 10.1016/j.trecan.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Deng L, Meng T, Chen L, Wei W, Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther. 2020;5:11. doi: 10.1038/s41392-020-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrigan JA, Jacq X, Martin NM, Jackson SP. Deubiquitylating enzymes and drug discovery: emerging opportunities. Nat Rev Drug Discov. 2018;17:57–78. doi: 10.1038/nrd.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J, Cho J, Song EJ. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch Pharm Res. 2020;43:1144–1161. doi: 10.1007/s12272-020-01281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poondla N, Chandrasekaran AP, Kim KS, Ramakrishna S. Deubiquitinating enzymes as cancer biomarkers: new therapeutic opportunities? BMB Rep. 2019;52:181–189. doi: 10.5483/BMBRep.2019.52.3.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaPlante G, Zhang W. Targeting the ubiquitin-proteasome system for cancer therapeutics by small-molecule inhibitors. Cancers (Basel) 2021;13:3079. doi: 10.3390/cancers13123079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu FS, Lin WC, Kuo KL, Chiu YL, Hsu CH, Liao SM, Dong JR, Liu SH, Chang SC, Yang SP, Chen YT, Chang RJ, Huang KH. PR-619, a general inhibitor of deubiquitylating enzymes, diminishes cisplatin resistance in urothelial carcinoma cells through the suppression of c-Myc: an in vitro and in vivo study. Int J Mol Sci. 2021;22:11706. doi: 10.3390/ijms222111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao SC, Chen YJ, Huang KH, Kuo KL, Yang TH, Huang KY, Wang CC, Tang CH, Yang RS, Liu SH. Induction of sirtuin-1 signaling by resveratrol induces human chondrosarcoma cell apoptosis and exhibits antitumor activity. Sci Rep. 2017;7:3180. doi: 10.1038/s41598-017-03635-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo KL, Liu SH, Lin WC, Chow PM, Chang YW, Yang SP, Shi CS, Hsu CH, Liao SM, Chang HC, Huang KH. The deubiquitinating enzyme inhibitor PR-619 enhances the cytotoxicity of cisplatin via the suppression of anti-apoptotic Bcl-2 protein: in vitro and in vivo study. Cells. 2019;8:1268. doi: 10.3390/cells8101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Li M, Sha B, Hu X, Sun Y, Zhu M, Xu Y, Li P, Wang Y, Guo Y, Li J, Shi J, Li P, Hu T, Chen P. Inhibition of deubiquitination by PR-619 induces apoptosis and autophagy via ubi-protein aggregation-activated ER stress in oesophageal squamous cell carcinoma. Cell Prolif. 2021;54:e12919. doi: 10.1111/cpr.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu MH, Lee CY, Huang TJ, Huang KY, Tang CH, Liu SH, Kuo KL, Kuan FC, Lin WC, Shi CS. MLN4924, a protein neddylation inhibitor, suppresses the growth of human chondrosarcoma through inhibiting cell proliferation and inducing endoplasmic reticulum stress-related apoptosis. Int J Mol Sci. 2018;20:72. doi: 10.3390/ijms20010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo KL, Ho IL, Shi CS, Wu JT, Lin WC, Tsai YC, Chang HC, Chou CT, Hsu CH, Hsieh JT, Chang SC, Pu YS, Huang KH. MLN4924, a novel protein neddylation inhibitor, suppresses proliferation and migration of human urothelial carcinoma: in vitro and in vivo studies. Cancer Lett. 2015;363:127–136. doi: 10.1016/j.canlet.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Kuo KL, Lin WC, Liu SH, Hsu FS, Kuo Y, Liao SM, Yang SP, Wang ZH, Hsu CH, Huang KH. THZ1, a covalent CDK7 inhibitor, enhances gemcitabine-induced cytotoxicity via suppression of Bcl-2 in urothelial carcinoma. Am J Cancer Res. 2021;11:171–180. [PMC free article] [PubMed] [Google Scholar]
- 25.Chow PM, Liu SH, Chang YW, Kuo KL, Lin WC, Huang KH. The covalent CDK7 inhibitor THZ1 enhances temsirolimus-induced cytotoxicity via autophagy suppression in human renal cell carcinoma. Cancer Lett. 2020;471:27–37. doi: 10.1016/j.canlet.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Kim MJ, Huang Y, Park JI. Targeting Wnt signaling for gastrointestinal cancer therapy: present and evolving views. Cancers (Basel) 2020;12:3638. doi: 10.3390/cancers12123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Wang L, Cheng X, Ge X, Wang P. An ultrasensitive system for measuring the USPs and OTULIN activity using Nanoluc as a reporter. Biochem Biophys Res Commun. 2014;455:178–183. doi: 10.1016/j.bbrc.2014.10.139. [DOI] [PubMed] [Google Scholar]
