Abstract
Objective: To observe the clinical value of prognostic nutritional index (PNI) combined with carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 242 in early prediction of anastomotic leakage after radical gastrectomy for gastric cancer. Methods: We retrospectively collected clinical data of 350 gastric cancer patients who underwent radical gastrectomy in Gansu Provincial Hospital of Traditional Chinese Medicine between January 2018 and May 2022. According to the occurrence of anastomotic leakage, patients were divided into an occurrence group (n=34) and a non-occurrence group (n=316). The clinical value of PNI combined with CEA and CA242 on the 3rd day after surgery in predicting anastomotic leakage was explored. Lasso regression analysis was used to screen predictive indicators of anastomotic leakage and establish a risk model. Results: In the 350 patients who underwent radical gastrectomy for gastric cancer, anastomotic leakage was observed in 34 cases, with an incidence rate of 9.7%. A higher proportion of patients in the occurrence group exhibited diabetes, hand-sewn anastomosis, advanced tumor node metastasis (TNM) staging, and intraoperative bleeding, when compared to those in the non-occurrence group (P<0.05). Moreover, on the 3rd postoperative day, patients in the occurrence group demonstrated a significantly lower PNI than those in the non-occurrence group, along with elevated levels of CEA and CA242 (P<0.05). The area under the curve (AUC) for PNI, CEA, and CA242 were 0.827, 0.601, and 0.504, respectively, while the AUC for the combination was 0.829. As per the LASSO regression analysis, history of diabetes and PNI were identified as key factors correlating with anastomotic leakage (P<0.05). Employing the risk score formula, we obtained individual risk scores for each sample. Notably, risk scores in the occurrence group significantly surpassed those in the non-occurrence group (P<0.0001). The AUC for the risk score in predicting patient lung infection was 0.854. The internal verification C-index emerged as 0.863 (0.806-0.920), indicating a good model fit. Furthermore, the DeLong test revealed a significantly greater AUC of the risk model, compared to the combination and PNI (P<0.05). Conclusion: CEA and CA242 are not promising predictive indicators for anastomotic leakage after surgery in patients with gastric cancer, but the prediction model we established can improve the predictive efficiency of anastomotic leakage in these patients.
Keywords: Prognostic nutritional index (PNI), carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 242, anastomotic leakage after radical gastrectomy for gastric cancer
Introduction
Gastric cancer is a highly prevalent malignant tumor worldwide, with its incidence ranking second among all types of malignant tumors and its mortality ranking third in China [1]. Despite significant advancements in modern chemotherapy and radiotherapy, radical gastrectomy remains the primary treatment choice for advanced gastric cancer [2]. Early postoperative complications include bleeding, anastomotic leakage, and intestinal obstruction. Among these, anastomotic leakage is the most severe and requires significant attention, as it can result in a mortality of up to 30% [3].
Although treatment outcomes for anastomotic leakage have improved, the mortality associated with this complication still persists at around 15% [4]. Early diagnosis and intervention are critical in enhancing patients’ postoperative quality of life and reducing the economic burden and hospital stay, as well as in decreasing mortality from postoperative complications [5,6]. However, surgery provokes a robust inflammatory response, manifesting as fever, leukocytosis, rapid breathing, and fast heartbeat, and other syndromes. This complicates the prediction of anastomotic leakage via clinical symptoms [7,8] and impedes the implementation of early interventions.
While auxiliary examinations like CT and B-ultrasound, as well as abdominal examinations, can diagnose anastomotic leakage [9], there are often misdiagnosis and false negatives. With the evolution of various anastomotic devices and the prevalent use of high-quality sutures, the reliability of detecting postoperative anastomotic leakage has significantly improved, making immediate postoperative leakage a rarity. Furthermore, due to individual variability, the timing of postoperative anastomotic leakage can be different, typically occurring around the 6th day after surgery but potentially appearing as late as 2 weeks or even 1 month post-surgery [10,11]. Thus, effective biochemical markers are needed for the early screening and monitoring of anastomotic leakage.
