Abstract
The incidence of early-onset pancreatic cancer (EOPC) among young population (<50 years) is rising in the last decade, with gender, medical overtreatment, and genetic factors as the risk factors in EOPC. Nevertheless, the role of genetic factors in the development of EOPC needs further exploration since the studies were carried out with small sample size and ambiguous evidence. Notable, the high incidence of pathogenic germline variant (PGV) appears to be involved in EOPC. Compared with average-age-onset pancreatic cancer (AOPC), EOPC patients display a distinctive genomic feature on several well-known tumor suppressor and oncogenic genes including, including SMAD4, RAS wild wild-type, CDKN2A BRCA1, BRCA2 and FOXC2, which is different from the findings of studies with AOPC and LOPC, suggesting the dynamic evolving entity of EOPC. In addition, the potential gender-related incidence found in several countries also suggests the involvement of genetic or socioenvironmental factors in the development of AOPC. Therefore, further prospective epidemiological and molecular studies are warranted to elucidate the shifting epidemiology of this disease and, most importantly, to better exploit the opportunities for the early diagnosis of the disease.
Keywords: Early-onset pancreatic cancer, genomic landscape, pancreatic ductal adenocarcinoma, pathogenic germline variant
Introduction
Pancreatic cancer (PC) is the 12th most common malignant tumor and the 7th most common cause of cancer death [1], posting a considerable economic and social burden with an annual estimate of death and disability-adjusted life years around 9.1 million worldwide in 2017 [2], due to its extremely aggressive nature and poor survival situation [3]. Among PC, the early-onset pancreatic cancer (EOPC), which is defined as the age at onset <50 years to 55 years, accounts for 5%-12% of all PC diagnosed, and its incidence has been rising significantly compared to the later onset pancreatic cancer [4-6].
In this minireview, we have summarized the epidemiology trends and the clinical features of EOPC, as well as have outlined the distinctive molecular characteristics of EOPC.
Epidemiology features and worldwide trends of EOPC
According to the US cancer statistics between 1995-2014, among the 12 obesity-related cancers in young adults (<50 years), the incidence of the 6 gastrointestinal tract-related cancers including PC has increased significantly in young generation [6]. PC is one of the main reasons of cancer-associated death and is predicted to be the second leading cause of cancer-associated death in the United States by 2040. It is projected that about 62,210 cases, including 32,970 men and 29,240 women in the US, will be diagnosed with pancreatic cancer with about 49,830 deaths (25,970 men and 23,860 women) in 2022 [7]. There has been a study using the data from the GLOBOCAN database to estimate the PC incidence and mortality in 184 countries and to evaluate the change in age-standardized rate (ASR) of incidence or mortality associated with 1% increase of a certain risk factor of pancreatic cancer. The results show that for individuals younger than 50 years, the Average Annual Percent Change (AAPC) of pancreatic cancer is increased in 8 countries: Germany, Sweden, the Netherlands, the United Kingdom, Canada, Czech Republic, Turkey, and Australia. Similar patterns are also observed in individuals younger than 40 years, in which the AAPC of pancreatic cancer increased in 4 countries: Netherlands, Canada, France, and the United Kingdom, respectively. Strikingly, a significant increase in the incidence of EOPC is observed in women compared with men in the same age group in 5 countries (Austria, the Czech Republic, Germany, the Netherlands, and the UK). The authors have further analyzed the lifestyle and metabolic risk factors that are associated with the incidence and the mortality of pancreatic cancer for each country in detail and found that the high prevalence of smoking, alcohol drinking, physical inactivity, obesity, and hypertension, as well as the high level of cholesterol contribute to the higher incidence and mortality [8].
Clinical characteristics and risk factors of EOPC
Notably, women are more susceptible than man to develop EOPC. A significant increase in AAPC occurs among women with EOPC (1.93%, 95% CI: 1.57%-2.28%, P<0.001) compared to that in men (0.77%, 95% CI: 0.50%-1.05%, P<0.001) with unequal trends (P=0.002). Women at 35-54 or 15-34 years show higher increasing rate of the disease than men [9]. Compared to that of LOPC, the annual age-adjusted incidence rate (AAIR) of EOPC increases greatly in females. In addition, the patients of EOPC experience a higher burden of pancreatic cancer, with more frequency of surgery although the treatment of radiation and chemotherapy is similar [10].
