Abstract
Hepatitis C virus (HCV) infection causes many cancers, including intrahepatic cholangiocarcinoma. Whether it increases the risk of extrahepatic cholangiocarcinoma (ECC) is unknown. A 10-year nationwide population-based cohort study of the Taiwan National Health Insurance Research Database (TNHIRD) was conducted. ECC was defined by ICD-9-CM code 156 or ICD-O-3 code C23-24. Risk factors and HCV core protein expression were surveyed in patients with ECC from a tertiary-care center. Out of 11,892,067 patients, three propensity score-matched TNHIRD cohorts were matched at a 1:4:4 ratio: HCV-treated (8,331 patients with interferon-based therapy >6 months), HCV-untreated (n=33,324), and HCV-uninfected cohorts (n=33,324). The cumulative incidence of ECC [HCV-treated: 0.088%, 95% confidence interval (CI): 0.035-0.198%; HCV-untreated: 0.095%, 0.047-0.179%; HCV-uninfected: 0.048%, 0.017-0.119%] was lowest in the HCV-uninfected cohort (P=0.0285) but was not different between the treated and untreated cohorts (P=0.5436). HCV infection [HCV-treated cohort: hazard ratio (HR): 3.618, 95% CI HR: 1.253-10.451; HCV-untreated cohort: 2.593, 95% CI HR: 1.077-6.241; reference: HCV-uninfected cohort] and age ≥49 years (HR: 5.139, 95% CI HR: 1.613-16.369) were associated with ECC development. Among the 855 hospitalized ECC patients (males: 57%; baseline age: 63.09±11.75 years, 2008-2018), the HCV Ab-positive rate was 8.4%. The HCV Ab-positive patients were more frequently female than their counterparts (66.7% vs. 40.8%, P=0.009). No HCV core-positive cells were found in the ECC tissues. In conclusion, HCV infection and age ≥49 years are potential risk factors for ECC. The HCV-associated ECC risk might not be reversed by interferon-based anti-HCV therapy nor associated with in situ HCV core-related carcinogenesis.
Keywords: HCV, extrahepatic cholangiocarcinoma, GB, age
Introduction
Hepatitis C virus (HCV) is a human pathogen responsible for acute and chronic liver disease [1]. There are an estimated 56.8 million viremic HCV infections globally [2]. In addition to hepatic complications such as steatosis, cirrhosis and hepatocellular carcinoma (HCC), HCV infection causes many extrahepatic complications, including mixed cryoglobulinemia [3], dyslipidemia, diabetes, obesity and cardiovascular events [1]. Several large, population-based case-control or cohort studies have demonstrated the associations between HCV infection and many extrahepatic cancers [4]. Of all the reported HCV-associated extrahepatic cancers, cholangiocarcinoma (CC), which is classified into intrahepatic cholangiocarcinoma (ICC) and extrahepatic cholangiocarcinoma (ECC) by anatomic location [5], has particularly drawn our attention. CC is an uncommon malignancy of the bile duct, occurring in nearly 2 out of 100,000 people [6], and the prognosis of CC is poor, with a 5-year survival rate ranging from 15% to 20% [7]. The incidence of CC varies between different regions of the world. Southeast Asia has the highest incidence, and Western countries have the lowest incidence [8]. Geographic variations in the incidence of CC are related to variations in risk factors. The established risk factors for CC include parasitic infection, primary sclerosing cholangitis, bile duct cysts and hepatolithiasis [9]. HCV infection is a risk factor for CC in areas not endemic for Opisthorchis viverrini infection [10]. Most links between CC and HCV infection have been well established in ICC [11], and the strongest evidence has been seen in the USA [12] and Japan [13], where HCV is endemic or hyperendemic. By contrast, the connection between HCV infection and ICC is negligible in Korea [14] and China [15] and is controversial in Thailand [16], where hepatolithiasis, hepatitis B virus (HBV) and fluke infection are more prevalent than HCV infection [14-16]. These findings are consistent with the notion that chronic inflammation in the biliary epithelium together with partial bile obstruction leads to ICC [17]. Of note, a case-control study of the US elderly population showed that HCV infection increased the adjusted odds ratio for ECC to 1.90 [18], although this adjusted odds ratio was lower than that (3.4) for ICC. Moreover, the HCV core protein is associated with CC invasion and metastasis [19] and is reported to be expressed in hilar CC tissue [20]. All of the above findings suggest the possibility that HCV infection accelerates the development of ECC.
We conducted a nationwide population-based cohort study to investigate the association of HCV infection with the development of ECC in Taiwan, an Asian country endemic for HCV infection [3] and hyperendemic for HBV infection [21]. The impact of HCV infection on the risk of ECC was investigated by comparing the cumulative incidences of ECC and ECC-associated mortalities among HCV-infected subjects with and without anti-HCV therapy and subjects without HCV infection. In parallel, the risk factors and HCV core protein expression in ECC were surveyed in patients with ECC from a tertiary referral center.