The prognostic nutritional index (PNI) is a measure reflecting a patient’s nutritional and immune status. It was first proposed by Buzby et al. in 1980 to assess nutritional status and surgical risk [12,13]. Although some research suggests a connection between PNI and anastomotic leakage, uncertainties still exist. For instance, a study by A-Lai et al. [14] found that preoperative PNI held no significant prognostic value for short-term prognosis of patients with post-esophagectomy anastomotic leakage. There is limited evidence regarding the predictive value of PNI for anastomotic leakage in patients with gastric cancer.
Consequently, this study aimed to analyze the clinical value of PNI with carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 242 in the early prediction of anastomotic leakage after radical gastrectomy for gastric cancer, thereby providing a reference for clinical practice.
Methods and materials
Ethical statement
This study was approved by the Medical Ethics Committee of Gansu Provincial Hospital of Traditional Chinese Medicine (ethical lot number: 2022 (A) 25).
Sample source
We retrospectively collected the clinical data of 350 patients with gastric cancer who underwent radical gastrectomy in Gansu Provincial Hospital of Traditional Chinese Medicine from January 2018 to May 2022. See Figure 1 for the study flow chart.
Figure 1.

Flow chart of risk factors for anastomotic leakage in patients with gastric cancer.
Inclusion and exclusion criteria
Inclusion criteria: patients with complete medical records; patients who were diagnosed with gastric cancer by digestive endoscopic biopsy before operation; patients who underwent radical resection of gastric cancer with D2 lymph node dissection; for patients with anastomotic leakage, and the complication developed between postoperative day 6 and day 30.
Exclusion criteria: patients who required surgery due to emergencies such as gastric perforation or bleeding; patients with distant metastasis or local invasion of adjacent organs, detected by imaging examination or other means; patients who underwent preoperative radiotherapy or chemotherapy; patients with long-term use of immunosuppressants or non-steroidal anti-inflammatory drugs; patients who underwent combined resection of other organs such as part of the pancreas or spleen; patients who only had palliative surgery; patients who experienced other infectious complications after the operation; patients who underwent intraperitoneal hyperthermic perfusion chemotherapy after the operation.
Diagnostic methods
The diagnostic criteria for anastomotic leakage were as follows. Patients experienced symptoms such as abdominal pain, fever, and signs of abdominal infection. There was evidence of digestive fluid or cloudy fluid discharge from the drainage tube or abdominal wall incision. Patients were asked to use oral melanin to help the observation of leakage from the drainage tube or abdominal wall. X-ray imaging of the upper gastrointestinal tract was performed to confirm the external leak of the digestive tract and to determine the size and occurrence of the anastomotic leak. A CT examination was conducted to assess the conditions around the anastomosis, and to observe whether there was contrast agent leakage together with the gastrointestinal tract imaging. Color ultrasound examination was performed to detect fluid accumulation in the thoracic and abdominal cavity. If there was fluid accumulation, puncture under the guidance of color ultrasound was carried out to extract the digestive fluid or pus.
Sample screening
Based on the inclusion and exclusion criteria, we included 350 among 425 patients. Among the included patients, 34 patients had anastomotic leakage, with an incidence of 9.7%.
Collection of clinical data
The patients’ clinical data were collected from the electronic medical records, including age, sex, body mass index (BMI), smoking history, history of hypertension or diabetes, surgical method, surgical resection range, pathological stage, Lauren classification, and pathological diagnosis. Laboratory indicators included CEA, CA242, and PNI on the third postoperative day. PNI = serum albumin value (g/L) + 5 × lymphocyte total count in peripheral blood (× 109/L).
Observation indicators
Primary observation indicators were the expression and predictive value of CEA, CA242, and PNI in patients with anastomotic leakage.
Secondary observation indicators included the clinical data of the two groups, predictors of anastomotic leakage screened by LASSO regression analysis, and a risk model.