Importantly, it should be noted that the current therapeutic approach for local-regional stage disease mismatches with the standard treatment guidelines for pancreatic cancer. Compared with average-age-onset pancreatic cancer (AOPC), more chemotherapy (38% vs. 29%), surgery (9% vs. 6.9%), chemoradiation (12% vs. 9.2%), and multimodal treatment (21% vs. 15%) have been applied to patients with EOPC. In contrast to 39% of patients with AOPC who receive no treatment, only 19% of patients with EOPC receive no treatment. However, regardless of treatment or without treatment, the overall survival of patients with EOPC is better than that of patients with AOPC across all stages of the disease [11]. Furthermore, smoking and heavy drinking have been confirmed to be correlated with the development of EOPC. Compared with late-onset pancreatic cancer (LOPC, >50 years old), patients with EOPC show more advanced TNM stage and higher neutrophil-to-lymphocyte ratios. In addition, the patients of EOPC are more likely to have hepatic metastases than patients with LOPC (42% vs. 23%, P=0.015), although the survival outcomes are similar between EOPC and LOPC. For patients with metastatic lesions, combination chemotherapy regimens are considered as the first-line treatment and are frequently applied to the treatment of patients with EOPC than LOPC patients (79% vs. 64%, P=0.028) [12].
Another clinical feature of patients with EOPC is the lack of significant involvement of genetic factors, in contrast to familial pancreatic cancer and hereditary pancreatic cancer syndromes. Patients with familial pancreatic cancer or hereditary pancreatic cancer syndromes are more likely to gradually evolve as pancreatic cancer at very early age [13,14]. According to an analysis of 1954 patients with pancreatic cancer, patients who have alcohol intake of >26 grams/d, tobacco exposure, obesity, and diabetes are markedly correlated with the higher incidence of EOPC [15].
Genomic perspectives of pancreatic cancer
It has been well documented that many pathways containing mutated genes are correlated with the development of pancreatic cancer, including ROBO/SLIT, TGF-β, KRAS, WNT, NOTCH signaling pathway, G1/S transition, chromatin, SWI-SNF modification, RNA processing, and DNA repairing. The subtypes of expression analysis include squamous tumors, pancreatic progenitor, immunogenic, and aberrantly differentiated endocrine exocrine (ADEX) that are associated with histopathological characteristics. Importantly, TP53 and KDM6A mutations are enriched in squamous tumor, which upregulates the TP63ΔN transcriptional network and determinates the hypermethylation of genes in pancreatic endodermal cells, leading to poor prognosis. On the other hand, some genes are preferentially expressed in patients with pancreatic cancers during the early development of pancreas, such as FOXA2/3, PDX1, MNX1. ADEX reveals that these genes regulate the networks related to KRAS activation, exocrine, and endocrine differentiation. In addition, the immune networks of immunogenic tumors are able to regulate signaling pathways that contribute to immune suppression [16].
Moreover, chromosomal rearrangements resulting in the functional disruption of genes are common in pancreatic cancer. Among these genes, TP53, SMAD4, CDKN2A, ARID1A, ROBO2, and novel candidate driver genes for pancreatic cancer, including KDM6A and PREX2, have been identified with a high frequency of mutation. Besides, focal amplifications are also detected in tumor samples, many of which harbor druggable oncogenes, including ERBB2, MET, FGFR1, CDK6, PIK3R3, and PIK3CA, although a low level of expression is found in individual patient [17].
Genomic landscape of EOPC
Pancreatic ductal adenocarcinoma (PDAC) is characterized by substantial genomic heterogeneity. For example, SMAD4 is frequently mutated in younger patients resulting in the dysregulation of the TGFβ signaling activity. In addition, high phospho-GSK3 level is also observed in patients with EOPC. However, no survival differences are observed between the different age groups. In contrast to AOPC, somatic gene variations in unique cellular pathways are frequently observed in patients with EOPC [18]. Notably, there is no difference in key driver genes such as KRAS, TP53, and CDKN2A, as well as the global methylation profiling between the AOPC and the late-onset PDAC, although the late-onset PDAC shows some features related to the age, such as the characteristics of enriched DNA repair gene, upregulation of oxidative stress defenses, and the improvement of proteome carbonylation [19].
Another study has also identified some molecular characteristics of EOPC. The results show that 31.9% patients have a pathogenic germline variant (PGV), while 27.5% patients harbor modifications on cancer susceptibility genes. Among PGV, BRCA1 (27.2%), BRCA2 (27.2%), PALB2 (9.1%) and CHEK2 (9.1%) are the most common PGVs. Interestingly, for patients who tested for PGVs, 27.5% of patients display a high and moderate penetrance. This study also demonstrates a higher incidence of RAS wild-type in EOPC (15.9%) and a lower incidence of RAS wild-type in AOPC (5.4% in MSK total cohort, <10% in TCGA cohort) [20].