Methods
Taiwan National Health Insurance Research Database (TNHIRD) samples and measurements
This population-based retrospective cohort study used nation-level data, including from the National Health Insurance (NHI) administrative database, the Cancer Registry Database, and the Death Registry Database. This mandatory, single-payer NHI program provides comprehensive coverage that includes ambulatory care, hospital services, laboratory tests, and prescription drugs. Over 99% of the population is enrolled in the program, and approximately 90% of healthcare organizations are contracted with the NHI Administration. Because HBV infection causes many hepatic complications, including cirrhosis [21] and CC [22], and cirrhosis is a risk factor for CC [23], patients diagnosed with HBV infection, cirrhosis and cirrhosis-associated complications in the observation period (2003-2012) or with ECCs before starting anti-HCV treatment (the baseline) were excluded.
The HCV-treated cohort included patients who had received HCV RNA tests and received ribavirin and peg-interferon (Peg-IFN) in 2003-2012. Their first HCV test was assumed to be the index date of diagnosis. The baseline for the HCV-treated cohort was 6 months after completing the combination therapy. Only those who had received anti-HCV therapy for ≥6 months were enrolled. HCV-untreated patients were those who had received an HCV test (HCV antibody or HCV RNA test) (their first HCV test was the index date), were diagnosed with HCV (ICD-9-CM codes: 070.41, 070.44, 070.51, 070.54, 070.70, 070.71, V02.62), were prescribed hepatoprotective agents (silymarin, liver hydrolysate, choline bitartrate, or ursodeoxycholic acid), but did not receive any anti-HCV therapy (ribavirin or Peg-IFN). HCV-uninfected subjects were those who had no HCV diagnosis or HCV test and received no hepatoprotective agents or anti-HCV therapy. The HCV-treated cohort, the HCV-untreated cohort, and the HCV-uninfected cohort were matched at a 1:4:4 ratio through a propensity score-matching method that indicated the probability of receiving the combination therapy, which was estimated by using a logistic model. The matching processes of the 3 cohorts are demonstrated in Supplementary Figure 1. The covariates in the model included age (20-39, 40-49, 50-59, ≥60), sex (male, female), NHI registration location (city, township, rural area), the Charlson Comorbidity Index (CCI) score (0, 1, ≥2) [24], and year of the index date (2003-2006, 2007-2009, 2010-2012). This method was used to assure that the HCV-treated cohort and the selected counterparts were comparable in observed characteristics. We used the following calculations to equalize the index-to-baseline time span within each set of matched counterparts: The baseline date of each HCV-treated patient was set as the date six months after treatment completion. Since the other two groups had no relevant treatment, each HCV-untreated and HCV-uninfected patient’s baseline date was their index date plus the index-to-baseline time span of their matched HCV-treated counterpart. The index date of the HCV-uninfected individuals was the date of a physician visit randomly selected from their claims database.
Outcomes of the NHIRD study were the development of ECC or ECC-associated mortality [ICD-9-CM code 156; International Classification of Diseases for Oncology (ICD-O-3): C23-24]. Types of cancer and dates of diagnosis were retrieved from the Cancer Registry Database. Subjects were followed until the date of the event, death, or the end of follow-up (December 31, 2012), whichever came first. For the HCV-treated group, only the events or mortality that occurred more than 6 months after the complement of anti-HCV therapy were recorded. The date of death was that in the Death Registry database.
Risk factor survey of hospitalized cancer patients with ECC
All patients older than 18 years old with a diagnosis of ECC who underwent surgery were consecutively recruited from a Taiwan tertiary referral center between July 2008 and June 2018. The baseline demographic data, including age, body mass index (BMI), diabetes mellitus (DM), hypertension, hyperlipidemia, hyperuricemia, acute coronary syndrome (ACS), cerebrovascular accident (CVA), smoking, drinking, betel nut chewing, HCV Ab, and hepatitis B surface antigen, of the enrolled patients were recorded and analyzed. DM, hypertension, hyperlipidemia, ACS, CVA and hyperuricemia were defined as described [24].