Statistical analysis
R language 4.1.1 software (R Foundation for Statistical Computing, Vienna, Austria) was used for data cleaning, data analysis, and model establishment. LASSO regression was used to screen predictive factors with non-zero coefficients, and the clinical value was verified by receiver operating characteristic (ROC) curve. The difference in the areas under the ROC curve was analyzed by Delong test. Graph Pad Prism 8.0 was used for data visualization. Measurement data were represented as (X±S) and analyzed by the t-test. Count data were expressed in percentage (%) and processed by chi-square test, representing by χ2. When P<0.05, the differences were considered statistically significant.
Results
Analysis of clinical data
Among the 350 included cases, 34 developed anastomotic leakage, with an incidence of 9.7%. Patients were subsequently divided into two groups: those with anastomotic leakage (occurrence group, n=34) and those without (non-occurrence group, n=316). Further analyses of the clinical data between the two groups revealed a significant higher proportion of patients with diabetes in the occurrence group compared to the non-occurrence group (P<0.05, see Table 1). However, no significant differences were found between the two groups in terms of age, sex, BMI, smoking history, and history of hypertension (P>0.05, see Table 1).
Table 1.
Comparison of the clinical data
| Variable | Occurrence group (n=34) | Non-occurrence group (n=316) | P value |
|---|---|---|---|
| Age | 0.355 | ||
| ≥60 years old | 19 | 205 | |
| <60 years old | 15 | 111 | |
| Sex | 0.577 | ||
| Male | 17 | 174 | |
| Female | 17 | 142 | |
| BMI | 0.492 | ||
| ≥25 kg/m^2 | 9 | 63 | |
| <25 kg/m^2 | 26 | 253 | |
| Smoking History | 0.266 | ||
| Present | 15 | 174 | |
| Absent | 19 | 142 | |
| History of Hypertension | 0.434 | ||
| Present | 13 | 142 | |
| Absent | 21 | 174 | |
| History of Diabetes | <0.0001 | ||
| Present | 16 | 57 | |
| Absent | 18 | 259 |
Note: BMI, Body mass index.
Analysis of surgical and pathological data
The occurrence group exhibited a higher number of patients with manual anastomosis, higher TNM staging, and intraoperative blood loss compared to the non-occurrence group (P<0.05, Table 2A). No statistical difference was found in the other variables, such as surgical method, resection range, tumor diameter, Lauren classification, and degree of differentiation (P>0.05, Table 2A).
Table 2A.
Analysis of surgical and pathological data
| Variable | Occurrence group (n=34) | Non-occurrence group (n=316) | P value |
|---|---|---|---|
| Surgical Method | 0.575 | ||
| Open Surgery | 16 | 133 | |
| Laparoscopic Surgery | 18 | 183 | |
| Extent of Resection | 0.611 | ||
| Proximal Gastrectomy | 1 | 16 | |
| Distal Gastrectomy | 26 | 253 | |
| Total Gastrectomy | 7 | 47 | |
| Anastomosis Method | 0.030 | ||
| Hand-sewn Anastomosis | 15 | 199 | |
| Stapler Anastomosis | 19 | 117 | |
| Tumor Diameter (mm) | 0.157 | ||
| ≥30 | 24 | 259 | |
| <30 | 10 | 57 | |
| TNM Staging | 0.014 | ||
| I | 4 | 92 | |
| II | 6 | 82 | |
| III | 24 | 142 | |
| Lauren Classification | 0.842 | ||
| Intestinal Type | 10 | 82 | |
| Diffuse Type | 4 | 47 | |
| Mixed Type | 20 | 186 | |
| Degree of Differentiation | 0.418 | ||
| Low-grade | 14 | 111 | |
| Medium, High-grade | 20 | 205 | |
| Intraoperative Blood Loss (mL) | 0.002 | ||
| ≥400 | 26 | 152 | |
| <400 | 9 | 164 |
Note: TNM, tumor node metastasis.