Furthermore, a bioinformatics analysis and the correlation with genomic and transcriptomic data have been carried out on the 269 advanced and 277 resectable PDAC tumor samples, in which the frequency and the expression patterns of somatic gene mutations that are commonly involved in PDAC, EOPC and all other groups are compared. Biallelic CDKN2A mutation is identified as the distinctive pattern in patients of EOPC. In addition, an increased expression of FOXC2, as well as the specific association between FOXC2 and EMT signaling pathways are also identified as the distinctive molecular features of EOPC [21].
As shown in Tables 1 and 2, we have summarized the information of pathogenic germline variant (PGV) in EOPC from previous publications and identified RAS wild wild-type, CDKN2A and SMAD4 are the most distinctive molecular features of EOPC (Tables 1 and 2). However, previous studies have reported that the most frequently mutated genes are BRCA1 (29/128) and BRCA2 (5/127), while the second most common mutations are detected in the MMR genes, such as MLH1 (2/33), MSH2 (3/34) and MSH6 (2/33) [22]. Other muted genes such as TP53 (2/35), CDKN2A (1/37), and STK11 (1/33) have also been identified. Hence, these researches suggesting that EOPC as an evolving entity, unique characteristics of pathogenic germline variant (PGV) of EOPC was completely distinguished with LOPC and AOPC.
Table 1.
Pathogenic germline variant (PGV) in EOPC
| Authors | EOPC | |
|---|---|---|
|
| ||
| Sample | Pathogenic germline variant | |
| Raffenne J, et al [19] 2021 | 53 | KRAS: 90.5% (48/53) |
| TP53: 69.8% (37/53) | ||
| CDKN2A: 20.7% (11/53) | ||
| SMAD4: 9.4% (5/53) | ||
| Varghese AM, et al [20] 2021 | 44 | KRAS: 83.3% (36/44) |
| BRCA1: 27.2% (12/44) | ||
| BRCA2: 27.2% (12/44) | ||
| PLAB2: 9.1% (4/44) | ||
| CHEK2: 9.1% (4/44) | ||
| CDKN2A: 6.8% (3/44) | ||
| Tsang ES, et al [21] 2021 | 117 | KRAS: 78.8% (92/117) |
| SMAD4: 18.6% (21/117) | ||
| CDKN2A: 85.0% (99/117) | ||
Table 2.
Comparison of the genomic landscape between EOPC and LOPC [19]
| Mutations | EOPC (n=53) | LOPC | P |
|---|---|---|---|
| KRAS n=(%) | 48 (90.5%) | 81 (91%) | 0.832 |
| TP 53 n=(%) | 37 (69.8%) | 63 (70.8%) | 0.946 |
| CDKN2A n=(%) | 11 (20.7%) | 14 (15.7%) | 0.594 |
| SMAD4 n=(%) | 5 (9.4%) | 22 (24.7%) | 0.043 |
Distinctive genomic feature and targeted therapy
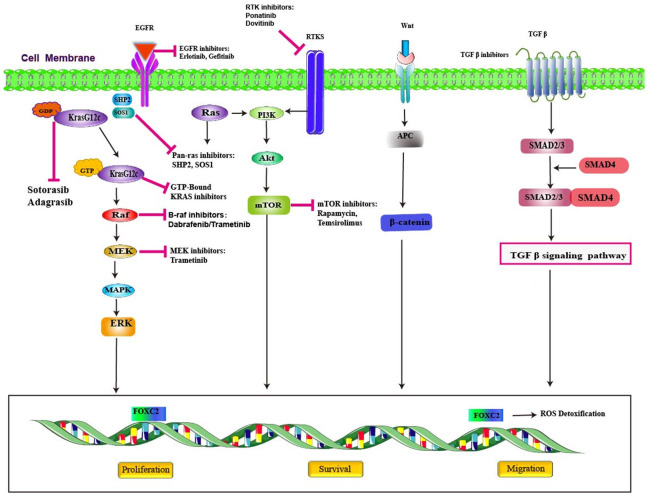
Kirsten rat sarcoma virus (KRAS) mutations are present in approximately 90% of pancreatic ductal adenocarcinoma [16,17]. The common types of KRAS are on exon 2 codons 12 and 13 with relative frequency of 71-80% [23,24] and mostly located at G12C, G12D and G12R in pancreatic cancer [25,26]. No significant differences observed in frequency in EOPC verses LOPC. KRAS protein is a small membrane-bound GTPase (GTP hydrolase), acting as a switch for a multitude of cellular signaling pathways (Figure 1). The balance between nucleotide hydrolysis and exchange determines the levels of active KRAS in cells. Bound to GDP, KRAS is in an “OFF” state. Upon GDP to GTP exchange, usually in response to growth factors and facilitated by guanine-nucleotide exchange factors (GEF) such as SOS1/SOS2, KRAS cycles to its activated “ON” state. KRAS activates effector pathways, including the MAPK and PI3K pathways, to promote cellular proliferation and survival [27]. The latest research identified that sotorasib, a KRAS G12C inhibitor, showed anticancer activity and had an acceptable safety profile in patients with KRAS G12C-mutated advanced pancreatic cancer who had received previous treatment [28]. Adagrasib, an another KRAS G12C inhibitor, demonstrated encouraging clinical activity and is well tolerated in this rare cohort of pretreated patients with KRASG12C-mutated solid tumors [29].