Immunohistochemical (IHC) staining of ECC
The IHC staining of the ECC from patients with chronic hepatitis C (CHC) and with past HCV infection were performed using paraffinized samples in the tissue bank of tertiary referral center with cases of ECC. CHC was defined as the presence of documented HCV antibodies and detectable HCV RNA for >24 weeks (n=10). Past HCV infection was defined as a positive HCV antibody but a negative HCV RNA (n=10). A total of 20 patients without any HCV infection served as controls. The baseline characteristics of the patients are listed in Supplementary Table 1. Liver tissue samples from HCV core transgenic mice served as positive controls [24]. IHC staining for HCV core protein (Virostat, Westbrook, ME, USA) in EBC was performed according to the manufacturer’s protocols. In brief, the cells were permeabilized with 0.1% Triton-100, incubated with HCV core antibody, washed, and then incubated with secondary antibody (Vector Laboratories, Burlingame, CA, USA). The intensity of protein expression was determined using ImageJ software (http://imagej.nih.gov/ij/, National Institutes of Health, Bethesda, MD, USA). The pathology of all IHC stains was reviewed by gastrointestinal pathologists at participating sites who were blinded to study participation and cancer prediction.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Science (SPSS package version 21, SPSS Inc., Chicago, IL, USA) or Statistical Analysis System (SAS version 9.4, SAS Institute Inc., Cary, NC, USA) software. Continuous variables were analyzed using Student’s t test, and categorical variables were analyzed using the chi-square test or Fisher’s exact test, as appropriate. Nonparametric tests were applied where indicated. Kaplan-Meier or univariate Cox regression analysis was used to assess the relationship among baseline variables and the development of ECC. Multivariate Cox regression models were used to assess the relationship between various dependent and independent variables by adjusting for all the independent variables with a p value <0.1 in the univariate analyses. Binary logistic regression analysis was performed to assess the variables that accounted for the emergence of events. Cumulative incidences of outcomes were estimated and compared by using the modified Kaplan-Meier method and the Gray method, with death being a competing risk event. A subdistribution hazards model, an extension of the Cox proportional hazards model taking competing mortality into consideration, was used to estimate the adjusted hazard ratio of developing ECC, adjusting for age, sex, NHI registration location, CCI score, year of the index date, and comorbid liver cirrhosis, chronic obstructive pulmonary disease (COPD), end-stage renal disease (ESRD), DM, hypertension, dyslipidemia, and cardiovascular events, including percutaneous coronary intervention, coronary artery bypass graft, myocardial infarction, heart failure, cardiogenic shock, peripheral vascular disease and stroke. Statistical significance was defined at the 5% level based on two-tailed tests of the null hypothesis.
Informed consent
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local institutional review board (IRB No.: 104-7005B).
Results
Baseline characteristics of cases in the TNHIRD
As shown in Figure 1, from January 1, 2003 to December 31, 2012, a total of 11,892,067 patients without HBV infection, cirrhosis or ECC were eligible for the current study. After matching the baseline factors, three cohorts matched at a 1:4:4 ratio, including 8,331 patients with HCV infection who received Peg-IFN with ribavirin for at least 6 months (HCV-treated cohort), 33,324 patients with HCV infection who had never been treated for HCV infection (HCV-untreated cohort), and 33,324 patients without HCV infection (HCV-uninfected cohort), were enrolled. The 3 cohorts were matched in propensity scores and did not differ in baseline demographic factors, residency, CCI score or index year, although baseline comorbidities were not similar. Compared with HCV-untreated cohorts, the HCV-treated cohort had higher rates of cirrhosis, a similar rate of COPD and lower rates of other comorbidities. Compared with the HCV-uninfected cohort, the HCV-treated cohort had higher rates of cirrhosis, COPD, ESRD, and hypertension, lower rates of dyslipidemia and stroke, and similar rates of DM and cardiovascular events (Table 1).
Figure 1.

Flow chart of subject selection of the TNHIRD study. TNHIRD: Taiwan National Health Insurance Research Database; HCV: hepatitis C virus; HBV: hepatitis B virus infection; Peg-IFN: pegylated interferon; PS: propensity score.