Expression of laboratory indicators and prediction of anastomotic leakage
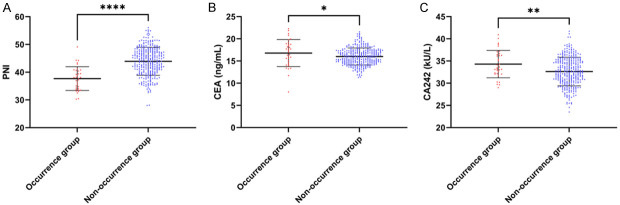
We scrutinized the levels of PNI, CEA, and CA242 on the 3rd day post-surgery in both the occurrence and non-occurrence groups. The results divulged that the PNI in the occurrence group was markedly lower than that in the non-occurrence group (P<0.0001, Figure 2). Concurrently, the levels of CEA and CA242 in the occurrence group were higher than those in the non-occurrence group (P<0.05, Figure 2). Further investigation using the ROC curve unveiled that the area under the curve (AUC) for PNI, CEA, and CA242 was 0.827, 0.601, and 0.504, respectively, and the AUC of the combination was 0.829 (Figure 3; Table 2B). Using the DeLong test, we then discovered that the AUC of the combination was significantly higher than that of CEA and CA242 alone (P<0.001, Figure 3A-D), but no significant difference was identified between the combination and PNI (P>0.05, Table 3).
Figure 2.
PNI, as well as CEA and CA242 levels on postoperative day 3. A. PNI; B. CEA; C. CA242. Note: PNI, Prognostic Nutritional Index; CEA, Carcinoembryonic Antigen; CA242, Carbohydrate Antigen 242, *P<0.05, **P<0.01, ****P<0.0001.
Figure 3.

AUC of PNI, CEA, and CA242 for predicting anastomotic leakage. A. AUC of PNI on postoperative day 3; B. AUC of CEA on postoperative day 3; C. AUC of CA242 on postoperative day 3; D. AUC of the combination of the 3 indicators. Note: PNI, Prognostic Nutritional Index; CEA, Carcinoembryonic Antigen; CA242, Carbohydrate Antigen 242; AUC, Area under the curve.
Table 2B.
ROC parameters
| Predictive Variable | Area under the curve | Confidence Interval | Cut-off Value | Sensitivity % | Specificity % | Youden’s Index |
|---|---|---|---|---|---|---|
| PNI | 0.827 | 0.758-0.895 | 40.87 | 73.42% | 82.35% | 55.77% |
| CEA | 0.601 | 0.480-0.723 | 17.65 | 81.65% | 47.06% | 28.70% |
| CA242 | 0.504 | 0.380-0.627 | 38.36 | 97.15% | 23.53% | 20.68% |
| Unite | 0.829 | 0.759-0.898 | 0.862 | 82.91% | 70.59% | 53.50% |
Note: ROC, receiver operating characteristic; PNI, Prognostic Nutritional Index; CEA, Carcinoembryonic Antigen; CA242, Carbohydrate Antigen 242.
Table 3.
Delong-test
| Test Results Comparison | Z value | P value | Area under curve difference | Standard error difference | Confidence Interval | |
|---|---|---|---|---|---|---|
|
| ||||||
| lower | upper | |||||
| PNI-CEA | -5.748 | <0.001 | -0.428 | 0.312 | -0.574 | -0.282 |
| PNI-CA242 | -4.879 | <0.001 | -0.33 | 0.312 | -0.463 | -0.198 |
| PNI-Unite | 0.134 | 0.893 | 0.002 | 0.261 | -0.027 | 0.031 |
| CEA-CA242 | 1.355 | 0.176 | 0.098 | 0.350 | -0.044 | 0.239 |
| CEA-Unite | 5.164 | <0.001 | 0.430 | 0.315 | 0.267 | 0.593 |
| CA242-Unite | 4.383 | <0.001 | 0.332 | 0.315 | 0.184 | 0.481 |
Note: PNI, Prognostic Nutritional Index; CEA, Carcinoembryonic Antigen; CA242, Carbohydrate Antigen 242.