Figure 1.
Promising targets and therapeutic inhibitors in pancreatic cancer.
CKDN2A, a tumor suppressor gene involved in the pathway that inhibits the cell cycle at the G1 checkpoint [30,31]. CKDN2A encodes the protein p16 (INK 4a), which acts as a cell cycle regulator, belongs to the cdkn2 cyclin-dependent kinase inhibitor family, is one of the crucial defenses against cancer development in number of human cancers (Figure 2). Loss of p16 (INK 4a) leads to cell immortalization, usually caused by hyper-methylation of CDKN2A promoter. The inactivation of p16 (INK 4a) in endothelial cells specifically causes defects in motility, morphogenesis and cytoskeletal organization [32]. The latest study found that Biallelic CDKN2A mutation is identified as the distinctive pattern in patients of EOPC [21]. Targeting different proteins in this pathway has been investigated and its clinical results will be published in the near future.
Figure 2.
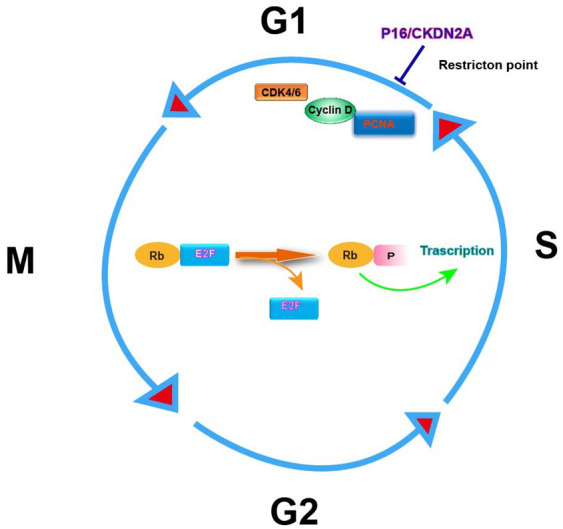
Diagrammatic representation of the involvement of p16 (INK 4a)/CKDN2A in cell cycle regulation. Abbreviations are as follows: CDK, cyclin-dependent kinase; PCNA, proliferating cell nuclear antigen; Rb, retinoblastoma gene product; E2F, transcription factor; P, phosphate.
FOXC2, an oncogenic transcription factor correlated with many different cancers. The real situation of FOXC2 mutations in pancreatic cancer still to be validated. Its role in pancreatic ductal adenocarcinoma is upregulating and enhancing growth and migration of cancer cells [33]. It interacts with beta-catenin and promote cell growth by activating beta-catenin/T-cell factor 4 signaling pathway (Figure 1). The beta-catenin/T-cell factor 4 interaction may serve as a therapeutic target for future drug development [34]. FOXC2 upregulation was associated with EOPC cohorts had been identified in newest research [21].
SMAD4 is an important tumor suppressor gene that is found to be inactivated in approximately 50% of pancreatic cancers and has been linked to a more aggressive clinical outcomes [35-38]. Apart from its role as tumor suppressor, it also acts as regulators of TGF-β pathway (Figure 1). In EOPC cohorts, SMAD4 serves as regulators of this pathway, it forms as complex after it is activated by a TGF-β protein. When SMAD4 is mutated, the cell proliferation process is left unchecked and rapid cell growth follows. Previous analyses have demonstrated that patients with EOPC had higher mutations rates of SMAD4 than those with LOPC and target therapy associated with SMAD4 still to be explored [19].