Table 1.
Baseline characteristics of the 3 HCV cohorts of TNHIRD
| (1) | (2) | (3) | p values | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HCV-treated | HCV-untreated | HCV-uninfected | (1, 2) | (1, 3) | (2, 3) | |
| N | 8,331 | 33,324 | 33,324 | |||
| Gender | ||||||
| Female, N, (%) | 4,397 (52.78) | 17,585 (52.77) | 17,588 (52.78) | 0.9883 | 1 | 0.9814 |
| Age range (years), N, (%) | ||||||
| 20-39 | 1,459 (17.51) | 5,833 (17.50) | 5,836 (17.51) | 1 | 1 | 1 |
| 40-49 | 2,175 (26.11) | 8,703 (26.12) | 8,700 (26.11) | |||
| 50-59 | 3,008 (36.11) | 12,032 (36.11) | 12,032 (36.11) | |||
| ≥60 | 1,689 (20.27) | 6,756 (20.27) | 6,756 (20.27) | |||
| Area, N, (%) | ||||||
| City | 1,789 (21.47) | 7,152 (21.46) | 7,156 (21.47) | 0.9996 | 1 | 0.9991 |
| Township | 2,597 (31.17) | 10,392 (31.18) | 10,388 (31.17) | |||
| Rural area | 3,945 (47.35) | 15,780 (47.35) | 15,780 (47.35) | |||
| CCI score, N, (%) | ||||||
| 0 | 3,967 (47.62) | 15,868 (47.62) | 15,868 (47.62) | 1 | 1 | 1 |
| 1 | 2,661 (31.94) | 10,644 (31.94) | 10,644 (31.94) | |||
| ≥2 | 1,703 (20.44) | 6,812 (20.44) | 6,812 (20.44) | |||
| Index_year, N, (%) | ||||||
| 2003-2006 | 4,397 (52.78) | 17,588 (52.78) | 17,588 (52.78) | 0.9989 | 1 | 0.9973 |
| 2007-2009 | 2,727 (32.73) | 10,914 (32.75) | 10,908 (32.73) | |||
| 2010-2012 | 1,207 (14.49) | 4,822 (14.47) | 4,828 (14.49) | |||
| Baseline factor, N, (%) | ||||||
| Liver cirrhosis | 926 (11.12) | 2,007 (6.02) | 18 (0.05) | <.0001 | <.0001 | <.0001 |
| COPD | 975 (11.7) | 3,985 (11.96) | 3,191 (9.58) | 0.5203 | <.0001 | <.0001 |
| ESRD | 59 (0.71) | 955 (2.87) | 102 (0.31) | <.0001 | <.0001 | <.0001 |
| DM | 1,609 (19.31) | 7,681 (23.05) | 6,197 (18.6) | <.0001 | 0.1335 | <.0001 |
| Hypertension | 2,506 (30.08) | 11,927 (35.79) | 9,237 (27.72) | <.0001 | <.0001 | <.0001 |
| Dyslipidemia | 1,007 (12.09) | 6,594 (19.79) | 6,072 (18.22) | <.0001 | <.0001 | <.0001 |
| Cardiovascular events | 221 (2.65) | 1,437 (4.31) | 896 (2.69) | <.0001 | 0.8556 | <.0001 |
| Stroke | 285 (3.42) | 1,706 (5.12) | 1,651 (4.95) | <.0001 | <.0001 | 0.33 |
HCV: hepatitis C virus; TNHIRD: Taiwan National Health Insurance Research Database; CCI: Charlson Comorbidity Index; COPD: Chronic obstructive pulmonary disease; ESRD: end stage renal disease; DM: diabetes.
Cumulative incidences of ECC and mortality
The HCV-treated, untreated, and uninfected cohorts were followed up until death for a duration (mean ± standard deviation) of 4.77±1.82 years, 4.82±2.0 years, and 4.99±1.91 years, respectively, with the longest observation being 10 years. The incidence of ECC was lowest in the HCV-uninfected cohort, as ECC occurred cumulatively in 0.088% [95% confidence interval (CI): 0.035-0.198%], 0.095% (95% CI: 0.047-0.179%), and 0.048% (95% CI: 0.017-0.119%) of the HCV-treated, untreated, and uninfected cohorts, respectively (Table 2; Figure 2). Antiviral treatment was not associated with a reduced risk of ECC, as the cumulative incidences were similar between the treated and untreated cohorts (P=0.5436). The cumulative incidences of ECC-associated mortality (Figure 3; Supplementary Table 2) and overall mortality (Supplementary Table 3) were lowest in the HCV-uninfected cohort. The HCV-treated cohort had a similar cumulative incidence of ECC-associated mortality (0.038%, 95% CI: 0.011-0.109% vs. 0.133%, 95% CI: 0.040-0.371%, P=0.9203) (Figure 3; Supplementary Table 2) but had lower overall mortality (14.259%, 95% CI: 12.054-16.645% vs. 28.122%, 95% CI: 25.940-30.340%, P<0.0001) (Supplementary Table 3) than the HCV-untreated cohort.
Table 2.
Cumulative incidences of ECC among (1) HCV-treated, (2) HCV-untreated and (3) HCV-uninfected cohorts
| (1) HCV-treated | (2) HCV-untreated | (3) HCV-uninfected | p values | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N=8,331 | N=33,324 | N=33,324 | (1, 2, 3) | (1, 2) | (1, 3) | (2, 3) | |
| Follow-up years, mean ± SD | 4.766±1.82 | 4.822±2.00 | 4.988±1.91 | ||||
| Event number, N (%) | 6 (0.07) | 19 (0.06) | 7 (0.02) | ||||
| Competing mortality, N (%) | 386 (4.63) | 4,405 (13.22) | 1,458 (4.38) | ||||
| CI, % (95% CI) | 0.088 (0.035-0.198) | 0.095 (0.047-0.179) | 0.048 (0.017-0.119) | 0.0285 | 0.5436 | 0.0132 | 0.0209 |
ECC: extrahepatic cholangiocarcinoma; HCV: hepatitis C virus; SD: standard deviation; CI: cumulative incidence; 95% CI: 95% confidence interval of cumulative incidence.
Figure 2.

Cumulative incidence of ECC among the 3 TNHIRD cohorts, including HCV-treated, HCV-untreated and HCV-uninfected cohorts.
Figure 3.