Establishment of LASSO regression risk model
We employed LASSO regression to assess the factors for early prediction of anastomotic leakage after radical gastrectomy for gastric cancer. The LASSO regression analysis indicated that the history of diabetes and PNI were associated with anastomotic leakage (P<0.05). Based on this, we opted for lambda.1se (0.05409) for further analysis (Figure 4A, 4B). Utilizing lambda.1se, we devised a risk score formula: 0.493005278 + history of diabetes * 0.018411352 + PNI * (-0.009226992). Relying on the risk scoring formula, we calculated the risk score for each sample. Upon comparing these scores, we noticed that the risk score of the occurrence group was significantly higher than that of the non-occurrence group (P<0.0001, Figure 4C). The risk score also demonstrated its potential to predict anastomotic leakage in patients, with an AUC of 0.854 (Figure 4D). Additionally, we internally validated the nomogram model using the Bootstrap method (after repeated sampling of raw data 1,000 times). The results showed that the internal verification C-index was 0.863 (0.806-0.920), and the calibration curves fit well to the ideal curve (Figure 4E).
Figure 4.

Establishment of the LASSO regression risk model. A. Selection of the best lambda in the LASSO model. B. Plot of log(λ) versus error curve. C. Risk model scores for each patient. D. Area under the curve of the risk model in predicting anastomotic leakage in patients. E. Correction curve for predicting anastomotic leakage in patients. Note: LASSO, Least Absolute Shrinkage and Selection Operator, ****P<0.0001.
Comparison of the combined curve with the regression model curve
At the end of the study, we compared the ROC curve of the combination of PNI, CEA, and CA242 with the ROC curve of the risk model constructed by LASSO regression using the Delong test. The results showed that the AUC of the risk model was significantly greater than the combined curve and PNI (P<0.05, Table 4), indicating that the LASSO regression risk model had a higher predictive value for anastomotic leakage in patients.
Table 4.
Risk model Delong-test
| Test Results Comparison | Z value | P value | Area under curve difference | Standard error difference | Confidence Interval | |
|---|---|---|---|---|---|---|
|
| ||||||
| lower | upper | |||||
| PNI-Risk | 10.512 | <0.001 | 0.681 | 0.260 | 0.554 | 0.808 |
| Unite-Risk | 10.662 | <0.001 | 0.683 | 0.261 | 0.557 | 0.808 |
Note: PNI, Prognostic Nutritional Index.
Discussion
Gastric cancer not only holds a prominent position among common malignant tumors, but also acts as one of the primary catalysts for global cancer mortality [15]. The only curative treatment considered for gastric cancer is surgical resection, which is supplemented by lymph node dissection, including total gastrectomy [16], proximal gastrectomy, and distal gastrectomy. Following gastric cancer surgery, there is a risk of anastomotic leakage. Studies [17] have established that postoperative anastomotic leakage is an independent risk factor impacting the prognosis of the patient. For patients afflicted with anastomotic leakage, early detection and prompt treatment can markedly decrease the mortality. The majority of anastomotic leakages manifest within 5 to 7 days after the surgery, and the diagnosis primarily hinges on the patient’s clinical manifestations [18]. However, at this juncture, the anastomotic leakage would have already formed, and certain patients’ clinical manifestations might not be conspicuous, making it challenging to diagnose the anastomotic leakage solely based on clinical manifestations.
In this investigation, we conducted a retrospective analysis on gastric cancer patients who had undergone radical gastrectomy and D2 lymph node dissection. Previous research conducted by Kim et al. [18] revealed that out of 4916 gastric cancer patients who underwent gastrectomy, 115 patients (equating to 2.3%) developed anastomotic leakage. Another study by Shen et al. [19] reported 34 cases of anastomotic leakage (amounting to 13.13%) in a survey of 259 patients. Our statistics demonstrated an incidence of 9.7% in anastomotic leakage, which aligns with the findings of the above-mentioned research. Through detailed analysis of the clinical and pathological data, it was identified that the percentage of patients suffering from diabetes, manual anastomosis, high TNM staging, and intraoperative bleeding in the occurrence group was noticeably higher than those in the non-occurrence group. This suggests that diabetes, anastomosis method, TNM staging, and intraoperative blood loss were associated with the occurrence of anastomotic leakage in patients with gastric cancer.