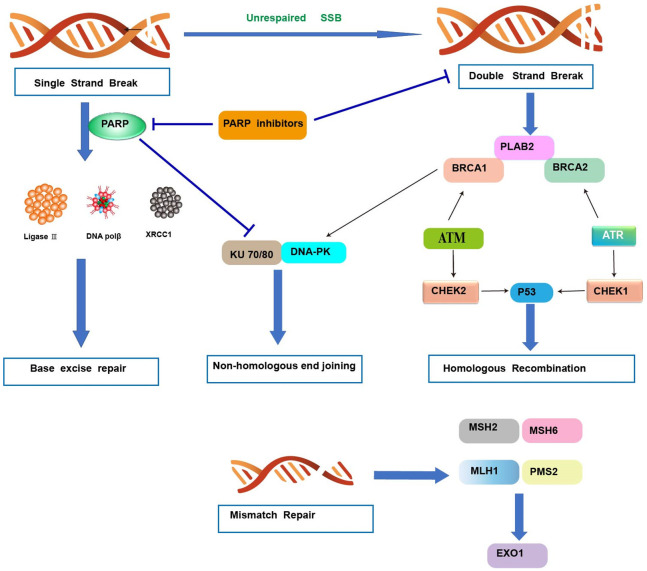
BRCA1/2 are tumor suppressor genes that are well known for their role in breast and ovarian cancers. They have also been described in pancreatic cancers. Pathogenic BRCA2 and BRCA1 mutations are found in approximately 2%, and ≤1% of pancreatic cancers, respectively [39-41]. BRCA genes are critical in DNA repair pathways, particularly in homologous recombination, which has a serious impact on genomic stability and can contribute to cancerous cell proliferation. However, BRCA1 also plays a fundamental role in cell cycle checkpoint control, ubiquitination, control of gene expression, and chromatin remodeling, while BRCA2 also plays a role in transcription and immune system response (Figure 3). Therefore, mutations in these genes lead to multiple defects in cells that may be utilized when treating cancer [42-44]. However, other studies have found no significant increase in BRCA1/2 mutations among EOPC cohorts when compared to those with LOPC [18]. The primary PARP inhibitor recommended by the National Comprehensive Cancer Network (NCCN) for BRCA-mutated pancreatic cancer is olaparib [45]. Rucaparib is another PARP inhibitor that is currently approved for ovarian and prostate cancer, tested as maintenance therapy in BRCA mutants [46]. Veliparib is another PARP inhibitor that recently showed promise in advanced BRCA-mutated ovarian cancer when combined with first-line chemotherapy, significantly increasing PFS [47]. AZD 5305 is a novel, highly selective PARP inhibitor currently being tested in the ongoing phase I/IIa PETRA trial (NCT04644068) [48].
Figure 3.
DNA damage repair mechanisms. Single-strand break (SSB) repair: PARP1 detects single-strand DNA breaks and facilitates the formation of a negatively charged, branched polymer that recruits XRCC1, Ligase 3, and DNA POL β to the site of damage for ligation and repair. Inhibition of PARP1 at this stage leads to an accumulation of SSBs that ultimately results in DSBs. Double-strand break (DSB) repair: nonhomologous end joining-DNA ends are bound by Ku proteins, which are stabilized by PARP1, and form the DNA-PK (DNA-dependent protein kinase) complex following recruitment of DNA-PKcs (catalytic subunit of DNA-PK). XCCR4 and Artemis are recruited, which stabilize and recruit other repair factors to the site of damage. Homologous recombination: damage is detected by the MRN complex, which recruits and activates ATM. ATM can activate the PALB2, BRCA1, and BRCA2 complex, ATR, or CHEK2, depending on cell cycle phase. RAD51 is activated and conducts a search for a homologous template used for repair, which activates other factors necessary for repair. Ultimately, P53 is stabilized by either CHEK1 or CHEK2 and proofs the repair. Mismatch repair: a MSH2/MSH6 heterodimer recognizes and localizes mismatched base pair errors and forms a complex with MLH1 and PMS2. PARP, poly (ADP-ribose) polymerase; PARPi, PARP inhibitor; DNA polβ/δ/ε, DNA polymerase beta/delta/epsilon; XRCC1, X-ray repair cross-complementing protein 1; DNA-PKcs, DNA-dependent protein kinase catalytic subunit; KU 70/80, a.k.a XRCC6/5 (X-ray repair cross-complementing protein 6/5); ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3-related.