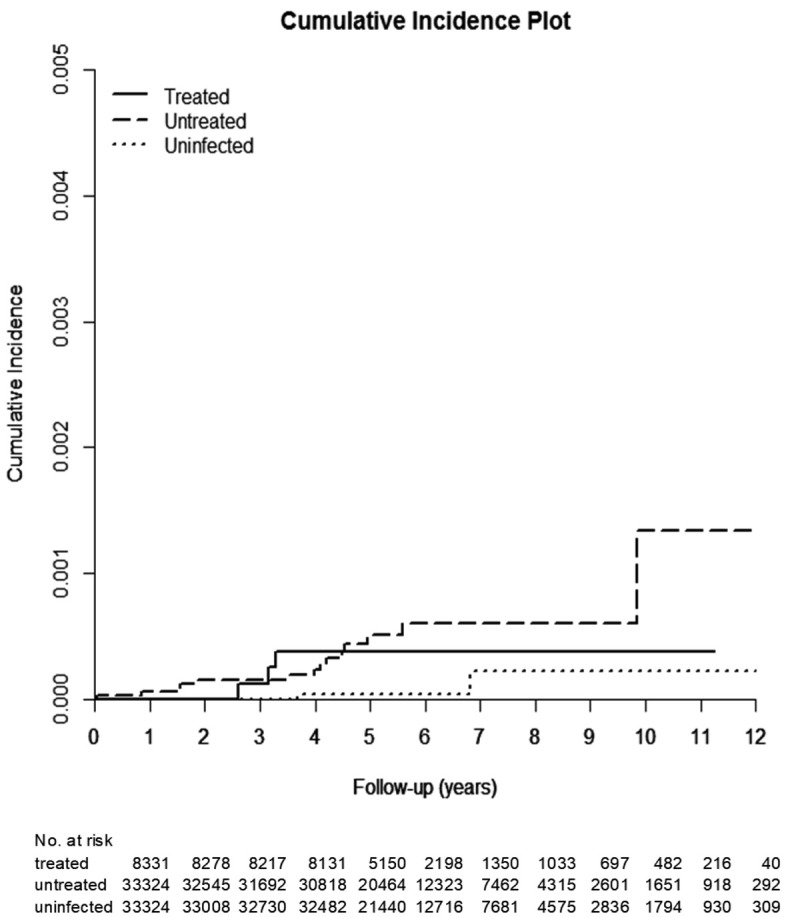
Cumulative incidence of ECC-associated mortality among the 3 TNHIRD cohorts, including HCV-treated, HCV-untreated and HCV-uninfected cohorts.
Factors associated with the cumulative incidence of ECC
The HCV-treated and HC-untreated cohorts had similar hazard ratios (HRs) for developing ECC. However, both HCV-treated (HR: 3.618, 95% CI HR: 1.253-10.451, P=0.018) and HCV-untreated cohorts (HR: 2.593, 95% CI HR: 1.077-6.241, P=0.034) had higher HRs for ECC than the HCV-uninfected cohort. Age ≥49 years was also associated with a higher HR (HR: 5.139, 95% CI HR: 1.613-16.369, P=0.006) for ECC (Figure 4).
Figure 4.
Forest plot of factors associated with incident ECC in the 3 TNHIRD cohorts. HR: hazard ratio; CI: confidence interval; HCV: hepatitis C virus; COPD: chronic obstructive pulmonary disease; ESRD: end-stage renal disease; DM: diabetes.
Risk factors for hospitalized patients with ECC
Among the 855 hospitalized patients with ECC (mean age: 63.09±11.75 years; median: 64.68 years), males (n=487, 57%) accounted for the majority. Of the 855 patients, 438 (51.2%), 204 (23.9%), 139 (16.3%), and 74 (8.7%) had ampulla vater cancer, gall bladder cancer, common bile duct cancer and hilar cancer, respectively. The positive rate of HCV Ab in the enrolled patients was 8.4%. Most risk factors had similar proportions between the HCV Abpositive and HCV Ab-negative patients, but the HCV Abpositive patients were more frequently female than their counterparts (66.7% vs. 40.8%, P=0.009) (Table 3). Specifically, the HCV Ab positivity rates were 10.3% for patients with GB cancer, 5.6% for those with hilar cancer, 9.4% for those with ampullar vater cancer and 3.4% for those with common bile duct cancer.
Table 3.
Prevalence rates of baseline factors in hospitalized patients with ECC
| HCV Ab-positive patients (N=72) | HCV Ab-negative patients (N=783) | p values | |
|---|---|---|---|
| Female, N (%) | 48 (66.7) | 320 (40.8) | 0.009 |
| Age (years) | 62.79±10.44 | 62.35±11.86 | 0.85 |
| BMI (kg/m2) | 24.47±4.34 | 23.19±4.44 | 0.208 |
| DM, N (%) | 18 (25.0) | 169 (21.6) | 0.393 |
| H/T, N (%) | 21 (29.2) | 312 (39.8) | 0.232 |
| Hyperlipidemia, N (%) | 3 (4.2) | 48 (6.1) | 0.594 |
| HU, N (%) | 5 (6.9) | 35 (4.5) | 0.504 |
| ACS, N (%) | 5 (6.9) | 37 (4.7) | 0.569 |
| CVA, N (%) | 3 (4.2) | 37 (4.7) | 0.785 |
| Smoking, N (%) | 5 (6.9) | 185 (23.6) | 0.067 |
| Alcohol, N (%) | 10 (13.8) | 180 (23.0) | 0.457 |
| Betel nut, N (%) | 3 (4.2) | 42 (5.4) | 0.785 |
| HBV infection, N (%) | 10 (13.8) | 74 (9.5) | 0.176 |
N: number; ECC: extrahepatic cholangiocarcinoma; BMI: body mass index; DM: diabetes; H/T: hypertension; DL: dyslipidemia; HU: hyperuricemia; ACS: acute coronary syndrome; CVA: cerebrovascular accident; HBV infection: hepatitis B virus surface antigen positivity.