In addition, we identified variations in PNI, CEA, and CA242 in patients with anastomotic leakage. Both CEA and CA242 are frequent tumor markers and are extensively utilized for cancer screening and monitoring [20]. These markers can be detected via blood or other biological samples to identify digestive system tumors [21]. This research did not find a direct correlation of CEA and CA242 with anastomotic leakage. It has been discovered that postoperative anastomotic leakage can trigger inflammatory responses and infections, which could potentially impact the levels of CEA and CA242 in the blood [22]. These tumor markers might escalate due to inflammation and infection, but it does not imply that CEA or CA242 itself caused the anastomotic leakage. Prior studies revealed that CEA and CA242 served as prognostic factors for gastric cancer patients, and anastomotic leakage could result in a poor prognosis for the patients [23,24]. Therefore, there might be an indirect relationship between CEA and CA242 and anastomotic leakage.
To further pinpoint the factors and predictive indicators that influence the anastomosis of gastric cancer patients, we deployed LASSO regression, which is a linear regression analysis method. The primary attribute of LASSO is that it contracts the coefficients of the model during the regression analysis process and can push certain coefficients to zero [25]. This feature makes LASSO regression an effective method for feature selection, allowing us to identify the most crucial predictive variables [26]. In our regression, we found that diabetes and PNI emerged as predictive factors for anastomotic leakage in gastric cancer patients. At the end of the study, we compared the predictive value of laboratory indicators with the risk model for anastomotic leakage. We discovered that the AUC for PNI predicting anastomotic leakage was substantially larger than that for other single laboratory indicators, and its predictive value was not different from the combination of PNI, CEA, and CA242. This indicates that the detection of CEA and CA242 does not enhance the predictive value of PNI for anastomotic leakage [27]. This also validates that the relationship between CEA and CA242 and anastomotic leakage was indirect. Nonetheless, we discovered that the AUC of the risk model was significantly greater than that of PNI prediction, signifying that the predictive value of the risk model for anastomotic leakage was superior to that of PNI [28].
This study still have its limitations. Firstly, we only recorded the occurrence of anastomotic leakage in patients within one month following surgery, but anastomotic leakage could take place after one month. The short follow-up time resulted in a small sample size in occurrence group, and whether it induces bias in data analysis requires verification. Secondly, as a single-center study, our sample size was limited. If we establish a training set, it could lead to a sharp decline in sample size. Therefore, the generalizability of the model requires more sample data to substantiate.