Given the poor prognosis of pancreatic cancer, it is critical to identify unique features of EOPC that may lead to the identification of new molecular targets and the development of new therapeutics.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. 2019;4:934–947. doi: 10.1016/S2468-1253(19)30347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28:645–656. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 4.Muniraj T, Jamidar PA, Aslanian HR. Pancreatic cancer: a comprehensive review and update. Dis Mon. 2013;59:368–402. doi: 10.1016/j.disamonth.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Ordonez JE, Hester CA, Zhu H, Augustine M, Porembka MR, Wang SC, Yopp AC, Mansour JC, Zeh HJ 3rd, Polanco PM. Clinicopathologic features and outcomes of early-onset pancreatic adenocarcinoma in the United States. Ann Surg Oncol. 2020;27:1997–2006. doi: 10.1245/s10434-019-08096-y. [DOI] [PubMed] [Google Scholar]
- 6.Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4:e137–e147. doi: 10.1016/S2468-2667(18)30267-6. [DOI] [PubMed] [Google Scholar]
- 7.Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4:e214708. doi: 10.1001/jamanetworkopen.2021.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Lok V, Ngai CH, Zhang L, Yuan J, Lao XQ, Ng K, Chong C, Zheng ZJ, Wong MCS. Worldwide burden of, risk factors for, and trends in pancreatic cancer. Gastroenterology. 2021;160:744–754. doi: 10.1053/j.gastro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Gaddam S, Abboud Y, Oh J, Samaan JS, Nissen NN, Lu SC, Lo SK. Incidence of pancreatic cancer by age and sex in the US, 2000-2018. JAMA. 2021;326:2075–2077. doi: 10.1001/jama.2021.18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaPelusa M, Shen C, Arhin ND, Cardin D, Tan M, Idrees K, Geevarghese S, Chakravarthy B, Berlin J, Eng C. Trends in the incidence and treatment of early-onset pancreatic cancer. Cancers (Basel) 2022;14:283. doi: 10.3390/cancers14020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saadat LV, Chou JF, Gonen M, Soares KC, Kingham TP, Varghese AM, Jarnagin WR, D’Angelica MI, Drebin JA, O’Reilly EM, Wei AC. Treatment patterns and survival in patients with early-onset pancreatic cancer. Cancer. 2021;127:3566–3578. doi: 10.1002/cncr.33664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda T, Sasaki T, Inoue Y, Okamoto T, Mori C, Mie T, Furukawa T, Yamada Y, Kasuga A, Matsuyama M, Ozaka M, Takahashi Y, Saiura A, Sasahira N. Early-onset pancreatic cancer: clinical characteristics and survival outcomes. Pancreatology. 2022;22:507–515. doi: 10.1016/j.pan.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi H, Kobayashi S, Gotoh K, Noda T, Doki Y. Characteristics of early-onset pancreatic cancer and its association with familial pancreatic cancer and hereditary pancreatic cancer syndromes. Ann Gastroenterol Surg. 2020;4:229–233. doi: 10.1002/ags3.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brune KA, Lau B, Palmisano E, Canto M, Goggins MG, Hruban RH, Klein AP. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. 2010;102:119–26. doi: 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McWilliams RR, Maisonneuve P, Bamlet WR, Petersen GM, Li D, Risch HA, Yu H, Fontham ET, Luckett B, Bosetti C, Negri E, La Vecchia C, Talamini R, Bueno de Mesquita HB, Bracci P, Gallinger S, Neale RE, Lowenfels AB. Risk factors for early-onset and very-early-onset pancreatic adenocarcinoma: a pancreatic cancer case-control consortium (PanC4) analysis. Pancreas. 2016;45:311–6. doi: 10.1097/MPA.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grützmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA Australian Pancreatic Cancer Genome Initiative. Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM Australian Pancreatic Cancer Genome Initiative. Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Aharon I, Elkabets M, Pelossof R, Yu KH, Iacubuzio-Donahue CA, Leach SD, Lowery MA, Goodman KA, O’Reilly EM. Genomic landscape of pancreatic adenocarcinoma in younger versus older patients: does age matter? Clin Cancer Res. 2019;25:2185–2193. doi: 10.1158/1078-0432.CCR-18-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raffenne J, Martin FA, Nicolle R, Konta M, Blum Y, Torrisani J, Puleo F, Bachet JB, Svrcek M, Bardier-Dupas A, Emile JF, Demetter P, Radman M, Van Laethem JL, Hammel P, Rebours V, Paradis V, Couvelard A, Cros J. Pancreatic ductal adenocarcinoma arising in young and old patients displays similar molecular features. Cancers (Basel) 2021;13:1234. doi: 