The 5-year cumulative incidence of mortality in hospitalized patients with ECC was 55.2%. We also stratified the hospitalized ECC patients by ECC type to estimate the impact of HCV infection on survival. The cumulative incidences of mortality in patients with various ECCs, including vater cancer, gall bladder cancer, common bile duct cancer and hilar cancer, were 48.7%, 52.6%, 39.4%, and 100%, respectively. There were no differences in the cumulative incidence of survival of various types of ECC patients with vs. without HCV Ab positivity (vater cancer, HCV Ab (+) vs. HCV Ab (-): 49% vs. 42.3%, P=0.524; gall bladder cancer, HCV Ab (+) vs. HCV Ab (-): 52% vs. 58.3%, P=0.495; common bile duct cancer, HCV Ab (+) vs. HCV Ab (-): 38.4% vs. 50%, P=0.075; hilar cancer, HCV Ab (+) vs. HCV Ab (-): 100% vs. 100%, P=0.599).
IHC ECCs in hospitalized patients
No HCV core-positive cells were seen in the ECC tissues for either CHC or past HCV infection patients (Supplementary Figure 2).
Discussion
The most compelling results of the current study are as follows: (1) The cumulative incidences of ECC and the associated mortalities were lowest in the HCV-uninfected cohort but similar between the HCV-treated and untreated cohorts. (2) The cumulative incidences of overall mortality were lowest in the HCV-uninfected cohort, followed by the HCV-treated and HCV-untreated cohorts. (3) HCV infection and age ≥49 years were independently associated with the development of ECC. (4) Among hospitalized ECC patients, the HCV Ab positivity rates were 8.4%. (5) No HCV core-positive cells were seen in ECC tissue by IHC.
The finding that the HCV-treated cohort had the highest cirrhosis rate but the lowest dyslipidemia rate among the 3 TNHIRD cohorts was consistent with the ideas that HCV infection causes cirrhosis and hypolipidemia [1] and that only patients with significant fibrosis are reimbursed for interferon-based anti-HCV therapy in Taiwan [23]. The differences in these baseline comorbidities thus support the reliability of the TNHIRD study results.
The HCV-uninfected cohort had the lowest cumulative incidence of ECC among the 3 cohorts, HCV infection was independently associated with the development of ECC, and the HCV Ab-positive rate of the hospital cases of ECC was 8.4%, which was higher than that of the general population in Taiwan (2.7%) [25], all suggesting that HCV infection is a potential risk factor for ECC in Taiwan. Although the evidence linking HCV infection to ICC has been solidified by many studies [11,12,26], the evidence of a connection between HCV infection and ECC is relatively limited [18,27], and most of it has confirmed the stronger link between HCV infection and ICC than with ECC [18,27]. Although a previous study showed the presence of the HCV core in hilar ECC [20], our IHC staining failed to demonstrate any HCV core-positive cells in the tumor tissue, and the local HCV core-associated oncogenesis effect could not be confirmed. The mechanisms causing the extrahepatic effects of HCV infection are likely multifactorial, and HCV-associated extrahepatic biliary oncogenesis may include endocrine effects or a heightened immune reaction with systemic effects [28]. Interestingly, in contrast to the male dominance of CC [29], hospitalized HCV Ab-positive ECC patients were more frequently female than HCV Ab-negative ECC patients. Given that diabetes has been associated with an increased risk of CC in women [30], the fact that HCV Ab-positive ECC patients were mostly female supports the potential link between HCV-associated ECC risk and metabolic alteration, though the small number of HCV-Ab-positive cases might have led to statistically similar diabetes rates between HCV Ab-positive and HCV Ab-negative patients. In addition to the fact that our TNHIRD analysis excluded any patients with HBV infection to avoid the interference of HBV, all the data support the association between HCV infection and ECC development, even in a country hyperendemic for HBV infection.
Given that the cumulative incidence of ECC was similar between the HCV-treated and untreated cohorts, the HCV-associated risk in ECC development might not be attenuated by viral clearance once the process of biliary carcinogenesis had been initiated. Although most HCV infections are currently curable with potent, direct-acting antiviral agents (DAAs) [3], special caution is still needed to prevent the development of ECC in HCV-infected patients who have achieved a sustained virological response (SVR) after anti-HCV therapy. Prevention of HCV infection might be more crucial than curing HCV infection for eliminating the HCV-associated risk of ECC. Additionally, the poor prognosis of ECCs [7] might account for the negligible differences in cumulative incidence of mortality between hospitalized patients with and without HCV infection. Again, the impact of HCV infection on ECC might be crucial during early oncogenesis, such as during cancer initiation, rather than in late cancer progression [31]. Additionally, the phenomenon that CC is rarely diagnosed before 40 years of age [30] is consistent with the finding that age ≥49 years was independently associated with the development of ECC, and the mean age of the hospital patients was 63 years.