In conclusion, CEA and CA242 are not promising predictive indicators for anastomotic leakage after gastric cancer surgery, while the predictive model we established can improve the predictive efficiency of anastomotic leakage in gastric cancer patients.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Guerrini GP, Esposito G, Magistri P, Serra V, Guidetti C, Olivieri T, Catellani B, Assirati G, Ballarin R, Di Sandro S, Di Benedetto F. Robotic versus laparoscopic gastrectomy for gastric cancer: the largest meta-analysis. Int J Surg. 2020;82:210–228. doi: 10.1016/j.ijsu.2020.07.053. [DOI] [PubMed] [Google Scholar]
- 3.Tsou CC, Lo SS, Fang WL, Wu CW, Chen JH, Hsieh MC, Shen KH. Risk factors and management of anastomotic leakage after radical gastrectomy for gastric cancer. Hepatogastroenterology. 2011;58:218–223. [PubMed] [Google Scholar]
- 4.Ichikawa D, Kurioka H, Yamaguchi T, Koike H, Okamoto K, Otsuji E, Shirono K, Shioaki Y, Ikeda E, Mutoh F, Yamagishi H. Postoperative complications following gastrectomy for gastric cancer during the last decade. Hepatogastroenterology. 2004;51:613–617. [PubMed] [Google Scholar]
- 5.Hayashi M, Yoshikawa T, Yura M, Otsuki S, Yamagata Y, Morita S, Katai H, Nishida T. Predictive value of the surgical Apgar score on postoperative complications in advanced gastric cancer patients treated with neoadjuvant chemotherapy followed by radical gastrectomy: a single-center retrospective study. BMC Surg. 2020;20:150. doi: 10.1186/s12893-020-00813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo HM, Lee HH, Shim JH, Jeon HM, Park CH, Song KY. Negative impact of leakage on survival of patients undergoing curative resection for advanced gastric cancer. J Surg Oncol. 2011;104:734–740. doi: 10.1002/jso.22045. [DOI] [PubMed] [Google Scholar]
- 7.Matsui R, Inaki N, Tsuji T, Fukunaga T. Relationship between fat mass indices and postoperative complications after laparoscopic gastrectomy in patients with gastric cancer: a propensity score matching analysis. Anticancer Res. 2022;42:4841–4848. doi: 10.21873/anticanres.15989. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Q, Li X, Duan B, Li X, Liu S, Xu B, Shi S, Zhang J, Qin H, Duan X, Pu Y. Clinical significance of controlling nutritional status score (CONUT) in evaluating outcome of postoperative patients with gastric cancer. Sci Rep. 2022;12:93. doi: 10.1038/s41598-021-04128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang GN, Lu JY, Xu L, Sun XY, Xiao Y. Overlap gastroduodenostomy in totally laparoscopic distal gastrectomy for gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2019;22:1064–1069. doi: 10.3760/cma.j.issn.1671-0274.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Zhao S, Zhang L, Gao F, Wu M, Zheng J, Bai L, Li F, Liu B, Pan Z, Liu J, Du K, Zhou X, Li C, Zhang A, Pu Z, Li Y, Feng B, Tong W. Transanal drainage tube use for preventing anastomotic leakage after laparoscopic low anterior resection in patients with rectal cancer: a randomized clinical trial. JAMA Surg. 2021;156:1151–1158. doi: 10.1001/jamasurg.2021.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makuuchi R, Irino T, Tanizawa Y, Bando E, Kawamura T, Terashima M. Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg Today. 2019;49:187–196. doi: 10.1007/s00595-018-1726-8. [DOI] [PubMed] [Google Scholar]
- 12.Ding P, Guo H, Sun C, Yang P, Kim NH, Tian Y, Liu Y, Liu P, Li Y, Zhao Q. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol. 2022;22:121. doi: 10.1186/s12876-022-02199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271:693–700. doi: 10.1097/SLA.0000000000002985. [DOI] [PubMed] [Google Scholar]
- 14.A-Lai GH, Deng HY, Song TN, Luo J, Zhuo ZG, Shen X, Lin YD. Preoperative prognostic nutritional index shows no significant prognostic value for short-term outcomes of anastomosis-leakage patients after cancerous esophagectomy. Ann Palliat Med. 2019;8:698–707. doi: 10.21037/apm.2019.11.08. [DOI] [PubMed] [Google Scholar]