10.3390/cancers13061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varghese AM, Singh I, Singh R, Kunte S, Chou JF, Capanu M, Wong W, Lowery MA, Stadler ZK, Salo-Mullen E, Saadat LV, Wei AC, Reyngold M, Basturk O, Benayed R, Mandelker D, Iacobuzio-Donahue CA, Kelsen DP, Park W, Yu KH, O’Reilly EM. Early-onset pancreas cancer: clinical descriptors, genomics, and outcomes. J Natl Cancer Inst. 2021;113:1194–1202. doi: 10.1093/jnci/djab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsang ES, Topham JT, Karasinska JM, Lee MKC, Williamson LM, Mendis S, Denroche RE, Jang GH, Kalloger SE, Moore RA, Mungall AJ, Bathe OF, Tang PA, Notta F, Wilson JM, Laskin J, O’Kane GM, Knox JJ, Goodwin RA, Loree JM, Jones SJM, Marra MA, Gallinger S, Schaeffer DF, Renouf DJ. Delving into early-onset pancreatic ductal adenocarcinoma: how does age fit in? Clin Cancer Res. 2021;27:246–254. doi: 10.1158/1078-0432.CCR-20-1042. [DOI] [PubMed] [Google Scholar]
- 22.Campa D, Gentiluomo M, Obazee O, Ballerini A, Vodickova L, Hegyi P, Soucek P, Brenner H, Milanetto AC, Landi S, Gao X, Bozzato D, Capurso G, Tavano F, Vashist Y, Hackert T, Bambi F, Bursi S, Oliverius M, Gioffreda D, Schöttker B, Ivanauskas A, Mohelnikova-Duchonova B, Darvasi E, Pezzilli R, Małecka-Panas E, Strobel O, Gazouli M, Katzke V, Szentesi A, Cavestro GM, Farkas G Jr, Izbicki JR, Moz S, Archibugi L, Hlavac V, Vincze Á, Talar-Wojnarowska R, Rusev B, Kupcinskas J, Greenhalf B, Dijk F, Giese N, Boggi U, Andriulli A, Busch OR, Vanella G, Vodicka P, Nentwich M, Lawlor RT, Theodoropoulos GE, Jamroziak K, Zuppardo RA, Moletta L, Ginocchi L, Kaaks R, Neoptolemos JP, Lucchesi M, Canzian F. Genome-wide association study identifies an early onset pancreatic cancer risk locus. Int J Cancer. 2020;147:2065–2074. doi: 10.1002/ijc.33004. [DOI] [PubMed] [Google Scholar]
- 23.Windon AL, Loaiza-Bonilla A, Jensen CE, Randall M, Morrissette JJD, Shroff SG. A KRAS wild type mutational status confers a survival advantage in pancreatic ductal adenocarcinoma. J Gastrointest Oncol. 2018;9:1–10. doi: 10.21037/jgo.2017.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.AACR Project GENIE Consortium. AACR project GENIE: powering precision medicine through an international consortium. Cancer Discov. 2017;7:818–31. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cefalì M, Epistolio S, Palmarocchi MC, Frattini M, De Dosso S. Research progress on KRAS mutations in colorectal cancer. J Cancer Metastasis Treat. 2021;7:26. [Google Scholar]
- 26.Li J, Gan S, Blair A, Min K, Rehage T, Hoeppner C, Halait H, Brophy VH. A highly verified assay for KRAS mutation detection in tissue and plasma of lung, colorectal, and pancreatic cancer. Arch Pathol Lab Med. 2019;143:183–89. doi: 10.5858/arpa.2017-0471-OA. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann MH, Gerlach D, Misale S, Petronczki M, Kraut N. Expanding the reach of precision oncology by drugging all KRAS mutants. Cancer Discov. 2022;12:924–937. doi: 10.1158/2159-8290.CD-21-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strickler JH, Satake H, George TJ, Yaeger R, Hollebecque A, Garrido-Laguna I, Schuler M, Burns TF, Coveler AL, Falchook GS, Vincent M, Sunakawa Y, Dahan L, Bajor D, Rha SY, Lemech C, Juric D, Rehn M, Ngarmchamnanrith G, Jafarinasabian P, Tran Q, Hong DS. Sotorasib in KRAS p.G12C-mutated advanced pancreatic cancer. N Engl J Med. 2023;388:33–43. doi: 10.1056/NEJMoa2208470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bekaii-Saab TS, Yaeger R, Spira AI, Pelster MS, Sabari JK, Hafez N, Barve M, Velastegui K, Yan X, Shetty A, Der-Torossian H, Pant S. Adagrasib in advanced solid tumors harboring a KRASG12C mutation. J. Clin. Oncol. 2023:JCO2300434. doi: 10.1200/JCO.23.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schutte M, Hruban RH, Geradts J, Maynard R, Hilgers W, Rabindran SK, Moskaluk CA, Hahn SA, Schwarte-Waldhoff I, Schmiegel W, Baylin SB, Kern SE, Herman JG. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 1997;57:3126–30. [PubMed] [Google Scholar]
- 31.McWilliams RR, Wieben ED, Rabe KG, Pedersen KS, Wu Y, Sicotte H, Petersen GM. Prevalence of CDKN2A mutations in pancreatic cancer patients: implications for genetic counseling. Eur J Hum Genet. 2011;19:472–78. doi: 10.1038/ejhg.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang V, Lu R, Mirchia K, Van Ziffle J, Devine P, Lee J, Phillips JJ, Perry A, Raleigh DR, Lucas CG, Solomon DA. Loss of p16 expression is a sensitive marker of CDKN2A homozygous deletion in malignant meningiomas. Acta Neuropathol. 2023;145:497–500. doi: 10.1007/s00401-023-02544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui L, Dang S, Qu J, Mao Z, Wang X, Zhang J, Chen J. FOXC2 is up-regulated in pancreatic ductal adenocarcinoma and promotes the growth and migration of cancer cells. Tumour Biol. 2016;37:8579–85. doi: 10.1007/s13277-015-4607-4. [DOI] [PubMed] [Google Scholar]