In line with the similar cumulative incidences of ECC between the HCV-treated and untreated cohorts, the HCV-untreated cohort had similar ECC-associated mortality to that of the HCV-treated cohort. However, the HCV-untreated cohort had the highest overall mortality, followed by that of HCV-treated and then by that of HCV-uninfected cohorts. This pattern might be caused by HCV-associated events, including cirrhosis, HCC or cardiometabolic events [1], other than ECC-associated complications. This phenomenon indicates the importance of prescribing efficient anti-HCV therapy, such as potent DAAs [3], to HCV-infected patients to decrease their overall mortality, regardless of their risk for ECC.
There are limitations to the current study. First, because linking the results from the TNHIRD to the laboratory results of individual patients was forbidden for privacy protection, no correlation of SVR with ECC could be tested. We are confident of the antiviral efficacy in the HCV-treated cohort since interferon-based therapy for HCV infection generally achieves an SVR rate up to 90% in Taiwan, where a favorable genetic variation in interferon lambda 3 for interferon-based anti-HCV therapy is prevalent [24]. Second, the few patients in the TNHIRD database developed ECC, which might lead to some statistical biases. A total of 855 hospitalized patients with ECC showed consistent results, which dampen these biases. Third, some ECC risk factors, such as alcohol drinking, were not included in the TNHIRD analyses. We surveyed these factors in our hospitalized patients to fill this gap. Fourth, the precise mechanism of the increased risk of ECC in HCV-infected patients was undetermined. Future prospective studies in other independent cohorts with many ECC cases, comprehensive risk factor surveys, identifiable SVR following DAA therapy and sophisticated molecular investigations are required to elucidate the fundamental mechanisms underlying the findings described herein.
Taken together, the evidence shows that HCV infection and age ≥49 years are associated with the development of ECC in Taiwan. Interferon-based anti-HCV therapy might not attenuate the risk of ECC development. The HCV-associated risk in ECC is potentially associated with indirect effects other than in situ HCV core-related oncogenesis.
Acknowledgements
The authors thank Mr. Chun-Kai Liang, from the Department of Gastroenterology and Hepatology, Chang Gung Memorial Hospital, Taiwan, for his assistance with IHC performance. This study was supported by grants from the Chang Gung Medical Research Program (CMRPG3I0413, CMRPG3L1191, CMRPG3M0211, and CMRPG1K0111-3); and the National Science Council, Taiwan (MOST 111-2629-B-182-001-, and 111-2314-B-182A-156-) to M.L.C.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Chang ML. Metabolic alterations and hepatitis C: from bench to bedside. World J Gastroenterol. 2016;22:1461–76. doi: 10.3748/wjg.v22.i4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396–415. doi: 10.1016/S2468-1253(21)00472-6. [DOI] [PubMed] [Google Scholar]
- 3.Chang ML, Cheng JS, Chuang YH, Pao LH, Wu TS, Chen SC, Chang MY, Chien RN. Evolution of cryoglobulinemia in direct-acting antiviral-treated Asian hepatitis C patients with sustained virological responses: a 4-year prospective cohort study. Front Immunol. 2022;13:823160. doi: 10.3389/fimmu.2022.823160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison RD, Tong X, Moorman AC, Ly KN, Rupp L, Xu F, Gordon SC, Holmberg SD Chronic Hepatitis Cohort Study (CHeCS) Investigators. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006-2010. J Hepatol. 2015;63:822–8. doi: 10.1016/j.jhep.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammad-Alizadeh AH, Ghobakhlou M, Shalmani HM, Zali MR. Cholangiocarcinoma: an-eight-year experience in a tertiary-center in Iran. Asian Pac J Cancer Prev. 2012;13:5381–4. doi: 10.7314/apjcp.2012.13.11.5381. [DOI] [PubMed] [Google Scholar]
- 7.Flemming JA, Zhang-Salomons J, Nanji S, Booth CM. Increased incidence but improved median overall survival for biliary tract cancers diagnosed in Ontario from 1994 through 2012: a population-based study. Cancer. 2016;122:2534–43. doi: 10.1002/cncr.30074. [DOI] [PubMed] [Google Scholar]
- 8.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, Geier A, Calvisi DF, Mertens JC, Trauner M, Benedetti A, Maroni L, Vaquero J, Macias RI, Raggi C, Perugorria MJ, Gaudio E, Boberg KM, Marin JJ, Alvaro D. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–80. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 9.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–84. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivatanakul P, Honjo S, Kittiwatanachot P, Jedpiyawongse A, Khuhaprema T, Miwa M. Hepatitis viruses and risk of cholangiocarcinoma in northeast Thailand. Asian Pac J Cancer Prev. 2010;11:985–8. [PubMed] [Google Scholar]