- 15.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 16.Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21:4012. doi: 10.3390/ijms21114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koedam TWA, Bootsma BT, Deijen CL, van de Brug T, Kazemier G, Cuesta MA, Furst A, Lacy AM, Haglind E, Tuynman JB, Daams F, Bonjer HJ COLOR COLOR II study group. Oncological outcomes after anastomotic leakage after surgery for colon or rectal cancer: increased risk of local recurrence. Ann Surg. 2022;275:e420–e427. doi: 10.1097/SLA.0000000000003889. [DOI] [PubMed] [Google Scholar]
- 18.Kim YI, Lee JY, Khalayleh H, Kim CG, Yoon HM, Kim SJ, Yang H, Ryu KW, Choi IJ, Kim YW. Efficacy of endoscopic management for anastomotic leakage after gastrectomy in patients with gastric cancer. Surg Endosc. 2022;36:2896–2905. doi: 10.1007/s00464-021-08582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Y, Wang H, Feng M, Tan L, Wang Q. The effect of narrowed gastric conduits on anastomotic leakage following minimally invasive oesophagectomy. Interact Cardiovasc Thorac Surg. 2014;19:263–268. doi: 10.1093/icvts/ivu151. [DOI] [PubMed] [Google Scholar]
- 20.Bjorkman K, Mustonen H, Kaprio T, Kekki H, Pettersson K, Haglund C, Bockelman C. CA125: a superior prognostic biomarker for colorectal cancer compared to CEA, CA19-9 or CA242. Tumour Biol. 2021;43:57–70. doi: 10.3233/TUB-200069. [DOI] [PubMed] [Google Scholar]
- 21.Rao H, Wu H, Huang Q, Yu Z, Zhong Z. Clinical value of serum CEA, CA24-2 and CA19-9 in patients with colorectal cancer. Clin Lab. 2021;67 doi: 10.7754/Clin.Lab.2020.200828. [DOI] [PubMed] [Google Scholar]
- 22.Schietroma M, Romano L, Schiavi D, Pessia B, Mattei A, Fiasca F, Carlei F, Giuliani A. Systemic inflammation response index (SIRI) as predictor of anastomotic leakage after total gastrectomy for gastric cancer. Surg Oncol. 2022;43:101791. doi: 10.1016/j.suronc.2022.101791. [DOI] [PubMed] [Google Scholar]
- 23.Jing JX, Wang Y, Xu XQ, Sun T, Tian BG, Du LL, Zhao XW, Han CZ. Tumor markers for diagnosis, monitoring of recurrence and prognosis in patients with upper gastrointestinal tract cancer. Asian Pac J Cancer Prev. 2014;15:10267–10272. doi: 10.7314/apjcp.2014.15.23.10267. [DOI] [PubMed] [Google Scholar]
- 24.Tian SB, Yu JC, Kang WM, Ma ZQ, Ye X, Cao ZJ, Yan C. Combined detection of CEA, CA19-9, CA242 and CA50 in the diagnosis and prognosis of resectable gastric cancer. Asian Pac J Cancer Prev. 2014;15:6295–6300. doi: 10.7314/apjcp.2014.15.15.6295. [DOI] [PubMed] [Google Scholar]
- 25.Tang G, Qi L, Sun Z, Liu J, Lv Z, Chen L, Huang B, Zhu S, Liu Y, Li Y. Evaluation and analysis of incidence and risk factors of lower extremity venous thrombosis after urologic surgeries: a prospective two-center cohort study using LASSO-logistic regression. Int J Surg. 2021;89:105948. doi: 10.1016/j.ijsu.2021.105948. [DOI] [PubMed] [Google Scholar]
- 26.McEligot AJ, Poynor V, Sharma R, Panangadan A. Logistic LASSO regression for dietary intakes and breast cancer. Nutrients. 2020;12:2652. doi: 10.3390/nu12092652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li SJ, Wang ZQ, Li YJ, Fan J, Zhang WB, Che GW, Liu LX, Chen LQ. Diabetes mellitus and risk of anastomotic leakage after esophagectomy: a systematic review and meta-analysis. Dis Esophagus. 2017;30:1–12. doi: 10.1093/dote/dox006. [DOI] [PubMed] [Google Scholar]
- 28.Yu WQ, Gao HJ, Shi GD, Tang JY, Wang HF, Hu SY, Wei YC. Development and validation of a nomogram to predict anastomotic leakage after esophagectomy for esophageal carcinoma. J Thorac Dis. 2021;13:3549–3565. doi: 10.21037/jtd-21-209. [DOI] [PMC free article] [PubMed] [Google Scholar]