- 34.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Ansari D, Althini C, Ohlsson H, Andersson R. Early-onset pancreatic cancer: a population-based study using the SEER registry. Langenbecks Arch Surg. 2019;404:565–71. doi: 10.1007/s00423-019-01810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergmann F, Aulmann S, Wente MN, Penzel R, Esposito I, Kleeff J, Friess H, Schirmacher P. Molecular characterisation of pancreatic ductal adenocarcinoma in patients under 40. J Clin Pathol. 2006;59:580–84. doi: 10.1136/jcp.2005.027292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blackford A, Serrano OK, Wolfgang CL, Parmigiani G, Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Eshleman JR, Goggins M, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Cameron JL, Olino K, Schulick R, Winter J, Herman JM, Laheru D, Klein AP, Vogelstein B, Kinzler KW, Velculescu VE, Hruban RH. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res. 2009;15:4674–9. doi: 10.1158/1078-0432.CCR-09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn SA, Schutte M, Hoque AT, Moskaluk CA, da Costa LT, Rozenblum E, Weinstein CL, Fischer A, Yeo CJ, Hruban RH, Kern SE. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–53. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 39.Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, Siddiqui A, Witmer PD, Tamura K, Song TJ, Navarro Almario JA, Brant A, Borges M, Ford M, Barkley T, He J, Weiss MJ, Wolfgang CL, Roberts NJ, Hruban RH, Klein AP, Goggins M. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J. Clin. Oncol. 2017;35:3382–3390. doi: 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young EL, Thompson BA, Neklason DW, Firpo MA, Werner T, Bell R, Berger J, Fraser A, Gammon A, Koptiuch C, Kohlmann WK, Neumayer L, Goldgar DE, Mulvihill SJ, Cannon-Albright LA, Tavtigian SV. Pancreatic cancer as a sentinel for hereditary cancer predisposition. BMC Cancer. 2018;18:697. doi: 10.1186/s12885-018-4573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, Lilyquist J, Na J, Moore R, Antwi SO, Bamlet WR, Chaffee KG, DiCarlo J, Wu Z, Samara R, Kasi PM, McWilliams RR, Petersen GM, Couch FJ. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA. 2018;319:2401–2409. doi: 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2012;12:68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowery MA, Wong W, Jordan EJ, Lee JW, Kemel Y, Vijai J, Mandelker D, Zehir A, Capanu M, Salo-Mullen E, Arnold AG, Yu KH, Varghese AM, Kelsen DP, Brenner R, Kaufmann E, Ravichandran V, Mukherjee S, Berger MF, Hyman DM, Klimstra DS, Abou-Alfa GK, Tjan C, Covington C, Maynard H, Allen PJ, Askan G, Leach SD, Iacobuzio-Donahue CA, Robson ME, Offit K, Stadler ZK, O’Reilly EM. Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst. 2018;110:1067–1074. doi: 10.1093/jnci/djy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Y, Guo M. Synthetic lethality strategies: beyond BRCA1/2 mutations in pancreatic cancer. Cancer Sci. 2020;111:3111–3121. doi: 10.1111/cas.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 46.Reiss KA, Mick R, O’Hara MH, Teitelbaum U, Karasic TB, Schneider C, Cowden S, Southwell T, Romeo J, Izgur N, Hannan ZM, Tondon R, Nathanson K, Vonderheide RH, Wattenberg MM, Beatty G, Domchek SM. Phase II study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J. Clin. Oncol. 2021;39:2497–2505. doi: 10.1200/JCO.21.00003. [DOI] [PubMed] [Google Scholar]
- 47.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat Ben-Baruch N, Werner TL, Cloven NG, Oaknin A, DiSilvestro PA, Morgan MA, Nam JH, Leath CA 3rd, Nicum S, Hagemann AR, Littell RD, Cella D, Baron-Hay S, Garcia-Donas J, Mizuno M, Bell-McGuinn K, Sullivan DM, Bach BA, Bhattacharya S, Ratajczak CK, Ansell PJ, Dinh MH, Aghajanian C, Bookman MA. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403–2415. doi: 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta M, Iyer R, Fountzilas C. Poly(ADP-Ribose) polymerase inhibitors in pancreatic cancer: a new treatment paradigms and future implications. Cancers (Basel) 2019;11:1980. doi: 10.3390/cancers11121980. [DOI] [PMC free article] [PubMed] [Google Scholar]