- 28.Altun M, Kramer HB, Willems LI, McDermott JL, Leach CA, Goldenberg SJ, Kumar KG, Konietzny R, Fischer R, Kogan E, Mackeen MM, McGouran J, Khoronenkova SV, Parsons JL, Dianov GL, Nicholson B, Kessler BM. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem Biol. 2011;18:1401–1412. doi: 10.1016/j.chembiol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Cui K, Prochownik EV, Li Y. The deubiquitinase USP21 stabilizes MEK2 to promote tumor growth. Cell Death Dis. 2018;9:482. doi: 10.1038/s41419-018-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Chen Z, Tang Q, Wang Z, Lu J, You Y, Wang H. USP21 promotes self-renewal and tumorigenicity of mesenchymal glioblastoma stem cells by deubiquitinating and stabilizing FOXD1. Cell Death Dis. 2022;13:712. doi: 10.1038/s41419-022-05163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullard M, Lavaud M, Regnier L, Tesfaye R, Ory B, Redini F, Verrecchia F. Ubiquitin-specific proteases as therapeutic targets in paediatric primary bone tumours? Biochem Pharmacol. 2021;194:114797. doi: 10.1016/j.bcp.2021.114797. [DOI] [PubMed] [Google Scholar]
- 32.Shukla N, Somwar R, Smith RS, Ambati S, Munoz S, Merchant M, D’Arcy P, Wang X, Kobos R, Antczak C, Bhinder B, Shum D, Radu C, Yang G, Taylor BS, Ng CK, Weigelt B, Khodos I, de Stanchina E, Reis-Filho JS, Ouerfelli O, Linder S, Djaballah H, Ladanyi M. Proteasome addiction defined in ewing sarcoma is effectively targeted by a novel class of 19S proteasome inhibitors. Cancer Res. 2016;76:4525–4534. doi: 10.1158/0008-5472.CAN-16-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Tan Z, Yang Y. Negative feedback and modern anti-cancer strategies targeting the ER stress response. FEBS Lett. 2020;594:4247–4265. doi: 10.1002/1873-3468.14000. [DOI] [PubMed] [Google Scholar]
- 34.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 35.Seiberlich V, Goldbaum O, Zhukareva V, Richter-Landsberg C. The small molecule inhibitor PR-619 of deubiquitinating enzymes affects the microtubule network and causes protein aggregate formation in neural cells: implications for neurodegenerative diseases. Biochim Biophys Acta. 2012;1823:2057–2068. doi: 10.1016/j.bbamcr.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2015;47:146–147. doi: 10.1093/abbs/gmu128. [DOI] [PubMed] [Google Scholar]
- 37.Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79:1019–1031. doi: 10.1158/0008-5472.CAN-18-2738. [DOI] [PubMed] [Google Scholar]
- 38.Barbosa R, Acevedo LA, Marmorstein R. The MEK/ERK network as a therapeutic target in human cancer. Mol Cancer Res. 2021;19:361–374. doi: 10.1158/1541-7786.MCR-20-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Yu C, Kang R, Kroemer G, Tang D. Cellular degradation systems in ferroptosis. Cell Death Differ. 2021;28:1135–1148. doi: 10.1038/s41418-020-00728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seiberlich V, Borchert J, Zhukareva V, Richter-Landsberg C. Inhibition of protein deubiquitination by PR-619 activates the autophagic pathway in OLN-t40 oligodendroglial cells. Cell Biochem Biophys. 2013;67:149–160. doi: 10.1007/s12013-013-9622-8. [DOI] [PubMed] [Google Scholar]
- 41.Soji K, Doi S, Nakashima A, Sasaki K, Doi T, Masaki T. Deubiquitinase inhibitor PR-619 reduces Smad4 expression and suppresses renal fibrosis in mice with unilateral ureteral obstruction. PLoS One. 2018;13:e0202409. doi: 10.1371/journal.pone.0202409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohberger B, Steinecker-Frohnwieser B, Stuendl N, Kaltenegger H, Leithner A, Rinner B. The proteasome inhibitor bortezomib affects chondrosarcoma cells via the mitochondria-caspase dependent pathway and enhances death receptor expression and autophagy. PLoS One. 2016;11:e0168193. doi: 10.1371/journal.pone.0168193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandhanayingyong C, Kim Y, Staples JR, Hahn C, Lee FY. MAPK/ERK signaling in osteosarcomas, ewing sarcomas and chondrosarcomas: therapeutic implications and future directions. Sarcoma. 2012;2012:404810. doi: 10.1155/2012/404810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu M, Ying J, Lin C, Wang Y, Huang K, Zhou Y, Teng H. Baicalin induces apoptotic death of human chondrosarcoma cells through mitochondrial dysfunction and downregulation of the PI3K/Akt/mTOR pathway. Planta Med. 2019;85:360–369. doi: 10.1055/a-0791-1049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.