- 11.Pol S, Vallet-Pichard A, Hermine O. Extrahepatic cancers and chronic HCV infection. Nat Rev Gastroenterol Hepatol. 2018;15:283–290. doi: 10.1038/nrgastro.2017.172. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: a population-based study of U.S. veterans. Hepatology. 2009;49:116–23. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, Takemura S, Tanaka H, Yamazaki O, Hirohashi K, Tanaka T. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95:592–5. doi: 10.1111/j.1349-7006.2004.tb02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, Kwon S, Lee SK, Seo DW, Kim MH, Suh DJ. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103:1716–20. doi: 10.1111/j.1572-0241.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632–5. doi: 10.3748/wjg.14.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barusrux S, Nanok C, Puthisawas W, Pairojkul C, Poovorawan Y. Viral hepatitis B, C infection and genotype distribution among cholangiocarcinoma patients in northeast Thailand. Asian Pac J Cancer Prev. 2012;13(Suppl):83–7. [PubMed] [Google Scholar]
- 17.Gatto M, Alvaro D. Cholangiocarcinoma: risk factors and clinical presentation. Eur Rev Med Pharmacol Sci. 2010;14:363–7. [PubMed] [Google Scholar]
- 18.Mahale P, Torres HA, Kramer JR, Hwang LY, Li R, Brown EL, Engels EA. Hepatitis C virus infection and the risk of cancer among elderly US adults: a registry-based case-control study. Cancer. 2017;123:1202–1211. doi: 10.1002/cncr.30559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T, Li D, Cheng L, Wu H, Gao Z, Liu Z, Jiang W, Gao YH, Tian F, Zhao L, Wang S. Epithelial-mesenchymal transition induced by hepatitis C virus core protein in cholangiocarcinoma. Ann Surg Oncol. 2010;17:1937–44. doi: 10.1245/s10434-010-0925-3. [DOI] [PubMed] [Google Scholar]
- 20.Chen RF, Li ZH, Zou SQ, Chen JS. Effect of hepatitis C virus core protein on modulation of cellular proliferation and apoptosis in hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:71–4. [PubMed] [Google Scholar]
- 21.Chang ML, Cheng JS, Chien RN, Liaw YF. Hepatitis flares are associated with better outcomes than no flare in patients with decompensated cirrhosis and chronic hepatitis B virus infection. Clin Gastroenterol Hepatol. 2020;18:2064–2072. e2. doi: 10.1016/j.cgh.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Spradling PR, Xing J, Zhong Y, Rupp LB, Moorman AC, Lu M, Teshale EH, Schmidt MA, Daida YG, Boscarino JA, Gordon SC. Incidence of malignancies among patients with chronic hepatitis B in US health care organizations, 2006-2018. J Infect Dis. 2022;226:896–900. doi: 10.1093/infdis/jiac011. [DOI] [PubMed] [Google Scholar]
- 23.Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Hepatol. 2020;72:95–103. doi: 10.1016/j.jhep.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Cheng JS, Chen TC, Chen TD, Ku HP, Huang SW, Wu TS, Chien RN, Chang ML. Association between breast cancer and hepatitis C: a joint study of hospitalized patients and nationwide cohorts. Transl Res. 2022;245:117–129. doi: 10.1016/j.trsl.2022.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Cheng YL, Wang YC, Lan KH, Huo TI, Huang YH, Su CW, Lin HC, Lee FY, Wu JC, Lee SD. Anti-hepatitis C virus seropositivity is not associated with metabolic syndrome irrespective of age, gender and fibrosis. Ann Hepatol. 2015;14:181–9. [PubMed] [Google Scholar]
- 26.Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016–21. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Hu B, Zhou ZQ, Guan J, Zhang ZY, Zhou GW. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: evidence from a systematic review and meta-analysis of 16 case-control studies. World J Surg Oncol. 2015;13:161. doi: 10.1186/s12957-015-0583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negro F, Forton D, Craxì A, Sulkowski MS, Feld JJ, Manns MP. Extrahepatic morbidity and mortality of chronic hepatitis C. Gastroenterology. 2015;149:1345–60. doi: 10.1053/j.gastro.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Tomimatsu M, Ishiguro N, Taniai M, Okuda H, Saito A, Obata H, Yamamoto M, Takasaki K, Nakano M. Hepatitis C virus antibody in patients with primary liver cancer (hepatocellular carcinoma, cholangiocarcinoma, and combined hepatocellular-cholangiocarcinoma) in Japan. Cancer. 1993;72:683–8. doi: 10.1002/1097-0142(19930801)72:3<683::aid-cncr2820720310>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Huang YJ, Wu AT, Chiou HY, Chuang MT, Meng TC, Chien LN, Yen Y. Interactive role of diabetes mellitus and female sex in the risk of cholangiocarcinoma: a population-based nested case-control study. Oncotarget. 2017;8:6642–6651. doi: 10.18632/oncotarget.14254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Smits R, Hao H, He C. Wnt/β-Catenin signaling in liver cancers. Cancers (Basel) 2019;11:926. doi: 10.3390/cancers11070926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



